SUMMARY
Pneumonia due to either Streptococcus pneumoniae (Sp) or Staphylococcus aureus (Sa) accounts for most mortality after influenza and acute respiratory illness (ARI). Because carriage precedes infection, we estimated Sp and Sa carriage to examine the co-colonization dynamics between Sp, Sa and respiratory viruses in the presence of ARI in the oropharynx. We tested oropharyngeal specimens of community subjects (aged ⩾2 years) with ARI for the presence of influenza A and B, 11 other common respiratory viruses, Sp and Sa, using real-time PCR. A total of 338 participants reported 519 ARI episodes of which 119 (35%) carried Sp, 52 (13%) carried Sa and 25 (7%) carried both. Thirty-five subjects tested positive for influenza, of which 14 (40%) carried Sp and six (17%) carried Sa, significantly more than in the influenza-negative group (P = 0·03 and P = 0·04, respectively). In subjects infected by any virus compared to those with no virus, Sp carriage (39·2% vs. 27·9%, P = 0·03) but not Sa carriage (11·6% vs. 14%, P = 0·6) was more frequent. For children, when Sa was present, Sp carriage tended to be less frequent than expected given the presence of viral infection, but not significantly [observed relative risk 1·14, 95% confidence interval (CI) 0·4–3·1; with a relative excess risk due to interaction of –0·11]. Independent of age, Sp carriers were more likely to return that season with subsequent ARI (odds ratio 2·14, 95% CI 1·1–4·3, P = 0·03). Both Sp and Sa carriage rates in the oropharynx increase during influenza infection in children. However, no negative interaction between Sp and Sa was observed. Sp carriers are more likely to suffer subsequent ARI episodes than non-carriers.
Key words: Influenza, oropharyngeal carriage, pneumonia, Staphylococcus aureus, Streptococcus pneumoniae
INTRODUCTION
Each year, about 30 000 Americans die from influenza. During the 2009 H1N1 pandemic, children and young adults were about 10 times more likely to die than during seasonal influenza [1]. However, most fatalities were attributed to secondary bacterial infection predominately caused by Streptococcus pneumoniae (Sp) [1, 2] and, to a lesser extent, Staphylococcus aureus (Sa), rather than influenza [3]. Both species are common commensals of the nasopharynx and oropharynx [3, 4]; Sp and influenza have well-characterized interactions, Sa and influenza less so. The duration and frequency of pneumococcal colonization varies by age and exposure to young children, the group at highest risk (30–80%) of carriage [5], but all individuals are likely colonized with Sp at some point [6]. Pneumococcal colonization rates are lower in older children and adults (8–15%) [7, 8], and children seem to carry Sp for longer periods than adults [9]. Sa carriage in the nares is also common (on average 32·4% of the US population), although neonates and youths aged between 7 and 19 years have the highest rates [10]. Carriage of Sa in the nares and oropharynx are overall similar, and throat swabs may even be more sensitive than nasal swabs for detecting Sa carriage [11].
Animal models [12, 13], epidemiological studies [4, 14] and previous work from our group [1] indicate that the transmission and pathogenicity of Sp is strongly affected by influenza infection. Influenza increases susceptibility and severity of pneumococcal invasive disease and facilitates pneumococcal colonization and growth [4, 12–16]. Sp colonization may also modify risk of upper respiratory viral infection and viral infection may facilitate transmission of Sp [8, 11, 17]. Sa colonization is also increased by concurrent influenza infection [18]. Sa was a major cause of mortality in each of the recent influenza pandemics – surpassing the mortality attributable to Sp in the 1957 and 1968 pandemics [19]. One possible explanation for the role of Sp and Sa in post-viral pneumonia is that both bacteria are better able to colonize the lower respiratory tract than other commensals, and compete for the same ecological niches. Further, in vitro [20] and epidemiological studies [21–23] suggest a negative interaction between Sp and Sa carriage in the nasopharynx. However, this negative interaction seems to be specific to certain pneumococcal serotypes, and may not be sustained with viral infection [18, 22, 24]. Additionally, although carriage in the two sites may be concordant, no study has specifically analysed co-colonization in the oropharynx, which may be more likely to be a source of lower-respiratory infections than the nasopharynx.
Despite being a common complication of influenza infection, the overall incidence of pneumonia is relatively low in the general population (~1/1000 cases of influenza). This has made it difficult to study the interactions of influenza, Sp, and Sa in human populations. Hence, much of the understanding of these complex co-colonization dynamics comes from animal models. However, since bacterial pneumonia requires aspiration of the infecting bacteria into the lungs, the same mechanisms that ultimately lead to pneumonia may also lead to increases in colonization rates and colonizing bacterial load in the oropharynx. High Sp colonization density is associated with invasive pneumococcal pneumonia [14]. In a study conducted among college students, Sp colonization, but not Sa, was higher in those with viral acute respiratory illness (ARI) than in healthy contacts [25]. Similarly, a Portuguese study conducted in 585 daycare children aged <3 years found the prevalence of Sp, but not Sa, increased with the number of respiratory symptoms [24].
Therefore, we chose to study the carriage rates and relative abundances of Sp and Sa in the oropharynx as an intermediate outcome towards the understanding of the increased risk of invasive pneumococcal or staphylococcal infections associated with ARI. We hypothesized that (i) the negative interaction between Sp and Sa may not be sustained during viral infection, and (ii) that in the presence of virus – particularly influenza, both Sp and Sa overgrow. Because rates of colonization by both bacteria are age-dependent, we took advantage of an ongoing prospective cohort study of ARI conducted in households including young children and their parents, to estimate the rate of carriage and relative abundance of Sp and Sa in these different age groups by detection of influenza or other respiratory viruses. We also conducted an exploratory analysis that assessed whether Sp colonization was associated with repeated ARI.
