Abstract
Background:
Binge-eating disorder (BED) is a common psychiatric condition with adverse psychological and metabolic consequences. Lisdexamfetamine (LDX) is the only approved BED drug treatment. New drugs to treat BED are urgently needed.
Methods:
A comprehensive review of published psychopathological, pharmacological and clinical findings.
Results:
The evidence supports the hypothesis that BED is an impulse control disorder with similarities to ADHD, including responsiveness to catecholaminergic drugs, for example LDX and dasotraline. The target product profile (TPP) of the ideal BED drug combines treating the psychopathological drivers of the disorder with an independent weight-loss effect. Drugs with proven efficacy in BED have a common pharmacology; they potentiate central noradrenergic and dopaminergic neurotransmission. Because of the overlap between pharmacotherapy in attention deficit hyperactivity disorder (ADHD) and BED, drug-candidates from diverse pharmacological classes, which have already failed in ADHD would also be predicted to fail if tested in BED. The failure in BED trials of drugs with diverse pharmacological mechanisms indicates many possible avenues for drug discovery can probably be discounted.
Conclusions:
(1) The efficacy of drugs for BED is dependent on reducing its core psychopathologies of impulsivity, compulsivity and perseveration and by increasing cognitive control of eating. (2) The analysis revealed a large number of pharmacological mechanisms are unlikely to be productive in the search for effective new BED drugs. (3) The most promising areas for new treatments for BED are drugs, which augment noradrenergic and dopaminergic neurotransmission and/or those which are effective in ADHD.
Keywords: Binge-eating disorder, binge eating, obesity, attention deficit hyperactivity disorder, drugs, animal models
Introduction
Binge-eating disorder (BED) is a psychiatric disorder characterised by loss of control leading to frequent, compulsive episodes of excessive eating (binges). BED differs from anorexia nervosa (AN) or bulimia nervosa (BN) because it is not associated with the regular use of inappropriate compensatory behaviour (e.g. purging, fasting and excessive exercise). As discussed in the review, BED is a major causal factor in obesity and is an independent risk factor for a wide range of metabolic, physical and other psychiatric disorders. Lisdexamfetamine (LDX) (Vyvanse®) has been approved to treat BED in the USA and a limited number of other countries. However, with only one medication available in some countries and none in others, new drugs are urgently needed to provide physicians with prescribing choices when treating this disorder.
In view of the extensive overlap between BED and obesity, we compare the diagnostic criteria for these two disorders, discuss their neurobiology and pathology, and the pharmacology of drugs used to treat them. The following objectives are addressed:
➢ Differentiate the neurobiology of BED and obesity.
➢ Define the pharmacology of current drugs that are effective in BED.
➢ Define the pharmacological target profile of the ideal BED drug.
➢ Evaluate drug-candidates for BED in non-clinical and clinical development.
➢ Propose research avenues for developing improved drugs.
In a complementary article (Heal and Gosden, 2021), we reviewed results from clinical trials in BED including not only anti-obesity drugs but also drugs for attention deficit hyperactivity disorder (ADHD), depression, epilepsy and substance use disorders to re-evaluate the evidence for efficacy. For that reason, we have only briefly summarised the findings here (Table 1).
Table 1.
Summary of the evidence from clinical trials in treating binge-eating disorder.
| Drug | Mode of action | Therapeutic indication | Weight-loss efficacy | Efficacy in binge-eating disorder | References |
|---|---|---|---|---|---|
| Lisdexamfetamine | Noradrenaline and dopamine releasing agent | ADHD | Yes | Yes | See text |
| Binge-eating disorder a | |||||
| Dasotraline | Noradrenaline and dopamine reuptake inhibitor | ADHD b | Yes | Yes | See text |
| Binge-eating disorder b | |||||
| Phentermine | Noradrenaline and dopamine releasing agent | Obesity c | Yes | No data as monotherapy | |
| ADHD d | |||||
| Atomoxetine | Noradrenaline reuptake inhibitor | ADHD | No | Weak efficacy, insufficient data | McElroy et al. (2007a) |
| Bupropion | Dopamine reuptake inhibitor + weak noradrenaline reuptake inhibitor | Major depressive disorder | Yes | Weak efficacy, insufficient data | White and Grillo (2013) and Calandra et al. (2012) |
| Smoking cessation | |||||
| ADHD e | |||||
| Amodafinil/modafinil | Enigmatic | Narcolepsy | No | No, insufficient data | McElroy et al. (2015b) |
| Very weak dopamine reuptake inhibitor | ADHD e | ||||
| Sibutramine | Noradrenaline and serotonin reuptake inhibitor | Obesity f | Yes | No | Appolinario et al. (2003), Mitchell et al. (2003), Milano et al. (2005), and Wilfley et al. (2008) |
| Duloxetine | Noradrenaline and serotonin reuptake inhibitor | Major depressive disorder | Yes | No | Guerdjikova et al. (2012) |
| Generalised anxiety disorder | |||||
| Diabetic peripheral neuropathy | |||||
| Chronic musculoskeletal pain | |||||
| Fluvoxamine | Selective serotonin reuptake inhibitor | Obsessive-compulsive disorder | Yes | Yes/No, insufficient data | Hudson et al. (1998) and Pearlstein et al. (2003) |
| Fluoxetine | Selective serotonin reuptake inhibitor | Major depressive disorder | Yes | No | Leombruni et al. (2008), Devlin et al. (2000), Greeno and Wing (1996), and Arnold et al. (2002) |
| Obsessive-compulsive disorder | |||||
| Bulimia nervosa | |||||
| Panic disorder | |||||
| Sertraline | Selective serotonin reuptake inhibitor | Major depressive disorder | Yes | Yes, insufficient data | Leombruni et al. (2008) |
| Obsessive-compulsive disorder | |||||
| Post-traumatic stress disorder | |||||
| Social anxiety disorder | |||||
| Premenstrual dysphoric disorder | |||||
| Citalopram | Selective serotonin reuptake inhibitor | Major depressive disorder | Yes | Yes, insufficient data | McElroy et al. (2003b) |
| Escitalopram | Selective serotonin reuptake inhibitor | Major depressive disorder | Yes | No, insufficient data | Guerdjikova et al. (2008) |
| Generalised anxiety disorder | |||||
| Vortioxetine | Selective serotonin reuptake inhibitor + 5HT1A agonist + 5HT1B partial agonist + 5HT1D/5HT3/5HT7 antagonist | Major depressive disorder | No | No, insufficient data | Sanchez et al. (2015) |
| Naltrexone | μ-opioid partial agonist/δ-opioid antagonist | Alcohol dependence | No | ??, no data as monotherapy | |
| Samidorphan | μ-opioid antagonist/δ- and κ-opioid partial agonist | Major depressive disorder with buprenorphine (ALKS-5461) g | No | No | McElroy et al. (2013) |
| Psychosis with olanzapine (ALKS-3831) g | |||||
| GSK1521498 | μ-, δ- and κ-opioid inverse agonist | No approved indication | No | No, insufficient data | Ziauddeen et al. (2013) |
| Rimonabant | μ-opioid partial agonist/δ-opioid antagonist | Obesity f | Yes | No | Pataky et al. (2013) |
| Lamotrigine | Voltage-gated Na+/Ca++ channel blocker | Epilepsy | No | No | Guerdjikova et al. (2009) |
| Topiramate | Complex carbonic anhydrase CA-II and CA-IV inhibitor | Epilepsy | Yes | ??, insufficient data | McElroy et al. (2003a, 2007b) |
| Zonisamide | Voltage-gated Na+/Ca++ channel blocker Carbonic anhydrase CA-II and CA-V inhibitor | Epilepsy | Yes | No, insufficient data | McElroy et al. (2006) |
| Acamprosate | NMDA receptor modulator/voltage-gated Ca++ channel blocker | Alcohol dependence | No | No | McElroy et al. (2011) |
| Baclofen | GABAB receptor agonist | Severe muscle spasticity | No | No | Corwin et al. (2012) |
| Drug combinations | |||||
| Phentermine + topiramate | See above | Obesity | Yes | Yes, insufficient data | Safer et al. (2020) and Guerdjikova et al. (2018) |
| Phentermine + fenfluramine | See above + 5-HT releasing agent | Obesity f | Yes | ??, no data | Alger et al. (1999) |
| Phentermine + fluoxetine | See above | No approved indication for this combination | Yes | ??, no data | Devlin et al. (2000) |
| Naltrexone + bupropion | See above | Obesity | Yes | Yes, insufficient data | Guerdjikova et al. (2017) |
ADHD: attention deficit hyperactivity disorder; GABA: gamma-aminobutyric acid; NMDA: N-methyl-D-aspartate.
Not approved for BED in Europe.
Not approved for this indication in the USA or Europe.
Withdrawn from the market in Europe.
Pharmacological mechanism of action predicts efficacy in ADHD + case report of efficacy.
Clinical trials reports of moderate efficacy in ADHD.
Withdrawn from the market.
In late-stage development.
In this review, we discuss similarities in the psychopathology of ADHD and BED and the pharmacology of drugs that have proved to be effective in treating both disorders. An analysis of the successes and failures of drug trials BED and ADHD will be used to provide insights into pharmacological mechanisms relevant to BED and its treatment.
Clinical characteristics of binge-eating disorder versus obesity
BED was first recognised as a discrete eating disorder in the American Psychiatric Association (APA): Diagnostic and Statistical Manual of Mental Disorders, Edition 5 (APA, 2013: DSM-V); its symptoms are defined under five criteria:
Criterion 1: Recurrent episodes of binge eating (BE). BE episodes are characterised by eating an excessive amount of food in a discrete period combined with a sense of lack of control.
- Criterion 2: BE episodes are associated with three (or more) of:
- Eating much more rapidly than normal.
- Eating until feeling uncomfortably full.
- Eating large amounts of food when not hungry.
- Eating alone because of embarrassment over how much is eaten.
- Feeling disgusted, depressed or very guilty after overeating.
Criterion 3: Marked distress regarding BE.
Criterion 4: BE occurs ⩾1 day/week for 3 months (APA, 2013: DSM-V).
Criterion 5: BE is not associated with the regular use of inappropriate compensatory behaviour (e.g. purging, fasting and excessive exercise) and does not occur exclusively during the course of AN or BN.
The severity rating of BED is defined in APA: DSM-V as ranging from Mild (1–3 episodes/week) to Extreme (⩾14 episodes/week).
It is important to emphasise that none of the BED diagnostic criteria refer to weight, the metabolic sequelae of obesity, or its risk factors. The inference is the effectiveness of BED treatments is based exclusively on enabling the patient to regain self-control, reduce the impulsive, compulsive and perseverative drive to binge-eat and decrease the frequency and severity of BE episodes.
BED is the most common eating disorder with a lifetime prevalence rate in the young >1% compared with 0.3% for AN and ~1% for BN (Cossrow et al., 2016; Hoek and van Hoeken, 2003). A more recent meta-analysis estimated the lifetime prevalence of BED at 2.22% compared with 0.21% and 0.81% for AN and BN, respectively (Qian et al., 2013). An analysis of BED and BN rates across 14 countries, which included those with different average income levels, produced similar lifetime prevalence rates of 1.9% and 1.0%, respectively, with no difference due to income classification (Kessler et al., 2013). BED is slightly more common in females than males (Hay et al., 2015; Hudson et al., 2007; Kessler et al., 2013). With ~40% comorbidity between the two disorders, BED is strongly associated with obesity (Fairburn et al., 2000; Goldschmidt et al., 2011; Hudson et al., 2007; Kessler et al., 2013) and BED in adolescence predicts the development of obesity with an odds ratio (OR) = 3.58 (Micali et al., 2015). Nonetheless, a significant proportion (17%–30%) of BED sufferers have normal body weights (body mass index (BMI) 18.0–25 kg/m2) (Fairburn et al., 2000; Goldschmidt et al., 2011; Kessler et al., 2013), and according to Kessler et al. (2013) and Hudson et al. (2007), the majority (~60%) are in the normal weight/overweight categories (BMI 18.5–29.9 kg/m2). Evidence that BED is a causal factor in extreme obesity comes from the Longitudinal Assessment of Bariatric Surgery-2 (LABS-2) before/after surgery (Mitchell et al., 2015). Of 2266 severely obese subjects, 15.7% subjects satisfied the criteria for BED, 17.7% for night eating syndrome, but only 2% for BN (Mitchell et al., 2015).
BED has a strong association and comorbidity with other psychiatric conditions. Obese BED subjects have greater concerns about their appearance and body weight and exhibit greater body dissatisfaction than those without BED (Lynch et al., 2008; Sonneville et al., 2012). There is also an association between BED and anxiety or substance use disorders (Wonderlich et al., 2009). Mitchell et al. (2015) reported that taking medication for psychiatric or emotional problems, having symptoms of alcohol use disorder, lower self-esteem and greater depressive symptoms were among the factors that independently increased the odds of BED. In addition to psychiatric disorders, BED is independently associated with an increased risk of physical comorbidities including back/neck pain, chronic headaches and other types of chronic pain, as well as the cardiometabolic diseases like type 2 diabetes and hypertension, but not heart attacks or strokes (Kessler et al., 2013).
The primary endpoint in BED trials is a reduction in the frequency of binge episodes. Although most patients in these trials are obese because of the severity of their BED (Citrome et al., 2019; McElroy et al., 2015a, 2016a; Navia et al., 2017), subjects are enrolled exclusively on a confirmed BED diagnosis generally with BMI inclusion criteria of ⩾18 to ⩽45. Consistent with the focus on BE episode frequency, decreased appetite and weight loss are treated as part of the safety and tolerability assessment, not as indices of clinical benefit (Citrome et al., 2019; McElroy et al., 2015a, 2016a; Navia et al., 2017).
In contrast to BED, the diagnosis and treatment of obesity focuses on weight, adiposity and a reduction of risk factors for cardiometabolic disease and cancer. The primary efficacy endpoint for new anti-obesity drugs is a decrease in body weight (absolute and/or categorical analyses), and an important secondary measure is a reduction in waist circumference or waist/hip ratio (Center for Drug Evaluation and Research (CDER)/Food and Drug Administration (FDA), 2007; Committee for Medicinal Products for Human Use (CHMP)/European Medicines Agency (EMA), 2014). The key assumption is a reduction in weight and visceral adiposity translate into a decreased risk of developing type 2 diabetes, and suffering strokes, heart attacks and some cancers, while simultaneously, directly treating comorbidities like osteoarthritis of the knee and sleep apnoea (Albaugh et al., 2021; Center for Drug Evaluation and Research (CDER)/Food and Drug Administration (FDA), 2007; Chao et al., 2020; Colman, 2012; Committee for Medicinal Products for Human Use (CHMP)/European Medicines Agency (EMA), 2014; Hemmingsson, 2011; Malik et al., 2021; Vincent et al., 2012; Yee et al., 2007). The objective of the therapeutic intervention is to reduce the disease burden and ultimately to increase patients’ life expectancy and quality of life. Since none of the approved drugs alters metabolic rate, weight reduction is solely driven by reduced food consumption that is decreased appetite or increased satiety. What is absent from these primary and secondary outcome measures are items to explore effects on abnormal eating patterns or eating disorders, for example BED, night-time eating or BN. The reason is simple. It is because anti-obesity drugs were not designed or developed with a view to producing weight loss in patients with eating disorders by treating the underpinning psychopathology of their conditions.
Psychopathology of binge-eating disorder
The diagnostic criterion ‘a sense of lack of control of eating during the episode’ implies that BED is a classical impulse control disorder. It also illustrates the compulsive nature of the behaviour. The criteria of ‘eating alone because of being embarrassed by how much one is eating’ and ‘feeling disgusted with oneself, depressed, or very guilty after overeating’ are indices of BED’s emotional impact. Binges are defined discrete hyperphagic episodes within a short time-frame. The classification of BED as ranging from ‘mild: 1–3 episodes/week’ to ‘extreme: ⩾14 episodes/week’ demonstrates it is a frequent and recurrent behaviour, which can justifiably be described as perseverative.
Psychiatric risk factors for BED include conduct problems, negative affect, anxiety, impulse control and substance abuse disorders and perfectionism (Hilbert et al., 2011, 2014; Hudson et al., 2007; Kessler et al., 2013; McCuen-Wurst et al., 2018). Moreover, there is an emerging body of clinical evidence to show that a loss of impulse control in BED is a causal factor in bingeing on palatable foods (Colles et al., 2008; Galanti et al., 2007; Nasser et al., 2004; Schag et al., 2013; Svaldi et al., 2014; Wu et al., 2013). McElroy et al. (2016b) investigated different facets of impulsiveness in BED patients and found they exhibited deficits in motor and non-planning impulsiveness, but not attentional impulsiveness. Intolerance of delayed reward and enhanced delay discounting are established indices of impulsive choice and enhanced delay-discounting is exhibited in BED (Davis et al., 2010; Mole et al., 2015; Stojek et al., 2014). Mole et al. (2015) studied delay discounting in obese subjects with/without BED and showed both groups exhibited greater delay discounting, that is increased cognitive impulsivity, compared with normal, healthy volunteers. Stojek et al. (2014) showed dysregulated eating behaviours were associated with enhanced delay discounting. Moreover, increased intolerance of delayed reward predicted higher levels of dietary restraint and weight and shape concerns (Stojek et al., 2014). Negative urgency in delay discounting (increased delay discounting in negative mood states) predicted BE as well as concerns about body weight and body shape (Stojek et al., 2014).
Neural circuits and neurotransmitter systems involved in BED have been investigated by functional magnetic resonance imaging (fMRI) and positron emission tomography (PET). However, the findings need to be viewed cautiously because several of the studies conflate BED with obesity, (Aviram-Friedman et al., 2018; Wang et al., 2011) or BN (Fischer et al., 2017) rather than specifically targeting BED by using weight- or BMI-matched non-BED subjects as controls.
Schienle et al. (2009) performed fMRI on female subjects who were (i) overweight with BED, (ii) overweight healthy controls, (iii) normal-weight healthy controls or (iv) normal-weight BN patients. Following an overnight fast, participants’ brain activation in response to visual exposure to high-caloric food, unpleasant and neutral images were measured. Although all groups experienced food pictures as very pleasant with increased activation in orbitofrontal cortex (OFC), anterior cingulate cortex (ACC) and insula, BED subjects reported enhanced reward sensitivity and stronger medial OFC responses than all other groups. In contrast, BN subjects displayed greater arousal, ACC activation and insula activation than the other groups. Additional evidence of abnormalities in reward signalling and executive cognitive control come from the finding that patients with BED and BN exhibited aberrant functional connectivity in the dorsal ACC within the salience network, as well as in the medial prefrontal cortex (PFC) within the default mode network compared with BMI-matched control groups (Stopyra et al., 2019). Interestingly, the functional connectivity within each network differed between the BED and BN groups (Stopyra et al., 2019).
