Abstract
Objective
Little is known about the transmission of coronavirus disease 2019 (COVID-19) in young children. This study aimed to clarify the risk of COVID-19 transmission among this population.
Methods
Between July 2020 and April 2022, 1660 0 to 3-year-old children underwent a nasopharyngeal swab for later reverse transcription-polymerase chain reaction testing at a mass screening test center in Japan. Their disease transmission rate and clinical symptoms were evaluated according to the predominant variant strains of that season.
Results
The secondary transmission rate after close contact of the Delta B.1.617.2 (17.4%) and Omicron B.1.1.529 (39.2%) variants was significantly higher than that of the conventional strains (B.1.1.284 and B.1.1.214; 4.5%) during the pandemic. The increased transmissibility with the Delta and Omicron variants was independent of close contact or location. The prevalence rates of cough, fatigability, and fever were similar in young children infected by the Delta and Omicron variants.
Conclusions
COVID-19 transmission in children aged 0 to 3 years increased by 3 to 4 fold during the Delta outbreak and by 8 to 10 fold during the Omicron outbreak compared with the conventional strain outbreak. The symptoms in young children were not different between the Delta and Omicron variants.
Keywords: Coronavirus disease 2019, young child, symptom, transmission, variant strain, Omicron, Delta
Introduction
The pandemic of coronavirus disease 2019 (COVID-19) caused by severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) remains a major global health concern in 2022.1,2 This pandemic is further complicated by the intermittent emergence of consequential variant strains. 3 Japan faced the fifth wave of a nationwide COVID-19 infection with the Delta variant (Phylogenetic Assignment of Named Global Outbreak [PANGO] lineage B.1.617.2) in August 2021, 4 during which the majority of adults in the country underwent two vaccinations against COVID-19.5,6 In parallel with the spread of the Delta variant and the smooth progression of mass vaccination campaigns among adults, the spread of COVID-19 among non-adults, especially infants and young children, has been a focus of attention.7,8 In the sixth wave with the Omicron variant (lineage B.1.1.529) since January 2022, the transmission of infection in young children from classmates at preschools/schools and at homes from other family members was further focused on.9,10 During these waves, the number of cluster outbreaks at preschools/schools apparently increased, and subsequent child-to-parent transmission could also have increased compared with that in the preceding seasons. During the pandemic prior to the of arrival of the Delta variant, the secondary transmission rate of variants at preschools or schools was lower than that outside preschools/schools. However, whether this situation also applies to the Delta and Omicron variants is yet to be determined by studies including large populations. This population-based, observational study reports the status of COVID-19 infection among young children aged 0 to 3 years between July 2020 and April 2022 in Japan. This study aimed to clarify the secondary transmission risk of COVID-19 in young children before the Delta outbreak and during the Delta and Omicron outbreaks.
Patients and methods
Participants
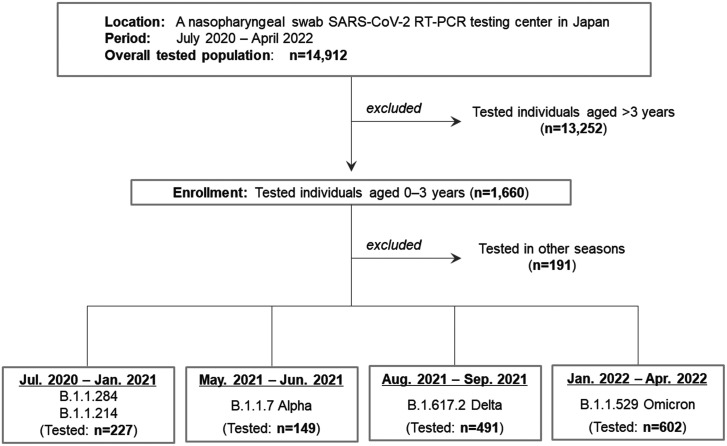
This study enrolled young children aged 0 to 3 years. The children had a nasopharyngeal swab performed to test for SARS-CoV-2 using reverse transcription-polymerase chain reaction (RT-PCR) at a mass screening test center in Sendai City (population of approximately 1.1 million) between July 2020 and April 2022. 11 This screening test center was the largest in the city for testing small children by the nasopharyngeal swab test. This center diagnosed approximately half of all patients with COVID-19 aged 0 to 3 years in the city during the study period. Overall, the testing center performed the nasopharyngeal swab RT-PCR test on 14,912 citizens during the study period, among whom 1660 (11.1%) were young children aged 0 to 3 years. This testing rate in this age group among the overall tested individuals at the center was higher than that expected from the age distribution. The reason for this high testing rate is probably because most of the young children aged 0 to 3 years in the city, who were judged by the local governments to have required RT-PCR testing, were preferentially guided to visit the above-mentioned screening test center. With regard to those in other age groups, there was another large mass-screening RT-PCR test center in the city. During the entire study period, none of the enrolled children aged 0 to 3 years were vaccinated for COVID-19. 12 To compare the transmission risk between different types of preschools/schools, RT-PCR-tested elementary school students and junior high school students who had contact with patients with COVID-19 at preschools/schools were further enrolled. The study design of the present study is shown in Figure 1.
