Abstract
Background
Chronic cough (CC) represents a significant health burden. This study assessed the prevalence of CC (defined as per international guidelines as cough duration >8 weeks) in Spanish adults and compared characteristics between CC and non-CC cohorts.
Methods
CC cohorts were compiled using data from adult respondents to the 2020 Spanish cross-sectional online National Health and Wellness Survey (NHWS). Using propensity scores, respondents experiencing CC during their lifetime and the previous 12 months were matched 3:1 to respondents without CC and their health characteristics were compared. The number of Spanish adults affected with CC was estimated using weighted CC prevalence.
Results
CC during their lifetime or the previous 12 months was experienced by 579 (8.2%) and 389 (5.5%) of 7074 NHWS respondents, of whom 233 (38.5%) and 171 (44.0%), respectively, had physician-diagnosed CC. Based on weighted prevalence rates, lifetime and 12-month CC were estimated to affect ≈3.3 million and ≈2.2 million Spanish adults, respectively. Relative to the non-CC cohort, the 12-month CC cohort consistently demonstrated poorer health status, poorer mental health, greater healthcare utilization, and lower productivity at work and home.
Conclusion
This study contributes novel data regarding the prevalence of CC in Spain, suggests that CC is underdiagnosed, and reflects that CC and related comorbidities inflict a significant health burden in the affected population.
Keywords: Chronic cough, epidemiology, prevalence, survey
Introduction
Cough is a common reason for visiting primary healthcare professionals (HCPs) worldwide 1 and a significant cause of morbidity. 2 The estimated global prevalence of chronic cough (CC), regardless of definition, is 9.6%, ranging from 2.3% and 4.4% in Africa and Asia to 11.0–18.1% in America, Europe, and Oceania. 2 Using the international guideline definition of CC (cough lasting ≥8 weeks,3,4 CC was more prevalent in Europe (12.0% 5 and 7.2% 6 in the UK 5 and Finland 6 ) than in Asia (2.2% 7 and 2.6% 8 in Japan 7 and Korea 8 ), and Africa (1.1% in Nigeria 9 ).
The absence of International Classification of Diseases (ICD) codes for CC prevents the use of health record databases for quantifying CC prevalence. Research conducted in clinical settings and specialist cough centers may provide different results than in the overall population. In Spain, evidence regarding the epidemiology of CC at the population-level is lacking. Because CC negatively impacts health status and quality of life (QoL),2,7,10–15 emotional well-being (associated with anger/frustration, 14 depression,14–16 anxiety14,15) daily living7,15 and social activities, 14 insights on the frequency and impact of CC are of interest for healthcare planning.
This study aimed to estimate the prevalence of CC in Spanish adults using data from a web-based survey administered to a panel representative of the general adult population of Spain, and compare characteristics between CC and matched non-CC cohorts.
Methods
The National Health and Wellness Survey (NHWS) is a cross-sectional survey conducted in numerous countries, including Spain, using a self-administered, internet-based questionnaire addressed to a sample of the general population aged ≥18 years. Post-hoc analyses have shown that the NHWS typically captures data from ≈0.02% of adults from a given country. The NHWS protocol and questionnaire were approved by the Pearl Institutional Review Board.
Potential NHWS respondents were recruited through an existing, general-purpose (non-healthcare-specific) web-based panel. All panelists explicitly agreed to be panel members. A targeted quota sampling procedure was implemented to ensure that the sex and age of the NHWS sample were representative of the Spanish adult population. Inclusion criteria for the NHWS were age ≥18 years, the ability to read and write in Spanish, and informed consent.
The 2020 Spanish NHWS was conducted between 30 December 2019 and 20 April 2020, using a Spanish-language survey version translated from original and reviewed by expert physicians. The covariates captured by the NHWS included demographic variables, general health characteristics, health status, experienced and/or diagnosed comorbidities, depression and anxiety symptoms, work productivity, and healthcare utilization. All information was self-reported by respondents, and no clinical charts or medical reports were reviewed.
Comorbidity burden was assessed using the Charlson comorbidity index (CCI),17,18 which represents a weighted sum of multiple comorbid conditions predictive of mortality (higher scores indicate a greater comorbidity burden). The original CCI, plus a modified version excluding chronic obstructive pulmonary disease (COPD), a condition frequently associated with CC, were used.
Health status was assessed using the Medical Outcomes Study 12-item Short Form Survey (SF-12v2). 19 Better health status is represented by higher domain (physical functioning, physical role limitations, bodily pain, general health, vitality, social functioning, emotional role limitations, and mental health), physical component summary (PCS), and mental component summary (MCS) scores. A score of 50 (±10), was assumed to be the population norm. The SF-12v2 response pattern was converted to SF-6D Utility Scores, which range from 0 (health state equivalent to death) to 1 (perfect health).
Depression and anxiety symptoms over the prior 2 weeks were evaluated respectively by the 9-item Patient Health Questionnaire (PHQ-9) 20 and 7-item Generalized Anxiety Disorder scale (GAD-7). 21 Each item is scored from 0–3 for a total PHQ-9 score of 0–27 (scores ≥10 suggest major depressive disorders) 22 and a total GAD-7 score of 0–21 (scores of 5, 10, and 15 are the cutoff for mild, moderate, and severe anxiety symptoms, respectively).
Work productivity over the previous 7 days was assessed by the 6-item Work Productivity and Activity Impairment (WPAI) questionnaire, which measured health-related absenteeism (percentage of work time missed), presenteeism (percentage of impairment experienced while at work), overall work productivity loss (absenteeism plus presenteeism), and activity impairment (percentage of impairment in daily activities outside of work). 23 All respondents provided activity impairment data, but only full- or part-time respondents provided work-related data.
Sleep problems (self-reported or physician-diagnosed) in the previous 12 months were assessed via direct questions to the respondents. To assess healthcare utilization, respondents were asked to report any-cause hospitalizations, and any visits to HCPs, relevant specialists, and emergency rooms (ER) during the previous 6 months.
