Abstract
BACKGROUND
Fibromyalgia (FM) syndrome is mainly characterized by widespread pain, sleeping disorders, fatigue, and cognitive dysfunction. In many cases, gastrointestinal distress is also reported, suggesting the potential pathogenic role of the gut microbiota (GM). The GM is deeply influenced by several environmental factors, especially the diet, and recent findings highlighted significant symptom improvement in FM patients following various nutritional interventions such as vegetarian diet, low-fermentable oligosaccharides, disaccharides, monosaccharides, and polyols based diets, gluten-free diet, and especially an ancient grain supplementation. In particular, a recent study reported that a replacement diet with ancient Khorasan wheat led to an overall improvement in symptom severity of FM patients.
AIM
To examine the effects of ancient Khorasan wheat on the GM, inflammation, and short-chain fatty acid production in FM patients.
METHODS
After a 2-wk run-in period, 20 FM patients were enrolled in this randomized, double-blind crossover trial. In detail, they were assigned to consume either Khorasan or control wheat products for 8 wk and then, following an 8-wk washout period, crossed. Before and after treatments, GM characterization was performed by 16S rRNA sequencing while the fecal molecular inflammatory response and the short-chain fatty acids (SCFAs) were respectively determined with the Luminex MAGPIX detection system and a mass chromatography-mass spectrometry method.
RESULTS
The Khorasan wheat replacement diet, in comparison with the control wheat diet, had more positive effects on intestinal microbiota composition and on both the fecal immune and SCFAs profiles such as the significant increase of butyric acid levels (P = 0.054), candidatus Saccharibacteria (P = 9.95e-06) and Actinobacteria, and the reduction of Enterococcaceae (P = 4.97e-04). Moreover, the improvement of various FM symptoms along with the variation of some gut bacteria after the Khorasan wheat diet have been documented; in fact we reported positive correlations between Actinobacteria and both Tiredness Symptoms Scale (P < 0.001) and Functional Outcome of Sleep Questionnaire (P < 0.05) scores, between Verrucomicrobiae and both Widespread Pain Index (WPI) + Symptom Severity scale (SS) (P < 0.05) and WPI (P < 0.05) scores, between candidatus Saccharibacteria and SS score (P < 0.05), and between Bacteroidales and Sleep-Related and Safety Behaviour Questionnaire score (P < 0.05).
CONCLUSION
The replacement diet based on ancient Khorasan wheat results in beneficial GM compositional and functional modifications that positively correlate with an improvement of FM symptomatology.
Keywords: Fibromialgya, Gut microbiota, Khorasan wheat, Fibromialgya symptoms, Ancient wheat
Core Tip: Fibromyalgia (FM) syndrome is mainly characterized by widespread pain but in many cases, gastrointestinal distress is also reported, suggesting the potential pathogenic role of the intestinal microbiota. Since gut microbiota is deeply influenced by diet, significant symptom improvement has been reported in FM patients following various nutritional interventions such as vegetarian diet, low-fermentable oligosaccharides, disaccharides, monosaccharides, and polyols based diets, gluten-free diet, and especially an ancient grain based diet. We reported that a replacement diet based on ancient Khorasan wheat, compared to a control wheat diet, resulted in beneficial gut microbiota compositional and functional modifications that positively correlated with an improvement of FM symptomatology.
INTRODUCTION
Fibromyalgia (FM) is a systemic syndrome of unclear etiology, characterized by widespread pain and tenderness, sleeping disorders, fatigue, and cognitive dysfunction[1]. It has been hypothesized that low-grade systemic inflammation, a preponderance of pro-oxidative state, and an insufficient antioxidant capability – as demonstrated by the low levels of some minerals and vitamins in FM patients – could contribute to the disease development, by reducing the pain threshold and inducing fatigue and mood disorders[2]. In many cases, gastrointestinal distress is also reported, suggesting a potential involvement of the gut microbiota (GM), as demonstrated by the rather frequent dysbiosis found in FM subjects[3,4].
The GM is a complex ecosystem composed of hundreds of thousands of microorganisms involved in various useful functions that range from the host organism’s defense to homeostasis maintenance and the metabolite production, such as short-chain fatty acids (SCFAs) that show anti-inflammatory properties[5]. The GM is deeply influenced by several environmental factors, especially the diet, which seems to be involved also in the FM syndrome. Recent evidence documented a significant symptom improvement in FM patients following various nutritional interventions such as vegetarian diet, low-fermentable oligosaccharides, disaccharides, monosaccharides, and polyols based diets or gluten-free diet[6].
In recent years, the potential health benefits of ancient grains have emerged and we have shown that the consumption of products made with Triticum turgidum ssp. turanicum (Khorasan) improves both the inflammatory profile and the gastrointestinal symptoms in different patient groups[7]. Furthermore, our recent study demonstrated that a replacement diet with ancient Khorasan grain led to an overall improvement in symptom severity and sleep pattern of FM patients[8]. However, as far as we know, no studies are available to evaluate the possible beneficial effects of ancient grain consumption on the GM of FM patients. Therefore, the aim of this present study was to examine whether a replacement diet with ancient Khorasan wheat could influence the GM composition, the fecal molecular immune profile, and SCFAs production in patients suffering of fibromyalgia syndrome.
MATERIALS AND METHODS
Study design and participants
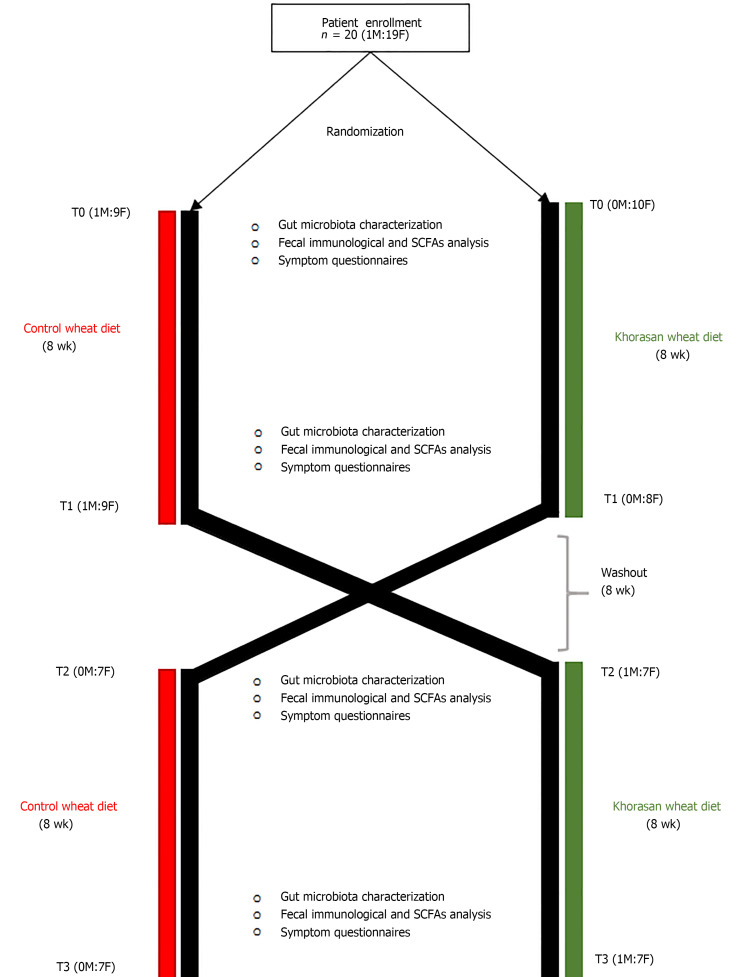
Participants were considered eligible for inclusion in this randomized, double-blind crossover trial (Figure 1) if they were aged 18-70 years and had a documented diagnosis of FM. The exclusion criteria were as follows: celiac disease, gluten sensitivity (assessed on the basis of medical evaluation, taking into account both the negative results of blood tests for celiac disease and the absence of celiac symptoms during a diet containing gluten) or wheat allergy, any known organic disease or clinical alarm signs, pregnancy or breast-feeding, and use of probiotics or antibiotics in the past 2 mo.
Figure 1.
Workflow of the crossover intervention study.
The experimental wheat used in this study was organic Khorasan wheat (Triticum turgidum subsp. Turanicum), KAMUT brand, supplied by Kamut Enterprises of Europe. KAMUT®, a registered trademark of Kamut International, Ltd., and Kamut Enterprises of Europe, bv, guarantees that the wheat is pure ancient Khorasan wheat and is organically grown and processed. The organically cultivated modern variety named "palesio" was used as control wheat.