A series of fMRI studies have investigated neural connectivity and functioning during executive control and reward processing in BED patients (Balodis et al., 2013a, 2013b, 2014). Relative to BMI-matched obese and lean controls, obese BED subjects were hypoactive in brain areas involved in self-regulation and impulse control with diminished activity in ventromedial PFC (vPFC), inferior frontal gyrus (IFG), and insula during Stroop test performance. Dietary restraint scores were negatively correlated with right IFG and vPFC activation in BED subjects, but not in either control group (Balodis et al., 2013a). In a monetary win/loss paradigm, obese subjects with BED exhibited diminished bilateral ventral striatal activity during anticipatory reward/loss processing relative to BMI-matched obese subjects, but not lean controls (Balodis et al., 2013b). The relatively diminished fronto-striatal activity occurred in both anticipatory and outcome phases and during win/loss conditions indicating a generalised pattern of diminished fronto-striatal processing of rewards and losses in BED (Balodis et al., 2013b). These derangements were evidently core to BED because they persisted in treatment-resistant patients, but not in successfully treated BED subjects (Balodis et al., 2014).
Research into CNS neurotransmitter systems involved in altered connectivity and neural function in BED has focused on the dopaminergic and endogenous opioid systems because of their pivotal role in reward, motivation and cognitive control. Eating disorders have been linked with dopaminergic dysregulation in the CNS (Geiger et al., 2009; Johnson and Kenny, 2010; Pothos et al., 1995). Endogenous opioids that are important in motivational aspects of feeding are dysregulated in BED and BN (Bencherif et al., 2005; Davis et al., 2009; Nathan and Bullmore, 2009). The implicated brain areas are striatum, including ventral striatum and nucleus accumbens (ABC) which play a pivotal role in motivation, emotional responding and reward processing (Balleine, 2007; Delgado, 2007; Valbrun and Zvonarev, 2020). PFC mediates attention, cognitive function and decision-making (Arnsten, 2001, 2011; Robbins and Arnsten, 2009) and is anatomically linked to striatum via the striato–cortical pathway (Arnsten, 2001), and hypothalamus, which integrates central and peripheral signals to regulate ingestive behaviour and thermogenesis (Clapham, 2012; Harrold et al., 2012; Parker and Bloom, 2012). 5-Hydroxytryptamine (5-HT; serotonin) function has recently been identified as being dysregulated in BED with increased serotonin reuptake transporter (SERT) binding in the parieto–occipital cortical regions in BED subjects, with parallel decreases in ABC, inferior temporal gyrus and lateral OFC (Majuri et al., 2017). Although brain imaging techniques have provided a wealth of information on the neuronal systems and transmitters involved in CNS disorders, there are important technical limitations to imaging research. Some of the findings discussed are based on small numbers of subjects (e.g. Balodis et al., 2013a, 2013b; Schienle et al., 2009). Furthermore, noradrenergic systems are involved in the mode of action of LDX and dasotraline and potentially also in the psychopathology of BED; however, as there are no good noradrenergic PET tools, its potential contribution is often overlooked.
Viewing the evidence overall, BED subjects show enhanced responsiveness to palatable food cues, with reduced ability to integrate and compute the positive and negative outcomes that inevitably result from their bingeing sessions. BED subjects have substantial decrements in their ventral striatal reward pathways and diminished ability to recruit fronto-cortical impulse-control circuits to implement dietary restraint. The findings also reveal that the pattern of dysregulated reward processing in limbic brain structures, combined with decreased saliency, impulse control and cognitive decision-making in cortical regions is unique to BED and not shared with other eating disorders or obesity.
The Yale–Brown Obsessive–Compulsive Scale (YBOCS), which was introduced by Goodman et al. (1989a, 1989b), has been modified for BED (YBOCS-BE) by including additional items related to impulsivity, behavioural restraint, and distress (Deal et al., 2015). The efficacy of LDX in BED made it possible to determine the validity of the YBOCS-BE scale by a post hoc assessment of the association between LDX-induced decreases of BE frequency and changes in the YBOCS-BE scores (Citrome et al., 2018; McElroy et al., 2016b; Yee et al., 2019). The results showed YBOCS-BE is a valid scale for assessing the obsessive, compulsive and impulsive features of BED (Citrome et al., 2018; McElroy et al., 2016b; Yee et al., 2019). Although the argument is somewhat circular because of LDX’s ability to reduce impulsivity and increase cognitive control in ADHD, nonetheless it supports the hypothesis that efficacy in BED is dependent on treating its core obsessive, compulsive and impulsive behaviours.
Most mental health risk factors for BED, including conduct problems, negative affect, anxiety and impulse control and substance abuse disorders (Hilbert et al., 2011, 2014; Hudson et al., 2007; Kessler et al., 2013; McCuen-Wurst et al., 2018), are prevalent and commonly comorbid with ADHD (De Alwis et al., 2014; Eme, 2013; Ishii et al., 2003; Pliszka, 1998). Impulsivity is a core symptom in ADHD and attention deficit disorder (ADD) and manifests itself as a loss of control causing the subject to engage in risk-taking behaviours despite an awareness of the adverse consequences that may follow, for example alcohol, nicotine and illicit drug use, dangerous driving, criminal activities, etc. Impulsive choice as revealed by enhanced delay discounting is exhibited by subjects with ADHD (Anokhin et al., 2011; Jackson and Mackillop, 2016; Mostert et al., 2015; Shiels et al., 2009). Furthermore, there is often a perseverative component to these actions in ADHD, and for drug and alcohol abuse, an increasingly compulsive component as the disorder progresses. It is, therefore, unsurprising that the prevalence of substance use disorders, their rate of development and severity are several-fold higher in people with ADHD than the general population (Chen et al., 2018; Ilbegi et al., 2018; Molina et al., 2018; Polyzoi et al., 2018; Romo et al., 2018). ADHD is also associated with higher rates of eating disorders and behavioural addictions (gambling, compulsive buying disorder and Internet addiction) (Romo et al., 2018) and anxiety and depression are frequently comorbid with ADHD (Chen et al., 2018; Polyzoi et al., 2018). This synopsis reveals not only substantial overlap between the psychopathology of BED and ADHD but also a clear association between these two disorders. Furthermore, LDX and dasotraline, which have clinically proven efficacy in treating BED (Citrome et al., 2019; McElroy et al., 2015a, 2016a; Navia et al., 2017), are either approved to treat ADHD, that is LDX, or have shown clinically significant efficacy in ADHD in randomised, placebo-controlled trials, that is dasotraline (Findling et al., 2019; Koblan et al., 2015; Wigal et al., 2020).
Target product profile of the ideal binge-eating disorder drug
Although BED is often a causal factor in obesity the neurobiological drivers of excessive food consumption are very different, and therefore, the pharmacological characteristics of the ‘ideal’; drug to treat BED will be unique to this psychiatric disorder. We propose the following target product profile (TPP) for the ideal BED drug.
The ‘ideal’ drug should:
➢ Prevent the incidence of uncontrolled BE episodes.
➢ Reduce impulsive, compulsive and perseverative symptoms of BED.
➢ Increase cognitive restraint over food consumption.
➢ Restore healthy eating patterns.
➢ Reduce bodyweight to help overweight/obese subjects achieve a healthy BMI.
➢ The weight-loss effect should not be so powerful that it causes large decreases in BMI in normal weight subjects with BED.
➢ Reduce food intake by increasing satiety not by suppressing appetite thereby disrupting normal meal patterns.
➢ It should be safe when used long term.
The ‘ideal’ drug should not:
➢ Produce pharmacological tolerance that would result in dose-escalation.
➢ Cause psychological or physical dependence.
➢ Be subject to human abuse.
➢ Be a controlled drug.
The neural circuits that fail to adequately regulate food consumption in obesity are different from those responsible for compulsive and perseverative bingeing in BED. Therefore, it logically follows that the pharmacological mechanisms of drugs, which are effective in obesity may not work in BED and vice versa. Furthermore, the psychopathology and neurobiology of BED is unique and distinct other eating disorders, implying that pharmacological mechanisms that are effective in BED may not be effective in AN and BN and vice versa.
An independent weight-loss effect has been included in the TPP of the ideal BED drug. As discussed later in the review, the actions of LDX and dasotraline include an independent effect to reduce food intake by decreasing appetite or increasing satiety. If patients in the LDX and dasotraline clinical trials are representative of those seeking treatment, their BMI values show that the majority are obese or severely obese (Citrome et al., 2019; Grilo et al., 2020; McElroy et al., 2015a, 2016a, 2020); they require clinically meaningful weight loss as part of their treatment. Evidence shows that the degree of achieved weight loss increases along with initial BMI (Aftab et al., 2014; De Pergola et al., 2020; Dhurandhar et al., 2019), suggesting that this independent weight-loss effect would be more in patients who are severely obese than those patients in the overweight and normal weight ranges.
In the following sections, we review the pharmacology of drugs and drug-candidates that have been clinically evaluated in BED to elucidate the mechanisms, which offer promise for new medications and those which are unlikely to be effective.
Lisdexamfetamine and dasotraline
LDX is the only approved drug to treat BED. Although dasotraline was in pre-registration for adult BED in the USA, its development was recently discontinued (Sunovion Press Release, 2020). The FDA had already issued a Complete Response Letter for the New Drug Application (NDA) declining to approve dasotraline for the treatment of ADHD without further clinical trials to establish its efficacy and safety in August 2018 (Sunovion Press Release, 2018). When Sunovion announced it was discontinuing development of dasotraline in ADHD and BED, it stated that that further clinical studies would be needed to support a regulatory approval for dasotraline in both indications (Sunovion Press Release, 2020). On this basis, the company took the decision not to invest further in developing this drug.
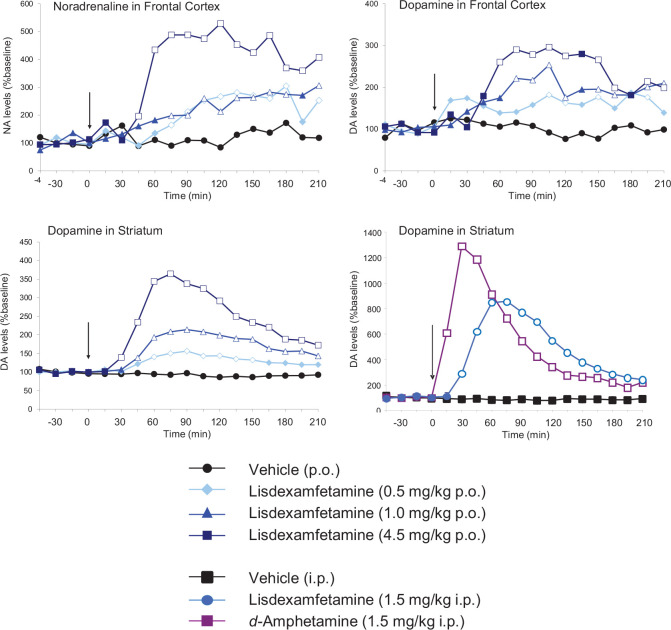
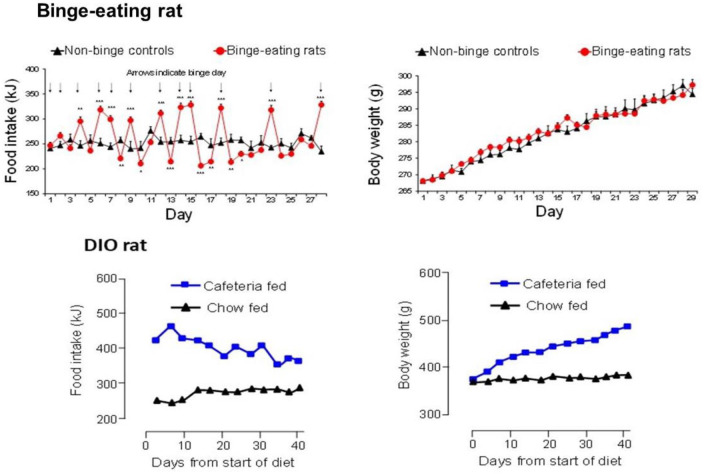
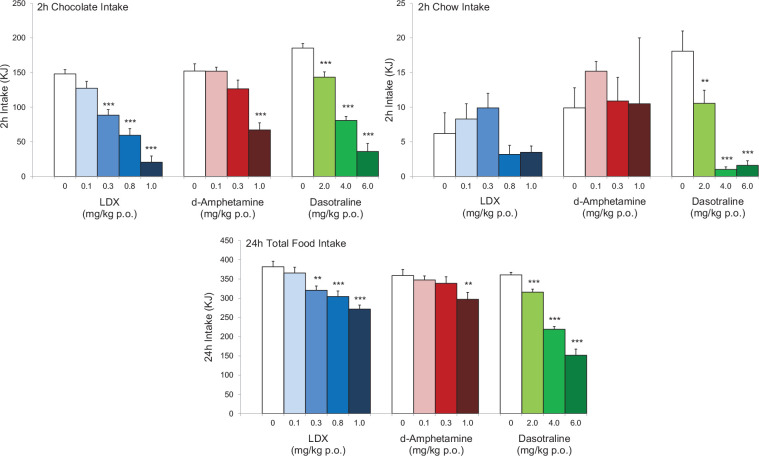
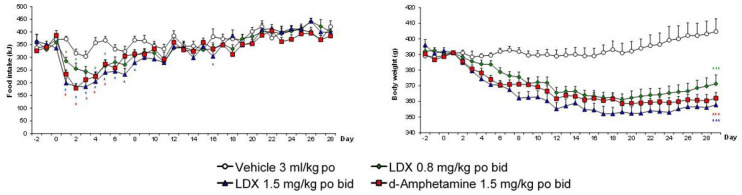
These drugs share some key features. Both drugs have catecholaminergic mechanisms. LDX is a d-amphetamine prodrug comprising d-amphetamine covalently bonded to L-lysine. The active moiety, d-amphetamine, is a close analogue of the catecholamine neurotransmitters, dopamine and noradrenaline (norepinephrine), and by mimicking their chemical structures, it serves as a competitive substrate for the dopamine and noradrenaline reuptake transporters (DAT and NET, respectively) and the vesicular monoamine transporter-2 (VMAT-2) (see review by Heal et al., 2013a). d-Amphetamine is translocated into presynaptic terminals by these ATP-driven carrier systems and where it displaces dopamine and noradrenaline from the cytosolic (newly synthesised) and vesicular storage pools. They are expelled into the synaptic cleft by reversal of DAT and NET’s direction of transport (‘reverse transport’) (Heal et al., 2013a). The rapid surge of synaptic dopamine and noradrenaline produced by d-amphetamine (Géranton et al., 2003; Heal et al., 2009, 2013a; Rowley et al., 2012, 2014; Wortley et al., 1999) underpin its efficacy in ADHD. Potentiating mesolimbic dopaminergic neurotransmission is partly responsible for its efficacy, and at supratherapeutic doses, make d-amphetamine a stimulant substance of abuse (Heal et al., 2009, 2013a; Rowley et al., 2012, 2014). LDX is highly unusual because it is metabolised by a rate-limited enzymatic hydrolysis in red blood cells (Pennick, 2010; Sharman and Pennick, 2014). The pharmacokinetics (PK) of LDX profoundly influence its pharmacological actions resulting in more gradual and sustained increases in dopamine and noradrenaline concentrations in the PFC and striatum compared with immediate-release d-amphetamine (IR-d-amphetamine) (Figure 1) (Hutson et al., 2014; Rowley et al., 2012, 2014). LDX has a much longer duration of action than IR-d-amphetamine and is considerably less stimulant in animals (Ermer et al., 2016; Hutson et al., 2014; Rowley et al., 2012, 2014) and humans (Jasinski and Krishnan, 2009a, 2009b). Unlike IR-d-amphetamine, its potency cannot be increased by switching from the oral to intravenous or intranasal routes (Heal et al., 2013a; Hutson et al., 2014). In a rat BED model (Figure 2, (Binge-eating rat)) (Vickers et al., 2015), LDX and its active metabolite, d-amphetamine, dose-dependently reduced chocolate BE (Figure 3). They also preferentially suppressed the consumption of chocolate (the highly palatable food that elicited the BE) compared with normal chow. Because rats consume ~40% of their daily kJ intake in these binges, they also markedly reduce 24 h food consumption (Figure 3). LDX and IR-d-amphetamine also reduce food intake and bodyweight in a rat dietary-induced obesity (DIO) model (Figure 2, (DIO rat)) (Dickinson et al., 2001; Heal and Jagger, 2005). The large initial reduction of food intake produces steep weight loss that is followed by sustained weight loss (Figure 4). Based on these findings LDX was predicted to have a dual action in BED (i) to suppress BE and (ii) to reduce overall food consumption to induce weight loss.
Figure 1.
Effects of lisdexamfetamine (LDX) on catecholaminergic neurotransmission in the frontal cortex and striatum. Extracellular concentrations of dopamine and noradrenaline were investigated by intracerebral microdialysis in freely moving rats. Results are adjusted means; n = 5–8 rats/group. To demonstrate pharmacological equivalence, the doses of LDX dimesylate and d-amphetamine hemi-sulphate are expressed in terms of d-amphetamine free base. The vertical arrow indicates time of drug administration. Microdialysate samples were collected at 15 min intervals and concentrations of dopamine and noradrenaline were measured by high-performance liquid chromatography (HPLC) with electrochemical detection. Data were analysed by analysis of covariance (ANCOVA) followed by the multiple t-test (d-amphetamine) and Williams’ test (LDX).
Source: Data abstracted from Rowley et al. (2012, 2014).
Significant differences are denoted by the open symbols.
Figure 2.
Comparison of the daily patterns of food intake and weight gain in binge eating (BE) and dietary-induced obese (DIO) female rats. BE is established in freely fed rats by giving them unpredictable, intermittent, 2 h access to powdered chocolate. Opportunities for chocolate binges are shown by the arrows in the top left panel. Rats develop a characteristic saw-tooth pattern of daily food intake with hyperphagia on chocolate binge days followed by voluntary restriction of food intake on non-binge days. Full details of the rat BE model are reported in Vickers et al. (2015). This highly abnormal pattern of BE induces impulsive and compulsive behaviours (Heal et al., 2016; Vickers et al., 2017), but not an obese phenotype (top right panel). The DIO rats are given ad libitum access to powdered chocolate as well as high-fat chow and ground salted peanuts. These rats show consistent hyperphagia over time (bottom left panel) and develop a profoundly obese phenotype (bottom right panel), which reaches a weight plateau after ~12 weeks on the diet. Full details of the DIO rat are reported in Dickinson et al. (2001).
Source: Results abstracted from Vickers et al. (2015) and data on file.
Figure 3.