Cohort selection
NHWS respondents were asked questions pertaining to CC (defined in the survey as daily cough lasting >8 weeks, according to the European Respiratory Society guidelines available at the time of study design and data collection 4 ). Individuals reporting that they had experienced CC at any point in their lifetime or during the previous 12 months formed the lifetime and 12-month CC cohorts, respectively. Individuals reporting CC diagnosed by a physician in their lifetime or the previous 12 months formed the diagnosed lifetime and diagnosed 12-month CC cohorts, respectively.
Statistical analysis
Descriptive data are reported as means (± standard deviation) for quantitative variables, and frequencies for categorical variables. The prevalence of CC was calculated using the total number of NHWS respondents as the denominator. The weighted prevalence of CC was estimated using post-stratification Horvitz-Thompson sampling weights based on the age and sex distribution in Spain according to data from the US Census’ International Database (https://www.census.gov/programs-surveys/international-programs/about/idb.html#). The weighted prevalence of CC was projected to the Spanish adult population to estimate the number of Spanish adults affected by CC.
To compare outcomes, respondents of the 12-month CC cohort were matched 1:3 to NHWS respondents without CC in the previous 12 months using a propensity score calculated using age, sex, modified CCI score, marital status, household income, and a marital status/household income interaction term. These variables were selected to account for differences in healthcare and general health status between adults of different sexes, ages, and socioeconomic status, and to account for comorbid conditions not associated with CC.
Chi-squared tests were used to compare proportions, and Student t-tests were used to compare continuous variables. Two-tailed p-values of <0.05 were considered statistically significant. Statistical analyses were performed using SPSS (v23), R (v4.0.2), and SAS (v9.4).
Results
Sample
The NHWS Spanish sample included 7074 respondents, 3487 males (49.3%) and 3587 females (50.7%) (Table 1). The mean respondent age was 46.6 (±15.0) years; respondents were generally well distributed across age categories (Table 1), except the ≥75-year age category, which had 131 respondents (1.9%). Most (62.1%) respondents were employed. Current, former and never smokers accounted for 31.1%, 31.2%, and 37.7% of respondents. One-third (34.3%) of respondents were overweight (body mass index [BMI] 25 to <30 kg/m2), and 14.6% were obese (BMI ≥30 kg/m2).
Table 1.
Unweighted prevalence of chronic cough (experienced or diagnosed by a physician) in Spain.
| NHWS respondents (n) | Lifetime chronic cough | 12-month chronic cough | ||||
|---|---|---|---|---|---|---|
| EXP, n (%) a | DIA, n (%) a | DIA/EXP (%) b | EXP, n (%) a | DIA, n (%) a | DIA/EXP (%) b | |
| Total (7074) | 579 (8.2) | 223 (3.2) | 38.5 | 389 (5.5) | 171 (2.4) | 44.0 |
| Gender | ||||||
| Female (3587) | 320 (8.9) | 123 (3.4) | 38.4 | 222 (6.2) | 97 (2.7) | 43.7 |
| Male (3487) | 259 (7.4) | 100 (2.9) | 38.6 | 167 (4.8) | 74 (2.1) | 44.3 |
| p-value | 0.022 | 0.177 | 0.010 | 0.111 | ||
| Age (years) | ||||||
| 18–29 (1109) | 115 (10.4) | 35 (3.2) | 30.4 | 64 (5.8) | 26 (2.3) | 40.6 |
| 30–39 (1289) | 133 (10.3) | 40 (3.1) | 30.1 | 81 (6.3) | 28 (2.2) | 34.6 |
| 40–49 (1634) | 124 (7.6) | 51 (3.1) | 41.1 | 92 (5.6) | 42 (2.6) | 45.7 |
| 50–64 (1865) | 142 (7.6) | 70 (3.8) | 49.3 | 105 (5.6) | 56 (3.0) | 53.3 |
| 65–74 (1046) | 53 (5.1) | 21 (2.0) | 39.6 | 38 (3.6) | 15 (1.4) | 39.5 |
| ≥75 (131) | 12 (9.2) | 6 (4.6) | 50.0 | 9 (6.9) | 4 (3.1) | 44.4 |
| p-value | <0.001 | 0.181 | 0.098 | 0.171 | ||
| Smoking status | ||||||
| Current/former (4409) | 419 (9.5) | 169 (3.8) | 40.3 | 293 (6.6) | 135 (3.1) | 46.1 |
| Never (2665) | 160 (6.0) | 54 (2.0) | 33.8 | 96 (3.6) | 36 (1.4) | 37.5 |
| p-value | <0.001 | <0.001 | <0.001 | <0.001 | ||
DIA: diagnosed by a physician; EXP: experienced; NA: not available; NHWS: National Health and Wellness Survey.
aValues indicate, by variable, the number and percentage (in brackets) of cohort members with chronic cough; percentages are based on the number of NHWS respondents with that variable.
bValues indicate the percentage of DIA members within the EXP cohort.
Chronic cough prevalence
During their lifetime, CC was experienced by 579 respondents (8.2%), and 233 (3.2%) reported physician-diagnosed CC (Table 1). CC during the previous 12 months was experienced by 389 respondents (5.5%), and 171 (2.4%) had physician-diagnosed CC during this period. A physician diagnosis of CC was received by 38.5% and 44.0% of respondents who experienced CC during their lifetime or the previous 12 months, respectively (Table 1).
For lifetime CC, the weighted prevalence of experiencing CC was 8.2%, estimated to affect ≈3.35 million adults in Spain, and of being diagnosed with CC was 3.2%, estimated to affect ≈1.30 million (Table 2). For 12-month CC, the weighted prevalence of experiencing CC was 5.5%, estimated to affect ≈2.2 million Spanish adults, and of being diagnosed with CC was 2.4%, affecting ≈1 million. The weighted prevalence of 12-month CC was slightly higher in women than in men (6.0% vs. 4.9%; p = 0.049), but no othersignificant between-gender differences were shown (Table 2).
