Abstract
Due to poor self-regenerative potential of articular cartilage, stem cell-based regeneration becomes a hopeful approach for the treatment of articular cartilage defects. Recent studies indicate that neural crest-derived cells (NCDCs) have the potential for repairing articular cartilage with even greater chondrogenic capacity than mesoderm-derived cells (MDCs): a conventional stem cell source for cartilage regeneration. Given that NCDCs originate from a different germ layer in the early embryo compared with MDCs that give rise to articular cartilage, a mystery remains regarding their capacity for articular cartilage regeneration. In this review, we summarize the similarities and differences between MDCs and NCDCs including articular and nasal chondrocytes in cell origin, anatomy, and chondrogenic differentiation and propose that NCDCs might be promising cell origins for articular cartilage regeneration.
Keywords: Neural crest-derived cells, Mesoderm-derived cells, Cartilage regeneration, Stem cells, Nasal chondrocytes, Articular chondrocytes
Introduction
Articular cartilage defects exist in many common clinical disorders, such as degenerative osteoarthritis, joint trauma, and osteochondritis dissecans [1–3]. Since articular cartilage has limitations in self-regenerative potential, these disorders need serial invasive procedures ranging from bone drilling to joint replacement [4]. Given the inherent disadvantages of the above methods, a biological approach, cell-based regeneration, has been developed and applied to animal models as well as to human beings [5]. For example, autologous chondrocyte implantation (ACI), described clinically by Brittberg et al. in 1994 [6], has been used widely in the past two decades. ACI could improve clinical scores of patients with cartilage defects; however, no significant superiority was shown compared with other simpler one-stage procedures, such as microfracture [1].
Some mesoderm-derived cells (MDCs), such as mesenchymal stromal/stem cells (MSCs), are a promising stem cell source for cartilage engineering and regeneration. Recent literature shows that neural crest-derived cells (NCDCs), including nasal chondrocytes and neural crest-derived stem cells (NCSCs), also potentially have the ability to repair articular cartilage with even greater chondrogenic capacity than MDCs [7, 8]. It is well known that articular cartilage is derived from the mesoderm; however, NCDCs originating from a different germ layer in the early embryo [9] are a contributor to articular cartilage in terms of both development and regeneration. Interestingly, earlier reports indicate that some nerve tissue-related tumors could generate divergent cartilage clinically [10]. These findings raise an interesting question about whether NCDCs possess a similar capacity for articular cartilage regeneration as MDCs. Given that NCDCs give rise to nasal cartilage, in this review, the similarities and differences between MDCs and NCDCs including articular and nasal chondrocytes are compared in cell origin, anatomy, and chondrogenic differentiation, along with an update of potential signaling pathways and key molecules during development and regeneration. We conclude that nasal chondrocytes possess greater chondrogenic capacity than articular chondrocytes and propose that NCDCs might be a promising stem cell source for articular cartilage regeneration.
Chondrocyte origin
In the early stage, called blastulation, of embryonic development, the germ cell gradually forms a bilaminar germ disk of which the upper layer is called the epiblast [11]. During gastrulation, the epiblast differentiates into three germ layers, ectoderm, mesoderm, and endoderm [12]. Throughout subsequent neurulation [13], the ectoderm starts to give rise to the neural plate and non-neural epithelial tissue [14], and the mesoderm divides into three groups which are nascent paraxial mesoderm, intermediate mesoderm, and lateral plate mesoderm [15]. A diagram in Fig. 1 illustrates the origin of these two chondrocytes: lateral plate mesoderm generates the articular chondrocytes while nasal chondrocytes are derived from the cranial neural crest [16–18].
Fig. 1.
A brief schematic shows the origin of NCs (nasal chondrocytes) and ACs (articular chondrocytes). In the left upper cycle, throughout serial steps including morula formation, blastulation, and gastrulation, the germ cell gradually forms the three layer plates of ectoderm, mesoderm, and endoderm [94]. In the middle cycle, the ectoderm starts to give rise to neural plate and non-neural epithelial tissue; cells between those two parts develop into the neural crest [13, 14]. At the same time, notochord, neural groove, and neural tube successively appear. Later, despite the fact that paraxial mesoderm divides into blocks of cells called somites, lateral plate mesoderm generates the articular chondrocytes, while nasal chondrocytes are derived from the neural crest [16–18]. The right upper cycle shows the mesenchymal cells from mesoderm give rise to ACs with formation of interzone and cavitation
Articular chondrocytes
During early embryogenesis, the lateral plate mesoderm generates mesenchymal condensations that differentiate into limb buds under regulation by conserved developmental genes, such as HOX (homeobox) genes [19], followed by formation of cartilage anlagen of future bones. Consequently, the interzone phase starts at the designated locations when articular chondrocytes and intra-articular tissues are differentiated [20, 21].
