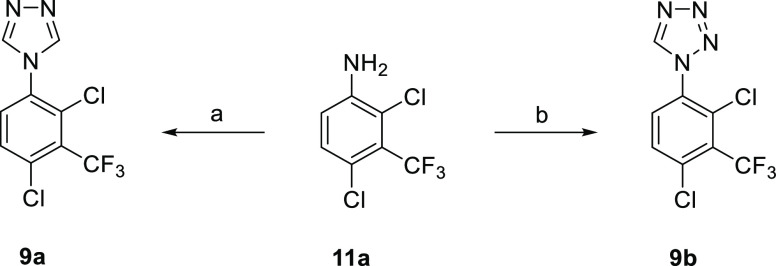
Abstract
Notum is a carboxylesterase that suppresses Wnt signaling through deacylation of an essential palmitoleate group on Wnt proteins. There is a growing understanding of the role Notum plays in human diseases such as colorectal cancer and Alzheimer’s disease, supporting the need to discover improved inhibitors, especially for use in models of neurodegeneration. Here, we have described the discovery and profile of 8l (ARUK3001185) as a potent, selective, and brain-penetrant inhibitor of Notum activity suitable for oral dosing in rodent models of disease. Crystallographic fragment screening of the Diamond-SGC Poised Library for binding to Notum, supported by a biochemical enzyme assay to rank inhibition activity, identified 6a and 6b as a pair of outstanding hits. Fragment development of 6 delivered 8l that restored Wnt signaling in the presence of Notum in a cell-based reporter assay. Assessment in pharmacology screens showed 8l to be selective against serine hydrolases, kinases, and drug targets.
Introduction
The Wnt signaling pathway is a complex, evolutionarily conserved system from Drosophila and Caenorhabditis elegans through to mammals, which plays key roles in both embryonic development and the adult animal. These roles include cell fate determination and maintenance, and cell division in many different tissues.1 The “canonical Wnt signaling pathway” is characterized by the secreted activator (or agonist) Wnt proteins, which initiate an intracellular signaling cascade by binding to the cell surface receptor (a member of the Frizzled family of cell surface proteins, complexed with the coreceptor LDL-receptor-related protein family 5/6).1 The intracellular signaling cascade utilizes β-catenin and GSK3β to activate gene transcription via T-cell factor (TCF)/lymphoid enhancing factor (LEF) transcription factors. This signaling system is tightly regulated by a complex network of modulators and feedback inhibitory proteins, including the family of dickkopf (Dkk) proteins2 and the carboxylesterase Notum.3,4 Given the pleiotropic and essential function of Wnt signaling, it is unsurprising that aberrant Wnt signaling can contribute to disease in humans, including certain cancers, diabetes, and osteoporosis.1,5 In the mammalian brain, Wnt signaling performs several important roles, including the maintenance of synapse function,6 modulating microglial signaling and survival,7 neurogenesis,8 and maintaining the integrity and function of the blood–brain barrier (BBB).9,10 In Alzheimer’s disease (AD), when the Wnt signaling tone is disrupted by an increase in the inhibitory Dkk proteins, this leads to synapse loss, which may contribute to cognitive impairment.11 In stroke, loss of Wnt signaling leads to BBB breakdown, contributing to the pathology.12,13 In a model of multiple sclerosis, reactivation of Wnt signaling at the BBB partially restores functional BBB integrity and limits immune cell infiltration into the CNS.14 Therefore, augmenting Wnt signaling in these disorders of the central nervous system (CNS) has therapeutic potential.
Notum is a negative regulator of the Wnt signaling pathway. It is a secreted protein that functions as a carboxylesterase, removing a palmitoleate ester from a conserved serine residue in Wnt proteins that is essential for its binding and activation of the cell surface coreceptors.3 While first identified in Drosophila, its function in mammals, in both healthy and diseased, is being increasingly explored. The key tools to enable this progress are fit-for-purpose small-molecule inhibitors of Notum carboxylesterase activity.15,16
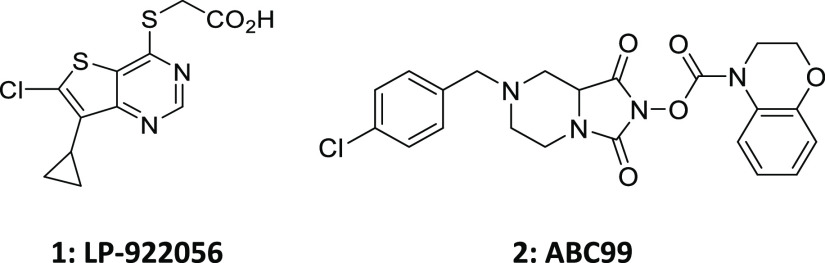
First-generation inhibitors of Notum activity were LP-922056 (1)17 and the irreversible inhibitor ABC99 (2)18 that have proved to be valuable research tools in exploring the link between pharmacological inhibition of Notum activity and disease association in rodent models (Figure 1). LP-922056 is a potent inhibitor of Notum suitable for chronic, oral dosing in rodents discovered through the optimization of a high-throughput screening (HTS) hit.17 LP-922056 was used to show that inhibition of Notum activity is a potential novel anabolic therapy for strengthening the cortical bone and preventing non-vertebral fractures.19 Notum has recently been identified as a key mediator for people at high risk of developing colorectal cancer.20,21 Pharmacological inhibition of Notum with LP-922056 abolished the ability of Apc-mutant cells to expand and form intestinal adenomas.21 ABC99 is a potent and selective covalent inhibitor of Notum activity developed by competitive activity-based protein profiling (ABPP) from a library of activated carbamates.18 Inhibition of Notum produced by Paneth cells in mice with ABC99 restored the regeneration of aged intestinal epithelium.22 In the brain, Notum regulates neurogenesis in the subventricular zone (SVZ) by modulating Wnt signaling, and inhibition of Notum with ABC99 leads to an activation of Wnt signaling and increased proliferation in the SVZ.8
Figure 1.
First-generation Notum inhibitors used in disease association studies.
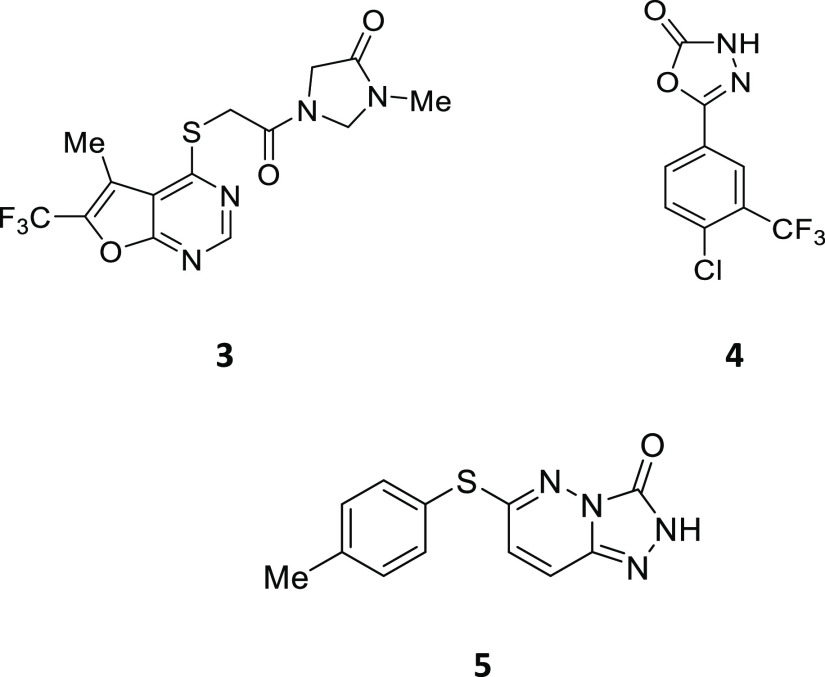
A number of structurally distinct Notum inhibitors have been reported from hit-to-lead programs (Figure 2).23−27 However, most of these inhibitors have limited brain penetration as directly measured in mouse pharmacokinetic (PK) studies;24,26−28 this restricts their use to peripheral (non-CNS) indications. Only ABC99 has been reported to reach the brain and activate the Wnt pathway as assessed by qRT-PCR of Axin2 in the ventricular-SVZ tissue following an intraperitoneal (i.p.) injection to Nestin-CFP mice.8
Figure 2.
Representative chemical structures of Notum inhibitors.
Our objective was to identify a screening hit that could be optimized to a potent, selective, and brain-penetrant inhibitor of Notum activity suitable for chronic, oral delivery. Such an inhibitor would complement LP-922056 and ABC99 as it could be administered in rodent models of neurodegenerative disease.
Fragment-based drug discovery (FBDD) is an established method for hit finding of biologically active compounds and is now supported by multiple complementary screening technologies.29,30 The concept of a “fragment” has essentially remained consistent with an emphasis on small, low-molecular-weight organic molecules with high aqueous solubility as described by Congreve et al.31 In contrast, there is an increase in the application of FBDD to more challenging, non-traditional targets such as GPCRs and ion channels, and to phenotypical screens including parasites.29 The creation of fragment libraries with a clearly defined purpose beyond chemical diversity has also received considerable attention. Examples of such libraries include reactive covalent fragments,32 3D fragments,33 natural product-like compounds,34 very small fragments such as FragLites and Minifrags,35 halogen-enriched fragments,36 and synthetically poised fragment libraries.37
The original Diamond-SGC Poised Library (DSPL) was created by Cox et al. as a 768-membered fragment library that was designed to allow rapid follow-up synthesis to provide quick structure–activity relationships (SARs) around fragment screening hits. Each member of the collection contained at least one functional group that could be created with reliable chemical reactions, that is, the fragment library was created to be structurally diverse and synthetically enabled.37,38
Herein, we describe the discovery of crystallographic fragment screening hits 6a and 6b from the DSPL and their optimization to identify 1-phenyl-1,2,3-triazoles as a new Notum inhibitor chemotype. We present the profile of lead compound 8l that supports it to be a potent, selective, and brain-penetrant inhibitor of Notum activity.
Results and Discussion
In order to find hit compounds for the development of new Notum inhibitors, we chose to conduct a crystallographic fragment screen. The structure of Notum has a clearly defined lipophilic pocket adjacent to the catalytic triad that accommodates a C16-lipid palmitoleate group of Wnt.3,39 The size and topography of this pocket gave encouragement that suitable fragments could be found to bind as the pocket has been assessed to be classically druggable.40
An X-ray crystallography-based fragment screen was performed at the XChem platform of Diamond Light Source (Oxford, U.K.). The highly automated XChem platform, in combination with synchrotron beamline I04-1, enabled us to efficiently screen 768 compounds from the DSPL fragment library for binding to Notum. Compounds were individually soaked into crystals of Notum and high-resolution structures were determined for each of these putative Notum–fragment complexes. The electron density maps provided evidence of compound binding associated with the active site of Notum for 59 of the crystal structures (hit rate: 7.7%).23,26,41 The complete output of the hits from this crystallographic screen with the DPSL will be published separately.42,43 Hit validation was then performed by demonstration that these binders were able to inhibit Notum carboxylesterase activity in a biochemical assay (up to 1 mM).
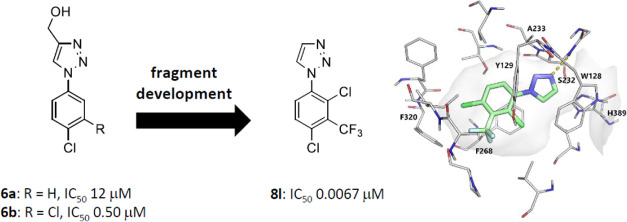
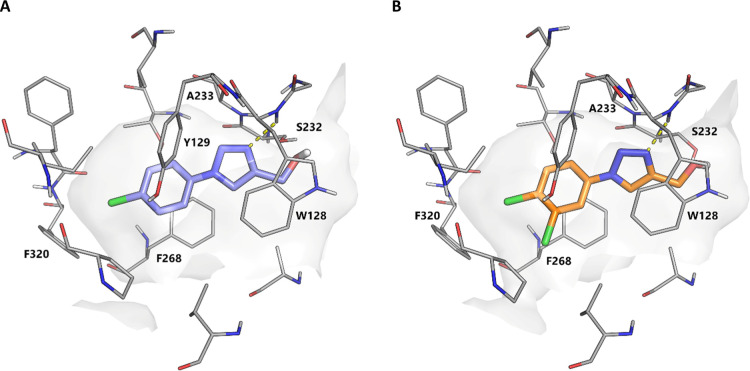
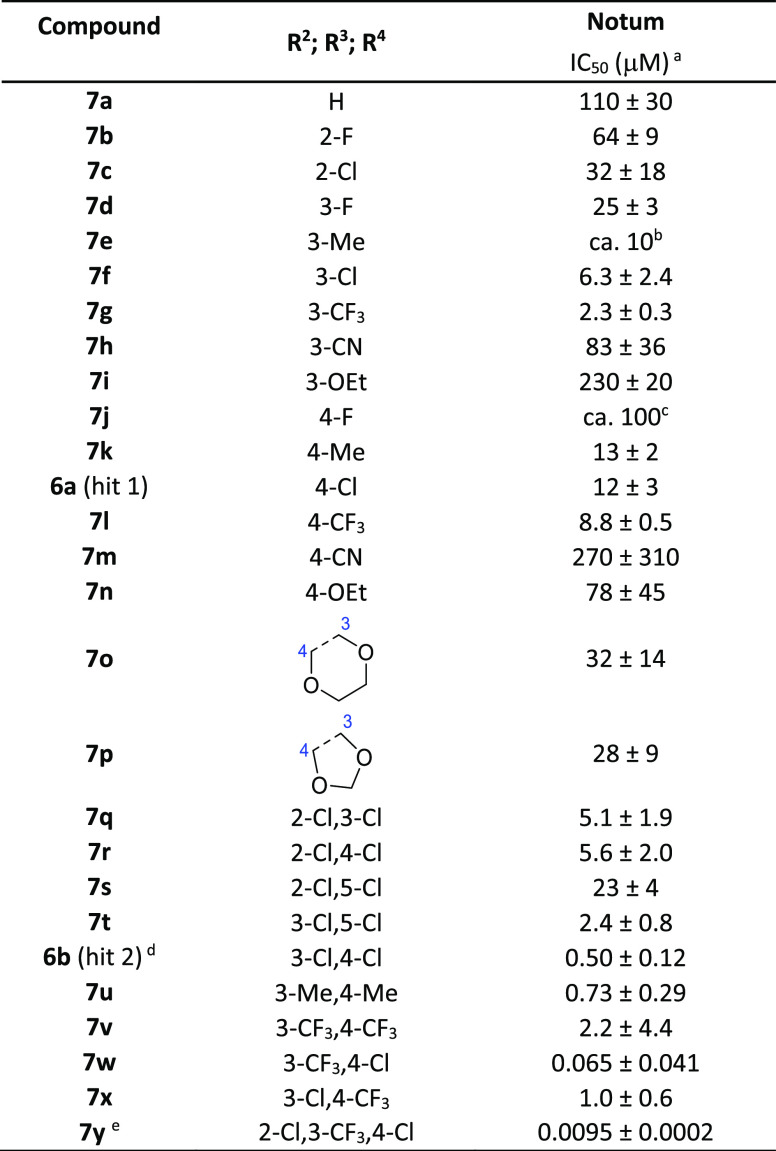
A pair of outstanding hits were 4-(hydroxymethyl)triazoles 6a (IC50 12 μM)26 and 6b (IC50 0.5 μM), which also highlighted some early beneficial SAR (Figure 3). A 3,4-Cl2 disubstituted phenyl ring conferred a substantial improvement in Notum inhibition when compared to a single 4-Cl (23-fold). Both the Notum-6a and -6b X-ray structures showed that the ligand formed a H-bond between N3 of the triazole with the backbone of Trp128. The increase in potency of 6b over 6a can be attributed to the improved occupancy of the palmitoleate pocket by the 3,4-Cl2 disubstituted phenyl ring (Figures 4 and S1). Specific interactions in the pocket include the potential stacking of the ligand with Trp128, Tyr129, and Phe268 as well as interactions by the phenyl ring substituents with Phe320.
Figure 3.
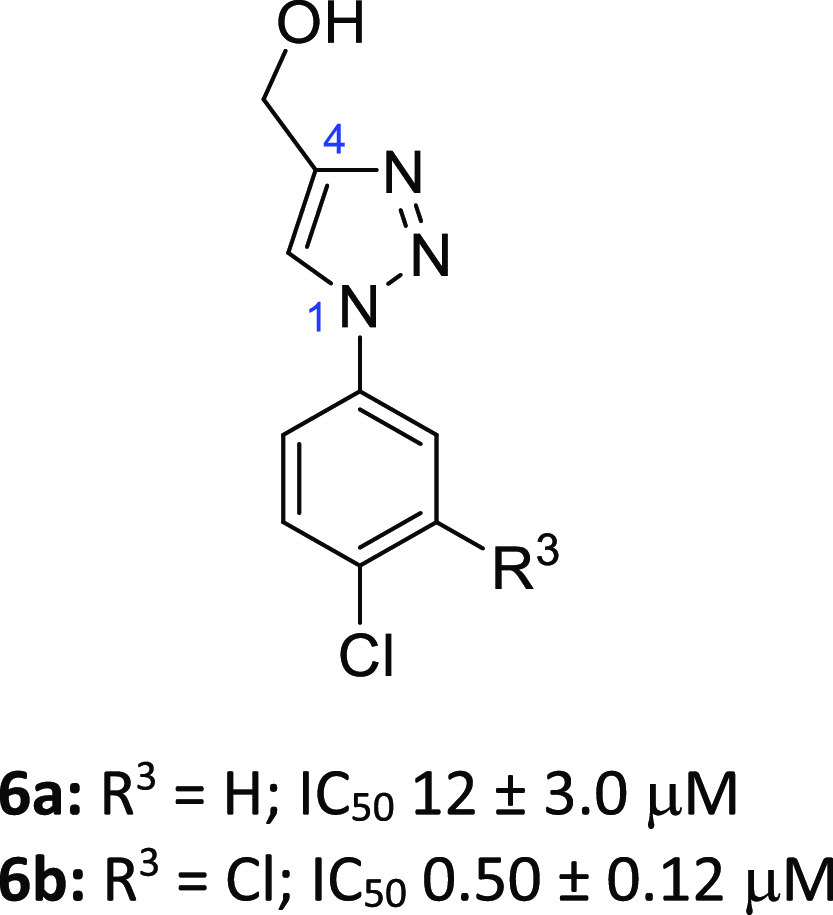
Preferred hits 6a and 6b from the crystallographic screen of the DSPL fragment library.
Figure 4.
(A) Structure of 6a (PDB: 6ZUV). N3 of the triazole forms a hydrogen bond with the backbone of Trp128 (dashed line, 2.36Å) (ref (26)). (B) Structure of 6b (PDB: 7B89). The compound forms a hydrogen bond with the backbone of Trp128 (dashed line, 2.21 Å). Select binding site residues are shown. Water molecules have been removed for clarity. The surface of the Notum binding pocket is outlined (gray).
Triazole 6b has molecular and physicochemical properties consistent with lead-like chemical space44 (MW 2444; clogP, 2.2; LogD7.4, 2.4; TPSA 51 Å2) and scored high when assessed by design metrics (LE 0.59; LLE 4.1),45,46 including a favorable prediction of brain penetration (CNS MPO 5.6/6.0; BBB Score 4.9/6.0).47,48 Triazole 6b was further evaluated in standard in vitro ADME assays and showed good aqueous solubility, moderate stability in liver microsomes, and excellent cell permeability (Table 1).
Table 1. Notum Inhibition SAR of Substituted (1-Phenyl-1H-1,2,3-triazol-4-yl)methanols 6 and 7.
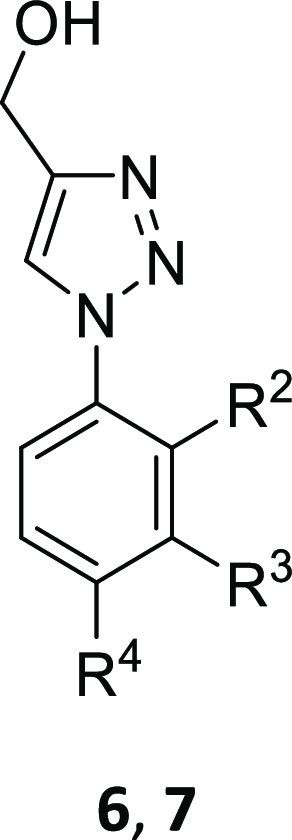
All values are mean ± s.d. of n = 2–13 experiments quoted to 2 s.f.
