SUMMARY
In October 2013, public health authorities were notified of a suspected outbreak of gastroenteritis in students and guests following a catered function at a university residential college. A retrospective cohort study was undertaken to examine whether foods served at the function caused illness. A total of 56 cases of gastroenteritis, including seven laboratory-confirmed cases of Campylobacter jejuni infection, were identified in 235 eligible respondents. Univariate analysis showed a significant association with a chicken liver pâté entrée [relative risk (RR) 3·64, 95% confidence interval (CI) 2·03–6·52, P < 0·001], which retained significance after adjustment for confounding via multivariable analysis (adjusted RR 2·80, 95% CI 1·26–6·19, P = 0·01). C. jejuni and C. coli were also isolated in chicken liver pâté recovered from the college's kitchen. Subsequent whole genome multilocus sequence typing (wgMLST) of clinical and food-derived C. jejuni isolates showed three genetically distinct sequence types (STs) comprising ST528, ST535 (both clinically derived) and ST991 (food derived). The study demonstrates the value of utilizing complementary sources of evidence, including genomic data, to support public health investigations. The use of wgMLST highlights the potential for significant C. jejuni diversity in epidemiologically related human and food isolates recovered during outbreaks linked to poultry liver.
Key words: Campylobacter, molecular epidemiology, outbreaks, whole genome sequencing, zoonotic foodborne diseases
INTRODUCTION
Campylobacter sp., are the most commonly reported cause of bacterial gastroenteritis worldwide and present as a significant challenge to public health and food safety [1]. In Australia, campylobacteriosis is the most common enteric condition notified to health authorities, with a reported incidence of 101 cases/100 000 population in 2012 [2].
Infection with Campylobacter sp., usually results in an acute self-limiting gastroenteritis, characterized by diarrhoea and abdominal pain, with accompanying fever and symptoms such as general malaise and myalgia [3]. The incubation period is typically between 2 and 5 days, with illness duration lasting from several days to 2 weeks [3]. Transmission is predominantly regarded as being foodborne, with food production animals, especially poultry but also ruminant species being recognized as important reservoirs for human infection [4].
From a public health perspective, a noted feature of campylobacteriosis is the infrequency of outbreak events relative to disease incidence [5]. In recent years there has been an increase in published outbreak reports, particularly those linked to poultry-derived liver products such as pâté and parfaits [6–8]. At the same time the increasing availability of sequence-based typing and advances in bioinformatics have enabled greater insight into the characterization of Campylobacter jejuni [9]. However the potential for genomic diversity even in epidemiologically linked isolates requires further appreciation and carries potential implications for public health surveillance [7].
On 30 October 2013, a medical practitioner reported several cases of gastroenteritis in students from a university residential college, including one laboratory-confirmed case of campylobacteriosis. Contact with the college and rapid interviewing of unwell students identified a catered function as a potential source of illness.
METHODS
Epidemiological investigations
Initial interviewing of cases was conducted using a rapid hypothesis-generating questionnaire, which included a 3-day food history. Findings from these interviews revealed that all cases had attended a large catered function held at the college on the evening of 25 October 2013. A retrospective cohort study was undertaken to test the hypothesis that one or more foods served at this function were responsible for causing illness. Ethics approval was not sought as the investigation was conducted as part of a public health response.
Lists of function attendees and their contact details, including university email addresses and mobile telephone numbers, were obtained via the residential college. A parallel environmental investigation was conducted that included interviewing of catering staff and sampling of residual foods. Interviews with function attendees were conducted by telephone using a standard questionnaire that captured details of symptoms, onset and exposure dates and times, and consumption of specific food and beverages based on the menu for the function.
A clinical case was defined as any person who attended the function on 25 October 2013 and subsequently developed signs or symptoms consistent with gastroenteritis, including diarrhoea, abdominal pain and/or vomiting between 26 October and 4 November 2013. Clinical cases were encouraged to provide a faecal sample to assist with the epidemiological investigation. A laboratory-confirmed case was defined as per a clinical case, in addition to having a faecal sample positive for C. jejuni subspecies jejuni.
Questionnaire data were entered into Microsoft Excel (Microsoft Corp., USA) for descriptive analysis and Stata v. 14 (StataCorp, USA) for univariate and multivariable analyses. Statistical analyses included the calculation of relative risks (RRs), adjusted relative risks (aRRs), associated 95% confidence intervals (CIs), and P values using a two-tailed Fisher's exact test. For the multivariable analysis, regression analysis was performed using a general linear model. A Wilcoxon rank sum test and a two-sample test of proportions were used to assess differences in age between cases and non-cases and differences in the proportion of male and female cases.
