SUMMARY
Tuberculosis (TB) in older people is a significant public health problem in low TB-incidence countries. Older persons have increased TB incidence, higher reactivation and mortality. A delay in diagnosis and initiation of TB treatment in patients with atypical clinical and radiological features is a significant factor of widespread transmission. This study aimed to evaluate the cost-effectiveness of interferon-gamma release assays [IGRAs; QuantiFERON®-TB Gold In-Tube (QFT) and T-SPOT®.TB (T-SPOT)] compared to the tuberculin skin test (TST) and chest X-ray (CXR) examination for TB screening for nursing homes. Decision trees and Markov models were constructed using a societal perspective on a lifetime horizon. Seven strategies: no screening, TST, QFT, T-SPOT, TST followed by QFT, TST followed by T-SPOT, and CXR were considered. QFT [US$ 401·9, 4·36 707 QALY (year 2014 values)] was the most cost-effective at the willingness-to-pay level of US$ 50 000/QALY gained. TST followed by QFT was the most cost-effective in residents with comorbidities. CXR was less cost-effective. Cost-effectiveness was sensitive to latent TB infection (LTBI) rate and bacillus Calmette-Guérin vaccination rate. Effective LTBI screening using IGRA is recommended to prevent TB transmission not only in nursing homes but also in local communities in low-incidence countries.
Key words: Cost-effectiveness study, public health, screening programme, transmission, tuberculosis (TB)
INTRODUCTION
Tuberculosis (TB) in older people (aged ⩾65 years) is a significant public health problem in low TB-incidence countries, especially in nursing homes and long-term care facilities [1–6]. Ageing is accompanied with TB risk factors; malnutrition, sarcopenia, progressive reduction in cell-mediated immune function and co-existing illnesses such as diabetes mellitus, chronic renal failure, malignancy and antineoplastic chemotherapy [1–4]. Elderly persons have increased incidence of TB, higher TB reactivation and higher TB mortality. Active TB at the time of arrival at the nursing home, reactivation of latent tuberculosis infection (LTBI) and transmission within the nursing home are causes of TB in the elderly. The low treatment completion proportion and high risk of adverse reaction due to LTBI treatment are also seen in the elderly population [3, 4]. A delay in diagnosis and initiation of TB treatment with atypical clinical and radiological features is a significant factor for widespread transmission in nursing homes and long-term care facilities [5]. A TB outbreak needs large-scale contact screening to detect LTBI and to control TB in nursing-home residents, healthcare workers, their staff and persons in the local community after prolonged TB exposure [1, 2, 6].
Two Mycobacterium tuberculosis-specific interferon-gamma release assays (IGRAs), QuantiFERON®-TB Gold In-Tube (QFT; Qiagen, Germany) and T-SPOT®.TB (T-SPOT; Oxford Immunotec, UK), are available instead of the tuberculin skin test (TST) as new methods for diagnosing LTBI. IGRAs neither influence by bacillus Calmette-Guérin (BCG) vaccination nor have booster phenomenon, unlike TST. They have excellent accuracy with higher sensitivities and specificities than those of TST, especially in BCG-vaccinated individuals [7–9]. However, the purchase cost of IGRAs are higher than those of TST and chest X-ray (CXR) examination.
The global proportion of older people increased from 9·2% in 1990 to 11·7% in 2013 and will continue to grow as a proportion of the world's population, reaching 21·1% by 2050 [10]. Cost-effectiveness regarding the use of IGRAs for TB screening for nursing homes warrants evaluation as a TB policy control measure.
In this study, cost-effectiveness of TB screening using IGRA (QFT or T-SPOT), compared to TST, TST followed by QFT or T-SPOT, CXR for active TB screening, and no screening was assessed to evaluate the optimal entry method for older persons to nursing homes.
METHODS
Target population
The target population was a hypothetical cohort of 84-year-old residents and those with comorbidities such as HIV infection, diabetes mellitus and chronic kidney disease in nursing homes using a societal perspective on a lifetime horizon. Nursing homes are defined as institutions that provide healthcare to people who are unable to manage independently in the community. The average age at nursing-home entry is 84 years and the average time spent living there is 4 years [11]. In Japan almost all the elderly have received BCG vaccination.
As this was a modelling study with all inputs and parameters derived from published literature, ethical approval was not required.
Decision trees and Markov models
The following seven clinical states were included in this model to represent the possible clinical states in the target populations: (i) well (no LTBI, no TB); (ii) LTBI; (iii) LTBI, taking LTBI treatment without complication; (iv) LTBI, taking LTBI treatment with liver dysfunction; (v) drug-sensitive TB (DS-TB) during TB treatment and before; (vi) multidrug resistant tuberculosis (MDR-TB) during MDR-TB treatment and before; (vii) dead. Decision-analytical calculations were performed using TreeAge Pro Healthcare Module 2012 (TreeAge Software Inc., USA). Each cycle length was 1 year.
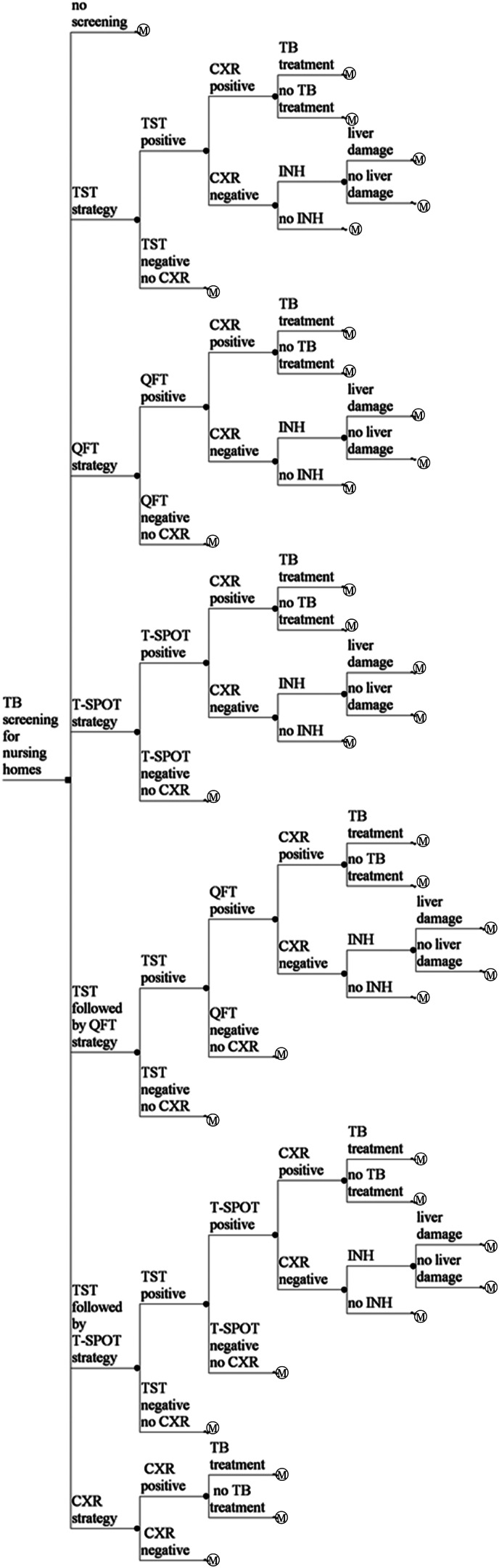
Decision trees and Markov models were developed for seven strategies; no screening, TST, QFT, T-SPOT, TST followed by QFT, TST followed by T-SPOT, and CXR (Fig. 1). Per-person cost and effectiveness were calculated. The incremental cost effectiveness ratio (ICER) of each screening arm was applied and compared. The rates of adherence of chemoprophylaxis, liver dysfunction induced by chemoprophylaxis, and completion of DS-TB and MDR-TB treatments were considered. Markov models that took into account comorbidities such as HIV infection, diabetes mellitus and chronic kidney disease were also constructed with the lower test sensitivities and their relative risks of reactivation rates.
