Abstract
Traumatic Brain injury affects at least 1.7 million people in the United States alone each year. The majority of injuries are categorized as mild but these still produce lasting symptoms that plague the patient and the medical field. Currently treatments are aimed at reducing a patient’s symptoms, but there is no effective method to combat the source of the problem, neuronal loss. We tested a mild, closed head traumatic brain injury model for the effects of modulation of the antioxidant transcription factor Nrf2 by the chemical activator, tert-butylhydroquinone (tBHQ). We found that post-injury visual memory was improved by a 7 day course of treatment and that the level of activated caspase-3 in the hippocampus was reduced. The injury-induced memory loss was also reversed by a single injection at 30 min after injury. Since the protective stress response molecule, HSP70, can be upregulated by Nrf2, we examined protein levels in the hippocampus, and found that HSP70 was elevated by the injury and then further increased by the treatment. To test the possible role of HSP70, model neurons in culture exposed to a mild injury and treated with the Nrf2 activator displayed improved survival that was blocked by the HSP70 inhibitor, VER155008. Following mild traumatic brain injury, there may be a partial protective response and patients could benefit from directed enhancement of regulatory pathways such as Nrf2 for neuroprotection. Published by Elsevier Ltd. on behalf of IBRO.
Keywords: traumatic brain injury, transcription factors, Nrf2, HSP70
INTRODUCTION
There are reported 1.7 million people who suffer traumatic brain injuries each year in the United States (CDC, 2011). Injuries range from mild to severe with approximately 80% presenting with mild injuries (Kraus and Nourjah, 1988). Traumatic brain injuries have become the signature affliction of deployed forces with most of these categorized as mild injuries (Hoge et al., 2008). While these traumas are not life threatening they still have a substantial impact on the individual initiating cognitive and other health problems (Rimel et al., 1981; Dacey et al., 1986; McAllister et al., 1999; Vakil, 2005; McAllister, 2011). Magnetic resonance imaging and single-photon emission scans have shown that mild injury causes brain lesions with atrophy apparent even 6 months post trauma (Hofman et al., 2001). This is recapitulated in animal studies where extended loss is noted after trauma (Smith et al., 1995). One of the most common symptoms that results from mild traumatic brain injury (mTBI) is memory loss (Binder, 1986; Warden, 2006; Halbauer et al., 2009). This may result from damage to the hippocampus (Rempel-Clower et al., 1996), which is sensitive to mechanical injury (Kotapka et al., 1991; Hicks et al., 1993; Rempel-Clower et al., 1996; Umile et al., 2002). Among the different aspects of memory, working memory or short-term memory is often impaired following mTBI (McAllister et al., 1999; Halbauer et al., 2009). Visual memory is a form of working memory that is specifically affected in patients. It has been observed that mild TBI patients have visual memory deficits, as measured by the Shum visual learning test, but they do not show any difference in spatial memory measured by an electronic maze test (Shum et al., 2000). Memory deficits following traumatic injury have been recapitulated in animal models. TBI induced in mice (Tashlykov et al., 2009) has shown neuronal loss in the hippocampus and associated cognitive deficits and similar results have been found in the rat (Hicks et al., 1993; Smith et al., 1994). Importantly, animal models have shown that closed head injury results in cognitive deficits (Zohar et al., 2003) when evaluated for visual memory (Biegon et al., 2004; Edut et al., 2011), spatial memory (Rubovitch et al., 2010; Baratz et al., 2011), non-spatial memory (Zohar et al., 2011), or anxiety (Edut et al., 2011). A chronic loss of memory, even slight, complicates several other cognitive functions.
Traumatic brain injury is a complex disease with heterogeneous actions and dysregulation throughout the brain resulting in neuronal loss. The primary injury results in a focal injury at the site of impact with local neuronal loss. It is not just these immediate events that result in neuronal deficit, but the pathobiology that occurs following the primary injury. These processes include disruption of cellular homeostasis by calcium release, oxidative stress, and apoptosis signaling cascades that persist for days or weeks following injury. It is the multifactorial dysregulations that occur following the primary injury, often called the secondary injury that results in extended neuronal loss as well as diffuse axonal injury. Diffuse axonal injury is a serious condition where the axon is damaged resulting in the degradation of neuronal circuitry. This loss of axonal branches alters neurotransmission and can be a major contributor to functional loss. The actual axonal damage is thought to be a result of secondary injury involving signaling cascades (Smith et al., 2003). This damage can be very significant even in mild injuries and is not detectable until hours or days following injury.
It is the secondary injury that provides targets for the treatment of traumatic brain injury. If sufficient neurons can be preserved then the cognitive deficit will be reduced along with prolonged symptoms observed in patients and this may eliminate the need for more invasive treatments in the future. One pharmacological target is the regulation of transcription factor signaling, which is altered by the secondary injury. Modest alterations of these factors can significantly alter downstream signaling to reduce detrimental signaling and promote pro-survival pathways allowing for neuronal preservation (Kane and Citron, 2009). Nuclear factor (erythroid-derived 2)-like 2 (Nrf2) is one transcription factor of interest and it is known to activate downstream antioxidant and oxidative stress genes. Nrf2 is a transcription factor that is sequestered in the cytoplasm and tethered to Kelch-like ECH-associated protein 1 (KEAP1). When Nrf2 is activated, it disassociates and translocates to the nucleus where it induces downstream targets by binding canonical antioxidant response elements (Rushmore et al., 1991; Lee et al., 2003; Johnson et al., 2008). Nrf2-deficient mice display an increase in apoptosis and inflammatory signaling following moderate closed head TBI (Jin et al., 2009). The downstream gene heat shock protein 70 (HSP70) acts through several mechanisms by which it protects protein function and stability in a cell. To elicit the effects of Nrf2 a chemical activator, tert-butylhydroquinone (tBHQ) can be administered (Shih et al., 2005). This compound is a metabolite of the antioxidant butylated hydroxyanisole, can cross the blood-brain barrier, and has produced neuroprotection in several models of disease (Kraft et al., 2004; Jakel et al., 2007; Shih et al., 2007).
The health impacts of TBI are extreme and the economical burden of these injuries is an estimated 60 billion dollars in the US alone (CDC, 2011). The need for effective treatments is clear. We show that the use of tBHQ, an activator of Nrf2, as a treatment following traumatic brain injury can improve visual memory in mice. This may be the result of an increase in HSP70 and the decrease in activated caspase-3.