Microbiological investigations
Bacterial strains and identification/speciation
Campylobacter identification and speciation were confirmed by characteristic appearance on culture media, and testing for: oxidase, antibiotic susceptibility to nalidixic acid and cephalothin, and hydrolysis of hippurate [10]. Hippurate-negative strains were submitted for polymerase chain reaction assays for the detection of the hippuricase gene and the aspartokinase gene to identify C. jejuni and C. coli, respectively [11].
Whole genome sequencing (WGS) and bioinformatics analysis
WGS and comparative genomics were applied to human and food-derived outbreak isolates to determine the sequence-level similarities of the epidemiologically linked C. jejuni cases and food isolates, as we hypothesized that significant variability could be found in epidemiologically linked cases.
After extraction of genomic DNA, sequencing libraries were created using the Nextera XT DNA preparation kit (Illumina, USA). Sequencing was performed on the NextSeq 500 platform with 150-bp paired-end chemistry. The sequenced reads were mapped to the complete C. jejuni reference sequence RM1221 (NCBI accession no. NC_003912·7), and single nucleotide polymorphisms (SNPs) were detected using Snippy v. 3.0 (https://github.com/tseemann/snippy), with a minimum mapping coverage threshold of 10 and a base-call stringency of 90%. A phylogenetic tree was constructed using core genome SNPs using FastTree v. 2.1.8 [12]. The multilocus sequence type (MLST) was inferred in silico from assembled WGS reads using MLST v. 2.0 (https://github.com/tseemann/mlst).
RESULTS
Epidemiological investigations
Descriptive results
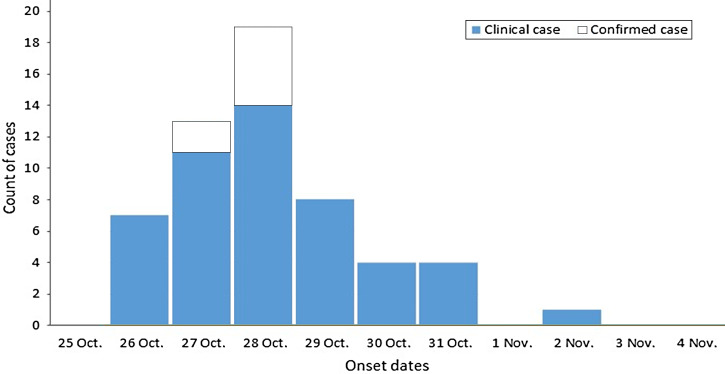
A total of 269 persons were identified as having attended the function on 25 October 2013. Of these, 87% (235/269) were interviewed as part of the cohort study. In total, 56 clinical and laboratory-confirmed cases of gastroenteritis were identified in the study respondents, giving an attack rate of 24% (56/235). Included in these cases were seven persons with microbiologically confirmed C. jejuni infection. Faecal samples were collected between 3 and 7 days after the function with a median incubation period of 61 h (range 39–74 h) in those providing samples. The epidemic curve (Fig. 1) shows the onset dates for clinical and confirmed cases, with a median incubation time of 61 h (range 5–188 h). Duration of illness was not calculated as most cases were symptomatic at the time of questionnaire completion. There was no statistically significant difference in age between cases and non-cases (P = 0·37), nor in the proportion of male and female cases (P = 0·28). The most frequently reported symptoms were diarrhoea (82%), abdominal pain (79%), nausea (61%), and fever (59%). Eighteen cases reported visiting a general medical practitioner, including one who presented to a hospital emergency department. No hospital admissions were reported.
Fig. 1.
Campylobacter-associated gastroenteritis cases by date of onset, Australia, October and November 2013 (n = 56).
Analytical results
Table 1 shows the results for the univariate epidemiological analysis. Increased risk of illness was shown for chicken liver pâté being eaten as an entrée (RR 3·64 95% CI 2·03–6·52, P < 0·001). An inverse association with illness was demonstrated if the alternative entrée option, the beetroot salad, had been consumed (RR 0·51 95% CI 0·31–0·83, P = 0·005). Following multivariable analysis only the chicken liver pâté retained statistical significance as an exposure variable (aRR 2·80, 95% CI 1·26–6·19, P = 0·01).
