Abstract
Physical activity (PA) counseling is under-utilized in primary care for patients with type 2 diabetes mellitus (T2D), despite improving important health outcomes, including physical function. We adapted evidence-based PA counseling programs to primary care patients, staff, and leader’s needs, resulting in “Be ACTIVE” comprised of shared PA tracker data (FitBit©), six theory-informed PA coaching calls, and three in-person clinician visits. In a pilot randomized pragmatic trial, we evaluated the feasibility, acceptability, and effectiveness of Be ACTIVE. Sedentary patients with T2D were randomized to Be ACTIVE versus an enhanced control condition. Mixed methods assessments of feasibility and acceptability included costs. Objective pilot effectiveness outcomes included PA (primary outcome, accelerometer steps/week), the Short Physical Performance Battery (SPPB) physical function measure, and behavioral PA predictors. Fifty patients were randomized to Be ACTIVE or control condition. Acceptability was >90% for patients and clinic staff. Coaching and PA tracking costs of ~$90/patient met Medicare reimbursement criteria. Pre–post PA increased by ~11% (Be ACTIVE) and ~6% in controls (group difference: 1574 ± 4391 steps/week, p = .72). As compared to controls, Be ACTIVE participants significantly improved SPPB (0.9 ± 0.3 vs. −0.1 ± 0.3, p = .01, changes >0.5 points prevent falls clinically), and PA predictors of self-efficacy (p = .02) and social-environmental support (p < .01). In this pilot trial, Be ACTIVE was feasible and highly acceptable to stakeholders and yielded significant improvements in objective physical function consistent with lower fall risk, whereas PA changes were less than anticipated. Be ACTIVE may need additional adaptation or a longer duration to improve PA outcomes.
Keywords: Implementation, Type 2 diabetes mellitus, Physical activity, Physical function, Primary care, acceptability
Lay Summary
We report results from a pragmatic and behavioral theory-based physical activity (PA) coaching program, termed “Be ACTIVE,” for patients with type 2 diabetes that was designed to improve PA and function for patients and to be reimbursable and feasible for primary care teams. As compared to those who did not receive coaching, patients who received Be ACTIVE had physical function improvements that lowered their risk of falls. Be ACTIVE was delivered with fidelity and was highly acceptable to the key primary care stakeholders of patients, clinic staff coaches, and clinicians. Patients particularly liked the focus on setting goals to do enjoyable activities, the accountability of wearing a PA monitor, and the support of their coach. Clinical care professionals felt that their role of encouraging behavior change (coach) and safety monitoring (clinician) aligned well with their clinical expertise, and was professionally rewarding. Coaches felt the program helped them guide many patients to overcome preexisting negative perceptions of PA and develop intrinsic motivations to be active. The costs of clinic coach time and PA tracker rental needed to deliver the 12-week program could be reimbursed by the Medicare Chronic Disease Management programs, albeit with a patient co-payment required.
Implications.
Practice: This study is proof of concept that a pragmatic physical activity (PA) coaching program, such as Be ACTIVE, can be delivered in primary care in a way that is acceptable to patients with diabetes, clinicians and clinic staff, that is reimbursable by Medicare, and that improves clinically important measures of physical function.
Policy: As health systems consider further how to efficiently improve health outcomes for patients with diabetes and other chronic diseases, it is important to consider the relative benefits, costs, and insurance reimbursement options for implementing PA coaching programs such as Be ACTIVE.
Research: Our findings from developing and testing Be ACTIVE with primary care program end-users identified salient priorities that would be relevant to others seeking to implement behavioral interventions in primary care: attention to efficient fit with clinic staff work flow, implementation costs, and reimbursement options (clinics/staff), and patient-reported outcomes such as physical function.
BACKGROUND
People with type 2 diabetes (T2D) have much lower rates of physical activity (PA) than the general population [1–3]. This is a serious health problem because insufficient PA is closely associated with premature mortality, physical disability, and decreased quality of life [1–3]. A recent national report showed that 40% of women and 25% of men with T2D experience major impairments in physical function that are a barrier to increasing PA [3]. Common comorbidities include osteoarthritis and peripheral neuropathy that make walking painful and increase the risk of falls [3–5]. Thus, it is difficult for patients with T2D to form new PA habits without a formal PA behavioral intervention [6, 7]. Prior PA behavioral interventions delivered in health systems have been too time and resource-intensive to be practically sustained in clinical practices [7]. Evidence-based PA interventions are needed that are feasible, replicable, and effective.
Our recent systematic review identified several effective PA counseling programs delivered in health systems that improved PA for adults with T2D and that were highly pragmatic for real-world use—hereafter termed “pragmatic evidence-based programs (EBPs)” [7]. However, our review found that even pragmatic EBPs were rarely sustained after grant funding ceased, thus signifying a pressing need for implementation strategies to address feasibility, costs, and financial reimbursement [7]. So, a key question emerged from our review: how can pragmatic EBPs be adapted to work in real-world primary care? Our overarching purposes in this study were to develop a PA coaching intervention (Be ACTIVE) to be feasible and acceptable for the primary care setting, to yield clinically important improvements in PA and physical function, and to report the pilot effectiveness and implementation outcomes from testing Be ACTIVE in a pilot pragmatic trial.
METHODS
Rationale for the Be ACTIVE Intervention package
To develop Be ACTIVE, our research team first identified common intervention elements of the pragmatic EBPs identified in our systematic review [7], and then engaged stakeholders of primary care staff and patients to adapt the way these intervention elements were delivered to fit their needs. This is in keeping with the recommendations of standard implementation science process models to adapt the implementation delivery methods (also termed “implementation strategies”) to the stakeholders’ context [8]. First, we identified common behavioral change techniques [9] among these pragmatic EBPs that aligned with multiple behavioral theories [10–12]: goal-setting compatible with intrinsic motives to select enjoyable forms of PA, self-monitoring with PA tracking devices, accountability from others/coaches monitoring with patients’ awareness, problem-solving and social support. Next, we identified common pragmatic delivery approaches among these pragmatic EBPs that tailored delivery to the busy primary care context, including the use of PA counseling checklists to promote efficiency. We then used an applied approach to obtain feedback on these delivery approaches from program end-users; we obtained input from clinic staff coaches and clinicians during their intervention materials use training. In addition, five patients provided input as the principal investigator beta-tested the intervention coaching scripts with them. In addition to informing our program delivery, this process also identified key outcomes that were important to our stakeholders: costs of implementation (staff), insurance reimbursement (staff), and physical function improvements (patients).