EXPERIMENTAL PROCEDURES
Animals
Male ICR mice were originally purchased from HSD Jerusalem or Harlan Indianapolis, IN and were bred and raised in our vivarium. The mice weighing 30–40 g were housed five per cage under a constant 12-h light/dark cycle. The mice were maintained at 22 °C ± 0.5 °C. Food and water were supplied ad libitum. All experiments were performed in accordance with local and national guidelines.
Treatment
Treatment solution: 100 mg tBHQ (tert-butylhydroquinone) was dissolved in 1 ml of 100% dimethyl sulfoxide (DMSO) and was stored at 2 °C as stock. Preparation of the injection solution was carried out by a 1:100 dilution of the stock in phosphate buffered saline (PBS), producing 1 mg/ml tBHQ, 1% DMSO in PBS. To achieve higher doses, a 1:50 dilution was also prepared to yield 2 mg/ml tBHQ, 1% DMSO in PBS. The injection solution was prepared freshly every day. The vehicle solution was a matching concentration of DMSO in PBS.
Mice were divided into four injection groups: (1) Sham + DMSO, (2) Sham + tBHQ, (3) mTBI + DMSO, and (4) mTBI + tBHQ. tBHQ (at 33.4 or 16.7 mg/kg) or DMSO vehicle were administered as treatment by IP injection beginning 30 min post trauma and involved either a single injection or six additional daily injections. The administration was based on effective doses reported in a rodent cerebral ischemia model (Shih et al., 2005). Behavioral tests were performed at 7 or 30 days post trauma (Fig. 1). The behavioral tests included Object recognition, Y maze, Elevated plus maze and passive avoidance and were performed one after the other. Each mouse was used only once and only at one time point.
Fig. 1.
Experimental timeline. Thirty minutes following a mild closed head injury or sham injury, mice were treated with tBHQ by IP injection. Some mice were treated with multiple injections, which were administered daily for 6 days afterward for a maximum of 7 injections. Tissues were harvested at 1 and 3 days post injury for biochemical analysis. Behavioral analysis was performed at 7 and 30 days following injury.
Closed-head mTBI injury
mTBI was induced using the concussive closed-head trauma instrument (Zohar et al., 2003) that causes behavioral and neuronal loss (Tashlykov et al., 2007). This device is composed of a metal tube (inner diameter 13 mm) placed vertically over a sponge that supports the head of the mouse. Mild closed-head trauma injury was caused by a 30 g cylindrical metal weight dropped 80 cm through the metal tube onto the right temporal side of the skull, with the skin and fur intact, in this established model that does not produce systemic complications (Zohar et al., 2003; Milman et al., 2005). Prior to the injury, the mice were lightly anesthetized with isoflurane. Sham mice were also lightly anesthetized with isoflurane without being injured.
Behavioral tests
Novel object recognition.
A Novel object task was used to evaluate recognition memory (Hammond et al., 2004). The open field was a 59 × 59 cm arena, surrounded by 20 cm black Plexiglas walls. The floor of the arena was also black and divided into 36 identical squares by white gridlines. Each mouse was placed in an empty arena for 5 min habituation. After 24 h, the mice were placed for 5 min into the arena with two identical objects, A and B (e.g., bottles), positioned 40 cm from each other and 10 cm from the walls. On the next day (day 3), the mice were placed again for 5 min into the arena with object A (the same as on the second day) and object C (a “new” object; e.g., a coffee can).
The arena and the objects were cleaned with 70% ethanol between each trial. Exploration of an object was defined as rearing on the object or sniffing it at a distance of less than 2 cm and/or touching it with the nose. Discrimination of visual novelty was assessed by a preference index (Dix and Aggleton, 1999): (time exploring the “new” object – time exploring the “old” object)/(total time exploring the objects).
Y maze.
The Y maze test was used to assess spatial memory (Conrad et al., 1996). The maze was made of black Plexiglas and consisted of three identical arms (8 × 30 × 15 cm at angles of 120°) that created a Y-shaped maze. One of the arms was defined as a ‘start arm’. The mice were put in the ‘start arm’ at the first trial that lasted 5 min and one of the two other arms was chosen randomly as a ‘novel arm’ which remained closed through the first trial. After this session, the mouse was returned to his home cage for 2 min habituation. In the second, trial, lasting 2 min, the mice were placed again in the start arm with all of the arms open. Between each trial and between each mouse the maze was cleaned with a 70% ethanol solution. The time of the mouse in each of the three arms was measured but the difference in the time between the novel and the old arm was used to assess memory. Discrimination of spatial novelty was calculated by a preference index (Dix and Aggleton, 1999): (time in the new arm − time old arm)/(time in the new arm + time in the old arm).
Elevated plus maze.
The elevated plus-maze task was used to evaluate anxiety-like behavior (Hogg, 1996). The Elevated plus maze consisted of two open arms (30 × 5 × 15 cm) and two closed arms (30 × 5 × 15 cm) all with an open roof. The open and closed arms were constructed of white and black Plexiglas respectively. The maze was elevated 60 cm above the floor. In this test the mice were placed in the center of the maze, facing one of the open arms. The time spent in each of the two arm types and the number of arm entries, were measured during a 5 min test period. Arm entries were defined as the entry of all four paws into the arm. The maze was cleaned with 70% ethanol between each mouse.
Rotarod.
Motor skills were tested 7 days following injury by rotarod performance on a Stoelting 57620. Mice were trained on the rotarod allowing them to become accustomed with the apparatus, and were tested following a 30 min rest period. Time spent on a rotating dowel that accelerates from 4 to 40 rpm over 5 min was measured. The mean time a mouse was able to stay on the dowel was calculated from 3 trials and normalized to baseline performance for each mouse.
Passive avoidance.
The passive avoidance task was used to assess simple non-spatial learning ability (Milman et al., 2005). The passive avoidance apparatus (San Diego Instruments, San Diego, USA) was made of black Plexiglas (48 × 22 × 22 cm) with a grid floor and two compartments: dark and lighted. A door separated the passage between the two compartments. A lamp illuminated the lighted compartment and the dark compartment was covered and not lighted at all. In this trial the mice were placed in the lighted compartment and after 30 s, the door was opened. When the mice entered the dark compartment, the door was closed, and the mice received an electric foot shock (1 mA for 3 s). After 24 h the mice were placed again in the lighted compartment with the passageway open during the whole time of the trial. If the mouse entered the dark compartment in the period of three minutes, we considered that as an entrance (and a memory failure), and recorded the time until entry. No shock was delivered during the second day. This data was collected as binary data and statistical significance was determined using the Chi square test.
