Abstract
Background:
An improved understanding of the clinico-epidemiology of bronchiolitis hospitalizations, a clinical surrogate of respiratory syncytial virus (RSV) disease, is critical to inform public health strategies for mitigating the in-patient burden of bronchiolitis in early life.
Methods:
A retrospective chart review was conducted of all bronchiolitis first admissions (N = 295) to the Children’s Hospital at Dartmouth-Hitchcock, CHaD, between 1 November 2010 and 31 October 2017 using the relevant International Classification of Diseases (ICD)-9 and ICD-10 codes for this illness. Abstracted data included laboratory confirmation of RSV infection, severity of illness, duration of hospitalization, age at admission in days, weight at admission, prematurity, siblings, and relevant medical pre-existing conditions.
Results:
Admissions for bronchiolitis were strongly associated with age of the child, the calendar month of an infant’s birth, and the presence of older children in the family. Medical risk factors associated with admission included premature birth and underlying cardiopulmonary disease.
Conclusion:
The very early age of hospitalization emphasizes the high penetration of RSV in the community, by implication the limited protection afforded by maternal antibody, and the complexity of protecting infants from this infection.
Plain Language Summary
Although risks for respiratory syncytial virus (RSV)/bronchiolitis hospitalization are well described, few studies have examined, with precision, the age-related frequency and severity of RSV/bronchiolitis. We also explore the implications of RSV clinico-epidemiology for our understanding of the pathogenesis of the disease and development of optimal approaches to prevention.
Keywords: respiratory syncytial virus, bronchiolitis, hospitalization
Introduction
Bronchiolitis is a clinical syndrome characterized by rhinorrhea, cough, wheeze, and hypoxia 1 and is a major contributor to the burden of hospitalization among young children in the United States.2,3 The pathology is marked by acute inflammation and exfoliation of mucosal epithelial tissues of the lower respiratory tract. 4 Although infections with a range of bacterial and viral agents can trigger bronchiolitis, respiratory syncytial virus (RSV) is by far the most common cause in children less than 2 years of age. 5 Globally, it is estimated that RSV-associated acute lower respiratory infections claim the lives of 118,200 children under-5 each year. 6 The financial costs of the more than 3 million annual cases of RSV requiring routine in-patient and intensive care place significant burdens on affected families and health care infrastructures.7,8
Ultimately, developing a comprehensive approach to bronchiolitis prevention requires a detailed understanding of the epidemiology of RSV as it relates to the duration of protection afforded by maternal antibody and the ages at which young children can be effectively immunized. Toward this end, this study reviewed the records of all infants and children hospitalized with bronchiolitis at the Children’s Hospital at Dartmouth-Hitchcock (CHaD), between November 2010 and October 2017, encompassing seven successive RSV seasons.
Methods
Study setting
This study reviewed the medical records of infants and children less than 3 years of age hospitalized at CHaD between 1 November 2010 and 31 October 2017. Records were abstracted for admissions with the following International Classification of Diseases (ICD) primary diagnostic codes corresponding to bronchiolitis – ICD-9 466.11 or 518.81 or ICD-10 J21.0, J21.9, J96.00, J96.01, and R06.00. CHaD, based in rural Grafton County, New Hampshire, is the only comprehensive children’s hospital serving the state of New Hampshire and eastern Vermont. Ninety-three percent of individuals residing in the hospital catchment area identify as white, and 27% of children belong to families with incomes less than 200% of the federal poverty level. 9
Definitions
To avoid confounding statistics for the number of cases with the number of children, only the first admission for each child was considered. One hundred and ninety-three were referred from regional hospital emergency rooms. Thirty of these had already been hospitalized at their local hospital before transfer. All patients were rigorously screened at the DHMC Emergency Department before admission. A secondary clinical endpoint for this investigation was admission to the CHaD pediatric intensive care unit (PICU). Admission to the PICU was predicated on children meeting strict and objective criteria of (1) a fraction of inspired oxygen (FiO2) greater than 0.50 to maintain oxygen saturations above 0.90 or (2) a high flow of oxygen (i.e. >8 liters/min) to achieve a WARM respiratory score of at least 5. 10 Those classified as having cardiopulmonary disease had hemodynamically significant defects requiring ongoing cardiology care.
American Academy of Pediatrics 2014 Clinical Practice Guidelines state that ‘when clinicians diagnose bronchiolitis on the basis of history and physical examination, radiographic or laboratory studies should not be obtained routinely’. 11 Thus, microbiologic confirmation was not routinely available after 2014.
Data abstraction
Data were abstracted from charts by an experienced research nurse, and de-identified data were coded using a standardized electronic form. Collected variables included confirmation of RSV infection when available, severity of illness (i.e. general in-patient versus PICU stay), duration of hospitalization, age at admission, weight at admission, prematurity (defined as birth at less than 37 weeks gestational age), 12 twin births, day care attendance, presence of other children (<18 years of age) in family, relevant pre-existing conditions, and history of palivizumab receipt. The Committee for the Protection of Human Subjects (CPHS) Dartmouth College approved the study (STUDY00029638) and granted a waiver of informed consent for this retrospective review. The data were carefully de-identified for final analysis.