MATERIAL AND METHODS
Study population
We prospectively studied the subset of participants in The Household Influenza Vaccine Effectiveness (HIVE) study aged ⩾2 years that developed ARI. Between October 2013 and May 2014, ARI was actively monitored in 290 southeastern Michigan households with at least two children, as described previously [26]. Households were contacted weekly to identify members with ARI defined by the presence of an illness of <7 days duration with ⩾2 of the following symptoms: cough, fever or feverishness, nasal congestion, chills, headache, body aches, or sore throat. Specimens were collected only from those meeting the ARI case definition. Participants could be included on more than one visit if another ARI occurred. The HIVE study collected sociodemographic data, and medical record/state registry documentation of both the 2013–2014 influenza and pneumococcal vaccines [Prevnar7® (PCV7; Wyeth Pharmaceuticals, USA) and Prevnar13® (PCV13; Pfizer Inc., USA) and/or the polysaccharide pneumococcal vaccine, Pneumovax23® (PPV23; Merck, USA)]. Participants were considered fully immunized for influenza if they had been vaccinated during the current influenza season before the onset of ARI and for pneumococcus if they had received three PCV doses in the first year of life or one after age 12 months for PCV7 or PCV13, and one dose in the last 10 years for PPV23 [27]. Health system medical records were reviewed to document high-risk health conditions for complications of influenza, following the Advisory Committee on Immunization Practices (ACIP) [28]. The HIVE study, and this study protocol, were approved by the University of Michigan Medical School Institutional Review Board.
Sample collection and conservation
Participants with ARI had a throat and nasal swab collected which were combined together and conserved in 1 ml of universal transport media (UTM, Copan, USA) at −80 °C for viral testing. A second throat swab was collected and conserved separately in 1 ml UTM for bacterial testing.
Detection of Sp and Sa
Total bacterial DNA was extracted from the throat sample using a commercially available protocol (Qiagen Dneasy, USA). The presence of Sp and Sa was assessed by quantitative real-time PCR (qPCR). For Sp, the lytA gene was targeted using lytA-CDC primers, as described previously [29]. For Sa, the well-conserved and specific nuc gene was amplified [30]. Quantitative PCR reactions were carried out in a total volume of 20 µl, using a commercially available SYBR Green PCR master mix (Ssofast EvaGreen Supermix, Bio-Rad, USA), 200 nm primers and 5 µl DNA. The lytA-CDC gene fragment was obtained from PCR from a reference Sp strain (S. pneumoniae ATCC 49619). Assay conditions were optimized to reduce the amount of primer dimer in order to maximize the bacteria-specific signal in the qPCR assay. The limit of detection was 103 genomic copies/ml. All assays were conducted in triplicate and the mean values used for analysis. A no-template control and Sp-positive (ATCC 49619) and Sa-positive (MRSA 1268 strain) DNA controls were included in every run. Negative samples were defined as those with cycle threshold values >40. Serotypes for the Sp-positive samples were determined by multiplex PCR, as described previously [31]. Isolates were typed for all serotypes found in PCV7 and PCV13 and additional common clinically relevant serotypes (11A, 15A, 15B, 16F, 20, 22F, 33F, 35B). Positive controls were used for all serotypes tested, from our laboratory collection and the Active Bacterial Core surveillance [ABCs/Emerging Infections Programs (EIP)] network.
Viral testing
Combined nasal and throat swab specimens were tested for influenza (in real-time) and 11 other common respiratory viruses (after conservation at −80 °C) by real-time reverse transcription–PCR (RT–PCR) on an ABI 7500 RT-PCR platform (Life Technologies, USA). RNA and DNA/RNA was extracted for influenza and additional respiratory virus testing using the Qiagen QIAamp Viral RNA Mini kit and MinElute Virus Vacuum kit (Qiagen, Germany), respectively. The Super-Script III Platinum One-Step Quantitative RT–PCR system and the AgPath-ID One-Step RT–PCR system (ThermoFisher Scientific, USA), plus primers and probes developed by the Centers for Disease Control and Prevention Influenza and Viral, Gastroenteritis, and Respiratory Viruses Divisions were used for detection of influenza A and B, respiratory syncytial viruses (RSVs), human metapneumoviruses (HMPVs), parainfluenza viruses 1–3, rhinoviruses, adenoviruses, and four coronaviruses (229E, OC43, HKU1, NL63), respectively, as described previously [32].
Laboratory testing was performed in the investigators' microbiology and respiratory virus laboratories at the University of Michigan School of Public Health.
Statistical analysis
Patients' characteristics were compared between adults and children, and Sp and Sa carriers and non-carriers using χ2 test or Fisher's exact test for categorical variables and Student's t test or Wilcoxon rank-sum test for continuous variables. We used similar age groups as other studies in order to be able to compare our results with previously published works [21, 23]. In order to account for the longitudinal data (multiple observations per subject) and household correlation (multiple subjects per household) we used generalized estimating equation (GEE) models with an exchangeable correlation structure and robust standard errors to estimate the association between Sp detection and the following variables: age group, detection of influenza or other respiratory viruses, and Sa carriage. Since detection of Sp was a relatively common outcome, log-binomial regression models were fit to estimate the relative risk (RR). Daycare or school was not included in multivariate models because it was strongly associated with age group. We also fitted a logistic regression model to determine whether visiting the clinic more than twice with ARI during the season was independently associated with carriage of Sp or Sa on the first visit, age group or participants' daytime activity. Both models were tested for the whole population, and separately for children.
We evaluated co-colonization between respiratory virus detection and Sa carriage at any point during surveillance by describing departures from additivity and multiplicativity on the risk scale. Departures from additivity were evaluated by first calculating the RR of Sp in those with a virus detected compared to those with no virus detected, stratified by Sa carriage. These estimates were compared to the expected joint effect by estimating the relative excess risk due to interaction (RERI) and 95% confidence intervals (CIs), calculated using the delta method [33]. A negative RERI value implies antagonism [34]. To evaluate departures from multiplicativity we included a product term in multivariable regression models. For all analyses a P value <0·05 was considered to be statistically significant. Multivariable regression models were performed using SAS software v. 9.3 (SAS Institute Inc., USA), RERI estimates and 95% CIs were estimated using the epiR package in R v. 3.1.3 (R Foundation, Austria).