Table 1.
Relative risks for food exposures following a Campylobacter jejuni outbreak, Australia, October 2013
| Exposed | Unexposed | ||||||||
|---|---|---|---|---|---|---|---|---|---|
| Menu item | No. ill | Total no. | AR (%) | No. ill | Total no. | AR (%) | RR | 95% CI | P value |
| Cold canapés | 36 | 131 | 27·48 | 20 | 104 | 19·23 | 1·43 | 0·88–2·31 | 0·14 |
| Hot canapés | 19 | 75 | 25·33 | 37 | 160 | 23·13 | 1·10 | 0·68–1·77 | 0·71 |
| Any canapés | 38 | 147 | 25·85 | 18 | 88 | 20·45 | 1·26 | 0·78–2·07 | 0·35 |
| Chicken liver pâté with port wine jelly and crostini | 44 | 118 | 37·29 | 12 | 117 | 10·26 | 3·64 | 2·03–6·52 | <0·001 |
| Baby beet salad with micro herbs and feta | 19 | 118 | 16·10 | 37 | 117 | 31·62 | 0·51 | 0·31–0·83 | 0·005 |
| Any entrée | 54 | 208 | 25·96 | 2 | 27 | 7·41 | 3·50 | 0·91–13·56 | 0·03 |
| Spatchcock with Dutch carrots and red wine jus | 31 | 111 | 27·93 | 25 | 124 | 20·16 | 1·39 | 0·87–2·20 | 0·16 |
| Moroccan lamb on rosti with onion jam and glaze | 36 | 137 | 26·28 | 20 | 98 | 20·41 | 1·29 | 0·80–2·08 | 0·30 |
| Vegetarian main meal option | 2 | 15 | 13·33 | 54 | 220 | 24·55 | 0·54 | 0·15–2·01 | 0·32 |
| Any main meal | 55 | 226 | 24·34 | 1 | 9 | 11·11 | 2·19 | 0·34–14·10 | 0·36 |
| Rose brûlée with biscotti | 30 | 107 | 28·04 | 26 | 128 | 20·31 | 1·38 | 0·87–2·18 | 0·17 |
| Chocolate marquise with raspberry | 33 | 135 | 24·44 | 23 | 100 | 23·00 | 1·06 | 0·67–1·69 | 0·80 |
| Any dessert | 50 | 195 | 25·64 | 6 | 40 | 15·00 | 1·71 | 0·79–3·71 | 0·15 |
AR, Attack rate; RR, relative risk; CI, confidence interval.
Chicken liver pâté preparation
On 31 October, the kitchen was inspected and leftover pâté samples were collected. An interview with the catering manager revealed that the pâté had been prepared by an agency chef, using a new recipe. The pâté preparation began 2 days prior to the function, with the chicken livers being soaked in milk and then left overnight in a cool room. The following day they were drained and sautéed in a pan, along with onion, garlic butter and port wine. The livers were described as being ‘medium cooked’, having a slightly pink inside. A temperature probe was not used to assess their internal temperature following this cooking step. The livers were then seasoned and blended, before a mixture of clarified butter and vegetable oil was added. The pâté mixture was then transferred to moulds and placed in a cool room. The pâté remained in the cool room until ~30 min prior to dinner service.
Microbiological investigations
Eight stool samples were obtained from cases, with seven being culture positive for C. jejuni subspecies jejuni. Four isolates from chicken liver pâté underwent confirmation, speciation and molecular analysis. Two were identified as being C. jejuni and two as C. coli. All environmental swabs taken from the kitchen on 31 October 2013 were negative for Campylobacter sp., Salmonella sp. and Escherichia coli.