Table 2.
Weighted prevalence of chronic cough (experienced or diagnosed by a physician) in Spain.
| Spanish population (n) | Lifetime chronic cough | 12-month chronic cough | ||
|---|---|---|---|---|
| EXP, n (%) a | DIA, n (%) a | EXP, n (%) a | DIA, n (%) a | |
| Adults (40,663,139) | 3,345,015 (8.2) | 1,296,104 (3.2) | 2,236,153 (5.5) | 980,074 (2.4) |
| Gender | ||||
| Female (20,767,414) | 1,792,882 (8.6) | 714,049 (3.4) | 1,256,228 (6.0) | 551,295 (2.7) |
| Male (19,895,725) | 1,552,133 (7.8) | 582,055 (2.9) | 979,925 (4.9) | 428,779 (2.2) |
| p-value | 0.229 | 0.247 | 0.049 | 0.191 |
| Age, years | ||||
| 18–29 (6,025,059) | 652,365 (10.8) | 196,785 (3.3) | 351,639 (5.8) | 145,701 (2.4) |
| 30–39 (6,790,933) | 719,511 (10.6) | 215,580 (3.2) | 437,452 (6.4) | 149,101 (2.2) |
| 40–49 (8,600,286) | 634,864 (7.4) | 261,698 (3.0) | 470,306 (5.5) | 214,450 (2.5) |
| 50–64 (10,149,558) | 779,304 (7.7) | 382,797 (3.8) | 577,281 (5.7) | 306,462 (3.0) |
| 65–74 (8,181,336) | 464,631 (5.7) | 192,074 (2.3) | 337,713 (4.1) | 135,911 (1.7) |
| ≥75 (915,967) | 94,340 (10.3) | 47,170 (5.1) | 61,762 (6.7) | 28,449 (3.1) |
| p-value | <0.001 | 0.341 | 0.267 | 0.372 |
| Smoking status | ||||
| Current/former (25,229,919) | 2,428,625 (9.6) | 964,116 (3.8) | 1,696,750 (6.7) | 764,453 (3.0) |
| Never smoked (15,433,220) | 916,390 (5.9) | 331,988 (2.2) | 539,403 (3.5) | 215,621 (1.4) |
| p-value | <0.001 | <0.001 | <0.001 | <0.001 |
DIA: diagnosed; EXP: experienced; NA: not available; NHWS: National Health and Wellness Survey.
aValues indicate, by variable, the estimated number of adults in Spain with chronic cough and (in brackets) the weighted prevalence in the overall NHWS population.
The non-weighted (Table 1) and weighted (Table 2) prevalence of lifetime and 12-month CC were significantly (p < 0.001) higher in current/former smokers than in never-smokers. In the cohorts self-reporting CC, more current/former smokers than non-smokers had physician-diagnosed CC (Table 1).
The weighted prevalence of lifetime CC differed significantly between age categories (p<0.001), being higher in those aged 18–39 and ≥75 years than in those aged 40–74 years (≈10% vs. ≈7%; Table 2). In the cohort self-reporting lifetime CC, physician-diagnosed CC was less common in individuals aged 18–39 years than in those aged >40 years (≈30% vs. ≈40–50%; Table 1).
General health and comorbidities
The cohort self-reporting CC in the past 12 months and the matched non-CC cohort of NHWS respondents had similar distributions of all covariates included in the propensity score algorithm. Mean ages in the 12-month CC and the matched non-CC cohorts were similar (45.1 and 45.3 years), as was the gender distribution (percentage of females: 57.1% and 58.2%, respectively). There were no significant differences between the 12-month CC and non-CC cohorts concerning weight-related parameters: mean BMI of 25.5 (±5.2) kg/m2 versus 25.2 (±5.2) kg/m2 (p=0.394), 30.3% versus 32.6% of cohort members were overweight, and 16.7% versus 13.3% were obese. The percentage of employed respondents was similar between the 12-month CC cohort (65.8%) and the matched non-CC cohort (61.7%).
Smoking status differed significantly (p < 0.001) between the 12-month CC and non-CC cohorts. Current smokers accounted for 50.1% versus 30.9% of the 12-month CC and non-CC cohorts, respectively, previous smokers for 25.2% versus 30.8%, and never smokers for 24.7% versus 38.3%.
CCI scores indicated that comorbidities were significantly (p < 0.05) more common in the 12-month CC than in the non-CC cohort (Table 3). Digestive disorders (specifically gastroesophageal reflux disease/acid reflux, heartburn), neurologic disease, respiratory diseases (specifically allergies, asthma, COPD, chronic bronchitis), and rheumatoid arthritis were significantly more common in the CC cohort than in the non-CC cohort.
Table 3.
Physician-diagnosed comorbidities during the previous 12 months.