Protein analysis
Mice were killed by cervical dislocation and the brains were rapidly dissected within five minutes and snap frozen in liquid N2 and stored at −80 °C. Proteins were extracted from the right cerebral cortex (including cingulate cortex) and hippocampal regions with RIPA buffer supplemented with protease inhibitors (Pierce Biotechnology, Rockford, IL). Protein was quantitated with a micro-Bio-Rad assay (Hercules, CA) and separated in a 4–20% SDS polyacrylamide gel (Pierce). Proteins were transferred onto a nitrocellulose membrane (LI-COR Biosciences, Lincoln, NE), blocked in Odyssey blocking buffer (LI-COR Biosciences), and incubated with primary antibody overnight at 4 °C. The membrane was then incubated with fluorescent IR dye secondary antibodies at 23 °C for 1 h, and visualized on a LI-COR Odyssey IR scanner. The primary antibodies were HSP70 1:500 (sc-33575; Santa Cruz Biotechnology, Santa Cruz, CA), Nrf2 1:200 (sc-722), c-Jun 1:1,000 (sc-1694), cleaved Caspase-3 (ASP 175) 1:750 dilution (#9661; Cell Signaling, Beverly, MA), and glyceraldehyde-3-phosphate dehydrogenase (GAPDH) 1:2,000 (MAB374; Millipore, Billerica, MA). Secondary antibodies were goat antirabbit IR Dye 680 1:20,000 (#926–32221; LI-COR) and goat anti-mouse IR Dye 800 1:20,000 (#926–32210; LI-COR).
Cell culture
Human neuroblastoma SH-SY5Y immortalized model neurons were obtained from ATCC (Manassas, VA) and maintained in Dulbecco’s modified Eagle’s medium supplemented with 10% fetal bovine serum (Invitrogen/Gibco, Carlsbad, CA), 0.25 U/ml penicillin and 50 μg/ml streptomycin (Invitrogen/Gibco). Cells were maintained in humidified 5% CO2 at 37 °C and grown until they were 90% confluent. 350,000 cells were plated per well into a 6-well Bioflex plate (Flexcell international, Hillsborough, NC) with a collagen type I coating. Bioflex plates have a silastic membrane that allows the cells to stretch mimicking the torsional impact-induced injury most common in TBI (Weber et al., 2001; Arundine et al., 2004; Lau et al., 2006).
In vitro treatment.
Cells were treated with 10 μM tBHQ (Sigma, St. Louis, MO). Stock solution was 200 mM dissolved in DMSO and diluted in media prior to adding to the cells for a final concentration of 10 μM. The HSP70 inhibitor, VER155008 (Tocris Bioscience/R&D systems, Minneapolis, MN), was dissolved in DMSO to 100 mM and diluted in media prior to adding to the cells for a final concentration of 10 μM. Treatment groups included tBHQ, tBHQ + VER155008, control (vehicle alone), or vehicle + VER155008 added 2 h prior to injury. Cell viability was assayed at 7 h after the treatment was introduced.
In vitro mild traumatic injury.
The Cell Injury Controller II (Virginia Commonwealth University) (Ellis et al., 1995) was used to deliver a biaxial stretch injury to the adherent SH-SY5Y cells growing on the Bioflex plates. A measured nitrogen pulse was delivered to the plate producing a controlled stretch of the silastic membrane as previously described (Kane et al., 2011). The cells received injury pulses for 99 ms. Cells were injured according to our mild injury protocol a total of 4 times, each one hour apart, and then allowed to recover for 2 h prior to analysis. We previously found that the injury effects are two-fold, inducing loss of neurites and cell death (Kane et al., 2011).
Cell viability.
Analysis of cell death was performed by staining with Annexin Alexa Fluor 488 (Invitrogen), propidium iodide (Sigma), and Hoechst 33342 (Invitrogen). Stain and Annexin-binding buffer (1 mM HEPES, 1.4 M NaCl, 25 mM CaCl2, pH 7.4) was added to the cells in media and incubated in the dark for 15 min. Staining was analyzed on an Olympus Fluoview 1000 laser scanning confocal microscope. Quantitation was performed with ImageJ (Abramoff et al., 2004).
Data analysis
All behavioral results are given as mean ± SEM and were analyzed with SPSS 17 software (Genius Systems, Petah Tikva, Israel). One-way ANOVA was performed to compare all groups, followed by LSD post hoc tests. ANOVA was used to test the results of the Y maze test, elevated plus maze test, and the Novel object recognition test. Results of the passive avoidance test were analyzed by the non-parametric Chi square test. Biochemical analyses are presented as mean ± SD and were analyzed by One-way ANOVA followed by the Student–Newman–Keuls Multiple Comparisons Test with InStat software (GraphPad, La Jolla, CA).
RESULTS
Cognitive performance and tBHQ treatment
Mice were subjected to a mild closed head injury at the right temporal region of the skull and treated 30 min post injury by inner peritoneal injection of either tBHQ or vehicle (1% DMSO). Mice were treated once per day for 6 days following injury and behavioral tests were performed to measure cognitive ability at 7 and 30 days post injury (Fig. 1).
The object recognition test demonstrated that mice subjected to a closed head injury spent significantly less time exploring the “new” object than sham mice indicating an impairment in visual memory 7 days post injury. The tBHQ treatment of injured mice resulted in a significant increase in time spent exploring the “new” object compared to those treated with vehicle at 7 days post trauma (Fig. 2A). A similar trend was also evident at 30 days post injury (Fig. 2B). A lower dose of tBHQ (16.7 mg/kg) was not sufficient to observe any difference between treated and untreated injured mice at 7 or 30 days post injury (data not shown). These experiments indicated that daily treatments of tBHQ administered following injury transiently increased visual memory.
Fig. 2.
Novel object recognition behavioral test. Injured and sham mice were treated with either vehicle or 33.4 mg/kg tBHQ for 6 days post injury and were analyzed for their ability to identify a “new” object (visual memory) at 7 days (n = 7–15) and (B) 30 days post injury (n = 6–9). The preference index equals (time near the “new” object − time near the “old” object)/(time near the “new” object + time near the “old” object). A significant decrease in preference index was observed in mTBI animals compared to sham animals at both 7 days (A) and 30 days (B) post injury. Treatment with tBHQ rescued this deficit 7 days post injury (A) and profound but no significant difference between the mTBI and mTBI + treatment groups could be observed at 30 days post injury. *P < 0.05.