Core elements of the Be ACTIVE Intervention package
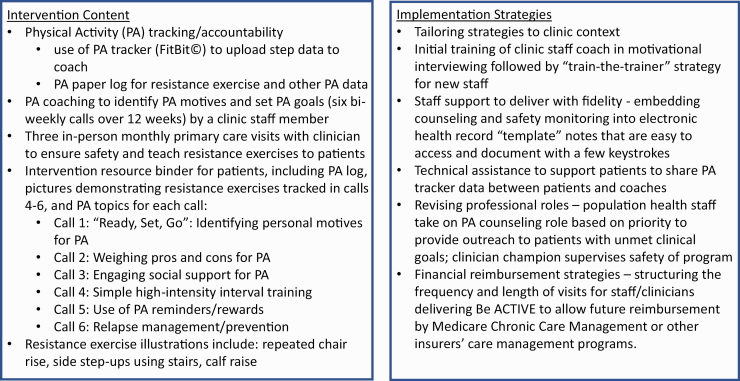
The result of this stakeholder-engaged intervention development and planning process yielded our new “Be ACTIVE” intervention package. We depict the core Be ACTIVE intervention elements and core implementation strategies in Fig. 1, and briefly summarize here from the perspective of a clinic’s organizational capacity: (1) to prepare for the PA coaching role, existing clinic staff members received brief training in motivational interviewing; (2) a trained clinic staff coach delivered six phone calls to each enrolled patient with a PA behavior change theme drawn from the evidence-based Active Living Every Day program (see Fig. 1) [13]; (3) in each phone call, coaches also used the core behavior change techniques [9] identified from our systematic review (i.e., goal-setting, self-monitoring, accountability from others’ monitoring with awareness, problem-solving, and social support); (4) coaches used a checklist embedded in the electronic health record to efficiently prompt their behavioral coaching approach; (5) each patient received a PA tracker device (FitBit©) to track aerobic PA, and a workbook with a PA log to track their PA goals and actual PA; (6) an existing primary care clinician supervised the intervention safety during 3 monthly visits, as patients with T2D are at risk for injury with exercise.
Fig. 1.
Summary of the Be ACTIVE Intervention content and implemented strategy tested.
Study design: pilot pragmatic trial
In the pilot trial, consented participants were block-randomized in a 1:1 fashion to Be ACTIVE versus an enhanced control condition for a 12-week intervention period. We used randomization blocks of n = 4 across 2 age strata (age 50–64; age 65–80) and gender (male/female), to minimize intervention selection bias by age or gender. The details of the Be ACTIVE intervention are shown in Fig. 1, including 6 bi-weekly coaching calls from an existing clinic staff coach and 3 monthly in-person visits with a clinician. Individuals randomized to the enhanced control condition received printed educational materials included the prevailing US PA guidelines at that time [14] and 3 monthly mailings on diet and other T2D education topics. The Colorado Multiple Institutional Review Board approved these processes.
Population
Our pragmatic eligibility criteria were representative of sedentary people with T2D: age 50–85 years, perform <3 days/week of moderate-intensity exercise for 20 min or more [15]. Exclusion criteria targeted safety concerns, including <6-month life expectancy, moderate risk of falls (cannot hold semi-tandem stance >10 s) [16], unsafe blood pressure levels (>170/95) or unsafe glucose levels (hemoglobin A1c >9%) to perform moderate-intensity exercise, or dementia based on either their clinician’s diagnosis or a Folstein Mini-Mental Status Examination score <24) [17]. An automated health record report identified potential patients with T2D based on problem list and laboratory data, and our research assistants confirmed eligibility with patients for PA behavior/fall risk, and with the primary care team for life expectancy and dementia. If participants were planning physical therapy or completing physical therapy at the time of recruitment, we delayed their enrollment until physical therapy was completed, to perform baseline testing after the benefits of physical therapy had accrued. We consented to eligible participants between 12/2015 and 1/2019.
Settings
For feasibility, we recruited two clinics near the facility where we needed to conduct our research evaluation assessments of cardiorespiratory fitness and physical function outcomes (i.e., these assessments were not part of the Be ACTIVE intervention). These sites are both academic primary care/General Internal Medicine clinics that serve ~15,000 racially diverse patients. As these clinics are certified as patient-centered medical homes that have a proactive health promotion focus, they have existing wellness-focused clinic staff who could serve as coaches, although their coaching time needed to be supported by our pilot grant. In our system, these were junior staff members who served a care manager function, and they had a Bachelor’s or Master’s degree in a health promotion field (e.g., social work). There were no existing PA coaching programs in these clinics prior to this pilot. In each site, we identified a primary care clinician champion: one Physician Assistant and one Doctor of Medicine (MD) volunteered.
Outcome measures
Implementation outcomes and mixed methods analytic plan
Our convergent mixed methods [18] approach simultaneously assessed qualitative and quantitative implementation outcomes with stakeholders [19]. A research staff member conducted in-person interviews with patients and videoconference interviews with clinic coaches and clinicians, using a separate semistructured interview guide developed to elicit perspectives about the intervention from patients, coaches, and clinicians, respectively. Patients were interviewed after completion of all other study visits, and coaches were interviewed ~4 weeks after the study ended. The primary implementation outcomes were acceptability and feasibility [20] of Be ACTIVE to patients and clinic staff, and secondary RE-AIM implementation outcomes included: Reach (% eligible patients participating; why participated); and Implementation (fidelity to protocol; needs for adaptation; costs in time and money). Relevant to future adopters, we considered costs from the clinic perspective [21], using an approach to express coaching time costs averaged across all coaching calls as a pro-rated portion of staff salary and benefits in 2017 US dollars (USD) [22]. Coaching time costs were self-reported by the coach for each call in a cost collection template. The template categories of costs included time preparing for the call, delivering the coaching, and scheduling the next call. We also assessed PA tracker costs to the clinic as the costs for the trackers used (FitBit© Zip) by the intervention patients—patients returned the tracker at study completion. A team member (IL or KC) unaffiliated with the intervention conducted patient interviews and our qualitative analyst (SL) conducted clinic interviews. Audio files were transcribed verbatim.