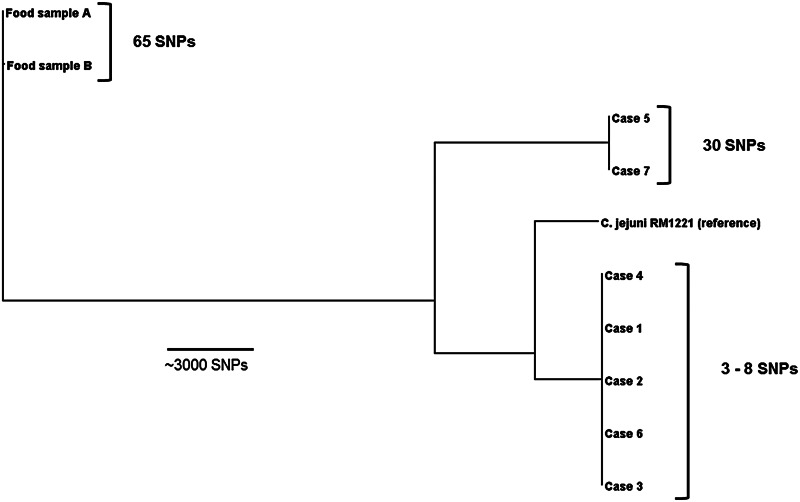
To further define the relationship between human and food isolates of C. jejuni, nine isolates underwent WGS and phylogenetic analysis (Fig. 2). This demonstrated three distinct clusters of isolates (five case isolates of MLST ST528 C. jejuni) that differed by a maximum of eight SNPs from each other, two ST535 case isolates that differed by 30 SNPs, and two ST991 pâté isolates that differed by 65 SNPs. The WGS analysis confirmed that the human case isolates were genetically distinct from the food isolates (Table 2).
Fig. 2.
Phylogenetic tree of nine Campylobacter jejuni outbreak isolates based on core-genome single nucleotide polymorphisms (SNPs), Australia 2013.
Table 2.
Core single nucleotide polymorphism distances between Campylobacter jejuni clinical and food outbreak isolates, Australia, October 2013
| Identifier | Pâté sample A | Pâté sample B | Case 1 | Case 2 | Case 3 | Case 4 | Case 5 | Case 6 | Case 7 | Reference |
|---|---|---|---|---|---|---|---|---|---|---|
| Pâté sample A | 0 | 65 | 22 944 | 22 940 | 22 943 | 22 942 | 23 241 | 22 941 | 23 242 | 22 924 |
| Pâté sample B | 65 | 0 | 22 957 | 22 953 | 22 956 | 22 955 | 23 262 | 22 956 | 23 263 | 22 943 |
| Case 1 | 22 944 | 22 957 | 0 | 4 | 7 | 8 | 13 123 | 5 | 13 130 | 5004 |
| Case 2 | 22 940 | 22 953 | 4 | 0 | 5 | 6 | 13 121 | 3 | 13 126 | 5002 |
| Case 3 | 22 943 | 22 956 | 7 | 5 | 0 | 7 | 13 122 | 4 | 13 127 | 5005 |
| Case 4 | 22 942 | 22 955 | 8 | 6 | 7 | 0 | 13 121 | 5 | 13 126 | 5004 |
| Case 5 | 23 241 | 23 262 | 13 123 | 13 121 | 13 122 | 13 121 | 0 | 13 122 | 30 | 12 847 |
| Case 6 | 22 941 | 22 956 | 5 | 3 | 4 | 5 | 13 122 | 0 | 13 127 | 5003 |
| Case 7 | 23 242 | 23 263 | 13 130 | 13 126 | 13 127 | 13 126 | 30 | 13 127 | 0 | 12 847 |
| Reference | 22 924 | 22 943 | 5004 | 5002 | 5005 | 5004 | 12 847 | 5003 | 12 847 | 0 |
DISCUSSION
Our epidemiological analysis demonstrated that chicken liver pâté, served as an entrée, was the food vehicle responsible for an outbreak of campylobacteriosis at a university college. We found a significant association between consuming chicken liver pâté and development of gastroenteritis. This finding was supported by microbiological evidence yielding C. jejuni in clinical specimens and the recovery of both C. jejuni and C. coli from leftover pâté sampled from the residential college's kitchen. Further, the food safety investigation determined that the chicken livers used in the pâté had likely been prepared in a manner that would have allowed Campylobacter sp. to survive the cooking process.
The epidemiology of campylobacteriosis in industrialized countries is characterized by the infrequency of reported outbreaks relative to the overall incidence of disease [5, 13]. When outbreaks do occur, the types of food vehicles that are implicated have been shown to vary internationally, with Australia identifying chicken meat as a major food vehicle [5], while elsewhere dairy products, particularly raw milk, and untreated drinking water have been identified as causes of outbreaks [13, 14]. Commentary on the increase in outbreaks of campylobacteriosis linked to offal, specifically poultry livers [15, 16] supports the establishment of liver pâté and similar dishes, as an increasingly important food vehicle during such events.