Figure 1.
Flow diagram of the study design. Individuals who had a nasopharyngeal swab RT-PCR test at a large screening test center in Japan between July 2020 and April 2022 were initially recruited. In this population, young children aged 0 to 3 years were enrolled. The study period was then divided into the following four seasons: July 2020 to January 2021 (B.1.1.284 and B.1.1.214 outbreaks), May 2021 to June 2021 (B.1.1.7 Alpha outbreak), August 2021 to September 2021 (B.1.617.2 Delta outbreak), and January 2022 to April 2022 (B.1.1.529 Omicron outbreak). Secondary transmission rates stratified by contact closeness or locations and symptoms in RT-PCR test-positive young children were then compared between the periods.
SARS-CoV-2, severe acute respiratory syndrome coronavirus 2; RT-PCR, reverse transcription-polymerase chain reaction.
Nasopharyngeal swab RT-PCR test
Almost all young children aged 0 to 3 years were tested on the basis of their recent contact history with patients infected with COVID-19. Nasopharyngeal swab tests were scheduled to be performed 4 to 5 days after making contact with patients with COVID-19. The presence of COVID-19-related symptoms was checked at the swab testing site. The RT-PCR test was performed by detecting the nucleocapsid protein set no. 2 (N2) gene. The primer/probe set developed by the National Institute of Infectious Diseases in Japan (NIID_2019-nCoV_N_F2, R2, and P2) was used.13,14 During the study period, none of the children aged <10 years were vaccinated against COVID-19 in the city.
Evaluated variables
Contact closeness with patients with COVID-19 was objectively assessed by local government staff before the nasopharyngeal swab test. High-risk contact was judged by local government staff on basis of the fulfillment of all of the following four criteria: 1) contact from 2 days before to 14 days after the onset of symptoms or positive RT-PCR test results; 2) not wearing masks; 3) <1-m distance; and 4) ≥15 minutes of contact. These criteria were not changed during the study period. The place of contact was classified into household contact, contact at preschools, and others. The presence of COVID-19-related clinical symptoms, such as cough, fatigability, and fever, was assessed at the swab test site. Therefore, the clinical symptoms evaluated in the present study were the symptoms that occurred 4 to 5 days after contacting patients with COVID-19. The secondary transmission rate in each subgroup was calculated by dividing the number of RT-PCR test-positive young children by the total number of the RT-PCR tested young children.
Study periods with different prevailing variants
The study period was divided into the following four periods with different predominant SARS-CoV-2 strains: July 2020 to January 2021 (predominance of conventional strains: B.1.1.284 and B.1.1.214 PANGO lineages), May 2021 to June 2021 (predominance of B.1.1.7 Alpha), August 2021 to September 2021 (predominance of B.1.617.2 Delta), and January 2022 to April 2022 (predominance of B.1.1.529 Omicron). The RT-PCR test results and clinical symptoms at 4 to 5 days after the enrolled young children were in contact patients with COVID-19 were compared between these four seasons with different prevailing COVID-19 variant strains.
