Abstract
The availability of Bruton tyrosine kinase (BTK) inhibitors has brought about a paradigm shift in the treatment of patients with B-cell lymphomas and chronic lymphocytic leukemia. BTK was clinically validated as a target by the efficacy of the first-in-class inhibitor ibrutinib. The extended survival conferred by BTK inhibitors has brought long-term tolerability to the foreground. To minimize toxicities thought to be attributable to off-target kinase inhibition, a next generation of BTK inhibitors with greater selectivity was developed. In the United States, zanubrutinib, a next-generation BTK inhibitor, has been approved for treating adults with mantle cell lymphoma who have received at least one prior therapy, for adults with Waldenström macroglobulinemia, and for adults with relapsed or refractory marginal zone lymphoma who have received at least one anti-CD20–based therapy. Because few head-to-head comparative trials of BTK inhibitors have so far been reported, no BTK ‘inhibitor of choice’ can be identified. Zanubrutinib has promising efficacy in its approved indications and appears to have reduced cardiac toxicities, particularly atrial fibrillation, which may influence the choice of BTK inhibitor treatment by prescribers. Further studies are needed to inform on optimal treatment sequencing of zanubrutinib and its combination with other agents. Here, we summarize existing clinical evidence for its efficacy and safety in mantle cell lymphoma, Waldenström macroglobulinemia, marginal zone lymphoma, chronic lymphocytic leukemia/small lymphocytic lymphoma, and other B-lymphoproliferative indications.
Plain Language Summary
Zanubrutinib is a drug that was shown to effectively treat cancer of B cells without causing excessive serious side effects
Patients with certain B-cell malignancies (cancers of white blood cells) benefit from treatment with Bruton tyrosine kinase (BTK) inhibitors, drugs that block the BTK protein and keep cancer from growing and spreading. Patients experience extended survival with ibrutinib, the first-generation BTK inhibitor approved by US Food and Drug Administration (FDA); however, one in five patients quit treatment because of harmful side effects. Ibrutinib-related side effects such as increased risk of bleeding, atrial fibrillation (abnormal heart rhythm), and high blood pressure are thought to be caused by ibrutinib blocking other proteins besides the intended target protein BTK. To reduce these side effects, zanubrutinib, a next-generation BTK inhibitor, was designed to block BTK more specifically than ibrutinib. Results of clinical studies on zanubrutinib treatment appear promising in patients with several types of B-cell malignancies, including mantle cell lymphoma (MCL), Waldenström macroglobulinemia (WM), marginal zone lymphoma (MZL), chronic lymphocytic leukemia, and small lymphocytic lymphoma. There are not yet enough clinical data to determine which BTK inhibitor is most effective in treating B-cell malignancies without causing harmful side effects. Early data from the phase 3 ALPINE clinical study suggest that zanubrutinib works better than ibrutinib, and fewer patients experience side effects and quit treatment. Zanubrutinib is currently approved for use for treatment of adult patients with MCL who have received at least one prior therapy, for adults with WM, and for adults with MZL who have received at least one anti-CD20–based therapy.
Keywords: B-cell malignancies, Bruton tyrosine kinase, BTK inhibitor, chronic lymphocytic leukemia, mantle cell lymphoma, marginal zone lymphoma, Waldenström macroglobulinemia, zanubrutinib
Introduction
Bruton tyrosine kinase (BTK)-mediated intracellular signaling of the B-cell receptor (BCR) is critical to normal B-cell development, expansion, and survival. 1 BTK, a member of the tyrosine kinase expressed in carcinoma (TEC) kinase family, is a nonreceptor tyrosine kinase and an essential component of the BCR signalosome. 2 During BCR signaling, BTK phosphorylation leads signal transduction via multiple pathways, culminating in the nuclear translocation of transcription factors, including NF-κB. 3 Activated BCR signaling contributes to the initiation and maintenance of B-cell malignancies. Aberrant BCR signaling has been strongly linked to lymphomagenesis 4 and plays a critical role in the pathobiology of chronic lymphocytic leukemia (CLL), 5 mantle cell lymphoma (MCL), 6 and other hematologic malignancies.7–10
BTK is an essential component of BCR signaling, as demonstrated by the observed effects of mutations interfering with its function in both animals and humans. 4 BTK was clinically validated as a target in B-cell malignancies by the first-in-class inhibitor ibrutinib,11,12 which currently has six US Food and Drug Administration (FDA)-approved indications, including CLL, MCL, Waldenström macroglobulinemia (WM), and marginal zone lymphoma (MZL). 13 Although it represents a significant advance in the treatment of these diseases, ibrutinib intolerance leading to treatment discontinuation remains a concern, particularly since continued administration for an indefinite course is recommended. Large, randomized trials of ibrutinib in CLL for which long-term (⩾ 5-year) data are available have reported discontinuation rates of 41–72%, with approximately 20% of patients having discontinued ibrutinib due to adverse events (AEs).14–17 Similar findings have been reported with MCL 18 and WM. 19 Real-world ibrutinib discontinuation rates in CLL appear to be higher than those in most trial reports (e.g. 49% at 30 months).20,21 Subsequent outcomes for patients who progress on ibrutinib have generally been poor. 22 Patients with CLL resistant to both BTK inhibitors (BTKis) and BCL2-targeted agents have particularly short survival, 23 although novel treatments such as chimeric antigen receptor (CAR) T-cell therapy may improve this.
In addition to BTK, ibrutinib is known to inhibit a number of other kinases at clinically relevant concentrations 24 and the off-target inhibition of TEC kinase is believed to contribute to the increased risk of bleeding.25–28 Similarly, off-target kinase inhibition can be associated with atrial fibrillation and explains ibrutinib-associated hypertension, seen in as many as 71.6% of patients who were normotensive at baseline. 29 Randomized trials and meta-analyses have linked ibrutinib to an increased risk of atrial fibrillation.28,30,31 Among patients with CLL, atrial fibrillation is the AE that most frequently leads to ibrutinib discontinuation in randomized trials and real-world practice.15,32 Although the mechanism of ibrutinib-associated atrial fibrillation is not entirely clear, off-target inhibition of TEC kinase is possibly involved, potentially via inhibition of cardiac PI3 K-Akt signaling. 33 Among other ibrutinib-associated AEs, the mechanistic basis for its increased infection risk is uncertain but may be related to off-target inhibition of interleukin-2-inducible T-cell kinase. 25 Off-target epidermal growth factor receptor (EGFR) inhibition may explain the diarrhea and rash observed in patients treated with ibrutinib.25,34–36
A more selective BTKi might minimize many of these off-target toxicities and may improve long-term treatment tolerability. In addition, a BTKi with greater potency could potentially minimize disease resistance and relapses, which are common reasons for ibrutinib discontinuation.16,18 Because BTK inhibition is key in treating B-cell malignancies as established by ibrutinib, the search began for a new generation of BTKis with more favorable properties.
The next BTKi to receive regulatory approval was acalabrutinib (ACP-196), which was approved by the FDA for use in relapsed or refractory (R/R) MCL in 2017 and for CLL/small lymphocytic lymphoma (SLL) in 2019.37,38 In a randomized, open-label, phase 3 head-to-head trial comparing acalabrutinib with ibrutinib in patients with previously treated CLL, acalabrutinib demonstrated noninferior progression-free survival (PFS; median 38.4 months in both arms). 39 All-grade atrial fibrillation rates were 9.4% versus 16.0% for acalabrutinib and ibrutinib, respectively (p = 0.02), but grade ⩾ 3 atrial fibrillation rates were higher (4.9% versus 3.8%) in the acalabrutinib arm. Any-grade (8.6% versus 22.8%) and grade ⩾ 3 hypertension (4.1% versus 8.7%) were significantly less frequent for acalabrutinib, but rates of grade ⩾ 3 infections (30.8% versus 30.0%) and major bleeding events (4.5% versus 5.3%) were similar between acalabrutinib and ibrutinib, respectively.
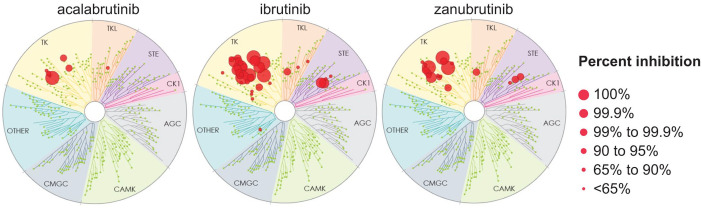
Zanubrutinib (BGB-3111) is the most recent next-generation, covalent, and irreversible BTKi approved for use in the United States, with first FDA approval granted in November 2019 for its use in previously treated MCL. Zanubrutinib is a potent, irreversible inhibitor designed for maximum BTK occupancy and minimal off-target kinase inhibition.40,41 Findings from in vivo and cellular assays have shown that zanubrutinib displays greater selectivity for BTK relative to TEC- and EGFR-family kinases than ibrutinib (Figure 1). In preclinical testing, zanubrutinib was associated with favorable antitumor activity compared with ibrutinib. 40 Zanubrutinib displayed favorable pharmacokinetic (PK) and pharmacodynamic (PD) properties in early clinical studies (Table 1). Its mean plasma half-life was 2–4 h following a single oral dose and was comparable in Asian and non-Asian patients. 42 Of note, zanubrutinib is primarily metabolized by CYP3A (cytochrome P450, family 3, subfamily A); dose reductions are therefore recommended for patients receiving concomitant moderate or strong CYP3A inhibitors, while co-administration of moderate or strong CYP3A inducers should be avoided.42–44
Figure 1.
Kinome profiling of acalabrutinib, ibrutinib, and zanubrutinib at a single dose of 1 mM (KINOMEscan; Eurofins DiscoverX, Fremont, CA).
Source: Adapted with permission from Kaptein et al. 45
Table 1.
Comparison of selected biochemical, pharmacokinetic, and pharmacodynamic properties for ibrutinib, acalabrutinib, and zanubrutinib.
| Ibrutinib | Acalabrutinib | Zanubrutinib | |
|---|---|---|---|
| FDA-approved indication(s)13,37,43 | CLL, MCL, MZL, WM, GVHD | CLL, MCL | MCL, WM, MZL |
| FDA-approved dose(s)13,37,43 | 420 or 560 mg QD | 100 mg BID | 160 mg BID or 320 mg QD |
| BTK IC50, nM 45 | 1.5 | 5.1 | 0.5 |
| TEC selectivity, fold | 6.7 | 25 | 88 |
| EGFR selectivity, fold | 3.5 | 196 | 42 |
| Half-life, hours | ~4–6 | ~0.6–2.8 | ~2–4 |
| Median BTK lymph node occupancy at trough46,47 | 420 mg QD: > 90% | 200 mg QD: 90% 100 mg BID: 95.8% |
320 mg QD: 94% 160 mg BID: 100% |
BID, twice daily; BTK, Bruton tyrosine kinase; CLL, chronic lymphocytic leukemia; EGFR, epidermal growth factor receptor; FDA, US Food and Drug Administration; GVHD, graft-versus-host disease; MCL, mantle cell lymphoma; MZL, marginal zone lymphoma; QD, once daily; TEC, tyrosine kinase expressed in carcinoma; WM, Waldenström macroglobulinemia.