We used a qualitative content analysis approach to allow our implementation outcomes not only to guide the deductive development of codes but also to identify codes inductively [23]. A qualitatively trained analyst (SL) and the principal investigator (AGH) jointly reviewed transcripts and developed the codes together, using ATLAS.ti version 8. Initial codes were deductively based on themes related to Effectiveness (perceived outcomes), Feasibility, Acceptability, and other implementation outcome domains, and the codebook was expanded based on codes that inductively emerged from the data. Transcripts were jointly reviewed and coded by SL and AGH until no new codes were identified and there was a strong code assignment agreement, at which point the codebook was finalized, and the remainder were coded by SL. All codes were merged in ATLAS.ti. Coders and our qualitative methods expert met regularly to discuss emergent codes and themes, and resolve any coding discrepancies.
Mixed methods analysis:
To better draw inferences for our implementation outcomes, we integrated the quantitative data with the qualitative themes and representative quotes [18]. Specifically, we used a joint data display to evaluate the extent to which the qualitative and quantitative data corroborated each other, and to allow the qualitative data to guide our understanding of the quantitative data.
Pilot effectiveness outcomes and analytic plan:
All primary and secondary pilot effectiveness outcomes were measured at baseline and immediately post-intervention. The primary outcome of objective PA was measured by an accelerometer (Actigraph GT3X+, LLC, Pensacola, FL). We used standards for accelerometer activity as very light, light, moderate, or vigorous intensity, respectively, according to standard methods [23]. In addition, following standard thresholds for accelerometer wear time to ensure sufficient data to estimate usual PA, each patient’s accelerometer data needed to include ≥3 “valid days” of wear time ≥10 h/day [24]. Using an adapted version of the Troiano algorithm for sedentary populations, accelerometer wear time was auto-calculated by the GT3X+ as a period during which individuals had 2-minute “spikes” in activity at least every 90 min (i.e., wear length interval = 90 minutes; spike level = 2 min) [24, 25]. The use of linear mixed-effects models allowed us to analyze PA data across all valid days at baseline and post-intervention assessment time points for patients who met these wear time thresholds. We considered steps/day and minutes of combined moderate-vigorous intensity exercise as co-primary PA outcome measures, based on their clinical relevance [26, 27].
Secondary outcomes of physical function were purposefully selected to be clinically relevant to risk of falls/disability, and sensitive to change for individuals with moderate functional impairment (Short Physical Performance Battery (SPPB)), or minimal/no functional impairment: timed 400-m walk assessment [28, 29]) and leg extension power [30]. There were no changes in the outcome assessment techniques or timing after the initiation of the research study testing procedures. In discussion with our exercise physiology study team member (JSL), after publishing the protocol on clinicaltrials.gov but before initiating testing procedures, we added leg extension power as a secondary functional outcome and eliminated grip strength as a secondary functional outcome, as leg power is typically more sensitive to change than grip strength [30–32]. Exploratory outcome measures included: glycemic control by Hemoglobin A1c [33], and cardiorespiratory fitness by modified Balke treadmill and metabolic cart protocol (MGC Diagnostics©) [34]. Participants independently completed paper surveys in our research evaluation facility to assess behavioral predictors of improvements in PA and physical function outcomes. As Be ACTIVE includes behavior change techniques from social cognitive theory, we measured social-environmental support [35], self-efficacy for PA [36–38], as well as depressive symptoms (Center for Epidemiologic Studies Depression scale, CESD), cognitive function (behavioral dyscontrol scale), and arthritis pain symptoms according to the Western Ontario and McMaster Universities Arthritis Index (WOMAC) [39–41].
As this was a pilot feasibility trial, we aimed to enroll 50 participants to ensure a stable effect size for our primary/secondary outcomes to power future trials and to estimate the clinical importance of these effects. Our block-stratified randomization sequence balanced covariates of age and gender between the two study arms—the randomization key was kept in a secure drive by a nonstudy staff member. We used an intention to treat approach to analyze all baseline and postintervention data from consented participants with linear mixed-effects models to account for correlations within patients [42]. R statistical software was used for all analyses [43].
RESULTS
We identified 267 eligible participants (Supplementary Fig. S1). Of these, 212 declined to participate, and 55 patients consented to participate, of whom 5 were ineligible to enroll based on a screening history and physical (Reach = 50/262 = 19%). Baseline demographics and other measures were not significantly different between study groups (Table 1). As compared to intervention participants (age: 65.5 ± 7.6 years and 46% female), there were similar age and gender demographics among the control participants (age of 66.5 ± 7.6 years and 46% female) and the eligible patients who declined participation (age of 67.6 ± 8.2 years and 56% female), respectively (p > .05 for each with comparison to the intervention group). The study population was racially diverse (~50% non-white) with good glycemic control (mean HbA1c ~7.0%) and had high rates of some physical function impairment at baseline (SPPB < 12). Among the n = 50 enrolled, 47 completed the intervention, and 3 dropped out of the Be ACTIVE group due to unrelated medical issues (i.e., spinal fracture in a motor vehicle accident, major depressive episode with suicidal ideation, exacerbation of chronic knee arthritis pain).
Table 1.