After a 2-wk run-in period, eligible participants were randomly assigned to consume either Khorasan or control wheat products (500 g/wk of pasta, 150 g/d of bread, 500 g/mo of crackers, and 1 kg/mo of biscuits) for 8 wk and then, following an 8-wk washout period, crossed.
During both intervention periods, all participants were encouraged to maintain their usual eating habits, but they were not allowed to eat other cereal-based products. Instead, during the washout period participants were allowed to eat all foods according to their usual eating habits. In the second intervention period, the group assigned to consume the control grain products in the first intervention period was assigned to consume the Khorasan products, and vice versa.
At the beginning and at the end of each intervention period, we collected stool samples, in order to perform GM characterization, SCFAs evaluation, and immunological analysis. The following validated self-administered questionnaires were used for evaluation: Widespread Pain Index (WPI), Symptom Severity scale (SS), FM severity scale (WPI + SS), FM Impact Questionnaire (FIQ), Fatigue Severity Scale (FSS), Tiredness Symptoms Scale (TSS), Sleep-Related and Safety Behaviour Questionnaire (SRSBQ), Restorative Sleep Questionnaire-Daily, and Functional Outcome of Sleep Questionnaire (FOSQ).
In general, as previously reported[8], both interventions resulted in an amelioration of all questionnaires scores; however, a statistically significant decrease in WPI + SS, FOSQ, and FIQ scores was observed only after the Khorasan diet.
The study was approved by the local Ethics Committee “Comitato Etico Regionale per la Sperimentazione Clinica della Regione Toscana, Sezione AREA VASTA CENTRO” (SPE 12.630), and adhered to the principles of the Declaration of Helsinki and the Data Protection Act.
Microbiota characterization
Total DNA was extracted using the DNeasy PowerLyzer PowerSoil Kit (Qiagen, Hilden, Germany) from frozen (-80 °C) stool samples, according to the manufacturer’s instructions. Briefly, 0.25 g of stool samples were added to a bead beating tube and homogenized with TissueLyser II for 5 min at 30 Hz. Genomic DNA was captured on a silica membrane in a spin column format, washed, and subsequently eluted. The quality and quantity of extracted DNA were assessed with a Qubit Fluorometer (Thermo Fisher Scientific, Waltham, United States) and then frozen at -20 °C.
Subsequently, DNA samples were sent to IGA Technology Services (Udine, Italy) where amplicons of the variable V3-V4 region of the bacterial 16S rRNA gene were sequenced by producing paired-end reads (2 × 300 cycles) on the Illumina MiSeq platform, according to the Illumina 16S Metagenomic Sequencing Library Preparation protocol.
Afterwards, the raw data were processed following the software pipeline MICCA (MICrobial Community Analysis) as we previously described[9].
Briefly, paired end reads were assembled, maintaining a minimum overlap of 20 bp and an edit distance in the maximum overlap of 2 bp. Next, the sequences were cut to remove the primers and all the reads having a length lower than 350 bp and with an error rate higher than or equal to 0.5 were removed. The cleaned reads were merged into a single file and transformed into a FASTA file. The operational taxonomic units (OTUs) were generated by setting a 97% identity threshold and performing an automatic removal of chimeras and so the longest sequence of each OTU was used for the taxonomic assignment, i.e., using the Ribosomal Database Project classifier that is able to obtain classification and confidence for taxonomic ranks up to genus.
SCFA determination by gas chromatography-mass spectrometry analysis
The qualitative and quantitative evaluation of fecal SCFAs was performed using the Agilent gas chromatography-mass spectrometry (GC-MS) system composed of a 5971 single quadrupole mass spectrometer, a 5890 gas-chromatograph, and a 7673 autosampler, through our previously described GC-MS method[10].
Just before the analysis, fecal samples were thawed and combined with 10 mM sodium bicarbonate solution (1:1 w/v) in a 1.5 mL centrifuge tube. Then, the obtained suspension was sonicated for 5 min and centrifuged at 5000 rpm for 10 min, and then the supernatant was collected. Finally, SCFAs were extracted as follows: an aliquot of 100 µL of sample solution (corresponding to 0.1 mg of stool sample) was added to 50 μL of internal standards mixture, 1 mL of tert-butyl methyl ether, and 50 µL of 1.0 M HCl solution in a 1.5 mL centrifuge tube. Subsequently, each tube was shaken in a vortex apparatus for 2 min and centrifuged at 10000 rpm for 5 min, and finally the solvent layer was transferred to an autosampler vial and analyzed three times.
Evaluation of fecal molecular inflammatory response
Fecal samples were collected into 1.5 mL centrifuge tubes, added with phosphate-buffer saline w/o calcium w/o magnesium (1:2 w/v), stirred in a vortex apparatus for 2 min, sonicated for 5 min, and then centrifuged at 14000 rpm for 5 min. The supernatants were then transferred in 1.5 mL centrifuge tubes and stored at -20 °C.
The inflammatory response was evaluated through Milliplex custom kit Human Cytokines panel A for Luminex MAGPIX detection system (Affymetrix, eBioscience), following the manufacturers' instructions for supernatants.
In detail, the levels of interleukin (IL)-1α, IL-1β, IL-17A, IL-4, and interferon (IFN)-γ were assessed. Cytokine levels were estimated using a 5-parameter polynomial curve (ProcartaPlex Analyst 1.0) and values under the lower limit of quantitation were considered as 0 pg/mL.
Statistical analysis
Parametric variables are expressed as the mean ± SD whereas non parametric continuous variables are presented as the median and interquartile range. Statistical analyses on the bacterial communities were performed in R using the packages phyloseq 1.26.1, DESeq2 1.22.2, and other packages satisfying their dependencies, in particular, vegan 2.5-5. For the cluster analysis of the entire community, the OTU table was first normalized using the total OTU counts of each sample and then adjusted using square root transformation.
The differential analysis of abundance at the OTUs as well as at different taxonomic ranks (created using the tax_glom function in phyloseq) was performed with DESeq2 using the paired Wilcoxon signed-rank test to compare pre- and post- intervention data. Spearman correlations were performed in R with the package Hmisc 4.5.0, while heatmaps were prepared using the package ggplot2 3.3.5.
Furthermore, GraphPad Prism (v.5) was used for paired statistical analysis of both the fecal molecular inflammatory profile and the SCFA levels before and after each diet.
RESULTS
Characteristics of the study population
Twenty FM patients (1 male and 19 females) with a mean age of 48.9 ± 12.3 years and a mean BMI of 25.30 ± 4.67 kg/m2 provided written informed consent and were enrolled in this randomized double-blind crossover trial.
As showed in Figure 1, after randomization, ten patients (1M:9F) with a mean age of 51.70 ± 12.93 years and a mean BMI of 25.66 ± 5.59 kg/m2 were assigned to consume control wheat products while the other ten patients (0M:10F) with a mean age of 46.20 ± 11.52 years and a mean BMI of 24.95 ± 3.81 kg/m2 were assigned to consume Khorasan wheat products. Subsequently, after the washout period, the intervention groups were crossed and, because of the retirement of five subjects, seven patients (0M:7F) consumed control wheat products and eight patients (1M:7F) consumed Khorasan wheat products.
Effects of dietary interventions on gut microbiota composition
First of all, aiming to examine the impact of both a control wheat diet (CD) [pre- (CD0) and post- (CD1)] and Khorasan wheat diet (KD) [pre- (KD0) and post- (KD1)] on the GM composition, we performed the analysis of alpha diversity; however, no significant differences were reported for Shannon index, Chao1 index, or Evenness (data not shown).
Taxonomic analysis, detailed in Table 1, revealed the presence of 14 phyla (> 99% reads), 24 classes (> 99% reads), 35 orders (> 98% of reads), 60 families (> 94% reads), and 142 genera (> 84% reads).
Table 1.
Summary of taxonomic analysis of obtained operational taxonomic units
|
Rank
|
Count
|
Reads
|
Reads %
|
OTU
|
OTU %
|
| Phylum | 14 | 1842915 | 99.78942 | 946 | 94.97992 |
| Class | 24 | 1828920 | 99.03162 | 911 | 91.46586 |
| Order | 35 | 1826444 | 98.89755 | 903 | 90.66265 |
| Family | 60 | 1745260 | 94.50164 | 773 | 77.61044 |
| Genus | 142 | 1568830 | 84.94838 | 447 | 44.87952 |
OTU: Operational taxonomic unit.