In its first-in-human study BGB-3111-AU-003 (NCT02343120), near-complete (> 95%), sustained BTK occupancy in lymph nodes and peripheral blood mononuclear cells was observed at a twice-daily (BID) dose of 160 mg in patients with B-cell malignancies, 48 an encouraging result despite unknown clinical significance. This result was consistent with a PK study that found zanubrutinib trough concentrations sevenfold higher than its IC50 during the 24-h dosing period when administered at that dosage, indicating favorable exposure coverage. 49
After receiving breakthrough therapy designation in January 2019, zanubrutinib received accelerated approval for the treatment of adults with MCL who have received at least one prior therapy. 43 Here, we review the clinical studies supporting this approval as well as key trials in CLL and other lymphoproliferative diseases. Although some AE data are provided for each trial, tolerability issues are more extensively discussed in the pooled safety analysis section that follows the efficacy sections.
Zanubrutinib efficacy in MCL
BGB-3111-AU-003
This was a global, first-in-human, open-label, dose-escalation/indication-expansion trial50,51 (Table 2). The dose-escalation part of the study (part 1) was designed to determine the recommended phase 2 dose. Part 2 enrolled patients in disease-specific cohorts with either treatment-naïve (TN) or R/R B-cell malignancies, including MCL, CLL/SLL, and WM. Key exclusion criteria were prior BTKi exposure and a requirement for concurrent strong CYP3A inhibitors/inducers or QT-prolonging medications.
Table 2.
Summary of reported results from key clinical trials of zanubrutinib in lymphoproliferative disorders.
| Trial name | Phase | Line | N | Patients | Treatment/median duration of treatment | Follow-up a | Efficacy | Safety |
|---|---|---|---|---|---|---|---|---|
| Mantle cell lymphoma | ||||||||
| NCT0234312050,51
BGB-3111-AU-003 |
1/2 | R/R | 32 | Age ⩾ 18 years, no prior BTKi | Zanu 160 mg BID or 320 mg QD/15.4 mo | 18.8 | 25% CR, 59% PR mDOR 18.5 mo mPFS 21.1 mo 24-mo OS 64% |
Gr ⩾ 3 AEsb,c: anemia 13%, neutropenia 9%, pneumonia 9%, myalgia 9%; 25% discontinued due to AEs; atrial fibrillation/flutter 6% |
| TN | 8 | 8.6 | 38% CR, 50.0% PR | Any-Gr AEs c : diarrhea 35%, petechia/purpura/contusion 31%; 21% discontinued due to AEs | ||||
| NCT0320697052,53
BGB-3111-206 |
2 | R/R | 86 | Age 18–75 years, ⩾ 1 prior treatment, no prior BTKi, Chinese trial | Zanu 160 mg BID/NA | 18.4 | 69% CR, 15% PR mDOR 19.5 mo mPFS 22.1 mo |
Gr ⩾ 3 AEs b : neutropenia d 20%, lung infection/pneumonia 9%; 9% discontinued due to AEs; no atrial fibrillation reported |
| Waldenström macroglobulinemia | ||||||||
| ASPEN54,55
NCT03053440 BGB-3111-302 |
3 | TN R/R |
101 | Age ⩾ 18 years, R/R with ⩾ 1 prior therapy or TN unsuitable for standard ICT; no prior BTKi | Zanu 160 mg BID/NA | 19.4 | In TN: 0% CR, 26% VGPR, 47% PR, 18-mo PFS 78% In R/R: 0% CR, 29% VGPR, 49% PR, 18-mo PFS 86% |
Gr ⩾ 3 AEs b : neutropenia/neutrophil count decreased 20%, hypertension 6%; 4% discontinued due to AEs; no atrial fibrillation/flutter reported |
| TN R/R |
98 | Ibr 420 mg QD/NA | 19.4 | In TN: 0% CR, 17% VGPR, 50% PR, 18-mo PFS 94% In R/R: 0% CR, 20% VGPR, 61% PR, 18-mo PFS 82% |
Gr ⩾ 3 AEs b : hypertension 11%, neutropenia/neutrophil count decreased 8%; 9% discontinued due to AEs; Gr ⩾ 3 atrial fibrillation/flutter 4% | |||
| NCT03332173
56
BGB-3111-210 |
2 | R/R | 44 | Age ⩾ 18 years, at least 1 prior therapy, Chinese trial | Zanu 160 mg BID/NA | 33.0 | CR 0%, VGPR 33%, PR 37% 24-mo PFS 61% mDOMR NR |
Gr ⩾ 3 AEs b : neutrophil count decreased 32%, thrombocytopenia 21%, pneumonia 21%; 14% discontinuation due to AEs; no atrial fibrillation/flutter reported |
| NCT02343120e,57
BGB-3111-AU003 |
1/2 | R/R | 49 | Age ⩾ 18 years, no prior BTKi | Zanu 160 mg BID or 320 mg QD (except for 3 pts starting at <320 mg zanu/day)/NA | 35.8 | CR 2.0%, VGPR 49.0%, PR 28.6% 24-mo PFS 76.2% 24-mo OS 91.5% |
Gr ⩾ 3 AEsb,c: neutropenia
d
15.6% anemia 9.1%; 14% discontinuation due to AEs; atrial fibrillation 6.1% |
| TN | 24 | Zanu 160 mg BID or 320 mg QD/NA | 23.5 | CR 0%, VGPR 33.3%, PR 54.2% 24-mo PFS 91.5% 24-mo OS 100% |
Gr ⩾ 3 AEsb,c: neutropenia d 15.6%; anemia 9.1%; 13% discontinuation due to AEs; atrial fibrillation 4.2% | |||
| Marginal zone lymphoma | ||||||||
| MAGNOLIA
58
NCT03846427 BGB-3111-214 |
2 | R/R | 68 | Age ⩾ 18 years, at least 1 therapy including ⩾ 1 anti-CD20 | Zanu 160 mg BID/NA | 6.8 | CR 15%, PR 45% mDOR and mPFS NR |
Gr ⩾ 3 AEs b : neutropenia 73%; 2.9% discontinuation due to AEs; atrial flutter 1.5% |
| NCT03520920
59
BGB-3111-213 |
2 | R/R | 5 | Age ⩾ 18 years, at least 1 prior therapy, Chinese trial | Zanu 160 mg BID + R 375 mg/m2 on D1, 8, 15, 22 (C1), then D1 of C4, C6, C8, and C10/NA | 10.3 | CR 20%, PR 40% mPFS NE 12-mo PFS 75% |
Gr ⩾ 3 AEsb,c: neutrophil count decrease 14.6%, white blood cell count decrease 9.8%; no discontinuations due to TEAEs |
| Chronic lymphocytic leukemia/small lymphocytic lymphoma | ||||||||
| ALPINE60,61
NCT03734016 BGB-3111-305 |
3 | R/R | 207 | Age ⩾ 8 years, ⩾ 1 prior treatment, no prior BTKi therapy | Zanu 160 mg BID/NA | 15.3 f | CR/CRi 1.9%, PR/nPR 76.3% 12-mo PFS 94.9% 12-mo OS 97.0% |
Gr ⩾ 3 AEs b : neutropenia 18.6%, infections 12.7%; 7.7% discontinued due to AEs; atrial fibrillation/flutter 2.5% |
| 208 | Ibr 420 mg QD/NA | 15.4 f | CR/CRi 1.4%, PR/nPR 61.1% 12-mo PFS 84.0% 12-mo OS 92.7% |
Gr ⩾ 3 AEs b : infections 17.9%, neutropenia 15.0%; 13.0% discontinued due to AEs; atrial fibrillation/flutter 10.1% | ||||
| SEQUOIA
62
NCT03336333 BGB-3111-304 (Arms A & B) |
3 | TN | 240 | Without del(17p), either aged ⩾ 65 years or 18–65 years and unsuitable for FCR | Zanu 160 mg BID/NA | 26.4 | CR 6.6% ORR 94.6% 24-mo PFS 85.5% 24-mo OS 94.3% |
Gr ⩾ 3 AEs b : neutropenia 11.3%, thrombocytopenia 1.7%; 8.3% discontinued due to AEs: atrial fibrillation 3.3% |
| 227 | B (90 mg/m2 D1 & D2) + R (375 mg/m2 C1, then 500 mg/m2C2-C6)/NA | 25.9 | CR 15.1% ORR 85.3% 24-mo PFS 69.5% 24-mo OS 94.6% |
Gr ⩾ 3 AEs b : neutropenia 51%, thrombocytopenia 7.0%; 13.7% discontinued due to AEs; atrial fibrillation 2.6% | ||||
| SEQUOIA
63
NCT03336333 BGB-3111-304 (Arm C) |
3 | TN | 109 | With centrally confirmed del(17p), either aged ⩾ 65 years or 18–65 years and unsuitable for FCR | Zanu 160 mg BID/NA | 18.2 | CR/CRi 3.7%, PR/PR-L 90.8% 12-mo DOR 92.8% 24-month PFS 88.9% 18-mo OS 95.1% |
Gr ⩾ 3 AEs b : neutropenia/decreased neutrophil count 12.9%, pneumonia (3.7%); 3.7% discontinued due to AEs; atrial fibrillation/flutter 2.8% |
| SEQUOIA
64
NCT03336333 BGB-3111-304 (Arm D) |
3 | TN | 49 | With centrally confirmed del(17p), either aged ⩾ 65 years or 18–65 years and unsuitable for FCR | Zanu 160 mg BID, V starting at C4, ramped up to 400 mg QD/NA | 12.0 | 13.9% CR/CRi, 77.8% PR, 5.6% PR-L |
Gr ⩾ 3 AEs b : neutropenia ~15%, diarrhea ~6%; 2.0% discontinued due to AEs; atrial fibrillation 2.9% |
| BOVene,65
NCT03824483 |
2 | TN | 39 | Age ⩾ 18 years, untreated, but prior local RT and short-course corticosteroids allowed | Zanu (160 mg BID) + G (1000 mg IV D1, 8, 15 of C1, then D1 C2-8) + V (targeting 400 mg PO daily)/NA | 14 + | uMRD (10-4) 92% (PB), 84% (BM); CR/CRi 49%, OR 100%; 77% achieved prespecified MRD endpoint and discontinued treatment |
Gr ⩾ 3 AEs b : neutropenia 15%, thrombocytopenia 5%, rash 5%, pneumonia 5% |
| NCT04116437e,66
BGB-3111-215 |
2 | R/R | 17 | Age ⩾ 18 years, CLL/SLL intolerant to but not progressing on ibr or acala | Zanu 160 mg BID or 320 mg QD/3.0 mo | NA | 10/10 efficacy-evaluable patients had SD or better; 6/10 had deepening of response since Zanu initiation | Gr ⩾ 3 AEs b : neutropenia, 5.9%, syncope 5.9%; 96.8% of AEs associated with prior BTKi intolerance did not recur; 94% of patients remained on treatment |
| NCT02569476e,67
BGB-3111-GA101 |
1b | TN R/R |
45 | Age ⩾ 18 years, TN, or R/R | Zanu 160 mg BID or 320 mg QD + G/29 mo | 29 | In TN: 30% CR, 70% PR. In R/R: 28% CR, 64% PR. 24-mo event-free rate for DOR 90% mPFS NR |
Gr ⩾ 3 AEs b : neutropenia 31%, pneumonia 9%; 9% discontinued due to AEs; no atrial fibrillation/flutter reported |
| NCT02343120e,48
BGB-3111-AU-003 |
1 | TN R/R |
94 | Age ⩾ 18 years, no prior BTKi | Zanu 40 mg QD–320 mg QD or 160 mg BID/NA | 13.7 | In TN: 4.5% CR, 95.5% PR/PR-L. In R/R: 1.8% CR, 92.9% PR/PR-L |
Gr ⩾ 3 AEs b : neutropenia 6.4%, hypertension 2.1%; 2.1% discontinued due to AEs; atrial fibrillation 1.1% |
| Diffuse large B-cell lymphoma | ||||||||
| NCT03145064
68
BGB-3111-207 |
2 | R/R | 41 | Age ⩾ 18 years, non-GCB DLBCL ineligible for intensive CT and BM transplantation, Chinese trial | Zanu 160 mg BID/NA | 6.8 | CR 17.1%, PR 12.2% mDOR 4.5 mo mPFS 2.8 mo mOS 8.4 mo |
Gr ⩾ 3 AEs in 48.8%; 9.8% discontinuations due to AEs; no atrial fibrillation reported |
| NCT02795182
69
BGB-3111-A317 |
1b | R/R | 27 | Age ⩾ 18 years, R/R DLBCL | Zanu 160 mg BID + tislelizumab IV 200 mg Q3W/3.0 mo | 4.1 | CR 15%, PR 22% | Gr ⩾ 3 AEsb,c: neutropenia 13%, anemia 9%; 13% discontinuations due to AEs |
| NCT03520920
59
BGB-3111-213 |
2 | R/R | 20 | Age ⩾ 18 years, non-GCB DLBCL with ⩾ 1 prior therapy, Chinese trial | Zanu 160 mg BID + R 375 mg/m2 on D1, 8, 15, 22 (C1), then D1 of C4, C6, C8, and C10/NA | 10.3 | CR 5%, PR 30% mDOR 8.8 mo mPFS 3.4 mo |
Gr ⩾ 3 AEsb,c: neutrophil count decrease 14.6%, white blood cell count decrease 9.8%; no discontinuations due to TEAEs; 15% Gr 5 AEs in DLBCL pts |
| Follicular lymphoma | ||||||||
| NCT02569476e,67
BGB-3111-GA101 |
1 | R/R | 36 | Age ⩾ 18 years, R/R FL | Zanu 160 mg BID or 320 mg QD + G/20 mo | 20.1 | CR 39%, PR 33% 24-mo DOR 62% mPFS 25 mo |
Gr ⩾ 3 AEs b : neutropenia 14%, hypertension 8%; 3% discontinuations due to AEs; no atrial fibrillation reported |
Acala, acalabrutinib; AE, adverse event; B, bendamustine; BID, twice daily; BM, bone marrow; BTKi, Bruton tyrosine kinase inhibitor; C, cycle; CR, complete response; CRi, CR with incomplete hematologic recovery; CT, chemotherapy; D, day; DLBCL, diffuse large B-cell lymphoma; DOR, duration of response; FCR, fludarabine-cyclophosphamide-rituximab; G, obinutuzumab; GCB, germinal center B-cell; Gr, grade; ibr, ibrutinib; ICT, immunochemotherapy; m, median; mDOMR, median duration of major response; mo, months; MRD, minimal residual disease; NA, not available; NE, not estimable; nPR, nodular partial response; NR, not reached; OR, overall response; OS, overall survival; PB, peripheral blood; PFS, progression-free survival; PO, by mouth; PR, partial response; PR-L, PR with lymphocytosis; pts, patients; Q3W, once every 3 weeks; QD, once daily; R, rituximab; R/R, relapsed or refractory; RT, radiotherapy; SD, stable disease; TEAE, treatment-emergent adverse event; TN, treatment naïve; uMRD, undetectable minimal residual disease; V, venetoclax; VGPR, very good partial response; Zanu, zanubrutinib.