Baseline demographics and selected descriptive covariates of sample
| Demographic variable | Enhanced usual care (n = 22) | Intervention (n = 28) |
p-value |
|---|---|---|---|
| Age, years (mean, SD) | 66.5 (7.1) | 65.5 (7.6) | .61 |
| Gender (n, % female) | 13 (59.1%) | 13 (46.4%) | .55 |
| Race | .28 | ||
| White/Caucasian (n, %) | 12 (54.5%) | 14 (50%) | |
| Black/African American (n, %) | 4 (18.2%) | 10 (35.7%) | |
| Asian (n, %) | 1 (4.5%) | 2 (7.1%) | |
| Alaskan Native/Native American | 0 | 1 (3.6%) | |
| Ethnicity (% Hispanic) | 4 (20.0%) | 2 (8.0%) | .38 |
| Weight (kg) | 92.0 (21.4) | 90.6 (21.7) | .84 |
| Body Mass Index (kg/m2) | 32.8 (6.0) | 31.6 (6.7) | .52 |
| Hemoglobin A1c (%) | 6.7 (0.8) | 6.9 (1.3) | .43 |
| Systolic blood pressure (mm/Hg) | 125.6 (10.5) | 128.1 (9.4) | .39 |
| Diastolic blood pressure (mm/Hg) | 80.0 (8.1) | 83.6 (8.6) | .13 |
| Depressive symptoms by CESD | 10.2 (8.3) | 10.3 (8.3) | .96 |
| Endurance Self-efficacy to walk without stopping (range: 0–1,200) | 584.1 (288.9) | 695.0 (372.7) | .24 |
| Self-efficacy: Motivation to conduct physical activity(PA) amidst competing demands (0–60) | 43.8 (8.6) | 41.2 (9.8) | .32 |
| Self-efficacy for PA in presence of diabetes (0–800) | 587.3 (183.2) | 592.1 (158.6) | .92 |
| % participants with baseline functional impairment (SPPB< 12) | 17 (77.3) | 17 (60.7) | .35 |
| Baseline fitness level (VO2peak, ml/kg/min) | 18.3 (3.0) | 18.5 (4.7) | .84 |
CESD Center for Epidemiologic Studies Depression scale; PA physical activity; Data reported as mean (SD) for continuous variables, and n (%) for categorical values; Missing <5% data for each variable reported.
The mixed-methods evaluation results demonstrated highly favorable acceptability ratings: patients—92%; coaches/clinicians—100% (Table 2). Major acceptability themes for patients, coaches, and clinicians are described in Table 2, and included developing a “healthy aging” mindset for patients and professional satisfaction for clinic staff/clinicians. Fidelity was excellent: coaches—88%; clinicians—96% (Supplementary Table S1). In terms of safety, patients identified as low-risk for safety concerns in their initial clinician visit had no additional safety concerns arise during the subsequent in-person visits.
Table 2.
Quantitative and qualitative stakeholder perspectives on the Acceptability of Be ACTIVE
| Quantitative acceptability data | Qualitative themes and representative quotations | |
|---|---|---|
| Reach | 20% of eligible patients joined | |
| Would Recommend program (Patients) |
92% of patients would recommend Be ACTIVE to a friend or family member High retention rates: 88% High adherence to wearing the PA tracker: 97 ± 11% (mean ± SD of days the tracker was actually worn) |
Support and accountability provided by the coach was invaluable
“The most important thing was when you have a real person, when you sluff off on your Fitbit it doesn’t bark at you. (Coach) didn’t bark, but that personal interaction rendered accountability”. (D29) “(Coach) was asking specifically what I wanted to accomplish and how to accomplish that, so it made me stop and think about exactly what I was going to do. It kind of gave me something to motivate me and hold me accountable.” (D34) Valued the Physical Activity (PA) tracker to provide an accountable measure of progress “Before the program I would not walk but now I have a meter and I motivate myself to get up and walk. I don’t have to do it I want to do it.“(D5) Appreciated that clinicians/coaches guided them to be active safely “It was helpful because it reminded you what you should and should not do to be safe and healthy in doing your exercises.” (D70) Many perceived functional improvements and a “healthy aging” mindset as a benefit: “I’m able to move around in the house more. My walks…make me feel good generally.” (D2) “I found that activity helps as opposed to hurt you. Others are like ‘I’m old - I can’t do this.’ Where I’m like, ‘I’m old - I better get out of here and do it.’” (D48) Some perceived benefits of improved overall health/type 2 diabetes (T2D) care: “[I am motivated to be active for] keeping blood sugar at a lower level - it used to jump when I did not walk and did not do any exercise, but I noticed the more I walked the lower my sugar levels would go down and they stayed down.” (D2) “I got off a couple medications since the study started.” (D7) |
| Would Not Recommend (Patients) |
8% of patients |
Felt the program was missing more intense exercise training options relevant for them
“This program didn’t have what I needed. I needed a physical therapist or a personal trainer. For people who really aren’t doing anything at all, maybe this program would help.” (D6) |
| Would Recommend program (Coaches and Clinicians) |
100% (n = 4 coaches, n = 2 clinicians) would recommend the clinic continue to offer Be ACTIVE |
All coaches and clinicians felt that their role of encouraging behavior change (coach) and safety monitoring (clinician) aligned well with their clinical expertise, and was professionally rewarding.
“[For] people that have maybe less of a social network to have them achieve their goals… that every two-week call (with me) is a big anchor for them. I think that is rewarding.” (CO2) “We got to build rapport with the patients, we really got to know them. They appreciated our outreach…a lot of our other programs we (were) cold calling patients.” (CO4) “It was all pretty relevant. Stretching, strengthening, step counting - all basics of getting up and moving. Doing it safely and knowing how to prevent an injury. That’s all necessary.” (CL1) Coaches found these 3 themes most beneficial about the program: (1) Overcoming negative perceptions of what “counts” as PA “I had a patient who told me on the first call I hate working out, I don’t want to do it. (I said) taking your grandson on a walk, playing with him at the park can increase your step count. She started loving it, and her attitude from call 1 to call 6 was completely different.” (CO1) “…just trying to broaden the definition of activity (beyond being) in a gym. and heavily sweating… (I would say) no, it can be like walking around the block with your dog….” (CO3) (2) Accountability “…they were happy to report when they did well and a little embarrassed, even though that wasn’t from me, when they didn’t…hit the goal for themselves.” (CO4) (3) PA tracker “raises awareness of current PA levels” “I think the [PA tracker] certainly helped motivate them. It wasn’t just “oh I walked a little bit longer” they could actually see how much…further they walked. (CO2) |
PA physical activity; participant coding designated parenthetically after quotes uses D* to designate quotes from a patient; CO* to designate quotes from a coach, and CL* to designate quotes from a clinician.