Statistical analysis
Comparisons of the prevalence of events between the two groups were performed using the chi-square test or Fisher’s exact test, according to the number of events in each group. Comparisons of the prevalence of events between three or more groups were performed using the chi-square test. To evaluate the diagnostic value of each evaluated COVID-19-related symptom, the positive likelihood ratio (PLR), which was calculated as , was obtained for each symptom by using the data from RT-PCR test-positive and -negative young children. PLR values > 5.0 were considered to have strong diagnostic values. Statistical significance was set at p <0.05. Statistical analyses were performed using R statistical software (version 4.0.5; R Foundation, Vienna, Austria).
Ethics
This study was approved by the institutional review board of Tohoku University School of Medicine (approval number: THK 2021-1-705). The requirement for written informed consent from the parents was waived by the institutional review board. Informed consent was obtained in an opt-out manner. 15 An overview of the study design and the opt-out process are described on the website of the Tohoku University Graduate School of Medicine. The reporting of this study conforms to STROBE guidelines. 16
Results
Secondary transmission in different seasons
We included 185 (61.1%) girls and 118 (38.9%) boys. The rate of girls appeared to be higher than that of boys. Among the enrolled 1660 young children, 303 (18.3%) showed a positive RT-PCR test. The secondary transmission rates in the young children by season with different prevailing strains and by contact situations are shown in Table 1. The secondary transmission risk in young children with the B.1.617.2 Delta or B.1.1.529 Omicron variant was significantly higher than that in young children with the former strains before the B.1.1.7 Alpha outbreak (i.e., B.1.1.284 and B.1.1.214) was confirmed (both p <0.0001). The secondary transmission rate after high-risk contact in young children was 4.5% during the B.1.1.284 and B.1.1.214 outbreaks, but this was not significantly different from that during the B.1.1.7 Alpha outbreak (8.8%, Fisher’s exact test). However, the secondary transmission rate after high-risk contact in young children during the B.1.617.2 Delta (p = 0.0016) and B.1.1.529 Omicron (p <0.0001, Fisher’s exact test) outbreaks was significantly higher than that during the former strains. The increased transmissibility with Delta and Omicron was independent of the contact level or location.
Table 1.
Demographic and clinical data of young children aged 0 to 3 years who underwent a nasopharyngeal swab for SARS-CoV-2 RT-PCR testing.
| July 2020 toJanuary 2021 | May 2021 toJune 2021 | August 2021 toSeptember 2021 | January 2022 toApril 2022 | p-value | |
|---|---|---|---|---|---|
| Predominant PANGOlineages in the locality | B.1.1.284 B.1.1.214 | B.1.1.7 (Alpha) | B.1.617.2 (Delta) | B.1.1.529 (Omicron) | – |
| Children tested by RT-PCR aged 0–3 years, n | 227 | 149 | 491 | 602 | – |
| RT-PCR (+) cases, n (%) | 4 (1.8) | 6 (4.0) | 64 (13.0) | 208 (34.6) | <0.0001 |
| RT-PCR (–) cases, n (%) | 223 (98.2) | 143 (96.0) | 427 (87.0) | 394 (65.4) | |
| RT-PCR (+) after high-risk contact, n (%) | 4/89 (4.5) | 5/57 (8.8) | 49/282 (17.4) | 189/482 (39.2) | <0.0001 |
| RT-PCR (+) after low-risk contact, n (%) | 0/133 (0.0) | 1/88 (1.1) | 15/204 (7.4) | 15/112 (13.4) | <0.0001 |
| RT-PCR (+) after household contact, n (%) | 3/47 (6.4) | 2/17 (11.8) | 36/121 (29.8) | 183/442 (41.4) | <0.0001 |
| RT-PCR (+) after contact at preschools, n (%) | 1/180 (0.6) | 1/88 (1.1) | 20/325 (6.2) | 20/144 (13.9) | <0.0001 |