No screening
TST strategy. A nursing-home resident undergoes TST testing. If TST induration diameter is ⩾5 mm in those without BCG vaccination and ⩾10 mm in those with BCG vaccination, the resident undergoes CXR. If active TB is suspected based on CXR, and subsequent smears, cultures and drug sensitivity test of sputum examination are performed, the resident is treated with the standard 6-month protocol for DS-TB or the protocol for MDR-TB. If active TB is not detected by CXR, the resident receives 9-month isoniazid (INH) chemoprophylaxis. If TST induration diameter is <5 mm in those without BCG vaccination and <10 mm in those with BCG vaccination, the resident does not require follow-up. The proportion of residents for whom the TST was performed and read was 1·0.
IGRA (QFT or T-SPOT) strategy. A nursing-home resident undergoes IGRA testing. If the IGRA is positive, active TB is suspected based on CXR and subsequent smears, and cultures and drug sensitivity test of sputum examination are performed, the resident is treated with the standard 6-month protocol for DS-TB or the protocol for MDR-TB. If the IGRA is positive and active TB is not detected by CXR, the resident receives 9-month INH chemoprophylaxis. If the IGRA is negative, the resident does not require follow-up.
TST followed by IGRA (QFT or T-SPOT) strategy. A nursing-home resident undergoes TST testing. If TST induration diameter is ⩾5 mm in those without BCG vaccination and ⩾10 mm in those with BCG vaccination, the resident undergoes IGRA testing. If the IGRA is positive, active TB is suspected based on CXR and subsequent smears, and cultures and drug sensitivity test of sputum examination are performed, the resident is treated with the standard 6-month protocol for DS-TB or the protocol for MDR-TB. If the IGRA is positive and active TB is not detected by CXR, the resident receives 9-month INH chemoprophylaxis. If the IGRA is negative, the resident does not require follow-up. If TST induration diameter is <5 mm in those without BCG vaccination and <10 mm in those with BCG vaccination, the resident does not require follow-up.
CXR strategy. A nursing-home resident undergoes a CXR test. If CXR is positive, active TB is suspected based on CXR and subsequent smears, cultures and drug sensitivity test of sputum examination are performed, the resident is treated with the standard 6-month protocol for DS-TB or the protocol for MDR-TB. If CXR is negative, the resident does not require follow-up.
Fig. 1.
Simplified illustration of the decision trees. A square node represents the decision node. A circular node represents a chance node. Branches from a chance node represent possible outcomes. An M○ node represents a Markov node. QFT, QuantiFERON®-TB Gold In-Tube; TB, tuberculosis; T-SPOT, T-SPOT®.TB; TST, tuberculin skin test; CXR, chest X-ray examination; INH, 9-month INH chemoprophylaxis protocol for latent tuberculosis infection.
Probabilities, costs, effectiveness, utilities and other assumptions
All data were collected using Medline. A search of the literature published from 1980 to 2 April 2016 was undertaken to use incremental cost-effectiveness analysis.
Prevalence of LTBI and TB, probability of TB patients having MDR-TB, relative risk of TB in the elderly, adherence rate of chemoprophylaxis, probability of hepatotoxicity induced by chemoprophylaxis, efficacy of chemoprophylaxis protocol, the completion rates of DS-TB and MDR-TB treatments, recurrence rates of DS-TB and MDR-TB after treatment and mortality rates of DS-TB and MDR-TB were derived from the published literature [2, 12–23]. The BCG vaccination rate was 0·93 in Japan in 2012 [24]. Age-specific all-cause mortality rates were obtained from Japanese life tables. Data from the meta-analyses, which included studies from numerous low-incidence countries, were used to determine the sensitivities and specificities of TST, QFT and T-SPOT [7–9, 26–29]. The sensitivity and specificity of CXRs were obtained from the published literature [30]. The lower test sensitivities and the relative risks of reactivation rates in the elderly with comorbidities such as HIV infection, diabetes mellitus and chronic kidney disease were also obtained from the published literature [23, 26–29].
Cost data were collected using a societal perspective. All costs were adjusted to 2014 Japanese yen, using the medical care component of the Japanese consumer price index and were converted to US dollars (US$), using the Organisation for Economic Cooperation and Development (OECD) purchasing power parity rate in 2014 (1 US$ = ¥105·8) [31, 32]. The cost of TST screening included labour costs for two physician visits and the TST reagents. The costs of QFT and T-SPOT screening included the screening kits, one physician visit, and the labour costs for laboratory technicians [31, 33]. The cost of CXR screening included the material cost of CXR, one physician visit, and the labour costs for radiology technicians [31, 33]. The costs of TB treatment, 9-month INH chemoprophylaxis and treatment of liver dysfunction caused by chemoprophylaxis were determined from the national fee schedule in Japan [31] (Table 1). The costs of smears, cultures and drug sensitivity testing of sputum examinations were also considered [31]. All costs were discounted at a fixed annual rate of 3%. Per-person costs were calculated for each strategy.
Table 1.