Median follow-up time in months.
The most common AEs of grade ⩾ 3 are shown.
Safety events are reported for the full study population, including patients with other indications/lines.
Includes preferred terms neutropenia, febrile neutropenia, and neutrophil count decrease.
Study also enrolled patients with other malignancies and/or lines. Only results for patients in the category indicated are shown here.
Per investigator assessment at interim analysis.
No dose-limiting toxicity was encountered in part 1; the recommended phase 2 dose of 160 mg BID or 320 mg once daily (QD) was based on PK considerations. At the December 2018 cutoff, 50 among the 37 patients with R/R MCL, the median age was 70 years, the median number of prior therapies was one (range = 1–4), and 35.1% had high-risk MCL international prognostic index (MIPI) scores. At a median follow-up of 19.4 months, the overall response rate (ORR) in the R/R population was 87%, including 30% achieving a complete response (CR). The median duration of response (mDOR) was 15.4 months and the median PFS was 17.3 months. Overall, 10 patients (19%) had discontinued zanubrutinib treatment due to AEs; 14 patients with R/R disease (38%) remained on the study. Atrial fibrillation/flutter was reported in four patients (7.5%) in the overall (37 R/R + 16 TN) safety population.
Among the 11 patients with TN MCL, at a median follow-up of 8.3 months the ORR was 82%, including 27% CR. 51 The mDOR had not been reached and eight patients (73%) remained on the study.
BGB-3111-206
BGB-3111-206 (NCT03206970) was an open-label, single-arm study of zanubrutinib in patients with R/R MCL that was conducted in China (Table 2). 52 Patients received 160 mg of oral zanubrutinib BID and had not previously received a BTKi. The 86 patients were predominantly men (77.9%). Their median age was 60.5 years, the median number of prior therapies was two (range = 1–4), and 33.7% had received three or more prior therapies. A total of 27.8% had TP53-mutated disease and 38.4% had high-risk MIPI-b scores.
At a median follow-up of 35.3 months, 70 the ORR was 83.7%, with 77.9% of patients achieving CR. The median PFS was 33.0 months and median overall survival (OS) was not reached. Although the ORR was nearly identical to that of the R/R population in the AU-003 trial (83.7% versus 84%), the CR rate in this trial was considerably higher (77.9% versus 25%).
Cross-trial comparisons are by nature problematic, but it seems notable that there were many differences in the two study populations aside from race (100% Asian in this study versus 8.1% Asian in AU-003). Although the BGB-3111-206 study population was generally younger (median age 60.5 versus 70.5 years), patients had more median prior therapies (2 versus 1), more bulky disease (43% versus 21.9% > 5 cm), more refractory disease (52.3% versus 25.0%), and a slightly higher percentage of high-risk MIPI score (38.4% versus 31.3%). Of note, positron emission tomography (PET) imaging was required in all patients for response assessment in BGB-3111-206, but this was not the case in AU-003, which was designed before the publication of the Lugano classification in 2014. 71 Thus, the latter study used computed tomography imaging with PET scans performed only at investigators’ discretion. Only eight patients had postbaseline PET scans in AU-003, of which four had their responses upgraded from non-CR to CR, suggesting that metabolic CRs may have been missed in at least some patients in that study. As a further point of reference, studies of ibrutinib in R/R MCL that did not require routine PET scans for all patients have reported CR rates of 21% 12 and 27%, 72 whereas a study of acalabrutinib in R/R MCL that also did not require routine PET scans at all assessments reported a CR rate of 40%. 73
In the BGB-3111-206 study, ORRs were high in all analyzed subgroups, including patients with more than three prior therapies and those in the blastoid variant, high-risk MIPI-b, and refractory disease subgroups. In contrast to the results from the AU-003 R/R MCL study, however, in BGB-3111-206 the ORR was significantly lower in patients aged ⩾ 65 years than in those aged < 65 years [59.1%, 95% confidence interval (CI) = 36.4–79.3 versus 92.2%, 95% CI = 82.7–97.4; p = 0.0009]. Similarly, CR rates were lower between patient age groups (45.5% versus 76.6%; p = 0.01).
Adverse events of grade ⩾ 3 were primarily hematologic. As of the data cutoff date (8 September 2020), no patients had experienced atrial fibrillation/flutter, a second primary malignancy (SPM), or tumor lysis syndrome. Discontinuation due to AEs occurred in 9.3% of patients, most commonly due to pulmonary events (lung infection, pneumonia, and interstitial lung disease; n = 1 each). A recently published pooled analysis of 112 patients with R/R MCL from AU-003 and BGB-3111-206 (median follow-up 24.7 and 24.9 months, respectively) reported a 1.8% rate of atrial fibrillation (0.9% grade ⩾ 3), with 12.5% of patients discontinuing zanubrutinib due to AEs. 74
Data from the BGB-3111-AU-003 and BGB-3111-206 studies provided the basis for FDA-accelerated approval of zanubrutinib in November 2019 for the treatment of adults with R/R MCL who have received at least one prior therapy. 43 As a condition of expedited approval, both the FDA and the Chinese National Medical Products Administration mandated that efficacy be validated in a postapproval confirmatory phase 3 trial (BGB-3111-306; NCT04002297). 75
Zanubrutinib efficacy in WM
ASPEN study
ASPEN (BGB-3111-302; NCT03053440) is an ongoing, randomized, open-label phase 3 study comparing zanubrutinib and ibrutinib in patients with WM requiring treatment. 54 It is the first and largest head-to-head trial of BTKis to date. Patients either had R/R disease (⩾ 1 prior therapy) or were TN and unsuitable for standard immunochemotherapy; patients who had prior BTKi treatment were excluded. In ASPEN, patients with MYD88L265P disease were randomized 1:1 to zanubrutinib 160 mg BID or ibrutinib 420 mg QD (arms A and B). Patients with MYD88 wild-type WM were enrolled in a nonrandomized study arm (arm C) and received zanubrutinib 160 mg BID. 55 The primary endpoint was the proportion of patients achieving very good partial response (VGPR) or CR as assessed by the independent review committee (IRC).
In the randomized trial, the ibrutinib population comprised 99 patients (81 R/R, 18 TN) and the zanubrutinib population comprised 102 patients (83 R/R, 19 TN). Baseline characteristics were generally balanced, but more patients were aged > 75 years (33% versus 22%) and more patients had anemia at baseline (hemoglobin ⩽ 11 g/dL in 66% versus 54%) in the zanubrutinib arm. The median number of prior therapies in the R/R population was one in both arms. At a median follow-up of 19.4 months, no patient in either arm achieved a CR; VGPR rates for zanubrutinib versus ibrutinib were 29% (95% CI = 20–40) versus 20% (95% CI = 12–30; p = 0.12) in R/R patients and 26% (95% CI = 9–51) versus 17% (95% CI = 4–41; p = 0.54) in TN patients. Among all patients (TN and R/R), the difference in response rates (28% and 19% for zanubrutinib and ibrutinib, respectively) was not statistically significant (p = 0.09). Eighteen-month PFS rates for zanubrutinib versus ibrutinib were 86% versus 82% in R/R patients and 78% versus 94% in TN patients. OS rates at 18 months were 97% and 93% for zanubrutinib and ibrutinib, respectively.
Treatment discontinuations due to AEs were more common in the ibrutinib arm (9% versus 4%). The rate of any-grade atrial fibrillation/flutter was approximately 10-fold higher in ibrutinib-treated patients (15% versus 2%) and atrial fibrillation/flutter of grade ⩾ 3 was reported in 4% of patients in the ibrutinib arm versus 0% of patients in the zanubrutinib arm. Cumulative rates of atrial fibrillation/flutter, hemorrhage, major hemorrhage, hypertension, pneumonia, and diarrhea were all higher for ibrutinib than zanubrutinib, whereas neutropenia was higher with zanubrutinib. Rates of infections were similar in both arms.
Thus, whereas ASPEN failed to show a statistically significant improvement in response to zanubrutinib compared with ibrutinib, the findings established the efficacy of zanubrutinib in WM and highlighted its important safety advantages, particularly regarding cardiac toxicity. Based on these results, zanubrutinib was approved for use in patients with WM by the FDA in August 2021.