| RT-PCR (+) after contact at other places, n (%) | None | 3/44 (6.8) | 8/45 (17.8) | 4/10 (40.0) | 0.0245 |
| Symptoms 4–5 days after contact in young children aged 0–3 years with COVID-19 (RT-PCR +), n (%) | |||||
| Cough | 0/4 (0.0) | 2/6 (33.3) | 19/64 (29.7) | 91/208 (43.8) | 0.0772 |
| Appearance of fatigability | 0/4 (0.0) | 0/6 (0.0) | 6/64 (9.4) | 23/208 (11.1) | 0.7200 |
| Body temperature ≥37.0°C | 0/4 (0.0) | 1/6 (16.7) | 19/64 (29.7) | 65/208 (31.3) | 0.4991 |
| Body temperature ≥37.5°C | 0/4 (0.0) | 0/6 (0.0) | 9/64 (14.1) | 30/208 (14.4) | 0.6438 |
| Symptoms 4–5 days after contact in young children aged 0–3 years (RT-PCR –), n (%) | |||||
| Cough | 57/223 (25.6) | 40/142 (28.2) | 101/427 (23.7%) | 103/394 (26.1) | 0.7102 |
| Appearance of fatigability | 2/136 (1.5) | 4/141 (2.8) | 4/427 (0.9) | 7/394 (1.8) | 0.4362 |
| Body temperature ≥37.0°C | 46/222 (20.7) | 35/141 (24.8) | 84/427 (19.7) | 70/394 (17.8) | 0.3365 |
| Body temperature ≥37.5°C | 4/222 (1.8) | 5/141 (3.5) | 10/427 (2.3) | 6/394 (1.5) | 0.5169 |
All children aged 0 to 3 years in the present study underwent nasopharyngeal swab tests to collect samples for SARS-CoV-2 RT-PCR testing. The p values are the results of the chi-square test between the four periods.
SARS-CoV-2, severe acute respiratory syndrome coronavirus 2; RT-PCR, reverse transcription-polymerase chain reaction; PANGO, Phylogenetic Assignment of Named Global Outbreak; COVID-19, coronavirus disease 2019.
COVID-19-related symptoms
The data on clinical manifestation in RT-PCR test-positive and RT-PCR test-negative young children at 4 to 5 days after contact with patients with COVID-19 during each of the four pandemic periods are shown in Table 1. The numbers of RT-PCR test-positive young children during the B.1.1.284, B.1.1.214, and B.1.1.7 Alpha outbreaks were too small to evaluate their clinical characteristics. During the Delta and Omicron outbreaks, the clinical manifestations in RT-PCR test-positive and RT-PCR-negative young children remained at similar levels. Only the prevalence of cough symptoms in the Omicron outbreak was slightly higher than that in the Delta outbreak (p = 0.045, chi-square test).
When we compared the symptoms between RT-PCR test-positive and RT-PCR negative young children during the Delta or Omicron outbreak, the presence of fatigability (PLR: 10.01, 95% confidence interval [CI] [2.90–34.50] during the Delta outbreak and 6.22 [2.72–14.26] during the Omicron outbreak) had a higher predictive effect on RT-PCR test positivity than the presence of a cough (1.26 [0.83–1.90] during the Delta outbreak and 1.67 [1.33–2.10] during the Omicron outbreak). The body temperature had a higher predictive effect on RT-PCR test positivity when the cutoff was set to ≥37.5°C (PLR: 6.00 [2.54–14.21] during the Delta outbreak and 9.74 [4.01–22.39] during the Omicron outbreak) than when the cutoff was set to ≥37.0°C (1.51 [0.99–2.30] during the Delta outbreak and 1.76 [1.31–2.36] during the Omicron outbreak).
Secondary transmission at preschools
To clarify whether the risk of transmission at preschools/schools was different between young children aged 0 to 3 years and other older children, secondary transmission at preschools/schools in different seasons was compared between the age groups (Table 2).The secondary transmission rate during the Delta (p = 0.0006) and Omicron (p <0.0001, Fisher’s exact test) outbreaks was significantly higher than that in seasons with the former strains before B.1.1.7 Alpha in preschool children. A similar trend during the Omicron outbreak was also found in children who had contact with patients with COVID-19 at elementary schools (p = 0.0003) and junior high schools (p = 0.0622, Fisher’s exact test).
Table 2.