Comparison of the effects lisdexamfetamine (LDX), its active metabolite, d-amphetamine and dasotraline on binge-eating (BE) behaviour in rats. The figures show the effects of LDX, d-amphetamine and dasotraline on the consumption of chocolate and chow (normal diet) in a 2 h BE session and the overall food consumption in the 24-h period including the BE session. To demonstrate pharmacological equivalence, the doses of LDX dimesylate and d-amphetamine hemisulphate are expressed in terms of d-amphetamine-free base. The data for LDX and d-amphetamine were abstracted from Vickers et al. (2015) and the data for dasotraline from Heal et al. (2018). The results show that all of the compounds produce marked reductions in chocolate bingeing and 24 h food intake predicting that they will reduce BE and produce weight loss.
Results are mean ± SEM for n = 8–29 rats/group. Significantly different from control **p < 0.01, ***p < 0.001.
Figure 4.
Effects of lisdexamfetamine (LDX) and its active metabolite, d-amphetamine, on daily food intake and bodyweight of dietary-induced obese (DIO) female rats. To demonstrate pharmacological equivalence, the doses of lisdexamfetamine dimesylate (LDX) and d-amphetamine hemisulphate are expressed in terms of d-amphetamine free base. Daily food consumption results were analysed by analysis of covariance (ANCOVA) with average baseline intake as covariate. Bodyweight data were analysed by ANCOVA with body weight on day 1 as covariate.
Source: Data abstracted from Heal et al. (2013b).
Results are adjusted means + SEMs, n = 9 rats/group. Significantly different from vehicle control: *p < 0.05, ***p < 0.001.
LDX significantly reduced the number of BE days/week relative to placebo in randomised, controlled trials and increased the percentage of subjects who were in remission (McElroy et al., 2015a, 2016a). Beneficial effects of LDX on BED psychopathology included significant decreases in the YBOCS-BE obsessional and compulsive scales, and at the highest dose, significant reductions in the Barratt Impulsiveness Scale, version 11 (BIS-11) self-reported questionnaire scores for non-planning and motor impulsivity (McElroy et al., 2016b). Although the lowest 30 mg/day dose of LDX did not significantly reduce the number of BE days/week, it produced substantial weight loss (McElroy et al., 2016a). Since placebo-treated patients experienced no weight loss, the results indicate that LDX has a dual mode of action, that is, it reduces food intake by suppressing appetite or enhancing satiety and is efficacious in BE at higher doses, that is, 50 and 70 mg/day. In summary, there is consensus between the findings from the non-clinical models and clinical trials demonstrating that the non-clinical models have excellent predictive validity for discovering new BED drug-candidates.
Lisdexamfetamine is approved by the FDA for the treatment of moderate to severe BED in adults. As a d-amphetamine prodrug, LDX is a schedule two controlled drug (C-II) and carries a ‘boxed warning’ in its Product Label stating that CNS stimulants (amphetamines and methylphenidate-containing products) have a high potential for abuse and dependence. It instructs prescribers to assess the risk of abuse prior to prescribing and monitor for signs of abuse and dependence while on therapy. The most common adverse events (AEs) associated with the use of LDX (incidence ⩾5% and at a rate at least twice placebo) were dry mouth, insomnia, decreased appetite, increased heart rate, constipation, feeling jittery and anxiety.
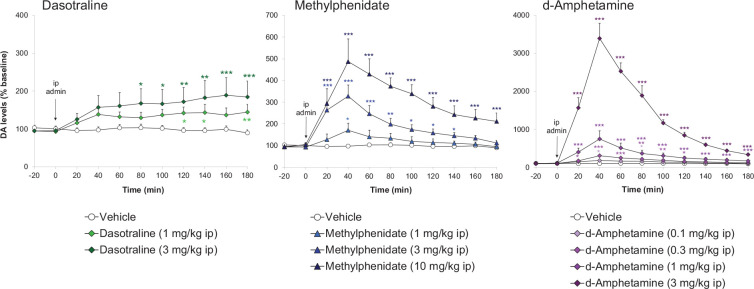
Dasotraline [(1R,4S)-4-(3,4-dichlorophenyl)-1,2,3,4-tetra-hydronaphthalen-1-amine] is a potent catecholamine reuptake inhibitor (DAT: IC50 = 3 nM and NET: IC50 = 4 nM) with weaker effects on the 5-HT transporter (SERT: IC50 = 15 nM) (Koblan et al., 2016). Dasotraline is slowly absorbed after oral administration in humans with a tmax of 10–12 h and a very long t1/2 (terminal elimination half-life) of 47–77 h (Chen et al., 2016; Hopkins et al., 2016; Koblan et al., 2015). It takes 2 weeks of daily dosing to reach steady state plasma concentrations (Chen et al., 2016; Koblan et al., 2015). Microdialysis measurements of ACB dopamine efflux were consistent with human PK (Heal et al., 2017; Rowley et al., 2017), that is small, dose-dependent increases that were slow in onset and sustained for many hours (Figure 5). Dasotraline is clearly different from the stimulants, d-amphetamine and methylphenidate, which produce rapid, large short-lasting increases in dopamine efflux (Figure 5). There is an important difference between the mechanism of releasing agents and reuptake inhibitors. The former are transporter substrates that expel neuronal monoamines by firing-independent reverse transport, whereas the latter are transporter blockers, which potentiate and prolong synaptic monoamines after firing-dependent exocytosis (Heal et al., 2013a). Tetrodotoxin blocks neuronal firing and it abolished dasotraline’s ability to increase synaptic monoamine concentrations, thereby demonstrating that dasotraline is a reuptake inhibitor not a monoamine releaser (Heal et al., 2017). In our rat BED model (Figure 2 (Binge-eating rat)) (Vickers et al., 2015), dasotraline dose-dependently suppressed chocolate bingeing with a much smaller effect on the consumption of normal chow (Figure 3) (Heal et al., 2018) predicting it would be effective in treating BED in humans. Dasotraline’s ability to independently evoke weight loss cannot be predicted without information on its effect in animal models of obesity.
Figure 5.
Comparison dasotraline, d-amphetamine and methylphenidate on extracellular dopamine concentrations in rat nucleus accumbens. Dopamine (DA) concentrations in the dialysates were quantified by high-performance liquid chromatography (HPLC) with electrochemical detection. Results were back-transformed, adjusted mean ± SEM (n = 6–9 rats/dose group). Drug doses are reported as free base and the time of administration is indicated by the vertical arrow. Data were log-transformed and analysed by the analysis of covariance (ANCOVA) with log(baseline) as covariate followed by the Williams’ test.
Source: Data abstracted from Rowley et al. (2017).
The graphs for dasotraline, methylphenidate and d-amphetamine are plotted using different scales for dopamine efflux.
Significant differences versus the vehicle group are denoted by: *p < 0.05, **p < 0.01, ***p < 0.001.
The efficacy of dasotraline in treating BED has been demonstrated in randomised, controlled trials (Citrome et al., 2019; Navia et al., 2017, 2018; Tsai et al., 2019). Dasotraline significantly reduced the psychopathological symptoms of BED with large falls in the YBOCS-BE obsession and compulsion scores (Navia et al., 2018). Although impulsivity scores were not reported, dasotraline-treated subjects showed a marked and significant increase in the dietary restraint score on the Eating Disorder Examination Questionnaire Brief Version (EDE-Q7) scale (Navia et al., 2018). Dasotraline (4 and 6 mg/day) produced significant reductions in YBOCS-BE total score from week 2 to week 12 (Tsai et al., 2019). Like LDX, dasotraline clearly reduces appetite and/or increases satiety because subjects experienced 4.78 kg decrease in bodyweight, whereas placebo controls experienced 0.4 kg weight gain despite a reduction of 3.76 BE days/week (Navia et al., 2017). A post hoc analysis of completers found dasotraline-induced weight loss increased with the patients’ BMI (Citrome et al., 2019). However, there was also evidence that some normal weight subjects experienced substantial weight decreases (Citrome et al., 2019).
Although LDX is approved and dasotraline discontinued, it is nonetheless worthwhile to assess their similarities and differences. Both drugs have a catecholaminergic mode of action, they increase noradrenergic and dopaminergic neurotransmission in the frontal cortex (FC), increase cognitive control and reduce impulsivity in ADHD. Both drugs also stimulate the mesolimbic dopaminergic reward pathway. The differences are (i) mechanism of action, that is, d-amphetamine is a catecholamine releasing agent while dasotraline is a potent, competitive catecholamine reuptake blocker with a very slow off-rate, and (ii) their PK profiles, that is, LDX has ⩽14-h duration of action while dasotraline produces continuous NET and DAT blockade. Consistent with their similar pharmacological mechanisms, LDX and dasotraline exhibit similar efficacy in reducing BE frequency and its underpinning psychopathology (Citrome et al., 2019; McElroy et al., 2015a, 2016a, 2016b; Navia et al., 2017, 2018; Tsai et al., 2019).
Placebo responses in these clinical trials revealed that substantially reducing binge frequency did not result in weight loss (Citrome et al., 2019; McElroy et al., 2015a, 2016a; Navia et al., 2017). Therefore, reduced calorie intake from fewer BE episodes was compensated by the subjects increasing their meal sizes or snacking. If treating BED does not materially improve obesity or its metabolic comorbidities, drugs need to have an independent weight-loss effect to be of maximum benefit to patients with BED who are overweight or obese.
Pharmacological approaches to treat binge-eating disorder: Insights from clinical trials with older drugs
There have been many drug trials in BED employing anti-obesity drugs and those approved for psychiatric or neurological disorders, which have been reported to cause weight loss. In a complementary article (Heal and Gosden, 2021), we have reviewed the results from these trials to dissect out which drugs are effective in treating the core psychopathology of BED as opposed to simply reducing weight loss. The clinical trial results are summarised in Table 1. In this review, we focus on the successes and failures to provide insights into pharmacological mechanisms relevant to BED and its treatment and particularly focus on the possible link between efficacy in treating ADHD and BED.
Monoaminergic drugs
Phentermine is a β-phenylethylamine, catecholamine releasing agent that differs from d-amphetamine by a single methyl group on the side chain of the molecule making it an obvious choice to evaluate in BED. Phentermine is a powerful noradrenaline and dopamine releasing agent (Rothman et al., 2001), administration of phentermine produces substantial increases in dopamine efflux in the ACB at anorectic doses and marked locomotor activation (Rowley et al., 2000). It is a stimulant reinforcer in animals (Stafford et al., 2001) and humans (Brauer et al., 1996; Carter et al., 2018). In addition to its approved obesity indication, phentermine has been reported to be efficacious in ADHD (Rothman, 1996). Although the evidence predicts phentermine would be effective in treating BED and its underlying psychopathology, no reports on phentermine as monotherapy exist; only those describing its use combined with topiramate (Qsymia®), fenfluramine, fluoxetine or topiramate (Table 1). The evidence from these drug combination trials indicates phentermine reduced the frequency and severity of BED and its underlying psychopathology (reductions in the YBOCS-BE and BIS scores and sub-scale scores). As discussed below, the evidence from monotherapy trials with the selective serotonin (5-HT) reuptake inhibitors (SSRIs) and topiramate predict that although these drugs produce weight loss, efficacy against BED psychopathology is probably mediated by phentermine’s catecholaminergic pharmacology.
Sibutramine (Meridia®, Reductil®), which has been withdrawn as an anti-obesity drug, is a potent noradrenaline and 5-HT reuptake inhibitor (Buckett et al., 1988; Heal and Cheetham, 1997; Luscombe et al., 1990) with minimal dopamine reuptake inhibition (Heal et al., 1992; Luscombe et al., 1990; Rowley et al., 2000). Noradrenaline and 5-HT reuptake inhibition operate synergistically to reduce food intake (Jackson et al., 1997a) via activation of α1-adrenergic and 5-HT2C receptors (Jackson et al., 1997b). Like ADHD drugs, sibutramine increases synaptic noradrenaline concentrations in the FC (Wortley et al., 1999). Because synaptic dopamine levels in FC are regulated by NET rather than DAT (Heal et al., 2009), it is predicted that sibutramine would also increase synaptic dopamine concentrations, c.f. atomoxetine (Bymaster et al., 2002). Unlike the stimulants used to treat ADHD, sibutramine does not influence mesolimbic dopaminergic reward mechanisms (Heal et al., 1992; Rowley et al., 2000). Sibutramine has been evaluated in four placebo-controlled trials in BED (Appolinario et al., 2003; Milano et al., 2005; Mitchell et al., 2003; Wilfley et al., 2008) where it consistently produced weight loss but had minimal effects on BED psychopathology (Table 1).
Duloxetine (Cymbalta®) is a noradrenaline+5-HT reuptake inhibitor antidepressant with a more balanced effect on noradrenaline and 5-HT reuptake (Guerdjikova et al., 2012) than sibutramine, which is more potent on noradrenaline. Duloxetine increases synaptic dopamine and noradrenaline concentrations in rat FC (Kihara and Ikeda, 1995; Umehara et al., 2013), but the effect is small, and duloxetine is minimally effective in treating ADHD (Park et al., 2014). In clinical trials, dasotraline produced a small weight loss, but it was ineffective in BED (Table 1).
Armodafinil (Nuvigil®) is the active, R(-)-enantiomer of modafinil (Provigil®). Armodafinil is approved to treat narcolepsy and other conditions involving excessive sleepiness. Although the parent racemate, modafinil, is stimulant, its pharmacology is enigmatic, and to date, no definitive mode of action has been demonstrated (Heal et al., 2009). Modafinil is a weak dopamine reuptake inhibitor (Madras et al., 2006; Volkow et al., 2009). However, with extremely low potency, that is Ki/IC50 values in the micromolar range (Cao et al., 2016; Karabacak et al., 2015; Minzenberg and Carter, 2008), it is debatable whether this mechanism is clinically irrelevant (Heal et al., 2009). Modafinil produces small increases in synaptic dopamine and noradrenaline concentrations in FC (Rowley et al., 2014), which is surprising because it has no NET blocking effect (Cao et al., 2016). Because of its micromolar DAT affinity, modafinil produces very small increases of extracellular dopamine in the striatum (Rowley et al., 2014). Consistent with its ability to increase noradrenergic and dopaminergic neurotransmission in the FC, modafinil is effective in ADHD (Wigal et al., 2006), but its efficacy is slower in onset and lower than the stimulants (Cortese et al., 2018; Wigal et al., 2006). The poor quality of the results from a single, small trial where armodafinil was administered to obese subjects with BED precludes a realistic assessment of its potential efficacy (Table 1).
Bupropion is approved for major depression (Wellbutrin®), as an aid to smoking cessation (Zyban®), and in combination with naltrexone for obesity (Contrave®). Bupropion is a weak DAT inhibitor (Hyttel, 1982; Richelson and Pfenning, 1984). Bupropion and its major metabolite have only micromolar affinities for NET (Ascher et al., 1995; Hyttel, 1982; Richelson and Pfenning, 1984). Nonetheless, NET inhibition appears to contribute to its pharmacological effects. Bupropion increases extracellular dopamine concentrations in PFC in microdialysis experiments (Li et al., 2002; Zocchi et al., 2003). It has been evaluated in ADHD, but the evidence is mixed with some trials showing efficacy (Casat et al., 1987; Wilens et al., 2001, 2005) and others not (Daviss et al., 2001). What is evident is the efficacy of bupropion in ADHD is substantially lower than the stimulants (Heal et al., 2012). Bupropion has been evaluated in BED where it produced weight loss, especially when administered in combination with naloxone (Contrave®), but at best bupropion is only marginally effective in treating BED psychopathology (Table 1).
SSRIs were initially developed as antidepressants but are now used in a broad range of psychiatric disorders. The selectivity of 5-HT versus noradrenaline reuptake inhibition varies from moderate (10–20-fold), for example fluoxetine, though selective (~100-fold), for example fluvoxamine and sertraline, to totally selective (>100-fold), for example citalopram (Bolden-Watson and Richelson, 1993; Richelson and Pfenning, 1984). Hypothalamic 5-HT plays an important role in the regulation of food intake (Halford et al., 2007) and various SSRIs have been shown to reduce hunger and/or decrease food intake in animals and humans (Greeno and Wing, 1996; Grignaschi et al., 1992; Halford et al., 2007; Ward et al., 1999). In part, these observations prompted the evaluation of several SSRIs as treatments for obesity. Although they produced short-term weight loss (Levine et al., 1989; Ward et al., 1999), tolerance developed (Abell et al., 1986; Goldstein et al., 1995; Ward et al., 1999) making them unsuitable as obesity treatments. Serotonergic drugs have never been thought to be effective in ADHD, which is a catecholaminergic disorder. This view is supported by paroxetine’s lack of efficacy in a randomised, double-blind, placebo-controlled trial in adults with ADHD (Weiss et al., 2006). The results from trials with SSRIs in BED are contradictory but viewing the data overall points to the conclusion that they are ineffective in treating BED psychopathology and are only efficacious in situations where their mild anti-obesity effect reduces daily food intake (Table 1). This view is supported by recent results with the antidepressant drug, vortioxetine (Trintellix®) showing no efficacy or weight loss in BED patients (Sanchez et al., 2015) (Table 1). Vortioxetine (Trintellix®) is a SSRI with 5-HT1A agonist, 5-HT1B partial agonist and 5-HT1D, 5-HT3 and 5-HT7 receptor antagonist properties.
Atomoxetine (Strattera®) is a highly selective, nanomolar potency, noradrenaline reuptake inhibitor (Bolden-Watson and Richelson, 1993; Wong et al., 1982) that is approved to treat ADHD in children, adolescents and adults. Atomoxetine is a classical monoamine reuptake inhibitor, which lacks the ability to evoke firing-dependent (c.f. methylphenidate) or firing-independent catecholamine release (c.f. d-amphetamine) (Heal et al., 2009, 2012, 2013a). Consequently, its potentiating effects on synaptic dopamine and noradrenaline concentrations in the FC are moderate and gradual in onset (Bymaster et al., 2002). As a non-stimulant, atomoxetine does not increase the synaptic concentration of dopamine in the mesolimbic reward system (Bymaster et al., 2002) further differentiating it from stimulant ADHD drugs. These differences in pharmacology and pharmacodynamics give atomoxetine a slow and gradual upward efficacy trajectory in ADHD that is very different from the rapid effects of the stimulants (Heal et al., 2009, 2012, 2013a). Atomoxetine has been evaluated in one, small trial in BED where it produced modest improvements in psychopathology with clinically insignificant weight loss (Table 1).
Antiepileptic drugs
Epilepsy is strongly associated with increased risk of cognitive and behavioural disorders including ADHD (Dunn and Kronenberger, 2005; Dunn et al., 2003; Hamoda et al., 2009; Pellock, 2004; Schubert, 2005). The reason for this association is unclear though it may be due to underlying neurodevelopmental vulnerability, the effects of chronic seizures and subclinical epileptiform activity (Hamoda et al., 2009). When assessing antiepileptic drugs as BED treatment, it is important to consider that some anticonvulsants, for example lamotrigine, produce relatively few cognitive adverse effects, whereas others, for example topiramate, produce substantial cognitive impairment (Schubert, 2005).