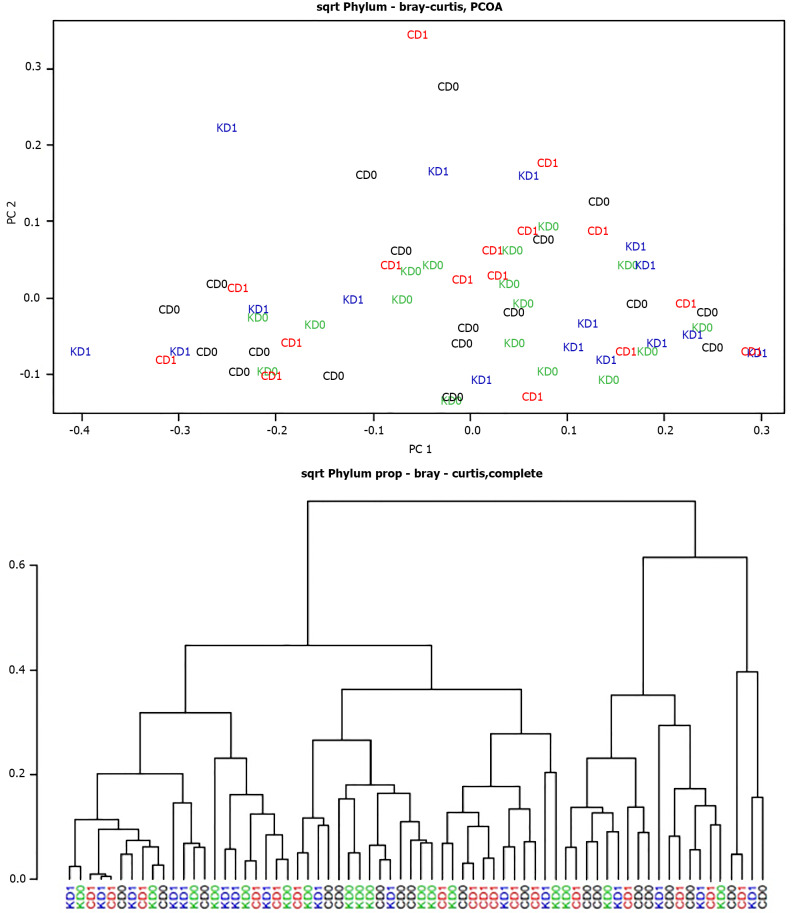
Furthermore, the cluster analysis and the principal coordinate analysis computed using the Bray-Curtis dissimilarity metric did not highlight any clear separation between the pre- and post-CD groups or the pre- and post-KD groups at the phylum level (Figure 2), neither to other taxonomic ranks.
Figure 2.
Cluster analysis and principal coordinate analysis of pre- and post-control-wheat-diet samples and pre- and post-Khorasan-wheat-diet samples. A: Cluster analysis; B: Principal coordinate analysis. CD0: Pre-control wheat diet; CD1: Post-control wheat diet; KD0: Pre-Khorasan wheat diet; KD1: Post-Khorasan wheat diet.
Subsequently, in order to evaluate the impact of both the dietary interventions on microbial abundances, we performed differential analysis at all taxonomic ranks.
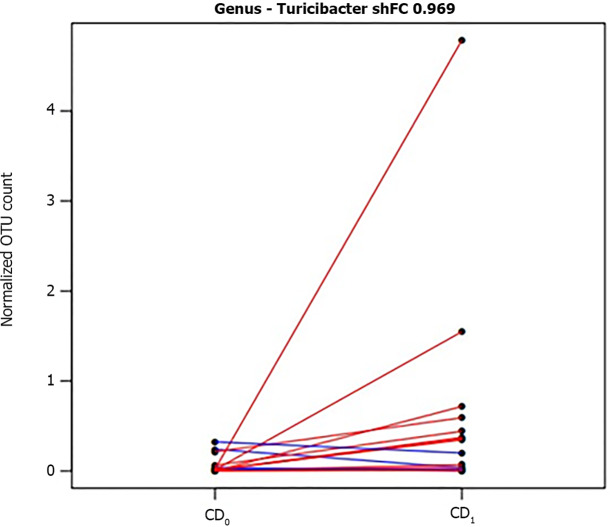
In detail, among taxonomic levels, no significant changes were observed except for genus level; in particular, as shown in Figure 3, CD resulted in a significant increase in the abundance of Turicibacter spp. (shrinked FC = 0.969, P = 0.014).
Figure 3.
Segment plot showing significantly different taxa between pre- and post-control diet. Lines link paired samples and red or blue colors respectively highlight the increase or decrease of normalized abundance for the indicated rank. Plot title reports shrinked fold change (according to the DESeq2 function lfcShrink) between pre- and post-control-wheat-diet samples. CD0: Pre-control wheat diet; CD1: Post-control wheat diet.
Evaluation of fecal SCFAs profiles before and after dietary interventions
We analyzed the abundances of fecal SCFAs (acetic, propionic, butyric, isobutyric, isovaleric 2-methylbutyric, and valeric acids) before and after both diets and no influence on their amounts was found after CD or KD (Table 2).
Table 2.
Short-chain fatty acid variations according to the provided diet
|
SCFAs (µmol/g)
|
CD0
|
CD1
|
P
value
|
KD0
|
KD1
|
P
value
|
| Acetic acid | 24.04 ± 19.34 | 27.21 ± 20.69 | 0.463 | 24.07 ± 19.75 | 26.77 ± 14.91 | 0.431 |
| Propionic acid | 6.81 ± 4.86 | 6.68 ± 5.16 | 0.939 | 6.02 ± 6.31 | 8.43 ± 5.73 | 0.891 |
| Butyric acid | 4.49 ± 9.51 | 6.15 ± 6.67 | 0.375 | 4.29 ± 6.61 | 5.43 ± 8.34 | 0.403 |
| Isobutyric acid | 0.91 ± 0.50 | 0.84 ± 0.91 | 0.82 | 0.87 ± 0.88 | 0.90 ± 0.51 | 0.547 |
| 2-Methylbutyric acid | 0.62 ± 0.39 | 0.54 ± 0.65 | 0.899 | 0.41 ± 0.52 | 0.48 ± 0.34 | 0.174 |
| Isovaleric acid | 0.76 ± 0.32 | 0.69 ± 0.80 | 0.705 | 0.58 ± 0.61 | 0.57 ± 0.29 | 0.579 |
| Valeric acid | 0.94 ± 0.84 | 1.31 ± 1.07 | 0.82 | 0.52 ± 1.69 | 1.18 ± 1.49 | 0.352 |
| Total | 38.53 ± 33.26 | 44.35 ± 40.98 | 0.596 | 45.32 ± 29.69 | 44.62 ± 33.69 | 0.403 |
Data are presented as the median (interquartile range). P values were calculated using the Wilcoxon signed-rank test. CD0: Pre-control wheat diet; CD1: Post-control wheat diet; KD0: Pre-Khorasan wheat diet; KD1: Post-Khorasan wheat diet.
Since these analyses could be in part influenced by the total amount of each SCFA, we repeated the same comparisons on the SCFA percentage compositions and no statistically significant differences were found.