| Comorbidity-related outcome | 12-month chronic cough cohort (n = 389) | Matched non-chronic cough cohort (n = 1164) | p value |
|---|---|---|---|
| CCI Original | |||
| Mean score (SD) | 0.77 (1.19) | 0.47 (1.06) | <0.001 |
| No. of comorbidities, n (%) a | |||
| 0 | 214 (55.0) | 869 (74.7) | <0.001 |
| 1 | 108 (27.8) | 162 (13.9) | |
| ≥2 | 67 (17.2) | 133 (11.4) | |
| CCI Modified (without COPD) | |||
| Mean score (SD) | 0.50 (1.05) | 0.43 (1.01) | 0.235 |
| No. of comorbidities, n (%) a | |||
| 0 | 277 (71.2) | 893 (76.7) | 0.042 |
| 1 | 69 (17.7) | 149 (12.8) | |
| ≥2 | 43 (11.1) | 122 (10.5) | |
| Comorbidity (responder n b ), n (%) c | |||
| Cancer, any d (1539) | 14 (3.6) | 54 (4.7) | 0.457 |
| Digestive disease, any e (1530) | 124 (32.4) | 193 (16.8) | <0.001 |
| GERD or acid reflux (1544) | 33 (8.5) | 42 (3.6) | <0.001 |
| Heartburn (1544) | 76 (19.6) | 95 (8.2) | <0.001 |
| Liver disease (1535) | 5 (1.3) | 14 (1.2) | 0.893 |
| Cardiovascular disease, any f (1540) | 40 (10.4) | 124 (10.7) | 0.876 |
| Neurological disease, any g (1540) | 14 (3.6) | 14 (1.2) | 0.005 |
| Rheumatoid arthritis (1540) | 24 (6.2) | 39 (3.4) | 0.025 |
| Respiratory disease, anyh (1542) | 267 (69.0) | 391 (33.6) | <0.001 |
| Allergies, any (1542) | 148 (38.2) | 342 (29.6) | 0.002 |
| Asthma (1549) | 66 (17.0) | 78 (6.7) | <0.001 |
| Rhinitis (1541) | 17 (4.4) | 27 (2.3) | 0.055 |
| COPD (1544) | 21 (5.4) | 13 (1.1) | <0.001 |
| Chronic bronchitis (1543) | 86 (22.3) | 33 (2.7) | <0.001 |
| Emphysema (1544) | 4 (1.0) | 8 (0.7) | 0.742 |
| Chronic cough, diagnosed (1541) | 171 (44.2) | 10 (0.9) | <0.001 |
CCI: Charlson Comorbidity Index; COPD: chronic obstructive pulmonary disease; GERD: gastroesophageal reflux disease; NHWS: National Health and Wellness Survey; SD: standard deviation.
aValues in the cohort columns indicate the absolute number and percentage (in brackets) of cohort members with variable; percentages were calculated based on the total number of members in the cohort.
bProvides the total number of responders to each question in both cohorts (maximum possible 1553); the remaining respondents declined to respond to this question.
cValues in the cohort columns indicate the absolute number and percentage (in brackets) of cohort members with variable; percentages were based on the number of cohort members who responded to the specific comorbidity question.
dIncludes any tumor, leukemia, lymphoma, and metastatic solid tumors.
eIncludes GERD, acid reflux, heartburn, irritable bowel syndrome (with constipation and/or diarrhea), ulcerative colitis, and active/peptic stomach or duodenal ulcer.
fIncludes congestive heart failure, ischemic heart disease, cerebrovascular disease (transient ischemic attack, stroke), and peripheral vascular disease.
gIncludes dementia, current hemiplegia, multiple sclerosis, and Parkinson’s disease.
hIncludes asthma, chronic bronchitis, COPD, emphysema, hay fever, physician-diagnosed chronic cough and allergies.
Respondents with past 12-month CC had significantly poorer health status than those in the non-CC cohort, as shown by their 3.8 and 3.3 point lower mean SF-12v2 PCS and MCS scores, and 2.7–4.3 point lower mean domain scores (Table 4; all p < 0.001).
Table 4.
Health status, depression, and anxiety in the previous 12 months.
| Outcome, instrument | 12-month chronic cough cohort (n = 389) | Matched non-chronic cough cohort (n = 1164) | p value |
|---|---|---|---|
| Health status, SF-12-v2 | |||
| Physical Component Summary score, mean (SD) | 47.68 (8.25) | 51.44 (8.03) | <0.001 |
| Mental Component Summary score, mean (SD) | 42.42 (9.14) | 45.69 (9.31) | <0.001 |
| Domain scores, mean (SD) | |||
| Physical functioning score | 47.69 (9.79) | 51.87 (8.40) | <0.001 |
| Physical role limitation score | 43.79 (8.76) | 47.94 (8.82) | <0.001 |
| Bodily pain score | 45.07 (9.77) | 49.20 (9.45) | <0.001 |
| General health score | 46.35 (9.91) | 49.25 (9.02) | <0.001 |
| Vitality score | 49.80 (9.08) | 52.51 (8.99) | <0.001 |
| Social functioning score | 42.38 (9.10) | 46.40 (9.23) | <0.001 |
| Emotional role limitations score | 39.23 (10.75) | 43.58 (10.82) | <0.001 |
| Mental health score | 44.57 (9.09) | 47.82 (8.71) | <0.001 |
| Health utility, SF-6D | |||
| Utility score, mean (SD) | 0.65 (0.11) | 0.70 (0.12) | <0.001 |
| Depression, PHQ-9 a | |||
| Total score, mean (SD) | 8.84 (6.64) | 5.78 (5.88) | <0.001 |
| Classification of depression, n (%) a | |||
| None–minimal (score 0–4) | 118 (30.3) | 624 (53.6) | <0.001 |
| Mild (score 5–9) | 116 (29.8) | 289 (24.8) | |
| Moderate (score 10–14) | 84 (21.6) | 136 (11.7) | |
| Moderately severe (score 15–19) | 40 (10.3) | 74 (6.4) | |
| Severe (score 20–27) | 31 (8.0) | 41 (3.5) | |
| Anxiety, GAD-7 | |||
| Mean score (SD) | 6.91 (5.30) | 4.56 (4.53) | <0.001 |
| Classification of anxiety, n (%) a | |||
| None (score 0–4) | 145 (37.3) | 660 (56.7) | <0.001 |
| Mild (score 5–9) | 140 (36.0) | 344 (29.6) | |
| Moderate (score 10–14) | 66 (17.0) | 110 (9.5) | |
| Severe (score 15–21) | 38 (9.8) | 50 (4.3) | |
GAD-7: Generalized Anxiety Disorder-7 scale; NHWS: National Health and Wellness Survey; PHQ-9: Patient Health Questionnaire-9; SD: standard deviation; SF-12v2: Medical Outcomes 12-item Short Form Survey Instrument; SF-6D Short-Form Six-Dimension health index.
aValues indicate the number and percentage (in brackets) of cohort members with that classification; percentages are based on the total number of NHWS respondents in the cohort.