Statistical analysis
Infants admitted to the general ward versus the PICU were compared using a chi-square test for categorical variables (annual season, prematurity, plurality of birth, cardiopulmonary disease status, and palivizumab receipt). Nonparametric Wilcoxon rank sum tests were used for continuous variables (age and weight on admission). The predicted presence of an older child in the family was estimated from 2000 US census data. The average number of children per family in the United States was 1.86 children and for New Hampshire and Vermont was 1.83 and 1.81, respectively. 13 In the 2020 US Census, families with children were categorized as 38% single-child families, 40% two-child families, 15% three-child families, and 6% four or more–child families suggesting that an estimated 69% of children are the oldest child in their first year of life, while 31% will have an older sibling. 14 Data were extracted from the New Hampshire Birth Cohort as representative of a population based within the CHaD service area. Among the 2103 children in the New Hampshire Birth Cohort who were matched by sex and birthdate (i.e. within 1 month) to children hospitalized with RSV in this study, an estimated 56% were categorized as single-child families, 29% as two-child families, 10% as three-child families, and 5% as four or more–child families (Supplementary Table 1), suggesting that an estimated 75% of children are an oldest child in their first year of life, while 25% will have an older sibling.
Duration of hospitalization was compared by intensity of care (PICU versus general ward), prematurity, plurality of birth, cardiopulmonary disease status, and palivizumab receipt using Wilcoxon rank sum tests and by annual season, tertiles of age at admission, and tertiles of weight at admission by Kruskal–Wallis tests. All statistical analyses were performed in R version 3.6.3 (R Project for Statistical Computing; http://www.r-project.org).
Results
Two hundred and ninety-five children less than 3 years of age were admitted to CHaD with an initial episode of bronchiolitis over a full 7-year period from 1 November 2010 to 31 October 2017. Of the admitted children, 56% were male, and 96% were white. Only five (1.6%) of the primary hospitalizations occurred in children between the ages of 2 and 3 years at the time of admission. There were 12 occurrences when children were readmitted more than 2 weeks after their primary infection for a second episode or late complication of their initial bronchiolitis, 6 of these were in the same season and 6 in the ensuing season. There were five re-hospitalizations within 2 weeks of their initial illness suggesting unresolved primary illnesses. From 2010 to 2014 when testing for RSV was routine practice, 177 out of 181 bronchiolitis hospitalizations (98%) were microbiologically confirmed as being due to RSV. Twelve (4.5%) of the children hospitalized had previously received at least one dose of palivizumab. No deaths were recorded in this population.
Seasonal patterns of bronchiolitis hospitalizations
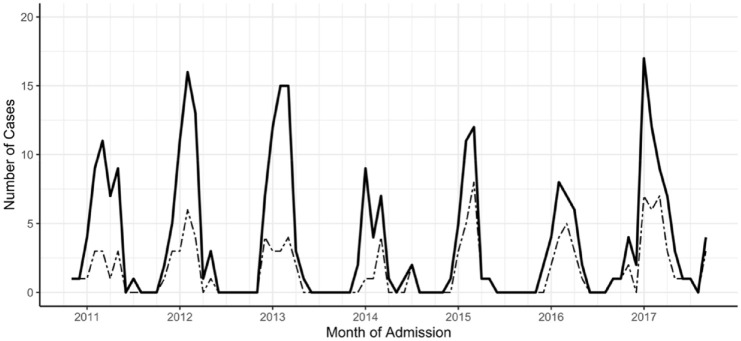
Peaks of bronchiolitis admissions occurred annually in the late winter (Figure 1). The composite epidemic curve of the seven successive seasons showed the peak months for admissions were January, February, and March, with 71% of admissions occurring in those 3 months alone. The intensity of the annual epidemics showed only minor year-to-year variation in total numbers of admissions (range: N = 26 in 2013–2014 to N = 60 in 2016–2017) and severity, as judged by percent of cases admitted to the PICU (range: 30% in 2010–2011 and 2012–2013 to 58% in 2014–2015) (Table 1). In total, bronchiolitis accounted for 1411 days of hospitalization, of which 935 (66%) were in the PICU. Duration of hospitalization did not vary significantly between the sequential seasons (Table 2).
Figure 1.
The distribution of initial bronchiolitis hospitalizations at CHaD during seven successive RSV seasons. Shown are total admissions (solid line, N = 295) and those that involved admissions to the PICU (dashed line, N = 121).
Table 1.