Analysis of fecal molecular inflammatory profile before and after dietary interventions
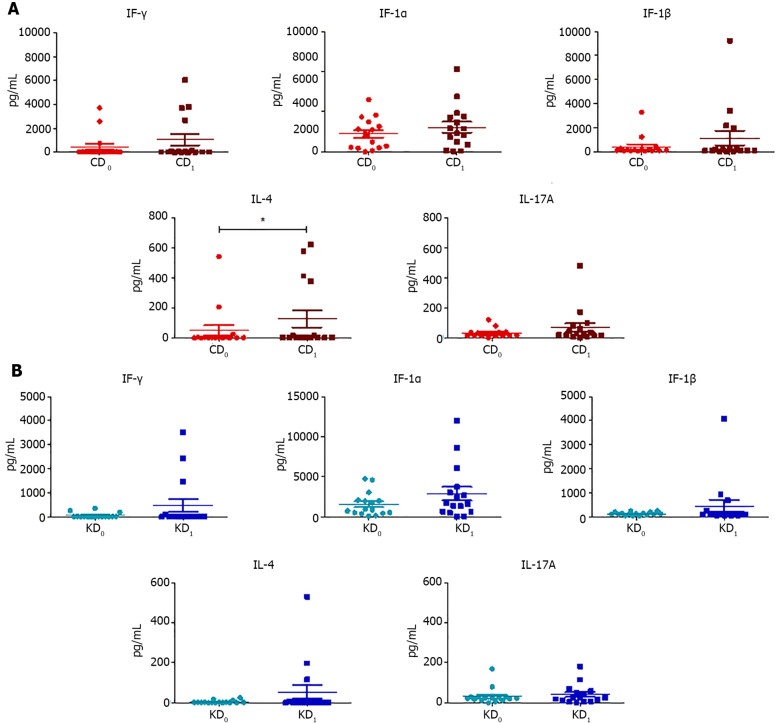
We evaluated the levels of fecal cytokines (IFN-γ, IL-1α, IL-1β, IL-4, and IL-17A) before and after both dietary interventions (Table 3) and we found that CD only resulted in a significant increase in IL-4 (P = 0.041) while no statistical differences were found after the KD (Figure 4).
Table 3.
Interleukins variations according to the provided diet
|
ILs (pg/mL) |
CD0
|
CD1
|
P
value
|
KD0
|
KD1
|
P
value
|
| IFN-γ | 19.00 ± 28.35 | 22.67 ± 125.37 | 0.141 | 15.22 ± 10.43 | 15.76 ± 29.06 | 0.205 |
| IL-1α | 1958.84 ± 2650.46 | 2291.12 ± 3412.85 | 0.171 | 933.79 ± 1516.38 | 1683.52 ± 2538.99 | 0.187 |
| IL-1β | 136.84 ± 123.32 | 167.83 ± 157.13 | 0.171 | 127.99 ± 68.04 | 123.09 ± 100.29 | 0.485 |
| IL-4 | 2.39 ± 5.31 | 2.64 ± 11.61 | 0.041 | 2.49 ± 1.76 | 2.04 ± 3.77 | 0.205 |
| IL-17A | 17.36 ± 20.39 | 29.67 ± 50.38 | 0.155 | 23.67 ± 12.83 | 26.03 ± 41.11 | 0.979 |
Data are showed as the median (interquartile range). P values were calculated using the Wilcoxon signed-rank test. IFN: Interferon; IL: Interleukin; CD0: Pre-control wheat diet; CD1: Post-control wheat diet; KD0: Pre-Khorasan wheat diet; KD1: Post-Khorasan wheat diet.
Figure 4.
Scatter plots representing the fecal concentrations of interferon-γ, interleukin-1α, interleukin-1β, interleukin-4, and interleukin-17A evaluated before and after control wheat diet and before and after Khorasan wheat diet. A: Before and after control wheat diet; B: Before and after Khorasan wheat diet. Analyses were assessed using the paired Wilcoxon signed-rank test and P values less than 0.05 were considered statistically significant. aP < 0.05, bP < 0.01, cP < 0.001. CD0: Pre-control wheat diet; CD1: Post-control wheat diet; KD0: Pre-Khorasan wheat diet; KD1: Post-Khorasan wheat diet; IL: Interleukin.
Evaluation of impact of dietary interventions on gut microbiota excluding the washout period
Finally, in order to evaluate the impact of both dietary interventions on GM composition excluding eventual “carry-over effects”, we focused on each single arm of both KD and CD.
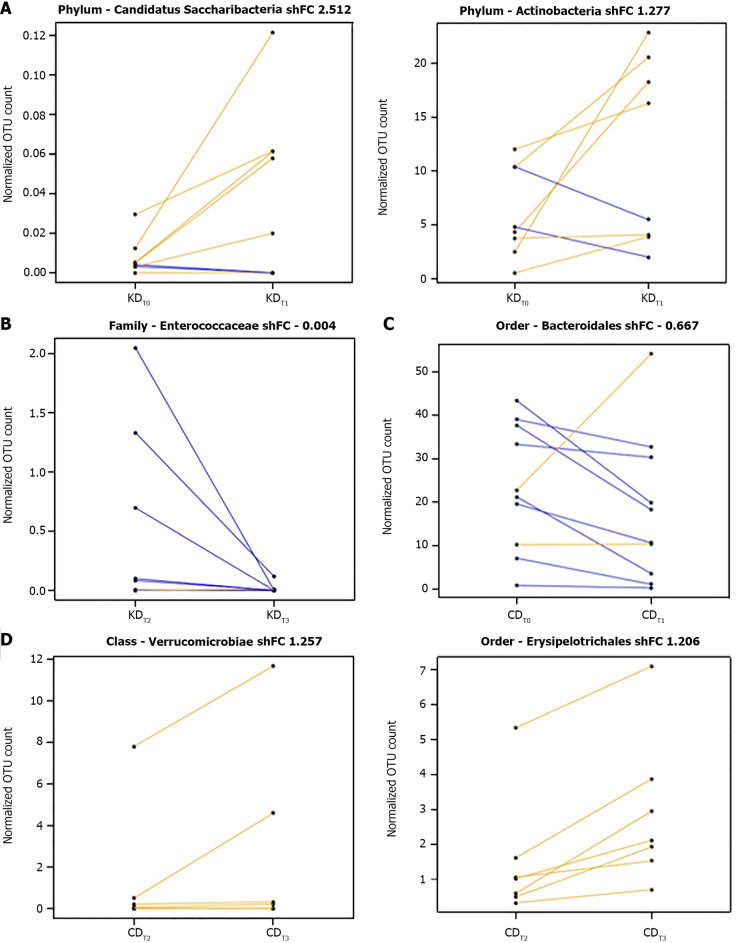
In particular, at the end of the KD first arm (KDT1), we found a significant increase in members of the phyla candidatus Saccharibacteria (log2FC = 2.679, P = 9.95e-06) and Actinobacteria (log2FC = 1.384, P = 9.63e-05) (Figure 5A), while at the end of the KD second arm (KDT3), a reduction of Enterococcaceae (log2FC = -4.829, P = 4.97e-04) was reported (Figure 5B).
Figure 5.
Segment plots showing significantly different taxa during the first arm or the second arm of the Khorasan wheat intervention and over the first arm or the second arm of the control wheat intervention. A: The first arm of the Khorasan wheat intervention; B: The second arm of the Khorasan wheat intervention; C: The first arm of the control wheat intervention; D: The second arm of the control wheat intervention. Lines link paired samples and yellow or blue colors respectively highlight the increase or decrease of normalized abundance for the indicated rank. Plot title reports Shrinked fold change (according to the DESeq2 function lfcShrink) between pre- and post-diet groups included in the two arms of either Khorasan wheat or control wheat interventions. CDT0: Pre-control-wheat-diet first arm; CDT1: Post-control-wheat-diet first arm; CDT2: Pre-control-wheat-diet second arm; CDT3: Post-control-wheat-diet second arm; KDT0: Pre-Khorasan-wheat-diet first arm; KDT1: Post-Khorasan-wheat-diet first arm; KDT2: Pre-Khorasan-wheat-diet second arm; KDT3: Post-Khorasan-wheat-diet second arm.
On the other hand, at the end of the CD first arm (CDT1), we reported a significant reduction of Bacteroidales members (log2FC = -0.916, P = 0.002) (Figure 5C) while the CD second arm (CDT3) resulted in significantly higher levels of the order Erysipelotrichales (log2FC = 1.342, P = 2.03e-5) and the class Verrucomicrobiae (log2FC = 1.975, P = 0.005) (Figure 5D).
Fecal SCFA assessment excluding the washout period
The analysis of the fecal SCFAs concentrations displayed an almost significant increase of butyric acid levels (P = 0.054) (Figure 6) at the end of the KDT1, while no differences were reported after the KDT3 and after both the CDT1 and CDT3.
Figure 6.