40–50% I@10 μM.
40–50% I@100 μM.
In vitro ADME data for 6b: LogD7.4 2.4; aqueous solubility, 100 μg/mL; mouse liver microsomes (MLM), Cli 88 μL/min/mg protein; human liver microsomes (HLM), Cli 12 μL/min/mg protein; MDCK-MDR1, AB/BA Papp 57/59 × 10–6 cm s–1; efflux ratio (ER), 1.0.
In vitro ADME data for 7y: aqueous solubility, 210 μg/mL; MLM, Cli 6.7 μL/min/mg protein; mouse hepatocytes, Cli 48 μL/min/106 cells; MDCK-MDR1, AB/BA Papp 46/28 × 10–6 cm s–1; ER, 0.61.
Hits 6 were an attractive starting point to improve Notum activity and develop PK properties for oral dosing to achieve good plasma exposure and brain penetration.
The optimization of 6 to deliver this profile was directed at three principle areas of these hit structures: (1) aryl ring 7 (Table 1); (2) the C4 substituent on triazole 8 (Table 2); and (3) further fine-tuning of the triazole 9 (Table 3). Inhibition of Notum activity was routinely measured in a biochemical assay with human Notum (81–451 Cys330Ser) as the enzyme and trisodium 8-octanoyloxypyrene-1,3,6-trisulfonate (OPTS) as the substrate.23−26 This assay was used to establish SAR and to identify preferred leads for additional studies. As Notum activity was developed, representative examples were screened in standard in vitro ADME assays to assess their suitability for progression to mouse PK studies.
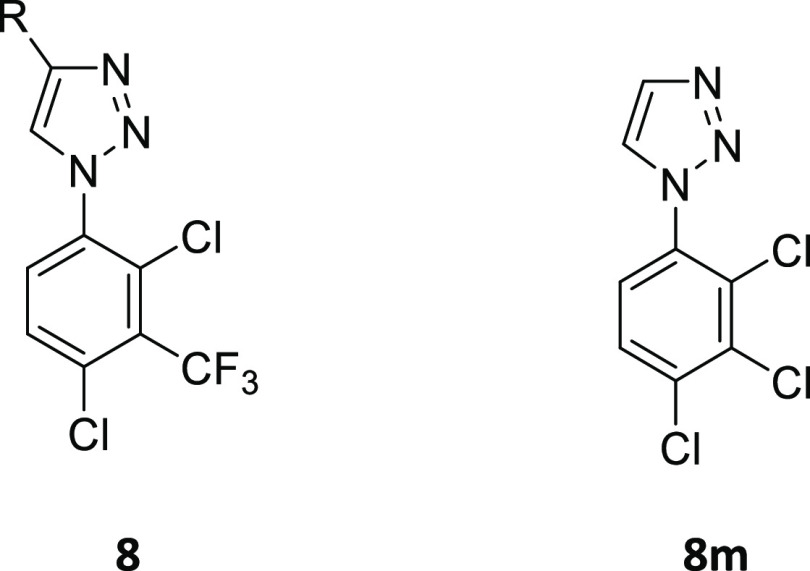
Table 2. Notum Inhibition SAR of Substituted 1-(2,4-Dichloro-3-(trifluoromethyl)phenyl)-1H-1,2,3-triazoles 8.
All values are mean ± s.d. of n = 2–6 experiments quoted to 2 s.f.
n > 20.
Table 3. Notum Inhibition, Aqueous Solubility, Microsomal Stability, and Cell Permeability of 7y, 8l, and 9.
| compound | 7y | 8l | 9a | 9b | 9c | d2-8l |
|---|---|---|---|---|---|---|
| Notum IC50 (nM)a | 9.5 ± 0.2 | 6.7 ± 1.6 | 1300 ± 210 | 140 ± 20 | 7.5 ± 1.6 | 4.6 ± 1.4 |
| aq. solubility (μg/mL)/(μM) | 210/680 | 68/240 | NDb | ND | 400/1400 | 44/155 |
| MLM/HLM Cli (μL/min/mg protein) | 6.7/1.7 | 3.2/1.5 | ND | ND | 4.9/ND | 5.4/ND |
| MDCK-MDR1 | ||||||
| AB/BA Papp (×10–6 cm s–1) | 46/28 | 31/39 | ND | ND | 2/5 | 48/35 |
| ER | 0.61 | 1.25 | 2.5 | 0.73 |
All values are mean ± s.d. of n = 3–6 experiments quoted to 2 s.f.
ND, not determined.
As before, trusted design metrics were calculated to aid compound design and assess progress, supported by structure-based drug design (SBDD) through inhibitor docking and X-ray structures. However, a significant change in our strategy was to discount any weakly acidic functional groups or heterocycles as these had failed to give sufficient brain penetration in mouse PK experiments (e.g., 4 and 5).26,27 Instead, lead compounds would need to be non-ionized, or weakly basic, at physiologically relevant pH to be advanced through the screening sequence.
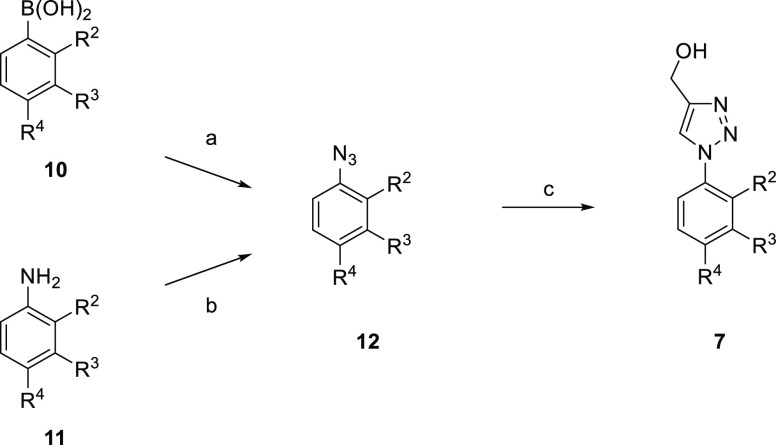
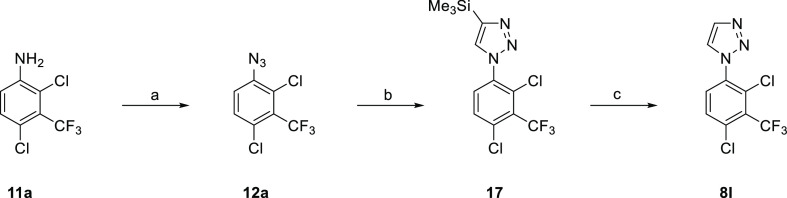
General synthetic methods to prepare inhibitors 7–9 (Tables 1–3) are shown in Schemes 1–5. Our initial efforts focused on exploration of the aryl ring SAR, thus a reliable synthesis of substituted (1-aryl-1H-1,2,3-triazol-4-yl)methanols 7 was required (Scheme 1). Target compounds 7 were readily prepared in two steps from commercially available starting materials. Either the prerequisite boronic acid 10 or aniline 11 was converted to the required aryl azide 12 and then subsequent copper(I)-catalyzed azide–alkyne cycloaddition (CuAAC) with propargyl alcohol afforded the desired triazoles 7.
Scheme 1. Preparation of (1-Phenyl-1H-1,2,3-triazol-4-yl)methanols 7.
Representative reagents and conditions: (a) NaN3 (2.0 equiv), CuSO4·5H2O (0.2 equiv), MeOH, 40 °C, 5 h; (b) (i) NaNO2 (1.2 equiv), CF3CO2H, 0 °C → RT, 1.5 h; (ii) H2O, RT → 0 °C, (iii) NaN3 (1.1 equiv), 0 °C → RT, 1 h; (c) HC≡CCH2OH (1.0 equiv), sodium l-ascorbate (0.4 equiv), CuSO4·5H2O (0.2 equiv), tBuOH-H2O, 50 °C, 2 h.
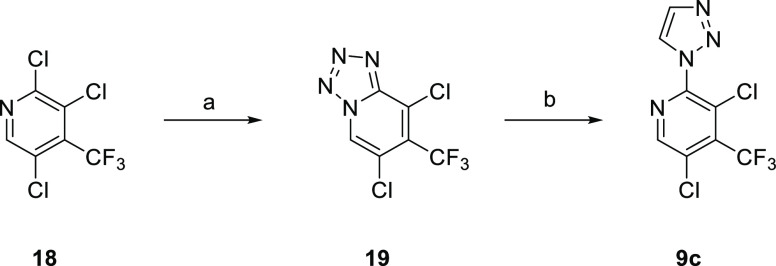
Scheme 5. Preparation of (1,2,3-Triazol-1-yl)pyridine 9c.
Reagents and conditions: (a) (i) NH2NH2·H2O (1.5 equiv), EtOH, 85 °C, 4 h; (ii) HCl, RT → 0 °C, NaNO2 (2.0 equiv), 30 min; (iii) NaOH, 0 °C → RT; (b) sodium l-ascorbate (1.0 equiv), CuSO4·5H2O (0.1 equiv), HC≡CSiMe3 (1.0 equiv), tBuOH-H2O, 40 °C, 16 h, 2%.
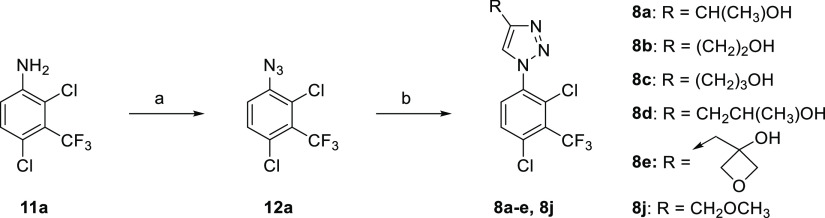
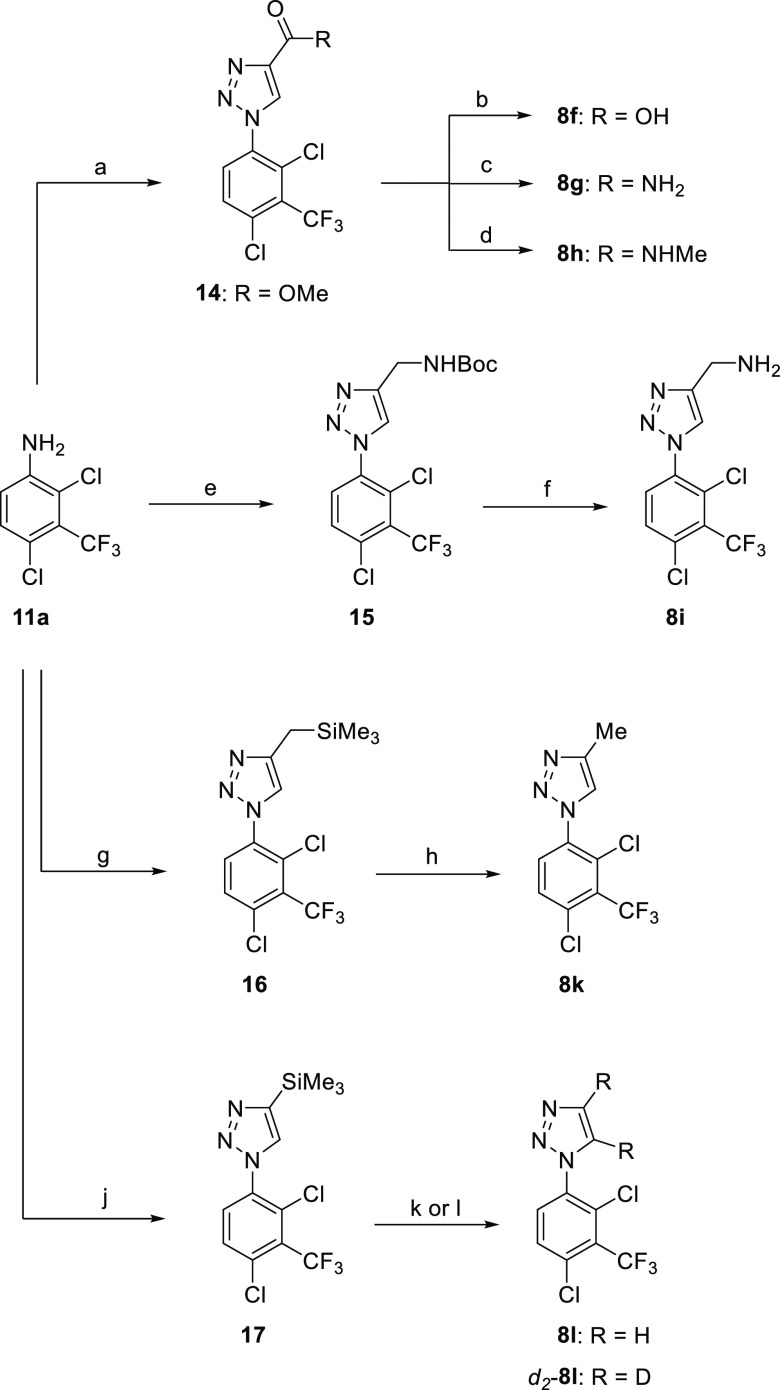
This approach (11 → 12 → 7) proved to be versatile and reliable and was applied to the synthesis of a number of inhibitors. 2,4-Dichloro-3-(trifluoromethyl)aniline (11a) was converted to the corresponding azide 12a, and reaction with the appropriate 1-alkyne 13 under CuAAC conditions gave a variety of 4-substituted 1,2,3-triazoles (8, 14–17) (Schemes 2 and 3). A number of inhibitors were prepared by functional group conversion and/or protecting group strategies. Methyl ester 14 was converted to the acid 8f by base hydrolysis, and to amides 8g and 8h by amination with NH3 and MeNH2, respectively. Acid deprotection of the Boc group from 15 gave methyl amine 8i, and K2CO3/TBAF deprotection deprotection of 16 furnished 4-methyl triazole 8k. CuAAC-promoted [2 + 3] cyclization of 12a with Me3SiC≡CH gave the corresponding 4-TMS triazole 17, and desilylation with K2CO3/MeOH afforded 8l. Desilylation of 17 with CD3OD and D2O as the solvents promoted deuteration at the C4 and C5 positions of the triazole and afforded d2-8l with high-isotope incorporation.
Scheme 2. Preparation of 1,2,3-Triazoles 8a–e, 8j.
Representative reagents and conditions: (a) (i) NaNO2 (2.0 equiv), aq. HCl, MeCN, RT, 1 h; (ii) RT → 0 °C, NaN3 (2.0 equiv), 0 °C, 1 h; or (i) NaNO2 (1.2 equiv), CF3CO2H, 0 °C → RT, 1.5 h; (ii) RT → 0 °C, H2O, NaN3 (1.1 equiv), 0 °C → RT, 1 h; (b) RC≡CH (13) (1.0 equiv), sodium l-ascorbate (0.4 equiv), CuSO4·5H2O (0.2 equiv), MeOH-tBuOH-H2O, 40 °C, 16 h.
Scheme 3. Preparation of 1,2,3-Triazoles 8f–i, 8k–l.
Reagents and conditions: (a) (i) NaNO2 (2.0 equiv), aq. HCl, MeCN, RT, 1 h; (ii) RT → 0 °C, NaN3 (2.0 equiv), 0 °C, 1 h; (iii) HC≡CCO2Me (1.0 equiv), sodium l-ascorbate (0.4 equiv), CuSO4·5H2O (0.2 equiv), MeOH-tBuOH-H2O, 40 °C, 16 h, 71%; (b) (i) aq. NaOH (2.0 equiv), MeOH, RT, 1 h; (ii) aq HCl, 95%; (c) NH3, MeOH, 70 °C, 3 h, 46%; (d) MeNH2, MeOH, 70 °C, 3 h, 64%; (e) NaNO2 (1.2 equiv), CF3CO2H, 0 °C → RT, 1.5 h; (ii) RT → 0 °C, aq. NaN3 (1.1 equiv), 0 °C → RT, 1 h; (iii) aq. NaOH; (iv) HC≡C-CH2NHBoc (1.0 equiv), sodium l-ascorbate (0.4 equiv), CuSO4·5H2O (0.2 equiv), tBuOH-H2O, 50 °C, 16 h, 8%; (f) HCl (excess), dioxane, 50 °C, 16 h, quant.; (g) (i) NaNO2 (1.2 equiv), CF3CO2H, 0 °C → RT, 1.5 h; (ii) RT → 0 °C, H2O, NaN3 (1.1 equiv), 0 °C → RT, 1 h; (iii) sodium l-ascorbate (0.4 equiv), CuSO4·5H2O (0.2 equiv), HC≡CCH2SiMe3 (1.0 equiv), tBuOH-H2O, 50 °C, 16 h; (h) (i) MeOH, K2CO3 (10 equiv), reflux, 2%; (ii) TBAF (2.0 equiv), THF, RT, 16 h; (j) (i) NaNO2 (4.7 equiv), CF3CO2H, 0 °C → RT, 1 h; (ii) RT → 0 °C, NaN3 (4.7 equiv), 0 °C → RT, 16 h; (iii) RT → 0 °C, aq. NaOH; (iv) HC≡CSiMe3 (2.0 equiv), sodium l-ascorbate (0.9 equiv), CuSO4·5H2O (0.5 equiv), MeOH-tBuOH-H2O, RT, 16 h; (k) MeOH, K2CO3 (10 equiv), RT, 24 h, 67%; (l) MeOH-d4, D20, K2CO3 (3.0 equiv), RT, 3 days, 67%.
Alternative heterocycles were prepared by standard methods (Scheme 4). Condensation of aniline 11a with 1,2-diformylhydrazine gave 1,2,4-triazole 9a, and the reaction of 11a with trimethyl orthoformate and NaN3 gave 1,2,3,4-tetrazole 9b. 2-Chloropyridine 18 was sequentially treated with hydrazine and NaNO2/HCl to give 19, and then CuAAC with HC≡CSiMe3 afforded 2-triazolepyridine 9c (Scheme 5). Tetrazolo[1,5-a]pyridine 19 acts as a synthon for the 2-azidopyridine in this reaction.
Scheme 4. Preparation of 1,2,4-Triazole 9a and 1,2,3,4-Tetrazole 9b.
Reagents and conditions: (a) ClSiMe3 (15 equiv), 1,2-diformylhydrazine (3.0 equiv), Et3N, pyridine, 0 °C → 130 °C, 20 h, 50%; (b) HC(OMe)3 (3.0 equiv), NaN3 (3.1 equiv), AcOH, 100 °C, 1.5 h, 10%.
We first explored the SAR of the aryl ring with a variety of single substituents at the ortho-, meta-, and para-positions. The unsubstituted phenyl 7a (IC50 110 μM) was prepared and screened as a benchmark to assess these changes, along with the original hits 6a and 6b. Substitutions were biased toward small, lipophilic groups, based upon the available structural information, and building upon previous Notum SAR from phenyl-azole scaffolds.25,26 The tactic of walking single Cl, Me, and CF3 groups around the phenyl ring proved to be fruitful, with the 3-Me (7e), 3-Cl (7f), 3-CF3 (7g), 4-Me (7k), and 4-CF3 (7l) analogues affording the largest improvements in potency over 7a (ca. 10 to 48-fold). A 2-Cl group (7c) afforded a modest increase in potency showing the potential benefit of substitution at this position. Inhibitors 7f, 7g, and 7l were superior to hit 6a.
Additive effects from multiple substituents were then investigated by preparing a set of dichlorophenyl triazole-matched molecular pairs (7q–t). This set of inhibitors was prioritized due to their synthetic accessibility from available commercial reagents and the precedent from 6b. The original hit 6b was preferred over all other isomers with activity following the order: 3,4-Cl2 (6b, IC50 0.5 μM) > 3,5-Cl2 (7t, IC50 2.4 μM) > 2,3-Cl2 (7q, IC50 5.1 μM) ≈ 2,4-Cl2 (7r, IC50 5.6 μM) > 2,5-Cl2 (7s, IC50 23 μM). Improvements could be achieved with alternative 3,4-disubstitutions when combinations of preferred substituents (Me, Cl, and CF3) were used with a clear preference for 3-CF3-4-Cl (7w, IC50 0.065 μM). Addition of a 2-Cl as a third substituent onto 7w as 2-Cl-3-CF3-4-Cl (7y) gave a further boost in potency with 7y (IC50 0.0095 μM) being 50-fold more active than hit 6b and 10,000-fold more active than the unsubstituted phenyl ring 7a.