To measure spatial memory, the Y maze behavioral test was performed on the mice. tBHQ treatment produced no detectable effect on the time spent in the novel arm at either 7 or 30 days post injury (data not shown). Another symptom of mTBI is increased anxiety-like behavior. To monitor the anxious-like behavior of mice the elevated plus maze was conducted. There was no difference in time spent in the open arms between mTBI and sham mice observed at 7 days post injury. Similarly there was no observed treatment effect (Fig. 3). The results indicated a normal anxious behavior of all the animals including the TBI and the tBHQ mice. Motor behavior was tested with a Rotarod and by 7 days, mice performance was unaffected by the injury (data not shown).
Fig. 3.
Elevated plus maze behavioral test. Anxiety-like behavior was monitored for injured and sham mice, treated with either vehicle or 33.4 mg/kg tBHQ for 6 days post injury, at 7 days (n = 6–9) and 30 days post trauma (n = 9–10). Injured and sham mice treated with either vehicle or 33.4 mg/kg tBHQ for 6 days post injury were monitored for anxiety-like behavior at 7 (n = 6–10) post mTBI. No significant difference between groups was observed at 7 days post trauma.
As a measure of non-spatial learning ability the passive avoidance test was performed. No difference was apparent in the frequency to enter the dark compartment between mTBI and sham mice, and no treatment effect was observed (Fig. 4).
Fig. 4.
Passive avoidance behavioral test. Injured and sham mice treated with either vehicle or 33.4 mg/kg tBHQ for 6 days post injury were monitored for their non-spatial learning ability. This test was performed 7 days (n = 6–8) following mTBI. No significant difference between groups was observed at days post trauma.
Activation of caspase-3 in the hippocampus
To determine the mechanism of how activated Nrf2 may improve visual memory we measured proteins known to contribute to neuronal loss (Table 1). Daily injections, that began at 30 min after injury, were performed until tissues were collected on either day 1 or 3 post injury (Fig. 1), the time points where we typically find regulatory changes. We observed an increase in c-Jun at day 1 and an increase in glial fibrillary acidic protein (GFAP) in the hippocampus at day 3. On day 3 following injury we observed an increase in caspase-3 activation in the hippocampus of the mice by western blot (Fig. 5). Activated caspase-3 (red, 19 kDa) was increased two fold in injured mice compared to sham. Mice treated with tBHQ showed a significant decrease in activated caspase-3 compared to injured vehicle-treated mice (Fig. 5A). The caspase signal was quantitated with ImageJ gel analysis and normalized to GAPDH expression (green, 38 kDa) (Fig. 5B).
Table 1.
Protein expression following injury. Proteins were isolated from the right hippocampus and right cortex of injured and sham mice treated with either vehicle or tBHQ at 1 (n = 4–5) and 3 (n = 5–6) days post injury. Specific proteins were measured using western immunoblots and only significant differences (P < 0.05) are shown involving 1.5–1.8-fold (↑) or ~2-fold (⇈) changes
| Hippocampus |
Cortex |
|||
|---|---|---|---|---|
| Day 1 | Day 3 | Day 1 | Day 3 | |
| c-Jun | ↑ in mTBI mice | No difference | No difference | No difference |
| GFAP | No difference | ↑ in mTBI | No difference | No difference |
| Nrf2 | No difference | No difference | ↑ in mTBI | No difference |
| HSP70 | No difference | ↑ in mTBI ⇈ by tBHQ + mTBI | No difference | No difference |
| Activated caspase-3 | Not measured | ⇈ in mTBI ↓ by tBHQ | Not measured | Not measured |
Fig. 5.
Activated caspase-3 expression following mTBI. Protein was harvested from the right hippocampus of injured and sham mice treated with either vehicle or 33.4 mg/kg tBHQ. Western blots were performed with antibodies specific to activated caspase-3. Expression was normalized to GAPDH. An increase in caspase-3 activation was observed in injured mice. Treatment with tBHQ reduced the activation of caspase-3 (n = 4–6, *P < 0.05).
HSP70
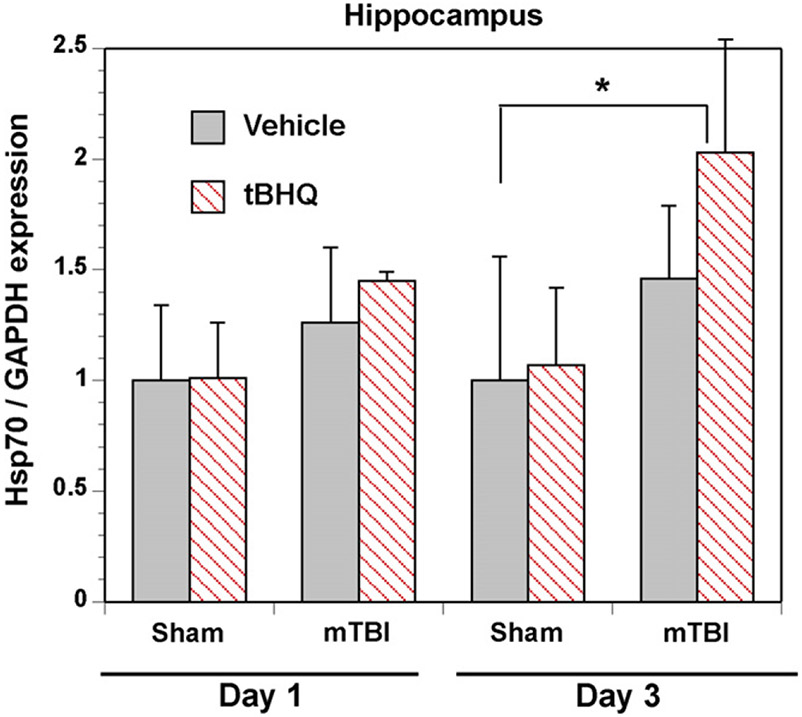
One downstream target of Nrf2 is HSP70, which is known for its role in protein stability and cellular protection. Protein expression was measured by western blot in the hippocampus (Fig. 6) and cortex of treated and control mice. Injured mice displayed an increase in HSP70 expression, compared to sham mice, in the hippocampus following treatment with tBHQ. Expression was quantitated with ImageJ and normalized to GAPDH. No difference in expression was observed between injured and sham in the cortex (Table 1).