Results from the ASPEN cohort comprising patients with MYD88 wild-type WM have also been reported. 55 Of these 26 patients, five had TN and 21 had R/R disease. At a median 17.9-month follow-up, 50% had achieved a major response [partial response (PR) or better], including 27% with VGPR as best response. The ORR was 81% (95% CI = 61–93) and the zanubrutinib safety profile was consistent with that seen for the zanubrutinib arm in the randomized ASPEN trial. Although the number of patients was limited, these results demonstrated the efficacy and tolerability of zanubrutinib in MYD88 wild-type WM, which is associated with poor prognosis.
BGB-3111-AU-003
Long-term results for zanubrutinib in WM have been reported for 24 and 53 patients with TN and R/R WM, respectively. 57 The median ages were 65 and 68 years, respectively, and patients with R/R disease had a median of two prior systemic therapies. All but four patients received zanubrutinib 160 mg BID or 320 mg QD. At the March 2018 cutoff date, among patients with R/R disease (median follow-up of 35.8 months) the ORR (minor response or better) was 93.9%, including 2% CR; the 24-month PFS rate was 76.2% and the 24-month OS was 91.5%. In patients with TN WM (median follow-up of 23.5 months), the ORR was 100% (0% CR), the 24-month PFS rate was 91.5%, and the 24-month OS was 100%. In the total study population, 72.7% of patients remained on treatment; 13.0% had discontinued due to AEs, of which one event was considered treatment related. Among all patients, atrial fibrillation/flutter was reported in 5.2%, including 3.9% of grade 1 or 2 and 1.3% of grade 3.
Other
The phase 2 BGB-3111-210 (NCT03332173) 56 trial is summarized in Table 2.
Zanubrutinib efficacy in MZL
MAGNOLIA
MAGNOLIA (BGB-3111-214; NCT03846427) was a phase 2, single-arm study of zanubrutinib in patients with MZL who had received at least one prior therapy including at least one CD20-directed regimen. 58 A total of 68 patients were enrolled and treated. Among these, the median age was 70 years, with 28% aged ⩾ 75 years, and the median number of prior therapies was two (range = 1–6). At a 10.7-month median follow-up, the ORR as assessed by investigators was 74%, with 24%, 50%, and 15% of patients achieving CR, PR, and stable disease, respectively. CR rates for extranodal, nodal, splenic, and unknown subtypes were 40%, 16%, 8%, and 25%, respectively. Responses were generally consistent across subgroups, including age, bulky disease, number of prior lines of therapy, and bone marrow (BM) involvement. The median PFS had not been reached. The 9-month PFS rate was 67%. One patient (1.5%) experienced grade ⩾ 3 atrial flutter, two patients discontinued zanubrutinib due to AEs, and one patient with preexisting cardiovascular disease had a grade 5 myocardial infarction.
Based on these results, in May 2021, the FDA granted priority review to a supplemental new drug application for zanubrutinib in adults with MZL who have previously received at least one anti-CD20–based therapy, 76 and accelerated approval for zanubrutinib in this indication was subsequently granted in September 2021.
BGB-3111-213
BGB-3111-213 (NCT03520920) is an ongoing phase 2 trial of zanubrutinib in combination with rituximab in indolent and aggressive lymphomas, including MZL, being conducted in China. 59 Early results are summarized in Table 2.
Zanubrutinib efficacy in chronic lymphocytic leukemia and small lymphocytic lymphoma
ALPINE study
ALPINE (BGB-3111-305; NCT03734016) is an ongoing, head-to-head phase 3, randomized, open-label, multicenter study comparing the efficacy and safety of zanubrutinib to ibrutinib in patients with R/R CLL/SLL (Table 2).60,61 The study randomizes adults with CLL/SLL who have had at least one prior therapy but no prior BTKi treatments 1:1 to zanubrutinib 160 mg BID or ibrutinib 420 mg QD. Results from the first interim analysis (data cutoff date: 20 December 2019), representing data for 207 and 208 patients randomized to zanubrutinib and ibrutinib, respectively, have been reported. 61 The median follow-up time were 15.3 months and 15.4 months, respectively.
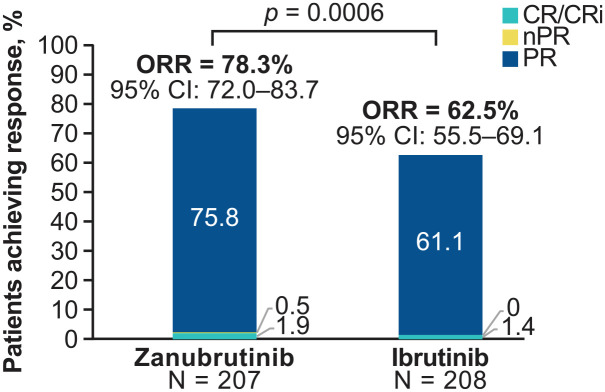
Baseline characteristics were generally similar, with a median of one prior therapy and a median age of 67 years in both arms. In the zanubrutinib and ibrutinib arms, 7.3% and 10.1%, respectively, had received more than three prior therapies, and 19.8% and 18.3% had del(17p) and/or mutant TP53, respectively. Some 87.4% (zanubrutinib) and 75.5% (ibrutinib) of patients remained on treatment. In the zanubrutinib arm, the ORR (defined as investigator-assessed CR + PR) was statistically superior to that in the ibrutinib arm [78.3% (95% CI = 72.0–83.7) versus 62.5% (95% CI 55.5–69.1); p = 0.0006] (Figure 2). Other responses for zanubrutinib and ibrutinib, respectively, were PR with lymphocytosis (PR-L) (10.1% versus 18.8%), stable disease (8.2% versus 13.5%), and progressive disease (0.5% versus 1.0%). Among patients with del(17p), the ORR was 83.3% for zanubrutinib (n = 24) and 53.8% for ibrutinib (n = 26). The ORR favored zanubrutinib in subgroups that included age (< 65 years versus ⩾ 65 years), sex, disease stage, number of prior lines of therapy (1–3 versus > 3), baseline del(17p)/TP53 mutation status, and bulky disease. Twelve-month PFS rates for zanubrutinib and ibrutinib were 94.9% and 84.0%, respectively [hazard ratio (HR) = 0.40 (95% CI = 0.2–0.69); p = 0.0007], and 12-month OS rates were 97.0% and 92.7%, respectively [HR = 0.54 (95% CI = 0.25–1.16); p = 0.108].
Figure 2.
Investigator-assessed responses at first interim analysis of the ALPINE trial of zanubrutinib versus ibrutinib in patients with relapsed or refractory CLL/SLL. 61 Two-sided superiority is shown.
CLL/SLL, chronic lymphocytic leukemia/small lymphocytic lymphoma; CR, complete response; CRi, complete response with incomplete hematologic recovery; nPR, nodular partial response; ORR, overall response rate (CR + PR); PR, partial response.
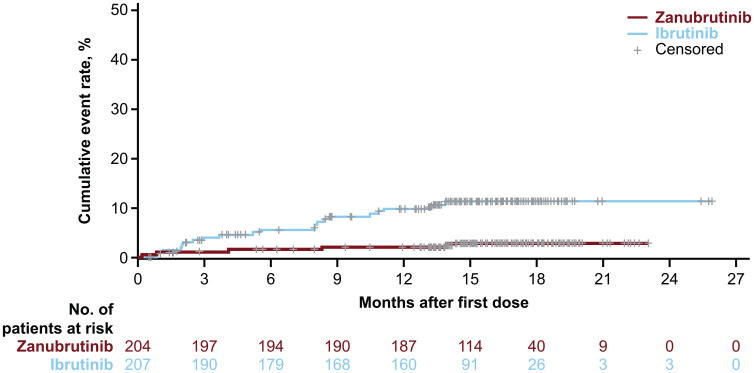
Treatment discontinuations due to AEs were reported in 7.7% and 13.0% of patients in the zanubrutinib and ibrutinib arms, respectively. ALPINE interim data indicated a striking difference between treatment arms for the incidence rate of a key secondary trial endpoint, atrial fibrillation/flutter. Atrial fibrillation/flutter of any grade (10.1% versus 2.5%) (Figure 3) and of grade ⩾ 3 (1.9% versus 1.0%) was more frequent in the ibrutinib arm.
Figure 3.
Cumulative incidence of atrial fibrillation/flutter by time from first dose at first interim analysis of the ALPINE study. 64
Overall, interim data from the large, randomized ALPINE trial demonstrated superior response rates as well as improved cardiac safety relative to ibrutinib in patients with R/R CLL. Limitations, however, include a relatively short follow-up time to date and the unblinded nature of the study with an investigator-assessed primary endpoint.
SEQUOIA study
The safety and efficacy of zanubrutinib in patients with TN CLL/SLL are being evaluated in another large comparative trial: the ongoing, multicenter, open-label, randomized phase 3 SEQUOIA study (BGB-3111-304; NCT03336333). 63 Patients without del(17p) are randomized 1:1 to zanubrutinib 160 mg BID (arm A) or six cycles of bendamustine/rituximab (arm B); however, considering the poor prognosis of patients with del(17p) undergoing chemotherapy, these patients are not randomized but instead receive zanubrutinib 160 mg BID (arm C) or zanubrutinib plus venetoclax (arm D) in separate cohorts.
In arms A and B at a median follow-up of 26.2 months, ORRs by independent review were 94.6% (6.6% CR) and 85.3% (15.1% CR) for zanubrutinib and bendamustine/rituximab, respectively. 62 Twenty-four-month PFS rates were 85.5% and 69.5% for zanubrutinib and bendamustine/rituximab, respectively (HR = 0.42; p < 0.0001). Significant zanubrutinib PFS benefit was seen in subgroups including unmutated IGHV (HR = 0.24; p < 0.0001) and chromosome 11q deletion (HR = 0.21). Estimated 24-month OS rates were 94.3% and 94.6% for zanubrutinib and bendamustine/rituximab, respectively. Zanubrutinib was associated with less frequent grade ⩾ 3 (52.5% versus 79.7%) and serious (36.7% versus 49.8%) AEs as well as hematologic AEs and discontinuations due to AEs (8.3% versus 13.7%). Rates of grade ⩾ 3 infections, hypertension, and bleeding were 16.3%, 6.3%, and 3.8%, respectively, in the zanubrutinib arm. Rates of any-grade and grade ⩾ 3 atrial fibrillation were 3.3% and 0.4%, respectively, for zanubrutinib, and 2.6% and 1.3%, respectively, for bendamustine/rituximab. The data therefore support the potential utility of zanubrutinib in the frontline management of patients with TN CLL/SLL.
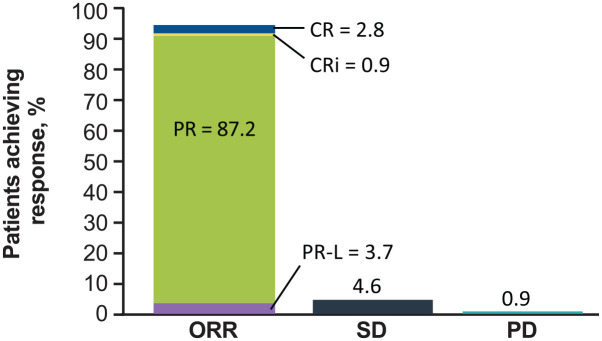
Detailed results have been reported for the noncomparative arm C, which enrolls patients with TN del(17p) CLL/SLL who are either aged ⩾ 65 years or unsuitable for fludarabine, cyclophosphamide, and rituximab therapy. 63 As of 20 February 2019 data cutoff date, 109 patients were enrolled in this arm of the global study. The median age was 70 years, 71.6% were men, 56.0% had cytopenia at baseline, and 37.2% had complex karyotype (⩾ 3 abnormalities). At a median follow-up of 18.2 months, the ORR was 94.5% (Figure 4); mDOR was not reached, and DOR was ⩾ 12 months in 92.8% of patients. Median PFS and OS were not reached; the 18-month PFS rate was 88.6% (95% CI = 79.0–94.0) and the 18-month OS rate was 95.1% (95% CI = 88.4–98.0). Most patients with baseline anemia (86.0%), neutropenia (75.0%), and thrombocytopenia (85.7%) experienced sustained improvements in their cytopenias.