RESULTS
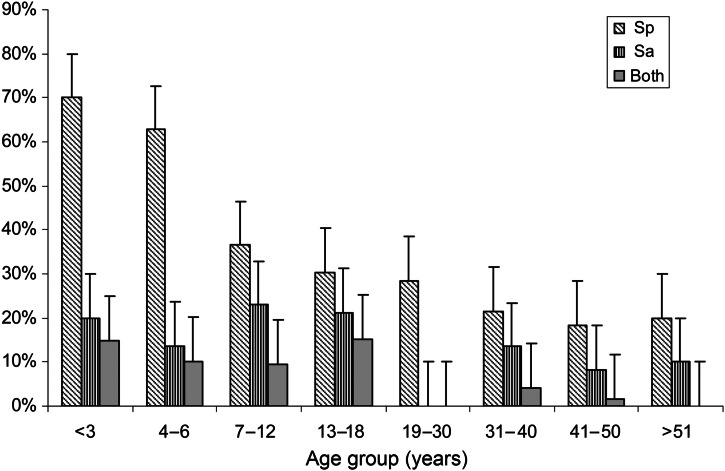
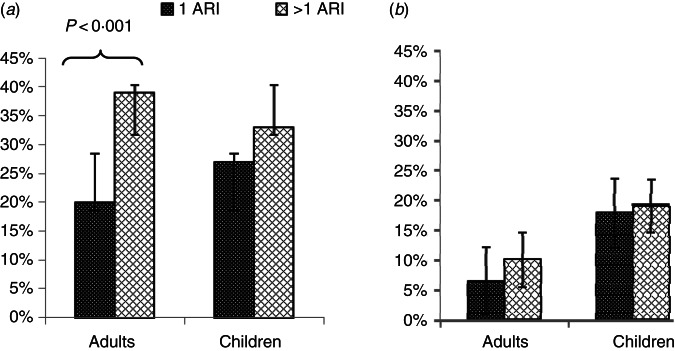
This analysis included 338 participants (152 adults, 186 children) with ARI residing in 143 different households. Overall, 119/338 (35·2%, 95% CI 30·1–40·4) participants carried Sp on at least one visit, 52 participants carried Sa (13·4%, 95% CI 11·9–19·6) and 25 (7·4%, 95% CI 5·1–10·7) carried both, with young children representing the majority of both Sp and Sa carriers (Table 1). Sp carriage was the highest (70%, 95% CI 48–85) for the 2–3 years age group and Sa carriage was highest (22%, 95% CI 7–22·7) for the 7–12 years age group (Fig. 1). Characteristics of subjects by Sp and/or Sa carriage are described in Table 2. At least one of the 13 viruses tested was detected in 224 participants (66·3%, 95% CI 61–71·1), including 31 (9%, 95% CI 6·3–12·7) with influenza A/H1N1 and four (1·2%, 95% CI 0·3–3·1) with influenza B/Yamagata. Of the 35 participants testing positive for influenza, 14 (40%, 95% CI 23·8–57·8) carried Sp and six (17%, 95% CI 6·2–32·3) carried Sa, significantly more than those carrying Sp, 105/303 (34·6%, 95% CI 29·3–40·3), and Sa, 22/303 (7·2%, 95% CI 4·6–10·7), in the influenza-negative group (P = 0·03 and P = 0·04 for Sp and Sa, respectively) (Fig. 2a). Sp, but not Sa, was carried significantly more often by participants with any virus detected than those with no virus detected (39·2% vs. 27·9%, P = 0·03 for Sp and 11·6% vs. 14%, P = 0·6 for Sa) (Fig. 2b).
Table 1.
Sociodemographic and medical characteristics of 338 participants with ARI in a household (n = 143 households) study of S. pneumoniae (Sp) and S. aureus (Sa) oropharyngeal carriage during the 2013–2014 influenza season in southeast Michigan
| All | Adults | Children | P value | |
|---|---|---|---|---|
| N | 338 | 152 | 186 | |
| Age, yr, mean (s.d.) [range] | 22·3 (16·8) [2–70] | 40 (6·5) [21–70] | 7·9 (3·8) [2–17] | |
| Male sex, n (%) | 160 | 54 | 106 | <0·001 |
| High risk*, n (%) | 72 (21·3) | 35 (23) | 26 (14) | 0·03 |
| Daytime activity | <0·001 | |||
| Daycare, n (%) | 34 (10·1) | 0 | 34 (18·2) | |
| School, n (%) | 137 (40·5) | 0 | 137 (73·6) | |
| Stay at home†, n (%) | 60 (17·7) | 45 (29·6) | 15 (8) | |
| Work, n (%) | 107 (31·6) | 107 (70·4) | ||
| No. of visits with ARI, mean (s.d.) | 1·54 (0·92) | 1·59 (0·97) | 1·49 (0·90) | 0·17 |
| Any complete pneumococcal vaccination‡, n (%) | 169 (50) | 20 (13·1) | 149 (80·1) | <0·001 |
| Complete PCV7 vaccination, n (%) | 82 (24·3) | 0 | 82 (44) | |
| Complete PCV13 vaccination, n (%) | 66 (19·5) | 0 | 66 (35·4) | |
| Complete PPV23 vaccination, n (%) | 22 (6·5) | 20 (13·1) | 2§ (1) | |
| Seasonal influenza vaccination, n (%) | 213 (63) | 97 (64) | 116 (62·3) | 0·98 |
| Tested positive for A respiratory virus, n (%) | 224 (66·2) | 96 (63·1) | 128 (68·8) | 0·27 |
| Influenza A/B, n (%) | 35 (10·3) | 18 (11·8) | 17 (9·1) | 0·40 |
ARI, Acute respiratory illness.
As defined by the Advisory Committee on Immunization Practices (ACIP) [28].
Including home schooling for children.
Pneumococcal vaccination was considered complete if the participant had received three PCV doses in the first year of life or at least one dose after age 12 months for PCV7 or PCV13, and at least one immunization in the last 10 years for PPV23 [27].
One child received both PCV7 and PPV23 vaccinations.
Fig. 1.
Proportion with carriage of Sp, Sa and both bacteria at once, by age group, among 338 participants with acute respiratory illness in a household (n = 143 households) study during the 2013–14 influenza season in southeast Michigan.
Table 2.