It is still unknown where interzone cells are derived from. They might be part of the initial mesenchymal condensations for cartilage anlagen [22], or a unique population of mesenchymal cells surrounding future joint sites and migrating into them [20]. Isolated interzone cells during in vitro culture expressed characteristic phenotypic markers, including GDF5 (growth differentiation factor 5), CD44, and Wnt14 [23, 24]. To determine the molecular mechanisms of articular chondrocyte formation, using a microinjection of the fluorescent dye DiI (1,1′-dioctadecyl-3,3,3′,3′-tetramethylindocarbocyanine perchlorate) in chick embryos, a study found that mesenchymal cells surrounding the joint migrated into the interzone and nascent joint, and became articular chondrocytes of long bone anlagen. However, cells with equal mobility a few hundred microns away were found scattered up and down the limb mesenchyme, but did not form part of the incipient joint [25].
Several studies genetically traced the origin of joint progenitor cells using conditional reporter mice [26, 27], and others focused on how the associated signals influence cell determination and functioning [28, 29]. Based on these reports, Decker and colleagues drew a comprehensive conclusion and provided an interesting model of interzone development [20]. Briefly, along the initial unsegmented SOX9 (SRY-Box 9)/COL2 (type II collagen)/DCX (doublecortin)-expressing cartilaginous anlagen, GDF5( +) cells would be triggered to determine the primal interzone through DCX expression. Cells located around the interzone could activate TGFBR2 (transforming growth factor beta type II receptor) expression, while anlagen-bound chondrocytes would start to express matrillin-1. GDF5( +) cells next to the cartilaginous anlagen with prior expression of SOX9/COL2 but without MATN1 (matrillin-1) expression would transform into articular chondrocytes. Many molecules, such as SOX11, OSR1/2 (oxidative stress responsive kinase 1/2), HIF1 (hypoxia-inducible factor 1), TGFB, HIP1 (Huntingtin-interacting protein 1), IHH (Indian Hedgehog), and PTHRP (parathyroid hormone-related protein), regulated the development process [21].
Nasal chondrocytes
At the junction of areas that are destined for the neural plate and non-neural epidermis, there is a group of cells called the neural crest, of which origination and specification timing are still controversial. Typically, the neural crest is considered to develop from the ectoderm, formed by interplay at the boundary between the neural plate and non-neural ectoderm [30]. BMPs (bone morphogenetic proteins) and Wnt proteins induce neural crest formation [31, 32]. However, some authors indicated that neural crest specification originates during gastrulation, earlier than previously proposed; in addition, PAX7 (paired box 7) plays a critical role during neural crest formation [33]. If the latter perspective is true, the neural crest originates from the epiblast rather than the ectoderm. Because the epiblast can generate all three germ layers, it is not surprising that the neural crest can differentiate into multiple cell lineages including craniofacial bone and cartilage, smooth muscle, melanocytes, peripheral and enteric neurons, and glia [34].
Owing to its migration ability, the neural crest has been found in multiple sites of the body, grouped into the cranial neural crest, trunk neural crest, and cardiac neural crest [35]. In addition, differences exist in groups for response to particular signals and differentiation preferences; for example, cranial neural crest cells have chondrogenic capacity while trunk cells do not [36, 37]. Most recently, using genetic lineage tracing in the Plp1CreERT2 mouse, researchers demonstrated that, in the development of murine embryo, some Schwann cell precursors detached from nerves and turned into MSCs, which developed further into chondrocytes and osteocytes. This finding occurred only in the course of embryonic formation, generating abundant craniofacial and trunk skeletal elements, but having no contribution to the formation of the limb skeleton [38].