Lamotrigine (Lamictal®) is an approved anticonvulsant and it is also used in bipolar disorder. Lamotrigine inhibits voltage-sensitive, sodium channels to prevent the high-frequency repetitive burst-firing that occurs during seizure spread (Rogawski and Löscher, 2004). It also inhibits high voltage-activated calcium (N- and P/Q-type) channels but does not affect low voltage-activated T-type calcium channels (Rogawski and Löscher, 2004). Although lamotrigine does not directly alter excitatory or inhibitory synaptic responses, its effect on action potentials translates into reduced transmitter output at synapses, particularly on glutamate release (Rogawski and Löscher, 2004). No randomised, placebo-controlled trials of lamotrigine in ADHD have been conducted but results from open-label trials in children and adults suggest it is weakly efficacious (Han et al., 2017; Öncü et al., 2014). The limited clinical trial data suggest that lamotrigine has no effect on BED psychopathology and no anti-obesity effect to deliver minimal efficacy (Table 1).
Topiramate, which is a derivative of the naturally occurring monosaccharide D-fructose, is approved to treat epilepsy (Topamax®) and combined with phentermine for obesity (Qsymia®). Topiramate has complex pharmacology with multiple actions. Like lamotrigine, topiramate inhibits voltage-sensitive sodium and high voltage-activated calcium (N- and P/Q-type) channels (Langtry et al., 1997; Rogawski and Löscher, 2004; Shank et al., 2000; White, 1999). Topiramate also enhances gamma-aminobutyric acid (GABA)A-evoked Cl− ion currents (Langtry et al., 1997; Shank et al., 2000; White, 1999), selectively inhibits kainate receptors, and to a lesser extent, AMPA receptors (Langtry et al., 1997; Rogawski and Löscher, 2004; Shank et al., 2000; White, 1999). Topiramate is also an inhibitor of carbonic anhydrase enzymes, CA-II and CA-IV (Langtry et al., 1997; Shank et al., 2000). Although this last mechanism does not contribute to its anticonvulsant effect, it is probably involved the drug’s action to reduce food intake and body weight. Since topiramate’s side effects include difficulty with attention, concentration and memory, speech problems and psychomotor slowing (Langtry et al., 1997), many of which are similar to the cognitive deficits in ADHD, topiramate is unsuitable as an ADHD treatment. Topiramate decreased the frequency and severity of BED with significant decreases in the BED psychopathology and substantial weight loss (Table 1). However, a careful review of the findings indicates topiramate’s effects on impulsivity and compulsivity probably result from a generalised increase in inhibitory neurotransmission rather than an improvement in cognitive control. Moreover, topiramate’s unacceptable AE profile at these dose levels led to all development in obesity and type-2 diabetes being halted. For this reason, topiramate is unsuitable as monotherapy in BED.
Zonisamide (Zonegran®) is a synthetic 1,2-benzisoxazole derivative (1,2-benzisoxazole-3-methanesulfonarnide) approved to treat various types of epileptic seizures. Zonisamide inhibits voltage-sensitive sodium, high voltage-activated (N- and P/Q-type) and low voltage, fast-acting, T-type calcium channels, which are pivotal in inhibiting repetitive neuronal firing and seizure spread (Leppik, 1999; Masuda et al., 1998). Zonisamide also inhibits carbonic anhydrase II and V (De Simone et al., 2005; Shank et al., 2008), which plays no role in its anti-seizure activity (Masuda et al., 1994), but is probably important in zonisamide’s weight-loss effect. Zonisamide reduced antipsychotic-induced weight gain in animals and humans (Ghanizadeh et al., 2013; Wallingford et al., 2008) and produced sustained weight loss in obese subjects (Gadde et al., 2012; Nguyen et al., 2013). In BED, zonisamide produced weight loss but failed to reduce BE frequency or BED psychopathology (Table 1). Zonisamide’s side effects were very burdensome. The findings are similar to those observed when topiramate, another carbonic anhydrase inhibitor, was evaluated in BED.
Miscellaneous CNS drugs
Rimonabant (Acomplia®) is a cannabinoid CB1 receptor antagonist/inverse agonist (Pertwee, 2005; Shire et al., 1996, 1999) anti-obesity drug that was withdrawn in 2007. In obese subjects with BED, rimonabant reduced weight but had no effect on BE frequency or BED psychopathology (Table 1).
Acamprosate (Campral®) is approved for alcohol dependence. It has a relatively simple chemical structure, but complex pharmacology. Recent research indicates it attenuates glutamatergic neurotransmission by acting as a co-agonist at the spermidine-sensitive modulatory site on the N-methyl-D-aspartate (NMDA) receptor (Mann et al., 2008; Mason and Heyser, 2010; Zornoza et al., 2003). Acamprosate also changes NMDA receptor subunit composition, is a high voltage-gated Ca++ channel blocker, and it enhances taurine release (Mann et al., 2008; Mason and Heyser, 2010; Zornoza et al., 2003). Acamprosate had no effect on BE frequency or BED psychopathology in obese subjects with this eating disorder (Table 1).
Naltrexone is a nanomolar affinity, partial agonist/antagonist at μ- and δ-opioid receptors (<20% intrinsic efficacy) and κ-opioid receptor partial agonist (~40% intrinsic efficacy) (Wentland et al., 2009). Naltrexone is approved for alcohol dependence (Vivitrol®) and combined with bupropion for obesity (Contrave®). Clinical trials of naltrexone as a weight loss agent have been conducted based on the hypothesis the endogenous opioid system is dysregulated in obesity but the drug was found to be ineffective as monotherapy (Atkinson et al., 1985; Mitchell et al., 1987). There are no trials in BED of naltrexone as monotherapy. As discussed above, naltrexone+bupropion may be moderately effective in treating BED (Table 1). However, the failure of other opioid receptor inhibitors in BED, that is samidorphan and GSK1521498, argues that this pharmacological mechanism delivers no additional benefit, that is, efficacy of the combination deriving exclusively from the catecholamine reuptake inhibitor, bupropion, leaving naltrexone to contribute nothing but AEs.
Baclofen (Lioresal®, Gablofen®) is a GABAB receptor agonist (Costantino et al., 2001; Misgeld et al., 1995) muscle relaxant that is approved to treat severe spasticity. In a small clinical trial in obese subjects with BED, baclofen failed to reduce BED psychopathology or produce weight loss (Table 1). This outcome is similar to clinical findings with other drugs that increase CNS inhibitory tone, for example topiramate, zonisamide and lamotrigine, or those that are effective in treating substance use disorders, for example naltrexone and nalmefene.
Pharmacological approaches to treat binge-eating disorder: Insights from clinical trials with novel drug-candidates
Samidorphan (ALKS-33) is in clinical development in combination with buprenorphine for major depression (ALKS-5461) and with olanzapine as an antipsychotic with reduced potential for weight gain (ALKS-3831). Samidorphan is a highly potent, μ-opioid receptor antagonist and a nanomolar potency δ- and κ-opioid receptor partial agonist (intrinsic efficacy ~35%) (Wentland et al., 2009). In severely obese, BED subjects, samidorphan did not reduce the frequency or severity of BE episodes, reduce the core psychopathology or decrease weight (Table 1). Adverse events produced by samidorphan were prominent and onerous leading to a large number of discontinuations (McElroy et al., 2013).
GSK1521498 is an inverse agonist of μ-, δ- and κ-opioid receptors (Ignar et al., 2011). It has nanomolar affinity for the μ-receptor with 10–20-fold selectivity over the δ- and κ-receptor subtypes (Ignar et al., 2011). In subjects with BED, GSK1521498 reduced the hedonic effect of palatable foods and decreased calorie intake in test meals, but it did not reduce subjects’ BE scores or produce weight loss (Table 1).
Overall, the results predict that opioid receptor antagonists are unlikely to be effective in BED.
Current knowledge about the pharmacology of binge-eating disorder
In our review of drug trials in BED, we found the quality of the data was often inadequate and the evidence to support the claimed efficacy of drugs from many pharmacological classes did not stand up to scrutiny (Heal and Gosden, 2021).
With a focus on pharmacological mechanisms, the evidence shows that catecholaminergic enhancing drugs are effective in BED. Thus, LDX and dasotraline that have proven efficacy in BED increase noradrenergic and dopaminergic neurotransmission in PFC through catecholamine release (LDX) or noradrenaline reuptake inhibition (dasotraline). This is combined with increased dopaminergic neurotransmission in ventral striatum and ACB through dopamine release (LDX) or reuptake inhibition (dasotraline). Furthermore, many pharmacological mechanisms can be discounted as being effective in BED including SSRIs, SNRIs, opioid antagonists, CB1 antagonists, calcium channel blockers, carbonic anhydrase inhibitors, NMDA, GABAB agonists and GABAA receptor modulators. To this list, we would add drugs which target hypothalamic systems regulating food intake.
Although BED is a causal factor in obesity, its neurobiology and neuropharmacology are almost totally distinct from those mediating obesity; in short, excessive food consumption is the only common factor in these two disorders. BED’s psychopathology is underpinned by a loss of behavioural and cognitive control resulting in repetitive, episodic consumption of excessive quantities of food. The disorder’s episodic nature explains why a significant proportion of BED sufferers are in the normal/overweight range. The brain areas implicated in BED’s psychopathology are higher cognitive centres of FC and PFC, and the mesolimbic reward system. It is why BED is responsive to catecholaminergic stimulants, which increase cognitive control but is not responsive to drugs that decrease food intake at the hypothalamic level, for example SNRIs, SSRIs, carbonic anhydrase inhibitor anti-epileptics or CB1 antagonists.
BED is an eating disorder with a psychopathology, neurophysiology and neuroanatomy that is distinct from BN. This has been shown by fMRI studies and by the observation that although the SSRIs are moderately effective in BN, they are ineffective in BED. Correspondingly, there is no evidence to show that the catecholaminergic stimulants, which are effective in BED, are of benefit in BN.
Evidence from animal and human studies indicates that dopaminergic and opioid dysregulation contributes to BED’s psychopathology. Analogies to neurobiological abnormalities in substance use disorders and similarities between the behavioural patterns of BE and drug abuse led to the ‘food addiction’ hypothesis and BED as an addiction disorder. ‘Food addiction’ is a highly controversial concept with proponents for (Avena et al., 2011; Corsica and Pelchat, 2010; Gearhardt et al., 2011) and against (Cassin and von Ranson, 2007; Kirschenbaum and Krawczyk, 2018; Ziauddeen and Fletcher, 2013) its existence. An in-depth critique of the hypothesis or taking sides in the debate is beyond the scope of this review. However, we take this opportunity to provide some evidence for consideration. Deficits in reward processing are present in ADHD (Carmona et al., 2009; Plichta et al., 2009; Scheres et al., 2007; Tomasi and Volkow, 2012) and are correlated with increased intolerance of delayed reward leading to increased impulsive responding (Carmona et al., 2009, 2012; Costa-Dias et al., 2013; Scheres et al., 2010; Ströhle et al., 2008). Although ADHD subjects have deficits in reward processing, are intolerant of delayed gratification, and at greater risk of developing a substance use disorders (Charach et al., 2011; Erskine et al., 2016; Faraone and Wilens, 2007; Wilens, 2004), it does not infer that ADHD is an addiction disorder. Similar logic also applies to BED’s psychopathology. Looking at the issue from the perspective of effective treatments points to the same conclusion. Three drugs, which are approved to treat substance use disorders, that is bupropion, acamprosate and naltrexone, have been evaluated as treatments for BED and none has shown clear evidence of efficacy. On the other hand, LDX, which failed to show benefit as a treatment for cocaine dependence (Mooney et al., 2015), reduces impulsivity in ADHD and BED and is an effective medication for treating both disorders. Based on the neurobiological and pharmacological evidence and the findings from a systematic review of drug trials in BED, we conclude that BED is an impulse control disorder; this conclusion has also been proposed by other researchers (Kessler et al., 2016; Reinblatt, 2015; Ural et al., 2017). Recently, Kaisari et al. (2018) have provided a valuable contribution to the hypothesis that there is a shared psychopathology between BED and ADHD by showing there were significant correlations between disinhibited eating and both the hyperactive/impulsive and the attentional symptoms of ADHD. One cautionary note is these associations were based on symptoms from self-reported questionnaires and not on cohorts of subjects who had received a definitive clinical diagnosis of ADHD with or without BED.
Research directions into new drugs to treat binge-eating disorder
The TPP of the ideal BED drug has been described earlier in this review. To achieve the TPP requires two separate strands of preclinical research. The first is to demonstrate the test compound selectively or preferentially reduces bingeing on palatable food and is effective against the psychopathology underpinning the disorder, that is, impulsivity, compulsivity and perseveration. The second is to demonstrate that test compound independently reduces food intake and produces sustained weight loss (see Figures 3 and 4). In our experience, the unpredictable, intermittent access to palatable food model described by Corwin (2004) is a robust test with excellent predictive validity for clinical efficacy (Vickers et al., 2015). The model’s strengths are the rats develop a syndrome that mirrors the core psychopathology of BED in terms of increased impulsivity/intolerance of delayed reward (Vickers et al., 2017), compulsive and perseverative bingeing behaviour when aware of the adverse consequences (Heal et al., 2016) and dysregulated neurochemistry in the brain’s reward systems (Davis et al., 2009; DiFeliceantonio et al., 2012; Heal et al., 2017; Tarazi et al., 2015). Since many BED subjects are in the normal weight/overweight range, substantial calorie restriction is not relevant to developing a BED phenotype. Depriving the rats of access to binge food, for example ground chocolate, is stressful enough to induce BED without resorting to other stressors, for example immobilisation or foot shocks. On the other hand, allowing rats regular or unlimited access to palatable food produces hyperphagia, but as shown by the examples of sibutramine, baclofen and naltrexone, efficacy in these models (Berner et al., 2009; Buda-Levin et al., 2005; Corwin and Wojniki, 2009; Popik et al., 2011) is not replicated in clinical trials (Table 1). In contrast, baclofen and sibutramine had no selective effect on BE in the unpredictable, intermittent access model and performed as non-selective inhibitors of food consumption (Vickers et al., 2015); these results were reflected by baclofen’s and sibutramine’s lack of efficacy in BED trials (Table 1).
We have evaluated many drugs and test compounds in the rat BE model (Heal et al., 2018; Hurley et al., 2020; Vickers et al., 2015). Our current view is efficacy in this model is an essential support for clinical evaluation, but it needs supplementing with efficacy in models of BED’s psychopathology, that is compulsive/perseverative responding (Heal et al., 2016) and impulsivity (Vickers et al., 2017). This view is supported by the finding that when LDX was repeatedly administered to BED rats, it maintained its ability to decrease chocolate bingeing, but it had no effect on food intake on non-binge days (Hurley et al., 2020). Therefore, to achieve the TPP criteria, the drug-candidate demonstrating weight loss and weight-loss maintenance in a rat DIO model is essential.
If BED is an impulse control disorder that is treatable with drugs that reduce impulsivity and increase cognitive control, then compounds which increase catecholaminergic drive in PFC and dopaminergic drive in ventral limbic system are key mediators of efficacy. The most efficacious ADHD drugs are d-amphetamine (and as the active moiety in LDX) (catecholamine releasing agent), methylphenidate (stimulant catecholamine reuptake inhibitor) and dasotraline (long-acting catecholamine reuptake inhibitor). We have previously reviewed various drug-candidates with novel mechanisms under evaluation in ADHD (Heal et al., 2012). Many of these drug-candidates directly or indirectly enhance catecholaminergic signalling in the PFC or increase cognitive control through other mechanisms. Table 2 provides an update on ADHD clinical trials. If efficacy in ADHD and BED are linked, the catecholamine reuptake inhibitor, centanafadine (EB1020), is strongly predicted to be efficacious in BED and the SNRI, viloxazine (SPN-812), may also be effective. The findings also predict that triple reuptake inhibitors, α4/β2 nicotinic agonists (partial or full), glutamate and AMPA modulators, or histamine H3 antagonists are unlikely to prove successful in search for novel BED drugs. However, it should be emphasised that these theoretical predictions need to be tested in clinical trials.
Table 2.
New molecular targets to treat binge-eating disorder – update on drug-candidates in development for ADHD.
| Drug | Mode of action | Company | Status in ADHD | References | Potential in BED |
|---|---|---|---|---|---|
| Centanafadine (EB1020) | Noradrenaline + dopamine reuptake inhibitor | Otsuka/Neurovance | Phase 3 | No published data | Efficacy predicted in BED, cf dasotraline or lisdexamfetamine |
| Positive findings in Phase 2 trials | |||||
| Viloxazine (SPN-812) | Noradrenaline reuptake inhibitor | Supernus Pharmaceuticals | Positive findings in 4 Phase 3 studies in patients aged 6 to 17 years with ADHD | Johnson et al. (2020) | Limited efficacy predicted in BED, cf atomoxetine |
| New drug application filed Nov 2019 | |||||
| Edivoxetine (LY22166840) | Noradrenaline reuptake inhibitor | Eli Lilly | Positive findings in Phase 2 trials | Lin et al. (2014) and Nery et al. (2017) | Limited efficacy predicted in BED, cf atomoxetine |
| Discontinued in 2013 | |||||
| GSK372475 (NS2359) | Triple monoamine reuptake inhibitor | GSK/NeuroSearch | Lack of efficacy | Wilens et al. (2008) | Not known |
| Discontinued | Prediction – inactive | ||||
| DOV102677 | Triple monoamine reuptake inhibitor | Dov Pharmaceuticals | Discontinued | No published data | Not known |
| Company wound up | Prediction – inactive | ||||
| SPD473 | Triple monoamine reuptake inhibitor | Shire Pharmaceuticals | Discontinued | No published data | Not known |
| Shire acquired by Takeda | Prediction – inactive | ||||
| Posanicline (ABT089) | α4/β2 partial agonist | Abbott/NeuroSearch | Lack of efficacy | Wilens et al. (2011), Bain et al. (2012), and Apostol et al. (2012) | Not known |
| Discontinued | Prediction – inactive | ||||
| NeuroSearch wound up | |||||
| AZD1446 (TC6683) | α4/β2 partial agonist | AZ/Targacept | Lack of efficacy | Jucaite et al. (2014) | Not known |
| Discontinued | Prediction – inactive | ||||
| Targacept acquired by catalyst | |||||
| ABT894 | α4/β2 agonist | Abbott/NeuroSearch | Lack of efficacy | Bain et al. (2013) | Not known |
| Discontinued | Prediction – inactive | ||||
| NeuroSearch wound up | |||||
| AZD3480 (TC1734) | α4/β2 agonist | AZ/Targacept | Minor efficacy | Potter et al. (2014) | Not known |
| Discontinued | Prediction – inactive | ||||
| Targacept acquired by catalyst | |||||
| Bavisant (JNJ31001074) | H3 antagonist | J&J | Lack of efficacy | Weissler et al. (2012) | Not known |
| Discontinued | Prediction – inactive | ||||
| Org26576 | AMPA modulator | Merck | Lack of efficacy | Adler et al. (2012) | Not known |
| Discontinued | Prediction – inactive |
ADHD: attention deficit hyperactivity disorder; BED: binge-eating disorder.