At this point in the program, mouse PK data for 7y was generated in vivo to determine the extent of plasma and brain exposure and to verify the correlation of the in vitro ADME data with in vivo outcomes in this series. Triazole 7y was selected as it combined excellent Notum activity (IC50 9.5 nM) with an attractive ADME profile as it possessed good aqueous solubility, microsomal stability, and cell permeability (Table 1 and Supporting Information). Following a single oral dose of 10 mg/kg, plasma exposure for 7y was low (Cmax 300 ng/mL; AUC(0→inf) 150 ng h/mL), which we attributed to higher clearance than predicted by MLM and highlighted the need to further improve metabolic stability (Figure S2). Our hypothesis for this disconnection between MLM stability and plasma clearance of 7y was due to phase 2 metabolism/conjugation of the OH group, and this was subsequently supported by the higher clearance of 7y in mouse hepatocytes (Cli 48 μL/min/106 cells). Hence, we designed inhibitors that explored direct modification and/or replacement of the −CH2OH (Table 2). Despite the poor plasma exposures, 7y displayed the highest brain-to-plasma ratio [Kp 1.2, based on AUC0–inf (total drug)] observed to date and was far superior to previous leads 1 (Kp ca. 0),283 (Kp 0.29),244 (Kp 0.16),26 and 5 (Kp ca. 0)27 where comparable studies were performed. This was viewed to be a significant advantage for this series.
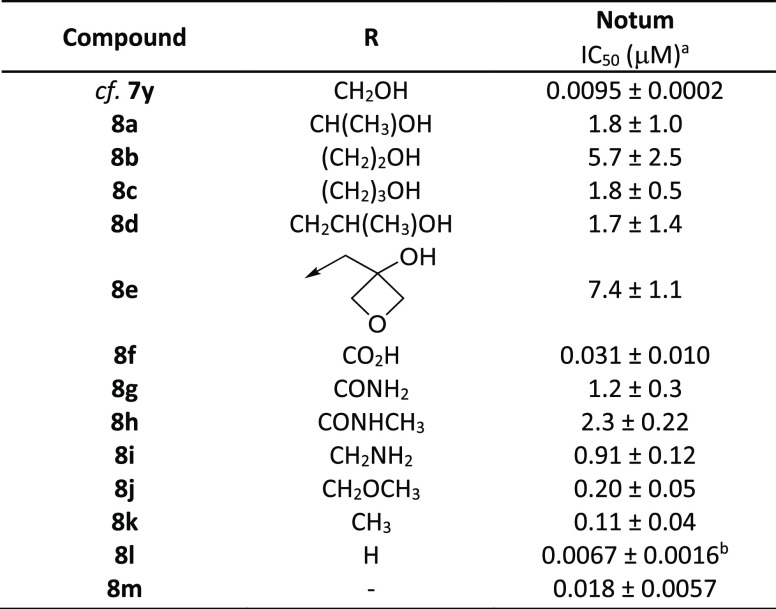
SAR of the C4-substituent of the triazole was studied with retention of the preferred aryl ring of 7y as this group conferred significant activity to the series and was superior to all other substitution patterns explored so far (Table 2). Substitution at the C5 position was not investigated as previous studies with related 4-Cl-phenyl azoles showed this to be unfavorable.26
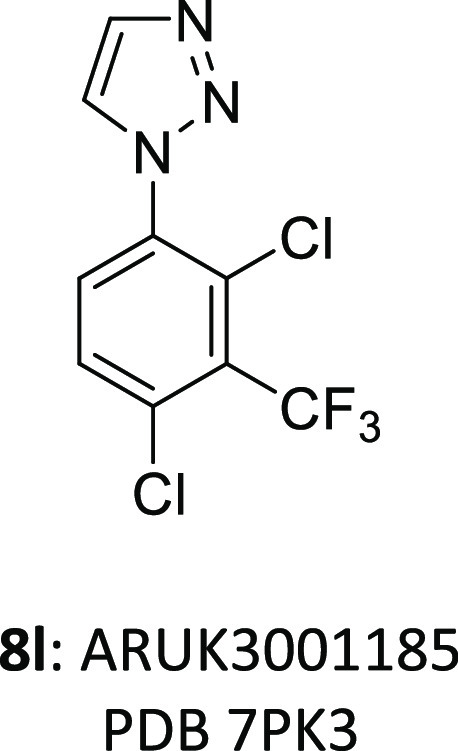
Modification of the −CH2OH through addition of the α-Me group (8a), elongation of the carbon chain (8b–8c), or a combination of both these approaches (8d–8e) leads to a dramatic reduction in potency (IC50 > 1 μM). Oxidation to the carboxylic acid 8f was tolerated with a modest reduction in potency (7y vs 8f), whereas activity was significantly diminished upon conversion to the corresponding primary 8g and secondary amides 8h. Primary amine 8i also had a significant reduction in potency as did “capping” the −CH2OH as the −CH2OCH3 ether 8j. Deletion of the −OH from 7y to give the 4-Me triazole 8k had a more modest effect upon the reduction in potency (12-fold) that was consistent with the loss of one H-bonding group from the ligand. So far, all changes to the C4 substituent had been unfavorable, then, somewhat unexpected, potent inhibition of Notum activity was entirely recovered by removal of the substituent as unsubstituted triazole 8l (IC50 6.7 nM) was the most potent inhibitor prepared in this series. Finally, revisiting the aryl ring substitution with 2,3,4-Cl38m, as a simpler and direct analogue of 8l, was slightly inferior.
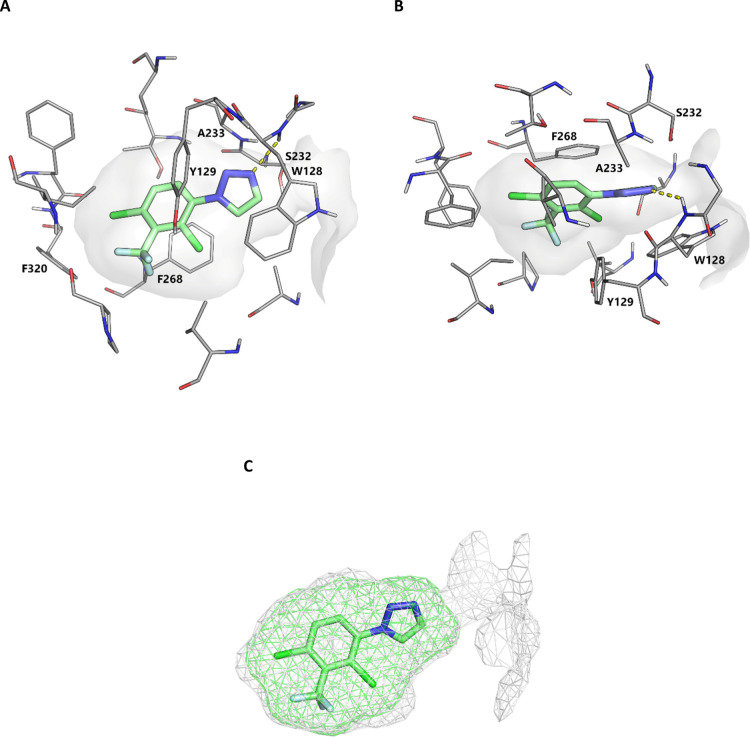
The Notum-8l X-ray structure is consistent with the ligand retaining the H-bond between N3 of the triazole and the backbone of Trp128. In addition, 8l has near complete occupancy of the palmitoleate pocket through the 2-Cl-3-CF3-4-Cl substituents on the aryl ring. The near optimal filling of the pocket is achieved while still positioning the triazole in a favorable position for a hydrogen bond with the backbone of Trp128 (Figure 5). This combination of features is likely why such high potency is observed.
Figure 5.
(A) Side view of structure of 8l (PDB: 7PK3). (B) Top view of the structure of 8l (PDB: 7PK3). The compound forms a hydrogen bond with the backbone of Trp128 (dashed line, 2.47 Å) and displays excellent occupancy of the lipophilic pocket. Select binding site residues shown within 4 Å of the compound. Water molecules have been removed for clarity. The surface of the Notum binding pocket outlined (gray). (C) Illustration of space occupied by 8l (green mesh, PDB: 7PK3) and the outline of the pocket (gray mesh, PDB: 7PK3).
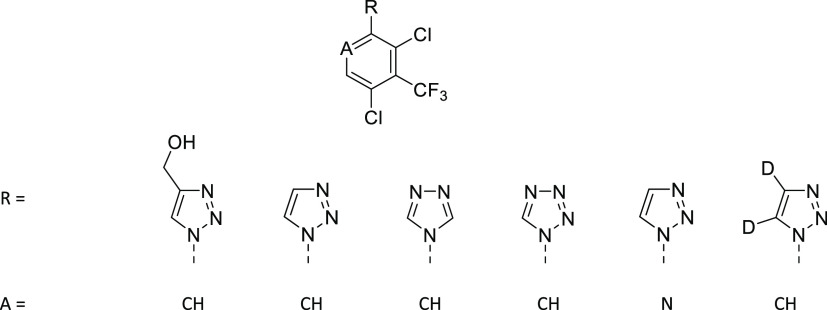
1,2,3-Triazole 8l was superior in potency to the isomeric 1,3,4-triazole 9a or the 1,2,3,4-tetrazole 9b (Table 3). These effects seem to be quite subtle as most key inhibitor–Notum interactions are available with these alternative 1-aryl azoles and may be due to a more optimal electronic interaction of 8l with Trp128 or simply that an N4 atom is detrimental.49 Modification of the phenyl ring of 8l to the corresponding pyridine 9c was equipotent and demonstrated that some modest polarity could be accommodated in the palmitoleate pocket. Dual deuteration of 8l at the C4 and C5 positions of the triazole gave d2-8l as a tool to investigate the metabolic fate of the parent molecule.
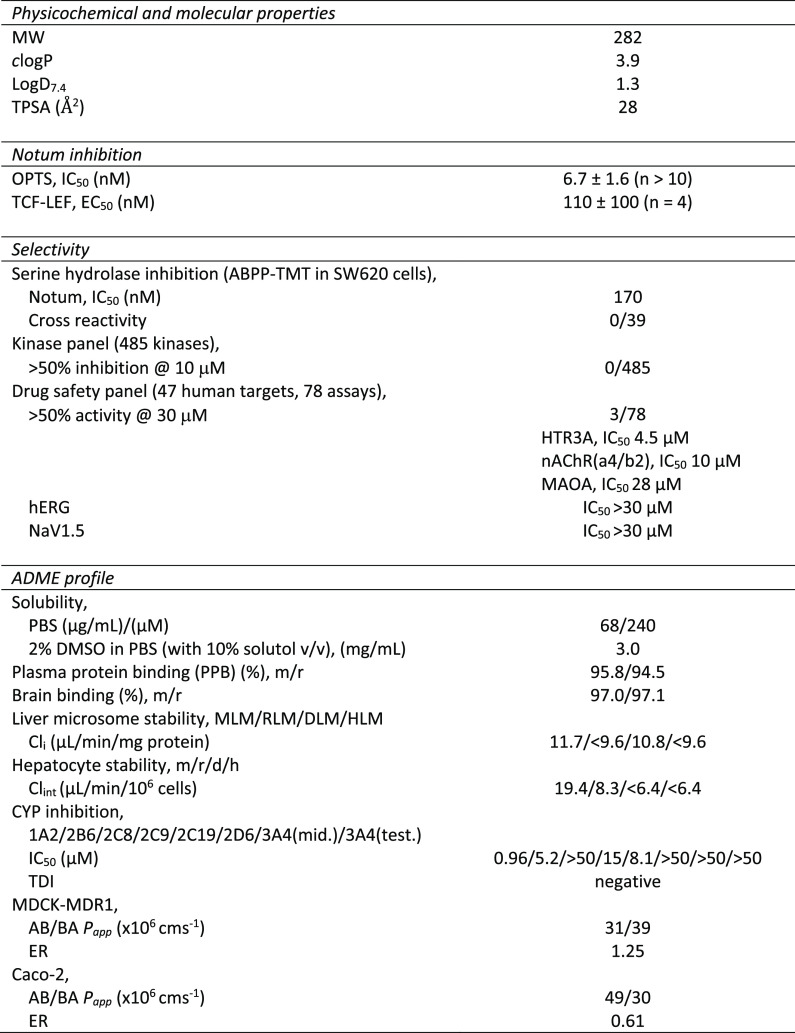
Triazole 8l has physicochemical and molecular properties consistent with drug-like space.50 It was noteworthy that 8l had a much lower measured distribution coefficient (LogD7.4 1.3 ± 0.05) than had been predicted by the calculated partition coefficient (clogP 3.9) despite being non-ionized at physiologically relevant pH. This disconnection could be attributed to the close proximity of the 2-Cl, 3-CF3, and 4-Cl substituents on the aromatic ring that results in a reduction in overall hydrophobic surface area when compared to summing their individual lipophilicity fragmental constants (π). These results highlight the importance of generating empirical data to correlate with calculated values. Evaluation of 8l against standard design metrics using Notum inhibition from the biochemical assay (IC50 6.7 nM, pIC50 8.2) and measured lipophilicity (LogD7.4 1.3) calculates 8l to have high LE (0.67), LLE (6.9), and a favorable prediction of brain penetration (CNS MPO 5.4/6.0; BBB Score 5.6/6.0).
Triazole 8l displayed satisfactory aqueous solubility in phosphate-buffered saline (PBS), good metabolic stability in both MLM and HLM, and high permeability in the MDCK-MDR1 cell transit assay with minimal evidence for recognition and efflux by the P-gp transporter (Table 3 and Supporting Information). In contrast, pyridine 9c performed poorly in the permeability assay although these results were confounded by low compound recovery. Comparison of MLM stability of 8l with d2-8l (Cli 3.2 vs 5.4 μL/min/mg protein) showed deuteration of the triazole afforded no improvement suggesting that CYP-mediated metabolism of the triazole C–H bonds was not a major route of clearance in this system.
Based on these data, 8l was selected as our nominated lead from this series for further profiling as it had the best combination of Notum activity and in vitro ADME properties including lipophilicity.
Lead 8l was then evaluated in additional in vitro pharmacology and ADME screens (Table 5). Triazole 8l restored Wnt signaling in the presence of Notum (EC50 110 nM; n = 4) in a in a cell-based TCF/LEF (Luciferase) reporter assay23,26,27 and gave standard S-shaped concentration–response curves up to 10 μM (Figure S9). Performing these experiments in the absence of Notum showed a maximal Wnt response at all concentrations tested (up to 10 μM; n = 4). The activation of Wnt signaling was due to direct on-target inhibition of Notum by 8l and not by assay interference or cell toxicity (up to 10 μM).
Table 5. Extended Profile of 8la.
Non-standard abbreviations: m, mouse; r, rat; d, dog; h, human; mid., midazolam; and test., testosterone.
The aqueous solubility of 8l could be significantly enhanced to 1 mg/mL with cosolvents bringing added flexibility to study design. Triazole 8l has good stability in liver microsomes and hepatocytes across mouse, rat, dog, and human, which predicts for low clearance across these species. Screening in HLM including eight standard substrates of cytochrome P450 (CYP450) enzymes showed moderate/weak inhibition of 1A2 (IC50 0.96 μM), 2B6 (IC50 5.2 μM), 2C9 (IC50 15 μM), and 2C19 (IC50 8.1 μM) but not 2C8, 2D6, or 3A4 (IC50 > 50 μM). Furthermore, there was no evidence for CYP time-dependent inactivation (TDI) as assessed by the preincubation of HLM with 8l in the presence and absence of NADPH, followed by the incubation with the discrete marker substrates (IC50 shift). There was good cell permeability across a Caco-2 monolayer consistent with good absorption from the gut. The hepatocyte data for 8l was encouraging as this deletion of the −CH2OH group from 7y seems to have removed the liability of this metabolic soft spot. Hence, 8l was advanced to rodent PK studies.
PK data for 8l was generated in vivo in mouse, and subsequently in rat, to evaluate plasma and brain exposure (Table 4 and Supporting Information). Following single intravenous administration of 8l to mouse, plasma clearance was low compared to liver blood flow and volume of distribution was moderate resulting in an elimination half-life of 2.4 h (Figure S3, Table S11). Following a single oral dose to mouse, 8l was rapidly absorbed and showed good oral bioavailability (66%) (Figure S4, Table S12). Good brain penetration was confirmed with drug levels in the brain similar to drug levels in the plasma (Kp 1.1). The brain-to-plasma ratio was 0.77 (Kp,uu) when free drug concentrations were calculated. This oral dose of 10 mg/kg achieved a concentration in the brain of Cmax ≈ 300 nM (free drug) that exceeded the Notum EC50 from the cell-based TCF/LEF assay, and brain levels were maintained above 100 nM for ∼4 h. Although encouraging, this dose, and the resulting level of brain exposure, may be insufficient to promote a sustained pharmacodynamic (PD) response, where Notum activity was reduced for an extended period of time. This will need to be determined empirically. Hence, we subsequently explored alternative dosing regimes to provide some flexibility in the determination of the required efficacious concentrations (Ceff) in rodent models of disease, and its relationship to Notum pharmacology (EC50), that is, establish the PK–PD relationship (vide infra).
Table 4. Rodent Plasma and Brain PK Parameters of 8la.
| mouseb | ratc | |
|---|---|---|
| Intravenous (i.v.) | ||
| dose (mg/kg) | 1.0d | 1.0d |
| CL (mL/min/kg) | 10.4 | 7.5 |
| Vdss (L/kg) | 2.0 | 2.0 |
| t1/2 (h) | 2.4 | 3.3 |
| mouseb | ratc | |||
|---|---|---|---|---|
| plasma | brain | plasma | brain | |
| Oral (p.o.) | ||||
| dose (mg/kg) | 10e | 10d | ||
| t1/2 (h) | 2.4 | 2.2 | 4.7 | 8.9 |
| Tmax (h) | 0.5 | 1.0 | 4.0 | 0.5 |
| Cmax (ng/mL or ng/g) | 2310 | 2900 | 3260 | 3650 |
| AUC0–inf (ng·h/mL or ng·h/g) | 10 800 | 11 600 | 32 000 | 46 100 |
| Fo (%) | 66 | 140 | ||
| brain-to-plasma ratio (Kp)f | 1.1 | 1.4 | ||
| free partition coefficient of brain (Kp,uu)f | 0.77 | 0.75 | ||
Values are mean; n = 3 per time point; terminal blood and brain levels were measured up to 24 h. All animals were normal throughout the study period.
Male C57BL6 mice.
Sprague–Dawley male rats.
Formulation A: dimethylsulfoxide (DMSO) (2% v/v) + 10% v/v Solutol in 1× PBS (98% v/v).
Formulation B: 0.1% Tween80 in water.
Kp was calculated by the ratio of brain concentration–time profile (AUC(0–inf),brain) to that of plasma concentration–time profile (AUC(0–inf),plasma). Kp,uu was calculated by multiplying Kp with the ratio of free fraction in brain homogenate to plasma (fu,brain/fu,plasma).
PK evaluation of 8l in rat also showed low plasma clearance and moderate volume of distribution with a half-life of 3.3 h (Table 4). Oral administration to rat showed good absorption, drug levels, and brain penetration (Kp 1.4). The oral bioavailability was supramaximal with Fo 140%. The plasma (and brain) concentration–time plots clearly show double Cmax peaks (Figure S7), which could indicate some contribution from excreted drug being reabsorbed from the gastrointestinal tract and/or an effect on gastric emptying times. On balance, these results established that 8l has PK properties compatible with evaluation in rodent models of disease.
Triazole 8l emerged as a promising lead with potent inhibition of Notum enzymic activity in both biochemical and cellular assays, along with excellent brain penetration in rodent. The wider pharmacology activity of 8l was then assessed against serine hydrolases, kinases, and representative drug safety targets to identify any off-target activity and to establish its selectivity.