In the analyses for Be ACTIVE feasibility, average times for coaching calls were ~25 minutes —equivalent to costs of ~$87.12 USD per patient for 6 calls over 12 weeks, as well as costs of PA tracker rental of $5.77 USD per patient (Supplementary Table S2). This was not a comprehensive cost analysis, and additional costs to recruit patients, coordinate PA data collection with patients, and to coordinate/train coaches were not estimated in this pilot study. Participation in the Medicare Chronic Care Management program at that time would have reimbursed $97.98 per patient for Be ACTIVE, including requiring $19.60 of that amount as patient co-pays (2017 USD). The qualitative data revealed key recommendations to improve the program, such as simplifying staff documentation, and providing a video training to patients on uploading PA data. See Supplementary Table S1 for details.
Pilot effectiveness outcome data
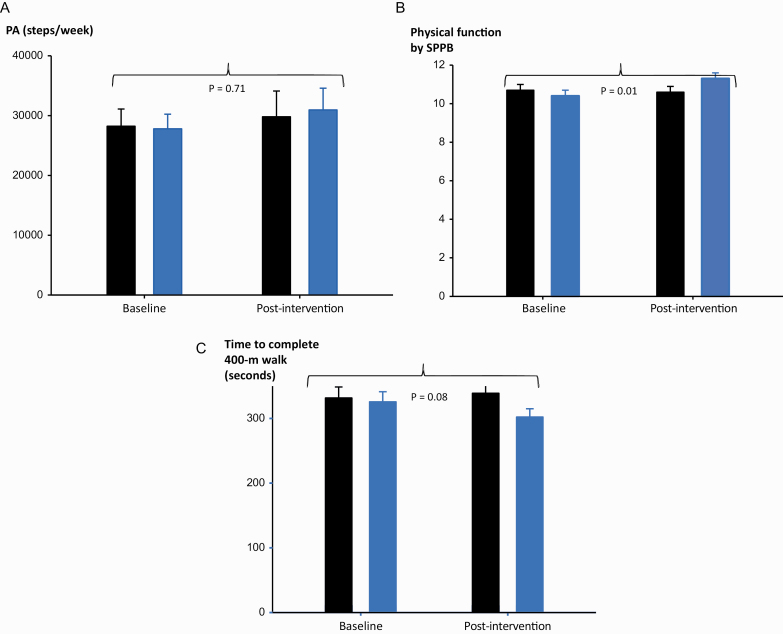
Absolute changes in pre-post PA increased (Fig. 2a), albeit not significantly, by ~11% in the Be ACTIVE group (+3155 steps/week, 95% CI: -−2169, +8478) and by ~6% in controls (+1581 steps/week, 95% CI: −5183, +8344), with a group difference of 1574 ± 4391 (mean ± SE, p = .72). Published data suggest that clinically important PA changes are ~4200 steps/week for populations with functional limitations (due to pulmonary disease) and ~7000 steps/week for healthy sedentary populations [26, 44]. Of note, participants wore the Actigraph© less than the 10 h/day “valid wear time” threshold on 43% of days in the 9-day sampling frame. Despite this large amount of missing data for discrete days, 92% of participants still had at least 3 valid days with ≥10 h/day wear time—the minimum standard for including an individual’s data for analysis [24]. Thus, we only had to exclude 8% of participants’ data from the PA outcome analysis due to insufficient wear time (baseline data: excluded n = 2 Be ACTIVE participants and n = 1 participant from the control group; post-intervention data: excluded n = 2 Be ACTIVE participants and n = 2 participants from the control group). However, we could typically only include 3–5 valid days of PA data to generate our participant PA estimates, leading to wider standard deviations and lower effect size estimates than if we had more “valid days” for our participants. Of note, there were no group differences in wear time (p = .90). Changes in moderate-to-vigorous intensity exercise levels were not significantly different between groups (group difference: −2.1 ± 17.0 min/week, mean ± SE, p = .90).
Fig. 2.
Be ACTIVE intervention increases Physical Function more than Physical Activity. Data shown compare group change between Be ACTIVE (Blue) vs. Enhanced Usual Care (Black) for outcomes of physical activity (PA, a), physical function by Short Physical Performance Battery (SPPB, b), and physical function by timed 400-meter walk (c).
In this pilot pragmatic trial, we aimed to obtain stable effect sizes for our primary and secondary outcomes, and were not powered to obtain statistically significant group differences. Our Cohen’s D effect sizes for our primary outcomes of PA and minutes/day of moderate-to-vigorous intensity exercise were .11 and <.10, respectively. For our secondary outcomes of physical function by SPPB, 400-m walk, and leg extension power, our Cohen’s D effect sizes were 0.6, 0.4, and 0.2, respectively. Despite limited statistical power in this small sample, Be ACTIVE yielded clinically important improvements in physical function [28, 29], as measured by the SPPB and 400-meter walk (Figs. 2b and c). SPPB scores increased by a clinically important >0.5-point difference in the Be ACTIVE group (+0.9, 95%CI: 0.1, 1.8) and decreased slightly (−0.1, 95% CI: −0.6, 0.4) in the control group (p = .01 for group difference over time). In addition, the mean change in 400-m walk time improved by a clinically important ≥20 s faster in Be ACTIVE versus control (Be ACTIVE: −23.4 s, 95% CI: −46.5, −0.3; control: +7.3, 95% CI: −17.6, 32.3; p = .08 for group difference over time).
Changes in behavioral and glycemic control outcomes are summarized in Supplementary Table S2, and are pertinent for significant improvements in the Be ACTIVE versus control group for self-efficacy to walk longer distances (p = .02), and social-environmental support (p < .01).
DISCUSSION
In this pilot pragmatic trial, both patients and clinic staff found Be ACTIVE to be highly acceptable and feasible. The coaching process led patients with T2D to change their “mindset” about PA and set personally enjoyable PA goals. In addition, Be ACTIVE led to significant improvements in behavioral constructs that predict regular PA, including self-efficacy and social support. In contrast, pre-post PA increased by ~11% (Be ACTIVE) and ~6% in controls with a group difference less than the minimal clinically important levels of 4200 steps/week for populations with functional limitations [44]. However, we did observe clinically important and statistically significant improvements in an objective physical function measure (SPPB) linked to lower all-cause mortality and lower risk of falls [28].