In the last 5 years, relatively few drug-candidates in BED have emerged in the research phase. As discussed below, several compounds that are claimed to prevent BE have been evaluated in normal rats exhibiting a normal hyperphagic response to highly palatable foods. These models are not relevant; testing in a rodent BED phenotype is essential.
The most promising among them is the trace amine-associated receptor-1 (TAAR-1) antagonist, RO5256390, which has been shown to reduce the operant responding of normal rats for palatable food without affecting chow intake (Ferragud et al., 2017). Selective inhibition of palatable food consumption sparing normal chow is encouraging. However, the rat model based on predictable, daily access to palatable food lacks the stress component required to generate a BED phenotype. Validation of RO5256390 as a promising treatment needs to explore its efficacy in a BED phenotype and in BED rats in models of impulsivity, for example delay discounting (Vickers et al., 2017), and compulsivity/perseveration, for example modified CAR model (Heal et al., 2016). A similar conclusion applies to cannabidiol which reduced sucrose self-administration in normal rats and mice (Bi et al., 2020). With no BED phenotype, the result demonstrates only an effect to decrease hyperphagia, not efficacy against the psychopathology of BED.
Cifani et al. (2020) have recently reported that sigma-1 antagonists show potential as drugs to treat BED. This result requires further replication because the fasting/stress BED model is claimed to have predictive validity partly based on the differential actions of sibutramine and topiramate (Cifani et al., 2009). As discussed above, although topiramate is reported to be efficacious in BED trials, the validity of the findings is doubtful, and the authors have not studied clinically proven drugs, that is LDX or dasotraline, in this model. Further caution about the predictive validity of the model comes from Hicks et al. (2020), who reported that prazosin reduced BE. This α1-adrenoceptor antagonist had no effect on BE in our model that had been validated with LDX and dasotraline (Vickers et al., 2015). These authors have also predicted efficacy in BED for salidroside (active principle in Rhodiola rosea extract) (Cifani et al., 2010), Hypericum perforatum extract (Di Bonaventura et al., 2012a), orexin antagonists (Piccoli et al., 2012), adenosine A2A antagonists (Di Bonaventura et al., 2012b) and oleoylethanolamide (Romano et al., 2020). Since the translational and predictive validity of this BED model is unproven, the effects demonstrate only an ability to decrease stress-induced palatable food intake, not necessarily efficacy in BED.
LY2940094, a nociceptin receptor antagonist, is claimed to be a potential BED treatment (Statnick et al., 2016). The only evidence is decreased fasting-induced feeding, reductions in palatable food intake in normal rats, and effects on food intake and weight gain in DIO rats. As discussed above, these findings demonstrate the potential of LY2940094 as an anti-obesity drug, and clinical trials have shown anti-obesity drugs are ineffective in BED (Table 1).
The 5-HT2C agonist, lorcaserin, has shown efficacy in various substance use disorder models. Lorcaserin and the 5-HT2A antagonist/inverse agonist, pimavanserin, were evaluated in a rat model of bingeing (Price et al., 2018). Both drugs produced small reductions in palatable food intake, but not binge-frequency. Their effects on normal chow intake were not determined so a selective effect on bingeing cannot be supported. Lorcaserin and another highly selective 5-HT2C agonist, CP-809101, decreased deprivation-induced feeding in rats (Higgins et al., 2016). In models of impulsivity, they decreased motor impulsivity in the 5-choice serial reaction time test (5-CSRT), but critically did not increase tolerance of delayed reward in delay discounting. The findings suggest lorcaserin-induced reductions of food consumption are due to its anti-obesity properties and 5-HT2C agonists will be ineffective in addressing BED’s psychopathology. This conclusion is also consistent with our hypothesis that BED is not an addiction disorder. Lorcaserin was withdrawn from sale in February 2020. The question whether 5-HT2C agonists are effective in BED can only be definitively answered by testing in an adequately controlled and powered clinical trial. However, as the weight-loss efficacy of lorcaserin was marginal in clinical terms and several other 5-HT2C agonists have been discontinued in development, it is unclear whether such a trial will ever be conducted.
Piracetam, an anticonvulsant with enigmatic pharmacology, is claimed to have promise in BED (Hussain and Krishnamurthy, 2018). In a starvation (no food for 48 h)/refeeding rat model of BED, piracetam and LDX (reference comparator) decreased bingeing on cookies. They also reduced chow intake, suggesting the effects were anorectic rather than selective for BE. What was most concerning was the oral doses studied: piracetam was administered at 200 mg/kg, and LDX at 100 mg/kg. As shown in Figure 3, LDX is orally active in BE models at doses between 0.3 and 1.0 mg/kg.
Very recently, Hurley et al. (2020) presented preliminary results on the serotonergic hallucinogen, psilocybin, which had been evaluated in our rat BED model. A single injection of psilocybin (1, 3 or 10 mg/kg ip) decreased chocolate bingeing 1 h after administration. The effect of the highest dose persisted for 24 h but was not maintained at 5 days. In contrast, daily administration of LDX (0.8 mg/kg po) consistently suppressed BE with no effect on food consumption on non-binge days. Since psilocybin is a drug that is intended to be administered infrequently in the clinic, the results suggest that it will not be useful as a treatment for BED.
Conclusions
In the last decade, we have learned that BED is a distinct eating disorder with a unique psycho- and neuropathology. The relatively high prevalence of BED coupled with the clinical evaluation of LDX and latterly dasotraline, which both demonstrated unequivocal efficacy in reducing the psychopathology of this disorder, have generated renewed interest in developing new drugs for this indication. Moreover, the availability of clinically effective drugs as well as those which failed to show efficacy in BED trials provides sufficient positive and negative controls to facilitate the development of animal models with translational validity for clinical outcomes.
One of the complexities is bingeing in BED is a manifestation of the psychopathology of the disorder. It is not due to a failure of the hypothalamic regulation of food intake. This point is clearly revealed by the failure of a large number of clinically effective anti-obesity drugs to show efficacy in BED in patient trials. Another is the discovery that in many instances remission in BED is not accompanied by weight loss in patients. If patients in clinical trials are representative of those seeking treatment, mean BMI values suggest that the majority are obese or severely obese. These patients require weight loss, which is the rationale for suggesting the TPP for a novel BED drug should include an independent effect to reduce food intake by decreasing appetite or increasing satiety.
Having reviewed historical clinical trials with drugs for diverse therapeutic indications and widely differing pharmacological mechanisms, we were surprised by the limited number that would meet current efficacy criteria (Heal and Gosden, 2021; summarised in Table 1).
Although there is a need for new drugs to support healthcare professionals in treating BED, the scope for drug targets appears to be restricted to compounds that markedly augment catecholaminergic neurotransmission. This conclusion is based on the diverse pharmacological mechanisms of drugs, which have already failed in BED trials together with those which we have tentatively predicted would fail because they have not proven to be effective in ADHD.
On a more positive note, behavioural phenotyping of compounds in animals identified TAAR-1 ligands as potential treatments for schizophrenia, a disorder that historically was only treatable with dopamine receptor antagonists. As described above, RO5256390 shows some promise as a potential BED treatment. We have animal models of BED including its core psychopathology with good predictive validity for clinical efficacy. Testing compounds in these validated animal models could, therefore, open a path to discovering new drugs which act through non-catecholaminergic mechanisms.
Footnotes
Author Note: David J Heal is now affiliated to Department of Pharmacy and Pharmacology, University of Bath, Bath, UK.
Declaration of conflicting interests: The author(s) declared the following potential conflicts of interest with respect to the research, authorship, and/or publication of this article: David J Heal and Sharon L Smith are shareholders and employees of DevelRx Ltd. DevelRx provides consultancy support to the pharmaceutical industry.
Funding: The author(s) received no financial support for the research, authorship, and/or publication of this article.
ORCID iD: David J Heal  https://orcid.org/0000-0002-6128-9632
https://orcid.org/0000-0002-6128-9632
References
- Abell CA, Farquhar DL, Galloway SM, et al. (1986) Placebo controlled double-blind trial of fluvoxamine maleate in the obese. J Psychosom Res 30: 143–146. [DOI] [PubMed] [Google Scholar]
- Adler LA, Kroon RA, Stein M, et al. (2012) A translational approach to evaluate the efficacy and safety of the novel AMPA receptor positive allosteric modulator Org 26576 in adult attention-deficit/hyperactivity disorder. Biol Psychiatry 72: 971–977. [DOI] [PubMed] [Google Scholar]
- Aftab SAS, Halder L, Piya MK, et al. (2014) Predictors of weight loss at 1 year after laparoscopic adjustable gastric banding and the role of presurgical quality of life. Obes Surg 24: 885–890. [DOI] [PubMed] [Google Scholar]
- Albaugh VL, Kindel TL, Nissen SE, et al. (2021) Cardiovascular risk reduction following metabolic and bariatric surgery. Surg Clin North Am 101: 269–294. [DOI] [PubMed] [Google Scholar]
- Alger SA, Malone M, Cerulli J, et al. (1999) Beneficial effects of pharmacotherapy on weight loss, depressive symptoms, and eating patterns in obese binge eaters and non-binge eaters. Obes Res 7: 469–476. [DOI] [PubMed] [Google Scholar]
- American Psychiatric Association (APA) (2013) Diagnostic and Statistical Manual of Mental Disorders, 5th edn. Arlington, VA: American Psychiatric Association. [Google Scholar]
- Anokhin AP, Golosheykin S, Grant JD, et al. (2011) Heritability of delay discounting in adolescence: A longitudinal twin study. Behav Genet 41: 175–183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Apostol G, Abi-Saab W, Kratochvil CJ, et al. (2012) Efficacy and safety of the novel α4β2 neuronal nicotinic receptor partial agonist ABT-089 in adults with attention-deficit/hyperactivity disorder: A randomized, double-blind, placebo-controlled crossover study. Psychopharmacology (Berl) 219: 715–725. [DOI] [PubMed] [Google Scholar]
- Appolinario JC, Bacaltchuk J, Sichieri R, et al. (2003) A randomized, double-blind, placebo-controlled study of sibutramine in the treatment of binge-eating disorder. Arch Gen Psychiatry 60: 1109–1116. [DOI] [PubMed] [Google Scholar]
- Arnold LM, McElroy SL, Hudson JI, et al. (2002) A placebo-controlled, randomized trial of fluoxetine in the treatment of binge-eating disorder. J Clin Psychiatry 63: 1028–1033. [DOI] [PubMed] [Google Scholar]
- Arnsten AF. (2001) Modulation of prefrontal cortical-striatal circuits: Relevance to therapeutic treatments for Tourette syndrome and attention-deficit hyperactivity disorder. Adv Neurol 85: 333–341. [PubMed] [Google Scholar]
- Arnsten AF. (2011) Catecholamine influences on dorsolateral prefrontal cortical networks. Biol Psychiatry 69: 89–99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ascher JA, Cole JO, Colin JN, et al. (1995) Bupropion: A review of its mechanism of antidepressant activity. J Clin Psychiatry 56: 395–401. [PubMed] [Google Scholar]
- Atkinson RL, Berke LK, Drake CR, et al. (1985) Effects of long-term therapy with naltrexone on body weight in obesity. Clin Pharmacol Ther 38: 419–422. [DOI] [PubMed] [Google Scholar]
- Avena NM, Bocarsly ME, Hoebel BG, et al. (2011) Overlaps in the nosology of substance abuse and overeating: The translational implications of “food addiction.” Curr Drug Abuse Rev 4: 133–139. [DOI] [PubMed] [Google Scholar]
- Aviram-Friedman R, Astbury N, Ochner CN, et al. (2018) Neurobiological evidence for attention bias to food, emotional dysregulation, disinhibition and deficient somatosensory awareness in obesity with binge eating disorder. Physiol Behav 184: 122–128. [DOI] [PubMed] [Google Scholar]
- Bain EE, Apostol G, Sangal RB, et al. (2012) A randomized pilot study of the efficacy and safety of ABT-089, a novel α4β2 neuronal nicotinic receptor agonist, in adults with attention-deficit/hyperactivity disorder. J Clin Psychiatry 73: 783–789. [DOI] [PubMed] [Google Scholar]
- Bain EE, Robieson W, Pritchett Y, et al. (2013) A randomized, double-blind, placebo-controlled phase 2 study of α4β2 agonist ABT-894 in adults with ADHD. Neuropsychopharmacology 38: 405–413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Balleine BW, Delgado MR, Hikosaka O. (2007) The role of the dorsal striatum in reward and decision-making. J Neurosci 27: 8161–8165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Balodis IM, Grilo CM, Kober H, et al. (2014) A pilot study linking reduced fronto-striatal recruitment during reward processing to persistent bingeing following treatment for binge-eating disorder. Int J Eat Disord 47: 376–384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Balodis IM, Kober H, Worhunsky PD, et al. (2013. b) Monetary reward processing in obese individuals with and without binge eating disorder. Biol Psychiatry 73: 877–886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Balodis IM, Molina ND, Kober H, et al. (2013. a) Divergent neural substrates of inhibitory control in binge eating disorder relative to other manifestations of obesity. Obesity (Silver Spring) 21: 367–377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bencherif B, Guarda AS, Colantuoni C, et al. (2005) Regional µ-opioid receptor binding in insular cortex is decreased in bulimia nervosa and correlates inversely with fasting behavior. J Nucl Med 46: 1349–1351. [PubMed] [Google Scholar]
- Berner LA, Bocarsly ME, Hoebel BG, et al. (2009) Baclofen suppresses binge eating of pure fat but not a sugar-rich or sweet-fat diet. Behav Pharmacol 20: 631–634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bi GH, Galaj E, He Y, et al. (2020) Cannabidiol inhibits sucrose self-administration by CB1 and CB2 receptor mechanisms in rodents. Addict Biol 25: e12783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bolden-Watson C, Richelson E. (1993) Blockade by newly-developed antidepressants of biogenic amine uptake into rat brain synaptosomes. Life Sci 52: 1023–1029. [DOI] [PubMed] [Google Scholar]
- Brauer LH, Johanson CE, Schuster CR, et al. (1996) Evaluation of phentermine and fenfluramine, alone and in combination, in normal, healthy volunteers. Neuropsychopharmacology 14: 233–241. [DOI] [PubMed] [Google Scholar]
- Buckett WR, Thomas PC, Luscombe GP. (1988) The pharmacology of sibutramine hydrochloride (BTS 54 524), a new antidepressant which induces rapid noradrenergic down-regulation. Prog Neuropsychopharmacol Biol Psychiatry 12: 575–584. [DOI] [PubMed] [Google Scholar]
- Buda-Levin A, Wojnicki FH, Corwin RL. (2005) Baclofen reduces fat intake under binge-type conditions. Physiol Behav 86: 176–184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bymaster FP, Katner JS, Nelson DL, et al. (2002) Atomoxetine increases extracellular levels of norepinephrine and dopamine in prefrontal cortex of rat: A potential mechanism for efficacy in attention deficit/hyperactivity disorder. Neuropsychopharmacology 27: 699–711. [DOI] [PubMed] [Google Scholar]
- Calandra C, Russo RG, Luca M. (2012) Bupropion versus sertraline in the treatment of depressive patients with binge eating disorder: Retrospective cohort study. Psychiatr Q 83: 177–185. [DOI] [PubMed] [Google Scholar]
- Cao J, Slack RD, Bakare OM, et al. (2016) Novel and high affinity 2-[(diphenylmethyl)sulfinyl] acetamide (Modafinil) analogues as atypical dopamine transporter inhibitors. J Med Chem 59: 10676–10691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carmona S, Hoekzema E, Ramos-Quiroga JA, et al. (2012) Response inhibition and reward anticipation in medication-naïve adults with attention-deficit/hyperactivity disorder: A within-subject case-control neuroimaging study. Hum Brain Mapp 33: 2350–2361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carmona S, Proal E, Hoekzema EA, et al. (2009) Ventro-striatal reductions underpin symptoms of hyperactivity and impulsivity in attention-deficit/hyperactivity disorder. Biol Psychiatry 66: 972–977. [DOI] [PubMed] [Google Scholar]
- Carter LP, Henningfield JE, Wang YG, et al. (2018) A randomized, double-blind, placebo-controlled, crossover study to evaluate the human abuse liability of solriamfetol, a selective dopamine and norepinephrine reuptake inhibitor. J Psychopharmacol 32: 1351–1361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Casat CD, Pleasants DZ, Van Wyck Fleet J. (1987) A double-blind trial of bupropion in children with attention deficit disorder. Psychopharmacol Bull 23: 120–122. [PubMed] [Google Scholar]
- Cassin SE, von Ranson KM. (2007) Is binge eating experienced as an addiction? Appetite 49: 687–690. [DOI] [PubMed] [Google Scholar]
- Center for Drug Evaluation and Research (CDER)/Food and Drug Administration (FDA) (2007) Guidance for industry developing products for weight management, revision 1, draft guidance. Available at: https://www.fda.gov/media/71252/download.
- Chao AM, Wadden TA, Berkowitz RI, et al. (2020) The risk of cardiovascular complications with current obesity drugs. Expert Opin Drug Saf 19: 1095–1104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Charach A, Yeung E, Climans T, et al. (2011) Childhood attention-deficit/hyperactivity disorder and future substance use disorders: Comparative meta-analyses. J Am Acad Child Adolesc Psychiatry 50: 9–21. [DOI] [PubMed] [Google Scholar]
- Chen Q, Hartman CA, Haavik J, et al. (2018) Common psychiatric and metabolic comorbidity of adult attention-deficit/hyperactivity disorder: A population-based cross-sectional study. PLoS One 13: e0204516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen Y‑L, Skende E, Lin J, et al. (2016) Absorption, distribution, metabolism, and excretion of [14C]-dasotraline in humans. Pharmacol Res Perspect 5: e00281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cifani C, Di Bonaventura EM, Botticelli L, et al. (2020) Novel highly potent and selective sigma1 receptor antagonists effectively block the binge eating episode in female rats. ACS Chem Neurosci 11: 3107–3116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cifani C, Di Bonaventura MVM, Vitale G, et al. (2010) Effect of salidroside, active principle of Rhodiola rosea extract, on binge eating. Physiol Behav 101: 555–562. [DOI] [PubMed] [Google Scholar]
- Cifani C, Polidori C, Melotto S, et al. (2009) A preclinical model of binge eating elicited by yo-yo dieting and stressful exposure to food: Effect of sibutramine, fluoxetine, topiramate, and midazolam. Psychopharmacology 204: 113–125. [DOI] [PubMed] [Google Scholar]
- Citrome L, Goldman R, Mandel M, et al. (2019) Effect of dasotraline on body weight in patients with binge-eating disorder. In: American Psychiatric Association meeting, San Francisco, 18–22 May 2019. Poster P7-079. [Google Scholar]
- Citrome L, Kando JC, Bliss C. (2018) Relationships between clinical scales and binge eating days in adults with moderate to severe binge eating disorder in two Phase III studies. Neuropsychiatr Dis Treat 14: 537–546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clapham JC. (2012) Central control of thermogenesis. Neuropharmacology 63: 111–123. [DOI] [PubMed] [Google Scholar]
- Colles SL, Dixon JB, O’Brien PE. (2008) Loss of control is central to psychological disturbance associated with binge eating disorder. Obesity 16: 608–614. [DOI] [PubMed] [Google Scholar]
- Colman E. (2012) Food and Drug Administration’s obesity drug guidance document: A short history. Circulation 125: 2156–2164. [DOI] [PubMed] [Google Scholar]
- Committee for Medicinal Products for Human Use (CHMP)/European Medicines Agency (EMA) (2014) Guideline on clinical evaluation of medical producta used in weight management. EMA/CHMP/311805/2014. Available at: https://www.ema.europa.eu/en/documents/scientific-guideline/guideline-clinical-evaluation-medicinal-products-used-weight-management-revision-1_en.pdf.