Selectivity against serine hydrolases was assessed by unbiased proteomic analysis in a human colorectal cancer cell line SW620 that has been characterized to have high Notum expression.51 Quantitative ABPP was performed using FP-biotin to label active serine hydrolases in concentrated conditioned media and cell lysates, combined with tandem mass tagging technology (TMT) for relative quantification.52 Triazole 8l showed concentration-dependent inhibition of Notum activity (IC50 170 nM in conditioned media) and demonstrated high selectivity in this system as there was no cross reactivity against any of the other 39 serine hydrolases detected (Figures 6, S11 and S12).18,52b
Figure 6.
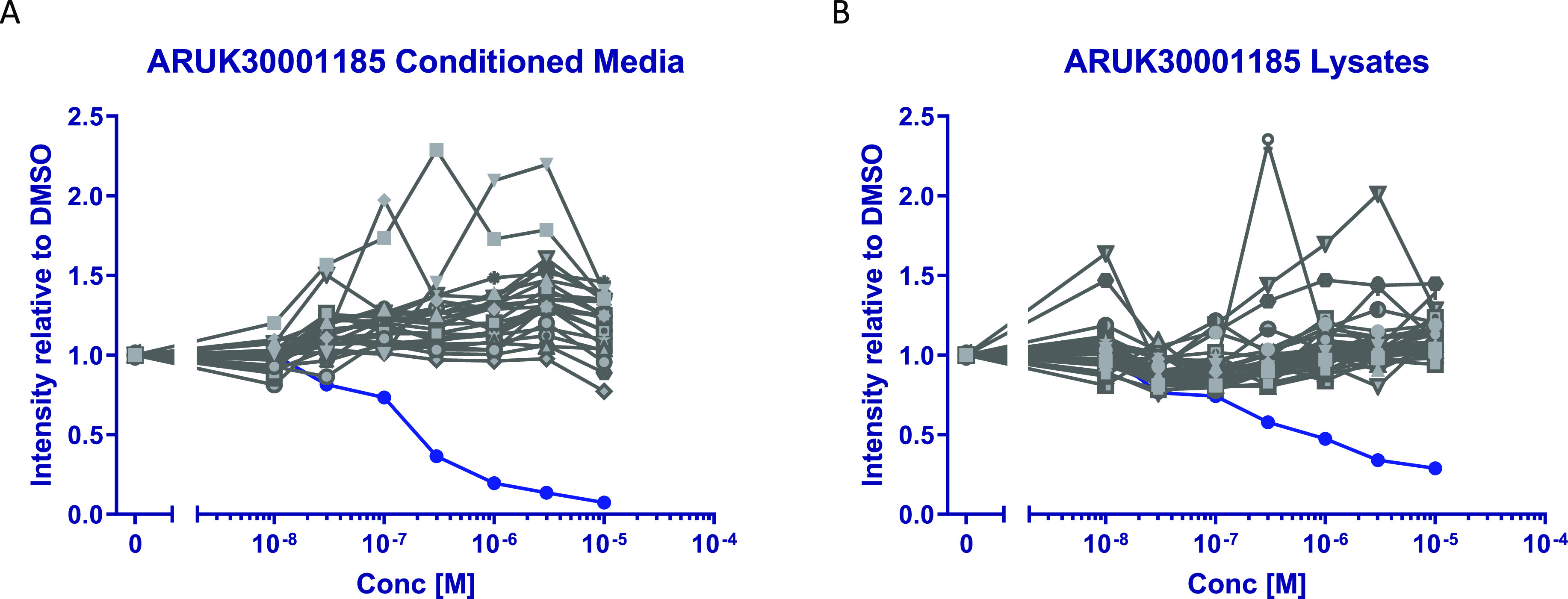
Concentration–response curves from proteomic analysis of 8l in SW620 cells. The response for Notum is shown in dark blue circles (•) and all other serine hydrolases in gray. (A) Concentrated conditioned media, Notum IC50 170 nM. (B) Cell lysates. Each sample set was preincubated with an eight-point concentration–response curve of 8l or DMSO, then labeled for 20 min with FP-Biotin (2 μM) to capture active serine hydrolases. Labeled proteins were enriched on NeutrAvidin agarose and then digested using LysC and trypsin. The subsequent peptides were isobarically labeled with eight channels of a TMT10plex and combined for final LC–MS/MS analysis. The MS proteomics data have been deposited to the ProteomeXchange Consortium via the PRIDE partner repository with the dataset identifier PXD031338.
Compound 8l showed excellent selectivity for Notum over a panel of 485 distinct kinases with <50% inhibition at 10 μM for all these kinases including casein kinase 1 (CK1) and GSK3 (Table 5). This was of critical importance to studies wishing to assess the function of Notum with 8l, as CK1 and GSK3 phosphorylate β-catenin leading to its degradation and thus reducing Wnt signaling.53 For some context, 8l showed the highest activity in this kinase panel at CDK14/cyclin Y with 43% inhibition at 10 μM (n = 2) (Table S20).
Compound 8l was screened for off-target pharmacology across multiple drug target classes in the DiscoverX Safety47 panel. This suite of 78 functional assays assesses pharmacological modulation of 47 human off-target liabilities that have been highlighted for the initial safety assessment of lead compounds.54 There was minimal activity at 75/78 targets having IC50/EC50 > 30 μM with only modest activity at HTR3A (IC50 4.5 μM), nAChR(a4/b2) (IC50 10 μM), and MAOA (IC50 28 μM) (Table 5). Liability through direct interaction with cardiac ion channels was low as 8l had no significant effect on either the hERG or the cardiac sodium channels (IC50 > 30 μM) (Table S21).
Having established 8l as a potent and selective inhibitor of Notum carboxylesterase activity, our efforts shifted to developing a multigram synthesis of 8l and identification of a suitable dosing regimen to investigate the role of Notum in mouse models of neurodegenerative disease.
The multigram synthesis of 8l essentially followed the discovery route but with additional precautions to minimize the risk of high energy intermediates on scale such as the azide 12a (Scheme 6). Differential scanning calorimetry (DSC) analysis of azide 12a determined an exotherm onset temperature at 125.8 and so 42 °C was established as the maximum safe handling temperature (Figure S15). Reactions were performed in smaller batches and then pooled for purification to give a single, homogenous product.
Scheme 6. Multigram Synthesis of 8l.
Reagents and conditions: (a) (i) NaNO2 (4.7 equiv), CF3CO2H, 0 °C, 0.5 h → RT, 1.5 h; (ii) NaN3 (4.7 equiv), H2O, 0 °C → RT, 16 h; (iii) NaOH, 0 °C; (b) HC≡CSiMe3 (2.0 equiv), sodium l-ascorbate (0.9 equiv), CuSO4·5H2O (0.5 equiv), MeOH-tBuOH-H2O, RT, 16 h; (c) K2CO3 (10 equiv), MeOH, RT, 24 h; overall yield, 67%.
Increasing the oral dose of 8l to mouse from 10 mg/kg to twice daily dosing of 30 mg/kg (2 × 30 mg/kg, p.o. at T = 0 and 9 h, formulation A) achieved higher and prolonged drug concentrations in the plasma and brain (Figure S5, Table S13). The sustained free drug levels of 8l in the brain now exceeded the Notum EC50 for ca. 20 h. This twice daily oral dosing of 8l was well tolerated in both wild-type and aged APP/PS1 mice for 7 days and was therefore deemed suitable for the in vivo pathfinder experiments. These studies are ongoing and will be reported in due course.
Conclusions
Crystallographic fragment screening of the DSPL for binding to Notum, supported by a biochemical enzyme assay to rank inhibition activity, proved to be successful in identifying chemically enabled hits. This screening strategy identified 58 hits that were shown to bind to the active site of Notum with 20 of these having IC50 < 100 μM. A pair of outstanding hits were 4-(hydroxymethyl)triazoles 6a (IC50 12 μM) and 6b (IC50 0.5 μM), and these were selected for fragment development to improve Notum activity and develop PK properties for oral dosing to achieve good plasma exposure and brain penetration.
Exploration of the substituents on the aryl ring that binds in the palmitoleate pocket of Notum gave potent early lead 7y (IC50 9.5 nM) and further optimization of the triazole group delivered 8l (IC50 6.7 nM). It is noteworthy that advanced lead 8l can be characterized as a fragment-sized molecule (MW 282; HAC 17) with high LE and LLE, and that inhibition of Notum activity was increased by over 15,000-fold by optimization of the substituents on the aryl ring (8l vs 7a). These findings highlight the benefits of FBDD when aligned with a suitable drug target.
The Notum-8l X-ray structure clearly showed that 8l has near complete occupancy of the palmitoleate pocket through the 2-Cl-3-CF3-4-Cl substituents on the aryl ring. Triazole 8l restored Wnt signaling in the presence of Notum (EC50 110 nM) in a cell-based TCF/LEF reporter assay confirming functional activity. Assessment in broader off-target and safety pharmacology screens showed 8l to be selective against serine hydrolases, kinases, and representative drug targets. PK studies with 8l in mouse and rat showed good plasma exposure and brain penetration, and was well tolerated.
In summary, triazole 8l (ARUK3001185) is a potent and selective inhibitor of Notum activity with good brain penetration suitable for oral dosing in rodent models of disease.
Experimental Section
Notum Protein Expression and Purification
Methods have been described in detail elsewhere.25
Notum OPTS Biochemical Assay
Methods have been described in detail elsewhere.23−26 Representative concentration–response curves are provided in the Supporting Information.
TCF/LEF Reporter (Luciferase) Cell-Based Assay
Methods have been described in detail elsewhere.23,26,27 Concentration–response curves are provided in the Supporting Information.
Proteomic Analysis in SW620 Cells
Cell Culture
SW620 cells were grown in Leibovitz’s L-15 medium supplemented with 10% FBS, Pen/Strep 10 U/mL, and 0.075% sodium bicarbonate.
Conditioned Medium Generation
SW620 cells were grown to around 80% confluence, washed three times with PBS, and then placed in serum-free media for 48 h. The medium was then removed, spun at 300G to remove any solids, and then concentrated in 10k MWCO protein concentrators (Pierce) by centrifugation.
Cell Lysate Generation
SW620 cells were grown to around 80% confluence, the medium was removed and cells were washed three times with PBS, and then lysed in the ice cold IP lysis buffer (Pierce). Lysates were clarified by centrifugation at 15k G for 10 min at 4 °C, then protein quantification was performed by the BCA assay (Pierce) and lysates were equalized to 1.5 mg/mL.
Proteomics Protocol
Either lysates or conditioned medium was treated with the appropriate concentration of each inhibitor or DMSO across a range of concentrations and then equilibrated at RT for 45 min. Following this, 2 μM FP-Biotin (Santa Cruz) was added to each sample for 20 min with agitation. Lysates were then precipitated using chloroform–methanol, and the protein pellet was washed two times with MeOH. Protein pellets were dissolved in 50 mM HEPES pH 8.0 with 2% SDS and 10 mM DTT, then the SDS concentration was adjusted to 0.2% with further 50 mM HEPES pH 8.0. Each solution was then added to an aliquot of equilibrated NeutrAvidin beads,55 which had been washed two times with 50 mM HEPES pH 8.0 with 0.2% SDS, for 16 h at RT with agitation. Beads were then pelleted by centrifugation, the supernatant was removed and then washed three times with 50 mM HEPES pH 8.0 with 0.2% SDS, followed by three washes with 50 mM HEPES pH 8.0. The beads were then incubated with 10 mM DTT in 50 mM HEPES pH 8.0 for 15 min at RT, followed by 20 mM iodoacetamide, which was incubated for 30 min in the dark. A further aliquot of 10 mM DTT was added to the beads, then they were pelleted by centrifugation, the supernatant was removed, the beads were washed with 50 mM HEPES pH 8.0, and all the supernatants were removed. LysC (Wako) was dissolved in 50 mM HEPES pH 8.0 to give a 1 μg/mL solution, of which 50 μL (50 ng of LysC) was added to the beads and incubated overnight at 37 °C. The beads were pelleted, the supernatant was removed, and then washed with additional 50 mM HEPES pH 8.0 to extract as many peptides as possible. 75 ng of trypsin (Pierce) was added from a 20 ng/μL solution in 50 mM HEPES pH 8.0 and incubated at 37 °C for 7 h. Each set of conditions were then labeled using eight channels of a TMT10plex (Thermo) following the manufacturer’s instructions, then combined. TMT-labeled tryptic peptides were filtered using 1 mm Empore C18 solid-phase extraction discs aka “stage tips” (3M) prior to vacuum centrifugation and resuspension in 0.1% trifluoroacetic acid.
On an Ultimate 3000 nanoRSLC HPLC (Thermo Scientific), 1–10 μL of sample was loaded onto a 20 mm × 75 μm Pepmap C18 trap column (Thermo Scientific) prior to elution via a 50 cm × 75 μm EasySpray C18 column into a Lumos Tribrid Orbitrap mass spectrometer (Thermo Scientific). A 70′ gradient of 6–40% B was used, followed by washing and re-equilibration (A = 2% MeCN, 0.1% formic acid; B = 80% MeCN, 0.1% formic acid).
The Orbitrap was operated in “Data Dependent Acquisition” mode, followed by MS/MS in “TopS” mode, with Orbitrap accumulation at R = 50,000 of HCD fragmented parent ions.
Raw files were processed using MaxQuant (maxquant.org) and Perseus (maxquant.net/perseus) with a recent download of the uniprot homo sapiens reference proteome database and a common contaminants database. A decoy database of reversed sequences was used to filter false positives, at a peptide false detection rate of 1%.
Analysis was performed using Perseus and filtered against contaminants, reversed, and proteins identified by the site. Protein groups were then filtered by those which contained valid values in at least six of the eight conditions. Serine hydrolases were identified by filtering for those proteins identified as such in these two reference publications18,52b with intensity values normalized relative to the DMSO control, and concentration–response curves and IC50 values were determined using GraphPad Prism 9.1.0.
The identity and quantification of each serine hydrolase identified in SW620 cells by FP-biotin ABPP for 8l (and 1) is provided in the Supporting Information. The MS proteomics data have been deposited to the ProteomeXchange Consortium via the PRIDE partner repository56 with the dataset identifier PXD031338.
Structural Biology
The methods of protein production, crystallization, data collection, and structure determination have been described in detail.26 Data collection and refinement statistics are presented in the Supporting Information.
To prepare the figures of the crystal structures, the PDB entry was processed using the Protein Preparation Wizard in Schrödinger Maestro 12.4 using the default settings and optimizing the hydrogen bond assignment. No changes were made to the position of heavy atoms. Final images were prepared using PyMol version 2.4.
ADME Assays
Molecular properties (MW, clogP, and TPSA) were calculated with ChemDraw Professional v16.0.1.4(77). Distribution coefficients (LogD7.4) were measured using the shake flask method.
Selected compounds were routinely screened for aqueous solubility in PBS (pH 7.4), transit performance in MDCK-MDR1 cell lines for permeability, and metabolic stability in MLM and HLM as a measure of clearance. Lead 8l was also screened for inhibition of representative CYP450 enzymes, permeability across the Caco-2 cell monolayer, and metabolic stability in liver microsomes and hepatocytes. Assay protocols and additional data are presented in the Supporting Information. ADME studies reported in this work were independently performed by Cyprotex (Macclesfield, U.K), GVK Biosciences (Hyderabad, India), and WuXi AppTec (Shanghai, China).
PK Studies
In vivo PK data in mouse and rat was independently generated at Charles River Laboratories (Groningen, Netherlands), GVK Biosciences (Hyderabad, India), and WuXi AppTec (Shanghai, China). PK was supported by the development of suitable formulations for the route of administration, and the measurement of plasma protein binding (PPB) and brain tissue binding for the calculation of free drug levels in these compartments. Study protocols and additional data are presented in the Supporting Information.
Kinase Selectivity Panel
Kinase selectivity screening was performed by Thermo Fisher Scientific (Paisley, U.K.) in their SelectScreen Biochemical Kinase Profiling Service. Assay information and data are presented in the Supporting Information.
Safety Pharmacology Studies
Screening for off-target pharmacology across multiple drug target classes was performed in the DiscoverX Safety47 panel as the SAFETYscan E/IC50 ELECT service by Eurofins (San Diego CA, U.S.A.). Assay information and data are presented in the Supporting Information.
Chemistry
General Information
Methods have been described in detail elsewhere.27a The purity of compounds 6–9 was evaluated by NMR spectroscopy and LCMS analysis. All compounds had purity ≥95% unless otherwise stated.
General Methods
*Caution* Azides are potentially explosive reagents and therefore all solutions containing azides were concentrated under reduced pressure behind a blast shield at RT. Furthermore, sodium azide reacts with acids to generate gaseous HN3, which is a toxic gas. All reactions were conducted behind a blast shield under a flow of nitrogen, and exit gases were passed through an aqueous base prior to their release into a ventilated fume hood.
Preparation of 1-Phenyl-1H-1,2,3-triazoles
General Method 1.1
*Caution* Step 1: Sodium azide (260 mg, 4.0 mmol, 2.0 equiv) and copper(II) sulfate pentahydrate (100 mg, 0.4 mmol, 0.2 equiv) were added to a solution of phenylboronic acid 10 (2.0 mmol, 1.0 equiv) in MeOH (10 mL). The reaction was stirred at 40 °C for 5 h, concentrated to ∼50% volume under reduced pressure, and then diluted with H2O (ca. 10 mL) and EtOAc (ca. 50 mL). The organic phase was separated, diluted with t-BuOH (1.7 mL), and then cautiously concentrated under reduced pressure at 25 °C to provide a solution of crude azide 12 in t-BuOH, which was used without further purification.
Step 2: Copper(II) sulfate pentahydrate (100 mg, 0.4 mmol, 0.22 equiv), sodium l-ascorbate (56 mg, 0.28 mmol, 0.15 equiv), H2O (1.7 mL), and propargyl alcohol (0.1 mL, 1.8 mmol, 1.0 equiv) were added to the solution of crude azide 12 from step 1. The reaction was stirred at 40 °C for 16 h, cooled to RT, diluted with H2O (ca. 10 mL), extracted with EtOAc (ca. 50 mL), and the organic phase was washed with H2O (ca. 10 mL) and brine (ca. 10 mL). The organic phase was dried, filtered, and concentrated under reduced pressure. The crude product was purified by column chromatography (0–5% MeOH in CH2Cl2) to give the triazoles 6b and 7.
General Method 1.2
*Caution* Step 1: Sodium nitrite (155 mg, 2.25 mmol, 1.2 equiv) was added portionwise to a solution of aniline 11 (1.87 mmol, 1.0 equiv) in TFA (2.0 mL) at 0 °C over 30 min. The reaction mixture was then allowed to warm to RT for 1.5 h before recooling to 0 °C. A solution of sodium azide (133 mg, 2.05 mmol, 1.1 equiv) in H2O (1.5 mL) was added dropwise over 5 min, stirred for 30 min, and then warmed to RT over 60 min. The mixture was basified to pH 8–9 by dropwise addition of aqueous NaOH (5 M), then diluted with t-BuOH (2.0 mL) to provide a solution of crude azide 12, which was used without further purification.
Step 2: Copper(II)sulfate pentahydrate (93 mg, 0.37 mmol, 0.2 equiv), sodium l-ascorbate (148 mg, 0.75 mmol, 0.4 equiv), and mono-substituted alkyne 13 (1.87 mmol, 1.0 equiv) were added to the solution from step 1. The reaction mixture was stirred at 50 °C for 16 h, cooled to RT, diluted with brine (ca. 10 mL), and then extracted with EtOAc (ca. 50 mL). The organic phase was dried, filtered, and concentrated under reduced pressure. The crude product was purified by column chromatography (0–5% MeOH in CH2Cl2) to give triazoles 7 and 8.
General Method 1.3
*Caution* Step 1: Aniline 11 (0.65 mmol, 1.0 equiv) was dissolved in H2O (0.5 mL) and MeCN (3.0 mL), then aqueous HCl (37%, 7.3 mL) was added at RT and stirred vigorously for 10 min. Sodium nitrite (90 mg, 1.3 mmol, 2.0 equiv) was added portionwise and the solution was stirred for 1 h, cooled to 0 °C, and then sodium azide (85 mg, 1.3 mmol, 2.0 equiv) was added portionwise. After 1 h, the solution was diluted with H2O (ca. 10 mL) and Et2O (ca. 50 mL). The organic phase was separated, diluted with t-BuOH (2.0 mL), and then cautiously concentrated under reduced pressure at 25 °C to provide a solution of crude azide 12 in t-BuOH, which was used without further purification.