Comparison of participants carrying S. pneumoniae (Sp) and S. aureus (Sa) at least at one visit with non-carriers in 338 participants with acute respiratory illness in a household (n = 143 households) study during the 2013–2014 influenza season in southeast Michigan
| Adults | Children | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Sp carriers | Non-Sp carriers | P | Sa carriers | Non-Sa carriers | P | Sp carriers | Non-Sp carriers | P | Sa carriers | Non-Sa carriers | P | |
| n (%) | 31 (20·4) | 121 (79·6) | 16 (10·5) | 136 (89·4) | 88 (47·3) | 98 (52·7) | 36 (19·3) | 150 (80·6) | ||||
| Age, yr, mean (s.d.) | 39·8 (7·9) | 40 (6·1) | 0·90 | 39·5 (5·1) | 40·0 (6·7) | 0·76 | 6·6 (3·5) | 9 (3·8) | <0·01 | 8·0 (3·8) | 7·9 (3·9) | 0·80 |
| Male sex, n (%) | 13 (42) | 42 (34·7) | 0·30 | 9 (56·3) | 45 (33·1) | 0·07 | 55 (62·5) | 51 (52) | 0·80 | 20 (55·6) | 86 (57·3) | 0·50 |
| High risk*, n (%) | 11 (38·7) | 20 (16·5) | 0·02 | 4 (25) | 31 (22·8) | 0·80 | 13 (14·8) | 13 (13·3) | 0·60 | 7 (19·4) | 19 (12·7) | 0·10 |
| Daytime activity | 0·21 | 0·30 | <0·01 | 0·69 | ||||||||
| Daycare, n (%) | 0 | 0 | 0 | 0 | 26 (29·5) | 8 (8·2) | 8 (22·2) | 26 (17·3) | ||||
| School, n (%) | 0 | 0 | 0 | 0 | 54 (61·4) | 83 (84·7) | 26 (72·2) | 111 (74) | ||||
| Stay at home, n (%) | 12 (38·7) | 33 (27·3) | 3 (18·8) | 42 (30·9) | 8 (9) | 7 (7·1) | 2 (5·6) | 13 (8·7) | ||||
| Work, n (%) | 19 (61·3) | 88 (72·7) | 13 (81·3) | 94 (69·1) | 0 | 0 | 0 | 0 | ||||
| Mean number of visits (s.d.) | 1·75 (1) | 1·55 (0·96) | 0·35 | 2 (1·46) | 1·54 (0·89) | 0·07 | 1·67 (1·1) | 1·34 (0·7) | 0·01 | 1·80 (1·17) | 1·42 (0·80) | 0·02 |
| Seasonal influenza vaccination, n (%) | 18 (58) | 79 (65·3) | 0·42 | 10 (62·5) | 87 (64) | 0·90 | 54 (61·4) | 62 (63·3) | 0·73 | 22 (61·1) | 94 (62·7) | 0·80 |
| Complete PCV7 vaccination†, n (%) | 0 | 0 | 0 | 0 | 33 (37·5) | 49 (50) | 0·08 | 18 (50) | 64 (42·7) | 0·43 | ||
| Complete PCV13 vaccination†, n (%) | 0 | 0 | 0 | 0 | 43 (48·9) | 23 (23·5) | <0·01 | 11 (30·6) | 55 (36·7) | 0·49 | ||
| Complete PPV23 vaccination†, n (%) | 5 (79·4) | 15 (48·4) | 0·60 | 1 (6·3) | 19 (14) | 0·45 | 0 | 2 | 0 | 2 (1·3) | 0·49 | |
| Tested positive for A respiratory virus, n (%) | 23 (74) | 74 (61·2) | 0·31 | 11 (71·5) | 85 (62·5) | 0·80 | 72 (81·8) | 65 (66·3) | 0·02 | 25 (69·4) | 102 (68) | 0·80 |
| Influenza A/B, n (%) | 4 (12·9) | 14 (11·6) | 0·84 | 2 (12·5) | 16 (11·8) | 0·90 | 10 (11·4) | 7 (7·1) | 0·41 | 5 (13·8) | 12 (8) | 0·2 |
| Tested positive for Sa, n (%) | 4 (12·9) | 8 (6·6) | 0·24 | 21 (23·8) | 15 (15·3) | 0·14 | ||||||
| Tested positive for Sp, n (%) | 4 (25) | 27 (19·9) | 0·63 | 21 (58·3) | 67 (44·7) | 0·14 | ||||||
Fig. 2.
Proportion with carriage of Sp, Sa or both bacteria at once, by influenza (a) and by virus detection status (b), in 338 participants with acute respiratory illness in a household (n = 143 households) study during the 2013–2014 influenza season in southeast Michigan (* P < 0·05). Sp and Sa bars represent both co-colonized individuals and single colonized individuals.
In children, both Sa carriage and influenza infection suggested an increase in risk of Sp carriage (RR 1·35, 95% CI 0·96–1·91; RR 1·47, 95% CI 1·01–2·14, respectively). However, when both Sa and influenza were present, Sp carriage tended to be less frequent than the expected additive effect of both Sa and influenza infection (observed RR 1·14, 95% CI 0·42–3·09 for an expected additive RR of 1·6) (Table 3). A similar departure from the expected joint effect was observed when all tested viruses were taken into account (for Sa alone: RR 1·76, 95% CI 0·81–3·80; for viruses alone: RR 1·31, 95% CI 0·99–1·75, observed RR for both Sa and virus 1·8, 95% CI 1·06–3·05, for an expected RR of 2·28). The negative RERI point estimate suggests that Sp carriage in children with ARI is less frequent than expected when Sa is also present, although this result is not statistically significant. A similar trend was not observed in adults, who were less likely than children to carry both Sp or Sa and to be infected by a virus. In the log-binomial adjusted regression model, only younger age was independently associated with Sp carriage (Table 4).
Table 3.