- Corsica JA, Pelchat ML. (2010) Food addiction: True or false? Curr Opin Gastroenterol 26: 165–169. [DOI] [PubMed] [Google Scholar]
- Cortese S, Adamo N, Del Giovane C, et al. (2018) Comparative efficacy and tolerability of medications for attention-deficit hyperactivity disorder in children, adolescents, and adults: A systematic review and network meta-analysis. Lancet Psychiatry 5: 727–738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Corwin RL. (2004) Binge-type eating induced by limited access in rats does not require energy restriction on the previous day. Appetite 42: 139–142. [DOI] [PubMed] [Google Scholar]
- Corwin RL, Boan J, Peters KF, et al. (2012) Baclofen reduces binge eating in a double-blind, placebo-controlled, crossover study. Behav Pharmacol 23: 616–625. [DOI] [PubMed] [Google Scholar]
- Corwin RL, Wojnicki FH. (2009) Baclofen, raclopride, and naltrexone differentially affect intake of fat and sucrose under limited access conditions. Behav Pharmacol 20: 537–548. [DOI] [PubMed] [Google Scholar]
- Cossrow N, Pawaskar M, Witt EA, et al. (2016) Estimating the prevalence of binge eating disorder in a community sample from the United States: Comparing DSM-IV-TR and DSM-5 criteria. J Clin Psychiatry 77: e968–e974. [DOI] [PubMed] [Google Scholar]
- Costa-Dias TG, Wilson VB, Bathula DR, et al. (2013) Reward circuit connectivity relates to delay discounting in children with attention-deficit/hyperactivity disorder. Eur Neuropsychopharmacol 23: 33–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Costantino G, Macchiarulo A, Guadix AE, et al. (2001) QSAR and molecular modeling studies of baclofen analogues as GABAB agonists. Insights into the role of the aromatic moiety in GABAB binding and activation. J Med Chem 44: 1827–1832. [DOI] [PubMed] [Google Scholar]
- Davis C, Patte K, Curtis C, et al. (2010) Immediate pleasures and future consequences. A neuropsychological study of binge eating and obesity. Appetite 54: 208–213. [DOI] [PubMed] [Google Scholar]
- Davis CA, Levitan RD, Reid C, et al. (2009) Dopamine for “wanting” and opioids for “liking”: A comparison of obese adults with and without binge eating. Obesity 17: 1220–1225. [DOI] [PubMed] [Google Scholar]
- Daviss WB, Bentivoglio P, Racusin R, et al. (2001) Bupropion sustained release in adolescents with comorbid attention-deficit/hyperactivity disorder and depression. J Am Acad Child Adolesc Psychiatry 40: 307–314. [DOI] [PubMed] [Google Scholar]
- Deal LS, Wirth RJ, Gasior M, et al. (2015) Validation of the Yale-Brown Obsessive Compulsive Scale modified for binge eating. Int J Eat Disord 48: 994–1004. [DOI] [PubMed] [Google Scholar]
- De Alwis D, Lynskey MT, Reiersen AM, et al. (2014) Attention-deficit/hyperactivity disorder subtypes and substance use and use disorders in NESARC. Addict Behav 39: 1278–1285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Delgado MR. (2007) Reward-related responses in the human striatum. Ann N Y Acad Sci 1104: 70–88. [DOI] [PubMed] [Google Scholar]
- De Pergola G, Zupo R, Lampignano L, et al. (2020) Higher body mass index, uric acid levels, and lower cholesterol levels are associated with greater weight loss. Endocr Metab Immune Disord Drug Targets 20: 1268–1281. [DOI] [PubMed] [Google Scholar]
- De Simone G, Di Fiore A, Menchise V, et al. (2005) Carbonic anhydrase inhibitors. Zonisamide is an effective inhibitor of the cytosolic isozyme II and mitochondrial isozyme V: Solution and X-ray crystallographic studies. Bioorg Med Chem Lett 15: 2315–2320. [DOI] [PubMed] [Google Scholar]
- Devlin MJ, Goldfein JA, Carino JS, et al. (2000) Open treatment of overweight binge eaters with phentermine and fluoxetine as an adjunct to cognitive-behavioral therapy. Int J Eat Disord 28: 325–332. [DOI] [PubMed] [Google Scholar]
- Dhurandhar NV, Kyle T, Stevenin B, et al. (2019) Predictors of weight loss outcomes in obesity care: Results of the national ACTION study. BMC Public Health 19: 1422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Di Bonaventura MVM, Cifani C, Lambertucci C, et al. (2012. b) A2A adenosine receptor agonists reduce both high-palatability and low-palatability food intake in female rats. Behav Pharmacol 23: 567–574. [DOI] [PubMed] [Google Scholar]
- Di Bonaventura MVM, Vitale G, Massi M, et al. (2012. a) Effect of Hypericum perforatum extract in an experimental model of binge eating in female rats. J Obes 2012: 956137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dickinson K, North TJ, Telford G, et al. (2001) Determination of body composition in conscious adult female Wistar utilising total body electrical conductivity. Physiol Behav 74: 425–433. [DOI] [PubMed] [Google Scholar]
- DiFeliceantonio AG, Mabrouk OS, Kennedy RT, et al. (2012) Enkephalin surges in dorsal neostriatum as a signal to eat. Curr Biol 22: 1918–1924. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dunn DW, Austin JK, Harezlak J, et al. (2003) ADHD and epilepsy in childhood. Dev Med Child Neurol 45: 50–54. [PubMed] [Google Scholar]
- Dunn DW, Kronenberger WG. (2005) Childhood epilepsy, attention problems, and ADHD: Review and practical considerations. Semin Pediatr Neurol 12: 222–228. [DOI] [PubMed] [Google Scholar]
- Eme R. (2013) Male adolescent substance use disorder and attention-deficit hyperactivity disorder: A review of the literature. ISRN Addict 2013: 815096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ermer JC, Pennick M, Frick G. (2016) Lisdexamfetamine dimesylate: Prodrug delivery, amphetamine exposure and duration of efficacy. Clin Drug Investig 36: 341–356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Erskine HE, Norman RE, Ferrari AJ, et al. (2016) Long-term outcomes of attention-deficit/hyperactivity disorder and conduct disorder: A systematic review and meta-analysis. J Am Acad Child Adolesc Psychiatry 55: 841–850. [DOI] [PubMed] [Google Scholar]
- Fairburn CG, Cooper Z, Doll HA, et al. (2000) The natural course of bulimia nervosa and binge eating disorder in young women. Arch Gen Psychiatry 57: 659–665. [DOI] [PubMed] [Google Scholar]
- Faraone SV, Wilens TE. (2007) Effect of stimulant medications for attention-deficit/hyperactivity disorder on later substance use and the potential for stimulant misuse, abuse, and diversion. J Clin Psychiatry 68(Suppl 11): 15–22. [PubMed] [Google Scholar]
- Ferragud A, Howell AD, Moore CF, et al. (2017) The trace amine-associated receptor 1 agonist RO5256390 blocks compulsive, binge-like eating in rats. Neuropsychopharmacology 42: 1458–1470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Findling RL, Adler LA, Spencer TJ, et al. (2019) Dasotraline in children with attention-deficit/hyperactivity disorder: A six-week, placebo-controlled, fixed-dose trial. J Child Adolesc Psychopharmacol 29: 80–89. [DOI] [PubMed] [Google Scholar]
- Fischer S, Breithaupt L, Wonderlich J, et al. (2017) Impact of the neural correlates of stress and cue reactivity on stress related binge eating in the natural environment. J Psychiatr Res 92: 15–23. [DOI] [PubMed] [Google Scholar]
- Gadde KM, Kopping MF, Wagner HR, et al. (2012) Zonisamide for weight reduction in obese adults: A 1-year randomized controlled trial. Arch Intern Med 172: 1557–1564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Galanti K, Gluck ME, Geliebter A. (2007) Test meal intake in obese binge eaters in relation to impulsivity and compulsivity. Int J Eat Disord 40: 727–732. [DOI] [PubMed] [Google Scholar]
- Gearhardt AN, White MA, Potenza MN. (2011) Binge eating disorder and food addiction. Curr Drug Abuse Rev 4: 201–207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Geiger BM, Haburcak M, Avena NM, et al. (2009) Deficits of mesolimbic dopamine neurotransmission in rat dietary obesity. Neuroscience 159: 1193–1199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Géranton SM, Heal DJ, Stanford SC. (2003) Differences in the mechanisms that increase noradrenaline efflux after administration of d-amphetamine: A dual-probe microdialysis study in rat frontal cortex and hypothalamus. Br J Pharmacol 139: 1441–1448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ghanizadeh A, Nikseresht MS, Sahraian A. (2013) The effect of zonisamide on antipsychotic-associated weight gain in patients with schizophrenia: A randomized, double-blind, placebo-controlled clinical trial. Schizophr Res 147: 110–115. [DOI] [PubMed] [Google Scholar]
- Goldschmidt AB, Le Grange D, Powers P, et al. (2011) Eating disorder symptomatology in normal-weight vs. obese individuals with binge-eating disorder. Obesity 19: 1515–1518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldstein DJ, Rampey AH Jr, Roback PJ, et al. (1995) Efficacy and safety of long-term fluoxetine treatment of obesity – Maximizing success. Obes Res 3(Suppl 4): 481S–490S. [DOI] [PubMed] [Google Scholar]
- Goodman WK, Price LH, Rasmussen SA, et al. (1989. a) The Yale-Brown Obsessive Compulsive Scale. I. Development, use, and reliability. Arch Gen Psychiatry 46: 1006–1011. [DOI] [PubMed] [Google Scholar]
- Goodman WK, Price LH, Rasmussen SA, et al. (1989. b) The Yale-Brown Obsessive Compulsive Scale. II. Validity. Arch Gen Psychiatry 46: 1012–1016. [DOI] [PubMed] [Google Scholar]
- Greeno CG, Wing RR. (1996) A double-blind, placebo-controlled trial of the effect of fluoxetine on dietary intake in overweight women with and without binge-eating disorder. Am J Clin Nutr 64: 267–273. [DOI] [PubMed] [Google Scholar]
- Grignaschi G, Neill JC, Petrini A, et al. (1992) Feeding pattern studies suggest that d-fenfluramine and sertraline specifically enhance the state of satiety in rats. Eur J Pharmacol 211: 137–142. [DOI] [PubMed] [Google Scholar]
- Grilo CM, McElroy SL, Hudson JI, et al. (2020) Efficacy and safety of dasotraline in adults with binge-eating disorder: A randomized, placebo-controlled, fixed-dose clinical trial. CNS Spectr. Epub ahead of print 19 May 2020. DOI: 10.1017/S1092852920001406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guerdjikova AI, McElroy SL, Kotwal R, et al. (2008) High-dose escitalopram in the treatment of binge-eating disorder with obesity: A placebo-controlled monotherapy trial. Hum Psychopharmacol 23: 1–11. [DOI] [PubMed] [Google Scholar]
- Guerdjikova AI, McElroy SL, Welge JA, et al. (2009) Lamotrigine in the treatment of binge-eating disorder with obesity: A randomized, placebo-controlled monotherapy trial. Int Clin Psychopharmacol 24: 150–158. [DOI] [PubMed] [Google Scholar]
- Guerdjikova AI, McElroy SL, Winstanley EL, et al. (2012) Duloxetine in the treatment of binge eating disorder with depressive disorders: A placebo-controlled trial. Int J Eat Disord 45: 281–289. [DOI] [PubMed] [Google Scholar]
- Guerdjikova AI, Walsh B, Shan K, et al. (2017) Concurrent improvement in both binge eating and depressive symptoms with naltrexone/bupropion therapy in overweight or obese subjects with major depressive disorder in an open-label, uncontrolled study. Adv Ther 34: 2307–2315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guerdjikova AI, Williams S, Blom TJ, et al. (2018) Combination phentermine-topiramate extended release for the treatment of binge eating disorder: An open-label, prospective study. Innov Clin Neurosci 15: 17–21. [PMC free article] [PubMed] [Google Scholar]
- Halford JC, Harrold JA, Boyland EJ, et al. (2007) Serotonergic drugs: Effects on appetite expression and use for the treatment of obesity. Drugs 67: 27–55. [DOI] [PubMed] [Google Scholar]
- Hamoda HM, Guild DJ, Gumlak S, et al. (2009) Association between attention-deficit/hyperactivity disorder and epilepsy in pediatric populations. Expert Rev Neurother 9: 1747–1754. [DOI] [PubMed] [Google Scholar]
- Han SA, Yang EJ, Song MK, et al. (2017) Effects of lamotrigine on attention-deficit hyperactivity disorder in pediatric epilepsy patients. Korean J Pediatr 60: 189–195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harrold JA, Dovey TM, Blundell JE, et al. (2012) CNS regulation of appetite. Neuropharmacology 63: 3–17. [DOI] [PubMed] [Google Scholar]
- Hay P, Girosi F, Mond J. (2015) Prevalence and sociodemographic correlates of DSM-5 eating disorders in the Australian population. J Eat Disord 3: 19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heal DJ, Cheetham SC. (1997) The pharmacology of sibutramine, the first serotonin and noradrenaline reuptake inhibitor to be developed for the treatment of obesity. La Lett Pharmacol 11(Suppl 10): 3–8. [Google Scholar]
- Heal DJ, Cheetham SC, Smith SL. (2009) The neuropharmacology of ADHD drugs in vivo: Insights on efficacy and safety. Neuropharmacology 57: 608–618. [DOI] [PubMed] [Google Scholar]
- Heal DJ, Frankland ATJ, Gosden J, et al. (1992) A comparison of the effects of sibutramine hydrochloride, bupropion and methamphetamine on dopaminergic function: Evidence that dopamine is not a pharmacological target for sibutramine. Psychopharmacology 107: 303–309. [DOI] [PubMed] [Google Scholar]
- Heal DJ, Goddard S, Brammer RJ, et al. (2016) Lisdexamfetamine reduces the compulsive and perseverative behaviour of binge-eating rats in a novel food reward/punished responding conflict model. J Psychopharmacol 30: 662–675. [DOI] [PubMed] [Google Scholar]
- Heal DJ, Gosden J. (2021) What treatments are effective in BED? A critical evaluation of the evidence from clinical trials. Int J Obes. Invited review. In press. [DOI] [PubMed] [Google Scholar]
- Heal DJ, Jagger E. (2005) Development, regulatory and marketing challenges for novel anti‑obesity therapies. In: Antel J, Finer N, Heal D, et al. (eds) Obesity and Metabolic Disorders. Amsterdam: IOS Press, pp.73–91. [Google Scholar]
- Heal DJ, Kulkarni RS, Pinder L, et al. (2017) Dasotraline is a monoamine reuptake inhibitor not a releasing agent as revealed by tetrodotoxin (TTX) sensitivity in microdialysis in the nucleus accumbens of freely-moving rats. In: Society for Neurosciences meeting, Washington, D.C., 11–15 November 2017, 201A7. Abstract 557.21. Neuroscience Meeting Planner. Available at: https://www.abstractsonline.com/pp8/#!/4376/presentation/21785. [Google Scholar]
- Heal DJ, Smith SL, Findling RL. (2012) ADHD: Current and future therapeutics. Curr Top Behav Neurosci 9: 361–390. [DOI] [PubMed] [Google Scholar]
- Heal DJ, Smith SL, Gosden J, et al. (2013. a) Amphetamine, past and present – A pharmacological and clinical perspective. J Psychopharmacol 27: 479–496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heal DJ, Vickers SP, Hackett D, et al. (2013. b). Metabolic effects of lisdexamfetamine in a rat model of human obesity with insulin resistance. Proceedings of the 26th ECNP Congress 5–9 October 2013, Barcelona, Spain. Eur Neuropsychoparmacol 23: S207. [Google Scholar]
- Heal DJ, Vickers SP, Hopkins SC, et al. (2018) Investigation of the effects of dasotraline in a validated rat model of binge-eating disorder. In: Proceedings of the 57th annual meeting of the American College of Neuropsychopharmacology (ACNP), Hollywood, FL, USA, 9–13 December 2018. Poster presentation. [Google Scholar]
- Hemmingsson E. (2011) Does medically induced weight loss improve obstructive sleep apnoea in the obese: Review of randomized trials. Clin Obes 1: 26–30. [DOI] [PubMed] [Google Scholar]
- Hicks C, Sabino V, Cottone P. (2020) The Alpha-1 adrenergic receptor antagonist prazosin reduces binge-like eating in rats. Nutrients 12: 1569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Higgins GA, Silenieks LB, Altherr EB, et al. (2016) Lorcaserin and CP-809101 reduce motor impulsivity and reinstatement of food seeking behavior in male rats: Implications for understanding the anti-obesity property of 5-HT2C receptor agonists. Psychopharmacology (Berl) 233: 2841–2856. [DOI] [PubMed] [Google Scholar]
- Hilbert A, Pike KM, Goldschmidt AB, et al. (2014) Risk factors across the eating disorders. Psychiatry Res 220: 500–506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hilbert A, Pike KM, Wilfley DE, et al. (2011) Clarifying boundaries of binge eating disorder and psychiatric comorbidity: A latent structure analysis. Behav Res Ther 49: 202–211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoek HW, van Hoeken D. (2003) Review of the prevalence and incidence of eating disorders. Int J Eat Disord 34: 383–396. [DOI] [PubMed] [Google Scholar]
- Hopkins SC, Sunkaraneni S, Skende E, et al. (2016) Pharmacokinetics and exposure-response relationships of dasotraline in the treatment of attention-deficit/hyperactivity disorder in adults. Clin Drug Investig 36: 137–146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hudson JI, Hiripi E, Pope HG, Jr, et al. (2007) The prevalence and correlates of eating disorders in the National Comorbidity Survey Replication. Biol Psychiatry 61: 348–358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hudson JI, McElroy SL, Raymond NC, et al. (1998) Fluvoxamine in the treatment of binge-eating disorder: A multicenter placebo-controlled, double-blind trial. Am J Psychiatry 155: 1756–1762. [DOI] [PubMed] [Google Scholar]
- Hurley S, Gilmour G, Soula A, et al. (2020) The effects of psilocybin on binge-like feeding behaviour in rats. In: Proceedings of the American College of Neuropsychopharmacology 59th annual meeting, 6–9 December 2020. Poster M51. [Google Scholar]
- Hussain Y, Krishnamurthy S. (2018) Piracetam attenuates binge eating disorder related symptoms in rats. Pharmacol Biochem Behav 169: 35–47. [DOI] [PubMed] [Google Scholar]