Baseline estimates for selected variables
| Baseline value | One-way sensitivity analysis range | Distribution in probability sensitivity analysis | References | |
|---|---|---|---|---|
| Prevalence of LTBI in nursing-home residents | 0·30 | 0·13–0·61 | Beta | [17, 18, 32] |
| Prevalence of active TB in nursing-home residents | 0·00 114 | 0·001–0·003 | Beta | [1, 17] |
| Probability of having MDR-TB in TB patients | 0·006 | 0–0·01 | Beta | [14] |
| Relative risk of TB in the elderly | 2·25 | 2·20–2·31* | Lognormal | [1] |
| Probability of recurrence of DS-TB after TB treatment | 0·0684 | 0·0182–0·1785* | Beta | [14, 15] |
| Probability of recurrence of MDR-TB after TB treatment | 0·0836 | 0·0273–0·204* | Beta | [21] |
| Relative risk of reactivation rate with HIV infection | 9·9 | 8·7–11·3 | Lognormal | [23] |
| Relative risk of reactivation rate with poorly controlled diabetes mellitus | 1·7 | 1·5–2·2* | Lognormal | [23] |
| Relative risk of reactivation rate with chronic kidney disease | 2·4 | 2·1–2·8* | Lognormal | [23] |
| Prevalence of active TB with HIV infection | 0·0113 | 0·0099–0·0129 | Beta | [1, 17, 23] |
| Prevalence of active TB with poorly controlled diabetes mellitus | 0·0019 | 0·0017–0·0025 | Beta | [1, 17, 23] |
| Prevalence of active TB with chronic kidney disease | 0·0027 | 0·0024–0·0032 | Beta | [1, 17, 23] |
| Proportion of patients had the TST performed and read | 1 | − | − | Assumption |
| Efficacy of 9H chemoprophylaxis protocol | 0·8 | 0·6–0·9 | Beta | [13, 22] |
| Adherence rate of 9H chemoprophylaxis protocol | 0·365 | 0–1 | Beta | [16] |
| Completion rate of DS-TB treatment | 0·5 | 0·3–0·7 | Beta | Assumption |
| Completion rate of MDR-TB treatment | 0·5 | 0·3–0·7 | Beta | [21] |
| Probability of developing active TB from LTBI | 0·0015 | 0·0004–0·0039 | Beta | [23] |
| Probability of drug-related hepatotoxicity by 9H chemoprophylaxis | 0·021 | 0·01–0·04 | Beta | [12] |
| TB mortality | 0·366 | 0·2–0·5 | Beta | [19] |
| Sensitivity of TST for LTBI | 0·77 | 0·71–0·82* | Beta | |
| Sensitivity of TST for LTBI with HIV infection | 0·43 | 0·37–0·5 | Beta | |
| Sensitivity of TST for LTBI with diabetes mellitus | 0·73 | 0·64–0·78 | Beta | |
| Sensitivity of TST for LTBI with chronic kidney disease | 0·68 | 0·58–0·78 | Beta | [7, 26–29] |
| Specificity of TST for LTBI (BCG-vaccinated) | 0·59 | 0·46–0·73* | Beta | |
| Specificity of TST for LTBI (non-BCG-vaccinated) | 0·97 | 0·95–0·99* | Beta | |
| Sensitivity of QFT for LTBI | 0·84 | 0·81–0·87* | Beta | |
| Sensitivity of QFT for LTBI with HIV infection | 0·61 | 0·54–0·67* | Beta | |
| Sensitivity of QFT for LTBI with diabetes mellitus | 0·80 | 0·71–0·83 | Beta | |
| Sensitivity of QFT for LTBI with chronic kidney disease | 0·75 | 0·65–0·85 | Beta | |
| Specificity of QFT for LTBI | 0·99 | 0·98–1·00* | Beta | |
| Sensitivity of T-SPOT for LTBI | 0·89 | 0·86–0·91* | Beta | [8, 9, 26–29] |
| Sensitivity of T-SPOT for LTBI with HIV infection | 0·65 | 0·56–0·74* | Beta | |
| Sensitivity of T-SPOT for LTBI with diabetes mellitus | 0·84 | 0·71–0·87 | Beta | |
| Sensitivity of T-SPOT for LTBI with chronic kidney disease | 0·79 | 0·69–0·89 | Beta | |
| Specificity of T-SPOT for LTBI | 0·98 | 0·94–0·99* | Beta | |
| Sensitivity of QFT for active TB | 0·80 | 0·75–0·84* | Beta | |
| Specificity of QFT for active TB | 0·79 | 0·75–0·82* | Beta | [25] |
| Sensitivity of T-SPOT for active TB | 0·81 | 0·78–0·84* | Beta | |
| Specificity of T-SPOT for active TB | 0·59 | 0·56–0·62* | Beta | |
| Sensitivity of CXR for active TB | 0·70 | 0·59–0·82 | Beta | |
| Specificity of CXR for active TB | 0·60 | 0·52–0·63 | Beta | [30] |
| Cost ($US 2014, 1 $US = ¥105·8) | ||||
| QFT | 59·5 | 22·5–97·1 | [31–33] | |
| T-SPOT | 59·5 | 22·5–97·1 | ||
| TST | 15·1 | 10·9–31·5 | ||
| CXR | 35·6 | 17·8–71·2 | ||
| Smears, cultures and drug sensitivity test of sputum examination | 156·8 | 78·4–313·6 | n.a. | |
| 9H chemoprophylaxis | 1219·3 | 390·2–1817·2 | ||
| Treatment of drug-induced hepatitis by chemoprophylaxis | 11 689 | 5845–23 378 | ||
| Treatment of DS-TB for 6 months | 14 612 | 7306–29 224 | ||
| Treatment of MDR-TB | 1 89 457 | 94 729–378 914 | ||
| Average physician income per hour | 49·4 | 24·7–98·8 | ||
| Average nurse income per hour | 14·1 | 7·1–28·2 | n.a. | |
| Average income per hour for radiology technician | 23·8 | 11·9–47·6 | ||
| Average income per hour for laboratory technician | 21·3 | 10·7–42·6 | ||
| Utility | ||||
| Well | 1 | |||
| LTBI | 1 | |||
| LTBI taking LTBI treatment without complication | 0·95 | |||
| LTBI taking LTBI treatment with liver dysfunction | 0·85 | n.a. | [34, 35] | |
| DS-TB during treatment and before | 0·80 | |||
| MDR-TB during treatment and before | 0·58 | |||
| Dead | 0 | |||
* 95% confidence interval.