Rates of hospitalization and intensity of care for bronchiolitis.
| General pediatric ward | Pediatric intensive care unit | p value | ||
|---|---|---|---|---|
| Total | Median (interquartile range) or N, % | |||
| RSV Season | 0.017 | |||
| 2010–2011 | 43 | 30, 70% | 13, 30% | |
| 2011–2012 | 51 | 33, 65% | 18, 35% | |
| 2012–2013 | 53 | 37, 70% | 16, 30% | |
| 2013–2014 | 26 | 18, 69% | 8, 31% | |
| 2014–2015 | 31 | 13, 42% | 18, 58% | |
| 2015–2016 | 31 | 14, 45% | 17, 55% | |
| 2016–2017 | 60 | 29, 48% | 31, 52% | |
| TOTAL | 295 | 174, 59% | 121, 41% | |
| Age at admission (months) | 295 | 4 (1, 8) | 5 (1, 13) | 0.603 |
| Weight on admission (kg) | 295 | 6.6 (4.7, 8.4) | 6.4 (4.3, 9.9) | 0.887 |
| Prematurity | 0.156 | |||
| Term birth | 236 | 144, 61% | 92, 39% | |
| Premature birth | 59 | 30, 51% | 29, 49% | |
| Plurality of birth | 0.273 | |||
| Singleton | 274 | 164, 60% | 110, 40% | |
| Twin | 21 | 10, 48% | 11, 52% | |
| Cardiopulmonary disease | 0.015 | |||
| Yes | 31 | 12, 39% | 19, 61% | |
| No | 264 | 162, 61% | 102, 39% | |
| Palivizumab receipt | 0.519 | |||
| At least one dose | 12 | 6, 50% | 6, 50% | |
| None | 283 | 168, 59% | 115, 41% | |
RSV, respiratory syncytial virus.
Table 2.
Potential correlates of duration of hospitalization for bronchiolitis.
| Duration of hospitalization, days | p value | ||
|---|---|---|---|
| Total | Median (interquartile range) | ||
| Admission to PICU | |||
| Yes | 121 | 5 (3, 8) | 0.0001 |
| No | 174 | 2 (1, 3) | |
| Annual season | 0.076 | ||
| 2010–2011 | 43 | 2 (1, 4) | |
| 2011–2012 | 51 | 3 (1, 5) | |
| 2012–2013 | 53 | 2 (1, 5) | |
| 2013–2014 | 26 | 2 (1, 3) | |
| 2014–2015 | 31 | 3 (2, 7) | |
| 2015–2016 | 31 | 4 (2, 6) | |
| 2016–2017 | 60 | 3 (2, 6) | |
| Age at admission (months) | 0.208 | ||
| <2.5 | 99 | 3 (2, 6) | |
| 2.5–7 | 98 | 3 (1, 6) | |
| 7–32 | 98 | 3 (1, 4) | |
| Weight at admission (kg) | 0.048 | ||
| 2.1–5.04 | 99 | 3 (2, 7) | |
| 5.06–7.8 | 98 | 3 (1, 6) | |
| 7.9–22.3 | 98 | 2 (1, 4) | |
| Prematurity | 0.200 | ||
| Term birth | 236 | 3 (1, 6) | |
| Premature birth | 59 | 3 (2, 8) | |
| Plurality of birth | 0.732 | ||
| Singleton | 274 | 3 (1, 6) | |
| Twin | 21 | 3 (1, 5) | |
| Cardiopulmonary disease | 0.0001 | ||
| Yes | 31 | 6 (2, 12) | |
| No | 264 | 3 (1, 5) | |
| Palivizumab receipt | 0.607 | ||
| At least one dose | 12 | 2 (2, 8) | |
| No | 283 | 3 (1, 6) |
PICU, pediatric intensive care unit.