- Hutson PH, Pennick M, Secker R. (2014) Preclinical pharmacokinetics, pharmacology and toxicology of lisdexamfetamine: A novel d-amphetamine pro-drug. Neuropharmacology 87: 41–50. [DOI] [PubMed] [Google Scholar]
- Hyttel J. (1982) Citalopram – Pharmacological profile of a specific serotonin uptake inhibitor with antidepressant activity. Prog Neuropsychopharmacol Biol Psychiatry 6: 277–295. [DOI] [PubMed] [Google Scholar]
- Ignar DM, Goetz AS, Noble KN, et al. (2011) Regulation of ingestive behaviors in the rat by GSK1521498, a novel μ-opioid receptor-selective inverse agonist. J Pharmacol Exp Ther 339: 24–34. [DOI] [PubMed] [Google Scholar]
- Ilbegi S, Groenman AP, Schellekens A, et al. (2018) Substance use and nicotine dependence in persistent, remittent, and late-onset ADHD: A 10-year longitudinal study from childhood to young adulthood. J Neurodev Disord 10: 42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ishii T, Takahashi O, Kawamura Y, et al. (2003) Comorbidity in attention deficit-hyperactivity disorder. Psychiatry Clin Neurosci 57: 457–463. [DOI] [PubMed] [Google Scholar]
- Jackson HC, Bearham MC, Hutchins LJ, et al. (1997. b) Investigation of the mechanisms underlying the hypophagic effects of the 5‑HT and noradrenaline reuptake inhibitor, sibutramine, in the rat. Br J Pharmacol 121: 1613–1618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jackson HC, Needham AM, Hutchins LJ, et al. (1997. a) Comparison of the effects of sibutramine and other monoamine reuptake inhibitors on food intake in the rat. Br J Pharmacol 121: 1758–1762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jackson JN, MacKillop J. (2016) Attention-deficit/hyperactivity disorder and monetary delay discounting: A meta-analysis of case-control studies. Biol Psychiatry Cogn Neurosci Neuroimaging 1: 316–325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jasinski DR, Krishnan S. (2009. a) Human pharmacology of intravenous lisdexamfetamine dimesylate: Abuse liability in adult stimulant abusers. J Psychopharmacol 23: 410–418. [DOI] [PubMed] [Google Scholar]
- Jasinski DR, Krishnan S. (2009. b) Abuse liability and safety of oral lisdexamfetamine dimesylate in individuals with a history of stimulant abuse. J Psychopharmacol 23: 419–427. [DOI] [PubMed] [Google Scholar]
- Johnson JK, Liranso T, Saylor K, et al. (2020) A phase II, double-blind, placebo-controlled, efficacy and safety study of SPN-812 (extended-release Viloxazine) in children with ADHD. J Atten Disord 24: 348–358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson PM, Kenny PJ. (2010) Dopamine D2 receptors in addiction-like reward dysfunction and compulsive eating in obese rats. Nat Neurosci 13: 635–641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jucaite A, Öhd J, Potter AS, et al. (2014) A randomized, double-blind, placebo-controlled crossover study of α4β2 nicotinic acetylcholine receptor agonist AZD1446 (TC-6683) in adults with attention-deficit/hyperactivity disorder. Psychopharmacology 231: 1251–1265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaisari P, Dourish CT, Rotshtein P, et al. (2018) Associations between core symptoms of attention deficit hyperactivity disorder and both binge and restrictive eating. Front Psychiatry 9: 103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karabacak Y, Sase S, Aher YD, et al. (2015) The effect of modafinil on the rat dopamine transporter and dopamine receptors D1–D3 paralleling cognitive enhancement in the radial arm maze. Front Behav Neurosci 9: 215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kessler RC, Berglund PA, Chiu WT, et al. (2013) The prevalence and correlates of binge eating disorder in the World Health Organization world mental health surveys. Biol Psychiatry 73: 904–914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kessler RM, Hutson PH, Herman BK, et al. (2016) The neurobiological basis of binge-eating disorder. Neurosci Biobehav Rev 63: 223–238. [DOI] [PubMed] [Google Scholar]
- Kihara T, Ikeda M. (1995) Effects of duloxetine, a new serotonin and norepinephrine uptake inhibitor, on extracellular monoamine levels in rat frontal cortex. J Pharmacol Exp Ther 272: 177–183. [PubMed] [Google Scholar]
- Kirschenbaum DS, Krawczyk R. (2018) The food addiction construct may do more harm than good: Weight controllers are athletes, not addicts. Child Obes 14: 227–236. [DOI] [PubMed] [Google Scholar]
- Koblan KS, Hopkins SC, Sarma K, et al. (2015) Dasotraline for the treatment of attention-deficit/hyperactivity disorder: A randomized, double-blind, placebo-controlled, proof-of-concept trial in adults. Neuropsychopharmacology 40: 2745–2752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koblan KS, Hopkins SC, Sarma K, et al. (2016) Assessment of human abuse potential of dasotraline compared to methylphenidate and placebo in recreational stimulant users. Drug Alcohol Depend 159: 26–34. [DOI] [PubMed] [Google Scholar]
- Langtry HD, Gillis JC, Davis R. (1997) Topiramate: A review of its pharmacodynamics and pharmacokinetic properties and clinical efficacy in the management of epilepsy. Drugs 54: 752–773. [DOI] [PubMed] [Google Scholar]
- Leombruni P, Pierò A, Lavagnino L, et al. (2008) A randomized, double-blind trial comparing sertraline and fluoxetine 6-month treatment in obese patients with binge eating disorder. Prog Neuropsychopharmacol Biol Psychiatry 32: 1599–1605. [DOI] [PubMed] [Google Scholar]
- Leppik IE. (1999) Zonisamide. Epilepsia 40(Suppl 5): S23–S29. [DOI] [PubMed] [Google Scholar]
- Levine LR, Enas GG, Thompson WL, et al. (1989) Use of fluoxetine, a selective serotonin-uptake inhibitor, in the treatment of obesity: A dose-response study (with a commentary by Michael Weintraub). Int J Obes 13: 635–645. [PubMed] [Google Scholar]
- Li SX, Perry KW, Wong DT. (2002) Influence of fluoxetine on the ability of bupropion to modulate extracellular dopamine and norepinephrine concentrations in three mesocorticolimbic areas of rats. Neuropharmacology 42: 181–190. [DOI] [PubMed] [Google Scholar]
- Lin DY, Kratochvil CJ, Xu W, et al. (2014) A randomized trial of edivoxetine in pediatric patients with attention-deficit/hyperactivity disorder. J Child Adolesc Psychopharmacol 24: 190–200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luscombe GP, Slater NA, Lyons MB, et al. (1990) Effect on radiolabelled-monoamine uptake in vitro of plasma taken from healthy volunteers administered the antidepressant sibutramine HCl. Psychopharmacology 100: 345–349. [DOI] [PubMed] [Google Scholar]
- Lynch WC, Heil DP, Wagner E, et al. (2008) Body dissatisfaction mediates the association between body mass index and risky weight control behaviors among white and native American adolescent girls. Appetite 51: 210–213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCuen-Wurst C, Ruggieri M, Allison KC. (2018) Disordered eating and obesity: Associations between binge-eating disorder, night-eating syndrome, and weight-related comorbidities. Ann N Y Acad Sci 1411: 96–105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McElroy SL, Arnold LM, Shapira NA, et al. (2003. a) Topiramate in the treatment of binge eating disorder associated with obesity: A randomized, placebo-controlled trial. Am J Psychiatry 160: 255–261. [DOI] [PubMed] [Google Scholar]
- McElroy SL, Guerdjikova AI, Blom TJ, et al. (2013) A placebo-controlled pilot study of the novel opioid receptor antagonist ALKS-33 in binge eating disorder. Int J Eat Disord 46: 239–245. [DOI] [PubMed] [Google Scholar]
- McElroy SL, Guerdjikova A, Kotwal R, et al. (2007. a) Atomoxetine in the treatment of binge-eating disorder: A randomized placebo-controlled trial. J Clin Psychiatry 68: 390–398. [DOI] [PubMed] [Google Scholar]
- McElroy SL, Guerdjikova AI, Mori N, et al. (2015. b) Armodafinil in binge eating disorder: A randomized, placebo-controlled trial. Int Clin Psychopharmacol 30: 209–215. [DOI] [PubMed] [Google Scholar]
- McElroy SL, Guerdjikova AI, Winstanley EL, et al. (2011) Acamprosate in the treatment of binge eating disorder: A placebo-controlled trial. Int J Eat Disord 44: 81–90. [DOI] [PubMed] [Google Scholar]
- McElroy SL, Hudson J, Capece JA, et al. (2007. b) Topiramate for the treatment of binge eating disorder associated with obesity: A placebo-controlled study. Biol Psychiatry 61: 1039–1048. [DOI] [PubMed] [Google Scholar]
- McElroy SL, Hudson J, Ferreira-Cornwell MC, et al. (2016. a) Lisdexamfetamine dimesylate for adults with moderate to severe binge eating disorder: Results of two pivotal phase 3 randomized controlled trials. Neuropsychopharmacology 41: 1251–1260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McElroy SL, Hudson JI, Grilo CM, et al. (2020) Efficacy and safety of dasotraline in adults with binge-eating disorder: A randomized, placebo-controlled, flexible-dose clinical trial. J Clin Psychiatry 81: 19m13068. [DOI] [PubMed] [Google Scholar]
- McElroy SL, Hudson JI, Malhotra S, et al. (2003. b) Citalopram in the treatment of binge-eating disorder: A placebo-controlled trial. J Clin Psychiatry 64: 807–813. [DOI] [PubMed] [Google Scholar]
- McElroy SL, Hudson JI, Mitchell JE, et al. (2015. a) Efficacy and safety of lisdexamfetamine for treatment of adults with moderate to severe binge-eating disorder: A randomized clinical trial. JAMA Psychiatry 72: 235–246. [DOI] [PubMed] [Google Scholar]
- McElroy SL, Kotwal R, Guerdjikova AI, et al. (2006) Zonisamide in the treatment of binge eating disorder with obesity: A randomized controlled trial. J Clin Psychiatry 67: 1897–1906. [DOI] [PubMed] [Google Scholar]
- McElroy SL, Mitchell JE, Wilfley D, et al. (2016. b) Lisdexamfetamine dimesylate effects on binge eating behaviour and obsessive-compulsive and impulsive features in adults with binge eating disorder. Eur Eat Disord Rev 24: 223–231. [DOI] [PubMed] [Google Scholar]
- Madras BK, Xie Z, Lin Z, et al. (2006) Modafinil occupies dopamine and norepinephrine transporters in vivo and modulates the transporters and trace amine activity in vitro. J Pharmacol Exp Ther 319: 561–569. [DOI] [PubMed] [Google Scholar]
- Majuri J, Joutsa J, Johansson J, et al. (2017) Serotonin transporter density in binge eating disorder and pathological gambling: A PET study with [11C]MADAM. Eur Neuropsychopharmacol 27: 1281–1288. [DOI] [PubMed] [Google Scholar]
- Malik PRA, Doumouras AG, Malhan RS, et al. (2021) Obesity, cancer, and risk reduction with bariatric surgery. Surg Clin North Am 101: 239–254. [DOI] [PubMed] [Google Scholar]
- Mann K, Kiefer F, Spanagel R, et al. (2008) Acamprosate: Recent findings and future research directions. Alcohol Clin Exp Res 32: 1105–1110. [DOI] [PubMed] [Google Scholar]
- Mason BJ, Heyser CJ. (2010) Acamprosate: A prototypic neuromodulator in the treatment of alcohol dependence. CNS Neurol Disord Drug Targets 9: 23–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Masuda Y, Ishizaki M, Shimizu M. (1998) Zonisamide: Pharmacology and clinical efficacy in epilepsy. CNS Drug Rev 4: 341–360. [DOI] [PubMed] [Google Scholar]
- Masuda Y, Noguchi H, Karasawa T. (1994) Evidence against a significant implication of carbonic anhydrase inhibitory activity of zonisamide in its anticonvulsive effects. Arzneimittelforschung 44: 267–269. [PubMed] [Google Scholar]
- Micali N, Solmi F, Horton NJ, et al. (2015) Adolescent eating disorders predict psychiatric, high-risk behaviors and weight outcomes in young adulthood. J Am Acad Child Adolesc Psychiatry 54: 652–659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Milano W, Petrella C, Casella A, et al. (2005) Use of sibutramine, an inhibitor of the reuptake of serotonin and noradrenaline, in the treatment of binge eating disorder: A placebo-controlled study. Adv Ther 22: 25–31. [DOI] [PubMed] [Google Scholar]
- Minzenberg MJ, Carter CS. (2008) Modafinil: A review of neurochemical actions and effects on cognition. Neuropsychopharmacology 33: 1477–1502. [DOI] [PubMed] [Google Scholar]
- Misgeld U, Bijak M, Jarolimek W. (1995) A physiological role for GABAB receptors and the effects of baclofen in the mammalian central nervous system. Prog Neurobiol 46: 423–462. [DOI] [PubMed] [Google Scholar]
- Mitchell JE, Gosnell BA, Roerig JL, et al. (2003) Effects of sibutramine on binge eating, hunger, and fullness in a laboratory human feeding paradigm. Obes Res 11: 599–602. [DOI] [PubMed] [Google Scholar]
- Mitchell JE, King WC, Courcoulas A, et al. (2015) Eating behavior and eating disorders in adults before bariatric surgery. Int J Eat Disord 48: 215–222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mitchell JE, Morley JE, Levine AS, et al. (1987) High-dose naltrexone therapy and dietary counseling for obesity. Biol Psychiatry 22: 35–42. [DOI] [PubMed] [Google Scholar]
- Mole TB, Irvine MA, Worbe Y, et al. (2015) Impulsivity in disorders of food and drug misuse. Psychol Med 45: 771–782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Molina BSG, Howard AL, Swanson JM, et al. (2018) Substance use through adolescence into early adulthood after childhood-diagnosed ADHD: Findings from the MTA longitudinal study. J Child Psychol Psychiatry 59: 692–702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mooney ME, Herin DV, Specker S, et al. (2015) Pilot study of the effects of lisdexamfetamine on cocaine use: A randomized, double-blind, placebo-controlled trial. Drug Alcohol Depend 153: 94–103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mostert JC, Onnink AMH, Klein M, et al. (2015) Cognitive heterogeneity in adult attention deficit/hyperactivity disorder: A systematic analysis of neuropsychological measurements. Eur Neuropsychopharmacol 25: 2062–2074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nasser JA, Gluck ME, Geliebter A. (2004) Impulsivity and test meal intake in obese binge eating women. Appetite 43: 303–307. [DOI] [PubMed] [Google Scholar]
- Nathan PJ, Bullmore ET. (2009) From taste hedonics to motivational drive: Central μ opioid receptors and binge-eating behaviour. Int J Neuropsychopharmacol 12: 995–1008. [DOI] [PubMed] [Google Scholar]
- Navia B, Hudson JI, McElroy SL, et al. (2017) Dasotraline for the treatment of moderate to severe binge eating disorder in adults: Results from a randomized, double-blind, placebo-controlled study. In: 170th annual meeting of the American Psychiatric Association (APA), San Diego, CA, USA, 20–24 May 2017. Poster. [Google Scholar]
- Navia B, Hudson JI, McElroy SL, et al. (2018) Dasotraline for treatment of adults with binge-eating disorder: Effect on behavioral outcomes. In: American Psychiatric Association annual meeting, New York, USA, 5–9 May 2018. Poster. [Google Scholar]
- Nery ESM, Bangs M, Liu P, et al. (2017) Long-term, open-label, safety study of edivoxetine monotherapy in children and adolescents with attention-deficit/hyperactivity disorder. J Child Adolesc Psychopharmacol 27: 700–707. [DOI] [PubMed] [Google Scholar]
- Nguyen ML, Pirzada MH, Shapiro MA. (2013) Zonisamide for weight loss in adolescents. J Pediatr Pharmacol Ther 18: 311–314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Öncü B, Er O, Çolak B, et al. (2014) Lamotrigine for attention deficit-hyperactivity disorder comorbid with mood disorders: A case series. J Psychopharmacol 28: 282–283. [DOI] [PubMed] [Google Scholar]
- Park P, Caballero J, Omidian H. (2014) Use of serotonin norepinephrine reuptake inhibitors in the treatment of attention-deficit hyperactivity disorder in pediatrics. Ann Pharmacother 48: 86–92. [DOI] [PubMed] [Google Scholar]
- Parker JA, Bloom SR. (2012) Hypothalamic neuropeptides and the regulation of appetite. Neuropharmacology 63: 18–30. [DOI] [PubMed] [Google Scholar]
- Pataky Z, Gasteyger C, Ziegler O, et al. (2013) Efficacy of rimonabant in obese patients with binge eating disorder. Exp Clin Endocrinol Diabetes 121: 20–26. [DOI] [PubMed] [Google Scholar]
- Pearlstein T, Spurell E, Hohlstein LA, et al. (2003) A double-blind, placebo-controlled trial of fluvoxamine in binge eating disorder: A high placebo response. Arch Womens Ment Health 6: 147–151. [DOI] [PubMed] [Google Scholar]
- Pellock JM. (2004) Understanding co-morbidities affecting children with epilepsy. Neurology 62(Suppl 2): S17–S23. [DOI] [PubMed] [Google Scholar]
- Pennick M. (2010) Absorption of lisdexamfetamine dimesylate and its enzymatic conversion to d-amfetamine. Neuropsychiatr Dis Treat 24: 317–327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pertwee RG. (2005) Inverse agonism and neutral antagonism at cannabinoid CB1 receptors. Life Sci 76: 1307–1324. [DOI] [PubMed] [Google Scholar]
- Piccoli L, Di Bonaventura MVM, Cifani C, et al. (2012) Role of orexin-1 receptor mechanisms on compulsive food consumption in a model of binge eating in female rats. Neuropsychopharmacology 37: 1999–2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Plichta MM, Vasic N, Wolf RC, et al. (2009) Neural hypo-responsiveness and hyperresponsiveness during immediate and delayed reward processing in adult attention-deficit/hyperactivity disorder. Biol Psychiatry 65: 7–14. [DOI] [PubMed] [Google Scholar]
- Pliszka SR. (1998) Comorbidity of attention-deficit/hyperactivity disorder with psychiatric disorder: An overview. J Clin Psychiatry 59(Suppl 7): 50–58. [PubMed] [Google Scholar]