T2D has been identified as a model of premature aging and impairment in physical function [45]; thus, pragmatic treatments to prevent functional decline are essential. Strikingly, >60% of this study population was relatively frail at baseline (i.e., SPPB < 12). Be ACTIVE has similar core components of regular walking and multi-muscle resistance exercises as other interventions shown to improve physical function in frail people, such as the OTAGO program [46]. A key difference between Be ACTIVE and OTAGO is the focus on engaging clinic and patient stakeholders to design Be ACTIVE in a way that will be feasible and acceptable. One other point relevant to patient stakeholders is the flexibility of the program for varying levels of functional impairment. To illustrate the flexibility of Be ACTIVE in this way, our coaching templates recommend that patients who have severe pain with walking consider non-weight-bearing activities, or select modest walking goals that are tailored to their ability level (e.g., 10%–20% increase from baseline step count).
Considering key aspects of program feasibility, such as program costs, is an important element of pragmatic trials. The potential reimbursements for this program through the Medicare Chronic Care Management program (~$97 USD) [47] were slightly higher than the program costs for the coaching (~$88 USD) and PA tracker (~$6 USD). However, that reimbursement approach requires patient to pay a co-payment, which the literature suggests patients are reluctant to do for primary prevention activities [48], and there were other costs borne by the research team to coordinate program aspects such as patient recruitment and PA data coordination that would need to be accounted for to sustain this program. Thus, other models of financial support within an institution would likely be necessary to successfully sustain Be ACTIVE, such as adding this program coaching role to the lifestyle behavioral counseling done by existing salaried behavioral health team members.
This was a pilot trial—it was not powered to yield statistically significant improvements in the outcomes studied but rather to obtain a robust estimate of the effect size of delivering Be ACTIVE for future studies. We observed moderately strong effect sizes for our secondary physical function outcomes (Cohen’s D of 0.2–0.6), but very limited effect sizes for PA in steps or moderate-vigorous intensity exercise (Cohen’s D of ~0.1). We also demonstrated statistically significant improvements in the SPPB physical function outcome, as well as significant improvements in self-efficacy and social-environmental support. However, a major question is why there was a weaker relative signal for the pilot effect size of Be ACTIVE on objective PA outcomes as compared with the stronger signal observed for objective physical function outcomes and reported PA self-efficacy. This was not expected—changes in physical function and PA self-efficacy are generally linked to PA behavior [12, 28, 29]. One possible explanation is a need for a longer intervention duration to obtain a sufficient “dose”—as due to the functional impairments of many participants, their initial PA goals were often modest, and they may need a longer period of coaching to make greater gains. In support of this notion, a post-hoc analysis of FitBit© data revealed a steady progression toward goals across the coaching calls (range: 60%–79% average progress towards goal, p > .05 for time trend). Prior studies have also found PA interventions >12 weeks are more effective than shorter programs [7]. Another possible explanation is that our missing PA accelerometer data widened the observed standard deviation, thus constraining the measured effect size. Future trials should add further safeguards to adherence for PA accelerometer data collection [24] to address this missing data issue.
There are several limitations of this pilot trial: its findings must be replicated with more varied sites, more efficient recruitment approaches are warranted to expand reach, and other reimbursement sources should be identified to promote feasibility. In addition, our methods did not allow us to assess for breaks in sedentary time—an important predictor of health, particularly among populations with physical function impairment [49]—breaks in sedentary time will be important to evaluate in our future work. Spillover contamination effects are a potential concern for all studies randomizing at the individual level. However, we expect minimal spillover effects in our case, as the clinic staff who delivered the PA coaching only interacted with participants in the intervention group. The clinician champion in each clinic may have seen control group patients as part of their routine clinical care, but their role in the intervention was solely to supervise safety rather than to provide PA coaching. Our use of coaching templates in the Epic electronic health record also limits its generalizability. Importantly, the templates we created aligned with the workflow for staff (which has been shown to promote sustainability), Epic© is currently the most prevalent electronic health record used in the United States [50], and our templates could be adapted for use in other electronic health records that allow users to create boilerplate templates. This pilot also has key strengths: pragmatic eligibility criteria, racially diverse participants, integrated mixed-methods evaluation, and a pre-implementation phase engaging patient and clinic stakeholders in the implementation strategies selected (Fig. 1).
CONCLUSIONS
Overall, this study represents an advance in developing and testing a pragmatic integrated PA coaching program derived from existing evidence-based and pragmatic PA interventions. Be ACTIVE has substantial potential to benefit patients with T2D based on the clinically important physical function improvements and the acceptability to key stakeholders demonstrated in this pilot work. The next step will be to refine and test the impact of Be ACTIVE on effectiveness and implementation outcomes in a fully-powered pragmatic trial.
Supplementary Material
Acknowledgments
This study was funded by the National Institutes of Health (K23 HL118133 funding to AGH). The content of this manuscript does not necessarily reflect NIH views. The authors would like to acknowledge and thank our research assistants for their assistance with data entry and the participant recruitment process, including Deirdre Rafferty and Katherine Littlefield.
Compliance with Ethical Standards
Conflict of Interest AGH, JEBR, and JGR report investigator-initiated funding from Merck, Inc. during the study funding period that was unrelated to the present study’s objectives or findings. All other authors report no conflicts of interest.
Authors’ Contributions: AGH, REG, JDR, JES-L, JEBR, JGR, and ALD conceived and planned the research. AGH, IML, KC, and SL carried out the experiments. All authors contributed to the interpretation of the results. AGH took the lead in writing the manuscript, and all authors provided critical feedback and edits on the manuscript.
Primary Data Findings reported have not been previously published and this manuscript is not being simultaneously submitted elsewhere. Data have not been previously reported elsewhere. The authors have full control of all primary data and agree to allow the Journal to review data if requested.
Ethical Approval All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards. This article does not contain any studies with animals performed by any of the authors.
Informed Consent Informed consent was obtained from all individual participants included in the study.
Transparency statement Trial Registration: Clinicaltrials.gov, NCT02473926, registered April 24, 2015.