Step 2: Sodium l-ascorbate (52 mg, 0.26 mmol, 0.4 equiv), mono-substituted alkyne 13 (0.65 mmol, 1.0 equiv), copper(II) sulfate pentahydrate (33 mg, 0.13 mmol, 0.2 equiv), MeOH (1.0 mL), and H2O (2.0 mL) were added to the solution of crude azide 12 from step 1, and the reaction was stirred at 40 °C for 16 h. The reaction was cooled to RT, diluted with H2O (ca. 10 mL), extracted with EtOAc (ca. 50 mL), and the organic phase was washed with H2O (ca. 10 mL) and brine (ca. 10 mL). The organic phase was dried, filtered, and concentrated under reduced pressure. The crude product was purified by column chromatography (0–5% MeOH in CH2Cl2) to give triazoles 7 and 8.
General Method 1.4
*Caution* Step 1: Sodium nitrite (72 mg, 1.0 mmol, 1.2 equiv) was added portionwise to a solution of aniline 11 (0.9 mmol, 1.0 equiv) in TFA (0.5 mL) at 0 °C over 30 min. The reaction mixture was then allowed to warm to RT for 1.5 h before recooling to 0 °C. H2O (0.1 mL), sodium azide (62 mg, 1.0 mmol, 1.1 equiv) was added portionwise over 30 min, and then warmed to RT over 1 h. The mixture was basified to pH 8–9 by dropwise addition of saturated aqueous NaHCO3, then diluted with CH2Cl2 (ca. 50 mL). The organic phase was separated, diluted with t-BuOH (0.5 mL), and then cautiously concentrated under reduced pressure at 25 °C to provide a solution of crude azide 12 in t-BuOH, which was used without further purification.
Step 2: Copper(II) sulfate pentahydrate (43 mg, 0.2 mmol, 0.2 equiv), sodium l-ascorbate (69 mg, 0.4 mmol, 0.4 equiv), H2O (0.5 mL), and mono-substituted alkyne 13 (0.9 mmol, 1.0 equiv) were added to the solution of crude azide 12 from step 1, and the reaction mixture was stirred at 50 °C for 2–16 h. The reaction was cooled to RT, diluted with H2O (ca. 10 mL), extracted with EtOAc (ca. 50 mL), and the organic phase was washed with H2O (ca. 10 mL) and brine (ca. 10 mL). The organic phase was dried, filtered, and concentrated under reduced pressure. The crude product was purified by column chromatography (0–5% MeOH in CH2Cl2) to give triazoles 7 and 8.
Notum Inhibitors
((4-Chlorophenyl)-1H-1,2,3-triazol-4-yl)methanol (6a)
Purchased from Key Organics, 4F-329S.
((3,4-Dichlorophenyl)-1H-1,2,3-triazol-4-yl)methanol (6b)
Purchased from Key Organics (4F-359S). It was prepared by General Method 1.1 from 3,4-dichlorophenylboronic acid and isolated as an off-white solid (110 mg, 11%).
1H NMR (600 MHz, DMSO-d6): δ 8.79 (s, 1H), 8.27 (d, J = 2.5 Hz, 1H), 7.97 (dd, J = 8.8, 2.5 Hz, 1H), 7.87 (d, J = 8.8 Hz, 1H), 5.36 (t, J = 5.6 Hz, 1H), 4.61 (d, J = 5.5 Hz, 2H); 13C NMR (151 MHz, DMSO-d6): δ 149.5, 136.3, 132.4, 131.8, 130.8, 121.6, 121.3, 119.9, 54.9; LCMS m/z: 244.1 [M + H]+.
(1-Phenyl-1H-1,2,3-triazol-4-yl)methanol (7a)
Prepared by General Method 1.1 from phenylboronic acid and isolated as an off-white solid (98 mg, 28%).
1H NMR (700 MHz, DMSO-d6): δ 8.68 (s, 1H), 7.96–7.84 (m, 2H), 7.64–7.54 (m, 2H), 7.56–7.41 (m, 1H), 5.32 (t, J = 5.6 Hz, 1H), 4.61 (dd, J = 5.6, 0.6 Hz, 2H); 13C NMR (176 MHz, DMSO-d6): δ 149.1, 136.8, 129.9, 128.5, 121.0, 119.9, 55.0; LCMS m/z: 176.1 [M + H]+.
((2-Fluorophenyl)-1H-1,2,3-triazol-4-yl)methanol (7b)
Prepared by General Method 1.2 from 2-fluoroaniline and propargyl alcohol and isolated as a light brown oil (48 mg, 12%).
1H NMR (400 MHz, methanol-d4): δ 8.31 (d, J = 2.4 Hz, 1H), 7.89–7.77 (m, 1H), 7.63–7.51 (m, 1H), 7.47–7.35 (m, 2H), 4.80–4.78 (m, 2H); LCMS m/z: 194.1 [M + H]+.
((2-Chlorophenyl)-1H-1,2,3-triazol-4-yl)methanol (7c)
Prepared by General Method 1.1 from 2-chlorophenylboronic acid and isolated as an off-white solid (30 mg, 7%).
1H NMR (600 MHz, DMSO-d6): δ 8.41 (s, 1H), 7.77 (dd, J = 8.0, 1.3 Hz, 1H), 7.67–7.56 (m, 3H), 5.35 (t, J = 5.6 Hz, 1H), 4.62 (d, J = 5.6 Hz, 2H); 13C NMR (151 MHz, DMSO-d6): δ 148.1, 134.7, 131.6, 130.6, 128.5, 128.5, 128.4, 125.0, 54.9; LCMS m/z: 210.1 [M + H]+.
((3-Fluorophenyl)-1H-1,2,3-triazol-4-yl)methanol (7d)
Prepared by General Method 1.2 from 3-fluoroaniline and propargyl alcohol and isolated as a colorless oil (12 mg, 3%).
1H NMR (700 MHz, DMSO-d6): δ 8.75 (s, 1H), 7.89–7.74 (m, 2H), 7.69–7.53 (m, 1H), 7.41–7.24 (m, 1H), 5.36 (t, J = 5.6 Hz, 1H), 4.61 (dd, J = 5.6, 0.6 Hz, 2H); 13C NMR (176 MHz, DMSO-d6): δ 162.4 (d, J = 245.0 Hz), 149.3, 138.0 (d, J = 10.5 Hz), 131.8 (d, J = 9.2 Hz), 121.2, 115.8 (d, J = 3.0 Hz), 115.2 (d, J = 21.0 Hz), 107.4 (d, J = 26.5 Hz), 54.9; LCMS m/z: 194.1 [M + H]+.
((3-Methylphenyl)-1H-1,2,3-triazol-4-yl)methanol (7e)
Prepared by General Method 1.2 from 3-methylaniline and propargyl alcohol and isolated as a clear colorless oil (7 mg, 2%).
1H NMR (400 MHz, methanol-d4): δ 8.43 (s, 1H), 7.70–7.67 (m, 1H), 7.65–7.60 (m, 1H), 7.46 (apparent t, J = 7.8 Hz, 1H), 7.35–7.30 (m, 1H), 4.78–4.75 (m, 2H), 2.47 (s, 3H); LCMS m/z: 190.2 [M + H]+.
((3-Chlorophenyl)-1H-1,2,3-triazol-4-yl)methanol (7f)
Prepared by General Method 1.1 from 3-chlorophenylboronic acid and isolated as an off-white solid (115 mg, 27%).
1H NMR (600 MHz, DMSO-d6): δ 8.77 (s, 1H), 8.05 (t, J = 2.0 Hz, 1H), 7.93 (ddd, J = 8.1, 2.1, 0.9 Hz, 1H), 7.62 (t, J = 8.1 Hz, 1H), 7.55 (ddd, J = 8.1, 2.0, 0.9 Hz, 1H), 5.34 (t, J = 5.6 Hz, 1H), 4.61 (d, J = 5.6 Hz, 2H); 13C NMR (151 MHz, DMSO-d6): δ 149.4, 137.8, 134.2, 131.7, 128.3, 121.2, 119.7, 118.5, 54.9; LCMS m/z: 210.1 [M + H]+.
((3-Trifluoromethylphenyl)-1H-1,2,3-triazol-4-yl)methanol (7g)
Prepared by General Method 1.2 from 3-trifluoromethylaniline and propargyl alcohol and isolated as a tan solid (175 mg, 39%).
1H NMR (700 MHz, DMSO-d6): δ 8.88 (s, 1H), 8.33–8.16 (m, 2H), 7.87–7.76 (m, 2H), 5.37 (t, J = 5.6 Hz, 1H), 4.63 (dd, J = 5.6, 0.6 Hz, 2H); 13C NMR (176 MHz, DMSO-d6): δ 149.5, 137.2, 131.3, 130.6 (q, J = 32.5 Hz), 125.0 (q, J = 3.7 Hz), 123.8, 123.6 (d, J = 272.6 Hz), 121.3, 116.5 (q, J = 3.9 Hz), 54.9; LCMS m/z: 244.2 [M + H]+.
((3-Cyanophenyl)-1H-1,2,3-triazol-4-yl)methanol (7h)
Prepared by General Method 1.1 from 3-cyanophenylboronic acid and isolated as an off-white solid (100 mg, 25%).
1H NMR (600 MHz, DMSO-d6): δ 8.80 (s, 1H), 8.48–8.38 (m, 1H), 8.30 (ddd, J = 8.3, 2.2, 0.8 Hz, 1H), 7.95 (dd, J = 6.7, 1.1 Hz, 1H), 7.81 (t, J = 8.0 Hz, 1H), 5.38 (t, J = 5.6 Hz, 1H), 4.63 (d, J = 5.5 Hz, 2H); 13C NMR (151 MHz, DMSO-d6): δ 149.6, 137.2, 132.1, 131.4, 124.6, 123.3, 121.3, 117.9, 112.8, 55.0; LCMS m/z: 201.1 [M + H]+.
((3-Ethoxyphenyl)-1H-1,2,3-triazol-4-yl)methanol (7i)
Prepared by General Method 1.1 from 3-ethoxyphenylboronic acid and isolated as an off-white solid (212 mg, 48%).
1H NMR (600 MHz, DMSO-d6): δ 8.70 (s, 1H), 7.52–7.41 (m, 3H), 7.09–6.95 (m, 1H), 5.31 (t, J = 5.6 Hz, 1H), 4.60 (d, J = 5.5 Hz, 2H), 4.13 (q, J = 7.0 Hz, 2H), 1.36 (t, J = 7.0 Hz, 3H); 13C NMR (151 MHz, DMSO-d6): δ 159.5, 149.1, 137.8, 130.9, 121.1, 114.6, 111.8, 105.9, 63.6, 55.0, 14.6; LCMS m/z: 220.2 [M + H]+.
((4-Fluorophenyl)-1H-1,2,3-triazol-4-yl)methanol (7j)
Prepared by General Method 1.2 from 4-fluoroaniline and propargyl alcohol and isolated as an off-white solid (115 mg, 32%).
1H NMR (700 MHz, DMSO-d6): δ 8.65 (s, 1H), 7.97–7.88 (m, 2H), 7.47–7.36 (m, 2H), 5.32 (t, J = 5.6 Hz, 1H), 4.60 (dd, J = 5.6, 0.6 Hz, 2H); 13C NMR (176 MHz, DMSO-d6): δ 161.5 (d, J = 245.5 Hz), 149.2, 133.3 (d, J = 2.8 Hz), 122.3 (d, J = 8.7 Hz), 121.2, 116.7 (d, J = 23.2 Hz), 55.0 (s); LCMS m/z: 194.2 [M + H]+.
((4-Methylphenyl)-1H-1,2,3-triazol-4-yl)methanol (7k)
Prepared by General Method 1.2 from 4-methylaniline and propargyl alcohol and isolated as a white solid (120 mg, 34%).
1H NMR (700 MHz, DMSO-d6): δ 8.61 (s, 1H), 7.80–7.73 (m, 2H), 7.37 (dd, J = 8.6, 0.6 Hz, 2H), 5.29 (t, J = 5.6 Hz, 1H), 4.62–4.54 (m, 2H), 2.36 (s, 3H); 13C NMR (176 MHz, DMSO-d6): δ 149.0, 138.0, 134.5, 130.2, 120.8, 119.8, 55.0, 20.5; LCMS m/z: 190.2 [M + H]+.
((4-Trifluoromethylphenyl)-1H-1,2,3-triazol-4-yl)methanol (7l)
Prepared by General Method 1.2 from 4-trifluoromethylaniline and propargyl alcohol and isolated as a white solid (294 mg, 65%).
1H NMR (700 MHz, DMSO-d6): δ 8.84 (s, 1H), 8.17 (d, J = 8.4 Hz, 2H), 7.97 (d, J = 8.5 Hz, 2H), 5.38 (t, J = 5.6 Hz, 1H), 4.63 (d, J = 5.3 Hz, 2H); 13C NMR (176 MHz, DMSO-d6): δ 149.6, 139.5, 128.5 (q, J = 32.4 Hz), 127.2 (q, J = 3.6 Hz), 123.8 (q, J = 272.2 Hz), 121.2, 120.3, 54.9; LCMS m/z: 244.2 [M + H]+.
((4-Cyanophenyl)-1H-1,2,3-triazol-4-yl)methanol (7m)
Prepared by General Method 1.1 from 4-cyanophenylboronic acid and isolated as an off-white solid (53 mg, 13%).
1H NMR (600 MHz, DMSO-d6): δ 8.85 (s, 1H), 8.21–8.12 (m, 2H), 8.12–8.03 (m, 2H), 5.38 (t, J = 5.6 Hz, 1H), 4.63 (d, J = 5.5 Hz, 2H); 13C NMR (151 MHz, DMSO-d6): δ 149.7, 139.7, 134.4, 121.3, 120.3, 118.2, 110.9, 54.9; LCMS m/z: 201.2 [M + H]+.
((4-Ethoxyphenyl)-1H-1,2,3-triazol-4-yl)methanol (7n)
Prepared by General Method 1.1 from 4-ethoxyphenylboronic acid and isolated as an off-white solid (224 mg, 51%).
1H NMR (600 MHz, DMSO-d6): δ 8.57 (s, 1H), 7.81–7.75 (m, 2H), 7.13–7.07 (m, 2H), 5.31 (t, J = 5.6 Hz, 1H), 4.59 (d, J = 5.6 Hz, 2H), 4.09 (q, J = 7.0 Hz, 2H), 1.35 (t, J = 7.0 Hz, 3H); 13C NMR (151 MHz, DMSO-d6): δ 158.4, 148.9, 130.1, 121.6, 121.0, 115.3, 63.6, 55.0, 14.6; LCMS m/z: 220.2 [M + H]+.
(1-(2,3-Dihydrobenzo[b][1,4]dioxin-6-yl)-1H-1,2,3-triazol-4-yl)methanol (7o)
Prepared by General Method 1.1 from 2,3-dihydro-1,4-benzodioxin-6-ylboronic acid and isolated as an off-white solid (124 mg, 27%).
1H NMR (700 MHz, DMSO-d6): δ 8.56 (s, 1H), 7.41 (d, J = 2.6 Hz, 1H), 7.35 (dd, J = 8.7, 2.6 Hz, 1H), 7.04 (d, J = 8.7 Hz, 1H), 5.29 (t, J = 5.6 Hz, 1H), 4.58 (dd, J = 5.6, 0.5 Hz, 2H), 4.37–4.27 (m, 4H); 13C NMR (176 MHz, DMSO-d6): δ 148.8, 143.8, 143.5, 130.5, 121.0, 117.9, 113.0, 109.1, 64.2, 64.1, 55.0; LCMS m/z: 234.2 [M + H]+.
(1-(Benzo[d][1,3]dioxol-5-yl)-1H-1,2,3-triazol-4-yl)methanol (7p)
Prepared by General Method 1.1 from 1,3-benzodioxol-5-ylboronic acid and isolated as an off-white solid (174 mg, 40%).
1H NMR (700 MHz, DMSO-d6): δ 8.56 (s, 1H), 7.49 (d, J = 2.2 Hz, 1H), 7.36 (dd, J = 8.4, 2.2 Hz, 1H), 7.09 (d, J = 8.4 Hz, 1H), 6.14 (s, 2H), 5.30 (t, J = 5.6 Hz, 1H), 4.59 (dd, J = 5.6, 0.6 Hz, 2H); 13C NMR (176 MHz, DMSO-d6): δ 148.9, 148.2, 147.3, 131.2, 121.1, 113.6, 108.6, 102.1, 101.9, 55.0; LCMS m/z: 220.1 [M + H]+.
((2,3-Dichlorophenyl)-1H-1,2,3-triazol-4-yl)methanol (7q)
Prepared by General Method 1.1 from 2,3-dichlorophenylboronic acid and isolated as an off-white solid (97 mg, 20%).
1H NMR (700 MHz, DMSO-d6): δ 8.45–8.44 (m, 1H), 7.91 (dd, J = 8.1, 1.5 Hz, 1H), 7.66 (dd, J = 8.0, 1.5 Hz, 1H), 7.61 (t, J = 8.0 Hz, 1H), 5.36 (t, J = 5.7 Hz, 1H), 4.63 (dd, J = 5.6, 0.6 Hz, 2H); 13C NMR (176 MHz, DMSO-d6): δ 148.2, 136.4, 132.9, 131.9, 129.0, 127.7, 127.3, 125.0 54.8; LCMS m/z: 244.1 [M + H]+.
((2,4-Dichlorophenyl)-1H-1,2,3-triazol-4-yl)methanol (7r)
Prepared by General Method 1.1 from 2,4-dichlorophenylboronic acid and isolated as an off-white solid (82 mg, 17%).
1H NMR (700 MHz, DMSO-d6): δ 8.41 (s, 1H), 7.99 (q, J = 2.3 Hz, 1H), 7.69 (ddd, J = 10.8, 8.5, 1.2 Hz, 2H), 5.44–5.11 (m, 1H), 4.62 (dd, J = 5.6, 0.6 Hz, 2H); 13C NMR (176 MHz, DMSO-d6): δ 148.2, 135.2, 133.8, 130.1, 129.7, 129.6, 128.6, 124.9, 54.8; LCMS m/z: 244.1 [M + H]+.
((2,5-Dichlorophenyl)-1H-1,2,3-triazol-4-yl)methanol (7s)
Prepared by General Method 1.1 from 2,5-dichlorophenylboronic acid and isolated as an off-white solid (90 mg, 18%).
1H NMR (700 MHz, DMSO-d6): δ 8.43 (d, J = 0.5 Hz, 1H), 7.87 (d, J = 2.5 Hz, 1H), 7.81 (d, J = 8.7 Hz, 1H), 7.75–7.70 (m, 1H), 5.36 (t, J = 5.6 Hz, 1H), 4.63 (dd, J = 5.6, 0.6 Hz, 2H); 13C NMR (176 MHz, DMSO-d6): δ 148.2, 135.6, 132.4, 131.9, 131.3, 128.1, 127.5, 124.9, 54.8; LCMS m/z: 244.1 [M + H]+.
((3,5-Dichlorophenyl)-1H-1,2,3-triazol-4-yl)methanol (7t)
Prepared by General Method 1.1 from 3,5-dichlorophenylboronic acid and isolated as an off-white solid (109 mg, 22%).
1H NMR (600 MHz, DMSO-d6): δ 8.85 (s, 1H), 8.09 (d, J = 1.8 Hz, 2H), 7.76 (t, J = 1.8 Hz, 1H), 5.41 (t, J = 5.5 Hz, 1H), 4.61 (d, J = 5.4 Hz, 2H); 13C NMR (151 MHz, DMSO-d6): δ 149.6, 138.4, 135.3, 133.7, 132.3, 127.8, 121.4, 118.5, 54.9; LCMS m/z: 244.1 [M + H]+.
((3,4-Dimethylphenyl)-1H-1,2,3-triazol-4-yl)methanol (7u)
Prepared by General Method 1.1 from 3,4-dimethylphenylboronic acid and isolated as an off-white solid (131 mg, 32%).