Depression and anxiety symptoms were more common in the 12-month CC cohort than in the non-CC cohort, as indicated by their significantly (p < 0.001) higher mean PHQ-9 and GAD-7 scores (Table 4). Significantly (p < 0.001) more individuals in the CC cohort than in the non-CC cohort had moderate-to-severe depression (39.9% vs. 21.6%) or moderate-to-severe anxiety symptoms (26.8% vs. 13.8%).
Sleeping disorders (experienced or physician-diagnosed) were significantly more common in the 12-month CC cohort than in the non-CC cohort, as were insomnia, narcolepsy (experienced only), sleep apnea, and other sleep difficulties (Table 5).
Table 5.
Sleep disorders in the previous 12 months.
| Sleep disorder | Both cohorts (n = 1553) | 12-month chronic cough cohort (n = 389) | Matched non-chronic cough cohort (n = 1164) | p value |
|---|---|---|---|---|
| Responded to question (n) a | n (%) b | n (%) b | ||
| Experienced in the previous 12 months | ||||
| Any | 1545 | 286 (73.6) | 513 (44.3) | <0.001 |
| Specific disorders | 1545 | |||
| Insomnia | 1545 | 186 (48.1) | 296 (25.6) | <0.001 |
| Narcolepsy | 1545 | 9 (2.3) | 5 (0.4) | 0.002 |
| Sleep apnea | 1545 | 59 (15.2) | 79 (6.8) | <0.001 |
| Other sleep difficulties | 1545 | 154 (39.8) | 243 (21.0) | <0.001 |
| Diagnosed by a physician in the previous 12 months | ||||
| Any | 1540 | 143 (37.1) | 209 (18.1) | <0.001 |
| Specific disorders | ||||
| Insomnia | 1539 | 99 (25.8) | 138 (11.9) | <0.001 |
| Narcolepsy | 1540 | 1 (0.3) | 1 (0.1) | 1.000 |
| Sleep apnea | 1539 | 35 (9.1) | 58 (5.0) | 0.004 |
| Other sleep difficulties | 1542 | 51 (13.2) | 71 (6.1) | <0.001 |
aThe remaining cohort members declined to answer these questions.
bPercentage based on the number of members of that cohort who responded to that specific question.
Activity/work impairment
As assessed by the WPAI, mean activity impairment in the previous 7 days was higher in the 12-month CC cohort than in the non-CC cohort (38.5% [±28.2] vs. 25.1% [±27.4]; p<0.001). Among actively working cohort members, the CC cohort had significantly (p<0.001) higher mean percentages of absenteeism (11.9% [±20.0] vs. 6.5% [±16.6]), presenteeism (34.6% [±27.9] vs. 21.6% [±26.3]), and total work productivity impairment (38.4% [±31.1] vs. 24.1% [±28.7]) than the non-CC cohort.
Healthcare utilization
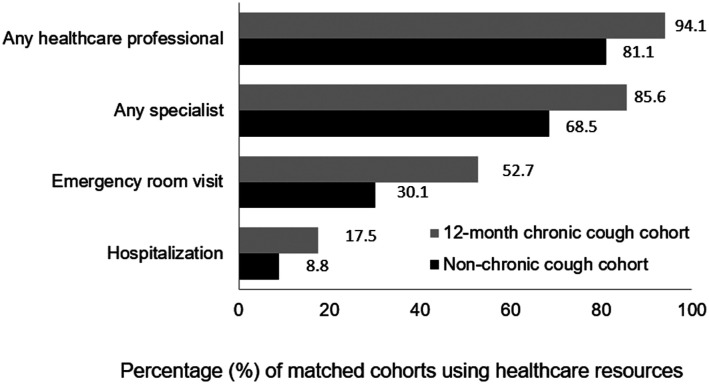
During the previous 6 months, significantly (p<0.001) more 12-month CC cohort than of non-CC cohort members used healthcare resources, including all-cause hospitalization, and visits to any HCP, relevant specialists, and ER (Figure 1). The CC cohort, relative to the non-CC cohort, had a significantly (p<0.001) higher mean number of visits to any HCP (7.6 [±9.9] vs. 4.1 [±4.7]), relevant specialists (4.4 [±5.8] vs. 2.3 [±3.2]), primary-care physicians (2.6 [±3.3] vs. 1.5 [±1.9]), and ER (1.4 [±2.1] vs. 0.7 [±2.2]).
Figure 1.
Use of all-cause healthcare resources in the previous 6 months. Any specialist is a summary of any of a group of specialties most relevant to persons with chronic cough, specifically general/family practitioner, internist, allergist, cardiologist, gastroenterologist, neurologist, nurse practitioner/physician assistant, otolaryngologist, psychiatrist, psychologist/therapist, and pulmonologist. All p < 0.001.
Discussion
Given the lack of epidemiological studies of CC in Spain, this study, based on the NHWS cross-sectional survey, contributes novel data regarding the estimated prevalence of CC in adults in Spain and the significant health burden experienced by this population. In this study, the weighted prevalence of lifetime and 12-month CC was 8.2% and 5.5% respectively. These figures are in line with recently published NHWS surveys from the USA 24 and Japan 25 using similar methodology, which reported a weighted prevalence of 12-month CC of 5.0% 24 and 4.3%, 25 respectively.