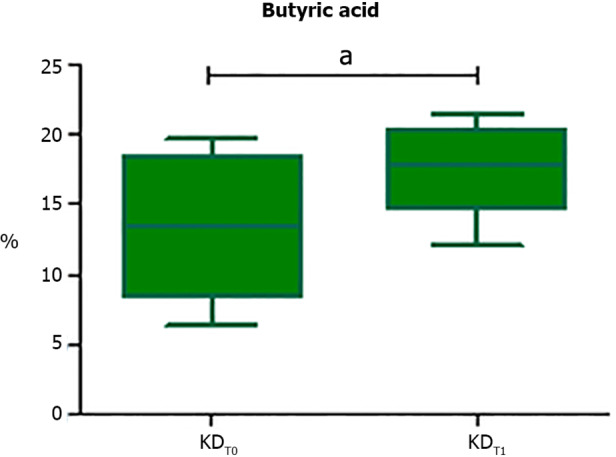
Box plot reporting statistically significant different butyric acid contents at the end of the Khorasan-wheat-diet first arm. Analyses were assessed using the paired Wilcoxon signed-rank test and P values less than 0.05 were considered statistically significant. aP < 0.05, bP < 0.01, cP < 0.001. KDT0: Pre-Khorasan-wheat-diet first arm; KDT1: Post-Khorasan-wheat-diet first arm.
Evaluation of fecal molecular inflammatory profile excluding the washout period
The assessment of the fecal cytokines concentration excluding eventual “carry-over effects” displayed that the CDT3 resulted in a significant increase of IL-1β levels (P = 0.031), whereas no significant differences were found at the end of the CDT1 or at the end of both the KDT1 and KDT3 (Figure 7).
Figure 7.
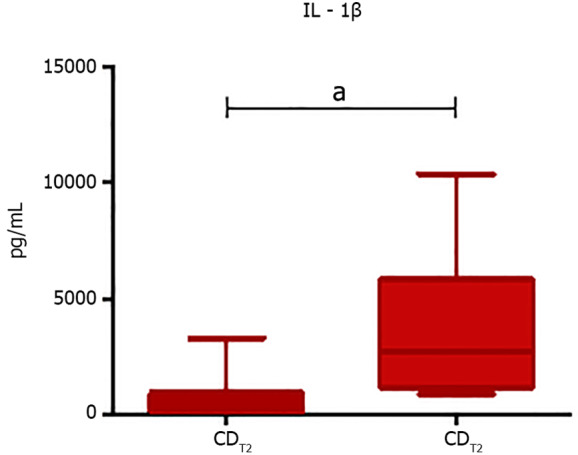
Box plot representing statistically significant different IL-1β levels at the end of the second control wheat diet arm. Analyses were assessed using the paired Wilcoxon signed-rank test and P values less than 0.05 were considered statistically significant. aP < 0.05, bP < 0.01, cP < 0.001. IL: Interleukin; CDT2: Pre-control-wheat-diet second arm; CDT3: Post-control-wheat-diet second arm.
Correlation between gut microbiota composition and questionnaire scores
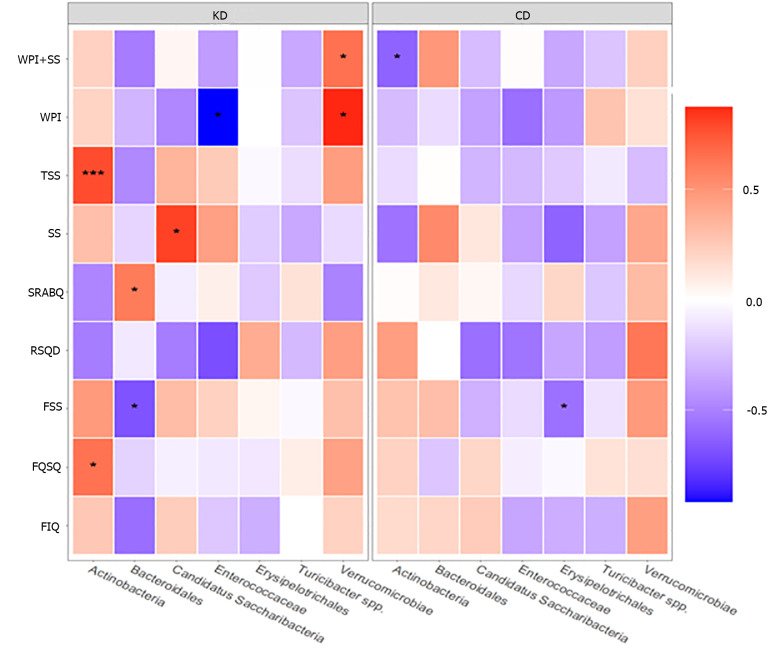
To examine the association between statistically different taxa (post/pre-intervention) and the results of the questionnaire scores (post/pre-intervention), we conducted Spearman correlation for each diet (Figure 8).
Figure 8.
Heatmap of Spearman correlations between statistically significant different taxa (post-pre) and changes in questionnaire scores (post-pre), according to the Khorasan wheat diet or control wheat diet. Red shades indicate positive correlations, whereas blue shades indicate negative correlations; the intensity of colors represents the degree of association. P values less than 0.05 were considered statistically significant. aP < 0.05, bP < 0.01, cP < 0.001. KD: Khorasan wheat diet; CD: Control wheat diet.
In detail, a positive correlation between the phylum Actinobacteria and both TSS (P < 0.001) and FOSQ (P < 0.05) scores and between the class Verrucomicrobiae and both WPI + SS (P < 0.05) and WPI (P < 0.05) scores was reported in patients following KD. Moreover, a positive correlation between the phylum candidatus Saccharibacteria and SS score (P < 0.05) and between the order Bacteroidales and SRSBQ score (P < 0.05) was reported after KD. On the other hand, we documented a negative correlation between Bacteroidales and FSS score (P < 0.05) and between Enteococcaceae and WPI score (P < 0.05).
In addition, regarding the patients who underwent CD intervention, negative correlations were found between Actinobacteria and WPI + SS score (P < 0.05) and between Erysipelothichales and FSS score (P < 0.05).
DISCUSSION
Different nutritional interventions provide benefits in fibromyalgia management[11], and we recently demonstrated that the replacement diet with ancient Khorasan wheat led to an overall improvement in symptom severity and sleep pattern of FM patients[8].
Currently, the relationship between FM syndrome and irritable bowel disease has been confirmed but the link between gut dysbiosis and FM is still poorly understood[4]. Furthermore, it has been documented that the worsening causes for the distinctive musculoskeletal pain associated with FM have been related to obesity and unhealthy diet[12,13].
Since the whole Khorasan-based diet seems to favor an healthy microbiota[14], the goal of our study was to examine, for the first time, its effects in FM patients.
In detail, we performed a randomized, double blind crossover trial where enrolled FM patients consumed control wheat products or Khorasan wheat products for 8 wk and then crossed.
Then we analyzed the effects of the diets on GM composition, the fecal SCFAs levels, the intestinal inflammatory profile, and symptom improvement in FM patients.
First, concerning the fecal microbiota analysis of FM patients, we found that both 8-wk interventions did not significantly modify either the microbial composition and diversity or the SCFAs levels. Whereas, looking at the changes in microbial abundances produced by each dietary intervention, KD did not result in modifications at any taxonomic level; instead, CD was associated with a significant increase of Turicibacter spp.
Previous studies documented that the abundance of the genus Turicibacter was markedly reduced in mice with high fat feeding[15,16] and recent findings reported its negative correlation with body fat[17,18]. In addition, Zhong et al[19] found a positive correlation between Turicibacter spp. and butyric acid, an SCFA famous for its anti-inflammatory properties; hence, CD seems to exert a slight beneficial effect on FM patients.
In addition, the evaluation of the fecal molecular inflammatory profile showed that CD resulted in an increased level of the anti-inflammatory IL-4, while no significant differences were reported after KD.
In contrast, current evidence suggests that, compared to healthy controls, FM patients showed lower plasma levels of IL-4[20,21]. Nevertheless, given its demonstrated role in the transcription enhancement of opioid receptors, a lower IL-4 abundance could be associated with an increase in pain perception[22].
Anyway, in general, both 8-wk dietary interventions displayed modest effects on GM composition and metabolic function (SCFAs) and on the fecal cytokine profile of FM patients.
Nonetheless, our findings are consistent with other previous studies[23,24] which demonstrated the remarkable stability, resilience, and inter-individual variability of the GM in response to short-term dietary modifications.
Finally, although our cross-over study, as it usually is, typically contains a washout period to exclude the repercussion of the modifications (carry-over effect) of one intervention phase on subsequent[25], given that we did not have the possibility to supervise FM patients in the meantime, we repeated all of the analysis at the end of each intervention arm.