Secondary transmission rates among children at preschools/schools.
| July 2020 toJanuary 2021 | August 2021 toSeptember 2021 | January 2021 toApril 2022 | p-value | |
|---|---|---|---|---|
| Predominant PANGO lineages in the locality | B.1.1.284B.1.1.214 | B.1.617.2 (Delta) | B.1.1.529 (Omicron) | – |
| Contact at preschools,n (%) | ||||
| RT-PCR (+) (overall) | 4/477 (0.8) | 20/443 (4.5) | 28/180 (15.6) | <0.0001 |
| RT-PCR (+)(0–3 years old) | 1/180 (0.6) | 20/325 (6.2) | 20/144 (13.9) | <0.0001 |
| RT-PCR (+)(4–6 years old) | 3/297 (1.0) | 0/118 (0.0) | 8/36 (22.2) | <0.0001 |
| Contact at elementary schools, n (%) | ||||
| RT-PCR (+) | 5/519 (1.0) | 0/246 (0.0) | 13/228 (5.7) | <0.0001 |
| Contact at junior high schools, n (%) | ||||
| RT-PCR (+) | 0/181 (0.0) | 0/79 (0.0) | 1/12 (8.3) | <0.0001 |
Children aged 0 to 3 years were not forced to wear masks in preschools, whereas children aged 4 to 6 years wore masks in preschools. The p values are the results of the chi-square test between the four periods. Percentages of RT-PCR test-positive individuals were calculated for each type of preschool/school.
PANGO, Phylogenetic Assignment of Named Global Outbreak; RT-PCR, reverse transcription-polymerase chain reaction.
Discussion
This study showed that the transmissibility of the Delta variant in young children aged 0 to 3 years was three to four times higher than that of the former strains before the Alpha outbreak and approximately two times higher than that of the Alpha variant. Furthermore, the transmissibility of the Omicron variant in young children appeared to be approximately twice as high as that of the Delta variant. The transmission rate of the Delta variant was reported to be 40% to 60% higher than that of the Alpha variant. 17 This study suggested that the increased rate of transmission with the Delta variant could be greater in young children than in other age groups. However, although the transmissibility among young children was remarkably elevated with the Delta variant, none of them became severe or were admitted to hospitals. Previous studies that evaluated the outcomes in children showed a milder disease course and better prognosis in children than in adults.18,19 This finding may be true even with the Delta and Omicron variants. A likely theory to explain the milder clinical course in children is that they have a strong innate immune response based on numerous viral infections without immunological memories. Such repeated attacks from numerous pathogens could train and boost the immune system against COVID-19 infection in children.18,20 Adult patients are more likely to show a suppressed or dysfunctional over-active immune response compared with children, possibly based on immune senescence and less frequent exposure to vaccinations or viral infections. 21
Another notable finding in this study was that an increase in secondary transmission at preschools/schools during the Delta outbreak was only found in young children aged 0 to 3 years. A conceivable theory to explain this finding is that the young children of this age group in Japan do not wear masks at preschools/schools. That children aged 4 to 6 years did not show such an increased transmission at preschools/schools with Delta supports this theory. Another conceivable theory is that young children aged 0 to 3 years may be more vulnerable to feco-oral transmission because of possible prolonged fecal shedding of the virus in children.22,23 During the Omicron outbreak, the transmission risk at elementary schools was similar to that at preschools. This finding suggests that only wearing masks may not have been sufficient to fully prevent transmission of COVID-19 during the Omicron outbreak because of the variant’s remarkably facilitated transmission efficiency. Further studies are warranted to scientifically evaluate these hypotheses and explain the remarkably increased transmissibility of the Delta and Omicron variants among small children.
This study suggested that decreasing transmission in young children with appropriate safety measures may benefit public health of the general population, especially during the Omicron outbreak with remarkably increased transmissibility. The safety of vaccinations against COVID-19 in young children aged 0 to 3 years is still unknown, and it should be carefully determined by evidence. Masking is recommended for children aged ≥2 years in the United States and in those aged ≥3 years in Japan.24,25 In both countries, there is agreement that young children aged 0 to 1 year should not be forced to wear masks from the viewpoint of safety. The safety and efficiency of forcing young children aged 2 to 3 years to wear masks remain controversial.26–28 The current priority in infection control measures among young children appears to be deciding the safety of vaccination or developing vaccines that can be safely used for young children.
Conclusions
The COVID-19 transmission risk among young children aged 0 to 3 years is 3 to 4 times higher with Delta and 8 to 10 times higher with Omicron than that with the former strains before Alpha. Clinical symptoms among SARS-CoV-2 RT-PCR test-positive young children are not remarkably different between the Delta and Omicron outbreaks. Although most children infected by the virus will not become severely infected, young children aged 0 to 3 years still have a high risk of transmission during Delta and Omicron outbreaks.