Observed and estimated relative risk of S. pneumoniae (Sp) carriage, according to S. aureus (Sa) status, and influenza or other virus exposure. Departures from additivity on the risk scale indicate biological interaction between Sa carriage and viral infection in terms of Sp carriage
| Observed | Estimated additive effect | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Neither (ref.) | Sa carriage | Virus detected | Both | |||||||
| Sp+/total | RR | Sp+/total | RR (95% CI) | Sp+/total | RR (95% CI) | Sp+/total | RR (95% CI) | RR | RERI (95% CI)† | |
| Influenza | ||||||||||
| Children | 61/139 | 1·00 | 19/32 | 1·35 (0·96–1·91) | 6/11 | 1·47 (1·01–2·14) | 2/4 | 1·14 (0·42–3·09) | 1·60 | −0·11 (−1·55 to 1·33) |
| Adults | 24/119 | 1·00 | 4/15 | 1·32 (0·53–3·29) | 3/16 | 1·15 (0·38–3·44) | 1/2 | 2·48 (0·59–10·37) | 1·25 | 1·49 (−2·25 to 5·25) |
| All ages | 85/258 | 1·00 | 23/47 | 1·49 (1·06–2·09) | 9/27 | 1·28 (0·84–1·96) | 3/6 | 1·52 (0·67–3·44) | 1·50 | −0·65 (−2·04 to 0·74) |
| Any virus* | ||||||||||
| Children | 14/43 | 1·00 | 4/7 | 1·76 (0·81–3·80) | 52/107 | 1·31 (0·99–1·75) | 17/29 | 1·80 (1·06–3·05) | 2·28 | −0·27 (−1·43 to 0·89) |
| Adults | 8/51 | 1·00 | 4/12 | 1·28 (0·20–8·23) | 19/85 | 1·28 (0·70–2·33) | 1/5 | 2·13 (0·76–5·91) | 1·70 | 0·04 (−2·88 to 2·95) |
| All ages | 22/94 | 1·00 | 21/41 | 1·78 (0·83–3·82) | 72/192 | 1·38 (1·06–1·79) | 5/12 | 2·19 (1·36–3·51) | 2·38 | −0·23 (−1·51 to 1·04) |
Sp+, Subjects carrying S. pneumoniae; RR, relative risk; CI, confidence interval; RERI, relative excess risk due to interaction.
Any virus includes all 13 virus tested for (influenza A/B, adenovirus; coronaviruses 229E, HKU1, NL63, and OC43; human metapneumovirus, parainfluenza 1, 2, 3; rhinovirus; respiratory syncytial virus).
The RERI estimates departures from additivity on the relative risk scale. A negative value implies antagonism, the null value is 0. RERI measures antagonism between virus detection (influenza or any virus) and Sa carriage in regards to Sp carriage and not antagonism between Sa and Sp.
Table 4.
Relative risk (RR) and 95% confidence interval (CI) of carriage of S. pneumoniae (Sp) by age group and detection of other pathogens in 519 episodes of acute respiratory illness from 338 participants in 143 households studied during the 2013–2014 influenza season in southeast Michigan
| Unadjusted model | Adjusted model‡ | |||
|---|---|---|---|---|
| Variable | RR (95% CI) | P | RR (95% CI) | P |
| Age group, years | ||||
| 2–3 | 4·50 (2·95–6·85) | <0·0001 | 4·36 (2·88–6·62) | <0·0001 |
| 4–6 | 3·54 (2·38–5·26) | <0·0001 | 3·41 (2·29–5·09) | <0·0001 |
| 7–12 | 2·01 (1·29–3·14) | <0·01 | 2·00 (1·28–3·13) | <0·01 |
| 13–17 | 1·78 (0·97–3·26) | 0·06 | 1·74 (0·95–3·21) | 0·07 |
| ⩾18 | Ref. | Ref. | Ref. | Ref. |
| Influenza detected* | 1·28 (0·84–1·96) | 0·25 | 1·34 (0·95–1·88) | 0·10 |
| Interaction between influenza and S. aureus (product term) | 1·58 (0·60–4·14) | 0·35 | 1·17 (0·60–2·10) | 0·73 |
| Other respiratory virus detected† | 1·29 (1·00–1·67) | 0·05 | 1·18 (0·92–1·52) | 0·20 |
| Interaction between other viruses and S. aureus (product term) | 0·84 (0·40–1·70) | 0·65 | 0·94 (0·47–1·85) | 0·85 |
| S. aureus | 1·02 (0·68–1·52) | 0·93 | 0·97 (0·68–1·37) | 0·85 |
Influenza A (pH1N1 and H3N2) and influenza B Yamagata viruses were detected by RT–PCR.
The following 11 viruses were detected by RT–PCR: adenovirus; coronaviruses 229E, HKU1, NL63, and OC43; human metapneumovirus; parainfluenza 1, 2, 3; rhinovirus; and respiratory syncytial virus.
Adjusted models included age group, Sa carriage, influenza detection, other respiratory virus detection, and product terms for the interaction between influenza and Sa and other respiratory viruses and Sa.
All 338 participants were included in the model, with no missing data. The participants' daytime activities (being in daycare, at school or at work) were not independent from age, they were therefore removed from the final regression model.
Sa-positive children were more likely than Sa-negative children to be infected with any virus (29/36, 80% vs. 107/186, 57·5%, P < 0·001). However, after adjustment for age and Sp carriage, this effect was minimal [odds ratio (OR) 1·3, 95% CI 0·75–2·18, P = 0·8]. Sp bacterial load was also higher in subjects with virus detected than those without (3·65 log genomic copies/ml vs. 3.3 log genomic copies/ml, P = 0·05). By contrast, Sa bacterial loads (3·91 vs. 3·98 log genomic copies/ml, P = 0·5) did not differ between virus-positive and virus-negative participants.
For 110 participants (54 adults, 56 children), ⩾2 ARI episodes occurred during the influenza season. Of these, 45 (41%) carried Sp (14 adults, 31 children) and nine (8·2%) carried Sa (three adults, six children) at least once. The probability of returning for a second ARI episode was significantly higher in adults carrying Sp on their first visit (39% for Sp carriers vs. 20% for non-Sp carriers, P < 0·001), but not for children (27% and 33%, respectively) (Fig. 3). After adjusting using logistic regression for age, household and daytime activity for children (staying at home, being at school, or daycare), Sp carriers were twice as likely as non-carriers (OR 2·14, 95% CI 1·07–4·26, P = 0·04) to return for a second visit. By contrast, Sa carriage was not associated with number of ARI episodes.