Interestingly, the subjects included in the first KD arm, before the unrestricted washout period diet, reported a significant increase of the phyla candidatus Saccharibacteria and Actinobacteria phyla and a significantly higher abundance of butyric acid.
As previously demonstrated, the Saccharibacteria members colonize the human oral cavity and are associated with oral mucosal infectious diseases[26]. However, most members of the Saccharibacteria phylum remain uncultivable and no in-depth metabolic analysis of these bacteria is yet available; hence, their potential role in the microbial community has not been fully elucidated[27].
On the other hand, Actinobacteria, which play a key role in the maintenance of gut homeostasis by producing SCFAs and modulating the immune response, were reduced in a cohort of FM patients[28,29].
Concerning the increase of fecal butyric acid abundance after the first KD arm, a recent study reported that FM patients showed lower levels of butyrate-producing bacteria, such as many members of the Lachnospiraceae family, than healthy subjects[30]. In addition, comparing the effect of a Kamut-based diet vs a modern wheat-based diet in healthy subjects, Saa and colleagues reported that the concentration of butyrate and its esters in stool and urine samples were increased after the Kamut-diet[31].
Butyric acid, which is secreted by the GM and absorbed in the blood stream, exerts anti-inflammatory effects and induces naive T cell differentiation into regulatory T cells (Tregs)[30]. Furthermore, it acts as a potent histone deacetylase inhibitor, thus exerting analgesic and antidepressant effects[32,33]. In addition, reports suggest that butyric acid improves pain behaviors in models of nerve injury-induced pain and ameliorates neuropathic pain by acting directly on the peripheral nerve system[34,35].
The KDT3 only resulted in a significant reduction of the Enterococcaceae family, whose members were instead over-represented in patients with chronic fatigue syndrome[36].
Given these different findings between the two arms of KD interventions, the possible carry-over effects of the washout period or of the CD intervention cannot be excluded.
In contrast, at the end of the CDT1, we found a significant decrease of Bacteroidales members; a reduction of this order was strongly associated with different disorders, especially in Crohn’s disease[37].
On the other hand, the CDT2 was surprisingly associated with a significant increase of the order Erysipelotrichales and the class Verrucomicrobiae, which have been respectively associated with irritable bowel syndrome and chronic pain severity[38,39].
Moreover, the CDT3 showed a significant increase of IL-1β, a potent pro-inflammatory cytokine that acts on nociceptors and results in an improvement in the transduction of pain through various ion channels. But, the IL-1β overexpression is also involved in many autoimmune disorders featuring pain such as inflammatory bowel disease, multiple sclerosis, and rheumatoid arthritis[40,41].
In addition, several studies have shown increased levels of IL-1β in FM patients compared to healthy controls, suggesting an association between IL-1β and some FM symptoms such as hyperalgesia, fatigue, sleep disorders, cognitive dysfunctions, and stress[42,43].
Finally, to evaluate the association between GM composition and FM symptoms, we performed Spearman correlations between the self-administered questionnaires and the differentially abundant taxa before and after both diets.
In detail, after KD, FM patients reported a positive association between an increasing abundance of Actinobacteria and the improvement of the TSS and FOSQ scores. In addition, we documented a positive correlation between the phylum candidatus Saccharibacteria and SS scores and a negative association between a reduced abundance of Enterococcaceae and an improved WPI score.
On the contrary, FM patients in the CD intervention reported a detrimental negative correlation between increasing Erysipelothichales and the amelioration of FSS score.
A significant relationship between the differential abundances of several gut bacteria and FM symptom indices such as pain intensity, WPI score, cognitive deficits, and fatigue was reported by Minerbi et al[44]. Moreover, Clos-Garcia et al[29] showed a correlation between some genera and FM pain indicators. Finally, several studies suggest that increased reactive oxygen species in FM patients, resulting in impaired mitochondrial function and reduced ATP in muscle and neural cells, might lead to chronic widespread pain in these patients[45]. Therefore, the improvement of various FM symptoms along with the variation of some gut bacteria after the KD intervention could be related to the reported antioxidant effects of the ancient Khorasan wheat[46].
Despite our present study having some limitations such as the limited number of enrolled patients, the low taxonomical resolution of 16S rRNA sequencing, the short period of dietary administrations, and the lack of patients’ supervision during the washout period, our study suggest that a replacement diet with Khorasan wheat products improves the symptoms of patients with fibromyalgia.
CONCLUSION
In conclusion, although further studies with a long-term administration of Khorasan wheat products in high numbers of FM patients are necessary, we demonstrated for the first time that an ancient Khorasan wheat diet results in some beneficial GM compositional and functional modifications that positively correlate with an improvement of fibromyalgia symptomatology.
ARTICLE HIGHLIGHTS
Research background
Fibromyalgia (FM) is a syndrome characterized by widespread pain, sleeping disorders, fatigue, and cognitive dysfunction. Frequently, gastrointestinal distress is also reported, suggesting the potential pathogenic role of the gut microbiota (GM).
Research motivation
The GM is deeply influenced by several factors, including diet, and recent findings highlighted a significant symptom improvement in FM patients following various nutritional interventions such as vegetarian diet, low-fermentable oligosaccharides, disaccharides, monosaccharides, and polyols based diets, gluten-free diet, and an ancient grain supplementation. Therefore, a replacement diet with ancient Khorasan grain could improve FM symptomatology.
Research objectives
The main objective of our study was to examine the effects of ancient Khorasan wheat on GM composition, fecal molecular immune profile, and short-chain fatty acids (SCFAs) production in FM patients.
Research methods
In this randomized, double-blind crossover trial, 20 FM patients were randomly assigned to consume either Khorasan or control wheat products for 8 wk and then, after an 8-wk washout period, crossed. At the beginning and at the end of each intervention period, symptom questionnaires and stool samples were collected and GM characterization was performed by 16S rRNA sequencing, while the fecal molecular inflammatory response and the SCFAs were respectively determined with a Luminex MAGPIX detection system and a mass chromatography-mass spectrometry method.
Research results
The Khorasan wheat replacement diet exhibited positive effects on GM composition and on both the fecal immune and SCFAs profiles. Moreover, we documented the improvement of various FM symptoms along with the variation of some gut bacteria after the Khorasan wheat diet.
Research conclusions
An ancient Khorasan wheat diet represents a good strategy to result in positive GM compositional and functional modifications that positively correlate with an improvement of FM symptomatology.
Research perspectives
We demonstrated that a replacement diet based on ancient Khorasan wheat seems to represent a non-invasive successful strategy to ameliorate FM patient’s symptomatology. We believe that our data will be a starting point for future studies on FM, especially with a long-term administration of Khorasan wheat products in high numbers of FM patients.
Footnotes
Institutional review board statement: The study was approved by the local ethics committee “Comitato Etico Regionale per la Sperimentazione Clinica della Regione Toscana, Sezione AREA VASTA CENTRO” (SPE 12.630), and adhered to the principles of the Declaration of Helsinki and the Data Protection Act.
Informed consent statement: Informed written consent was obtained from the patients for publication of this report and any accompanying images.
Conflict-of-interest statement: The authors declare no competing interests for this article.
Provenance and peer review: Invited article; Externally peer reviewed.
Peer-review model: Single blind
Peer-review started: November 26, 2021
First decision: January 8, 2022
Article in press: March 27, 2022
Specialty type: Nutrition and dietetics
Country/Territory of origin: Italy
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): B
Grade C (Good): C
Grade D (Fair): D
Grade E (Poor): 0
P-Reviewer: Almeida C, Portugal; Ren JY, China S-Editor: Zhang H L-Editor: Wang TQ P-Editor: Cai YX
Contributor Information
Simone Baldi, Department of Experimental and Clinical Medicine, University of Florence, Florence 50134, Italy.
Giuditta Pagliai, Department of Experimental and Clinical Medicine, University of Florence, Florence 50134, Italy; Unit of Clinical Nutrition, Careggi University Hospital, Florence 50134, Italy.
Monica Dinu, Department of Experimental and Clinical Medicine, University of Florence, Florence 50134, Italy; Unit of Clinical Nutrition, Careggi University Hospital, Florence 50134, Italy.
Leandro Di Gloria, Department of Experimental and Clinical Medicine, University of Florence, Florence 50134, Italy.
Giulia Nannini, Department of Experimental and Clinical Medicine, University of Florence, Florence 50134, Italy.