1H NMR (400 MHz, DMSO-d6): δ 8.58 (s, 1H), 7.70 (d, J = 2.1 Hz, 1H), 7.59 (dd, J = 8.1, 2.4 Hz, 1H), 7.33 (d, J = 8.2 Hz, 1H), 5.28 (t, J = 5.6 Hz, 1H), 4.69–4.51 (m, 2H), 2.32 (s, 3H), 2.28 (s, 3H); 13C NMR (151 MHz, DMSO-d6): δ 149.0, 138.1, 136.8, 134.7, 130.6, 120.8, 120.8, 117.1, 55.0, 19.5, 19.0; LCMS m/z: 204.2 [M + H]+.
((3,4-Bis(trifluoromethyl)phenyl)-1H-1,2,3-triazol-4-yl)methanol (7v)
Prepared by General Method 1.3 from 3,4-bis(trifluoromethyl)aniline and propargyl alcohol and isolated as a white solid (64 mg, 38%).
1H NMR (400 MHz, CDCl3): δ 8.29 (s, 1H), 8.13–8.03 (m, 3H), 4.94 (s, 2H), 2.34 (s, 1H); LCMS m/z: 312.0 [M + H]+.
((4-Chloro-3-(trifluoromethyl)phenyl)-1H-1,2,3-triazol-4-yl)methanol (7w)
Prepared by General Method 1.4 from 4-chloro-3-trifluoromethylaniline and propargyl alcohol and isolated as a white solid (1.18 g, 83%).
1H NMR (700 MHz, DMSO-d6): δ 8.89 (s, 1H), 8.35 (d, J = 2.5 Hz, 1H), 8.26 (dd, J = 8.7, 2.6 Hz, 1H), 7.95 (d, J = 8.7 Hz, 1H), 5.37 (t, J = 5.6 Hz, 1H), 4.62 (d, J = 5.5 Hz, 2H); 13C NMR (176 MHz, DMSO-d6): δ 149.6, 135.7, 133.3, 130.1, 127.9 (q, J = 31.6 Hz), 125.1, 122.3 (q, J = 273.4 Hz), 121.4, 119.1 (q, J = 5.4 Hz), 54.9; LCMS m/z: 278.1 [M + H]+.
((3-Chloro-4-(trifluoromethyl)phenyl)-1H-1,2,3-triazol-4-yl)methanol (7x)
Prepared by General Method 1.4 from 3-chloro-4-(trifluoromethyl)aniline and propargyl alcohol and isolated as a white solid (248 mg, 65%).
1H NMR (400 MHz, DMSO-d6): δ 8.92 (s, 1H), 8.38 (d, J = 1.9 Hz, 1H), 8.16 (dd, J = 8.6, 1.4 Hz, 1H), 8.08 (d, J = 8.7 Hz, 1H), 5.41 (t, J = 5.6 Hz, 1H), 4.63 (d, J = 5.4 Hz, 2H); 13C NMR (151 MHz, DMSO-d6): δ 149.8, 140.2, 132.4, 129.7 (q, J = 5.2 Hz), 125.9 (q, J = 31.4 Hz), 122.6 (q, J = 272.7 Hz), 122.2, 121.5, 118.4, 54.9; LCMS m/z: 278.1[M + H]+.
((2,4-Dichloro-3-(trifluoromethyl)phenyl)-1H-1,2,3-triazol-4-yl)methanol (7y)
Prepared by General Method 1.3 from 2,4-dichloro-3-(trifluoromethyl)aniline 11a and propargyl alcohol and isolated as a white solid (74 mg, 44%).
1H NMR (700 MHz, DMSO-d6): δ 8.43 (t, J = 0.6 Hz, 1H), 7.97 (q, J = 9.0 Hz, 2H), 5.38 (t, J = 5.6 Hz, 1H), 4.63 (dt, J = 6.3, 3.2 Hz, 2H); 13C NMR (176 MHz, DMSO-d6): δ 148.3, 136.1, 134.8, 132.8, 132.1, 130.6, 125.6 (q, J = 30.2 Hz), 125.4, 122.1 (q, J = 276.6 Hz), 54.8; LCMS m/z: 312.0 [M + H]+.
1-(1-(2,4-Dichloro-3-(trifluoromethyl)phenyl)-1H-1,2,3-triazol-4-yl)ethan-1-ol (8a)
Prepared by General Method 1.3 from 2,4-dichloro-3-trifluoromethylaniline 11a and 3-butyn-2-ol and isolated as a white solid (40 mg, 19%).
1H NMR (400 MHz, CDCl3): δ 7.87 (d, J = 0.6 Hz, 1H), 7.67–7.61 (m, 2H), 5.20 (q, J = 6.6 Hz, 1H), 2.51 (s, 1H), 1.68 (d, J = 6.6 Hz, 3H); LCMS m/z: 326.1 [M + H]+.
2-(1-(2,4-Dichloro-3-(trifluoromethyl)phenyl)-1H-1,2,3-triazol-4-yl)ethan-1-ol (8b)
Prepared by General Method 1.4 from 2,4-dichloro-3-(trifluoromethyl)aniline 11a and 3-butyn-1-ol and isolated as an off-white solid (55 mg, 19%).
1H NMR (600 MHz, DMSO-d6): δ 8.33 (d, J = 4.7 Hz, 1H), 8.00–7.90 (m, 2H), 4.76 (s, 1H), 3.71 (t, J = 6.3 Hz, 2H), 2.89 (dt, J = 6.9 Hz, J = 3.4 Hz, 2H); 13C NMR (151 MHz, DMSO-d6): δ 144.8, 136.1, 134.7, 132.7, 132.1, 130.8–129.7 (m), 125.7 (q, J = 29.8 Hz), 125.1, 122.1 (q, J = 276.9 Hz), 60.1, 29.0; LCMS m/z: 326.1 [M + H]+.
3-(1-(2,4-Dichloro-3-(trifluoromethyl)phenyl)-1H-1,2,3-triazol-4-yl)propan-1-ol (8c)
Prepared by General Method 1.3 from 2,4-dichloro-3-(trifluoromethyl)aniline 11a and 4-pentyn-1-ol and isolated as a clear, colorless oil (94 mg, 58%).
1H NMR (600 MHz, CDCl3): δ 7.71 (s, 1H), 7.63 (q, J = 8.7 Hz, 1H), 7.65 (d, J = 8.7 Hz, 1H), 7.62 (d, J = 8.7 Hz, 1H), 3.77 (t, J = 6.0 Hz, 1H), 2.95 (t, J = 7.4 Hz, 1H), 2.05–1.99 (m, 3H); 13C NMR (151 MHz, CDCl3): δ 147.9, 136.3, 136.0, 131.6, 131.1, 130.8, 128.0 (q, J = 31 Hz), 123.5, 122.1 (q, J = 278 Hz), 62.0, 31.9, 22.1; LCMS m/z: 340.2 [M + H]+.
1-(1-(2,4-Dichloro-3-(trifluoromethyl)phenyl)-1H-1,2,3-triazol-4-yl)propan-2-ol (8d)
Prepared by General Method 1.4 from 2,4-dichloro-3-(trifluoromethyl)aniline 11a and 4-pentyn-2-ol and isolated as an off-white, waxy solid (71 mg, 37%).
1H NMR (600 MHz, CDCl3): δ 8.01 (s, 1H), 7.67 (d, J = 8.8 Hz, 1H), 7.63 (d, J = 8.4 Hz, 1H), 4.42 (br s, 1H), 3.14–2.32 (m, 3H), 1.51–1.36 (m, 3H); 13C NMR (176 MHz, CDCl3): δ 136.7, 131.7 (q, J = 6.3 Hz), 131.5, 131.2, 131.0, 130.9, 130.8, 128.0 (q, J = 31.1 Hz), 122.2 (q, J = 277.2 Hz), 67.7, 34.1, 29.8; LCMS m/z: 340.1 [M + H]+.
3-((1-(2,4-Dichloro-3-(trifluoromethyl)phenyl)-1H-1,2,3-triazol-4-yl)methyl)oxetan-3-ol (8e)
Prepared by General Method 1.4 from 2,4-dichloro-3-(trifluoromethyl)aniline 11a and 3-prop-2-ynyloxetan-3-ol and isolated as a white solid (75 mg, 22%).
1H NMR (600 MHz, DMSO-d6): δ 8.33 (s, 1H), 7.98–7.93 (m, 2H), 4.76 (t, J = 5.3 Hz, 1H), 3.71 (td, J = 6.9, 5.3 Hz, 2H), 2.89 (dt, J = 6.9, 3.5 Hz, 2H), 1.25–1.22 (m, 2H); LCMS m/z: 368.1 [M + H]+.
1-(2,4-Dichloro-3-(trifluoromethyl)phenyl)-1H-1,2,3-triazole-4-carboxylic Acid (8f)
Methyl 1-(2,4-dichloro-3-(trifluoromethyl)phenyl)-1H-1,2,3-triazole-4-carboxylate (14) was prepared by General Method 1.3 from 2,4-dichloro-3-(trifluoromethyl)aniline 11a and methyl propiolate and isolated as a white solid (1.05 g, 71%).
1H NMR (700 MHz, DMSO-d6): δ 9.28 (s, 1H), 8.11 (d, J = 8.7 Hz, 1H), 8.03 (d, J = 8.7 Hz, 1H), 3.90 (s, 3H); LCMS: MS m/z: 340.0 [M + H]+.
Aqueous NaOH (2.65 mL, 1 M, 2.65 mmol, 2.0 equiv) was added to a solution of 14 (450 mg, 1.32 mmol, 1.0 equiv) in MeOH (30 mL). The reaction was stirred at RT for 1 h, acidified to pH 3 with aqueous HCl (2 M), extracted with EtOAc (2 × 100 mL), and washed with brine (50 mL). The organic phase was dried, filtered, and concentrated under reduced pressure to give 8f. The product was isolated as an off-white solid (410 mg, 95%).
1H NMR (700 MHz, DMSO-d6): δ 13.45 (s, 1H), 9.16 (s, 1H), 8.10 (d, J = 8.6 Hz, 1H), 8.05–7.98 (m, 1H); 13C NMR (176 MHz, DMSO-d6): δ 161.3, 140.1, 135.5, 135.3, 133.1, 132.2, 131.6, 130.9, 125.7 (q, J = 30.3 Hz), 122.1 (q, J = 276.7 Hz); LCMS m/z: 325.9 [M + H]+.
1-(2,4-Dichloro-3-(trifluoromethyl)phenyl)-1H-1,2,3-triazole-4-carboxamide (8g)
NH3 (1.0 mL, 2 M in MeOH, 2.0 mmol, 13.3 equiv) was added to 14 (50 mg, 0.15 mmol, 1.0 equiv) and heated to 70 °C for 3 h. The reaction was concentrated under reduced pressure, triturated with Et2O (ca. 2 mL), and then dried in vacuo to give 8g. The product was isolated as a white solid (22 mg, 46%).
1H NMR (700 MHz, DMSO-d6): δ 8.98 (s, 1H), 8.09 (d, J = 8.7 Hz, 1H), 8.07 (s, 1H), 8.01 (d, J = 8.7 Hz, 1H), 7.65 (s, 1H); 13C NMR (176 MHz, DMSO-d6): δ 160.9, 143.1, 135.5, 135.4, 133.0, 132.2, 130.8, 129.4, 125.7 (q, J = 30.2 Hz), 122.1 (q, J = 276.6 Hz); LCMS m/z: 325.0 [M + H]+.
1-(2,4-Dichloro-3-(trifluoromethyl)phenyl)-N-methyl-1H-1,2,3-triazole-4-carboxamide (8h)
MeNH2 (1.0 mL, 2 M in MeOH, 2.0 mmol, 13.3 equiv) was added to 14 (50 mg, 0.15 mmol, 1.0 equiv) and heated to 70 °C for 3 h. The reaction was concentrated under reduced pressure, triturated with Et2O (ca. 2 mL), and then dried in vacuo to give 8h. The product was isolated as a white solid (32 mg, 64%).
1H NMR (700 MHz, DMSO-d6): δ 9.00 (s, 1H), 8.68 (q, J = 4.4 Hz, 1H), 8.09 (apparent dd, J = 8.6, 0.5 Hz, 1H), 8.01 (apparent dd, J = 8.7, 0.6 Hz, 1H), 2.81 (d, J = 4.7 Hz, 3H); 13C NMR (176 MHz, DMSO-d6): δ 159.6, 143.1, 135.5, 135.4, 133.0, 132.2, 130.9, 129.0, 125.7 (q, J = 30.2 Hz), 122.1 (q, J = 276.7 Hz), 25.7; LCMS m/z: 339.0 [M + H]+.
(1-(2,4-Dichloro-3-(trifluoromethyl)phenyl)-1H-1,2,3-triazol-4-yl)methanamine Hydrochloride (8i)
Steps 1 and 2: 15 was prepared by General Method 1.2 from 2,4-dichloro-3-(trifluoromethyl)aniline 11a and tert-butyl prop-2-yn-1-ylcarbamate and isolated as a white solid (64 mg, 8%).
1H NMR (500 MHz, DMSO-d6): δ 8.34 (s, 1H), 8.01–7.90 (m, 2H), 7.42 (t, J = 5.5 Hz, 1H), 4.29 (d, J = 5.5 Hz, 2H), 1.39 (s, 9H); LCMS m/z: = 433.1 [M + Na]+.
Step 3: HCl (0.47 mL, 4 M in dioxane, 1.88 mmol, 11.8 equiv) was added to 15 (64 mg, 0.16 mmol, 1.0 equiv) in Et2O (2.0 mL) and heated to 50 °C for 16 h. The reaction was concentrated under reduced pressure to give 8i·HCl. The product was isolated as an off-white solid (54 mg, quant).
1H NMR (600 MHz, DMSO-d6): δ 8.65 (s, 1H), 8.51 (s, 3H), 8.00 (d, J = 8.8 Hz, 1H), 7.97 (d, J = 8.7 Hz, 1H), 4.26 (s, 2H); 13C NMR (151 MHz, DMSO-d6): δ 140.6, 135.7, 135.2, 132.8, 132.3, 130.5, 127.3, 125.8 (q, J = 30.2 Hz), 122.1 (q, J = 276.7 Hz), 33.7 (s, J = 18.5 Hz); LCMS m/z: 310.9 [M + H]+.
1-(2,4-Dichloro-3-(trifluoromethyl)phenyl)-4-(methoxymethyl)-1H-1,2,3-triazole (8j)
Prepared by General Method 1.3 from 2,4-dichloro-3-(trifluoromethyl)aniline 11a and 3-methoxyprop-1-yne and isolated as a white solid (79 mg, 51%).
1H NMR (600 MHz, DMSO-d6): δ 8.59 (s, 1H), 8.03 (d, J = 8.7 Hz, 1H), 7.98 (d, J = 8.7 Hz, 1H), 4.57 (s, 2H), 3.33 (s, 3H); 13C NMR (151 MHz, DMSO-d6): δ 144.1, 136.0, 135.0, 133.0, 132.2, 130.7, 126.7, 125.7 (q, J = 30.2 Hz), 122.1 (q, J = 276.7 Hz), 64.7, 57.5; LCMS m/z: 326.0 [M + H]+.
1-(2,4-Dichloro-3-(trifluoromethyl)phenyl)-4-methyl-1H-1,2,3-triazole (8k)
*Caution* Step 1: Sodium nitrite (180 mg, 2.61 mmol, 1.2 equiv) was added portionwise to a solution of 2,4-dichloro-3-(trifluoromethyl)aniline 11a (500 mg, 2.17 mmol, 1.0 equiv) in TFA (5 mL, 4.35 mmol) at 0 °C over 30 min. The reaction was then allowed to warm to RT for 1.5 h, H2O (0.1 mL) was added, and the mixture was then recooled to 0 °C. Sodium azide (155 mg, 2.39 mmol, 1.1 equiv) was added portionwise over 30 min, and the reaction mixture was then allowed to warm to RT over 1 h. The mixture was basified to pH 8–9 by dropwise addition of saturated aqueous NaCO3H, then diluted with EtOAc (ca. 50 mL). The organic phase was separated, diluted with t-BuOH (5.0 mL), and then cautiously concentrated under reduced pressure at 25 °C to provide a solution of crude azide 12a in t-BuOH, which was used without further purification.
Step 2: MeOH (5 mL), H2O (5 mL), sodium l-ascorbate (172 mg, 0.9 mmol, 0.4 equiv), trimethyl(propargyl)silane (244 mg, 2.2 mmol, 1.0 equiv), and copper(II) sulfate pentahydrate (109 mg, 0.43 mmol, 0.2 equiv) were added to the solution of crude azide 12a from step one, and the reaction mixture was stirred at 50 °C for 16 h. The reaction was cooled to RT, diluted with H2O (ca. 10 mL), extracted with EtOAc (ca. 50 mL), and the organic phase was washed with H2O (ca. 10 mL) and brine (ca. 10 mL). The organic phase was dried, filtered, and concentrated under reduced pressure to give 16, which was used immediately without further purification.
Step 3: K2CO3 (3.05 g, 21.7 mmol, 10.0 equiv) was added to crude 16 from step 2 in MeOH (15 mL) and heated to reflux for 16 h. The mixture was taken to pH 7 by addition of aqueous HCl (1 M), then diluted with EtOAc (ca. 50 mL). The organic phase was dried, filtered, and concentrated under reduced pressure. Due to incomplete desilylation, the crude material was suspended in THF (10 mL) and TBAF hydrate (1.22 g, 4.35 mmol, 2.0 equiv) in H2O (0.5 mL) was added. The reaction was stirred at RT for 16 h, diluted with EtOAc (ca. 50 mL), and then the organic phase was dried, filtered, and concentrated under reduced pressure. The crude product was purified by column chromatography, eluting with 0–30% EtOAc in cyclohexane to give 8k isolated as an off-white solid (12 mg, 2%).
1H NMR (700 MHz, CDCl3): δ 7.67 (d, J = 0.8 Hz, 1H), 7.64 (d, J = 8.6 Hz, 1H), 7.61 (d, J = 8.7 Hz, 1H), 2.47 (d, J = 0.8 Hz, 3H); 13C NMR (176 MHz, CDCl3): δ 143.7, 136.2 (q, J = 1.2 Hz), 136.1, 131.6, 131.0, 130.7, 128.0 (q, J = 31.1 Hz), 123.6, 122.2 (q, J = 277.2 Hz), 10.9; LCMS m/z: 296.0 [M + H]+.
1-(2,4-Dichloro-3-(trifluoromethyl)phenyl)-1H-1,2,3-triazole (8l)
*Caution* Step 1: 2,4-Dichloro-3-(trifluoromethyl)aniline 11a (1.0 g, 4.3 mmol, 1.0 equiv) was dissolved in TFA (20 mL), cooled to 0 °C, a solution of sodium nitrite (1.4 g, 20.6 mmol, 4.7 equiv) in H2O (2.8 mL) was added dropwise over 10 min, and stirred for a further 30 min. The ice bath was removed, the reaction was stirred at RT for 1 h, and then recooled to 0 °C, before a solution of sodium azide (1.3g, 20.6 mmol, 4.7 equiv) in H2O (5.4 mL, 20.6 mmol) was added dropwise over 30 min. The reaction was allowed to warm to RT over 16 h, before recooling to 0 °C. Aqueous NaOH (52 mL, 5 M) was then cautiously added to take the mixture to pH 8–9 to provide a solution of crude azide 12a, which was used without further purification.
Step 2: MeOH (10 mL), t-BuOH (10 mL), aqueous copper(II) sulfate pentahydrate (5.1 mL, 100 mg/mL, 2.1 mmol, 0.5 equiv), aqueous sodium l-ascorbate (4.1 mL, 1 M, 4.1 mmol, 0.9 equiv), and trimethylsilylacetylene (0.94 mL, 8.7 mmol, 2.0 equiv) were added to the solution of crude azide 12a from step one at RT. The reaction was stirred for 16 h, diluted with EtOAc (ca. 500 mL), and the organic phase was cautiously concentrated under reduced pressure at 25 °C to give crude 17.