Fig. 3.
Proportion with carriage of Sp (a) and Sa (b) on the first visit by number of ARI episodes (⩾1) and by age group, in 338 participants with acute respiratory illness in a household (n = 143 households) study during the 2013–2014 influenza season in southeast Michigan. Sp and Sa bars represent both co-colonized individuals and single colonized individuals.
Sp and Sa carriage in households
Participating households had a median of 4·5 subjects (range 4–8), with at least one adult and two children per household, but household size did not vary by carriage of Sp or Sa. Ninety (53·8%) households accounted for two-thirds (62·7%) of participants in which Sp was detected; children in these households were of younger age than households with no Sp (average age 6·7 vs. 9·7 years for Sp-negative households, P < 0·001). By contrast, the average age of children in the 45 (27%) households with at least one Sa carrier [141 (41·7%) participants] was not different from those where no Sa was detected (7·7 vs. 8·1 years, P = 0·4).
Sp serotypes
Serotypes were identified for 66 (45%) of the 148 Sp-positive samples (Supplementary Table S1). One adult (3·2%) and nine children (10·3%) carried more than one serotype concurrently. Only one adult (3%) and five children (3%) carried a serotype included in PCV7. Serotypes 35B (three adults, 11 children), 16 F (two adults, eight children) and 33 F (three adults, four children) were the most frequently detected. Thirteen subjects (four adults, nine children) carried the same serotype consistently during 2–4 visits, with an average follow-up time of 64 days (minimum 8 days between two visits, maximum 164). In three households, the same serotypes were carried by all participants: in one household, three children carried serotype 19A on the same date, in another household, three children carried serotype 35B and all tested positive for influenza B on the same date, and in a third household both a child and his parents carried 19A on the same date.
DISCUSSION
Our household study of 338 adults and children with ARI provides some of the first estimates of the prevalence of Sp and Sa carriage in the oropharynx in individuals with acute respiratory illness. We identified three major insights into the co-colonization dynamics of Sp, Sa and ARI. First, influenza and other common respiratory viruses were positively associated with Sp carriage and an association with Sa was observed only in subjects infected by influenza. Second, we did not observe a negative interaction between Sa and Sp colonization in the oropharynx. Third, those who carried Sp on their initial visit were more likely than non-carriers to return with subsequent ARI episodes, independently of age or household. Consistent with recent findings, we found a decrease in carriage of PCV vaccine serotypes in immunized children and non-immunized adults, but no decrease in overall carriage rates, indicating a switch in the serotype distribution since the implementation of PCV vaccination and efficient herd immunity [27, 35].
Overall carriage rates of Sp in children were similar to those found in the literature, while adults' rates were higher, probably because they lived in households with young children [5, 18]. However, Sa rates were significantly lower than those found in healthy populations [10, 18, 21] where the prevalence based on culture-dependent methods is close to 37% [11], with Sa carriage decreasing with age [36]. We are not the first to note a lower Sa prevalence with respiratory symptoms: in a Portuguese daycare study Sa prevalence was (13·9%), and Sa carriage decreased with severity of rhinitis symptoms. Similarly, in a previous work from our group, Sa recovery decreased with days since onset of ARI symptoms [25]. The understanding of this phenomenon is currently unclear, although many molecular pathways, such as increased mucus production, may explain the decrease in Sa detection in the oropharynx during ARI [37].
When the first PCV7 vaccination was approved and implemented, concern arose that decreasing Sp nasopharyngeal colonization might allow overgrowth of Sa and possibly be responsible for an increase in subsequent Sa infections [21, 23]. A 2004 Israeli study of 790 healthy children showed that Sp nasopharyngeal carriage, specifically of PCV7 vaccine-type strains, was negatively associated with Sa nasal carriage in children, even after controlling for age [18]. As the authors argue, this apparent negative correlation may be due to specific bacterial antagonisms or could be a consequence of confounding effects [22, 38]. Yet, although serotype replacement has largely occurred since PCV vaccination [27, 35], there has been no corresponding increase in incidence of Sa infections in the post-vaccine era. Later works, including a recent randomized controlled trial, have confirmed that the implementation of PCV vaccinations had no impact in terms of Sa carriage despite driving Sp serotype replacement [39].
Previous epidemiological and experimental findings of an antagonism between Sa and Sp co-colonization in the face of viral infection focused on the nares and the nasopharynx [20–23]. Although our sample size is small, and we did not have an ARI-negative control group, we did not observe an antagonism between Sa and Sp in the oropharynx. However, in children, who have the highest colonization rates of both Sa and Sp, the effect of viral infection on Sp colonization was lower, although not significantly, in the presence of Sa. In all age groups Sa carriage was lower than reported in the healthy US population, despite the use of a very sensitive detection method [10, 36].
In our sample, Sp colonization occurred more frequently in the oropharynx of participants infected by influenza and/or one of 11 other viruses than in those with no virus found, whereas Sa colonization was more frequent only in participants infected by influenza. Our inability to detect a viral infection may be due to the actual lack of viral infection, a high viral detection limit, or an infection with a virus not tested here. We screened for the 13 viruses most frequently associated with ARI in both adults and children and used a highly sensitive detection system [32, 40]; yet we cannot rule out that subjects may have been infected with another virus or with very low titres of one of the viruses screened for here, which constitutes a limitation to this work.
Our findings are consistent with a previous study from our group where Sp, but not Sa nor Haemophilus influenzae colonization was associated with detection of a respiratory virus in college students [25], and the Portuguese daycare study which showed an increase in Sp colonization, but not of Sa or H. influenzae, in children with respiratory symptoms [24]. The association of Sp colonization and viral detection may be the consequence of Sp proliferation during viral infection, as found in animal models [13, 41]. Importantly, higher Sp colonization densities are independently associated with invasive pneumococcal infection [14]. Other studies have found a positive association between Sp and other respiratory viruses, such as HMPV and RSV [12, 24, 42, 43]. This suggests specific, multifactorial, immunological consequences of viral infection that lead to increasing Sp growth following ARI. These may include aberrant innate immune responses to bacterial invaders in the immediate post viral state, uncontrolled pro- and anti-inflammatory cytokine production and diminished epithelial and mucosal defences [41].