Acknowledgements
The authors appreciate all medical staff and local government staff (Sendai City, Miyagi Prefecture) who joined and cooperated with the drive-through RT-PCR testing project. The authors also appreciate all tested young children and their parents for having approved the screening test to implement appropriate infection control measures in the locality.
Footnotes
Declaration of conflicting interest: The authors declare that they have no potential competing interests with respect to this study.
Funding: This research received no specific grant from any funding agency in the public, commercial or not-for-profit sectors.
Author contributions: TA collected the data, performed statistical analyses, and drafted the manuscript. TI supervised the process of the study, collected the data, and critically revised the manuscript.
ORCID iD: Tetsuya Akaishi https://orcid.org/0000-0001-6728-4966
References
- 1.The Lancet Infectious D. Transitioning to endemicity with COVID-19 research. Lancet Infect Dis 2022; 22: 297. 20220210. DOI: 10.1016/s1473-3099(22)00070-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Reis G, Dos Santos Moreira-Silva EA, Silva DCM, et al. Effect of early treatment with fluvoxamine on risk of emergency care and hospitalisation among patients with COVID-19: the TOGETHER randomised, platform clinical trial. Lancet Glob Health 2022; 10: e42–e51. 20211028. DOI: 10.1016/s2214-109x(21)00448-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Fontanet A, Autran B, Lina B, et al. SARS-CoV-2 variants and ending the COVID-19 pandemic. Lancet 2021; 397: 952–954. 2021/02/15. DOI: 10.1016/s0140-6736(21)00370-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.World Health Organization. Tracking SARS-CoV-2 variants, https://www.who.int/en/activities/tracking-SARS-CoV-2-variants/.
- 5.Song P, Karako T. The strategy behind Japan's response to COVID-19 from 2020–2021 and future challenges posed by the uncertainty of the Omicron variant in 2022. Biosci Trends 2022; 15: 350–352. 20211228. DOI: 10.5582/bst.2021.01560. [DOI] [PubMed] [Google Scholar]
- 6.Kodera S, Rashed EA, Hirata A. Estimation of Real-World Vaccination Effectiveness of mRNA COVID-19 Vaccines against Delta and Omicron Variants in Japan. Vaccines (Basel) 2022; 10: 430. DOI: 10.3390/vaccines10030430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Shoji K, Akiyama T, Tsuzuki S, et al. Comparison of the clinical characteristics and outcomes of COVID-19 in children before and after the emergence of Delta variant of concern in Japan. J Infect Chemother 2022; 28: 591–594. 20220120. DOI: 10.1016/j.jiac.2022.01.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kobayashi J, Takeuchi R, Shibuya F, et al. Recommendations for the urgent need to vaccinate school-aged and adolescent children against COVID-19 in the Asia-Pacific region. Trop Med Health 2021; 49: 74–74. DOI: 10.1186/s41182-021-00365-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Cloete J, Kruger A, Masha M, et al. Paediatric hospitalisations due to COVID-19 during the first SARS-CoV-2 omicron (B.1.1.529) variant wave in South Africa: a multicentre observational study. Lancet Child Adolesc Health 2022; 6: 294–302. 20220218. DOI: 10.1016/s2352-4642(22)00027-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Torjesen I. Covid-19: Omicron variant is linked to steep rise in hospital admissions of very young children. Bmj 2022; 376: o110. 20220114. DOI: 10.1136/bmj.o110. [DOI] [PubMed] [Google Scholar]
- 11.Sendai City Official Web Site. Population in Sendai city of the first day of every month., http://www.city.sendai.jp/koryu/foreignlanguage/en/index.html.
- 12.Japanese Ministry of Health, Labour and Welfare. Nationwide vaccination status against COVID-19 in Japan, https://www.mhlw.go.jp/stf/seisakunitsuite/bunya/vaccine_sesshujisseki.html (2022).