Step 3: MeOH (15 mL) and K2CO3 (6.0 g, 43.5 mmol) were added to crude 17, and the reaction was stirred at RT for 24 h, before H2O (ca. 20 mL) was then added until all the solid carbonate dissolved. MeOH was then removed under reduced pressure and the resultant aqueous mixture was diluted with EtOAc (ca. 500 mL). The organic phase was dried, filtered, and concentrated under reduced pressure. The crude product was purified by column chromatography (0–30% EtOAc in cyclohexane) to give 8l and isolated as a white solid (0.83 g, 67%).
mp 111–112 °C; IR νmax (film): 3164, 3141, 3082, 1248, 1198, 1132, 1026, 790 cm–1; 1H NMR (700 MHz, DMSO-d6): δ 8.61 (d, J = 1.2 Hz, 1H), 8.03 (d, J = 1.1 Hz, 1H), 8.01 (apparent dd, J = 8.6, 0.5 Hz, 1H), 7.98 (apparent dd, J = 8.6, 0.5 Hz, 1H); 13C NMR (176 MHz, DMSO-d6): δ 136.0, 134.9, 133.7, 132.9, 132.1, 130.6, 127.7, 125.7 (q, J = 30.2 Hz), 122.1 (q, J = 276.6 Hz); LCMS: MS m/z: 282.0 [M + H]+; HRMS C9H4Cl2F3N: calcd 281.9807 [M + H]+; found, 281.9807.
Spectroscopic and analytical data for 8l are presented in the Supporting Information.
1-(2,4-Dichloro-3-(trifluoromethyl)phenyl)-1H-1,2,3-triazole-4,5-d2 (d2-8l)
*Caution* Step 1: Sodium nitrite (360 mg, 5.22 mmol, 1.2 equiv) was added portionwise to a solution of 2,4-dichloro-3-(trifluoromethyl)aniline 11a (1.0 g, 4.35 mmol, 1.0 equiv) in TFA (10 mL) at 0 °C over 30 min. The reaction mixture was then allowed to warm to RT over 90 min. H2O (1.0 mL) was added and then the mixture was recooled to 0 °C. Sodium azide (310 mg, 4.78 mmol, 1.1 equiv) was added portionwise over 30 min, then the reaction was allowed to warm to RT over 1 h. The mixture was basified to pH 8–9 by dropwise addition of saturated aqueous NaHCO3 and then extracted with CH2Cl2 (ca. 250 mL). The organic phase was separated, diluted with t-BuOH (10.0 mL), and then cautiously concentrated under reduced pressure at 25 °C to provide a solution of crude azide 12a in t-BuOH, which was used without further purification.
Step 2: Copper(II) sulfate pentahydrate (217 mg, 0.87 mmol, 0.2 equiv), sodium l-ascorbate (345 mg, 1.74 mmol, 0.4 equiv), H2O (10 mL), and then trimethylsilylacetylene (0.6 mL, 4.35 mmol, 1.0 equiv) were added to the solution of crude azide 12a from step 1. The reaction mixture was stirred at RT for 16 h, diluted with H2O (20 mL), extracted with EtOAc (ca. 100 mL), and the organic phase was washed with H2O (ca. 20 mL) and brine (ca. 20 mL). The organic phase was dried, filtered, and concentrated under reduced pressure. The crude product was purified by column chromatography (0–30% EtOAc in cyclohexane) to give 17 (390 mg, 1 .1 mmol, 25%) as an oily solid.
1H NMR (400 MHz, DMSO-d6): δ 8.62 (s, 1 H), 8.01 (d, J = 8.8 Hz, 1 H), 7.96 (d, J = 8.8 Hz, 1 H), 0.33 (s, 9H).
Step 3: 17 (100 mg, 0.28 mmol, 1.0 equiv) and K2CO3 (117 mg, 0.85 mmol, 3.0 equiv) were dried for 2 h under vacuum (0.5 mbar, RT). >99% methanol-d4 (5.0 mL) was added under an argon atmosphere and stirred at RT for 3 days, before addition of >99% D2O (5.0 mL). The vessel was quickly transferred to a rotary evaporator and the organics were removed under reduced pressure, then the vessel was quickly resealed. Alumina-treated anhydrous CH2Cl2 (ca. 10 mL) was added, stirred for 15 min, and then the organic layer was removed via a syringe and concentrated under reduced pressure.
The crude product was purified by column chromatography (0–30% EtOAc in cyclohexane) to give d2-8l (>90% d2-isotope) and isolated as a white solid (60 mg, 67%).
1H NMR (700 MHz, DMSO-d6): δ 7.98 (apparent dd, J = 8.7, 0.5 Hz, 1H), 7.72 (apparent dd, J = 8.7, 0.6 Hz, 1H); 13C NMR (176 MHz, DMSO-d6): δ 153.3, 134.14–133.53 (m), 133.0 (q, J = 1.6 Hz), 131.6, 131.3, 128.3, 127.06–126.53 (m), 122.4 (q, J = 275.7 Hz), 121.8 (q, J = 29.5 Hz); LCMS m/z: 284.2 [M + H]+.
1-(2,3,4-Trichlorophenyl)-1H-1,2,3-triazole (8m)
Steps 1 and 2: azide 12 (R2 = R3 = R4 = Cl) was prepared by General Method 1.4 from 2,3,4-trichloroaniline (200 mg, 1.0 mmol, 1.0 equiv) and trimethyl(propargyl)silane and was used directly in step 3.
Step 3: Na2CO3 (5.0 g, 47.2 mmol, 47.2 equiv) was added to azide 12 in MeOH (5.0 mL) and stirred vigorously at RT for 16 h. Aqueous HCl (2 M, ca. 60 mL) was cautiously added; once gas evolution had ceased, the organics were removed under reduced pressure and the mixture was extracted with CH2Cl2 (ca. 60 mL). The organic phase was dried, filtered, and concentrated under reduced pressure. The crude product was purified by column chromatography (0–30% EtOAc in cyclohexane) to give 8m, which was isolated as an off-white solid (181 mg, 72%).
1H NMR (600 MHz, DMSO-d6): δ 8.62 (d, J = 0.9 Hz, 1H), 8.02 (d, J = 1.0 Hz, 1H), 7.93 (d, J = 8.7 Hz, 1H), 7.75 (d, J = 8.7 Hz, 1H); 13C NMR (151 MHz, DMSO-d6): δ 135.1, 134.6, 133.7, 131.7, 129.8, 129.5, 127.6, 127.4; LCMS: MS 248.0 m/z: [M + H]+.
4-(2,4-Dichloro-3-(trifluoromethyl)phenyl)-4H-1,2,4-triazole (9a)
Chlorotrimethylsilane (1.9 mL, 15.0 mmol, 15.0 equiv) was added dropwise to a solution of 2,4-dichloro-3-(trifluoromethyl)aniline 11a (230 mg, 1.0 mmol, 1.0 equiv), 1,2-diformylhydrazine (264 mg, 3.0 mmol, 3.0 equiv), and NEt3 (1.0 mL, 7.0 mmol, 7.0 equiv) in pyridine (9.0 mL) at 0 °C. The reaction was then heated to 115 °C for 2.5 h and then to 130 °C for 20 h. The reaction was cooled to RT, H2O (2.0 mL) was added, and then the mixture was concentrated under reduced pressure. The crude product was purified by reversed-phase chromatography (0–100% MeCN in H2O, 0.1% NH4OH modifier) to give 9a, which was isolated as a tan solid (140 mg, 50%).
1H NMR (700 MHz, DMSO-d6): δ 8.86 (s, 1H), 8.00 (d, J = 8.7, 1H), 7.96 (d, J = 8.7 Hz, 1H); 13C NMR (176 MHz, DMSO-d6): δ 143.3, 134.2, 133.4, 133.1, 132.0, 130.9, 125.5 (q, J = 30.2 Hz), 122.1 (q, J = 276.6 Hz); LCMS m/z: 282.1 [M + H]+.
1-(2,4-Dichloro-3-(trifluoromethyl)phenyl)-1H-tetrazole (9b)
*Caution* Trimethyl orthoformate (0.39 mL, 3.26 mmol, 3.0 equiv) and sodium azide (219 mg, 3.37 mmol, 3.1 equiv) were added to a solution of 2,4-dichloro-3-(trifluoromethyl)aniline 11a (250 mg, 1.09 mmol, 1.0 equiv) in AcOH (2.5 mL). The reaction was heated behind a blast shield to 100 °C for 90 min, then cautiously concentrated (ca. 1 mL solvent remaining) under reduced pressure at 25 °C. H2O (30 mL) was added to the resultant residue at RT and the resultant white crystalline precipitated was collected by filtration, washed with H2O (ca. 10 mL), and dried in vacuo to give 9b. The product was isolated as a white crystalline solid (31 mg, 0.11 mmol, 10%).
1H NMR (700 MHz, DMSO-d6): δ 9.90 (s, 1H), 8.15 (apparent dd, J = 8.7, 0.5 Hz, 1H), 8.06 (apparent dd, J = 8.7, 0.5 Hz, 1H); 13C NMR (176 MHz, DMSO-d6): δ 145.4 (apparent d, J = 16.9 Hz), 136.0 (apparent d, J = 1.2 Hz), 133.1, 132.7, 132.4, 130.9 (apparent d, J = 1.1 Hz), 125.9 (q, J = 30.4 Hz), 122.0 (q, J = 276.7 Hz); LCMS m/z: 283.0 [M + H]+.
3,5-Dichloro-2-(1H-1,2,3-triazol-1-yl)-4-(trifluoromethyl)pyridine (9c)57
*Caution* Step 1: Hydrazine monohydrate (60 μL, 1.2 mmol, 1.5 equiv) was added to a solution of 2,3,5-trichloro-4-trifluoromethyl pyridine 18 (200 mg, 0.80 mmol, 1.0 equiv) in EtOH (0.5 mL). The reaction was slowly warmed to 85 °C behind a blast shield over 4 h, concentrated under reduced pressure, and the resultant solid was suspended in aqueous HCl (1 M, 10 mL) at RT, then cooled to 0 °C. A solution of sodium nitrite (110 mg, 1 .6 mmol) in H2O (0.5 mL) was added over 30 min.
Step 2: The mixture was stirred for a further 30 min before cautious addition of aqueous NaOH (5 M) to adjust the pH to 8–9. H2O (2.0 mL), t-BuOH (2.0 mL), sodium l-ascorbate (159 mg, 0.8 mmol, 1.0 equiv), copper(II) sulfate pentahydrate (20 mg, 0.08 mmol, 0.1 equiv), and trimethylsilylacetylene (110 μL, 0.8 mmol, 1.0 equiv) were added and the reaction was heated to 40 °C for 16 h. The mixture was cooled to RT, diluted with EtOAc (ca. 50 mL), the organic phase was dried, filtered, and concentrated under reduced pressure. The crude product was purified by reversed-phase chromatography (0–100% MeCN in H2O, 0.1% formic acid modifier) to give 9c, which was isolated as a white solid (5 mg, 2%).
1H NMR (700 MHz, CDCl3): δ 8.66 (d, J = 0.6 Hz, 1H), 8.08 (d, J = 1.2 Hz, 1H), 7.90 (d, J = 1.2 Hz, 1H); 13C NMR (176 MHz, CDCl3): δ 149.2, 147.1, 136.7 (q, J = 32.1 Hz), 133.8, 132.0, 126.7, 125.1, 121.2 (q, J = 278.6 Hz); LCMS: MS m/z: 283.0 [M + H]+.
Acknowledgments
We thank our colleagues Jamie Bilsland, Veronique Birault, Dustin (Dusty) Flanagan, Ernest Palomer, Morgan Roberts, Laura Schuhmacher, Owen Sansom, David Steadman, and Ed Tate of our Notum Consortium for their support and advice. Our advisors from the Alzheimer’s Drug Discovery Foundation and Harrington Discovery Institute Therapeutic Development Center are gratefully acknowledged for their expert guidance: Peter Bernstein, Jeffery Klein, Robert Lipper, Meriel Owen, Frank Richardson, Ashley Roe, and Alessio Travaglia. The Cell Services and Structural Biology Science Technology Platforms (STPs) at the Francis Crick Institute are gratefully acknowledged for their provision and purification of recombinant Notum. We thank staff at Diamond Light Source for assistance with X-ray data collection and the UCL Department of Chemistry for spectroscopic and analytical services. We are grateful to our partners who performed research studies on our behalf: Charles River Laboratories (Netherlands), Cyprotex (U.K.), eurofins (U.S.A.), GVK Biosciences (now aragen) (India), Key Organics (U.K.), Reach Separations (U.K.), Thermo Fisher Scientific (U.K.), and WuXi AppTec (China).
Glossary
Abbreviations
- ABPP
activity-based protein profiling
- AD
Alzheimer’s disease
- ADME
absorption, distribution, metabolism, and elimination
- BBB
blood–brain barrier
- CDK14
cyclin-dependent kinase 14
- CK1
casein kinase 1
- CNS
central nervous system
- CYP450
cytochrome P450
- DMSO
dimethylsulfoxide
- DSPL
Diamond-SGC Poised Library
- ER
efflux ratio
- FBDD
fragment-based drug design
- GSK3β
glycogen synthase kinase 3β
- HLM
human liver microsomes
- HTS
high-throughput screen
- Kp
brain to plasma ratio
- Kp,uu
free partition coefficient of brain
- LE
ligand efficiency
- LLE
lipophilic ligand efficiency
- MLM
mouse liver microsomes
- MPO
multiparameter optimization
- MW
molecular weight
- OPTS
trisodium 8-octanoyloxypyrene-1,3,6-trisulfonate
- PBS
phosphate-buffered saline
- PDB
Protein Data Bank
- P-gp
P-glycoprotein
- PD
pharmacodynamics
- PK
pharmacokinetics
- RT
room temperature
- SAR
structure–activity relationship
- SBDD
structure-based drug design
- TBAF
tetra-n-butylammonium fluoride
- TCF/LEF
T-cell factor/lymphoid enhancing factor
- TDI
time-dependent inactivation
- THF
tetrahydrofuran
- TMS
trimethylsilyl
- TPSA
topological polar surface area
Supporting Information Available
The Supporting Information is available free of charge at https://pubs.acs.org/doi/10.1021/acs.jmedchem.2c00162.
Overlay of DSPL screening hits 6; ADME protocols and data; PK studies; Notum OPTS and TCF/LEF screening data concentration–response curves; ABPP optimization; serine hydrolase selectivity of 1; kinase selectivity panel; safety pharmacology studies; DSC for azide 12a; X-ray structure determination including data collection and refinement statistics; ligand electron-density maps; and spectroscopic and analytical data for 8l (PDF)
Identity and quantification of each serine hydrolase identified in SW620 cells by FP-biotin ABPP (XLSX)
Molecular formula strings and Notum IC50 data for 6–9 (CSV)
Accession Codes
Coordinates for X-ray structures of Notum crystallized with 6b (7B89) and 8l (7PK3) have been deposited in the Protein Data Bank. Authors will release the atomic coordinates upon article publication.
Author Contributions
The research was performed through contributions of all authors. N.J.W., J.S., P.W., and P.V.F. wrote the manuscript with additional contributions from Y.Z. and F.S. All authors have given approval to the final version of the manuscript.
This work was supported by Alzheimer’s Research UK (ARUK), the Francis Crick Institute, the Alzheimer’s Drug Discovery Foundation (ADDF), and Cancer Research UK. The ARUK UCL Drug Discovery Institute is core funded by Alzheimer’s Research UK (520909). The Francis Crick Institute receives its core funding from Cancer Research UK (FC001002), the UK Medical Research Council (FC001002), and the Wellcome Trust (FC001002). The Alzheimer’s Drug Discovery Foundation and Harrington Discovery Institute at University Hospitals in Cleveland granted an ADDF-Harrington Scholar Award to P.V.F. Structural analysis was performed by Y.Z., L.V., R.R.R., and E.Y.J. supported by Cancer Research UK (Programme Grant C375/A17721). The Wellcome Trust funds the Wellcome Centre for Human Genetics, University of Oxford (Centre Grant 203141/Z/16/Z).
The authors declare the following competing financial interest(s): N.J.W, W.M., J.S., Y.Z., H.L.W., B.N.A., E.D.B., L.V., R.R.R., E.Y.J and P.V.F. are co-inventors of patent application WO 2020043866, which describes inhibitors of Notum. The authors have no other relevant affiliations or financial involvement apart from those disclosed.