Figure 4.
Best response to zanubrutinib monotherapy in evaluable patients with treatment-naive CLL and 17p deletion in the SEQUOIA (BGB-3111-304) trial. ORR was defined as CR + CRi + PR + PR-L.
CLL, chronic lymphocytic leukemia; CR, complete response; CRi, CR with incomplete hematologic recovery; PD, progressive disease; PR, partial response; PR-L, PR with lymphocytosis; SD, stable disease.
Four patients (3.7%) discontinued treatment due to AEs. Of note, although 18.3% of patients received anticoagulants and 24.8% received antiplatelet therapy, grade ⩾ 3 bleeding occurred in only 4.6% of patients. Atrial fibrillation/flutter was reported in 2.8% of patients. There were two fatal AEs, one of which (sepsis in an 84-year-old woman with healthcare-associated pneumonia post-zanubrutinib) was assessed as related to zanubrutinib. Thus, results from arm C of SEQUOIA for patients with del(17p) compare favorably with those from previous studies of patients with TN CLL/SLL who were treated with chemoimmunotherapy, and the toxicity profile associated with zanubrutinib was consistent with that seen in other studies.
Arm D explores zanubrutinib in combination with venetoclax in patients with TN CLL/SLL with del(17p). To decrease the risk of TLS, venetoclax was first administered after three cycles of zanubrutinib, and venetoclax doses were then ramped up. 64 At the relatively short median follow-up time of 12.0 months, among the 36 patients evaluable, the ORR was 97.2% (13.9% CR/CR with incomplete hematologic recovery; 77.8% PR; 5.6% PR-L); no patient had PD. Responses appeared to deepen in patients treated for longer periods. No TLS was reported. In the safety population (n = 49), 32.7% of patients had a grade ⩾ 3 AE, most commonly neutropenia, and there was one fatal AE, lung carcinoma, unrelated to zanubrutinib or venetoclax. One patient (2.9%) experienced worsening of preexisting atrial fibrillation/flutter. More mature follow-up data are needed to further investigate the combination of zanubrutinib and venetoclax in this high-risk population.
BOVen study
Currently, BTKis are generally used until disease progression or intolerance. The investigator-sponsored, ongoing, multicenter, phase 2 BOVen (NCT03824483) study is examining zanubrutinib in combination with obinutuzumab and venetoclax as a minimal residual disease (MRD)-driven, time-limited therapy for patients with TN CLL/SLL (Table 2) as well as TP53-mutated MCL. After a minimum of eight cycles, patients with undetectable MRD (10–4 sensitivity) in two consecutive peripheral blood assays and one BM assay discontinue therapy and enter post-treatment surveillance. 65 Results for 39 patients with TN CLL have been reported. 65 In this population, CLL-International Prognostic Index risk scores were high/very high for 67% of patients, 72% of patients had unmutated IGHV, and 15.4% of patients had del(17p) or TP53 mutant disease. At a median follow-up of > 14 months, all patients had achieved an objective response, with 49% achieving CR or CR with incomplete hematologic recovery. Undetectable MRD status had led to treatment discontinuation – in this case the desired outcome – in 29 of 39 patients (77%).
BGB-3111-AU-003
This first-in-human phase 1 trial enrolled 22 and 72 patients with TN and R/R CLL/SLL, respectively, in the dose-expansion cohort, of whom 78 were evaluable for response. 48 Previously treated patients had a median of two prior therapies. At a median follow-up of 13.7 months, the ORR (defined as CR, PR, or PR-L), was 100% and 94.6% in the TN and R/R groups, respectively, and predominantly comprised PR in both (81.8% and 80.4%, respectively). At 12 months, the PFS rate was 100%. Favorable tolerability was noted, with only 2.1% of patients discontinuing due to AEs, and atrial fibrillation reported in one patient (1.1%). These encouraging early results supported further studies of zanubrutinib in CLL such as those described above.
Other
Several other key trials of zanubrutinib as a single agent or in combination with other agents for the treatment of patients with TN or R/R CLL/SLL are summarized in Table 2.
Zanubrutinib efficacy in other indications
Diffuse large B-cell lymphoma
Owing to the unmet need for better therapies for patients with R/R diffuse large B-cell lymphoma (DLBCL), 77 zanubrutinib is being investigated as a single agent and as part of combination therapy in this population. Trials that have reported results for these regimens are shown in Table 2.
Follicular lymphoma
BGB-3111-GA101 (NCT02569476), which investigates zanubrutinib in combination with obinutuzumab in patients with CLL, also included patients with R/R follicular lymphoma (FL) (Table 2). 67 At a median follow-up of 20 months, among these 36 patients (median = 2 prior therapies), the ORR was 72%, including 39% with a CR. Combined with reported favorable tolerability in the total (CLL and FL) trial population, these results supported further development of zanubrutinib combinations in FL.
Zanubrutinib safety
Zanubrutinib pooled safety analysis
Recently, an analysis of safety data from six pooled zanubrutinib studies has been reported. 78 The studies were BGB-3111-1002 (B-cell lymphomas), BGB-3111-205 (R/R CLL/SLL), BGB-3111-206 (R/R MCL), BGB-3111-302 (TN or R/R WM), BGB-3111-210 (R/R WM), and BGB-3111-AU-003 (B-cell malignancies). A total of 779 patients were treated with ⩾ 1 dose of zanubrutinib, 98% at either 320 mg QD or 160 mg BID.
The median age in these studies was 65 years, with 20% of patients aged ⩾ 75 years. Approximately 68% of patients were men and 88% had R/R disease with a median of two prior lines of therapy (range = 1–12). The most common malignancies were WM (33%), CLL/SLL (29%), and MCL (19%). The median duration of zanubrutinib treatment was 25.8 months; median overall dose intensity was 99%. At the time of analysis, 43% of patients had discontinued zanubrutinib treatment, most commonly due to disease progression (27%) or AEs (10%). All-grade treatment-emergent AEs (TEAEs) were reported in 98% of patients. The most common all-grade TEAE was upper respiratory tract infection (40%), and the most common grade ⩾ 3 TEAE was pneumonia (11%) (Table 3). Grade ⩾ 3 AEs were more common in patients aged ⩾ 75 years (76%) than in those aged 65 to < 75 (64%) or < 65 years (63%). 78
Table 3.
Nonhematologic treatment-emergent adverse events of all grades reported in ⩾ 10% or grade ⩾ 3 reported in ⩾ 2% of patients in the pooled analysis of single-agent zanubrutinib safety (n = 779). 78 .
| Adverse event | All-grade, % | Grade ⩾ 3, % |
|---|---|---|
| Upper respiratory tract infection | 40 | 2 |
| Rash a | 27 | 0.4 |
| Bruising a | 25 | 0.1 |
| Musculoskeletal pain a | 24 | 2 |
| Diarrhea | 23 | 2 |
| Cough | 21 | 0.1 |
| Pneumonia a | 21 | 11 |
| Urinary tract infection | 15 | 2 |
| Fatigue | 15 | 1 |
| Hematuria | 14 | 0.5 |
| Constipation | 14 | 0.4 |
| Headache | 13 | 1 |
| Pyrexia | 13 | 1 |
| Hypertension | 12 | 5 |
| Nausea | 11 | 0.4 |
| Sepsis a | 2 | 2 |
Includes multiple preferred terms within the Medical Dictionary for Regulatory Activities.
Grade 5 AEs (regardless of attribution to treatment) occurring in more than one patient were pneumonia (n = 9; 1%), multiple organ dysfunction syndrome (n = 5; 1%), sepsis (n = 4; 1%), and death, cause unspecified (n = 4; 1%). Of the 39 patients with grade 5 AEs, at least seven occurred in the setting of progressive disease. Thirteen of the grade 5 AEs (33%) were considered treatment related.
Data for zanubrutinib AEs of interest are shown in Table 4. The most frequent grade ⩾ 3 AE of interest was infection, with an exposure-adjusted incidence rate (EAIR) of 1.4/100 person-months. Infections were more common in patients with CLL/SLL (87%) and WM (80%) than for MCL (69%). 78 The second most frequent grade ⩾ 3 AE was neutropenia, with an EAIR of 1.2/100 person-months. All other grade ⩾ 3 AEs of interest occurred at EAIRs of < 1/100 person-months. The most common AEs of interest of all grades included grade 1–2 mucocutaneous bleeding (e.g. petechiae, purpura, and contusion). Bleeding events were more common in patients aged ⩾ 75 years (65%) and in those with CLL/SLL (72%) compared with MCL (47%) or WM (54%), whereas major hemorrhages were more common in WM (6%) and MCL (5%) than in CLL/SLL (2%). 78 Use of concurrent antiplatelet therapy weakly correlated with risk of grade ⩾ 3 hemorrhage (HR = 2.0; p = 0.1). Anticoagulant use up to and including the day of the event, however, was significantly associated with an increased risk of grade ⩾ 3 hemorrhage (HR = 10.1; p < 0.0001). Most AEs (e.g. neutropenia, atrial fibrillation/flutter, and major hemorrhage) had reached a plateau by approximately 24 months.
Table 4.
Summary of zanubrutinib adverse events of interest by category.
| AEI | EAIR a | All patients with Gr ⩾ 3 event, n (%) | |
|---|---|---|---|
| All-grade | Grade ⩾ 3 | ||
| Infections | 9.6 | 1.4 | 214 (27) |
| Opportunistic infections | 0.1 | 0.1 | 15 (2) |
| Hemorrhage b | 4.8 | 0.2 | 28 (4) |
| Major hemorrhage | 0.2 | 0.2 | 28 (4) |
| Neutropenia c | 2.1 | 1.2 | 183 (23) |
| Thrombocytopenia d | 1.1 | 0.3 | 61 (8) |
| Anemia e | 0.8 | 0.04 | 63 (8) |
| Second primary malignancies f | 0.6 | 0.2 | 40 (5) |
| Skin cancers | 0.4 | 0.1 | 13 (2) |
| Hypertension | 0.6 | 0.2 | 41 (5) |
| Atrial fibrillation and flutter | 0.1 | 0.03 | 6 (1) |
| Tumor lysis syndrome g | 0.02 | 0.02 | 3 (0.4) |
AEI, adverse event of interest; EAIR, exposure-adjusted incidence rate; Gr, grade; PT, preferred term.
EAIRs calculated as the first occurrence of each adverse event of interest per 100 person-months of zanubrutinib exposure.
Includes major hemorrhage.
Includes clinical adverse events reported under PTs neutropenia (n = 97), neutrophil count decreased (n = 178), febrile neutropenia (n = 14), and neutropenic sepsis (n = 1).
Includes clinical adverse events reported under the PTs thrombocytopenia (n = 58) and platelet count decreased (n = 97).
Includes clinical adverse events reported under the PTs anemia (n = 125) and hemoglobin decreased (n = 6).
Inclusive of skin cancers.
Two cases of tumor lysis syndrome occurred > 30 days after discontinuation of zanubrutinib for disease progression; both were assessed as grade ⩾ 3 and serious. In one patient, the event occurred in association with venetoclax exposure, a known precipitant of tumor lysis syndrome. A third patient experienced an event with onset 9 days after discontinuation of zanubrutinib for progression of mantle cell lymphoma on study day 150, which was unresponsive to medical management. The patient died 3 days after onset from complications of acute kidney injury.