BCG, Bacillus Calmette-Guérin; CXR, chest X-ray examination; DS-TB, drug-sensitive tuberculosis; IGRA, interferon-gamma release assay; LTBI, latent tuberculosis infection; MDR-TB, multidrug-resistant tuberculosis; QFT, QuantiFERON®-TB Gold In-Tube; TB, tuberculosis; T-SPOT, T-SPOT®.TB; TST, tuberculin skin test; 9H, 9-month isoniazid.
The main outcome measure of effectiveness was quality-adjusted life-years (QALYs). Health state utilities were calculated by using a utility weight of 0·58 for MDR-TB, 0·80 for DS-TB, 0·85 for LTBI (taking chemoprophylaxis with complication), 0·95 for LTBI (taking chemoprophylaxis without complication) and 1 each for LTBI and well (Table 1) [34, 35]. All clinical benefits were discounted at a fixed annual rate of 3%. Per-person QALYs were calculated for each strategy.
One-way sensitivity analyses and probability sensitivity analyses
One-way sensitivity analyses and probability sensitivity analyses were performed to determine which strategy yielded the greatest benefits and costs, using the ranges of probabilities, costs, relative risks and utilities. Each model variable was assigned a distribution based on the values in the literature and assumptions (Table 1). By Monte Carlo simulation distributions, the selected probabilities are in β distributions and the selected relative risks are in lognormal probabilities.
RESULTS
In the base-case analysis, QFT strategy was the most cost-effective at the willingness-to-pay level of US$ 50 000/QALY gained (US$ 401·9, 4·36 707 QALY; ICER 91·3 US$/QALY, year 2014 values). TST followed by QFT strategy (US$ 516·3, 4·36 900 QALY; ICER 59 129·9 US$/QALY) was less cost-effective than QFT strategy. CXR strategy (US$ 6683·3, 4·37 579 QALY; ICER 908 961·6 US$/QALY) was less cost-effective (Table 2). In analyses considered with higher risk of TB reactivation due to comorbidities such as HIV infection, diabetes mellitus and chronic kidney disease, TST followed by QFT strategy was more cost-effective than QFT strategy. CXR strategy was also less cost-effective (Table 2).
Table 2.
Results of seven strategies for TB screening of elderly nursing-home residents
| Strategy | Cost ($US 2014) |
Incremental Cost ($US) |
Effectiveness (QALY) |
Incremental effectiveness (QALY) |
ICER (US$/QALY) |
|---|---|---|---|---|---|
| Base case | |||||
| No screening | 123·5 | 0 | 1·31 737 | 0 | 0 |
| QFT | 401·9 | 278·5 | 4·36 707 | 3·04 970 | 91·3 |
| T-SPOT | 419·3 | 17·3 | 4·36 646 | −0·00 061 | Dominated |
| TST/QFT | 516·3 | 114·4 | 4·36 900 | 0·00 193 | 59 129·9 |
| TST/T-SPOT | 527·6 | 11·3 | 4·36 857 | −0·00 043 | Dominated |
| TST | 666·2 | 149·9 | 4·36 418 | −0·00 482 | Dominated |
| CXR | 6683·3 | 6167·0 | 4·37 579 | 0·00 678 | 908 961·6 |
| HIV infection | |||||
| TST/QFT | 491·7 | 0 | 4·34 437 | 0 | 0 |
| TST/T-SPOT | 499·0 | 7·3 | 4·34 419 | −0·00 019 | Dominated |
| QFT | 559·8 | 68·1 | 4·34 376 | −0·00 062 | Dominated |
| T-SPOT | 577·4 | 85·7 | 4·34 331 | −0·00 106 | Dominated |
| TST | 687·7 | 196·1 | 4·33 961 | −0·00 476 | Dominated |
| No screening | 761·7 | 270·0 | 1·34 383 | −3·00 054 | Dominated |
| CXR | 6801·0 | 6309·3 | 4·34 950 | 0·00 512 | 1 231 827·4 |
| Diabetes mellitus | |||||
| No screening | 171·2 | 0 | 1·31 935 | 0 | 0 |
| QFT | 408·4 | 237·2 | 4·36 556 | 3·04 621 | 77·9 |
| T-SPOT | 423·6 | 15·2 | 4·36 506 | −0·00 050 | Dominated |
| TST/QFT | 510·2 | 101·8 | 4·36 761 | 0·00 205 | 49 599·9 |
| TST/T-SPOT | 519·4 | 9·2 | 4·36 728 | −0·00 033 | Dominated |
| TST | 669·9 | 159·7 | 4·36 262 | −0·00 499 | Dominated |
| CXR | 6692·1 | 6181·9 | 4·37 382 | 0·00 621 | 995 725·1 |
| Chronic kidney disease | |||||
| No screening | 221·4 | 0 | 1·32 143 | 0 | 0 |
| QFT | 413·5 | 192·0 | 4·36 405 | 3·04 263 | 63·1 |
| T-SPOT | 428·9 | 15·4 | 4·36 356 | −0·00 049 | Dominated |
| TST/QFT | 499·7 | 86·3 | 4·36 619 | 0·00 213 | 40 410·5 |
| TST/T-SPOT | 508·6 | 8·9 | 4·36 588 | −0·00 031 | Dominated |
| TST | 669·7 | 170·0 | 4·36 100 | −0·00 519 | Dominated |
| CXR | 6701·4 | 6201·7 | 4·37 175 | 0·00 556 | 1 115 156·1 |
CXR, Chest X-ray examination; ICER, incremental cost-effectiveness ratio; QALY, quality-adjusted life-year; QFT, QuantiFERON®-TB Gold In-Tube; T-SPOT, T-SPOT®.TB; TST, tuberculin skin test.
One-way sensitivity analyses
In the base-case analysis, cost-effectiveness was sensitive to LTBI rate and BCG vaccination rate. TST followed by QFT strategy was more cost-effective than QFT strategy when the LTBI rate was >0·35 and when the BCG vaccination rate was <0·57 at the willingness-to-pay level of US$ 50 000/QALY gained (Tables 3, 4). In the analyses considered with the risk of TB reactivation due to comorbidities such as HIV infection, diabetes mellitus and chronic kidney disease, QFT strategy was more cost-effective than TST followed by QFT strategy when the LTBI rate was <0·18 in HIV-infected residents, 0·30 in diabetes mellitus residents, 0·26 in chronic kidney disease residents, and when the BCG vaccination rate was >0·95 in diabetes mellitus residents at the willingness-to-pay level of US$ 50 000/QALY gained.
Table 3.