Age and weight at time of hospitalization
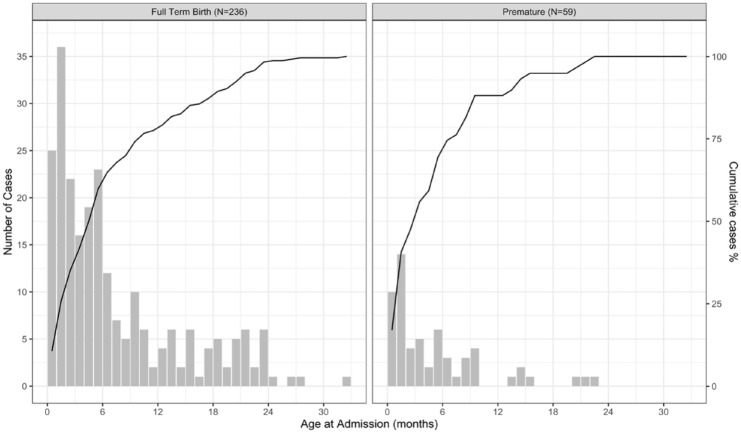
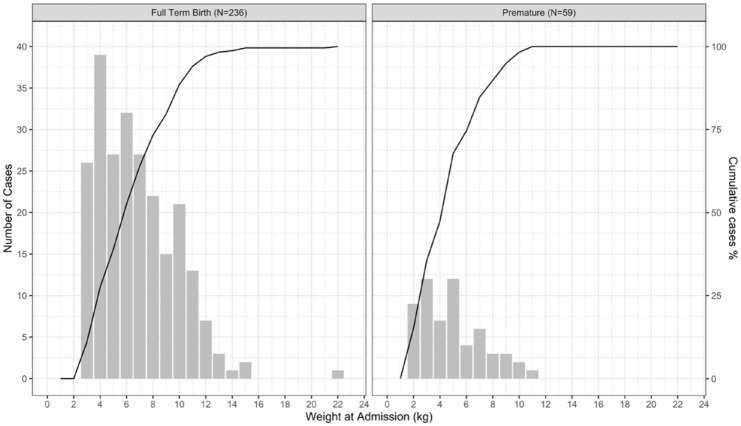
Examining the frequency of admissions by age revealed that beginning at 2 weeks following birth, admissions were most frequent within the first months of life with 111/295 (37%) of cases occurring by 3 months and 182/295 (61%) by 6 months of age. For premature infants, this trend was even more pronounced with 28/59 (47%) cases occurring by 3 months and 41/59 (69%) occurring by 6 months after birth (Figure 2). Hospitalization frequency as a function of weight on admission exhibited a linear increase up to approximately 10 kg, the median weight at 1 year of age, for both term and premature infants (Figure 3).
Figure 2.
Accrual of bronchiolitis hospitalizations at CHaD by age at admission during seven successive RSV seasons (N = 295) in term and premature infants; the line indicates the cumulative percentage of cases.
Figure 3.
Accrual of initial bronchiolitis hospitalizations at CHaD by weight at admission during seven successive RSV seasons (N = 295) in term and premature infants; the line indicates the cumulative percentage of cases.
Risk factors for hospitalization
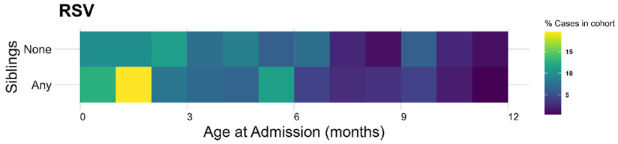
Of the 295 children admitted with bronchiolitis, 214 (72.5%) had an older sibling. This figure is very different from the 31% of children who had an older sibling estimated from the US census data and translates to a 2.4 times higher risk of bronchiolitis hospitalization if there are siblings in the family. Approached in another way we interrogated the New Hampshire Birth Cohort – a pregnancy cohort of maternal–infant dyads of New Hampshire children that are followed longitudinally. In this study even fewer children had an older sibling (25%) which translates to a 2.9-times higher risk of bronchiolitis hospitalization if there are older children in the family. The presence of an older sibling in the family also leads to hospitalization at an earlier age (Figure 4).
Figure 4.
A heat-map of age at hospitalization in the first year of life for our cohort based on the presence or absence of an older sibling in the family. The yellow color in the 2 months of life highlights the younger age of hospitalization with siblings in the family.
Functionally significant cardiopulmonary disease was present in 31/295 (10.5%) of children. Using less than 37 weeks of gestation as a definition of prematurity, 12 59/295 (20%) of the hospitalized children were born prematurely; 21/295 (7%) children had a twin sibling. On five occasions, both twins were hospitalized simultaneously.
The risk of being hospitalized with bronchiolitis was strongly influenced by the calendar month of birth. 15 Forty-seven percent of children hospitalized were born in the 4 months of October, November, December, and January. In contrast, only 22% of children hospitalized were born in April through July, despite them being predicted to have the lowest average level of persistent passively acquired maternal antibody on exposure and being alive for an entire RSV winter epidemic season during their first year of life (Supplemental Figure 1).
Severity of illness
Requiring admission to the PICU during hospitalization reflects severity of illness in a physiologically defined way. Of the hospital admissions for bronchiolitis, 121/295 (41%) required a PICU admission during their hospitalization. Neither weight, age, nor prematurity influenced the risk of admission to the PICU (Table 1). Admission to the PICU during hospitalization predicted a significantly longer hospitalization; overall, the median length of stay in ward versus PICU setting was 2 versus 5 days (Table 1). Duration of hospitalization also varied by weight on admission (p = 0.048), with the children in the highest tertile for weight on admission experiencing a shorter length of stay. However, duration of hospitalization did not vary by age or history of prematurity (Table 2). The children with pre-existing cardiopulmonary disease experienced more severe illness as reflected by higher rates of admission to PICU (Table 1) and longer duration of hospitalization (Table 2).