EAIRs for atrial fibrillation and flutter were low (grade 1 or 2; 0.1/100 person-months; grade ⩾ 3, 0.03/100 person-months). In addition, most patients experiencing atrial fibrillation/flutter had known risk factors, including hypertension (n = 9), atrial fibrillation/supraventricular tachycardia (n = 6), and dyslipidemias (n = 6). As expected, atrial fibrillation was more prevalent in older patients (9% in patients aged ⩾75 years versus 3% in patients aged ⩾ 65–75 years and 0.3% in patients aged < 65 years). SPMs were reported in 13% of patients, primarily comprising nonmelanoma skin cancers. Four patients (0.5%) died from complications of SPMs [acute myeloid leukemia, gastric adenocarcinoma, transformation to aggressive lymphoma, and recurrent metastatic cutaneous squamous cell carcinoma (n = 1 each)].
Ongoing zanubrutinib trials
A list of ongoing zanubrutinib trials in hematologic malignancies, which were currently recruiting as of 21 July 2021 according to the ClinicalTrials.gov website, is shown in Table 5. The studies are arranged by geographical region and decreasing numbers of patients expected to be enrolled.
Table 5.
Summary of clinical trials of zanubrutinib in hematologic malignancies listed in ClinicalTrials.gov as currently enrolling patients as of 30 September 2021.
| Trial identifier a | Phase | Sites | N | Line | Indication | Key inclusions/exclusions | Treatment | Est. primary completion date |
|---|---|---|---|---|---|---|---|---|
| Global | ||||||||
| NCT0333633363
SEQUOIA BGB-3111-304 |
3 | 160 | 710 | TN | CLL/SLL | Must be either age ⩾ 65 years or unsuitable for FCR | No del(17p): zanu versus BR (Arms A&B) Del(17p): zanu (Arm C); zanu + V (Arm D) |
10/2021 |
| NCT04002297
79
MANGROVE BGB-3111-306 |
3 | 122 | 500 | TN | MCL | Must be age ⩾ 65 years and transplant ineligible | Zanu + R versus BR | 9/2021 |
| NCT02914938 ME-401-002 |
1 | 24 | 177 | R/R | CLL/SLL, DLBCL, FL, MCL, MZL, high-grade NHL | No prior PI3K inhibitor No prior BTKi unless progressing or intolerant |
ME0401, ME-401 + zanu, ME-401 + R | 9/2021 |
| Asia/Pacific | ||||||||
| NCT04277637 BGB-11417-101 |
1 | 4 | 284 | R/R | CLL/SLL, DLBCL, FL, MCL, MZL, WM | No prior Bcl-2 inhibitor | BGB-11417 ± zanu | 8/2023 |
| NCT04282018 BGB-A317-3111-10188-101 |
1/2 | 6 | 150 | R/R | R/R CLL/SLL, FL, MCL, MZL, DLBCL |
No prior PI3k inhibitor | BGB-10188 ± zanu | 3/2025 |
| NCT04938297 ZR2 |
2 | 1 | 100 | TN R/R |
CNSL, DLBCL | No prior BTKi or len treatment | Zanu + len + R induction; then len or zanu maintenance |
10/2022 |
| NCT04436107 BGB-3111-110 |
1 | 10 | 67 | R/R | DLBCL | Must have prior CD20 and anthracycline treatment | Zanu + len ± R | 8/2022 |
| NCT04668365 HNSZLYYNHL05 |
2 | 1 | 59 | TN R/R |
DLBCL | Must have high-risk disease | Zanu + R-CHOP (TN); zanu + GemOx/DHAP/ICE/GDP, then maintenance zanu (R/R) |
12/2023 |
| NCT04463953 BDH-WM2020/4 |
2 | 1 | 55 | TN | WM | Patient must not have undergone standard treatment | Zanu + ixa + dexa | 5/2023 |
| NCT04172246 BGB-3111-211 |
1/2 | 15 | 53 | R/R | CLL/SLL, MCL, FL, MZL, and WM | – | Zanu | 7/2022 |
| NCT04736914 BGB-3111-2002-IIT |
2 | 1 | 47 | TN | MCL | Age 18–65 years, suitable for high-dose Ara-C | Zanu + R-CHOP or R-DHAOx (induction), ASCT, zanu (maintenance) | 1/2023 |
| NCT04624958 B2020-232-01 |
2 | 3 | 42 | TN | MCL | – | Zanu + R (induction); R-DHAOx (consolidation); zanu (maintenance) | 12/2025 |
| NCT04899453 PUMCH-NHL-007 |
2 | 1 | 42 | TN | PVRL | – | Zanu + R + MTX | 8/2022 |
| NCT04705129 RJ-PMBCL-1 |
2 | 1 | 40 | R/R | PMBCL, EBV + DLBCL NOS | Must have prior R, anthracycline therapies | Zanu + tislelizumab | 1/2023 |
| NCT04460248 ZR2 |
2 | 1 | 40 | TN | DLBCL | Must be age ⩾ 75 years unsuitable or unwilling to undergo CT | Zanu + len + R | 8/2021 |
| NCT04835870 ZR-CHOP |
2 | 1 | 20 | TN | Non-GCB DLBCL | – | Zanu + R-CHOP | 1/2024 |
| NCT04899570 PUMCH-NHL-008 |
2 | 1 | 20 | TN | IVLCL | – | Zanu + R-CHOP | 4/2023 |
| Europe | ||||||||
| NCT04271956 CLL-RT1 |
2 | 12 | 48 | R/R | CLL with RT | Must have < 2 prior treatments, not primary progressive | Zanu + tislelizumab | 8/2022 |
| NCT04515238 CLL2-BZAG |
2 | 2 | 40 | R/R | CLL | No prior progression on V or a BTKi | B (debulking), then zanu + GV induction and maintenance | 10/2022 |
| North America | ||||||||
| NCT03824483 BOVen 18-427 |
2 | 7 | 77 | TN | CLL/SLL, MCL | For MCL, must have TP53 mutation | Zanu + G + V | 2/2022 |
| NCT04116437 BGB-3111-215 |
2 | 29 | 90 | R/R | CLL/SLL, MCL, MZL, WM | Must be intolerant to prior ibr or acala | Zanu | 6/2025 |
| NCT04458610 NCI-2020-05053 |
2 | 1 | 60 | TN | CLL/SLL | – | Zanu + R | 5/2022 |
Ab, antibody; acala, acalabrutinib; B, bendamustine; BR, bendamustine and rituximab; BTKi, Bruton tyrosine kinase inhibitor; CHOP, cyclophosphamide, doxorubicin, vincristine and prednisone; CLL, chronic lymphocytic leukemia; CNSL, central nervous system lymphoma; CT, chemotherapy; dexa, dexamethasone; DHAOx, dexamethasone high-dose cytarabine, and oxaliplatin; DLBCL, diffuse large B-cell lymphoma; EBV, Epstein–Barr virus; FCR, fludarabine, cyclophosphamide, and rituximab; FL, follicular lymphoma; G, obinutuzumab; GCB, germinal center B-cell; GDP, gemcitabine, dexamethasone, and cisplatin; GemOx, gemcitabine and oxaliplatin; ibr, ibrutinib; ICE, ifosfamide, carboplatin, and etoposide; IVLCL, intravascular large B-cell lymphoma; ixa, ixazomib; len, lenalidomide; MCL, mantle cell lymphoma; MTX, methotrexate; MZL, marginal zone lymphoma; N, projected enrollment; NHL, non-Hodgkin lymphoma; NOS, not otherwise specified; PMBCL, primary mediastinal large B-cell lymphoma; PVRL, primary vitreoretinal lymphoma; R, rituximab; R/R, relapsed or refractory; RT, Richter transformation; SLL, small lymphocytic lymphoma; TN, treatment naïve; V, venetoclax; WM, Waldenström macroglobulinemia; Zanu, zanubrutinib.
Phase 1 pharmacokinetics trials were excluded. Trials are ordered by geographical region and projected numbers of patients. For more complete information, consult http://clinicaltrials.gov.
Other areas of research
Other questions remain to be addressed, including the safety and efficacy of zanubrutinib in other malignancies. New options for patients with B-cell malignancies, such as CAR T-cell therapy, warrant new studies to clarify the optimal place of zanubrutinib in treatment lines. The ZUMA-2 trial investigated CAR T-cell therapy in patients with R/R MCL whose previous therapies included BTKis, associating KTE-X19 with durable remissions in most patients with this difficult-to-treat malignancy. 80 At that time, zanubrutinib had not yet been approved, so all ZUMA-2 patients had been exposed to ibrutinib, acalabrutinib, or both. Because the FDA subsequently approved zanubrutinib for use in R/R MCL, it is likely that some patients have been or will be treated with zanubrutinib followed by CAR T-cell therapy, but data are not yet available. Other issues of optimal sequencing of CAR T-cell therapy and zanubrutinib in MCL and CLL remain to be resolved.
Finally, potential uses of zanubrutinib in nononcology indications such as chronic graft-versus-host disease, for which ibrutinib has been approved,13,81 as well as IgG4 disease, 82 remain to be explored but fall outside the scope of the current review.
Conclusion
The first-in-class BTKi ibrutinib has been associated with toxicities that may limit its use in certain patient populations. These groups include those on anticoagulants or antiplatelet therapy and patients with cardiovascular comorbidities. Next-generation BTKis (acalabrutinib and zanubrutinib) subsequently designed to improve tolerability by reducing off-target inhibition have received FDA approval in specific B-cell lymphoproliferative indications. Still more recently, preliminary efficacy and tolerability results in B-cell malignancies for a noncovalent, reversible BTKi, pirtobrutinib, have been reported. 83 Only a meager amount of comparative trial data exists for these agents.
Zanubrutinib is a BTKi with approximately four- to 60-fold improved selectivity relative to ibrutinib. Zanubrutinib has been FDA-approved for use in patients with WM, R/R MCL, and R/R MZL and is being investigated in other B-cell malignancies. Interim data from the randomized phase 3, ALPINE (NCT03734016) trial of zanubrutinib versus ibrutinib in patients with R/R CLL or SLL recently associated zanubrutinib with a superior response rate as well as improved cardiac safety and fewer treatment discontinuations due to AEs in this patient population. 61 A phase 3 randomized trial of the next-generation BTKi acalabrutinib versus ibrutinib in patients with R/R CLL reported acalabrutinib noninferiority, improved cardiac safety, and fewer discontinuations due to AEs. 39 Thus, although the mechanisms of ibrutinib-associated toxicities have not been fully elucidated, the selectivity that is engineered into next-generation BTKis appears to translate into improved tolerability for these agents in studies to date.
Further studies are needed to address the relative merits of next-generation BTKis as well as answer questions regarding optimal sequence, combination versus single-agent use, and duration of therapy. Current evidence is insufficient to identify a BTKi of choice, leaving the benefits and harms of each agent to be weighed by the clinician in choosing the most appropriate therapy.
Numerous phase 3 studies of zanubrutinib are now underway, which will begin to address these important issues. These include SEQUOIA (TN CLL/SLL), ALPINE (R/R CLL/SLL), MANGROVE (TN and stem cell transplant–ineligible older MCL), and ASPEN (TN and R/R WM). These and other ongoing and planned trials worldwide will be critical for determining the place of zanubrutinib in the treatment of B-cell lymphoproliferative disorders.
Acknowledgments
This work, including medical writing and editorial assistance, was supported by BeiGene USA, Inc. Writing and editorial support were provided by Robert Rydzewski, MS, CMPP, and submission support was provided by Laura Leistikow, BFA, both of Bio Connections, LLC (Chicago, IL), supported by BeiGene USA, Inc. (San Mateo, CA).