Fig. 6.
HSP70 expression following mTBI. Protein was harvested from the right hippocampus and the right frontal cortex of injured and sham mice treated with either vehicle or 33.4 mg/kg tBHQ at 1 day or 3 days following injury. Western blots were performed with antibodies specific to HSP70 and expression was normalized to GAPDH. An increase in HSP70 protein was observed in the hippocampus of injured mice (n = 5–6) 3 days following injury and this was further elevated by the Nrf2 activator (*P < 0.01). No differences were observed at 1 day post injury (n = 4–5). No significant differences were observable in the cortex on 1 (n = 4–5) or 3 days (n = 5–6) post injury.
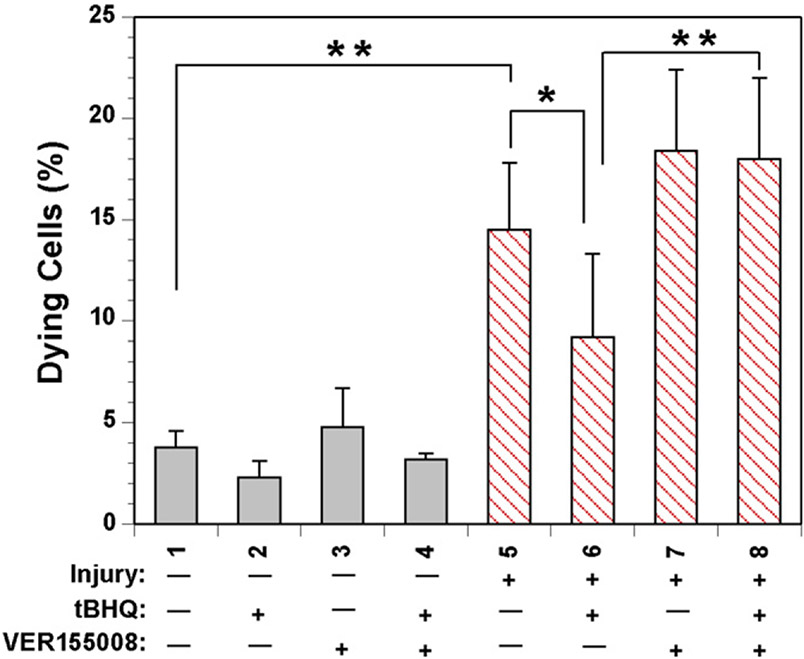
To test whether HSP70 plays a role in neuronal survival, SH-SY5Y model neurons were treated with the HSP70 inhibitor VER155008 (10 μM) and tBHQ (10 μM) and subjected to an in vitro traumatic injury mimicking the torsional rotation that is observed in traumatic brain injuries (Weber et al., 2001; Arundine et al., 2004; Lau et al., 2006). We have previously shown that the degree of injury achieved from this apparatus on immortalized cells can result in a loss of processes and cell bodies (Kane et al., 2011). Cell viability following injury was determined by staining with Hoechst, Annexin V, and propidium iodide. Cells that were treated with tBHQ showed a significant decrease in neuronal loss (Fig. 7, columns 6 vs. 5). However, treatment with VER155008 blocked the protection induced by tBHQ and these model neurons suffered maximal neuronal loss (Fig. 7, columns 8 vs. 6), indicating that the tBHQ-induced upregulation of HSP70 can be neuroprotective.
Fig. 7.
Viability of SH-SY5Y model neurons treated with tBHQ and HSP70 inhibitor. Undifferentiated human neuroblastoma SH-SY5Y cells were pretreated with vehicle, 10 μm VER155008, 10 μm tBHQ, or both VER155008 and tBHQ and subjected to multiple mild biaxial stretch injuries. Viability was measured 2 h following the injury by counting Annexin V-positive cells measured by confocal microscopy. A significant increase in Annexin staining was observed in injured cells. A decrease in Annexin staining was observed in injured cells treated with tBHQ compared to injured cells treated with vehicle (column 5 and 6). No significant difference in tBHQ treatment can be observed in the presence of HSP70 inhibitor (column 6 and 8). Data analysis was performed with ImageJ (sham n = 3, injured n = 6, *P < 0.01, **P < 0.001).
One treatment of tBHQ is sufficient to improve visual memory
Finally, to determine whether multiple treatments with tBHQ were necessary to achieve improved visual memory, mice underwent a traumatic injury or sham injury and were given a single IP injection at 30 min post injury. Novel object recognition testing showed that at 7 days post injury mice treated with tBHQ displayed improved visual memory (Fig. 8A). 30 days post injury mice had significantly reduced visual memory compared to sham mice (Fig. 8B). As observed with multiple injections, there was no treatment effect for spatial memory (not shown), increase in anxiety (Fig. 8C, D), or passive avoidance (Fig. 8E, F).
Fig. 8.
Novel object recognition test after single injection of treatment in vivo. Injured and sham mice treated with 1 injection of either vehicle or tBHQ (33.4 mg/kg) 30 min post injury and visual memory of a novel object was analyzed at 7 days (n = 9–18) and (B) 30 days post injury (n = 7–12). Preference index was calculated by (time near the “new” object-time near the “old” object)/time near the “new” object + time near the “old” object). A significant decrease in preference index was observed in mTBI animals compared to sham animals at both 7 days (A) and 30 days (B) post injury. Treatment of tBHQ rescued this deficit at 7 days (A) but not at 30 days (B) post injury. No difference in anxious-like behavior was observed between groups 7 days (C) or 30 days (D) post injury. No difference in non-spatial learning was observed between groups 7 days (E) or 30 days (F) post injury.
DISCUSSION
The transcription factor Nrf2 makes an ideal candidate to be altered for treatment as it can be chemically modulated by a compound that is currently in use in the food industry, can be administered by several methods, and is able to cross the blood-brain barrier. Using an array of tests we measured cognitive impairment, motor function, and anxiety following TBI. This model of mTBI only displayed cognitive deficits while motor function and anxiety were not affected. This battery of cognitive tests showed that mice treated with tBHQ following a mild closed head injury experienced improved visual memory 7 days later (Fig. 2A). Visual memory is impaired to a greater degree in mTBI mice 30 days following injury and 6 days of tBHQ treatment was not sufficient to significantly recover the deficits after 30 days indicating that this treatment dosage did not rescue long-term functional loss (Fig. 2B). Nonetheless a positive trend was observed, suggesting that with altered administration of treatment a long-term effect may be possible. This may indicate that loss of neuronal health is still incurred 7 days following injury, which is routinely observed using imaging techniques in patients (Smith et al., 1995; Hofman et al., 2001; Bazarian et al., 2007).