Discussion
A retrospective chart review was undertaken to define the age, risk factors, and duration of admission for bronchiolitis at CHaD, a smaller children’s hospital, located in a predominantly rural area in northern New England, with rigorous criteria for hospital admission and precisely specified markers of diminishing pulmonary reserve for receiving care in the PICU. Bronchiolitis is an illness presenting early in life that is strongly associated with RSV infection. In the early years of this study when testing was routine – up to 2014 – 98% of the cases were confirmed as being due to RSV. Subsequently, per guidelines of the American Academy of Pediatrics, virologic testing was not routinely performed. 11
Young children have very high risks of exposure to RSV during annual winter epidemics. 15 Estimates of the infection rate range from 40 to 70% during their first seasonal exposure.16,17 The role of birth order in determining risk of infection is explored in this article. By two different approaches the risk of bronchiolitis hospitalization has been estimated to be between 2.4 and 2.9 times higher for children who are not the first born. There are important selection biases in each of these calculations: for example, the census data are based on the national data of ‘own’ children, whereas the Birth Cohort is based on the willingness of the family to enroll. Nevertheless, the relative risks are very high, are supported by the finding of an earlier age of hospitalization, and are consistent with data from other surveys. 18 The earlier age of hospitalization in those with older siblings also speaks to the force of infection within the family setting (Figure 4).
Although we had data on day care exposure in the hospitalized group, it proved to be impossible to get accurate estimates of number of control children in the first year of life who were in day care or had an older sibling in day care or preschool in the CHaD catchment area. Definitions and sizes of these types of facilities vary widely but day care exposure is logically an additional risk factor.
As we have previously noted, a corollary of the seasonality of RSV and age at admission for bronchiolitis is that risk of hospitalization is dependent on month of birth. Our prior research indicates the risk of hospitalization is twofold higher if a child is born during the late fall-early winter months than at other times of year. 18 The striking seasonality of bronchiolitis (Supplemental Figure 1) and the subsequent relative risk of hospitalization by month of birth (Supplemental Figure 2) were replicated in this study.
The distribution of age at hospitalization by severity of illness is critical in defining preventive strategies and may give clues to the pathogenesis of bronchiolitis. By defining age at admission by number of days since birth, we refine the statement that RSV is a disease of the very young infant and contribute to the dialogue about the role of maternally transferred antibody in providing protection in the first months of life. After sparing in the first 2 weeks of life (potentially attributable to the 5- to 7-day incubation period of the disease and cocooning of the youngest infant from the general environment), the rate of hospitalization rose sharply with 37% of cases occurring in the first 3 months of life. Among prematurely born children, the rate of presentation with disease was even earlier in life, with 47% of admissions occurring by 3 months of age (Figure 3). These findings suggest a vaccine intervention targeted to be effective by 3 months of age might only prevent two-thirds of hospitalizations in infants born at term and one-half in premature infants.
Establishing causation for the early hospitalization in bronchiolitis is complex. It may be related to immunologic maturation and physiologic limitations in the size and function of the airway in the setting of a highly transmissible disease. The effect of weight on rates of admission for bronchiolitis had greater linearity than the effect of age suggesting that physical size was not the major determinant of admission. We speculate that immunologic immaturity plays more of a role in disease severity in young infants than weight – a surrogate for the diameter of the airways. The high frequency of hospitalizations in very young infants is perhaps evidence against the importance of protection from maternal antibodies as levels will be highest in aggregate in the newborn period with a steady decline with a half-life estimated at 28 days.
A further observation from Figures 2 and 3 is that at about 9 to 12 months of age and 10 kg of weight, the rate of admission shows a distinct flattening. A possible interpretation would be that at this age children have already lived through all or part of the previous year’s epidemic and that previously acquired immunity is decreasing the rates of severe disease.
Risk of hospitalization in premature infants (20% of children hospitalized) and in those with cardiopulmonary disease (10% of children hospitalized) was higher than would be expected based on regional rates of 8% of births being premature19,20 and 1% have underlying heart disease. 21 Consistent with published literature, 22 being a twin increased the risk of hospitalization with 7% of children admitted being twins – somewhat higher than the national percentage of twin births which is 3.4%. 23
Research has focused on the reduction of bronchiolitis-associated hospitalization through the amelioration of RSV-induced disease. A dogma in the RSV literature is that maternally derived or passively administered serum antibody lowers the risk of hospitalization in a dose-dependent fashion. 24 However, this conclusion is not uniformly supported in the published literature.25,26
Many of the current global approaches to preventing RSV hospitalization are predicated on protection being afforded by enhanced levels of maternal antibodies over the first months of life.
Several RSV vaccines targeting pregnant women are in development. 27 The argument has been made that maternal antibodies resulting from prior natural infection may not be reflective of highly neutralizing epitopes formed on recognition of uncleaved F protein. 28 The potential to design immunogens that express these epitopes may be a promising direction for maternal immunization.