Viral infection may also increase susceptibility to Sp acquisition, as suggested by the mechanistic transmission model previously published by our group [16], and a 2014 study which found that influenza and parainfluenza ARIs appeared to facilitate pneumococcal acquisition in young children [44]. Although the mechanisms remain unclear, knowing that Sp colonization is more frequent in the oropharynx during viral disease including influenza is an important step towards the understanding of the pathophysiology of pneumococcal infection following viral infection.
The co-colonization dynamics between Sp and respiratory viruses may work in both directions. Indeed, we found that participants carrying Sp on their first visit were more likely to return with another ARI episode, independently of age and of household (with the limit of the small number of households involved). This finding is consistent with results from animal models that found that Sp infection may increase influenza titres and viral release from infected cells [41], and in vitro data suggesting that exposure to Sp can increase susceptibility to HMPV infection [45]. Unfortunately, we did not have samples collected before the onset of ARI, which would have allowed us to characterize the impact of Sp colonization on the risk of ARI. We plan to specifically address this question in future studies.
In summary, our study complements the growing literature regarding the complex dynamics of co-colonization between Sp and Sa and respiratory viral illnesses in animal and in vitro models by adding results of co-colonization in the oropharynx from a household study in adults and children with ARI. Future studies should test for Sp carriage prior to the onset of ARI, so that the risk of ARI given Sp colonization can be estimated. Understanding these complex relationships is an essential step towards helping clinicians prevent pneumococcal and staphylococcal infections in the aftermath of acute respiratory viral infections.
ACKNOWLEDGEMENTS
This work was made possible thanks to an Advancing Science through Pfizer-Investigator Research (ASPIRE) Award 2013 awarded to Betsy Foxman. The Household Influenza Vaccine Effectiveness (HIVE) study is supported by the Centers for Disease Control and Prevention (U01 IP000474) and the National Institute of Allergy and Infectious Diseases (R01 AI097150).
V.dL. would like to thank the Philippe Foundation and the Assistance-Publique Hôpitaux de Paris for financial support.
DECLARATION OF INTEREST
None.
Supplementary material
For supplementary material accompanying this paper visit https://doi.org/10.1017/S0950268816001473.
click here to view supplementary material
REFERENCES
- 1.Morens DM, Taubenberger JK, Fauci AS. Predominant role of bacterial pneumonia as a cause of death in pandemic influenza: implications for pandemic influenza preparedness. Journal of Infectious Diseases 2008; 198: 962–970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Weinberger DM, et al. Impact of the 2009 influenza pandemic on pneumococcal pneumonia hospitalizations in the United States. Journal of Infectious Diseases 2012; 205: 458–465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Mertz D, et al. Exclusive Staphylococcus aureus throat carriage: at-risk populations. Archives of Internal Medicine 2009; 169: 172–178. [DOI] [PubMed] [Google Scholar]
- 4.Nilsson P, Ripa T. Staphylococcus aureus throat colonization is more frequent than colonization in the anterior nares. Journal of Clinical Microbiology 2006; 44: 3334–3339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Peerbooms PGH, et al. Nasopharyngeal carriage of potential bacterial pathogens related to day care attendance, with special reference to the molecular epidemiology of Haemophilus influenzae. Journal of Clinical Microbiology 2002; 40: 2832–2836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bogaert D, De Groot R, Hermans PWM. Streptococcus pneumoniae colonisation: the key to pneumococcal disease. Lancet Infectious Diseases 2004; 4: 144–154. [DOI] [PubMed] [Google Scholar]
- 7.Cardozo DM, et al. Prevalence and risk factors for nasopharyngeal carriage of Streptococcus pneumoniae among adolescents. Journal of Medical Microbiology 2008; 57: 185–189. [DOI] [PubMed] [Google Scholar]
- 8.Rawlings BA, Higgins TS, Han JK. Bacterial pathogens in the nasopharynx, nasal cavity, and osteomeatal complex during wellness and viral infection. American Journal of Rhinology & Allergy 2013; 27: 39–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Hill PC, et al. Transmission of Streptococcus pneumoniae in rural Gambian villages: a longitudinal study. Clinical Infectious Diseases 2010; 50: 1468–1476. [DOI] [PubMed] [Google Scholar]
- 10.Mainous AG, et al. Nasal carriage of Staphylococcus aureus and methicillin-resistant S. aureus in the United States, 2001–2002. Annals of Family Medicine 2006; 4: 132–137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Mertz D, et al. Throat swabs are necessary to reliably detect carriers of Staphylococcus aureus. Clinical Infectious Diseases 2007; 45: 475–477. [DOI] [PubMed] [Google Scholar]
- 12.Kukavica-Ibrulj I, et al. Infection with human metapneumovirus predisposes mice to severe pneumococcal pneumonia. Journal of Virology 2009; 83: 1341–1349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.McCullers JA, et al. Influenza enhances susceptibility to natural acquisition of and disease due to Streptococcus pneumoniae in ferrets. Journal of Infectious Diseases 2010; 202: 1287–1295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Wolter N, et al. High nasopharyngeal pneumococcal density, increased by viral coinfection, is associated with invasive pneumococcal pneumonia. Journal of Infectious Diseases 2014; 210: 1649–1657. [DOI] [PubMed] [Google Scholar]
- 15.Shrestha SS, et al. Estimating the burden of 2009 pandemic influenza A(H1N1) in the United States (April 2009-April 2010). Clinical Infectious Diseases 2011; 52 (Suppl. 1): S75–82. [DOI] [PubMed] [Google Scholar]
- 16.Shrestha S, et al. Identifying the interaction between influenza and pneumococcal pneumonia using incidence data. Science Translational Medicine 2013; 5: 191ra84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Diavatopoulos DA, et al. Influenza A virus facilitates Streptococcus pneumoniae transmission and disease. FASEB Journal 2010; 24: 1789–1798. [DOI] [PubMed] [Google Scholar]
- 18.van den Bergh MR, et al. Associations between pathogens in the upper respiratory tract of young children: interplay between viruses and bacteria. PLoS ONE 2012; 7: e47711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.McCullers JA. Do specific virus-bacteria pairings drive clinical outcomes of pneumonia? Clinical Microbiology & Infection 2013; 19: 113–118. [DOI] [PubMed] [Google Scholar]
- 20.Regev-Yochay G, et al. Interference between Streptococcus pneumoniae and Staphylococcus aureus: In vitro hydrogen peroxide-mediated killing by Streptococcus pneumoniae. Journal of Bacteriology 2006; 188: 4996–5001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Regev-Yochay G, et al. Association between carriage of Streptococcus pneumoniae and Staphylococcus aureus in children. Journal of the American Medical Association 2004; 292: 716–720. [DOI] [PubMed] [Google Scholar]
- 22.Regev-Yochay G, et al. The pneumococcal pilus predicts the absence of Staphylococcus aureus co-colonization in pneumococcal carriers. Clinical Infectious Diseases 2009; 48: 760–763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Bogaert D, et al. Colonisation by Streptococcus pneumoniae and Staphylococcus aureus in healthy children. Lancet 2004; 363: 1871–1872. [DOI] [PubMed] [Google Scholar]
- 24.Rodrigues F, et al. Relationships between rhinitis symptoms, respiratory viral infections and nasopharyngeal colonization with Streptococcus pneumoniae, Haemophilus influenzae and Staphylococcus aureus in children attending daycare. Pediatric Infectious Disease Journal 2013; 32: 227–232. [DOI] [PubMed] [Google Scholar]
- 25.Davis B, et al. Influenza-like illness is associated with increased Streptococcus pneumoniae carriage among otherwise healthy college students. Abstract no. 618. Program Abstracts of the Society for Epidemiologic Research. 47th Annual Meeting 2014.