One factor potentially contributing to the apparent increase in liver-associated campylobacteriosis outbreaks is a change in consumer eating habits, with consumption of raw and minimally cooked foods gaining more widespread acceptance [17]. Such a trend has perhaps evolved without sufficient awareness or understanding of potential risks associated with raw or minimally cooked foods, as evidenced by the increasing number of outbreaks of salmonellosis linked to foods containing raw eggs in Australia [18]. While poultry production and consumption has soared in Australia it remains difficult to obtain data describing domestic offal consumption, although it is reported that 95% of Australian chicken offal is exported [19].
The relative infrequency of outbreaks due to Campylobacter sp., compared to outbreak-prone foodborne pathogens, such as Salmonella sp., creates a significant challenge in highlighting and maintaining awareness in consumers, restaurants and the catering sector of risks associated with poultry-liver use. C. jejuni in particular, is a prolific surface contaminant of poultry meat but the organism's inherent susceptibility to heat suggests that effective cooking practices will kill most organisms, leaving cross-contamination as a more likely mechanism for infection [20, 21]. Pâté, however, represents one of the few food vehicles where internal contamination of the liver tissue is likely and undercooking can result in campylobacteriosis. [22]. For poultry livers used in pâté to be free of Campylobacter, they need to be held at an internal temperature >70 °C for 2–3 min [22]. However, an important consideration for those preparing pâté is ensuring that cooked livers retain ‘pinkness’, a feature regarded as being desirable to consumers; if cooked too long the livers will become grey, with their flavour and texture being compromised [22]. The use of such subjective measures to determine the readiness of cooked poultry liver could potentially result in the occurrence of an outbreak situation such as we have described.
Our results revealed a high degree of difference between the two human outbreak-associated sequence types, as well as distinct differences between these and the sequence type found in the pâté recovered from the kitchen. Despite the absence of a definitive genetic link between the clinical and food isolates, we do not believe this adversely impacts our investigation's conclusion that Campylobacter-contaminated chicken liver pâté was responsible for this outbreak. The small differences in SNPs in cases with the same sequence type, suggests a common source and further supports the closeness of the epidemiological association we have outlined.
Our findings also align with other studies employing molecular typing of outbreak isolates where recovery of differing C. jejuni strains from epidemiologically linked cases has been demonstrated [7, 23]. In this investigation, cases within the two distinct groups differed by more than 13 000 SNPs. Explanations for such differences in cases during outbreaks could include strain diversity at the reservoir level [24], and the impact of cross-contamination and strain mixing during slaughtering and processing [25] or at wholesale or retail sale points [26]. The microbiological processing of food samples in the laboratory may also not have uncovered the full genetic diversity within the source.
While there are a limited number of studies concerning C. jejuni MLST from patients from different parts of the world, the predominant lineages appear to show some regional differences [27]. Interrogation of the Campylobacter MLST website shows that both ST528 (CC354) and ST535 (CC460) have been identified previously with sporadic human infection in Australia, with a suggestion that ST528 may be endemic [28]. ST535 appears to have a more global distribution, with sporadic cases reported from Canada, Japan, Germany Thailand and the UK (PubMLST, accessed October 2015), further illustrating the complexities of C. jejuni's genetic epidemiology.
While MLST has been demonstrated to be a good predictor of the genome-level relatedness of C. jejuni isolates [29], the interpretation of outbreak-associated isolates, including the integration of epidemiological data with SNP differences, is less well established [30]. Examples, however, do include the investigation of outbreaks linked to improperly pasteurized milk and contaminated drinking water [30, 31], highlighting differing levels of genomic diversity in outbreak strains, presumably due to the transmission routes and underlying reservoirs. We believe our investigation is the first use of WGS to aid characterization of a C. jejuni outbreak caused by contaminated poultry liver, with our results supporting previous findings showing marked strain diversity in isolates linked to foodborne poultry liver outbreaks [7, 32]. However a dearth of local data describing common sequence types from clinical cases and reservoirs particularly poultry, presently limits our interpretations and understanding of the genetic epidemiology of campylobacteriosis in Australia.