The partial protection against hospitalization afforded by administration of palivizumab, a monoclonal antibody to the RSV F protein, and the encouraging preliminary results with nirsevimab are strong indications that serum antibody can have a protective role.29,30 No approaches to treatment of disease after onset of symptoms have altered the severity of illness. 1 Current preventive strategies focus on minimizing exposure and boosting infants’ humoral immunity to RSV to limit the progression of disease following exposure. Six infants in our review qualified for the palivizumab intervention on the basis of prematurity, while an additional six received the drug prophylactically based on cardiopulmonary disease for which they were on cardiac medication.
There also are ongoing efforts to immunize young children with live-attenuated RSV or vectors expressing RSV proteins. 31 An inactivated RSV vaccine caused enhanced illness on natural re-exposure eliminating killed vaccines from exploration. 32 Although many live vaccines are given in infancy, this also presents challenges of safety and the capacity of the young infant to mount a protective systemic and mucosal immune response. Nevertheless, these challenges are being taken up by the pharmaceutical and academic communities. 27
A limitation to our analysis is the lack of laboratory confirmation for RSV infection in the later years of the study. The high concordance of RSV with bronchiolitis in the first years studied (2010–2014, 98%) and the relative constancy of the epidemic curves throughout the 7 years are consistent with RSV being the dominant cause of the bronchiolitis hospitalizations. We recognize that other viruses cause bronchiolitis and RSV illness may be a pneumonia, which would not have been selected for in our chart review. The seasonal incidence of the bronchiolitis admissions (Figure 1) matches the described seasonality of RSV infection 4 and reassures us that attribution of bronchiolitis to RSV is a reasonable approximation. Another limitation of this review is the generalizability of the results. As the population was drawn from a predominately white rural population, the findings from this chart review may not be representative of the United States as a whole. However, our data mirror that from the Medicaid database in Tennessee, collected three decades before from a largely urban, non-white Medicaid population.18,28
In our study, young age was the primary driver of the risk of hospitalization. Thus, we could find no supportive evidence that the anticipated higher level of maternal antibody in the youngest children limited hospitalization. That statement must be tempered by the facts that this study did not incorporate a measure of individual immunity and young age may contribute increased risk of serious illness due either to impaired immune control of infection or mechanical blockage of the airways due to their small size in the very young.
Conclusion
The distribution of bronchiolitis cases demonstrates the very strong influence of age on risk of hospitalization and strongly suggests that levels of maternally derived serum antibody are not a major determinant of protection against severe RSV. Older children in the family play an important role in transmission of RSV to the index infant. Thus, the answer to prevention of bronchiolitis may lie in infant vaccination at as early an age as possible with the addition of vaccine-induced protection of the older pediatric population responsible for spreading infections to infants. Maternal vaccination for transplacental transfer of highly potent antibodies or infant prophylaxis with long-acting monoclonal antibodies may also play a role in disease prevention but is unlikely to substantively alter transmission or the frequency of infection.
Supplemental Material
Supplemental material, sj-docx-1-tai-10.1177_20499361221099447 for Bronchiolitis hospitalizations in rural New England: clues to disease prevention by Peter F. Wright, Anne G. Hoen, J. Dean Jarvis, Michael S. Zens, Erika F. Dade, Margaret R. Karagas, Juliana Taube and Elizabeth B. Brickley in Therapeutic Advances in Infectious Disease
Acknowledgments
Meave Otieno, a Geisel School of Medicine student, contributed to the data analysis. Dr Kristin E. Smith of the Dartmouth Department of Sociology was extremely helpful in conceptualizing the role of older children in the epidemiology of RSV.
Footnotes
Ethics approval and consent to participate: The Committee for the Protection of Human Subjects (CPHS) Dartmouth College approved the study (STUDY00029638) and granted a waiver of informed consent for this retrospective review.
Consent for publication: Approval from IRB included permission to publish data in a deidentified fashion
Author contribution(s): Peter F. Wright: Conceptualization; Data curation; Formal analysis; Funding acquisition; Investigation; Methodology; Project administration; Resources; Supervision; Validation; Visualization; Writing – original draft; Writing – review & editing.
Anne G. Hoen: Conceptualization; Data curation; Formal analysis; Funding acquisition; Investigation; Supervision; Visualization; Writing – review & editing.
J. Dean Jarvis: Data curation; Investigation; Validation; Writing – review & editing.
Michael S. Zens: Methodology; Software.
Erika F. Dade: Methodology; Software; Validation; Visualization.
Margaret R. Karagas: Resources; Writing – review & editing.
Juliana Taube: Data curation; Investigation; Methodology; Resources; Writing – original draft.
Elizabeth B. Brickley: Conceptualization; Data curation; Formal analysis; Methodology; Visualization; Writing – original draft; Writing – review & editing.
ORCID iD: Peter F. Wright  https://orcid.org/0000-0003-1950-6928
https://orcid.org/0000-0003-1950-6928
Funding: The authors disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: Dartmouth IDeA States Pediatric Clinical Trials Network supported the data collection and medical records review. Support was also received from an Elmer R. Pfefferkorn & Allan U. Munck Education and Research Fund award to Dr Anne Hoen.