References
- 1. Zhao G, Ford ES, Li C, Balluz LS. Physical activity in U.S. older adults with diabetes mellitus: Prevalence and correlates of meeting physical activity recommendations. J Am Geriatr Soc. 2011;59(1):132–137. [DOI] [PubMed] [Google Scholar]
- 2. Saint-Maurice PF, Troiano RP, Berrigan D, Kraus WE, Matthews CE. Volume of light versus moderate-to-vigorous physical activity: Similar benefits for all-cause mortality? J Am Heart Assoc. 2018;7(7):e008815. doi: 10.1161/JAHA.118.008815 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Gregg EW, Menke A. Diabetes and disability. In: Diabetes in America, 3rd ed., National Institute of Diabetes and Digestive and Kidney Diseases (NIDDK). 2018:34.31–34.15. https://www.niddk.nih.gov/about-niddk/strategic-plans-reports/diabetes-in-america-3rd-edition. [Google Scholar]
- 4. Rejeski WJ, Ip EH, Bertoni AG, et al. ; Look AHEAD Research Group . Lifestyle change and mobility in obese adults with type 2 diabetes. N Engl J Med. 2012;366(13):1209–1217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Kirk A, Mutrie N, MacIntyre P, Fisher M. Effects of a 12-month physical activity counselling intervention on glycaemic control and on the status of cardiovascular risk factors in people with Type 2 diabetes. Diabetologia. 2004;47(5):821–832. [DOI] [PubMed] [Google Scholar]
- 6. Korkiakangas EE, Alahuhta MA, Laitinen JH. Barriers to regular exercise among adults at high risk or diagnosed with type 2 diabetes: A systematic review. Health Promot Int. 2009;24(4):416–427. [DOI] [PubMed] [Google Scholar]
- 7. Luoma KA, Leavitt IM, Marrs JC, et al. How can clinical practices pragmatically increase physical activity for patients with type 2 diabetes? A systematic review. Transl Behav Med. 2017;7(4):751–772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Nilsen P. Making sense of implementation theories, models and frameworks. Implement Sci. 2015;10:53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Michie S, Richardson M, Johnston M, et al. The behavior change technique taxonomy (v1) of 93 hierarchically clustered techniques: Building an international consensus for the reporting of behavior change interventions. Ann Behav Med. 2013;46(1):81–95. [DOI] [PubMed] [Google Scholar]
- 10. Prohaska JO, DiClemente CC. Transtheoretical therapy: Toward a more integrative model of change. Psychother: Theory Res Pract. 1982;20:161–173. [Google Scholar]
- 11. Ryan RM, Deci EL. Self-determination theory and the facilitation of intrinsic motivation, social development, and well-being. Am Psychol. 2000;55(1):68–78. [DOI] [PubMed] [Google Scholar]
- 12. Allen NA. Social cognitive theory in diabetes exercise research: An integrative literature review. Diabetes Educ. 2004;30(5):805–819. [DOI] [PubMed] [Google Scholar]
- 13. Wilcox S, Dowda M, Leviton LC, et al. Active for life: Final results from the translation of two physical activity programs. Am J Prev Med. 2008;35(4):340–351. [DOI] [PubMed] [Google Scholar]
- 14. Physical Activity Guidelines Advisory Committee. Physical Activity Guidelines Committee Report.Washington, D.C.: U.S. Department of Health and Human Services, 2008. https://health.gov/sites/default/files/2019-10/CommitteeReport_7.pdf. Accessed February 17, 2022. [Google Scholar]
- 15. Blair SN, Applegate WB, Dunn AL, et al. Activity Counseling Trial (ACT): Rationale, design, and methods. Activity Counseling Trial Research Group. Med Sci Sports Exerc. 1998;30(7):1097–1106. [DOI] [PubMed] [Google Scholar]
- 16. Centers for Disease Control and Prevention. Stopping Elderly Accidents, Deaths, and Injuries (STEADI) Toolkit, 2019. https://www.cdc.gov/steadi/materials.html. Accessed February 17, 2022.
- 17. Ismail Z, Rajji TK, Shulman KI. Brief cognitive screening instruments: An update. Int J Geriatr Psychiatry. 2010;25(2):111–120. [DOI] [PubMed] [Google Scholar]
- 18. Creswell JW, Plano Clark VL, Gutman ML, Hanson WE. Advanced mixed methods research designs. In: Teddlie ATC, ed. Handbook of Mixed Methods in Social and Behavioral Research. Thousand Oaks, CA: Sage; 2003:209–240. [Google Scholar]
- 19. Creswell JW, Klassen AC, Plano Clark VL, Clegg Smith K. Best Practices for Mixed Methods Research in the Health Sciences, 2011. https://obssr.od.nih.gov/research-resources/mixed-methods-research. Accessed February 17, 2022.
- 20. Lewis CC, Proctor EK, Brownson RC. Measurement issues in dissemination and implementation research. In: Dissemination and Implementation Research in Health, 2nd ed. New York, NY: Oxford University Press; 2018:229–244. [Google Scholar]
- 21. Roberts SLE, Healey A, Sevdalis N. Use of health economic evaluation in the implementation and improvement science fields: A systematic literature review. Implement Sci. 2019;14(1):72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Keel G, Savage C, Rafiq M, Mazzocato P. Time-driven activity-based costing in health care: A systematic review of the literature. Health Policy. 2017;121(7):755–763. [DOI] [PubMed] [Google Scholar]
- 23. Hsieh HF, Shannon SE. Three approaches to qualitative content analysis. Qual Health Res. 2005;15(9):1277–1288. [DOI] [PubMed] [Google Scholar]
- 24. Migueles JH, Cadenas-Sanchez C, Ekelund U, et al. Accelerometer data collection and processing criteria to assess physical activity and other outcomes: A systematic review and practical considerations. Sports Med. 2017;47(9):1821–1845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. National Institutes of Health, National Cancer Institute. SAS Programs for Analyzing NHANES 2003-2004 Accelerometer Data. https://epi.grants.cancer.gov/nhanes-pam/. Accessed February 17, 2022.