No differences were found between the groups in the anxiety-like or Rotarod behavior. According to these results, we can assume that the cognitive performances are not affected by any anxiety or motor deficits and this reinforces the other behavioral results. A previous study in rats after controlled cortical injury indicated that another Nrf2 activator, sulforaphane, improved post injury performance in water maze tasks (Dash et al., 2009). Sulforaphane also activates the Nrf2-ARE antioxidant pathway similar to tBHQ. Sulforaphane treatment in rats activates this pathway and is neuroprotective following TBI (Hong et al., 2010) and subarachnoid hemorrhage (Chen et al., 2011). The mild closed head injury that we tested in mice induced significant visual memory defects. tBHQ reduced these impairments 7 days post trauma even when given as only a single injection.
To determine why the Nrf2 activator treatment is neuroprotective we measured several candidate proteins during a 3 day window post injury. As anticipated we observed some alterations in signaling and observed increases in c-Jun and GFAP although only mild alterations in levels were detected. This also indicates that the degree of injury is mild but that signaling changes are still occurring. We did identify an increase in activated caspase-3 expression in the hippocampus 3 days following mTBI. This is consistent with prior studies where the procaspase-3 was reduced after TBI while, as reported, the levels of activated caspase-3 are very low and not readily detected (Tweedie et al., 2007). Neuronal cell death, including in the hippocampus, is known in this injury model (Tashlykov et al., 2007). Caspase-3 is an executioner caspase in the apoptotic cascade leading to programmed cell death. Its relevance to neuronal loss is well characterized and treatment with tBHQ significantly reduced the levels of activated caspase-3. The hippocampus is sensitive to mTBI and neuronal loss in this region may account for the reduction in visual memory (Kotapka et al., 1991; Hicks et al., 1993). The reduction in caspase-3 activation in the hippocampus likely contributes to the neuronal preservation.
One mechanism for the reduction in activated caspase-3 following treatment with the Nrf2 activator, tBHQ, may be through increased expression of HSP70. Nrf2 is known to increase HSP70 expression. We previously found that Nrf2 activation upregulates HSP70 after model mild TBI in culture (Hatic et al., 2012). HSP70 is a chaperone protein that is induced following stress to stabilize proteins by promoting proper protein folding or promoting ubiquitinated proteolysis of degraded proteins for cellular protection. The most direct route of cellular protection is through its ability to indirectly inhibit caspase-3 by blocking activation through apoptosis protease-activating factor (Apaf-1) (Beere et al., 2000; Saleh et al., 2000). We observed an increase in HSP70 in the hippocampus 3 days following injury when tBHQ treatment was present. Tests with injured model neurons in culture and VER155008, a modulator of HSP70 that targets the ATPase-binding domain inactivating the chaperone protein, indicated that HSP70 inhibition blocks the neuroprotection induced by the Nrf2 activator.
To monitor the efficacy of one dose of the Nrf2 activator, mice were also injured and injected only once at 30 min following injury. The single dose of tBHQ administered after injury was sufficient to produce the recovery of visual memory at 7 days post injury. This suggests that minimal tBHQ may be required to preserve cognition following mTBI.
CONCLUSIONS
Modulation of Nrf2 activity is a promising treatment for mTBI. Administration of an activator after injury resulted in improved cognitive performance. The neuroprotection observed by Nrf2 is likely in part through the reduction in the activation of caspase-3 in the hippocampus. The specific mechanism by which tBHQ reduces activated caspase-3 is yet to be determined but could result from an increase in oxidative stress response genes such as HSP70. The method of administration and optimal dosage of tBHQ may further improve the cognitive outcome following mTBI and provide a potential treatment for patients.
Acknowledgments
The authors thank Andrea Smith for expert assistance with animal studies. This study was supported by the Department of Veterans Affairs (Veterans Health Administration, Office of Research and Development, Biomedical Laboratory Research and Development), the Florida Department of Health James and Esther King Biomedical Research Program, the Bay Pines Foundation, and the University of South Florida Signature Interdisciplinary Program in Neuroscience.
Abbreviations:
- Apaf-1
apoptosis protease activating factor
- DMSO
dimethyl sulfoxide
- GAPDH
glyceraldehyde-3-phosphate dehydrogenase
- GFAP
glial fibrillary acidic protein
- HSP70
heat shock protein 70
- mTBI
mild traumatic brain injury
- Nrf2
nuclear factor erythroid 2-like 2
- PBS
phosphate buffered saline
- tBHQ
tert-butylhydroquinone
Footnotes
AUTHOR DISCLOSURE STATEMENT
No competing financial interests exist. The contents do not represent the views of the Department of Veterans Affairs or the United States Government.