Conflict of interest statement: The authors declared the following potential conflicts of interest with respect to the research, authorship, and/or publication of this article: Dr Wright serves as a scientific consultant to Sanofi-Pasteur, GlaxoSmithKline, and Meissa Vaccines. The other authors have no conflicts of interest relevant to this article to disclose.
Availability of data and materials: Deidentified data will be shared for further analysis to assess overall impact of bronchiolitis
Supplemental material: Supplemental material for this article is available online.
Contributor Information
Peter F. Wright, Department of Pediatrics, Dartmouth-Hitchcock Medical Center, One Medical Center Drive, Lebanon, NH 03756, USA.
Anne G. Hoen, Department of Epidemiology, Geisel School of Medicine, Dartmouth College, Hanover, NH, USA
J. Dean Jarvis, Department of Pediatrics, Dartmouth-Hitchcock Medical Center, Lebanon, NH, USA.
Michael S. Zens, Department of Epidemiology, Geisel School of Medicine, Dartmouth College, Hanover, NH, USA
Erika F. Dade, Department of Epidemiology, Geisel School of Medicine, Dartmouth College, Hanover, NH, USA
Margaret R. Karagas, Department of Epidemiology, Geisel School of Medicine, Dartmouth College, Hanover, NH, USA
Juliana Taube, Bowdoin College, Brunswick, ME, USA.
Elizabeth B. Brickley, Department of Infectious Disease Epidemiology, London School of Hygiene & Tropical Medicine, London, UK
References
- 1. Meissner HC. Viral bronchiolitis in children. N Engl J Med 2016; 374: 62–72. [DOI] [PubMed] [Google Scholar]
- 2. Shay DK, Holman RC, Newman RD, et al. Bronchiolitis-associated hospitalizations among US children, 1980-1996. JAMA 1999; 282: 1440–1446. [DOI] [PubMed] [Google Scholar]
- 3. Zhou H, Thompson WW, Viboud CG, et al. Hospitalizations associated with influenza and respiratory syncytial virus in the United States, 1993-2008. Clin Infect Dis 2012; 54: 1427–1436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Johnson JE, Gonzales RA, Olson SJ, et al. The histopathology of fatal untreated human respiratory syncytial virus infection. Mod Pathol 2007; 20: 108–119. [DOI] [PubMed] [Google Scholar]
- 5. Miller EK, Gebretsadik T, Carroll KN, et al. Viral etiologies of infant bronchiolitis, croup, and upper respiratory illness during four consecutive years. Pediatr Infect Dis J 2013; 32: 950–955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Shi T, McAllister DA, O’Brien KL, et al. Global, regional, and national disease burden estimates of acute lower respiratory infections due to respiratory syncytial virus in young children in 2015: a systematic review and modeling study. Lancet 2017; 390: 946–958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Pelletier AJ, Mansbach JM, Camargo CA., Jr. Direct medical costs of bronchiolitis hospitalizations in the United States. Pediatrics 2006; 118: 2418–2423. [DOI] [PubMed] [Google Scholar]
- 8. Palmer L, Hall CB, Katkin JP, et al. Respiratory outcomes, utilization and costs 12 months following a respiratory syncytial virus diagnosis among commercially insured late-preterm infants. Curr Med Res Opin 2011; 27: 403–412. [DOI] [PubMed] [Google Scholar]
- 9. Dartmouth-Hitchcock Medical Center and Alice Peck Day Memorial Hospital. Dartmouth-Hitchcock and Alice Peck Day Memorial Hospital Community Health Needs Assessment. http://www.alicepeckday.org/assets/2015_APDH_Community_Health_Needs_Assessment.pdf (2015, accessed 15 August 2018).
- 10. Perlstein PH, Kotagal UR, Bolling C, et al. Evaluation of an evidence-based guideline for bronchiolitis. Pediatrics 1999; 104: 1334–1341. [DOI] [PubMed] [Google Scholar]
- 11. Ralston SL, Lieberthal AS, Meissner HC, et al. Clinical practice guideline: the diagnosis, management, and prevention of bronchiolitis. Pediatrics 2014; 134: e1474–e1502. http://pediatrics.aappublications.org/content/pediatrics/early/2014/10/21/peds.2014-2742.full.pdf (accessed 15 August 2018). [DOI] [PubMed] [Google Scholar]
- 12. Preterm Birth. Centers for Disease Control and Prevention. https://www.cdc.gov/reproductivehealth/maternalinfanthealth/pretermbirth.htm (2018, accessed 13 August 2018).