- 26. Kraus WE, Janz KF, Powell KE, et al. ; 2018 Physical Activity Guidelines Advisory Committee . Daily step counts for measuring physical activity exposure and its relation to health. Med Sci Sports Exerc. 2019;51(6):1206–1212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Sluik D, Buijsse B, Muckelbauer R, et al. Physical activity and mortality in individuals with diabetes mellitus: A prospective study and meta-analysis. Arch Intern Med. 2012;172(17):1285–1295. [DOI] [PubMed] [Google Scholar]
- 28. Guralnik JM, Simonsick EM, Ferrucci L, et al. A short physical performance battery assessing lower extremity function: association with self-reported disability and prediction of mortality and nursing home admission. J Gerontol. 1994;49(2):M85–M94. [DOI] [PubMed] [Google Scholar]
- 29. Guralnik JM, Ferrucci L, Pieper CF, et al. Lower extremity function and subsequent disability: Consistency across studies, predictive models, and value of gait speed alone compared with the short physical performance battery. J Gerontol A Biol Sci Med Sci. 2000;55(4):M221–M231. [DOI] [PubMed] [Google Scholar]
- 30. Bassey EJ, Short AH. A new method for measuring power output in a single leg extension: feasibility, reliability and validity. Eur J Appl Physiol Occup Physiol. 1990;60(5):385–390. [DOI] [PubMed] [Google Scholar]
- 31. Santanasto AJ, Glynn NW, Lovato LC, et al. ; LIFE Study Group . Effect of physical activity versus health education on physical function, grip strength and mobility. J Am Geriatr Soc. 2017;65(7):1427–1433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Zech A, Steib S, Sportwiss D, Freiberger E, Pfeifer K. Functional muscle power testing in young, middle-aged, and community-dwelling nonfrail and prefrail older adults. Arch Phys Med Rehabil. 2011;92(6):967–971. [DOI] [PubMed] [Google Scholar]
- 33. John WG. Hemoglobin A1c measurement: New precise immunoassay method involving latex particle agglutination. Clin Chem. 1996;42(11):1874–1875. [PubMed] [Google Scholar]
- 34. American College of Sports Medicine. Clinical exercise testing. In: Pescatello LS, ed. ACSM’s Guidelines for Exercise Testing and Prescription, 9th ed. Baltimore, MD: Lippincott Williams & Wilkins, 2013;114–137. [Google Scholar]
- 35. Wilson W, Ary DV, Biglan A, Glasgow RE, Toobert DJ, Campbell DR. Psychosocial predictors of self-care behaviors (compliance) and glycemic control in non-insulin-dependent diabetes mellitus. Diabetes Care. 1986;9(6):614–622. [DOI] [PubMed] [Google Scholar]
- 36. Hu L, McAuley E, Motl RW, Konopack JF. Influence of self-efficacy on the functional relationship between ratings of perceived exertion and exercise intensity. J Cardiopulm Rehabil Prev. 2007;27(5):303–8; quiz 309. [DOI] [PubMed] [Google Scholar]
- 37. Sallis JF, Pinski RB, Grossman RM, Patterson TL, Nader PR. The development of self-efficacy scales for health-related diet and exercise behaviors. Health Educ Res. 1988;3:10. [Google Scholar]
- 38. Lorig K, Chastain RL, Ung E, Shoor S, Holman HR. Development and evaluation of a scale to measure perceived self-efficacy in people with arthritis. Arthritis Rheum. 1989;32(1):37–44. [DOI] [PubMed] [Google Scholar]
- 39. Weissman MM, Sholomskas D, Pottenger M, Prusoff BA, Locke BZ. Assessing depressive symptoms in five psychiatric populations: A validation study. Am J Epidemiol. 1977;106(3):203–214. [DOI] [PubMed] [Google Scholar]
- 40. Grigsby J, Kaye K, Robbins LJ. Reliabilities, norms and factor structure of the Behavioral Dyscontrol Scale. Percept Mot Skills. 1992;74(3 Pt 1):883–892. [DOI] [PubMed] [Google Scholar]
- 41. Bellamy N, Buchanan WW, Goldsmith CH, Campbell J, Stitt LW. Validation study of WOMAC: A health status instrument for measuring clinically important patient relevant outcomes to antirheumatic drug therapy in patients with osteoarthritis of the hip or knee. J Rheumatol. 1988;15(12):1833–1840. [PubMed] [Google Scholar]
- 42. Fitzmaurice GM, Laird NM, Ware JH.. Applied Longitudinal Analysis. Hoboken, N.J.: Wiley-Interscience; 2004. [Google Scholar]
- 43. R Core Team. R: A Language and Environment for Statistical Computing, 2018. https://www.R-project.org/.
- 44. Demeyer H, Burtin C, Hornikx M, et al. The minimal important difference in physical activity in patients with COPD. PLoS One. 2016;11(4):e0154587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Huebschmann AG, Kohrt WM, Regensteiner JG. Exercise attenuates the premature cardiovascular aging effects of type 2 diabetes mellitus. Vasc Med. 2011;16(5):378–390. [DOI] [PubMed] [Google Scholar]
- 46. Kocic M, Stojanovic Z, Nikolic D, et al. The effectiveness of group Otago exercise program on physical function in nursing home residents older than 65 years: A randomized controlled trial. Arch Gerontol Geriatr. 2018;75:112–118. [DOI] [PubMed] [Google Scholar]
- 47. Centers for Medicare and Medicaid Services. Chronic Care Management Services, 2019. https://www.cms.gov/Outreach-and-Education/Medicare-Learning-Network-MLN/MLNProducts/Downloads/ChronicCareManagement.pdf.
- 48. Rezayatmand R, Pavlova M, Groot W. The impact of out-of-pocket payments on prevention and health-related lifestyle: A systematic literature review. Eur J Public Health. 2013;23(1):74–79. [DOI] [PubMed] [Google Scholar]
- 49. Barone Gibbs B, Aaby D, Siddique J, et al. Bidirectional 10-year associations of accelerometer-measured sedentary behavior and activity categories with weight among middle-aged adults. Int J Obes. 2020;44(3):559–567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Shull JG. Digital health and the state of interoperable electronic health records. JMIR Med Inform. 2019;7(4):e12712. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.