Although the molecular mechanisms directing the neural crest to mesenchymal differentiation potential remain poorly understood, a new model (Fig. 2) targeting cell fate decisions, revealed by Soldatov and coworkers [37], provided an approach for investigation of neural crest adjustment. They found that neural crest cells differentiate via a sequence of stereotypical lineage-restriction events that include co-expression and competition between genes that drive alternative fates.
Fig. 2.
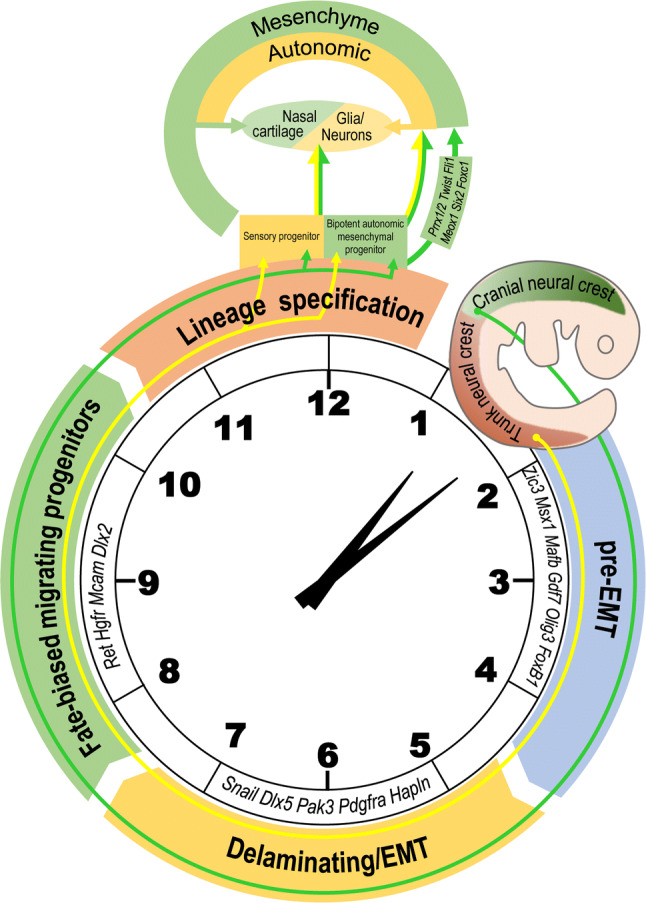
A new model shows how neural crest cells change into mesenchymal cells and subsequent nasal cartilage. The process could be divided into four events including the pre-EMT (epithelial to mesenchymal transition) stage, delaminating/EMT, fate-biased migrating progenitors, and lineage specification [37]. Conducted by transient regulation of specific genes at each point, cells could develop into sensory progenitors, biopotent autonomic mesenchymal progenitors, and the latter could further become mesenchyme and autonomic cells. During the lineage specification process, cells expressing PRRX1 and TWIST1 are biased mesenchymal cells, some of which would give rise to nasal cartilage
During the pre-EMT (epithelial to mesenchymal transition) stage, neural crest generating cells have not yet begun to delaminate from the neural tube with peak expression of neural plate border specifiers, i.e., ZIC3 (Zic Family Member 3), MSX1 (Msh homeobox 1), MAFB (v-maf avian musculoaponeurotic fibrosarcoma oncogene homolog B), and GDF7 [39–41], as well as neural tube markers, i.e., OLIG3 (Oligodendrocyte Transcription Factor 3) and FOXB1 (Forkhead Box B1). At that point, the delaminating subpopulation is characterized by expression of the vital EMT gene SNAIL1 (snail family zinc finger 1) [42] and absence of ATOH1 (Atonal BHLH Transcription Factor 1), along with sequential temporary up-regulation of a series of genes, including DLX5 (Distal-Less Homeobox 5), PAK3 (p21-activated kinase 3), PDGFRA (platelet-derived growth factor receptor alpha), and HAPLN (hyaluronan and proteoglycan link protein). After this stage, they become fate-biased migrating progenitors, expressing RET (ret proto-oncogene), HGFR (hepatocyte growth factor receptor), MCAM (melanoma cell adhesion molecule), and DLX2. During the lineage specification process, cells expressing PRRX1 (paired related homeobox 1) and TWIST1 (Twist-related protein 1) are biased mesenchymal cells; in addition, these cells also express many unbiased markers, such as PRRX2, FLI1 (friend leukemia integration 1 transcription factor), MEOX1 (mesenchyme homeobox 1), SIX2 (sine oculis-related homeobox 2), and FOXC1.