Sensitivity analysis of LTBI rate
| LTBI rate |
Strategy | Cost (US$ 2014) |
Effectiveness (QALY) |
Incremental cost (US$) |
Incremental effectiveness (QALY) |
ICER (US$/QALY) |
|---|---|---|---|---|---|---|
| 0·13 | No screening | 106·9 | 0·86 278 | 0·0 | 0·00 000 | 0·0 |
| 0·13 | QFT | 292·6 | 4·37 178 | 185·7 | 3·50 900 | 52·9 |
| 0·13 | T-SPOT | 304·7 | 4·37 144 | 12·1 | −0·00 034 | Dominated |
| 0·13 | TST/QFT | 420·0 | 4·37 260 | 127·3 | 0·00 082 | 156 009·1 |
| 0·13 | TST/T-SPOT | 426·7 | 4·37 238 | 6·7 | −0·00 021 | Dominated |
| 0·13 | TST | 615·3 | 4·36 761 | 195·3 | −0·00 499 | Dominated |
| 0·13 | CXR | 6683·3 | 4·37 560 | 6263·3 | 0·00 300 | 2 086 461·0 |
| 0·226 | No screening | 110·7 | 0·99 315 | 0·0 | 0·00 000 | 0·0 |
| 0·226 | QFT | 354·4 | 4·36 912 | 243·7 | 3·37 597 | 72·2 |
| 0·226 | T-SPOT | 369·4 | 4·36 863 | 15·0 | −0·00 049 | Dominated |
| 0·226 | TST/QFT | 474·4 | 4·37 057 | 120·0 | 0·00 145 | 82 906·6 |
| 0·226 | TST/T-SPOT | 483·6 | 4·37 023 | 9·3 | −0·00 034 | Dominated |
| 0·226 | TST | 644·0 | 4·36 567 | 169·7 | −0·00 490 | Dominated |
| 0·226 | CXR | 6683·3 | 4·37 571 | 6208·9 | 0·00 514 | 1 208 423·0 |
| 0·322 | No screening | 127·3 | 1·41 375 | 0·0 | 0·00 000 | 0·0 |
| 0·322 | QFT | 416·1 | 4·36 646 | 288·8 | 2·95 270 | 97·8 |
| 0·322 | T-SPOT | 434·1 | 4·36 582 | 18·0 | −0·00 064 | Dominated |
| 0·322 | TST/QFT | 528·8 | 4·36 854 | 112·7 | 0·00 208 | 54 208·0 |
| 0·322 | TST/T-SPOT | 540·6 | 4·36 808 | 11·9 | −0·00 046 | Dominated |
| 0·322 | TST | 672·8 | 4·36 374 | 144·0 | −0·00 480 | Dominated |
| 0·322 | CXR | 6683·3 | 4·37 581 | 6154·5 | 0·00 727 | 846 076·9 |
| 0·418 | No screening | 143·8 | 1·83 436 | 0·0 | 0·00 000 | 0·0 |
| 0·418 | QFT | 477·8 | 4·36 380 | 333·9 | 2·52 944 | 132·0 |
| 0·418 | T-SPOT | 498·8 | 4·36 300 | 21·0 | −0·00 079 | Dominated |
| 0·418 | TST/QFT | 583·2 | 4·36 651 | 105·4 | 0·00 271 | 38 880·5 |
| 0·418 | TST/T-SPOT | 597·6 | 4·36 593 | 14·4 | −0·00 058 | Dominated |
| 0·418 | TST | 701·5 | 4·36 180 | 118·4 | −0·00 471 | Dominated |
| 0·418 | CXR | 6683·3 | 4·37 592 | 6100·1 | 0·00 941 | 648 236·1 |
| 0·514 | No screening | 160·4 | 2·25 497 | 0·0 | 0·00 000 | 0·0 |
| 0·514 | QFT | 539·5 | 4·36 114 | 379·1 | 2·10 617 | 180·0 |
| 0·514 | T-SPOT | 563·5 | 4·36 019 | 24·0 | −0·00 095 | Dominated |
| 0·514 | TST/QFT | 637·6 | 4·36 448 | 98·1 | 0·00 334 | 29 345·1 |
| 0·514 | TST/T-SPOT | 654·6 | 4·36 377 | 17·0 | −0·00 070 | Dominated |
| 0·514 | TST | 730·3 | 4·35 986 | 92·7 | −0·00 461 | Dominated |
| 0·514 | CXR | 6683·3 | 4·37 602 | 6045·7 | 0·01 155 | 523 598·1 |
| 0·61 | No screening | 177·0 | 2·67 558 | 0·0 | 0·00 000 | 0·0 |
| 0·61 | QFT | 601·2 | 4·35 847 | 424·2 | 1·68 290 | 252·1 |
| 0·61 | T-SPOT | 628·2 | 4·35 738 | 26·9 | −0·00 110 | Dominated |
| 0·61 | TST/QFT | 692·0 | 4·36 245 | 90·8 | 0·00 397 | 22 840·3 |
| 0·61 | TST/T-SPOT | 711·6 | 4·36 162 | 19·6 | −0·00 083 | Dominated |
| 0·61 | TST | 759·0 | 4·35 793 | 67·1 | −0·00 452 | Dominated |
| 0·61 | CXR | 6683·3 | 4·37 613 | 5991·3 | 0·01 368 | 437 877·4 |
CXR, Chest X-ray examination; ICER, incremental cost-effectiveness ratio; LTBI, latent tuberculosis infection; QALY, quality-adjusted life-year; QFT, QuantiFERON®-TB Gold In-Tube; T-SPOT, T-SPOT®.TB; TST, tuberculin skin test.
Table 4.