Lavinia Curini, Department of Experimental and Clinical Medicine, University of Florence, Florence 50134, Italy.
Marco Pallecchi, Department of Neurosciences, Psychology, Drug Research and Child Health, Section of Pharmaceutical and Nutraceutical Sciences, University of Florence, Sesto Fiorentino 50019, Italy.
Edda Russo, Department of Experimental and Clinical Medicine, University of Florence, Florence 50134, Italy.
Elena Niccolai, Department of Experimental and Clinical Medicine, University of Florence, Florence 50134, Italy.
Giovanna Danza, Department of Biomedical, Experimental and Clinical Sciences “Mario Serio”, University of Florence, Florence 50134, Italy.
Stefano Benedettelli, Department of Agriculture, Food, Environment and Forestry, University of Florence, Florence 50144, Italy.
Giovanna Ballerini, Multidisciplinary Center for Pain Therapy, Reference Center for Fibromyalgia, Piero Palagi Hospital, USL Toscana Centro, Florence 50122, Italy.
Barbara Colombini, Department of Experimental and Clinical Medicine, University of Florence, Florence 50134, Italy.
Gianluca Bartolucci, Department of Neurosciences, Psychology, Drug Research and Child Health, Section of Pharmaceutical and Nutraceutical Sciences, University of Florence, Sesto Fiorentino 50019, Italy.
Matteo Ramazzotti, Department of Biomedical, Experimental and Clinical Sciences “Mario Serio”, University of Florence, Florence 50134, Italy.
Francesco Sofi, Department of Experimental and Clinical Medicine, University of Florence, Florence 50134, Italy; Unit of Clinical Nutrition, Careggi University Hospital, Florence 50134, Italy.
Amedeo Amedei, Department of Experimental and Clinical Medicine, University of Florence, Florence 50134, Italy; SOD of Interdisciplinary Internal Medicine, Careggi University Hospital, Florence 50134, Italy. aamedei@unifi.it.
Data sharing statement
No additional data are available.
References
- 1.Littlejohn G, Guymer E. Key milestones contributing to the understanding of the mechanisms underlying fibromyalgia. Biomedicines. 2020;8 doi: 10.3390/biomedicines8070223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Banfi G, Diani M, Pigatto PD, Reali E. T cell subpopulations in the physiopathology of fibromyalgia: evidence and perspectives. Int J Mol Sci. 2020;21 doi: 10.3390/ijms21041186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Malatji BG, Mason S, Mienie LJ, Wevers RA, Meyer H, van Reenen M, Reinecke CJ. The GC-MS metabolomics signature in patients with fibromyalgia syndrome directs to dysbiosis as an aspect contributing factor of FMS pathophysiology. Metabolomics. 2019;15:54. doi: 10.1007/s11306-019-1513-6. [DOI] [PubMed] [Google Scholar]
- 4.Erdrich S, Hawrelak JA, Myers SP, Harnett JE. Determining the association between fibromyalgia, the gut microbiome and its biomarkers: a systematic review. BMC Musculoskelet Disord. 2020;21:181. doi: 10.1186/s12891-020-03201-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Thursby E, Juge N. Introduction to the human gut microbiota. Biochem J. 2017;474:1823–1836. doi: 10.1042/BCJ20160510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Silva AR, Bernardo A, Costa J, Cardoso A, Santos P, de Mesquita MF, Vaz Patto J, Moreira P, Silva ML, Padrão P. Dietary interventions in fibromyalgia: a systematic review. Ann Med. 2019;51:2–14. doi: 10.1080/07853890.2018.1564360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Sofi F, Whittaker A, Gori AM, Cesari F, Surrenti E, Abbate R, Gensini GF, Benedettelli S, Casini A. Effect of Triticum turgidum subsp. turanicum wheat on irritable bowel syndrome: a double-blinded randomised dietary intervention trial. Br J Nutr. 2014;111:1992–1999. doi: 10.1017/S000711451400018X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Pagliai G, Colombini B, Dinu M, Whittaker A, Masoni A, Danza G, Amedei A, Ballerini G, Benedettelli S, Sofi F. Effectiveness of a Khorasan wheat-based replacement on pain symptoms and quality of life in patients with fibromyalgia. Pain Med. 2020;21:2366–2372. doi: 10.1093/pm/pnaa134. [DOI] [PubMed] [Google Scholar]
- 9.Niccolai E, Russo E, Baldi S, Ricci F, Nannini G, Pedone M, Stingo FC, Taddei A, Ringressi MN, Bechi P, Mengoni A, Fani R, Bacci G, Fagorzi C, Chiellini C, Prisco D, Ramazzotti M, Amedei A. Significant and conflicting correlation of IL-9 with Prevotella and Bacteroides in human colorectal cancer. Front Immunol. 2020;11:573158. doi: 10.3389/fimmu.2020.573158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Niccolai E, Baldi S, Ricci F, Russo E, Nannini G, Menicatti M, Poli G, Taddei A, Bartolucci G, Calabrò AS, Stingo FC, Amedei A. Evaluation and comparison of short chain fatty acids composition in gut diseases. World J Gastroenterol. 2019;25:5543–5558. doi: 10.3748/wjg.v25.i36.5543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Lowry E, Marley J, McVeigh JG, McSorley E, Allsopp P, Kerr D. Dietary interventions in the management of fibromyalgia: a systematic review and best-evidence synthesis. Nutrients. 2020;12 doi: 10.3390/nu12092664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Malatji BG, Meyer H, Mason S, Engelke UFH, Wevers RA, van Reenen M, Reinecke CJ. A diagnostic biomarker profile for fibromyalgia syndrome based on an NMR metabolomics study of selected patients and controls. BMC Neurol. 2017;17:88. doi: 10.1186/s12883-017-0863-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kaleth AS, Slaven JE, Ang DC. Obesity moderates the effects of motivational interviewing treatment outcomes in fibromyalgia. Clin J Pain. 2018;34:76–81. doi: 10.1097/AJP.0000000000000500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Fassarella M, Blaak EE, Penders J, Nauta A, Smidt H, Zoetendal EG. Gut microbiome stability and resilience: elucidating the response to perturbations in order to modulate gut health. Gut. 2021;70:595–605. doi: 10.1136/gutjnl-2020-321747. [DOI] [PubMed] [Google Scholar]
- 15.Dimova LG, Zlatkov N, Verkade HJ, Uhlin BE, Tietge UJF. High-cholesterol diet does not alter gut microbiota composition in mice. Nutr Metab (Lond) 2017;14:15. doi: 10.1186/s12986-017-0170-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Zhang J, Yi C, Han J, Ming T, Zhou J, Lu C, Li Y, Su X. Novel high-docosahexaenoic-acid tuna oil supplementation modulates gut microbiota and alleviates obesity in high-fat diet mice. Food Sci Nutr. 2020;8:6513–6527. doi: 10.1002/fsn3.1941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Dhakal S, McCormack L, Dey M. Association of the gut microbiota with weight-loss response within a retail weight-management program. Microorganisms. 2020;8 doi: 10.3390/microorganisms8081246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Guo X, Li J, Tang R, Zhang G, Zeng H, Wood RJ, Liu Z. High fat diet alters gut microbiota and the expression of Paneth cell-antimicrobial peptides preceding changes of circulating inflammatory cytokines. Mediators Inflamm. 2017;2017:9474896. doi: 10.1155/2017/9474896. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Zhong Y, Nyman M, Fåk F. Modulation of gut microbiota in rats fed high-fat diets by processing whole-grain barley to barley malt. Mol Nutr Food Res. 2015;59:2066–2076. doi: 10.1002/mnfr.201500187. [DOI] [PubMed] [Google Scholar]
- 20.Sturgill J, McGee E, Menzies V. Unique cytokine signature in the plasma of patients with fibromyalgia. J Immunol Res. 2014;2014:938576. doi: 10.1155/2014/938576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Uçeyler N, Valenza R, Stock M, Schedel R, Sprotte G, Sommer C. Reduced levels of antiinflammatory cytokines in patients with chronic widespread pain. Arthritis Rheum. 2006;54:2656–2664. doi: 10.1002/art.22026. [DOI] [PubMed] [Google Scholar]