Supplementary Material
References
- Nusse R.; Clevers H. Wnt/β-catenin signaling, disease, and emerging therapeutic modalities. Cell 2017, 169, 985–999. 10.1016/j.cell.2017.05.016. [DOI] [PubMed] [Google Scholar]
- Niehrs C. Function and biological roles of the Dickkopf family of Wnt modulators. Oncogene 2006, 25, 7469–7481. 10.1038/sj.onc.1210054. [DOI] [PubMed] [Google Scholar]
- Kakugawa S.; Langton P. F.; Zebisch M.; Howell S. A.; Chang T.-H.; Liu Y.; Feizi T.; Bineva G.; O’Reilly N.; Snijders A. P.; Jones E. Y.; Vincent J.-P. Notum deacylates Wnt proteins to suppress signalling activity. Nature 2015, 519, 187–192. 10.1038/nature14259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang X.; Cheong S.-M.; Amado N. G.; Reis A. H.; MacDonald B. T.; Zebisch M.; Jones E. Y.; Abreu J. G.; He X. NOTUM is required for neural and head induction via Wnt deacylation, oxidation, and inactivation. Dev. Cell 2015, 32, 719–730. 10.1016/j.devcel.2015.02.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ng L.; Kaur P.; Bunnag N.; Suresh J.; Sung I.; Tan Q.; Gruber J.; Tolwinski N. WNT signaling in disease. Cells 2019, 8, 826. 10.3390/cells8080826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McLeod F.; Salinas P. C. Wnt proteins as modulators of synaptic plasticity. Curr. Opin. Neurobiol. 2018, 53, 90–95. 10.1016/j.conb.2018.06.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zheng H.; Jia L.; Liu C.-C.; Rong Z.; Zhong L.; Yang L.; Chen X.-F.; Fryer J. D.; Wang X.; Zhang Y.-w.; Xu H.; Bu G. TREM2 promotes microglial survival by activating Wnt/β-catenin pathway. J. Neurosci. 2017, 37, 1772–1784. 10.1523/JNEUROSCI.2459-16.2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mizrak D.; Bayin N. S.; Yuan J.; Liu Z.; Suciu R. M.; Niphakis M. J.; Ngo N.; Lum K. M.; Cravatt B. F.; Joyner A. L.; Sims P. A. Single-cell profiling and SCOPE-Seq reveal lineage dynamics of adult ventricular-subventricular zone neurogenesis and NOTUM as a key regulator. Cell Rep. 2020, 31, 107805. 10.1016/j.celrep.2020.107805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou Y.; Wang Y.; Tischfield M.; Williams J.; Smallwood P. M.; Rattner A.; Taketo M. M.; Nathans J. Canonical WNT signaling components in vascular development and barrier formation. J. Clin. Invest. 2014, 124, 3825–3846. 10.1172/JCI76431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cho C.; Smallwood P. M.; Nathans J. Reck and Gpr124 are essential receptor cofactors for Wnt7a/Wnt7b-specific signaling in mammalian CNS angiogenesis and blood-brain barrier regulation. Neuron 2017, 95, 1221–1225. 10.1016/j.neuron.2017.08.032. [DOI] [PubMed] [Google Scholar]
- Palomer E.; Buechler J.; Salinas P. C. Wnt signaling deregulation in the aging and Alzheimer’s brain. Front. Cell. Neurosci. 2019, 13, 227. 10.3389/fncel.2019.00227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chang J.; Mancuso M. R.; Maier C.; Liang X.; Yuki K.; Yang L.; Kwong J. W.; Wang J.; Rao V.; Vallon M.; Kosinski C.; Zhang J. J. H.; Mah A. T.; Xu L.; Li L.; Gholamin S.; Reyes T. F.; Li R.; Kuhnert F.; Han X.; Yuan J.; Chiou S.-H.; Brettman A. D.; Daly L.; Corney D. C.; Cheshier S. H.; Shortliffe L. D.; Wu X.; Snyder M.; Chan P.; Giffard R. G.; Chang H. Y.; Andreasson K.; Kuo C. J. Gpr124 is essential for blood-brain barrier integrity in central nervous system disease. Nat. Med. 2017, 23, 450–460. 10.1038/nm.4309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tran K. A.; Zhang X.; Predescu D.; Huang X.; Machado R. F.; Göthert J. R.; Malik A. B.; Valyi-Nagy T.; Zhao Y.-Y. Endothelial β-catenin signaling is required for maintaining adult blood-brain barrier integrity and central nervous system homeostasis. Circulation 2016, 133, 177–186. 10.1161/CIRCULATIONAHA.115.015982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lengfeld J. E.; Lutz S. E.; Smith J. R.; Diaconu C.; Scott C.; Kofman S. B.; Choi C.; Walsh C. M.; Raine C. S.; Agalliu I.; Agalliu D. Endothelial Wnt/β-catenin signaling reduces immune cell infiltration in multiple sclerosis. Proc. Natl. Acad. Sci. U.S.A. 2017, 114, E1168–E1177. 10.1073/pnas.1609905114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bayle E. D.; Svensson F.; Atkinson B. N.; Steadman D.; Willis N. J.; Woodward H. L.; Whiting P.; Vincent J.-P.; Fish P. V. Carboxylesterase Notum is a druggable target to modulate Wnt signalling. J. Med. Chem. 2021, 64, 4289–4311. 10.1021/acs.jmedchem.0c01974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao Y.; Jolly S.; Benvegnu S.; Jones E. Y.; Fish P. V. Small molecule inhibitors of carboxylesterase Notum. Future Med. Chem. 2021, 13, 1001–1015. 10.4155/fmc-2021-0036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tarver J. E.; Pabba P. K.; Barbosa J.; Han Q.; Gardyan M. W.; Brommage R.; Thompson A. Y.; Schmidt J. M.; Wilson A. G. E.; He W.; Lombardo V. K.; Carson K. G. Stimulation of cortical bone formation with thienopyrimidine based inhibitors of Notum pectinacetylesterase. Bioorg. Med. Chem. Lett. 2016, 26, 1525–1528. 10.1016/j.bmcl.2016.02.021. [DOI] [PubMed] [Google Scholar]
- Suciu R. M.; Cognetta A. B.; Potter Z. E.; Cravatt B. F. Selective irreversible inhibitors of the Wnt deacylating enzyme NOTUM developed by activity-based protein profiling. ACS Med. Chem. Lett. 2018, 9, 563–568. 10.1021/acsmedchemlett.8b00191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brommage R.; Liu J.; Vogel P.; Mseeh F.; Thompson A. Y.; Potter D. G.; Shadoan M. K.; Hansen G. M.; Jeter-Jones S.; Cui J.; Bright D.; Bardenhagen J. P.; Doree D. D.; Movérare-Skrtic S.; Nilsson K. H.; Henning P.; Lerner U. H.; Ohlsson C.; Sands A. T.; Tarver J. E.; Powell D. R.; Zambrowicz B.; Liu Q. NOTUM inhibition increases endocortical bone formation and bone strength. Bone Res. 2019, 7, 2. 10.1038/s41413-018-0038-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Robertis M.; Arigoni M.; Loiacono L.; Riccardo F.; Calogero R. A.; Feodorova Y.; Tashkova D.; Belovejdov V.; Sarafian V.; Cavallo F.; Signori E. Novel insights into Notum and glypicans regulation in colorectal cancer. Oncotarget 2015, 6, 41237–41257. 10.18632/oncotarget.5652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Flanagan D. J.; Pentinmikko N.; Luopajärvi K.; Willis N. J.; Gilroy K.; Raven A. P.; Mcgarry L.; Englund J. I.; Webb A. T.; Scharaw S.; Nasreddin N.; Hodder M. C.; Ridgway R. A.; Minnee E.; Sphyris N.; Gilchrist E.; Najumudeen A. K.; Romagnolo B.; Perret C.; Williams A. C.; Clevers H.; Nummela P.; Lähde M.; Alitalo K.; Hietakangas V.; Hedley A.; Clark W.; Nixon C.; Kirschner K.; Jones E. Y.; Ristimäki A.; Leedham S. J.; Fish P. V.; Vincent J.-P.; Katajisto P.; Sansom O. J. NOTUM from Apc-mutant cells biases clonal competition to initiate cancer. Nature 2021, 594, 430–435. 10.1038/s41586-021-03525-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pentinmikko N.; Iqbal S.; Mana M.; Andersson S.; Cognetta A. B.; Suciu R. M.; Roper J.; Luopajärvi K.; Markelin E.; Gopalakrishnan S.; Smolander O.-P.; Naranjo S.; Saarinen T.; Juuti A.; Pietiläinen K.; Auvinen P.; Ristimäki A.; Gupta N.; Tammela T.; Jacks T.; Sabatini D. M.; Cravatt B. F.; Yilmaz Ö. H.; Katajisto P. Notum produced by Paneth cells attenuates regeneration of aged intestinal epithelium. Nature 2019, 571, 398–402. 10.1038/s41586-019-1383-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Atkinson B. N.; Steadman D.; Zhao Y.; Sipthorp J.; Vecchia L.; Ruza R. R.; Jeganathan F.; Lines G.; Frew S.; Monaghan A.; Kjær S.; Bictash M.; Jones E. Y.; Fish P. V. Discovery of 2-phenoxyacetamides as inhibitors of the Wnt-depalmitoleating enzyme NOTUM from an X-ray fragment screen. MedChemComm 2019, 10, 1361–1369. 10.1039/c9md00096h. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Atkinson B. N.; Steadman D.; Mahy W.; Zhao Y.; Sipthorp J.; Bayle E. D.; Svensson F.; Papageorgiou G.; Jeganathan F.; Frew S.; Monaghan A.; Bictash M.; Yvonne Jones E.; Fish P. V. Scaffold-hopping identifies furano[2,3-d]pyrimidine amides as potent Notum inhibitors. Bioorg. Med. Chem. Lett. 2020, 30, 126751. 10.1016/j.bmcl.2019.126751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mahy W.; Patel M.; Steadman D.; Woodward H. L.; Atkinson B. N.; Svensson F.; Willis N. J.; Flint A.; Papatheodorou D.; Zhao Y.; Vecchia L.; Ruza R. R.; Hillier J.; Frew S.; Monaghan A.; Costa A.; Bictash M.; Walter M. W.; Jones E. Y.; Fish P. V. Screening of a custom-designed acid fragment library identifies 1-phenylpyrroles and 1-phenylpyrrolidines as inhibitors of Notum carboxylesterase activity. J. Med. Chem. 2020, 63, 9464–9483. 10.1021/acs.jmedchem.0c00660. [DOI] [PubMed] [Google Scholar]
- Mahy W.; Willis N. J.; Zhao Y.; Woodward H. L.; Svensson F.; Sipthorp J.; Vecchia L.; Ruza R. R.; Hillier J.; Kjær S.; Frew S.; Monaghan A.; Bictash M.; Salinas P. C.; Whiting P.; Vincent J.-P.; Jones E. Y.; Fish P. V. 5-Phenyl-1,3,4-oxadiazol-2(3H)-ones are potent inhibitors of Notum carboxylesterase activity identified by the optimization of a crystallographic fragment screening hit. J. Med. Chem. 2020, 63, 12942–12956. 10.1021/acs.jmedchem.0c01391. [DOI] [PubMed] [Google Scholar]
- a Steadman D.; Atkinson B. N.; Zhao Y.; Willis N. J.; Frew S.; Monaghan A.; Patel C.; Armstrong E.; Costelloe K.; Magno L.; Bictash M.; Jones E. Y.; Fish P. V.; Svensson F. Virtual screening directly identifies new fragment-sized inhibitors of carboxylesterase Notum with nanomolar activity. J. Med. Chem. 2022, 65, 562–578. 10.1021/acs.jmedchem.1c01735. [DOI] [PubMed] [Google Scholar]; b Zhao Y.; Svensson F.; Steadman D.; Frew S.; Monaghan A.; Bictash M.; Moreira T.; Chalk R.; Lu W.; Fish P. V.; Jones E. Y. Structural Insights into Notum Covalent Inhibition. J. Med. Chem. 2021, 64, 11354–11363. 10.1021/acs.jmedchem.1c00701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Willis N. J.; Bayle E. D.; Papageorgiou G.; Steadman D.; Atkinson B. N.; Mahy W.; Fish P. V. An improved, scalable synthesis of Notum inhibitor LP-922056 using 1-chloro-1,2-benziodoxol-3-one as a superior electrophilic chlorinating agent. Beilstein J. Org. Chem. 2019, 15, 2790–2797. 10.3762/bjoc.15.271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- For recent reviews, see:; a Kirsch P.; Hartman A. M.; Hirsch A. K. H.; Empting M. Concepts and core principles of fragment-based drug design. Molecules 2019, 24, 4309. 10.3390/molecules24234309. [DOI] [PMC free article] [PubMed] [Google Scholar]; b Lamoree B.; Hubbard R. E. Current perspectives in fragment-based lead discovery (FBLD). Essays Biochem. 2017, 61, 453–464. 10.1042/ebc20170028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- For an excellent series of annual reviews, see:de Esch I. J. P.; Erlanson D. A.; Jahnke W.; Johnson C. N.; Walsh L. Fragment-to-lead medicinal chemistry publications in 2020. J. Med. Chem. 2022, 65, 84–99. 10.1021/acs.jmedchem.1c01803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Congreve M.; Carr R.; Murray C.; Jhoti H. A “Rule of Three” for fragment-based lead discovery?. Drug Discovery Today 2003, 8, 876–877. 10.1016/s1359-6446(03)02831-9. [DOI] [PubMed] [Google Scholar]
- a Lu W.; Kostic M.; Zhang T.; Che J.; Patricelli M. P.; Jones L. H.; Chouchani E. T.; Gray N. S. Fragment-based covalent ligand discovery. RSC Chem. Biol. 2021, 2, 354–367. 10.1039/d0cb00222d. [DOI] [PMC free article] [PubMed] [Google Scholar]; b Keeley A.; Petri L.; Ábrányi-Balogh P.; Keserű G. M. Covalent fragment libraries in drug discovery. Drug Discovery Today 2020, 25, 983–996. 10.1016/j.drudis.2020.03.016. [DOI] [PubMed] [Google Scholar]
- Morley A. D.; Pugliese A.; Birchall K.; Bower J.; Brennan P.; Brown N.; Chapman T.; Drysdale M.; Gilbert I. H.; Hoelder S.; Jordan A.; Ley S. V.; Merritt A.; Miller D.; Swarbrick M. E.; Wyatt P. G. Fragment-based hit identification: thinking in 3D. Drug Discovery Today 2013, 18, 1221–1227. 10.1016/j.drudis.2013.07.011. [DOI] [PubMed] [Google Scholar]
- Liu M.; Quinn R. J. Fragment-based screening with natural products for novel anti-parasitic disease drug discovery. Expert Opin. Drug Discovery 2019, 14, 1283–1295. 10.1080/17460441.2019.1653849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- a Wood D. J.; Lopez-Fernandez J. D.; Knight L. E.; Al-Khawaldeh I.; Gai C.; Lin S.; Martin M. P.; Miller D. C.; Cano C.; Endicott J. A.; Hardcastle I. R.; Noble M. E. M.; Waring M. J. FragLites – minimal, halogenated fragments displaying pharmacophore doublets. An efficient approach to druggability assessment and hit generation. J. Med. Chem. 2019, 62, 3741–3752. 10.1021/acs.jmedchem.9b00304. [DOI] [PubMed] [Google Scholar]; b O’Reilly M.; Cleasby A.; Davies T. G.; Hall R. J.; Ludlow R. F.; Murray C. W.; Tisi D.; Jhoti H. Crystallographic screening using ultra-low-molecular-weight ligands to guide drug design. Drug Discovery Today 2019, 24, 1081–1086. 10.1016/j.drudis.2019.03.009. [DOI] [PubMed] [Google Scholar]
- Heidrich J.; Sperl L. E.; Boeckler F. M. Embracing the diversity of halogen bonding motifs in fragment-based drug discovery – construction of a diversity-optimized halogen-enriched fragment library. Front. Chem. 2019, 7, 9. 10.3389/fchem.2019.00009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cox O. B.; Krojer T.; Collins P.; Monteiro O.; Talon R.; Bradley A.; Fedorov O.; Amin J.; Marsden B. D.; Spencer J.; von Delft F.; Brennan P. E. A poised fragment library enables rapid synthetic expansion yielding the first reported inhibitors of PHIP(2), an atypical bromodomain. Chem. Sci. 2016, 7, 2322–2330. 10.1039/c5sc03115j. [DOI] [PMC free article] [PubMed] [Google Scholar]
- See. https://www.diamond.ac.uk/Instruments/Mx/Fragment-Screening/Fragment-Libraries0/DSi-Poised-Library.html. The DSPL was decommissioned in 2017 and replaced by the Diamond-SGC-iNEXT Poised Library (DsiPL), which is aligned with the Enamine REAL Database (accessed on Jan 6, 2022).
- Zhao Y.; Schuhmacher L.-N.; Roberts M.; Kakugawa S.; Bineva-Todd G.; Howell S.; O’Reilly N.; Perret C.; Snijders A. P.; Vincent J.-P.; Jones E. Y.; Jones E. Y. Notum deacylates octanoylated ghrelin. Mol. Metab. 2021, 49, 101201. 10.1016/j.molmet.2021.101201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- As assessed by Schrödinger SiteMap, which is used to detect and score binding sites. The score is constructed and calibrated so that the average SiteScore for 157 investigated submicromolar sites is 1.0; a score >1 suggests a site of particular promise for druggability. Application of SiteMap to the palmitoleate binding site of Notum (PDB: 4UZQ) generated a SiteScore of 1.2. Schrödinger Release 2019-3: SiteMap, Schrödinger, LLC, New York, NY, 2019. See,Halgren T. A. Identifying and characterizing binding sites and assessing druggability. J. Chem. Inf. Model. 2009, 49, 377–389. 10.1021/ci800324m. [DOI] [PubMed] [Google Scholar]
- Zhao Y.; Ren J.; Hillier J.; Jones M.; Lu W.; Jones E. Y. Structural characterization of melatonin as an inhibitor of the Wnt deacylase Notum. J. Pineal Res. 2020, 68, e12630. 10.1111/jpi.12630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coordinates for these 59 X-ray structures have been published in the Protein Data Bank (PDB) (accessed Jan 13, 2022): 6R8P, 6TR7, 6ZUV, 6ZVL, 7B7W, 7B7X, 7B7Y, 7B84, 7B86, 7B87, 7B89, 7B8A, 7B8C, 7B8D, 7B8F, 7B8G, 7B8J, 7B8K, 7B8L, 7B8M, 7B8N, 7B8O, 7B8U, 7B8X, 7B8Y, 7B8Z, 7B98, 7B99, 7B9D, 7B9I, 7B9N, 7B9U, 7BA1, 7BAC, 7BAP, 7BC8, 7BC9, 7BCC, 7BCD, 7BCE, 7BCF, 7BCH, 7BCI, 7BCK, 7BCL, 7BD2, 7BD3, 7BD4, 7BD5, 7BD6, 7BD8, 7BD9, 7BDA, 7BDB, 7BDC, 7BDD, 7BDF, 7BDG, 7BDH and 7B01.
- Zhao Y.; Mahy W.; Willis N. J.; Woodward H. L.; Steadman D.; Bayle E. D.; Atkinson B. N.; James Sipthorp J.; Luca Vecchia L.; Ruza R. R.; Hillier J.; Harlos K.; Jeganathan F.; Constantinou S.; Costa A.; Kjær S.; Bictash M.; Salinas P. C.; Whiting P.; Vincent J.-P.; Fish P. V.; Jones E. Y.. Structure Analysis and Development of Notum Fragment Screening Hits. Unpublished results. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Teague S. J.; Davis A. M.; Leeson P. D.; Oprea T. The design of leadlike combinatorial libraries. Angew. Chem., Int. Ed. 1999, 38, 3743–3748. . [DOI] [PubMed] [Google Scholar]
- Hopkins A. L.; Groom C. R.; Alex A. Ligand efficiency: a useful metric for lead selection. Drug Discovery Today 2004, 9, 430–431. 10.1016/s1359-6446(04)03069-7. [DOI] [PubMed] [Google Scholar]
- Johnson T. W.; Gallego R. A.; Edwards M. P. Lipophilic efficiency as an important metric in drug design. J. Med. Chem. 2018, 61, 6401–6420. 10.1021/acs.jmedchem.8b00077. [DOI] [PubMed] [Google Scholar]
- Wager T. T.; Hou X.; Verhoest P. R.; Villalobos A. Moving beyond rules: the development of a central nervous system multiparameter optimization (CNS MPO) approach to enable alignment of druglike properties. ACS Chem. Neurosci. 2010, 1, 435–449. 10.1021/cn100008c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gupta M.; Lee H. J.; Barden C. J.; Weaver D. F. The Blood-brain barrier (BBB) score. J. Med. Chem. 2019, 62, 9824–9836. 10.1021/acs.jmedchem.9b01220. [DOI] [PubMed] [Google Scholar]
- Chopra N.; Kaur D.; Chopra G. Nature and hierarchy of hydrogen-bonding interactions in binary complexes of azoles with water and hydrogen peroxide. ACS Omega 2018, 3, 12688–12702. 10.1021/acsomega.8b01523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- a Leeson P. D.; Springthorpe B. The influence of drug-like concepts on decision-making in medicinal chemistry. Nat. Rev. Drug Discovery 2007, 6, 881–890. 10.1038/nrd2445. [DOI] [PubMed] [Google Scholar]; b Leeson P. D.; Bento A. P.; Gaulton A.; Hersey A.; Manners E. J.; Radoux C. J.; Leach A. R. Target-based evaluation of “drug-like” properties and ligand efficiencies. J. Med. Chem. 2021, 64, 7210–7230. 10.1021/acs.jmedchem.1c00416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scherf U.; Ross D. T.; Waltham M.; Smith L. H.; Lee J. K.; Tanabe L.; Kohn K. W.; Reinhold W. C.; Myers T. G.; Andrews D. T.; Scudiero D. A.; Eisen M. B.; Sausville E. A.; Pommier Y.; Botstein D.; Brown P. O.; Weinstein J. N. A gene expression database for the molecular pharmacology of cancer. Nat. Genet. 2000, 24, 236–244. 10.1038/73439. [DOI] [PubMed] [Google Scholar]
- a Liu Y.; Patricelli M. P.; Cravatt B. F. Activity-based protein profiling: The serine hydrolases. Proc. Natl. Acad. Sci. U.S.A. 1999, 96, 14694–14699. 10.1073/pnas.96.26.14694. [DOI] [PMC free article] [PubMed] [Google Scholar]; b Bachovchin D. A.; Cravatt B. F. The pharmacological landscape and therapeutic potential of serine hydrolases. Nat. Rev. Drug Discovery 2012, 11, 52–68. 10.1038/nrd3620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peters J. M.; McKay R. M.; McKay J. P.; Graff J. M. Casein kinase I transduces Wnt signals. Nature 1999, 401, 345–350. 10.1038/43830. [DOI] [PubMed] [Google Scholar]
- Bowes J.; Brown A. J.; Hamon J.; Jarolimek W.; Sridhar A.; Waldron G.; Whitebread S. Reducing safety-related drug attrition: the use of in vitro pharmacological profiling. Nat. Rev. Drug Discovery 2012, 11, 909–922. 10.1038/nrd3845. [DOI] [PubMed] [Google Scholar]
- Goya Grocin A.; Serwa R. A.; Morales Sanfrutos J.; Ritzefeld M.; Tate E. W. Whole proteome profiling of N-myristoyltransferase activity and inhibition using Sortase A. Mol. Cell. Proteomics 2019, 18, 115–126. 10.1074/mcp.ra118.001043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perez-Riverol Y.; Csordas A.; Bai J.; Bernal-Llinares M.; Hewapathirana S.; Kundu D. J.; Inuganti A.; Griss J.; Mayer G.; Eisenacher M.; Pérez E.; Uszkoreit J.; Pfeuffer J.; Sachsenberg T.; Yılmaz Ş.; Tiwary S.; Cox J.; Audain E.; Walzer M.; Jarnuczak A. F.; Ternent T.; Brazma A.; Vizcaíno J. A. The PRIDE database and related tools and resources in 2019: improving support for quantification data. Nucleic Acids Res. 2019, 47, D442–D450. 10.1093/nar/gky1106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reisinger A.; Bernhardt P. V.; Wentrup C. Synthesis of 1,3-diazepines and ring contraction to cyanopyridines. Org. Biomol. Chem. 2004, 2, 246–256. 10.1039/b311247k. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.