Sensitivity analysis of BCG vaccination rate
| BCG vaccination rate | Strategy | Cost (US$ 2014) |
Effectiveness (QALY) |
Incremental cost (US$) |
Incremental effectiveness (QALY) |
ICER (US$/QALY) |
|---|---|---|---|---|---|---|
| 0 | No screening | 123·5 | 1·31 737 | 0·0 | 0·00 000 | 0·0 |
| 0 | QFT | 401·9 | 4·36 707 | 278·5 | 3·04 970 | 91·3 |
| 0 | T-SPOT | 419·3 | 4·36 646 | 17·3 | −0·00 061 | Dominated |
| 0 | TST/QFT | 470·5 | 4·36 904 | 68·6 | 0·00 197 | 34 853·5 |
| 0 | TST | 474·9 | 4·36 758 | 4·4 | −0·00 146 | Dominated |
| 0 | TST/T-SPOT | 480·1 | 4·36 864 | 9·5 | −0·00 040 | Dominated |
| 0 | CXR | 6683·3 | 4·37 579 | 6212·8 | 0·00 675 | 920 317·9 |
| 0·2 | No screening | 123·5 | 1·31 737 | 0·0 | 0·00 000 | 0·0 |
| 0·2 | QFT | 401·9 | 4·36 707 | 278·5 | 3·04 970 | 91·3 |
| 0·2 | T-SPOT | 419·3 | 4·36 646 | 17·3 | −0·00 061 | Dominated |
| 0·2 | TST/QFT | 480·4 | 4·36 903 | 78·4 | 0·00 196 | 40 003·2 |
| 0·2 | TST/T-SPOT | 490·3 | 4·36 863 | 9·9 | −0·00 040 | Dominated |
| 0·2 | TST | 516·0 | 4·36 685 | 35·7 | −0·00 218 | Dominated |
| 0·2 | CXR | 6683·3 | 4·37 579 | 6202·9 | 0·00 676 | 917 866·1 |
| 0·4 | No screening | 123·5 | 1·31 737 | 0·0 | 0·00 000 | 0·0 |
| 0·4 | QFT | 401·9 | 4·36 707 | 278·5 | 3·04 970 | 91·3 |
| 0·4 | T-SPOT | 419·3 | 4·36 646 | 17·3 | −0·00 061 | Dominated |
| 0·4 | TST/QFT | 490·2 | 4·36 902 | 88·3 | 0·00 195 | 45 191·5 |
| 0·4 | TST/T-SPOT | 500·5 | 4·36 861 | 10·3 | −0·00 041 | Dominated |
| 0·4 | TST | 557·2 | 4·36 612 | 67·0 | −0·00 290 | Dominated |
| 0·4 | CXR | 6683·3 | 4·37 579 | 6193·1 | 0·00 677 | 915 419·5 |
| 0·6 | No screening | 123·5 | 1·31 737 | 0·0 | 0·00 000 | 0·0 |
| 0·6 | QFT | 401·9 | 4·36 707 | 278·5 | 3·04 970 | 91·3 |
| 0·6 | T-SPOT | 419·3 | 4·36 646 | 17·3 | −0·00 061 | Dominated |
| 0·6 | TST/QFT | 500·1 | 4·36 901 | 98·1 | 0·00 195 | 50 418·7 |
| 0·6 | TST/T-SPOT | 510·7 | 4·36 860 | 10·6 | −0·00 042 | Dominated |
| 0·6 | TST | 598·3 | 4·36 539 | 98·3 | −0·00 363 | Dominated |
| 0·6 | CXR | 6683·3 | 4·37 579 | 6183·2 | 0·00 677 | 912 978·2 |
| 0·8 | No screening | 123·5 | 1·31 737 | 0·0 | 0·00 000 | 0·0 |
| 0·8 | QFT | 401·9 | 4·36 707 | 278·5 | 3·04 970 | 91·3 |
| 0·8 | T-SPOT | 419·3 | 4·36 646 | 17·3 | −0·00 061 | Dominated |
| 0·8 | TST/QFT | 509·9 | 4·36 901 | 108·0 | 0·00 194 | 55 685·3 |
| 0·8 | TST/T-SPOT | 520·9 | 4·36 858 | 11·0 | −0·00 043 | Dominated |
| 0·8 | TST | 639·5 | 4·36 465 | 129·6 | −0·00 435 | Dominated |
| 0·8 | CXR | 6683·3 | 4·37 579 | 6173·4 | 0·00 678 | 910 542·2 |
| 1 | No screening | 123·5 | 1·31 737 | 0·0 | 0·00 000 | 0·0 |
| 1 | QFT | 401·9 | 4·36 707 | 278·5 | 3·04 970 | 91·3 |
| 1 | T-SPOT | 419·3 | 4·36 646 | 17·3 | −0·00 061 | Dominated |
| 1 | TST/QFT | 519·8 | 4·36 900 | 117·8 | 0·00 193 | 60 991·7 |
| 1 | TST/T-SPOT | 531·1 | 4·36 857 | 11·4 | −0·00 043 | Dominated |
| 1 | TST | 680·6 | 4·36 392 | 160·8 | −0·00 508 | Dominated |
| 1 | CXR | 6683·3 | 4·37 579 | 6163·6 | 0·00 679 | 908 111·4 |
BCG, Bacillus Calmette-Guérin; CXR, chest X-ray examination; ICER, incremental cost-effectiveness ratio; QALY, quality-adjusted life-year; QFT, QuantiFERON®-TB Gold In-Tube; T-SPOT, T-SPOT®.TB; TST, tuberculin skin test.
Hepatotoxicity by 9-month INH chemoprophylaxis had little impact on cost-effectiveness.
Probabilistic sensitivity analyses
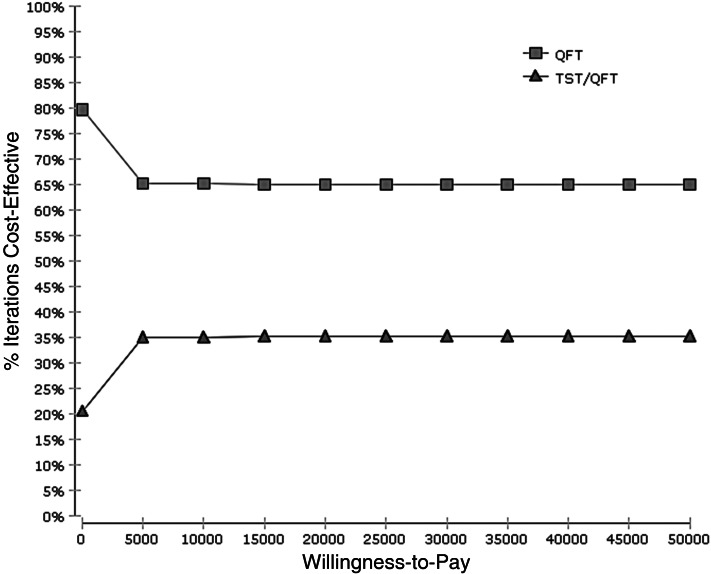
According to the Monte Carlo simulations for 10 000 trials, the cost-effectiveness acceptability curve in 84-year-old nursing-home residents demonstrated that the QFT strategy had more chance of being cost-effective than TST followed by QFT with 65% probability at the US$ 50 000 willingness-to-pay level (Fig. 2).
Fig. 2.
Cost-effectiveness acceptability curve. QFT, QuantiFERON®-TB Gold In-Tube strategy; TST/QFT, tuberculin skin test followed by QuantiFERON®-TB Gold In-Tube strategy.