REFERENCES
- Abramoff MD, Mgalhaes PJ, Ram SJ (2004) Image processing with ImageJ. Biophoton Intl 11:36–42. [Google Scholar]
- Arundine M, Aarts M, Lau A, Tymianski M (2004) Vulnerability of central neurons to secondary insults after in vitro mechanical stretch. J Neurosci 24:8106–8123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baratz R, Tweedie D, Rubovitch V, Luo W, Yoon JS, Hoffer BJ, Greig NH, Pick CG (2011) Tumor necrosis factor-alpha synthesis inhibitor, 3,6′-dithiothalidomide, reverses behavioral impairments induced by minimal traumatic brain injury in mice. J Neurochem 118:1032–1042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bazarian JJ, Zhong J, Blyth B, Zhu T, Kavcic V, Peterson D (2007) Diffusion tensor imaging detects clinically important axonal damage after mild traumatic brain injury: a pilot study. J Neurotrauma 24:1447–1459. [DOI] [PubMed] [Google Scholar]
- Beere HM, Wolf BB, Cain K, Mosser DD, Mahboubi A, Kuwana T, Tailor P, Morimoto RI, Cohen GM, Green DR (2000) Heat-shock protein 70 inhibits apoptosis by preventing recruitment of procaspase-9 to the Apaf-1 apoptosome. Nat Cell Biol 2:469–475. [DOI] [PubMed] [Google Scholar]
- Biegon A, Fry PA, Paden CM, Alexandrovich A, Tsenter J, Shohami E (2004) Dynamic changes in N-methyl-d-aspartate receptors after closed head injury in mice. Implications for treatment of neurological and cognitive deficits. Proc Natl Acad Sci USA 101:5117–5122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Binder LM (1986) Persisting symptoms after mild head injury: a review of the postconcussive syndrome. J Clin Exp Neuropsychol 8:323–346. [DOI] [PubMed] [Google Scholar]
- Centers for Disease Control and Prevention (2011) Surveillance for Traumatic Brain Injury–Related Deaths — United States, 1997-2007. MMWR 60:1–32. [PubMed] [Google Scholar]
- Chen G, Fang Q, Zhang J, Zhou D, Wang Z (2011) Role of the Nrf2-ARE pathway in early brain injury after experimental subarachnoid hemorrhage. J Neurosci Res 89:515–523. [DOI] [PubMed] [Google Scholar]
- Conrad CD, Galea LA, Kuroda Y, McEwen BS (1996) Chronic stress impairs rat spatial memory on the Y maze, and this effect is blocked by tianeptine pretreatment. Behav Neurosci 110:1321–1334. [DOI] [PubMed] [Google Scholar]
- Dacey RG Jr, Alves WM, Rimel RW, Winn HR, Jane JA (1986) Neurosurgical complications after apparently minor head injury. Assessment of risk in a series of 610 patients. J Neurosurg 65:203–210. [DOI] [PubMed] [Google Scholar]
- Dash PK, Zhao J, Orsi SA, Zhang M, Moore AN (2009) Sulforaphane improves cognitive function administered following traumatic brain injury. Neurosci Lett 460:103–107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dix SL, Aggleton JP (1999) Extending the spontaneous preference test of recognition: evidence of object-location and object-context recognition. Behav Brain Res 99:191–200. [DOI] [PubMed] [Google Scholar]
- Edut S, Rubovitch V, Schreiber S, Pick CG (2011) The intriguing effects of ecstasy (MDMA) on cognitive function in mice subjected to a minimal traumatic brain injury (mTBI). Psychopharmacology (Berl) 214:877–889 [DOI] [PubMed] [Google Scholar]
- Ellis EF, McKinney JS, Willoughby KA, Liang S, Povlishock JT (1995) A new model for rapid stretch-induced injury of cells in culture: characterization of the model using astrocytes. J Neurotrauma 12:325–339. [DOI] [PubMed] [Google Scholar]
- Halbauer JD, Ashford JW, Zeitzer JM, Adamson MM, Lew HL, Yesavage JA (2009) Neuropsychiatric diagnosis and management of chronic sequelae of war-related mild to moderate traumatic brain injury. J Rehabil Res Dev 46:757–796. [DOI] [PubMed] [Google Scholar]
- Hammond RS, Tull LE, Stackman RW (2004) On the delay-dependent involvement of the hippocampus in object recognition memory. Neurobiol Learn Mem 82:26–34. [DOI] [PubMed] [Google Scholar]
- Hatic H, Kane MJ, Saykally JN, Citron BA (2012) Modulation of Transcription Factor Nrf2 in an In Vitro Model of Traumatic Brain Injury. J Neurotrauma 29:1188–1196. [DOI] [PubMed] [Google Scholar]
- Hicks RR, Smith DH, Lowenstein DH, Saint Marie R, McIntosh TK (1993) Mild experimental brain injury in the rat induces cognitive deficits associated with regional neuronal loss in the hippocampus. J Neurotrauma 10:405–414. [DOI] [PubMed] [Google Scholar]
- Hofman PA, Stapert SZ, van Kroonenburgh MJ, Jolles J, de Kruijk J, Wilmink JT (2001) MR imaging, single-photon emission CT, and neurocognitive performance after mild traumatic brain injury. AJNR Am J Neuroradiol 22:441–449. [PMC free article] [PubMed] [Google Scholar]
- Hoge CW, McGurk D, Thomas JL, Cox AL, Engel CC, Castro CA (2008) Mild traumatic brain injury in U.S. Soldiers returning from Iraq. N Engl J Med 358:453–463. [DOI] [PubMed] [Google Scholar]
- Hogg S (1996) A review of the validity and variability of the elevated plus-maze as an animal model of anxiety. Pharmacol Biochem Behav 54:21–30. [DOI] [PubMed] [Google Scholar]
- Hong Y, Yan W, Chen S, Sun CR, Zhang JM (2010) The role of Nrf2 signaling in the regulation of antioxidants and detoxifying enzymes after traumatic brain injury in rats and mice. Acta Pharmacol Sin 31:1421–1430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jakel RJ, Townsend JA, Kraft AD, Johnson JA (2007) Nrf2-mediated protection against 6-hydroxydopamine. Brain Res 1144:192–201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jin W, Wang H, Yan W, Zhu L, Hu Z, Ding Y, Tang K (2009) Role of Nrf2 in protection against traumatic brain injury in mice. J Neurotrauma 26:131–139. [DOI] [PubMed] [Google Scholar]
- Johnson JA, Johnson DA, Kraft AD, Calkins MJ, Jakel RJ, Vargas MR, Chen PC (2008) The Nrf2-ARE pathway: an indicator and modulator of oxidative stress in neurodegeneration. Ann NY Acad Sci 1147:61–69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kane MJ, Citron BA (2009) Transcription factors as therapeutic targets in CNS disorders. Recent Patents CNS Drug Discov 4:190–199. [DOI] [PubMed] [Google Scholar]
- Kane MJ, Hatic H, Delic V, Dennis JS, Butler CL, Saykally JN, Citron BA (2011) Modeling the pathobiology of repetitive traumatic brain injury in immortalized neuronal cell lines. Brain Res 1425:123–131. [DOI] [PubMed] [Google Scholar]