- 26.Monto AS, et al. Frequency of acute respiratory illnesses and circulation of respiratory viruses in households with children over three surveillance seasons. Journal of Infectious Diseases 2014; 210: 1792–1799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Loughlin AM, et al. Direct and indirect effects of PCV13 on nasopharyngeal carriage of PCV13 unique pneumococcal serotypes in Massachusetts’ children. Pediatric Infectious Disease Journal 2014; 33: 504–510. [DOI] [PubMed] [Google Scholar]
- 28.Centers for Disease Control and Prevention (CDC). Prevention and control of seasonal influenza with vaccines. Recommendations of the Advisory Committee on Immunization Practices – United States, 2013–2014. Morbidity & Mortality Weekly Report 2013; 62: 1–43. [PubMed] [Google Scholar]
- 29.Carvalho M da GS, et al. Evaluation and improvement of real-time PCR assays targeting lytA, ply, and psaA genes for detection of pneumococcal DNA. Journal of Clinical Microbiology 2007; 45: 2460–2466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Brakstad OG, Aasbakk K, Maeland JA. Detection of Staphylococcus aureus by polymerase chain reaction amplification of the nuc gene. Journal of Clinical Microbiology 1992; 30: 1654–1660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Pai R, Gertz RE, Beall B. Sequential multiplex PCR approach for determining capsular serotypes of Streptococcus pneumoniae isolates. Journal of Clinical Microbiology 2006; 44: 124–131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Ohmit SE, et al. Influenza vaccine effectiveness in households with children during the 2012–2013 season: assessments of prior vaccination and serologic susceptibility. Journal of Infectious Diseases 2015; 211: 1519–1528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Hosmer DW, Lemeshow S. Confidence interval estimation of interaction. Epidemiology (Cambridge, Mass.) 1992; 3: 452–456. [DOI] [PubMed] [Google Scholar]
- 34.Knol MJ, et al. Estimating measures of interaction on an additive scale for preventive exposures. European Journal of Epidemiology 2011; 26: 433–438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.van Hoek AJ, et al. Pneumococcal carriage in children and adults two years after introduction of the thirteen valent pneumococcal conjugate vaccine in England. Vaccine 2014; 32: 4349–4355. [DOI] [PubMed] [Google Scholar]
- 36.Graham PL, Lin SX, Larson EL. A U.S. population-based survey of Staphylococcus aureus colonization. Annals of Internal Medicine 2006; 144: 318–325. [DOI] [PubMed] [Google Scholar]
- 37.Shuter J, Hatcher VB, Lowy FD. Staphylococcus aureus binding to human nasal mucin. Infection & Immunity 1996; 64: 310–318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Reiss-Mandel A, Regev-Yochay G. Staphylococcus aureus and Streptococcus pneumoniae interaction and response to pneumococcal vaccination: Myth or reality? Human Vaccines & Immunotherapeutics 2016; 12: 351–357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Lewnard JA, et al. Epidemiological markers for interactions among Streptococcus pneumoniae, Haemophilus influenzae, and Staphylococcus aureus in upper respiratory tract carriage. Journal of Infectious Diseases 2016; 213: 1596–1605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Sakthivel SK, et al. Comparison of fast-track diagnostics respiratory pathogens multiplex real-time RT-PCR assay with in-house singleplex assays for comprehensive detection of human respiratory viruses. Journal of Virological Methods 2012; 185: 259–266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Short KR, et al. Interactions between Streptococcus pneumoniae and influenza virus: a mutually beneficial relationship? Future Microbiology 2012; 7: 609–624. [DOI] [PubMed] [Google Scholar]
- 42.Stark JM, et al. Decreased bacterial clearance from the lungs of mice following primary respiratory syncytial virus infection. Journal of Medical Virology 2006; 78: 829–838. [DOI] [PubMed] [Google Scholar]
- 43.Weinberger DM, et al. Association between respiratory syncytial virus activity and pneumococcal disease in infants: a time series analysis of US hospitalization data. PLoS Medicine 2015; 12: e1001776. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Grijalva CG, et al. The role of influenza and parainfluenza infections in nasopharyngeal pneumococcal acquisition among young children. Clinical Infectious Diseases 2014; 58: 1369–1376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Verkaik NJ, et al. Streptococcus pneumoniae exposure is associated with human metapneumovirus seroconversion and increased susceptibility to in vitro HMPV infection. Clinical Microbiology & Infection 2011; 17: 1840–1844. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
For supplementary material accompanying this paper visit https://doi.org/10.1017/S0950268816001473.
click here to view supplementary material