A strength of our investigation was the high response rate in attendees (87%), underscoring the validity of our conclusion that liver pâté was the food vehicle responsible for this outbreak. We were able to commence interviews within 6 days of the implicated function, with the majority of respondents’ details collected over the following four working days. Contributing to this success was the agreement by the college to provide student mobile phone and email details, enabling contact via multiple communication media. With respect to bias, the closed setting and interconnectedness of students could have introduced ascertainment bias. However, our finding of an inverse association with illness and consumption of the alternate entrée option, suggests such an effect to have been unlikely. Similarly, recall bias is another potential issue, with students served the pâté potentially reporting this exposure differently to students who were served the alternate entrée but who may have then swapped this or otherwise sampled the pâté. Despite the common exposure, case misclassification also remains a possibility, with only 14% (8/57) of cases submitting stool samples for culture.
CONCLUSIONS
Poultry-liver-derived dishes, such as pâté and parfait, have become established food vehicles during outbreaks of campylobacteriosis. We believe this study represents the first use of WGS to aid characterization of a C. jejuni outbreak caused by contaminated poultry liver, and highlights the potential genetic diversity in epidemiologically linked food and human isolates. The study highlights the usefulness of employing complementary components of epidemiological evidence, including genomic data, to support public health inference.
ACKNOWLEDGEMENTS
We thank staff from the Australian Capital Territory (ACT)'s Communicable Disease Control section, Environmental Health section and microbiology staff from the ACT Government Analytical Laboratory and Canberra Hospital and Health Service for their assistance with the investigation. We also acknowledge the assistance provided by the staff and students of the college, whose cooperation was invaluable during the epidemiological investigation.
This publication made use of the Campylobacter MLST website (http://pubmlst.org/campylobacter/) developed by Keith Jolley and sited at the University of Oxford (Jolley & Maiden, 2010, BMC Bioinformatics, 11: 595). The development of this site has been funded by the Wellcome Trust.
This work was supported by the National Health and Medical Research Council (C.R.M.M. Public Health and Health Services Postgraduate Research Scholarship APP1074790). OzFoodNet is funded by the Australian Government Department of Health. Microbiological Diagnostic Unit Public Health Laboratory is funded by the Department of Health and Human Services, Victoria.
DECLARATION OF INTEREST
None.
REFERENCES
- 1.World Health Organisation, Food and Agriculture Organization of the United Nations, World Organisation for Animal Health. The global view of campylobacteriosis: report of an expert consultation, Utrecht, Netherlands, 9–11 July 2012. Geneva: World Health Organisation, 2013.
- 2.NNDSS Annual Report Writing Group. Australia's Notifiable Disease Status, 2012: Annual Report of the National Notifiable Diseases Surveillance System. Communicable Disease Intelligence 2015; 39: E46–E136. [DOI] [PubMed] [Google Scholar]
- 3.Heymann DL. Control of Communicable Diseases Manual, 19th edn. Washington, DC: American Public Health Association, 2008. [Google Scholar]
- 4.Blaser MJ. Epidemiologic and clinical features of Campylobacter jejuni infections. Journal of Infectious Disease 1997; 176 (Suppl. 2): S103–105. [DOI] [PubMed] [Google Scholar]
- 5.Unicomb LE, et al. Outbreaks of campylobacteriosis in Australia, 2001 to 2006. Foodborne Pathogens and Disease 2009; 6: 1241–1250. [DOI] [PubMed] [Google Scholar]
- 6.Edwards DS, et al. Campylobacteriosis outbreak associated with consumption of undercooked chicken liver pate in the East of England, September 2011: identification of a dose-response risk. Epidemiology and Infection 2014; 142: 352–357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Forbes KJ, et al. Campylobacter immunity and coinfection following a large outbreak in a farming community. Journal of Clinical Microbiology 2009; 47: 111–116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Inns T, Foster K, Gorton R. Cohort study of a campylobacteriosis outbreak associated with chicken liver parfait, United Kingdom, June 2010. Eurosurveillance 2010; 15(44). [DOI] [PubMed] [Google Scholar]
- 9.Cody AJ, et al. Real-time genomic epidemiological evaluation of human Campylobacter isolates by use of whole-genome multilocus sequence typing. Journal of Clinical Microbiology 2013; 51: 2526–2534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Bolton FJ, et al. Identification and biotyping of campylobacters. In: Board GR, Jones D, Skinner FA, eds. Identification Methods in Applied and Environmental Microbiology. Oxford, UK: Blackwell Scientific Publications, 1992, pp. 151–161. [Google Scholar]