DISCUSSION
This study demonstrated that using IGRA was more cost-effective for LTBI screening for nursing homes at the willingness-to-pay level of US$ 50 000/QALY gained. Highest specificity of QFT, and cost savings due to effective chemoprophylaxis by preventing TB reactivation in the elderly are the main reasons for the higher cost-effectiveness result of the QFT strategy. Cost-effectiveness was sensitive to LTBI rate and BCG vaccination rate. Hepatotoxicity by 9-month INH chemoprophylaxis had little impact on cost-effectiveness.
In this study, the main outcome measure of effectiveness was QALYs gained. The use of QALYs can combine the effects of quantity of life with quality of life in a single measure. ICER, which is calculated by using incremental costs and incremental QALYs gained can be compared to the willingness-to-pay level. Willingness to pay provides a measure of the societal value attached to a given health benefit when the values from a population are aggregated. Even if the differences in effectiveness between IGRA and TST on LTBI screening are very small, as in this case, the willingness-to-pay method using QALYs gained is very useful to evaluate cost-effectiveness.
Current entry TB screening is conducted by CXR as active TB screening in Japan. There is no data regarding the prevalence of hepatotoxicity by 9-month INH chemoprophylaxis in nursing-home residents in Japan. We derived the prevalence of hepatotoxicity and efficacy of the chemoprophylaxis protocol from the published literature. CXR examination only can detect active TB. When TB is detected by CXR screening for symptoms of TB in nursing-home residents, TB infection spreads in nursing residents and healthcare staff [36, 37]. Large-scale contact screening in nursing homes is needed [36, 37]. Some residents may die due to transmission of TB in nursing homes [5, 6, 36, 37]. This study demonstrates that active case-finding was not cost-effective and that preventive strategy with the diagnosis and treatment of LTBI was the most efficient strategy to control TB in nursing homes despite its hepatotoxicity in low TB-incidence countries.
A previous study reported the cost-effectiveness of QFT compared to CXR, and no screening compared to TB screening of the BCG-vaccinated elderly general population and demonstrated that the no-screening strategy offered the greatest cost saving for elderly populations in Japan [38]. In that study for the elderly general population, the results also demonstrated that the QFT strategy was more cost-effective than no screening when TB prevalence was >0·00 047 on the sensitivity analysis. The superiority of the QFT strategy in the present study is consistent with those results.
To the best of our knowledge, this study is the first cost-effectiveness analysis of IGRAs for TB screening of elderly nursing-home residents, compared to TST, TST followed by IGRAs, CXR and no screening using a Markov model.
Katsenos et al. showed that QFT had a significant additive value to single TST for detecting LTBI in institutionalized older adults [20]. Verma et al. demonstrated that LTBI screening with TST for the elderly was more cost-effective than CXR screening in long-term care facilities in Canada and concluded that TB screening strategies on entry to long-term care are costly [18]. We first demonstrated that using IGRA was more cost-effective than TST and CXR for entry TB screening to a nursing home.
There are several limitations to this study. First, sensitivities and specificities of TB screening kits (IGRA and TST), were obtained from meta-analyses of immunocompetent individuals, but not for older people with waning immunity. Further study of test sensitivities with waning immunity of the elderly is needed. Second, there is little data on LTBI rates using IGRAs in nursing-home residents. Further studies of the elderly based on IGRA testing are needed. Third, the harm from radiation exposure by repeating CXR was not considered in this model. Fourth, the use of rifapentine plus isoniazid for 3 months, which had a higher treatment completion rate, was not considered for chemoprophylaxis regimen of nursing-home residents in this model. Further, long-term safety monitoring research and a cost-effectiveness study using rifapentine plus isoniazid for 3 months is required. Fifth, the epidemiology of TB in the elderly needs to be dealt with in much more detail in order to make a more convincing case for TB policy change. Sixth, there is no method for diagnosing whether LTBI differentiates first infection with TB from reinfection. Seventh, there are little epidemiological studies of TB outbreaks in nursing homes. Finally, there are different costs and medical systems in each country. Further cost-effectiveness studies will be needed for each country using each cost.
CONCLUSIONS
QFT [US$ 401·9, 4·36 707 QALY (year 2014 values)] was the most cost-effective at the willingness-to-pay level of US$ 50 000/QALY gained. TST followed by QFT was the most cost-effective in residents with comorbidities. CXR was less cost-effective. Effective LTBI screening using IGRA is recommended to prevent TB transmission not only in nursing homes but also in local communities in low-incidence countries.
DECLARATION OF INTEREST
None.
REFERENCES
- 1.Thrupp L, et al. SHEA Long-Term-Care Committee. Tuberculosis prevention and control in long-term-care facilities for older adults. Infection Control & Hospital Epidemiology 2004; 25: 1097–1108. [DOI] [PubMed] [Google Scholar]
- 2.Hochberg NS, Horsburgh CR Jr. Prevention of tuberculosis in older adults in the United States: obstacles and opportunities. Clinical Infectious Diseases 2013; 56: 1240–1247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Pratt RH, et al. Tuberculosis in older adults in the United States, 1993–2008. Journal of the American Geriatrics Society 2011; 59: 851–857. [DOI] [PubMed] [Google Scholar]
- 4.Yoshikawa TT. Important infections in elderly persons. Western Journal of Medicine 1981; 135: 441–445. [PMC free article] [PubMed] [Google Scholar]
- 5.Rajagopalan S. Tuberculosis and aging: a global health problem. Clinical Infectious Diseases 2001; 33: 1034–1039. [DOI] [PubMed] [Google Scholar]
- 6.Rajagopalan S, Yoshikawa TT. Tuberculosis in long-term-care facilities. Infection Control & Hospital Epidemiology 2000; 21: 611–615. [DOI] [PubMed] [Google Scholar]
- 7.Pai M, Zwerling A, Menzies D. Systematic review: T-cell-based assays for the diagnosis of latent tuberculosis infection: an update. Annals of Internal Medicine 2008; 149: 177–184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Diel R, Loddenkemper R, Nienhaus A. Evidence-based comparison of commercial interferon-gamma release assays for detecting active TB: a metaanalysis. Chest 2010; 137: 952–968. [DOI] [PubMed] [Google Scholar]
- 9.Diel R, et al. Interferon-γ release assays for the diagnosis of latent Mycobacterium tuberculosis infection: a systematic review and meta-analysis. European Respiratory Journal 2011; 37: 88–99. [DOI] [PubMed] [Google Scholar]
- 10.Department of Economic and Social Affairs Population Division of the United Nations. World population, Aging 2013.New York, 2013. (http://www.un.org/en/development/desa/population/publications/pdf/ageing/WorldPopulationAgeing2013.pdf). Accessed 2 April 2016.