Similar to prior CC studies,5,6 being a current/former smoker was consistently associated with an increased prevalence of CC. A significantly higher weighted prevalence of CC in females than in males was shown only in the 12-month CC cohort; similar between-gender differences have been reported in other studies,9–11, 14,26 including the NHWS survey from the USA. 24 Age significantly influenced only the weighted prevalence of lifetime CC. Of interest, although respondents aged 18–39 years had higher rates of lifetime CC than those aged 40–74 years, rates of diagnosed CC were lower in younger individuals. Studies frequently describe CC as being more common in middle-aged populations (aged 40–65 years),6,10,11,14,26 but this study suggests that younger populations may have a higher relative prevalence of CC than previously described. This finding could be due in part to a higher percentage of underdiagnosis in this age group, who are less often in contact with the health system and less likely to seek medical care.
Matching the 12-month CC cohort to a non-CC cohort allowed this study to explore differences in these populations while controlling for differences in age, gender, CCI, marital status, and income. Comorbidities were more common in individuals with CC during the previous 12 months than those without CC, both overall and with regard to particular types of comorbidities, including diseases affecting the digestive, neurologic, and respiratory systems, and rheumatoid arthritis. Potential relationships have been suggested between CC and many other conditions, primarily gastrointestinal symptoms5,6,26–31 and respiratory conditions,6,11,26–31 as well as sleep apnea32–34 and laryngeal hypersensititivy. 35
Individuals with CC during the previous 12 months had poorer overall, physical, and mental health status than those without CC, as shown by their significantly lower SF-12v2 PCS, MCS, and domain scores. Similar results have been shown in other studies. For example, the condition was burdensome and affected daily living measures in CC patients in a US study, 10 adversely affected the QoL of 96% of CC patients in a European survey, 11 and decreased QoL scores in CC patients relative to healthy volunteers in a Chinese study 12 and matched non-CC respondents in Japanese 13 and Korean 15 surveys.
Furthermore, depression, anxiety, and sleep problems were significantly more common in individuals with CC in the previous 12 months than in those without CC, as has been previously shown. Depression was significantly associated with CC in elderly patients in Korea, 16 depression, anxiety, and sleep disturbances more common in patients with CC than in matched non-CC respondents in Japan, 13 and depression, anxiety, and disturbed sleep were each reported in 55–77% of patients with CC in the UK. 14 These aspects must be taken into account when studying patients with CC.
In addition, CC impacted the daily life and work of individuals to a significant extent. Relative to individuals without CC, those with 12-month CC had 1.4-fold higher impairment of daily activities and, in individuals who were currently working, a 1.6-fold higher impairment of total work productivity. This confirms the findings of other studies, in which CC significantly impaired daily activities and work productivity by 1.4-and 1.5-fold in Japan, 13 significantly affected the employment, social life, hobbies, and holidays of Swedish patients, 36 and affected the daily activities and social life of >60% of UK patients. 14
Finally, healthcare utilization for any reason was more common in patients with than without CC, including a 1.2-. 1.7-, and 2.0-fold higher proportion who visited any HCP or specialist, visited the ER, or who were hospitalized, respectively. Likewise, the Japanese survey showed that CC patients had higher numbers of visits to HCPs and the ER than matched non-CC respondents 13
The incremental burden of CC on comorbidities, health status, depression, anxiety, sleep problems, daily life and work activities, and healthcare utilization, indicate that there is an unmet need to improve overall health in patients with CC. Furthermore, CC may be difficult to treat successfully and is often persistent.11,37,38 Given the estimated prevalence of CC in Spain and other countries, CC represents a considerable burden to healthcare payers, and, when work impairment is included, to society. Better interventions and targeted treatments are needed to manage CC,38–40 which have the potential to improve the overall health status and QoL of patients, as well as reduce CC-related depression, anxiety, sleep problems, daily living, and work impairment, and healthcare utilization.
The current study has limitations that should be considered. In common with other internet-based, patient-reported surveys, the NHWS sample likely under-represents people without access to or familiarity with electronic media, including the elderly, institutionalized individuals, and those with severe comorbidities and disabilities that may make access to, and ability to participate in, online survey panels more difficult. As all information was self-reported by participants, it may be subject to recall and self-representation bias and cannot be verified.
Our sample can be considered representative of the adult Spanish population aged 18–75 years, but not of those aged ≥75 years, who accounted for only 1.9% of NHWS respondents. Smoking or the use of angiotensin-converting enzyme inhibitors (ACEi) did not exclude participation in the survey. Respondents were asked about smoking habits, but not specifically ACEi use, therefore, its impact on CC prevalence could not be analyzed. The calculation of CCI may be slightly low, as the NHWS does not capture the comorbidities of moderate/severe liver disease and paraplegia (usually included in the definition of hemiplegia).
Finally, it should be noted that many of the outcomes that were worse in patients with CC (health status, depression, anxiety, sleep disorders, healthcare utilization, and activity and work-productivity impairment) may be influenced by existing comorbidities, beyond those accounted for in the modified CCI used in matching, rather than by CC itself. Future research is warranted specifically in populations with refractory CC or unexplained CC. Patients with these conditions constitute a subset of the overall CC population with a greater burden of cough, and exploration of outcomes in this population where cough has been adequately diagnosed and managed may reveal additional insights on the burden of cough not related to underlying conditions. Further studies evaluating the influence of comorbidities and other potential confounding factors on these outcomes would be of value.
Conclusion
This study showed that CC is frequent in Spanish adults, with 8.2% (≈3.2 million) and 5.5% (≈2.2 million) experiencing CC lifetime or in the past 12 months, but it appears underdiagnosed, especially in younger age groups. Respondents afflicted with CC experience a significant health and QoL burden relative to those without CC, that might be related to CC itself, and also to comorbidities not included in the modified CCI.
Acknowledgements
Medical writing assistance was provided by Katherine A. Lyseng-Williamson, Content Ed Net, and funded by MSD Spain.
Footnotes
Author contributions: All authors have contributed significantly to the conception, design, or acquisition of data, or analysis and interpretation of data. All authors have participated in drafting, reviewing, and/or revising the manuscript and have approved its submission.