Anatomical comparison
Both articular cartilage and nasal cartilage are hyaline cartilages consisting of chondrocytes and the extracellular matrix (ECM), mainly composed of collagen and proteoglycans. Moreover, unlike the growth plate that is temporary cartilage during bone elongation, both are permanent cartilage in the body throughout life. Functionally, articular cartilage covers the ends of bones, ensuring joint motion and spreading biomechanical pressure to the underlying subchondral cancellous bone, while the nasal cartilage provides structure and support to the nose. This section provides a direct comparison of these two hyaline cartilages in composition, functionality, and structure.
Composition and functionality
The percentage of components in articular cartilage is site and age dependent. As for the wet weight of human articular cartilage [43, 44], water and dissolved electrolytes comprise 60–85%, collagen accounts for 10–30%, GAG (glycosaminoglycan) constitutes 3–10%, and chondrocytes make up approximately 10%. When it comes to volume, Alford and colleagues [45] found that ECM makes up approximately 95% and chondrocytes constitute only 2%. Other reports gave similar percentages of components with minor differences between exact numbers [46, 47].
In human nasal cartilage, as for wet weight, water accounts for 77.7% (range 73.9–81.4%), collagen makes up 7.7% (range 4.6–12.9%), and GAG constitutes 2.9% (range 1.4–4.5%); moreover, 24.9 million cells were found in each gram of wet weight [48]. Given there was no published literature measuring articular cartilage using unit of cells per gram, Quinn and coworkers [49] determined the chondrocyte density to be 9.6 ± 1.2 million cells/cm3 on average for the human medial femoral condyle; in addition, the cell density decreased from the superficial zone of 24.0 ± 7.5 million to the transitional and deep zones of 10.3 ± 1.1–7.7 ± 2.0 million cells/cm3, respectively. This outcome was consistent with a report from Stockwell and colleagues [50]. Given the density of cartilage around 1.1 g/cm3 [51], the data from the abovementioned two groups are comparable, indicating that cell density of nasal cartilage is higher than articular cartilage, but is similar to the superficial zone of articular cartilage.
The ratios of collagen to GAG affect biomechanical strength and rigidity of cartilage. Within human articular cartilage, the ratios differ relying on the area of the tissue and the mechanical load that the tissue experiences, with averages ranging from 3.1 to 4.9 [52]. In human nasal cartilage, the ratio is approximately 4.4, and is substantially higher in the dorsal part of the nasal septum, possibly suggesting that the dorsal part of the nasal septum would possess the highest mechanical toughness and stability [53].
Structure
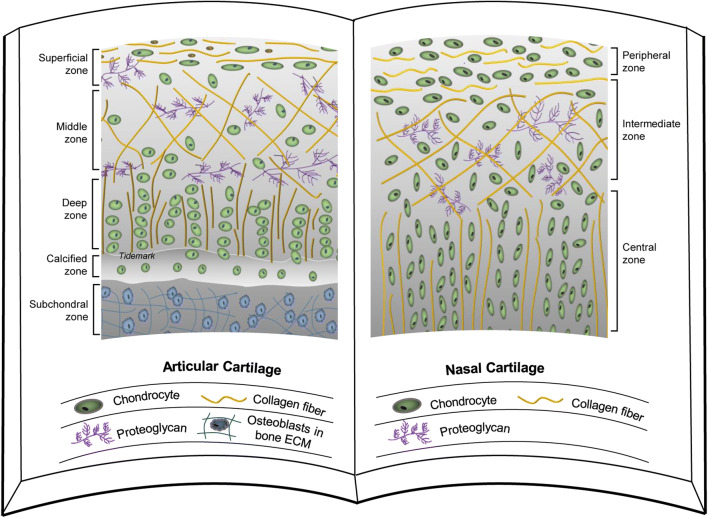
Typically, articular cartilage can be separated into four different zones according to functional and morphological features of chondrocytes, in terms of superficial, middle, deep, and calcified zones (Fig. 3) [54, 55]. The superficial chondrocytes are surrounded by thin collagen fibril organization that generally runs parallel to the articular surface and has the lowest percentage of aggrecan. The middle zone has more rounded cells with lower cell density and an extensive ECM rich in the proteoglycan aggrecan. The deep zone is located between the intermediate zone and a layer of calcified cartilage with the lowest level of cell density but maximal aggrecan content and fibril diameter despite the minimal collagen content. The chondrocytes in the calcified zone usually express the hypertrophic phenotype [56].