- Polyzoi M, Ahnemark E, Medin E, et al. (2018) Estimated prevalence and incidence of diagnosed ADHD and healthcare utilization in adults in Sweden – A longitudinal population-based register study. Neuropsychiatr Dis Treat 14: 1149–1161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Popik P, Kos T, Zhang Y, et al. (2011) Memantine reduces consumption of highly palatable food in a rat model of binge eating. Amino Acids 40: 477–485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pothos EN, Creese I, Hoebel BG. (1995) Restricted eating with weight loss selectively decreases extracellular dopamine in the nucleus accumbens and alters dopamine response to amphetamine, morphine, and food intake. J Neurosci 15: 6640–6650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Potter AS, Dunbar G, Mazzulla E, et al. (2014) AZD3480, a novel nicotinic receptor agonist, for the treatment of attention-deficit/hyperactivity disorder in adults. Biol Psychiatry 75: 207–214. [DOI] [PubMed] [Google Scholar]
- Price A, Brehm VD, Hommel JD, et al. (2018) Pimavanserin and lorcaserin attenuate measures of binge eating in male Sprague-Dawley rats. Front Pharmacol 9:1424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qian J, Hu Q, Wan Y, et al. (2013) Prevalence of eating disorders in the general population: A systematic review. Shanghai Arch Psychiatry 25: 212–223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reinblatt SP. (2015) Are eating disorders related to attention deficit/hyperactivity disorder? Curr Treat Options Psychiatry 2: 402–412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Richelson E, Pfenning M. (1984) Blockade by antidepressants and related compounds of biogenic amine uptake into rat brain synaptosomes: Most antidepressants selectively block norepinephrine uptake. Eur J Pharmacol 104: 277–286. [DOI] [PubMed] [Google Scholar]
- Robbins TW, Arnsten AF. (2009) The neuropsychopharmacology of fronto-executive function: Monoaminergic modulation. Annu Rev Neurosci 32: 267–287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rogawski MA, Löscher W. (2004) The neurobiology of antiepileptic drugs. Nat Rev Neurosci 5: 553–564. [DOI] [PubMed] [Google Scholar]
- Romano A, Di Bonaventura MVM, Gallelli CA, et al. (2020) Oleoylethanolamide decreases frustration stress-induced binge-like eating in female rats: A novel potential treatment for binge eating disorder. Neuropsychopharmacology 45: 1931–1941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Romo L, Ladner J, Kotbagi G, et al. (2018) Attention-deficit hyperactivity disorder and addictions (substance and behavioral): Prevalence and characteristics in a multicenter study in France. J Behav Addict 7: 743–751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rothman RB. (1996) Treatment of a 4-year-old boy with ADHD with the dopamine releaser phentermine. J Clin Psychiatry 57: 308–309. [PubMed] [Google Scholar]
- Rothman RB, Baumann MH, Dersch CM, et al. (2001) Amphetamine-type central nervous system stimulants release norepinephrine more potently than they release dopamine and serotonin. Synapse 39: 32–41. [DOI] [PubMed] [Google Scholar]
- Rowley HL, Butler SA, Prow MR, et al. (2000) Comparison of the effects of sibutramine and other weight-modifying drugs on extracellular dopamine in the nucleus accumbens of freely moving rats. Synapse 38: 167–176. [DOI] [PubMed] [Google Scholar]
- Rowley HL, Kulkarni R, Gosden J, et al. (2012) Lisdexamfetamine and immediate release d-amfetamine – Differences in pharmacokinetic/pharmacodynamic relationships revealed by striatal microdialysis in freely-moving rats with simultaneous determination of plasma drug concentrations and locomotor activity. Neuropharmacology 63: 1064–1074. [DOI] [PubMed] [Google Scholar]
- Rowley HL, Kulkarni RS, Gosden J, et al. (2014) Differences in the neurochemical and behavioural profiles of lisdexamfetamine methylphenidate and modafinil revealed by simultaneous dual-probe microdialysis and locomotor activity measurements in freely-moving rats. J Psychopharmacol 28: 254–269. [DOI] [PubMed] [Google Scholar]
- Rowley HL, Kulkarni RS, Pinder L, et al. (2017) Dasotraline – Evaluation of its dopamine reuptake characteristics in comparison to stimulants and non-stimulants by microdialysis in the nucleus accumbens of freely-moving rats. In: Society for Neurosciences meeting, Washington, 11–15 November 2017. Abstract 557.23. Neuroscience Meeting Planner. Available at: https://www.abstractsonline.com/pp8/#!/4376/presentation/21779. [Google Scholar]
- Safer DL, Adler S, Dalai SS, et al. (2020) A randomized, placebo-controlled crossover trial of phentermine-topiramate ER in patients with binge-eating disorder and bulimia nervosa. Int J Eat Disord 53: 266–277. [DOI] [PubMed] [Google Scholar]
- Sanchez C, Asin KE, Artigas F. (2015) Vortioxetine, a novel antidepressant with multimodal activity: Review of preclinical and clinical data. Pharmacol Ther 145: 43–57. [DOI] [PubMed] [Google Scholar]
- Schag K, Schonleber J, Teufel M, et al. (2013) Food-related impulsivity in obesity and binge eating disorder – A systematic review. Obes Rev 14: 477–495. [DOI] [PubMed] [Google Scholar]
- Scheres A, Milham MP, Knutson B, et al. (2007) Ventral striatal hypo-responsiveness during reward anticipation in attention-deficit/hyperactivity disorder. Biol Psychiatry 61: 720–724. [DOI] [PubMed] [Google Scholar]
- Scheres A, Tontsch C, Thoeny AL, et al. (2010) Temporal reward discounting in attention-deficit/hyperactivity disorder: The contribution of symptom domains, reward magnitude, and session length. Biol Psychiatry 67: 641–648. [DOI] [PubMed] [Google Scholar]
- Schienle A, Schäfer A, Hermann A, et al. (2009) Binge-eating disorder: Reward sensitivity and brain activation to images of food. Biol Psychiatry 65: 654–661. [DOI] [PubMed] [Google Scholar]
- Schubert R. (2005) Attention deficit disorder and epilepsy. Pediatr Neurol 32: 1–10. [DOI] [PubMed] [Google Scholar]
- Shank RP, Gardocki JF, Streeter AJ, et al. (2000) An overview of the preclinical aspects of topiramate: Pharmacology, pharmacokinetics, and mechanism of action. Epilepsia 41(Suppl 1): S3–S9. [PubMed] [Google Scholar]
- Shank RP, Smith-Swintosky VL, Maryanoff BE. (2008) Carbonic anhydrase inhibition. Insight into the characteristics of zonisamide, topiramate, and the sulfamide cognate of topiramate. J Enzyme Inhib Med Chem 23: 271–276. [DOI] [PubMed] [Google Scholar]
- Sharman J, Pennick M. (2014) Lisdexamfetamine prodrug activation by peptidase-mediated hydrolysis in the cytosol of red blood cells. Neuropsychiatr Dis Treat 10: 2275–2280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shiels K, Hawk LW, Reynolds B, et al. (2009) Effects of methylphenidate on discounting of delayed rewards in attention deficit/hyperactivity disorder. Exp Clin Psychopharmacol 17: 291–301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shire D, Calandra B, Bouaboula M, et al. (1999) Cannabinoid receptor interactions with the antagonists SR 141716A and SR 144528. Life Sci 65: 627–635. [DOI] [PubMed] [Google Scholar]
- Shire D, Calandra B, Delpech M, et al. (1996) Structural features of the central cannabinoid CB1 receptor involved in the binding of the specific CB1 antagonist SR 141716A. J Biol Chem 271: 6941–6946. [DOI] [PubMed] [Google Scholar]
- Sonneville KR, Calzo JP, Horton NJ, et al. (2012) Body satisfaction, weight gain and binge eating among overweight adolescent girls. Int J Obes 36: 944–949. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stafford D, LeSage MG, Rice KC, et al. (2001) A comparison of cocaine, GBR 12909, and phentermine self-administration by rhesus monkeys on a progressive-ratio schedule. Drug Alcohol Depend 62: 41–47. [DOI] [PubMed] [Google Scholar]
- Statnick MA, Chen Y, Ansonoff M, et al. (2016) A novel nociceptin receptor antagonist LY2940094 inhibits excessive feeding behavior in rodents: A possible mechanism for the treatment of binge eating disorder. J Pharmacol Exp Ther 356: 493–502. [DOI] [PubMed] [Google Scholar]
- Stojek MM, Fischer S, Murphy CM, et al. (2014) The role of impulsivity traits and delayed reward discounting in dysregulated eating and drinking among heavy drinkers. Appetite 80: 81–88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stopyra MA, Simon JJ, Skunde M, et al. (2019) Altered functional connectivity in binge eating disorder and bulimia nervosa: A resting-state fMRI study. Brain Behav 9: e01207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ströhle A, Stoy M, Wrase J, et al. (2008) Reward anticipation and outcomes in adult males with attention-deficit/hyperactivity disorder. Neuroimage 39: 966–972. [DOI] [PubMed] [Google Scholar]
- Sunovion Press Release (2018) FDA issues a complete response letter for new drug application for dasotraline for the treatment of ADHD. Available at: https://news.sunovion.com/press-releases/press-releases-details/2018/FDA-Issues-a-Complete-Response-Letter-for-New-Drug-Application-for-Dasotraline-for-the-Treatment-of-ADHD/default.aspx (accessed 20 July 2021).
- Sunovion Press Release (2020) Sunovion discontinues dasotraline program. Available at: https://news.sunovion.com/press-releases/press-releases-details/2020/Sunovion-Discontinues-Dasotraline-Program/default.aspx (accessed 20 July 2021).
- Svaldi J, Naumann E, Trentowska M, et al. (2014) General and food-specific inhibitory deficits in binge eating disorder. Int J Eat Disord 47: 534–542. [DOI] [PubMed] [Google Scholar]
- Tarazi FI, Choi YK, Gosden J, et al. (2015) Reduced expression of GAD65/67 mRNA and dopamine D1 and D2 receptors in binge-eating rats. In: 54th Annual meeting of the American College of Neuropsychopharmacology, 6–10 December 2015. Poster M48. [Google Scholar]
- Tomasi D, Volkow ND. (2012) Abnormal functional connectivity in children with attention-deficit/hyperactivity disorder. Biol Psychiatry 71: 443–450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsai J, Hudson JI, McElroy SL, et al. (2019) Efficacy and safety of dasotraline in adults with binge-eating disorder: A randomized, double-blind, fixed-dose trial. In: Poster presentation of the American Psychiatric Association, San Francisco, USA, 18–22 May 2019. [Google Scholar]
- Umehara M, Ago Y, Fujita K, et al. (2013) Effects of serotonin-norepinephrine reuptake inhibitors on locomotion and prefrontal monoamine release in spontaneously hypertensive rats. Eur J Pharmacol 702: 250–257. [DOI] [PubMed] [Google Scholar]
- Ural C, Belli H, Akbudak M, et al. (2017) Relation of binge eating disorder with impulsiveness in obese individuals. World J Psychiatry 7: 114–120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Valbrun LP, Zvonarev V. (2020) The opioid system and food intake: Use of opiate antagonists in treatment of binge eating disorder and abnormal eating behavior. J Clin Med Res 12: 41–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vickers SP, Goddard S, Brammer RJ, et al. (2017) Investigation of impulsivity in binge-eating rats in a delay-discounting task and its prevention by the d-amphetamine prodrug, lisdexamfetamine. J Psychopharmacol 31: 784–797. [DOI] [PubMed] [Google Scholar]
- Vickers SP, Hackett D, Murray F, et al. (2015) Effects of lisdexamfetamine in a rat model of binge-eating. J Psychopharmacol 29: 1290–1307. [DOI] [PubMed] [Google Scholar]
- Vincent HK, Heywood K, Connelly J, et al. (2012) Obesity and weight loss in the treatment and prevention of osteoarthritis. PM R 4(5 Suppl): S59–S67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Volkow ND, Fowler JS, Logan J, et al. (2009) Effects of modafinil on dopamine and dopamine transporters in the male human brain: Clinical implications. JAMA 301: 1148–1154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wallingford NM, Sinnayah P, Bymaster FP, et al. (2008) Zonisamide prevents olanzapine-associated hyperphagia, weight-gain, and elevated blood glucose in rats. Neuropsychopharmacology 33: 2922–2933. [DOI] [PubMed] [Google Scholar]
- Wang GJ, Geliebter A, Volkow ND, et al. (2011) Enhanced striatal dopamine release during food stimulation in binge eating disorder. Obesity 19: 1601–1608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ward AS, Comer SD, Haney M, et al. (1999) Fluoxetine-maintained obese humans: Effect on food intake and body weight. Physiol Behav 66: 815–821. [DOI] [PubMed] [Google Scholar]
- Weiss M, Hechtman L; Adult ADHD Research Group (2006) A randomized double-blind trial of paroxetine and/or dextroamphetamine and problem-focused therapy for attention-deficit/hyperactivity disorder in adults. J Clin Psychiatry 67: 611–619. [PubMed] [Google Scholar]
- Weissler RH, Pandina GJ, Daly EJ, et al. (2012) Randomized clinical study of a histamine H3 receptor antagonist for the treatment of adults with attention-deficit hyperactivity disorder. CNS Drugs 26: 421–434. [DOI] [PubMed] [Google Scholar]
- Wentland MP, Lou R, Lu Q, et al. (2009) Syntheses of novel high affinity ligands for opioid receptors. Bioorg Med Chem Lett 19: 2289–2294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- White HS. (1999) Comparative anticonvulsant and mechanistic profile of the established and newer antiepileptic drugs. Epilepsia 40(Suppl 5): S2–S10. [DOI] [PubMed] [Google Scholar]
- White MA, Grilo CM. (2013) Bupropion for overweight women with binge-eating disorder: A randomized, double-blind, placebo-controlled trial. J Clin Psychiatry 74: 400–406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wigal SB, Biederman J, Swanson JM, et al. (2006) Efficacy and safety of modafinil film-coated tablets in children and adolescents with or without prior stimulant treatment for attention-deficit/hyperactivity disorder: Pooled analysis of 3 randomized, double-blind, placebo-controlled studies. Prim Care Companion J Clin Psychiatry 8: 352–360. [PMC free article] [PubMed] [Google Scholar]
- Wigal SB, Hopkins SC, Koblan KS, et al. (2020) Efficacy and safety of dasotraline in children with ADHD: A laboratory classroom study. J Atten Disord 24: 192–204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilens TE. (2004) Attention-deficit/hyperactivity disorder and the substance use disorders: The nature of the relationship, subtypes at risk, and treatment issues. Psychiatr Clin North Am 27: 283–301. [DOI] [PubMed] [Google Scholar]
- Wilens TE, Gault LM, Childress A, et al. (2011) Safety and efficacy of ABT-089 in pediatric attention-deficit/hyperactivity disorder: Results from two randomized placebo-controlled clinical trials. J Am Acad Child Adolesc Psychiatry 50: 73–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilens TE, Haight BR, Horrigan JP, et al. (2005) Bupropion XL in adults with attention-deficit/hyperactivity disorder: A randomized, placebo-controlled study. Biol Psychiatry 57: 793–801. [DOI] [PubMed] [Google Scholar]
- Wilens TE, Klint T, Adler L, et al. (2008) A randomized controlled trial of a novel mixed monoamine reuptake inhibitor in adults with ADHD. Behav Brain Funct 4: 24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilens TE, Spencer TJ, Biederman J, et al. (2001) A controlled clinical trial of bupropion for attention deficit hyperactivity disorder in adults. Am J Psychiatry 158: 282–288. [DOI] [PubMed] [Google Scholar]
- Wilfley DE, Crow SJ, Hudson JI, et al. (2008) Efficacy of sibutramine for the treatment of binge eating disorder: A randomized multicentre placebo-controlled double-blind study. Am J Psychiatry 165: 51–58. [DOI] [PubMed] [Google Scholar]
- Wonderlich SA, Gordon KH, Mitchell JE, et al. (2009) The validity and clinical utility of binge-eating disorder. Int J Eat Disord 42: 687–705. [DOI] [PubMed] [Google Scholar]
- Wong DT, Threlkeld PG, Best KL, et al. (1982) A new inhibitor of norepinephrine uptake devoid of affinity for receptors in rat brain. J Pharmacol Exp Ther 222: 61–65. [PubMed] [Google Scholar]
- Wortley KE, Hughes ZA, Heal DJ, et al. (1999) Comparison of changes in the extracellular concentration of noradrenaline in rat frontal cortex induced by sibutramine or d-amphetamine: Modulation by α2‑adrenoceptors. Br J Pharmacol 127: 1860–1866. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu M, Giel KE, Skunde M, et al. (2013) Inhibitory control and decision making under risk in bulimia nervosa and binge-eating disorder. Int J Eat Disord 46: 721–728. [DOI] [PubMed] [Google Scholar]
- Yee BJ, Phillips CL, Banerjee D, et al. (2007) The effect of sibutramine-assisted weight loss in men with obstructive sleep apnoea. Int J Obes 31: 161–168. [DOI] [PubMed] [Google Scholar]
- Yee K, Serrano D, Kando J, et al. (2019) A psychometric analysis and revalidation of the Yale-Brown Obsessive Compulsive Scale modified for Binge Eating in adults with binge eating disorder. Qual Life Res 28: 3385–3394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ziauddeen H, Chamberlain SR, Nathan PJ, et al. (2013) Effects of the μ-opioid receptor antagonist GSK1521498 on hedonic and consummatory eating behaviour: A proof of mechanism study in binge-eating obese subjects. Mol Psychiatry 18: 1287–1293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ziauddeen H, Fletcher PC. (2013) Is food addiction a valid and useful concept? Obes Rev 14: 19–28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zocchi A, Varnier G, Arban R, et al. (2003) Effects of antidepressant drugs and GR-205171, a neurokinin-1 (NK1) receptor antagonist, on the response in the forced swim test and on monoamine extracellular levels in the frontal cortex of the mouse. Neurosci Lett 345: 73–76. [DOI] [PubMed] [Google Scholar]
- Zornoza T, Cano MJ, Polache A, et al. (2003) Pharmacology of acamprosate: An overview. CNS Drug Rev 9: 359–374. [DOI] [PMC free article] [PubMed] [Google Scholar]