- 11.Linton D, et al. PCR detection, identification to species level, and fingerprinting of Campylobacter jejuni and Campylobacter coli direct from diarrheic samples. Journal of Clinical Microbiology 1997; 35: 2568–2572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Price MN, Dehal PS, Arkin AP. FastTree 2 – approximately maximum-likelihood trees for large alignments. PLoS ONE 2010; 5: e9490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Taylor EV, et al. Common source outbreaks of Campylobacter infection in the USA, 1997–2008. Epidemiology and Infection 2013; 141: 987–996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Clark CG, et al. Characterization of waterborne outbreak-associated Campylobacter jejuni, Walkerton, Ontario. Emerging Infectious Diseases 2003; 9: 1232–1241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Little CL, et al. A recipe for disaster: outbreaks of campylobacteriosis associated with poultry liver pate in England and Wales. Epidemiology and Infection 2010; 138: 1691–1694. [DOI] [PubMed] [Google Scholar]
- 16.Merritt T, Combs B, Pingault N. Campylobacter outbreaks associated with poultry liver dishes. Communicable Disease Intelligence 2011; 35: 299–300. [DOI] [PubMed] [Google Scholar]
- 17.Newell DG, et al. Food-borne diseases – the challenges of 20 years ago still persist while new ones continue to emerge. International Journal of Food Microbiology 2010; 139 (Suppl. 1): S3–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Moffatt CR, Musto J. Salmonella and egg-related outbreaks. Microbiology Australia 2013; 34: 94–98. [Google Scholar]
- 19.Mifsud C. Chicken meat. Agricultural Commodities 2014; 4: 101–104. [Google Scholar]
- 20.Luber P, Bartelt E. Enumeration of Campylobacter spp. on the surface and within chicken breast fillets. Journal of Applied Microbiology 2007; 102: 313–318. [DOI] [PubMed] [Google Scholar]
- 21.Moffatt C. Correspondence: A multi-centre prospective case-control study of campylobacter infection in persons aged 5 years and older in Australia. Epidemiology and Infection 2008; 136: 1315–1316 (author reply 1316–1318). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Whyte R, Hudson JA, Graham C. Campylobacter in chicken livers and their destruction by pan frying. Letters in Applied Microbiology 2006; 43: 591–595. [DOI] [PubMed] [Google Scholar]
- 23.Moffatt CR, et al. Campylobacter jejuni gastroenteritis at an Australian boarding school: consistency between epidemiology, flaA typing, and multilocus sequence typing. Foodborne Pathogens and Disease 2010; 7: 1285–1290. [DOI] [PubMed] [Google Scholar]
- 24.Bull SA, et al. Sources of Campylobacter spp. colonizing housed broiler flocks during rearing. Applied and Environmental Microbiology 2006; 72: 645–652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Allen VM, et al. Campylobacter spp. contamination of chicken carcasses during processing in relation to flock colonisation. International Journal of Food Microbiology 2007; 113: 54–61. [DOI] [PubMed] [Google Scholar]
- 26.Kramer JM, et al. Campylobacter contamination of raw meat and poultry at retail sale: identification of multiple types and comparison with isolates from human infection. Journal of Food Protection 2000; 63: 1654–1659. [DOI] [PubMed] [Google Scholar]
- 27.McCarthy ND, et al. Molecular epidemiology of human Campylobacter jejuni shows association between seasonal and international patterns of disease. Epidemiology and Infection 2012; 140: 2247–2255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Mickan L, et al. Multilocus sequence typing of Campylobacter jejuni isolates from New South Wales, Australia. Journal of Applied Microbiology 2007; 102: 144–152. [DOI] [PubMed] [Google Scholar]
- 29.Carrillo CD, et al. A framework for assessing the concordance of molecular typing methods and the true strain phylogeny of Campylobacter jejuni and C. coli using draft genome sequence data. Frontiers in Cellular and Infection Microbiology 2012; 2: 57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Revez J, et al. Genomic variation between Campylobacter jejuni isolates associated with milk-borne-disease outbreaks. Journal of Clinical Microbiology 2014; 52: 2782–2786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Fernandes AM, et al. Partial failure of milk pasteurization as a risk for the transmission of campylobacter from cattle to humans. Clinical Infectious Diseases 2015; 61: 903–909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Abid M, et al. Duck liver-associated outbreak of campylobacteriosis among humans, United Kingdom, 2011. Emerging Infectious Diseases 2013; 19: 1310–1313. [DOI] [PMC free article] [PubMed] [Google Scholar]