Declaration of conflicting interests: The author(s) declared the following potential conflicts of interest with respect to the research, authorship, and/or publication of this article: JDO has received payment for consultancy services and presentations from Leti Pharma, Mundipharma, AstraZeneca, Chiesi, Novartis, GSK, MSD, Sanofi, and Teva. VP has received payment for consultancy services from Sanofi, for presentations from AstraZeneca, Chiesi, MSD and Boehringer Ingelheim, meeting travel assistance from Novartis and research grants from AstraZeneca, Chiesi and Menarini. VWL and AM are full time employees at Kantar Health LLC, New York, NY, USA. EF, JB and JS are full-time employees of Merck Sharp & Dohme Corp., a subsidiary of Merck & Co., Inc., Rahway, NJ, USA and shareholders in Merck & Co., Inc., Rahway, NJ, USA. LCC and MSJ are full-time employees of MSD Spain.
Funding: The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: This study was funded by Merck Sharp & Dohme Corp., a subsidiary of Merck & Co., Inc., Rahway, NJ, USA.
References
- 1.Finley CR, Chan DS, Garrison S, et al. What are the most common conditions in primary care? Systematic review. Can Fam Physician 2018; 64: 832–840. [PMC free article] [PubMed] [Google Scholar]
- 2.Song WJ, Chang YS, Faruqi S, et al. The global epidemiology of chronic cough in adults: a systematic review and meta-analysis. Eur Respir J 2015; 45: 1479–1481. DOI: 10.1183/09031936.00218714 [DOI] [PubMed] [Google Scholar]
- 3.Pratter MR, Brightling CE, Boulet LP, et al. An empiric integrative approach to the management of cough: ACCP evidence-based clinical practice guidelines. Chest 2006; 129: 222s–231s. DOI: 10.1378/chest.129.1_suppl.222S [DOI] [PubMed] [Google Scholar]
- 4.Morice AH, Fontana GA, Belvisi MG, et al. ERS guidelines on the assessment of cough. Eur Respir J 2007; 29: 1256–1276. DOI: 10.1183/09031936.00101006 [DOI] [PubMed] [Google Scholar]
- 5.Ford AC, Forman D, Moayyedi P, et al. Cough in the community: a cross sectional survey and the relationship to gastrointestinal symptoms. Thorax 2006; 61: 975–979. DOI: 10.1136/thx.2006.060087 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Lätti AM, Pekkanen J, Koskela HO. Defining the risk factors for acute, subacute and chronic cough: a cross-sectional study in a Finnish adult employee population. BMJ Open 2018; 8: e022950. DOI: 10.1136/bmjopen-2018-022950 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Fujimura M. Frequency of persistent cough and trends in seeking medical care and treatment-results of an internet survey. Allergol Int 2012; 61: 573–581. DOI: 10.2332/allergolint.11-OA-0368 [DOI] [PubMed] [Google Scholar]
- 8.Kang MG, Song WJ, Kim HJ, et al. Point prevalence and epidemiological characteristics of chronic cough in the general adult population: the Korean National Health and Nutrition Examination Survey 2010-2012Point prevalence and epidemiological characteristics of chronic cough in the general adult population. Medicine (Baltimore) 2017; 96: e6486. DOI: 10.1097/md.0000000000006486 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Desalu OO, Salami AK, Fawibe AE. Prevalence of cough among adults in an urban community in Nigeria. West Afr J Med 2011; 30: 337–341. [PubMed] [Google Scholar]
- 10.Zeiger RS, Schatz M, Hong B, et al. Patient-reported burden of chronic cough in a managed care organization. J Allergy Clin Immunol Pract 2021; 9: 1624–1637, e1610. DOI: 10.1016/j.jaip.2020.11.018 [DOI] [PubMed] [Google Scholar]
- 11.Chamberlain SA, Garrod R, Douiri A, et al. The impact of chronic cough: a cross-sectional European survey. Lung 2015; 193: 401–408. DOI: 10.1007/s00408-015-9701-2 [DOI] [PubMed] [Google Scholar]
- 12.Ma W, Yu L, Wang Y, et al. Changes in health-related quality of life and clinical implications in Chinese patients with chronic cough. Cough 2009; 5: 7. DOI: 10.1186/1745-9974-5-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kubo T, Tobe K, Okuyama K, et al. Disease burden and quality of life of patients with chronic cough in Japan: a population-based cross-sectional survey. BMJ Open Respir Res 2021; 8: e000764. DOI: 10.1136/bmjresp-2020-000764 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Everett CF, Kastelik JA, Thompson RH, et al. Chronic persistent cough in the community: a questionnaire survey. Cough 2007; 3: 5. DOI: 10.1186/1745-9974-3-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Won HK, Lee JH, An J, et al. Mechanisms and rationale for targeted therapies in refractory and unexplained chronic cough: the Korean National Health and Nutrition Examination survey 2010-2016. Allergy Asthma Immunol Res 2020; 12: 964–979. DOI: 10.4168/aair.2020.12.6.964 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Sohn KH, Song WJ, Kim SH, et al. Chronic cough, not asthma, is associated with depression in the elderly: a community-based population analysis in South Korea. Korean J Intern Med 2019; 34: 1363–1371. DOI: 10.3904/kjim.2018.187 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Charlson ME, Pompei P, Ales KL, et al. A new method of classifying prognostic comorbidity in longitudinal studies: development and validation. J Chronic Dis 1987; 40: 373–383. DOI: 10.1016/0021-9681(87)90171-8 [DOI] [PubMed] [Google Scholar]