- 13.https://www.shrm.org/ResourcesAndTools/hr-topics/benefits/Documents/tabST-F1-2000.pdf
- 14. https://www2.census.gov/programs-surveys/demo/tables/families/2020/cps-2020/tabf2-all.xls
- 15. Glezen WP, Taber LH, Frank AL, et al. Risk of primary infection and reinfection with respiratory syncytial virus. Am J Dis Child 1986; 140: 543–546. [DOI] [PubMed] [Google Scholar]
- 16. Nokes DJ, Okiro EA, Ngama M, et al. Respiratory syncytial virus epidemiology in a birth cohort from Kilifi District, Kenya: infection during the first year of life. J Infect Dis 2004; 190: 1828–1832. [DOI] [PubMed] [Google Scholar]
- 17. Kim HW, Arrobio JO, Brandt CD, et al. Epidemiology of respiratory syncytial virus in Washington, DC. I. Importance of the virus in different respiratory tract disease syndromes and temporal distribution of infection. Am J Epidemiol 1973; 98: 216–225. [DOI] [PubMed] [Google Scholar]
- 18. Boyce TG, Mellen BG, Mitchel EF, Jr, et al. Rates of hospitalization for respiratory syncytial virus infection among children in Medicaid. J Pediatr 2000; 137: 865–870. [DOI] [PubMed] [Google Scholar]
- 19. March of Dimes. 2017. Premature Birth Report Card: Vermont. https://www.marchofdimes.org/peristats/tools/reportcard.aspx?frmodrc=1®=50 (accessed 13 August 2018).
- 20. March of Dimes. 2017. Premature Birth Report Card: New Hampshire. https://www.marchofdimes.org/peristats/tools/reportcard.aspx?frmodrc=1®=33 (accessed 13 August 2018).
- 21. Hoffman JI, Kaplan S. The incidence of congenital heart disease. J Am Coll Cardiol 2002; 39: 1890–1900. [DOI] [PubMed] [Google Scholar]
- 22. Simoes EA, King SJ, Lehr MV, et al. Preterm twins and triplets: a high-risk group for severe respiratory syncytial virus infection. Am J Dis Child 1993; 147: 303–306. [DOI] [PubMed] [Google Scholar]
- 23. Martin JA, Hamilton BE, Osterman MJ, et al. Births: final data for 2015. Natl Vital Stat Rep 2017; 66: 1–69. [PubMed] [Google Scholar]
- 24. Glezen WP, Paredes A, Allison JE, et al. Risk of respiratory syncytial virus infection for infants from low-income families in relationship to age, sex, ethnic group, and maternal antibody level. J Pediatr 1981; 98: 708–715. [DOI] [PubMed] [Google Scholar]
- 25. Nyiro JU, Sande CJ, Mutunga M, et al. Absence of association between cord specific antibody levels and severe respiratory syncytial virus (RSV) disease in early infants: a case control study from coastal Kenya. PLoS ONE 2016; 11: e0166706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Chu HY, Tielsch J, Katz J, et al. Transplacental transfer of maternal respiratory syncytial virus (RSV) antibody and protection against RSV disease in infants in rural Nepal. J Clin Virol 2017; 95: 90–95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Mazur NI, Higgins D, Nunes MC, et al. The respiratory syncytial virus vaccine landscape: lessons from the graveyard and promising candidates. Lancet Infect Dis 2018; 18: e295–e311. https://www.thelancet.com/action/showPdf?pii=S1473-3099%2818%2930292-5 (accessed 20 August 2018). [DOI] [PubMed] [Google Scholar]
- 28. McLellan JS, Chen M, Leung S, et al. Structure of RSV fusion glycoprotein trimer bound to a prefusion-specific neutralizing antibody. Science 2013; 340: 1113–1117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. American Academy of Pediatrics Committee on Infectious Diseases and Bronchiolitis Guidelines Committee. Updated guidance for palivizumab prophylaxis among infants and young children at increased risk of hospitalization for respiratory syncytial virus infection. Pediatrics 2014; 134: e620–e638. [DOI] [PubMed] [Google Scholar]
- 30. Hammitt LL, Dagan R, Yuan Y, et al. Nirsevimab for prevention of RSV in healthy late-preterm and term infants. N Engl J Med 2022; 386: 837–846. [DOI] [PubMed] [Google Scholar]
- 31. Gerretsen HE, Sande CJ. Development of respiratory syncytial virus (RSV) vaccines for infants. J Infect 2017; 74(Suppl. 1): S143–S146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Kim HW, Canchola JG, Brandt CD, et al. Respiratory syncytial virus disease in infants despite prior administration of antigenic inactivated vaccine. Am J Epidemiol 1969; 89: 422–434. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental material, sj-docx-1-tai-10.1177_20499361221099447 for Bronchiolitis hospitalizations in rural New England: clues to disease prevention by Peter F. Wright, Anne G. Hoen, J. Dean Jarvis, Michael S. Zens, Erika F. Dade, Margaret R. Karagas, Juliana Taube and Elizabeth B. Brickley in Therapeutic Advances in Infectious Disease