Footnotes
Ethics approval and consent to participate: Not applicable.
Consent for publication: Not applicable.
Author contribution(s): Javier Muñoz: Conceptualization; Writing – original draft; Writing – review & editing.
Yucai Wang: Conceptualization; Writing – original draft; Writing – review & editing.
Preetesh Jain: Conceptualization; Writing – original draft; Writing – review & editing.
Michael Wang: Conceptualization; Writing – original draft; Writing – review & editing.
ORCID iD: Javier Muñoz  https://orcid.org/0000-0002-9060-8911
https://orcid.org/0000-0002-9060-8911
Funding: The authors received no financial support for the research, authorship, and/or publication of this article.
Conflict of interest statement: The authors declared the following potential conflicts of interest with respect to the research, authorship, and/or publication of this article: JM reports consultancy role with, research funding from, and speakers’ bureau for Pharmacyclics, Bayer, Gilead/Kite Pharma, Janssen, and Celgene; consultancy role with Pfizer, Alexion, Fosunkite, Innovent, Debiopharm, Epizyme, Karyopharm, and Genmab; consultancy role with Juno/Celgene; consultancy role with and speakers’ bureau for Bristol Myers Squibb, BeiGene; consultancy role with, honoraria from, and speakers’ bureau for Kyowa; consultancy role with, research funding from, honoraria from, and speakers’ bureau for Seattle Genetics; research funding from Merck, Portola, Incyte, and Millennium; research funding from and speakers’ bureau for Genentech; speakers’ bureau for Acrotech/Aurobindo, Verastem, AstraZeneca, Roche, and AbbVie. YW reports research funding from, and advisory role with Incyte and Loxo Oncology; research funding from InnoCare, Novartis, and Genentech; advisory role with Eli Lilly and TG therapeutics. PJ reports consultancy role with Eli Lilly, Incyte, Kite, research funding from AstraZeneca. MW reports research funding and honoraria from Acerta Pharma; consultancy role with, research funding, and honoraria from AstraZeneca, BeiGene, Janssen, and Kite Pharma; honoraria from Anticancer Association, CAHON, Chinese Medical Association, Clinical Care Options, Dava Oncology, Hebei Cancer Prevention Federation, Imbruvica, Imedex, Moffit Cancer Center, Mumbai Hematology Group, Newbridge Pharmaceuticals, OMI, Physicians Education Resources (PER), Scripps, and The First Affiliated Hospital of Zhejiang University; consultancy role with Bayer Healthcare, CSTone, DTRM Biopharma (Cayman) Limited, and Genentech; research funding from BioInvent, Celgene, and Molecular Templates; consultancy role with and honoraria from Epizyme and Miltenyi Biomedicine GmbH; consultancy role with and research funding from InnoCare, Juno, Loxo Oncology, Oncternal, Pharmacyclics, and VelosBio.
Availability of data and materials: Not applicable.
Contributor Information
Javier Muñoz, Program Director, Lymphoma, Mayo Clinic, 5881 E. Mayo Boulevard, Phoenix, AZ 85054, USA.
Yucai Wang, Mayo Clinic, 200 1st Street SW, Rochester, MN 55905, USA.
Preetesh Jain, The University of Texas MD Anderson Cancer Center, Houston, TX, USA.
Michael Wang, The University of Texas MD Anderson Cancer Center, Houston, TX, USA.
References
- 1. Fowler N, Davis E. Targeting B-cell receptor signaling: changing the paradigm. Hematology Am Soc Hematol Educ Program 2013; 2013: 553–560. [DOI] [PubMed] [Google Scholar]
- 2. Niemann CU, Wiestner A. B-cell receptor signaling as a driver of lymphoma development and evolution. Semin Cancer Biol 2013; 23: 410–421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Hendriks RW, Yuvaraj S, Kil LP. Targeting Bruton’s tyrosine kinase in B cell malignancies. Nat Rev Cancer 2014; 14: 219–232. [DOI] [PubMed] [Google Scholar]
- 4. Honigberg LA, Smith AM, Sirisawad M, et al. The Bruton tyrosine kinase inhibitor PCI-32765 blocks B-cell activation and is efficacious in models of autoimmune disease and B-cell malignancy. Proc Natl Acad Sci USA 2010; 107: 130750–131308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Herman SE, Gordon AL, Hertlein E, et al. Bruton tyrosine kinase represents a promising therapeutic target for treatment of chronic lymphocytic leukemia and is effectively targeted by PCI-32765. Blood 2011; 117: 6287–6296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Saba NS, Liu D, Herman SE, et al. Pathogenic role of B-cell receptor signaling and canonical NF-kappaB activation in mantle cell lymphoma. Blood 2016; 128: 82–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Yan Q, Huang Y, Watkins AJ, et al. BCR and TLR signaling pathways are recurrently targeted by genetic changes in splenic marginal zone lymphomas. Haematologica 2012; 97: 595–598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Davis RE, Ngo VN, Lenz G, et al. Chronic active B-cell-receptor signalling in diffuse large B-cell lymphoma. Nature 2010; 463: 88–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Hunter ZR, Yang G, Xu L, et al. Genomics, signaling, and treatment of Waldenstrom macroglobulinemia. J Clin Oncol 2017; 35: 994–1001. [DOI] [PubMed] [Google Scholar]
- 10. Noy A, de Vos S, Thieblemont C, et al. Targeting Bruton tyrosine kinase with ibrutinib in relapsed/refractory marginal zone lymphoma. Blood 2017; 129: 2224–2232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Byrd JC, Furman RR, Coutre SE, et al. Targeting BTK with ibrutinib in relapsed chronic lymphocytic leukemia. N Engl J Med 2013; 369: 32–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Wang ML, Rule S, Martin P, et al. Targeting BTK with ibrutinib in relapsed or refractory mantle-cell lymphoma. N Engl J Med 2013; 369: 507–516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Imbruvica® (package insert). Sunnyvale, CA: Pharmacyclics LLC, 2020. [Google Scholar]
- 14. O’Brien S, Furman RR, Coutre S, et al. Single-agent ibrutinib in treatment-naive and relapsed/refractory chronic lymphocytic leukemia: a 5-year experience. Blood 2018; 131: 1910–1919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Burger JA, Barr PM, Robak T, et al. Long-term efficacy and safety of first-line ibrutinib treatment for patients with CLL/SLL: 5 years of follow-up from the phase 3 RESONATE-2 study. Leukemia 2020; 34: 787–798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Munir T, Brown JR, O’Brien S, et al. Final analysis from RESONATE: up to six years of follow-up on ibrutinib in patients with previously treated chronic lymphocytic leukemia or small lymphocytic lymphoma. Am J Hematol 2019; 94: 1353–1363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Byrd JC, Furman RR, Coutre SE, et al. Ibrutinib treatment for first-line and relapsed/refractory chronic lymphocytic leukemia: final analysis of the pivotal phase Ib/II PCYC-1102 study. Clin Cancer Res 2020; 26: 3918–3927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Jain P, Kanagal-Shamanna R, Zhang S, et al. Long-term outcomes and mutation profiling of patients with mantle cell lymphoma (MCL) who discontinued ibrutinib. Br J Haematol 2018; 183: 578–587. [DOI] [PubMed] [Google Scholar]
- 19. Treon SP, Meid K, Gustine J, et al. Long-term follow-up of ibrutinib monotherapy in symptomatic, previously treated patients with Waldenstrom macroglobulinemia. J Clin Oncol 2021; 39: 565–575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Winqvist M, Andersson PO, Asklid A, et al. Long-term real-world results of ibrutinib therapy in patients with relapsed or refractory chronic lymphocytic leukemia: 30-month follow up of the Swedish compassionate use cohort. Haematologica 2019; 104: e208–e210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Mato AR, Nabhan C, Thompson MC, et al. Toxicities and outcomes of 616 ibrutinib-treated patients in the United States: a real-world analysis. Haematologica 2018; 103: 874–879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Jeon YW, Yoon S, Min GJ, et al. Clinical outcomes for ibrutinib in relapsed or refractory mantle cell lymphoma in real-world experience. Cancer Med 2019; 8: 6860–6870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Lew TE, Lin VS, Cliff ER, et al. Outcomes of patients with CLL sequentially resistant to both BCL2 and BTK inhibition. Blood Adv 2021; 5: 4054–4058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Berglof A, Hamasy A, Meinke S, et al. Targets for ibrutinib beyond B cell malignancies. Scand J Immunol 2015; 82: 208–217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Stephens DM, Byrd JC. How I manage ibrutinib intolerance and complications in patients with chronic lymphocytic leukemia. Blood 2019; 133: 1298–1307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Atkinson BT, Ellmeier W, Watson SP. Tec regulates platelet activation by GPVI in the absence of Btk. Blood 2003; 102: 3592–3599. [DOI] [PubMed] [Google Scholar]
- 27. Mock J, Kunk PR, Palkimas S, et al. Risk of major bleeding with ibrutinib. Clin Lymphoma Myeloma Leuk 2018; 18: 755–761. [DOI] [PubMed] [Google Scholar]
- 28. Pellegrini L, Novak U, Andres M, et al. Risk of bleeding complications and atrial fibrillation associated with ibrutinib treatment: a systematic review and meta-analysis. Crit Rev Oncol Hematol 2021; 159: 103238. [DOI] [PubMed] [Google Scholar]
- 29. Dickerson T, Wiczer T, Waller A, et al. Hypertension and incident cardiovascular events following ibrutinib initiation. Blood 2019; 134: 1919–1928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Brown JR, Moslehi J, O’Brien S, et al. Characterization of atrial fibrillation adverse events reported in ibrutinib randomized controlled registration trials. Haematologica 2017; 102: 1796–1805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Caldeira D, Alves D, Costa J, et al. Ibrutinib increases the risk of hypertension and atrial fibrillation: systematic review and meta-analysis. PLoS ONE 2019; 14: e0211228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Mato AR, Nabhan C, Barr PM, et al. Outcomes of CLL patients treated with sequential kinase inhibitor therapy: a real world experience. Blood 2016; 128: 2199–2205. [DOI] [PubMed] [Google Scholar]
- 33. McMullen JR, Boey EJ, Ooi JY, et al. Ibrutinib increases the risk of atrial fibrillation, potentially through inhibition of cardiac PI3K-Akt signaling. Blood 2014; 124: 3829–3830. [DOI] [PubMed] [Google Scholar]
- 34. Hirsh V, Blais N, Burkes R, et al. Management of diarrhea induced by epidermal growth factor receptor tyrosine kinase inhibitors. Curr Oncol 2014; 21: 329–336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Paydas S. Management of adverse effects/toxicity of ibrutinib. Crit Rev Oncol Hematol 2019; 136: 56–63. [DOI] [PubMed] [Google Scholar]
- 36. Perez-Soler R, Delord JP, Halpern A, et al. HER1/EGFR inhibitor-associated rash: future directions for management and investigation outcomes from the HER1/EGFR inhibitor rash management forum. Oncologist 2005; 10: 345–356. [DOI] [PubMed] [Google Scholar]