- 11.Ministy of Health, Labour and Welfare. The current institution services of the Long-term care insurance system in Japan. 2013. (http://www.mhlw.go.jp/file.jsp?id=146267&name=2r98520000033t91_1.pdf) [in Japanese]. Accessed 2 April 2016.
- 12.Fountain FF, et al. Isoniazid hepatotoxicity associated with treatment of latent tuberculosis infection: a 7-year evaluation from a public health tuberculosis clinic. Chest 2005; 128: 116–123. [DOI] [PubMed] [Google Scholar]
- 13.International Union Against Tuberculosis Committee on Prophylaxis. Efficacy of various durations of isoniazid preventive therapy for tuberculosis: five years of follow-up in the IUAT trial. Bulletin of the World Health Organization 1982; 60: 555–564. [PMC free article] [PubMed] [Google Scholar]
- 14.The Research Institute of Tuberculosis/JATA. The Tuberculosis Surveillance Center. Statistics of TB (http://www.jata.or.jp/rit/ekigaku/en). Accessed 2 April 2016.
- 15.Horsburgh CR Jr., et al. Revisiting rates of reactivation tuberculosis: a population-based approach. American Journal of Respiratory and Critical Care Medicine 2010; 182: 420–425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Horsburgh CR Jr., et al. Tuberculosis Epidemiologic Studies Consortium. Latent TB infection treatment acceptance and completion in the United States and Canada. Chest 2010; 137: 401–409. [DOI] [PubMed] [Google Scholar]
- 17.Wang CS, et al. The impact of age on the demographic, clinical, radiographic characteristics and treatment outcomes of pulmonary tuberculosis patients in Taiwan. Infection 2008; 36: 335–340. [DOI] [PubMed] [Google Scholar]
- 18.Verma G, Chuck AW, Jacobs P. Tuberculosis screening for long-term care: a cost-effectiveness analysis. International Journal of Tuberculosis and Lung Disease 2013; 17: 1170–1177. [DOI] [PubMed] [Google Scholar]
- 19.Tuberculosis Surveillance Center; RIT; JATA. Tuberculosis annual report 2012 – (4) Tuberculosis treatment and outcomes [in Japanese]. Kekkaku 2014; 89: 825–834. [PubMed] [Google Scholar]
- 20.Katsenos S, et al. Use of interferon-gamma release assay for latent tuberculosis infection screening in older adults exposed to tuberculosis in a nursing home. Journal of the American Geriatrics Society 2011; 59: 858–862. [DOI] [PubMed] [Google Scholar]
- 21.Ormerod LP. Multidrug-resistant tuberculosis (MDR-TB): epidemiology, prevention and treatment. British Medical Bulletin 2005; 73–74: 17–24. [DOI] [PubMed] [Google Scholar]
- 22.Lobue P, Menzies D. Treatment of latent tuberculosis infection: an update. Respirology 2010; 15: 603–22. [DOI] [PubMed] [Google Scholar]
- 23.Horsburgh CR. Jr. Priorities for the treatment of latent tuberculosis infection in the United States. New England Journal of Medicine 2004; 350: 2060–2067. [DOI] [PubMed] [Google Scholar]
- 24.Ministry of Health, Labour and Welfare. BCG vaccination rate in Japan (http://www.mhlw.go.jp/topics/bcg/other/5.html) [in Japanese]. Accessed 17 January 2016.
- 25.Sester M, et al. Interferon-γ release assays for the diagnosis of active tuberculosis: a systematic review and meta-analysis. European Respiratory Journal 2011; 37: 100–111. [DOI] [PubMed] [Google Scholar]
- 26.Kowada A. Cost effectiveness of the interferon-γ release assay for tuberculosis screening of hemodialysis patients. Nephrolgy Dialysis Transplantation 2013; 28: 682–688. [DOI] [PubMed] [Google Scholar]
- 27.Kowada A. Cost effectiveness of interferon-γ release assay for TB screening of HIV positive pregnant women in low TB incidence countries. Journal of Infection 2014; 68: 32–42. [DOI] [PubMed] [Google Scholar]
- 28.Dobler CC, Flack JR, Marks GB. Risk of tuberculosis among people with diabetes mellitus: an Australian nationwide cohort study. British Medical Journal Open 2012; 2 e000666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Faurholt-Jepsen D, et al. Diabetes is associated with lower tuberculosis antigen-specific interferon gamma release in Tanzanian tuberculosis patients and non-tuberculosis controls. Scandinavian Journal of Infectious Diseases 2014; 46: 384–391. [DOI] [PubMed] [Google Scholar]
- 30.Tattevin P, et al. The validity of medical history, classic symptoms, and chest radiographs in predicting pulmonary tuberculosis: derivation of a pulmonary tuberculosis prediction model. Chest 1999; 115: 1248–1253. [DOI] [PubMed] [Google Scholar]
- 31.Igakutsushin-sya. National fee schedule and Medical insurance reimbursement table in Japan [in Japanese]. Tokyo: Igakutsushin-sya, 2010. [Google Scholar]
- 32.World Health Organization. Guidelines on the Management of Latent Tuberculosis Infection. Geneva, World Health Organization, 2015. [PubMed] [Google Scholar]
- 33.Ministry of Health, Labor and Welfare. Basic survey on wage structure 2014 (http://www.e-stat.go.jp/SG1/estat/GL08020103.do?_toGL08020103_&tclassID=000001054146&cycleCode=0&requestSender=estat) [in Japanese]. Accessed 2 April 2016.
- 34.Guo N, Marra F, Marra CA. Measuring health-related quality of life in tuberculosis: a systematic review. Health and Quality of Life Outcomes 2009; 7: 14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Dion MJ, et al. Feasibility and reliability of health-related quality of life measurements among tuberculosis patients. Quality of Life Research 2004; 13: 653–665. [DOI] [PubMed] [Google Scholar]
- 36.Ijaz K, et al. Unrecognized tuberculosis in a nursing home causing death with spread of tuberculosis to the community. Journal of the American Geriatrics Society 2002; 50: 1213–1218. [DOI] [PubMed] [Google Scholar]
- 37.Chitnis AS, et al. Trends in tuberculosis cases among nursing home residents, California, 2000 to 2009. Journal of the American Geriatrics Society 2015; 63: 1098–1104. [DOI] [PubMed] [Google Scholar]
- 38.Kowada A, et al. Cost effectiveness of interferon-gamma release assay versus chest X-ray for tuberculosis screening of BCG-vaccinated elderly populations. Molecular Diagnosis & Therapy 2010; 14: 229–236. [DOI] [PubMed] [Google Scholar]