- 18.Witt EA, Goren A. A comparison of the utility of variants of the charlson comorbidity index (CCI) in predicting patient-reported health outcomes. Value in Health 2014; 17: A194, DOI: 10.1016/j.jval.2014.03.1134 10.1016/j.jval.2014.03.1134. [DOI] [Google Scholar]
- 19.Maruish ME. User’s manual for the SF-36v2 Health Survey. Lincoln, RI: Quality Metric Incorporated, 2011. [Google Scholar]
- 20.Kroenke K, Spitzer RL, Williams JB. The PHQ-9: validity of a brief depression severity measure. J Gen Intern Med 2001; 16: 606–613. DOI: 10.1046/j.1525-1497.2001.016009606.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Spitzer RL, Kroenke K, Williams JB, et al. A brief measure for assessing generalized anxiety disorder: the GAD-7. Arch Intern Med 2006; 166: 1092–1097. DOI: 10.1001/archinte.166.10.1092 [DOI] [PubMed] [Google Scholar]
- 22.Manea L, Gilbody S, McMillan D. Optimal cut-off scorefor diagnosing depression with the Patient Health Questionnaire (PHQ-9): a meta-analysis. CMAJ 2012; 184: E191–E196. DOI: 10.1503/cmaj.110829 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Reilly MC, Zbrozek AS, Dukes EM. The validity and reproducibility of a work productivity and activity impairment instrument. Pharmacoeconomics 1993; 4: 353–365. DOI: 10.2165/00019053-199304050-00006 [DOI] [PubMed] [Google Scholar]
- 24.Meltzer EO, Zeiger RS, Dicpinigaitis P, et al. Prevalence and burden of chronic cough in the United States. J Allergy Clin Immunol Pract 2021; 9: 4037–4044. DOI: 10.1016/j.jaip.2021.07.022 [DOI] [PubMed] [Google Scholar]
- 25.Tobe K, Kubo T, Okuyama K, et al. Web-based survey to evaluate the prevalence of chronic and subacute cough and patient characteristics in Japan. BMJ Open Respir Res 2021; 8: e000832. DOI: 10.1136/bmjresp-2020-000832 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Campi G, Noale M, Fabbrizzi A, et al. The demographic and clinical characteristics of an Italian population of adult outpatients with chronic cough. Aging Clin Exp Res 2020; 32: 741–746. DOI: 10.1007/s40520-019-01464-4 [DOI] [PubMed] [Google Scholar]
- 27.Kanemitsu Y, Niimi A, Matsumoto H, et al. Gastroesophageal dysmotility is associated with the impairment of cough-specific quality of life in patients with cough variant asthma. Allergol Int 2016; 65: 320–326. DOI: 10.1016/j.alit.2016.02.014 [DOI] [PubMed] [Google Scholar]
- 28.Shirai T, Mikamo M, Tsuchiya T, et al. Real-world effect of gastroesophageal reflux disease on cough-related quality of life and disease status in asthma and COPD. Allergol Int 2015; 64: 79–83. DOI: 10.1016/j.alit.2014.08.001 [DOI] [PubMed] [Google Scholar]
- 29.Morice A. Chronic cough: epidemiology. Chron Respir Dis 2008; 5: 43–47. DOI: 10.1177/1479972307084252 [DOI] [PubMed] [Google Scholar]
- 30.Chung KF, Pavord ID. Prevalence, pathogenesis, and causes of chronic cough. Lancet 2008; 371: 1364–1374. DOI: 10.1016/s0140-6736(08)60595-4 [DOI] [PubMed] [Google Scholar]
- 31.D’Urzo A, Jugovic P. Chronic cough. Three most common causes. Can Fam Physician 2002; 48: 1311–1316. [PMC free article] [PubMed] [Google Scholar]
- 32.Gouveia CJ, Yalamanchili A, Ghadersohi S, et al. Are chronic cough and laryngopharyngeal reflux more common in obstructive sleep apnea patients? Laryngoscope 2019; 129: 1244–1249. DOI: 10.1002/lary.27557 [DOI] [PubMed] [Google Scholar]
- 33.Sundar KM, Daly SE. Chronic cough and OSA: an underappreciated relationship. Lung 2014; 192: 21–25. DOI: 10.1007/s00408-013-9534-9 [DOI] [PubMed] [Google Scholar]
- 34.Good JT, Jr., Rollins DR, Kolakowski CA, et al. New insights in the diagnosis of chronic refractory cough. Respir Med 2018; 141: 103–110. DOI: 10.1016/j.rmed.2018.06.024 [DOI] [PubMed] [Google Scholar]
- 35.Sundar KM, Stark AC, Hu N, et al. Is laryngeal hypersensitivity the basis of unexplained or refractory chronic cough? ERJ Open Res 2021; 7: 00793–02020. DOI: 10.1183/23120541.00793-2020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Ternesten-Hasséus E, Larsson S, Millqvist E. Symptoms induced by environmental irritants and health-related quality of life in patients with chronic cough-A cross-sectional study. Cough 2011; 7: 6. DOI: 10.1186/1745-9974-7-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Koskela HO, Lätti AM, Purokivi MK. Long-term prognosis of chronic cough: a prospective, observational cohort study. BMC Pulm Med 2017; 17: 146. DOI: 10.1186/s12890-017-0496-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Kardos P, Blaiss M, Dicpinigaitis P. Addressing unmet needs for diagnosis and management of chronic cough in the primary care setting. Postgrad Med 2021; 133: 481–488. DOI: 10.1080/00325481.2021.1914944 [DOI] [PubMed] [Google Scholar]
- 39.Mazzone SB, McGarvey L. Mechanisms and rationale for targeted therapies in refractory and unexplained chronic cough. Clin Pharmacol Ther 2021; 109: 619–636. DOI: 10.1002/cpt.2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Muccino D, Green S. Update on the clinical development of gefapixant, a P2X3 receptor antagonist for the treatment of refractory chronic cough. Pulm Pharmacol Ther 2019; 56: 75–78. DOI: 10.1016/j.pupt.2019.03.006 [DOI] [PubMed] [Google Scholar]