- 37. Calquence® (package insert). Gaithersburg, MD: AstraZeneca, 2019. [Google Scholar]
- 38. Wu J, Zhang M, Liu D. Acalabrutinib (ACP-196): a selective second-generation BTK inhibitor. J Hematol Oncol 2016; 9: 21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Byrd JC, Hillmen P, Ghia P, et al. Acalabrutinib versus ibrutinib in previously treated chronic lymphocytic leukemia: results of the first randomized phase III trial. J Clin Oncol 2021; 39: 3441–3452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Guo Y, Liu Y, Hu N, et al. Discovery of zanubrutinib (BGB-3111), a novel, potent, and selective covalent inhibitor of Bruton’s tyrosine kinase. J Med Chem 2019; 62: 7923–7940. [DOI] [PubMed] [Google Scholar]
- 41. Ou YC, Preston RA, Marbury TC, et al. A phase 1, open-label, single-dose study of the pharmacokinetics of zanubrutinib in subjects with varying degrees of hepatic impairment. Leuk Lymphoma 2020; 61: 1355–1363. [DOI] [PubMed] [Google Scholar]
- 42. Mu S, Tang Z, Novotny W, et al. Effect of rifampin and itraconazole on the pharmacokinetics of zanubrutinib (a Bruton’s tyrosine kinase inhibitor) in Asian and non-Asian healthy subjects. Cancer Chemother Pharmacol 2020; 85: 391–399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Brukinsa® (package insert). Beijing, China: BeiGene Co. Ltd., 2019. [Google Scholar]
- 44. Wang K, Yao X, Zhang M, et al. Comprehensive PBPK model to predict drug interaction potential of zanubrutinib as a victim or perpetrator. CPT Pharmacometrics Syst Pharmacol 2021; 10: 441–454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Kaptein A, de Bruin G, Emmelot-van Hoek M, et al. Potency and selectivity of BTK inhibitors in clinical development for B-cell malignancies. Blood 2018; 132: 1871–1871.30082493 [Google Scholar]
- 46. Herman SE, Mustafa RZ, Gyamfi JA, et al. Ibrutinib inhibits BCR and NF-kappaB signaling and reduces tumor proliferation in tissue-resident cells of patients with CLL. Blood 2014; 123: 3286–3295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Sun C, Nierman P, Kendall EK, et al. Clinical and biological implications of target occupancy in CLL treated with the BTK inhibitor acalabrutinib. Blood 2020; 136: 93–105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Tam CS, Trotman J, Opat S, et al. Phase 1 study of the selective BTK inhibitor zanubrutinib in B-cell malignancies and safety and efficacy evaluation in CLL. Blood 2019; 134: 851–859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Ou YC, Tang Z, Novotny W, et al. Rationale for once-daily or twice-daily dosing of zanubrutinib in patients with mantle cell lymphoma. Leuk Lymphoma 2021; 62: 2612–2624. [DOI] [PubMed] [Google Scholar]
- 50. Tam CS, Opat S, Simpson D, et al. Zanubrutinib for the treatment of relapsed or refractory mantle cell lymphoma. Blood Adv 2021; 5: 2577–2585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Tam CS, Wang M, Simpson D, et al. Updated safety and efficacy data in the phase 1 trial of patients with mantle cell lymphoma (MCL) treated with Bruton tyrosine kinase (BTK) inhibitor zanubrutinib (BGB-3111). Hematol Oncol 2019; 37: 245–247. [Google Scholar]
- 52. Song Y, Zhou K, Zou D, et al. Treatment of patients with relapsed or refractory mantle-cell lymphoma with zanubrutinib, a selective inhibitor of Bruton’s tyrosine kinase. Clin Cancer Res 2020; 26: 4216–4224. [DOI] [PubMed] [Google Scholar]
- 53. Song Y, Zhou K, Zou D, et al. Safety and activity of the investigational Bruton tyrosine kinase inhibitor zanubrutinib (BGB-3111) in patients with mantle cell lymphoma from a phase 2 trial. Blood 2018; 132: 148–148.29866818 [Google Scholar]
- 54. Tam CS, Opat S, D’Sa S, et al. A randomized phase 3 trial of zanubrutinib vs ibrutinib in symptomatic Waldenstrom macroglobulinemia: the ASPEN study. Blood 2020; 136: 2038–2050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Dimopoulos M, Sanz RG, Lee HP, et al. Zanubrutinib for the treatment of MYD88 wild-type Waldenstrom macroglobulinemia: a substudy of the phase 3 ASPEN trial. Blood Adv 2020; 4: 6009–6018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. An G, Zhou D, Cheng S, et al. A phase II trial of the Bruton tyrosine-kinase inhibitor zanubrutinib (BGB-3111) in patients with relapsed/refractory Waldenstrom macroglobulinemia. Clin Cancer Res 2021; 27: 5492–5501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Trotman J, Opat S, Gottlieb D, et al. Zanubrutinib for the treatment of patients with Waldenstrom macroglobulinemia: 3 years of follow-up. Blood 2020; 136: 2027–2037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Opat S, Tedeschi A, Linton K, et al. Efficacy and safety of zanubrutinib in patients with relapsed/refractory marginal zone lymphoma: initial results of the MAGNOLIA (BGB-3111-214) trial. Blood 2020; 136: 28–30. [Google Scholar]
- 59. Zhang Q, Tao R, Li Z, et al. Zanubrutinib (BGB-3111) in combination with rituximab in patients with relapsed/refractory non-Hodgkin lymphoma. In: European Hematology Association 2020 virtual meeting, 2020, https://library.ehaweb.org/pdfviewer/web/viewer.html?file=https%3A//library.ehaweb.org/eha/download/poster%3Fcm_id%3D298264
- 60. Hillmen P, Brown JR, Eichhorst BF, et al. ALPINE: zanubrutinib versus ibrutinib in relapsed/refractory chronic lymphocytic leukemia/small lymphocytic lymphoma. Future Oncol 2020; 16: 517–523. [DOI] [PubMed] [Google Scholar]
- 61. Hillmen P, Eichhorst B, Brown JR, et al. First interim analysis of ALPINE study: results of a phase 3 randomized study of zanubrutinib vs. ibrutinib in patients with relapsed/refractory chronic lymphocytic leukemia/small lymphocytic lymphoma. In: European Hematology Association 2021 virtual meeting, 17-20 September 2021, https://www.beigenemedical.com/CongressDocuments/Hillmen_BGB-3111-305_iwCLL_Presentation_2021.pdf [Google Scholar]
- 62. Tam CS, Giannopoulos K, Jurczak W, et al. SEQUOIA: results of a phase 3 randomized study of zanubrutinib versus bendamustine + rituximab (BR) in patients with treatment-naïve (TN) chronic lymphocytic leukemia/small lymphocytic lymphoma (CLL/SLL). Blood 2021; 138: 396–396. [Google Scholar]
- 63. Tam CS, Robak T, Ghia P, et al. Zanubrutinib monotherapy for patients with treatment naive chronic lymphocytic leukemia and 17p deletion. Haematologica 2020; 106: 2354–2363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Tedeschi A, Ferrant E, Flinn IW, et al. Zanubrutinib in combination with venetoclax for patients with treatment-naïve (TN) chronic lymphocytic leukemia (CLL) or small lymphocytic lymphoma (SLL) with del(17p): early results from arm D of the SEQUOIA (BGB-3111-304) trial. Blood 2021; 138: 67–67. [Google Scholar]
- 65. Soumerai JD, Mato A, Carter J, et al. MRD-driven time limited therapy with zanubrutinib, obinutuzumab, and venetoclax (BOVen) in previously untreated chronic lymphocytic leukemia. In: 62nd American Society of Hematology annual meeting, virtual meeting 2020, 5–7 December 2020, p. 1307. [Google Scholar]
- 66. Shadman M, Sharman JP, Levy MY, et al. Phase 2 study of zanubrutinib in patients with relapsed/refractory B-cell malignancies intolerant to ibrutinib/acalabrutinib. Blood 2020; 136: 51–52. [Google Scholar]
- 67. Tam CS, Quach H, Nicol A, et al. Zanubrutinib (BGB-3111) plus obinutuzumab in patients with chronic lymphocytic leukemia and follicular lymphoma. Blood Adv 2020; 4: 4802–4811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68. Yang H, Xiang B, Song Y, et al. Zanubrutinib monotherapy for patients with relapsed or refractory non-germinal center diffuse large B-cell lymphoma: results from a phase II, single-arm, multicenter, study. J Clin Oncol 2020; 38: e20051. [Google Scholar]
- 69. Tam CS, Cull G, Opat S, et al. An update on safety and preliminary efficacy of highly specific Bruton tyrosine kinase (BTK) inhibitor zanubrutinib in combination with PD-1 inhibitor tislelizumab in patients with previously treated B-cell lymphoid malignancies. Blood 2019; 134: 1594–1594. [Google Scholar]
- 70. Song Y, Zhou K, Zou D, et al. Zanubrutinib (zanu) in patients (pts) with relapsed/refractory (R/R) mantle cell lymphoma (MCL): long-term efficacy and safety results from a phase 2 study. HemaSphere 2021; 5: 362 (Abstract book). [Google Scholar]
- 71. Cheson BD, Fisher RI, Barrington SF, et al. Recommendations for initial evaluation, staging, and response assessment of Hodgkin and non-Hodgkin lymphoma: the Lugano classification. J Clin Oncol 2014; 32: 3059–3068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72. Rule S, Dreyling M, Goy A, et al. Ibrutinib for the treatment of relapsed/refractory mantle cell lymphoma: extended 3.5-year follow up from a pooled analysis. Haematologica 2019; 104: e211–e214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73. Wang M, Rule S, Zinzani PL, et al. Acalabrutinib in relapsed or refractory mantle cell lymphoma (ACE-LY-004): a single-arm, multicentre, phase 2 trial. Lancet 2018; 391: 659–667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74. Zhou K, Zou D, Zhou J, et al. Zanubrutinib monotherapy in relapsed/refractory mantle cell lymphoma: a pooled analysis of two clinical trials. J Hematol Oncol 2021; 14: 167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75. Li G, Liu X, Chen X. Simultaneous development of zanubrutinib in the USA and China. Nat Rev Clin Oncol 2020; 17: 589–590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76. Staff. American Journal of Managed Care Staff. Marginal zone lymphoma, 2021. https://www.ajmc.com/view/fda-gives-zanubrutinib-priority-review-for-marginal-zone-lymphoma
- 77. Skarbnik AP, Goy AH. Mantle cell lymphoma: state of the art. Clin Adv Hematol Oncol 2015; 13: 44–55. [PubMed] [Google Scholar]
- 78. Tam CS, Dimopoulos M, Garcia-Sanz R, et al. Pooled safety analysis of zanubrutinib monotherapy in patients with B-cell malignancies. Blood Adv 2022; 6:1296–1308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79. Dreyling M, Tam CS, Wang M, et al. A phase III study of zanubrutinib plus rituximab versus bendamustine plus rituximab in transplant-ineligible, untreated mantle cell lymphoma. Future Oncol 2021; 17: 255–262. [DOI] [PubMed] [Google Scholar]
- 80. Wang M, Munoz J, Goy A, et al. KTE-X19 CAR T-cell therapy in relapsed or refractory mantle-cell lymphoma. N Engl J Med 2020; 382: 1331–1342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81. Jaglowski SM, Blazar BR. How ibrutinib, a B-cell malignancy drug, became an FDA-approved second-line therapy for steroid-resistant chronic GVHD. Blood Adv 2018; 2: 2012–2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82. Yamamoto M. B cell targeted therapy for immunoglobulin G4-related disease. Immunol Med 2021; 44: 216–222. [DOI] [PubMed] [Google Scholar]
- 83. Mato AR, Shah NN, Jurczak W, et al. Pirtobrutinib in relapsed or refractory B-cell malignancies (BRUIN): a phase 1/2 study. Lancet 2021; 397: 892–901. [DOI] [PMC free article] [PubMed] [Google Scholar]