- 22.Labuz D, Celik MÖ, Seitz V, Machelska H. Interleukin-4 induces the release of opioid peptides from M1 macrophages in pathological pain. J Neurosci. 2021;41:2870–2882. doi: 10.1523/JNEUROSCI.3040-20.2021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Zhang C, Björkman A, Cai K, Liu G, Wang C, Li Y, Xia H, Sun L, Kristiansen K, Wang J, Han J, Hammarström L, Pan-Hammarström Q. Impact of a 3-months vegetarian diet on the gut microbiota and immune repertoire. Front Immunol. 2018;9:908. doi: 10.3389/fimmu.2018.00908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Pagliai G, Russo E, Niccolai E, Dinu M, Di Pilato V, Magrini A, Bartolucci G, Baldi S, Menicatti M, Giusti B, Marcucci R, Rossolini GM, Casini A, Sofi F, Amedei A. Influence of a 3-month low-calorie Mediterranean diet compared to the vegetarian diet on human gut microbiota and SCFA: the CARDIVEG Study. Eur J Nutr. 2020;59:2011–2024. doi: 10.1007/s00394-019-02050-0. [DOI] [PubMed] [Google Scholar]
- 25.Evans SR. Clinical trial structures. J Exp Stroke Transl Med. 2010;3:8–18. doi: 10.6030/1939-067x-3.1.8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Bor B, Bedree JK, Shi W, McLean JS, He X. Saccharibacteria (TM7) in the human oral microbiome. J Dent Res. 2019;98:500–509. doi: 10.1177/0022034519831671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Figueroa-Gonzalez PA, Bornemann TLV, Adam PS, Plewka J, Révész F, von Hagen CA, Táncsics A, Probst AJ. Saccharibacteria as organic carbon sinks in hydrocarbon-fueled communities. Front Microbiol. 2020;11:587782. doi: 10.3389/fmicb.2020.587782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Binda C, Lopetuso LR, Rizzatti G, Gibiino G, Cennamo V, Gasbarrini A. Actinobacteria: a relevant minority for the maintenance of gut homeostasis. Dig Liver Dis. 2018;50:421–428. doi: 10.1016/j.dld.2018.02.012. [DOI] [PubMed] [Google Scholar]
- 29.Clos-Garcia M, Andrés-Marin N, Fernández-Eulate G, Abecia L, Lavín JL, van Liempd S, Cabrera D, Royo F, Valero A, Errazquin N, Vega MCG, Govillard L, Tackett MR, Tejada G, Gónzalez E, Anguita J, Bujanda L, Orcasitas AMC, Aransay AM, Maíz O, López de Munain A, Falcón-Pérez JM. Gut microbiome and serum metabolome analyses identify molecular biomarkers and altered glutamate metabolism in fibromyalgia. EBioMedicine. 2019;46:499–511. doi: 10.1016/j.ebiom.2019.07.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Furusawa Y, Obata Y, Fukuda S, Endo TA, Nakato G, Takahashi D, Nakanishi Y, Uetake C, Kato K, Kato T, Takahashi M, Fukuda NN, Murakami S, Miyauchi E, Hino S, Atarashi K, Onawa S, Fujimura Y, Lockett T, Clarke JM, Topping DL, Tomita M, Hori S, Ohara O, Morita T, Koseki H, Kikuchi J, Honda K, Hase K, Ohno H. Commensal microbe-derived butyrate induces the differentiation of colonic regulatory T cells. Nature. 2013;504:446–450. doi: 10.1038/nature12721. [DOI] [PubMed] [Google Scholar]
- 31.Saa DT, Turroni S, Serrazanetti DS, Rampelli S, Maccaferri S, Candela M, Severgnini M, Simonetti E, Brigidi P, Gianotti A. Impact of Kamut® Khorasan on gut microbiota and metabolome in healthy volunteers. Food Res Int. 2014;63:227–232. [Google Scholar]
- 32.Qiao M, Jiang QS, Liu YJ, Hu XY, Wang LJ, Zhou QX, Qiu HM. Antidepressant mechanisms of venlafaxine involving increasing histone acetylation and modulating tyrosine hydroxylase and tryptophan hydroxylase expression in hippocampus of depressive rats. Neuroreport. 2019;30:255–261. doi: 10.1097/WNR.0000000000001191. [DOI] [PubMed] [Google Scholar]
- 33.Zammataro M, Sortino MA, Parenti C, Gereau RW 4th, Chiechio S. HDAC and HAT inhibitors differently affect analgesia mediated by group II metabotropic glutamate receptors. Mol Pain. 2014;10:68. doi: 10.1186/1744-8069-10-68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Kukkar A, Singh N, Jaggi AS. Attenuation of neuropathic pain by sodium butyrate in an experimental model of chronic constriction injury in rats. J Formos Med Assoc. 2014;113:921–928. doi: 10.1016/j.jfma.2013.05.013. [DOI] [PubMed] [Google Scholar]
- 35.Bonomo RR, Cook TM, Gavini CK, White CR, Jones JR, Bovo E, Zima AV, Brown IA, Dugas LR, Zakharian E, Aubert G, Alonzo F 3rd, Calcutt NA, Mansuy-Aubert V. Fecal transplantation and butyrate improve neuropathic pain, modify immune cell profile, and gene expression in the PNS of obese mice. Proc Natl Acad Sci U S A. 2020;117:26482–26493. doi: 10.1073/pnas.2006065117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Sheedy JR, Wettenhall RE, Scanlon D, Gooley PR, Lewis DP, McGregor N, Stapleton DI, Butt HL, DE Meirleir KL. Increased d-lactic acid intestinal bacteria in patients with chronic fatigue syndrome. In Vivo. 2009;23:621–628. [PubMed] [Google Scholar]
- 37.Cénit MC, Matzaraki V, Tigchelaar EF, Zhernakova A. Rapidly expanding knowledge on the role of the gut microbiome in health and disease. Biochim Biophys Acta. 2014;1842:1981–1992. doi: 10.1016/j.bbadis.2014.05.023. [DOI] [PubMed] [Google Scholar]
- 38.Li S, Hua D, Wang Q, Yang L, Wang X, Luo A, Yang C. The role of bacteria and its derived metabolites in chronic pain and depression: recent findings and research progress. Int J Neuropsychopharmacol. 2020;23:26–41. doi: 10.1093/ijnp/pyz061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Barandouzi ZA, Lee J, Maas K, Starkweather AR, Cong XS. Altered gut microbiota in irritable bowel syndrome and its association with food components. J Pers Med. 2021;11 doi: 10.3390/jpm11010035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Ji RR, Chamessian A, Zhang YQ. Pain regulation by non-neuronal cells and inflammation. Science. 2016;354:572–577. doi: 10.1126/science.aaf8924. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Baral P, Udit S, Chiu IM. Pain and immunity: implications for host defence. Nat Rev Immunol. 2019;19:433–447. doi: 10.1038/s41577-019-0147-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Rodriguez-Pintó I, Agmon-Levin N, Howard A, Shoenfeld Y. Fibromyalgia and cytokines. Immunol Lett. 2014;161:200–203. doi: 10.1016/j.imlet.2014.01.009. [DOI] [PubMed] [Google Scholar]
- 43.Peck MM, Maram R, Mohamed A, Ochoa Crespo D, Kaur G, Ashraf I, Malik BH. The influence of pro-inflammatory cytokines and genetic variants in the development of fibromyalgia: a traditional review. Cureus. 2020;12:e10276. doi: 10.7759/cureus.10276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Minerbi A, Gonzalez E, Brereton NJB, Anjarkouchian A, Dewar K, Fitzcharles MA, Chevalier S, Shir Y. Altered microbiome composition in individuals with fibromyalgia. Pain. 2019;160:2589–2602. doi: 10.1097/j.pain.0000000000001640. [DOI] [PubMed] [Google Scholar]
- 45.Meeus M, Nijs J, Hermans L, Goubert D, Calders P. The role of mitochondrial dysfunctions due to oxidative and nitrosative stress in the chronic pain or chronic fatigue syndromes and fibromyalgia patients: peripheral and central mechanisms as therapeutic targets? Expert Opin Ther Targets. 2013;17:1081–1089. doi: 10.1517/14728222.2013.818657. [DOI] [PubMed] [Google Scholar]
- 46.Trozzi C, Raffaelli F, Vignini A, Nanetti L, Gesuita R, Mazzanti L. Evaluation of antioxidative and diabetes-preventive properties of an ancient grain, KAMUT® khorasan wheat, in healthy volunteers. Eur J Nutr. 2019;58:151–161. doi: 10.1007/s00394-017-1579-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
No additional data are available.