- 13.Shirato K, Nao N, Katano H, et al. Development of Genetic Diagnostic Methods for Detection for Novel Coronavirus 2019 (nCoV-2019) in Japan. Jpn J Infect Dis 2020; 73: 304–307. 2020/02/20. DOI: 10.7883/yoken.JJID.2020.061. [DOI] [PubMed] [Google Scholar]
- 14.Ishii T, Kushimoto S, Katori Y, et al. Predictors of SARS-CoV-2 Positivity Based on RT-PCR Swab Tests at a Drive-Through Outpatient Clinic for COVID-19 Screening in Japan. Tohoku J Exp Med 2021; 253: 101–108. 2021/02/05. DOI: 10.1620/tjem.253.101. [DOI] [PubMed] [Google Scholar]
- 15.Vellinga A, Cormican M, Hanahoe B, et al. Opt-out as an acceptable method of obtaining consent in medical research: a short report. BMC Med Res Methodol 2011; 11: 40. 20110406. DOI: 10.1186/1471-2288-11-40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Von Elm E, Altman DG, Egger M, et al. The Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) statement: guidelines for reporting observational studies. Ann Intern Med 2007; 147: 573–577. DOI: 10.7326/0003-4819-147-8-200710160-00010. [DOI] [PubMed] [Google Scholar]
- 17.Teyssou E, Delagrèverie H, Visseaux B, et al. The Delta SARS-CoV-2 variant has a higher viral load than the Beta and the historical variants in nasopharyngeal samples from newly diagnosed COVID-19 patients. J Infect 2021; 83: e1–e3. 2021/08/23. DOI: 10.1016/j.jinf.2021.08.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Panahi L, Amiri M, Pouy S. Clinical Characteristics of COVID-19 Infection in Newborns and Pediatrics: A Systematic Review. Arch Acad Emerg Med 2020; 8: e50. 2020/05/23. [PMC free article] [PubMed] [Google Scholar]
- 19.Ludvigsson JF. Systematic review of COVID-19 in children shows milder cases and a better prognosis than adults. Acta Paediatr 2020; 109: 1088–1095. 2020/03/24. DOI: 10.1111/apa.15270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Sinaei R, Pezeshki S, Parvaresh S, et al. Why COVID-19 is less frequent and severe in children: a narrative review. World J Pediatr 2021; 17: 10–20. 2020/09/27. DOI: 10.1007/s12519-020-00392-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Dhochak N, Singhal T, Kabra SK, et al. Pathophysiology of COVID-19: Why Children Fare Better than Adults? Indian J Pediatr 2020; 87: 537–546. 2020/05/16. DOI: 10.1007/s12098-020-03322-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Li X, Xu W, Dozier M, et al. The role of children in transmission of SARS-CoV-2: A rapid review. J Glob Health 2020; 10: 011101. 2020/07/03. DOI: 10.7189/jogh.10.011101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Xing YH, Ni W, Wu Q, et al. Prolonged viral shedding in feces of pediatric patients with coronavirus disease 2019. J Microbiol Immunol Infect 2020; 53: 473–480. 2020/04/12. DOI: 10.1016/j.jmii.2020.03.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Knopf A. Mask and vaccination guidance from AAP. The Brown University Child and Adolescent Behavior Letter 2021; 37: 9–10. 2021/07/05. DOI: 10.1002/cbl.30566. [Google Scholar]
- 25.Murray TS, Malik AA, Shafiq M, et al. Association of Child Masking With COVID-19-Related Closures in US Childcare Programs. JAMA Netw Open 2022; 5: e2141227–e2141227. DOI: 10.1001/jamanetworkopen.2021.41227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Esposito S, Principi N. To mask or not to mask children to overcome COVID-19. Eur J Pediatr 2020; 179: 1267–1270. 2020/05/11. DOI: 10.1007/s00431-020-03674-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Eberhart M, Orthaber S, Kerbl R. The impact of face masks on children-A mini review. Acta Paediatr 2021; 110: 1778–1783. 2021/02/04. DOI: 10.1111/apa.15784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Lubrano R, Bloise S, Testa A, et al. Assessment of Respiratory Function in Infants and Young Children Wearing Face Masks During the COVID-19 Pandemic. JAMA Netw Open 2021; 4: e210414. 2021/03/03. DOI: 10.1001/jamanetworkopen.2021.0414. [DOI] [PMC free article] [PubMed] [Google Scholar]