- Kotapka MJ, Gennarelli TA, Graham DI, Adams JH, Thibault LE, Ross DT, Ford I (1991) Selective vulnerability of hippocampal neurons in acceleration-induced experimental head injury. J Neurotrauma 8:247–258. [DOI] [PubMed] [Google Scholar]
- Kraft AD, Johnson DA, Johnson JA (2004) Nuclear factor E2-related factor 2-dependent antioxidant response element activation by tert-butylhydroquinone and sulforaphane occurring preferentially in astrocytes conditions neurons against oxidative insult. J Neurosci 24:1101–1112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kraus JF, Nourjah P (1988) The epidemiology of mild, uncomplicated brain injury. J Trauma 28:1637–1643. [DOI] [PubMed] [Google Scholar]
- Lau A, Arundine M, Sun HS, Jones M, Tymianski M (2006) Inhibition of caspase-mediated apoptosis by peroxynitrite in traumatic brain injury. J Neurosci 26:11540–11553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee JM, Calkins MJ, Chan K, Kan YW, Johnson JA (2003) Identification of the NF-E2-related factor-2-dependent genes conferring protection against oxidative stress in primary cortical astrocytes using oligonucleotide microarray analysis. J Biol Chem 278:12029–12038. [DOI] [PubMed] [Google Scholar]
- McAllister TW (2011) Neurobiological consequences of traumatic brain injury. Dialogues Clin Neurosci 13:287–300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McAllister TW, Saykin AJ, Flashman LA, Sparling MB, Johnson SC, Guerin SJ, Mamourian AC, Weaver JB, Yanofsky N (1999) Brain activation during working memory 1 month after mild traumatic brain injury: a functional MRI study. Neurology 53:1300–1308. [DOI] [PubMed] [Google Scholar]
- Milman A, Rosenberg A, Weizman R, Pick CG (2005) Mild traumatic brain injury induces persistent cognitive deficits and behavioral disturbances in mice. J Neurotrauma 22:1003–1010. [DOI] [PubMed] [Google Scholar]
- Rempel-Clower NL, Zola SM, Squire LR, Amaral DG (1996) Three cases of enduring memory impairment after bilateral damage limited to the hippocampal formation. J Neurosci 16:5233–5255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rimel RW, Giordani B, Barth JT, Boll TJ, Jane JA (1981) Disability caused by minor head injury. Neurosurgery 9:221–228. [PubMed] [Google Scholar]
- Rubovitch V, Edut S, Sarfstein R, Werner H, Pick CG (2010) The intricate involvement of the insulin-like growth factor receptor signaling in mild traumatic brain injury in mice. Neurobiol Dis 38:299–303. [DOI] [PubMed] [Google Scholar]
- Rushmore TH, Morton MR, Pickett CB (1991) The antioxidant responsive element. Activation by oxidative stress and identification of the DNA consensus sequence required for functional activity. J Biol Chem 266:11632–11639. [PubMed] [Google Scholar]
- Saleh A, Srinivasula SM, Balkir L, Robbins PD, Alnemri ES (2000) Negative regulation of the Apaf-1 apoptosome by Hsp70. Nat Cell Biol 2:476–483. [DOI] [PubMed] [Google Scholar]
- Shih AY, Erb H, Murphy TH (2007) Dopamine activates Nrf2-regulated neuroprotective pathways in astrocytes and meningeal cells. J Neurochem 101:109–119. [DOI] [PubMed] [Google Scholar]
- Shih AY, Li P, Murphy TH (2005) A small-molecule-inducible Nrf2-mediated antioxidant response provides effective prophylaxis against cerebral ischemia in vivo. J Neurosci 25:10321–10335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shum DH, Harris D, O’Gorman JG (2000) Effects of severe traumatic brain injury on visual memory. J Clin Exp Neuropsychol 22:25–39. [DOI] [PubMed] [Google Scholar]
- Smith DH, Lowenstein DH, Gennarelli TA, McIntosh TK (1994) Persistent memory dysfunction is associated with bilateral hippocampal damage following experimental brain injury. Neurosci Lett 168:151–154. [DOI] [PubMed] [Google Scholar]
- Smith DH, Meaney DF, Lenkinski RE, Alsop DC, Grossman R, Kimura H, McIntosh TK, Gennarelli TA (1995) New magnetic resonance imaging techniques for the evaluation of traumatic brain injury. J Neurotrauma 12:573–577. [DOI] [PubMed] [Google Scholar]
- Smith DH, Meaney DF, Shull WH (2003) Diffuse axonal injury in head trauma. J Head Trauma Rehabil 18:307–316. [DOI] [PubMed] [Google Scholar]
- Tashlykov V, Katz Y, Gazit V, Zohar O, Schreiber S, Pick CG (2007) Apoptotic changes in the cortex and hippocampus following minimal brain trauma in mice. Brain Res 1130:197–205. [DOI] [PubMed] [Google Scholar]
- Tashlykov V, Katz Y, Volkov A, Gazit V, Schreiber S, Zohar O, Pick CG (2009) Minimal traumatic brain injury induce apoptotic cell death in mice. J Mol Neurosci 37:16–24. [DOI] [PubMed] [Google Scholar]
- Tweedie D, Milman A, Holloway HW, Li Y, Harvey BK, Shen H, Pistell PJ, Lahiri DK, Hoffer BJ, Wang Y, Pick CG, Greig NH (2007) Apoptotic and behavioral sequelae of mild brain trauma in mice. J Neurosci Res 85:805–815. [DOI] [PubMed] [Google Scholar]
- Umile EM, Sandel ME, Alavi A, Terry CM, Plotkin RC (2002) Dynamic imaging in mild traumatic brain injury: support for the theory of medial temporal vulnerability. Arch Phys Med Rehabil 83:1506–1513. [DOI] [PubMed] [Google Scholar]
- Vakil E (2005) The effect of moderate to severe traumatic brain injury (TBI) on different aspects of memory: a selective review. J Clin Exp Neuropsychol 27:977–1021. [DOI] [PubMed] [Google Scholar]
- Warden D (2006) Military TBI during the Iraq and Afghanistan wars. J Head Trauma Rehabil 21:398–402. [DOI] [PubMed] [Google Scholar]
- Weber JT, Rzigalinski BA, Ellis EF (2001) Traumatic injury of cortical neurons causes changes in intracellular calcium stores and capacitative calcium influx. J Biol Chem 276:1800–1807. [DOI] [PubMed] [Google Scholar]
- Zohar O, Rubovitch V, Milman A, Schreiber S, Pick CG (2011) Behavioral consequences of minimal traumatic brain injury in mice. Acta Neurobiol Exp (Wars) 71:36–45. [DOI] [PubMed] [Google Scholar]
- Zohar O, Schreiber S, Getslev V, Schwartz JP, Mullins PG, Pick CG (2003) Closed-head minimal traumatic brain injury produces long-term cognitive deficits in mice. Neuroscience 118:949–955. [DOI] [PubMed] [Google Scholar]