Fig. 3.
A structural comparison between articular cartilage and nasal cartilage. Articular cartilage (left) [95] and nasal cartilage (right) [57] have a similar structure, which includes three layers of the peripheral zone, the intermediate zone, and the central zone. Nasal cartilage lacks a calcified zone and does not undergo endochondral ossification. The chondrocyte density in nasal cartilage is greater than that in articular cartilage
Interestingly, nasal cartilage can be distinguished clearly into three layers based on differences in chondrocytes and ECM (Fig. 3). In the peripheral layer are numerous small, flat cells, which run parallel to the cartilage surface, and are surrounded by a collagen rich content material. Cells in the intermediate zone are more ovoid and less numerous, and the axis goes more perpendicular to the surface of cartilage. The lowest cell density is found in the central zone, where the cells are spheroidal and more or less arranged in columns particular to the cartilaginous surface [57]. These zones and features in nasal cartilage are consistent with the outer three zones in articular cartilage. The calcified zone in articular cartilage is related to the transition between cartilage and subchondral bone, so it is not present in nasal cartilage.
Experimental findings
In this section, primary literature was reviewed by year focusing on the comparison of regenerative capacities between articular and nasal chondrocytes from bench to bedside (Table 1).
Table 1.
Experimental findings in chondrogenic potential between ACs and NCs
| References | Donor info | Experimental method | Main findings |
|---|---|---|---|
| [58] | Adult bovine (AC/NC: 16–20 mo) | 7-Day expansion with FGF2 (10 ng/mL) followed by 40-day incubation on PGA scaffold in differentiation medium with insulin (10 µg/mL) and AA (50 µg/mL) | Tissue constructs from NCs generated higher fraction of collagen II and GAG than those from ACs |
| Adult human (AC: 50–78 yo; NC: 39–75 yo) (n = 5) | Engineering of 40-day tissue constructs followed by subcutaneous implantation in nude mice for 3 or 6 weeks | NCs had better proliferation and chondrogenic capacity and could survive upon implantation | |
| [59] | Adult human (AC/NC: 32–60 yo) (n = 5) | Expansion with TGFβ1 (1 ng/mL), FGF2 (5 ng/mL), and PDGFBB (10 ng/mL) followed by 2-week incubation on HYAFF11 or polyurethane scaffolds in DM-I and then mechanical stimulation | Tissue constructs from NCs generated higher fraction of collagen II and GAG than those from ACs, and produced more SZP and hyaluronan upon stimulation with physical forces resembling joint loading |
| [60] | Adult human (NC) | NCs seeded in fibrin sealant followed by 3-week subcutaneous implantation in nude mice | Both implantations formed cartilage-like tissue in vivo |
| Adult rabbit (NC) | NCs seeded in fibrin sealant followed by 6-week intra-articular implantation to repair autologous osteochondral defects | ||
| [61] | Adult human (AC/NC: 37–84 yo) (n = 5) | Expansion with TGFβ1 (1 ng/mL) and FGF2 (5 ng/mL) followed by 2- or 4-week incubation on collagen I meshes in DM-I and treatment with IL-1β (50 pg/mL) or by 1- or 2-week pellet culture in DM-II and treatment with low oxygen (5% O2) | Addition of IL-1β resulted in a loss of GAG and collagen II, but NC tissue constructs displayed more effective recovery upon IL-1β withdrawal; NCs also exhibited less influence from low oxygen culture than ACs |
| [62] | Young porcine (AC/NC: 3–6 mo) (n = 6) | Monolayer culture followed by culture on PGA scaffold | NCs exhibited higher vitality with ECM rich in PG, collagen, and ratio of collagen II/I |
| Adult human (AC/NC: 45–85 yo) (n = 6) | Monolayer culture | Both cell types yielded comparable chondrogenic differentiation expression | |
| [63] | Adult human (AC/NC) (n = 6) | Seeded onto collagen I/III membrane and grown in differentiation medium for 1 week followed by 5-week subcutaneous implantation in nude mice | Lack of HOX expression differentiated NCs from ACs; serially cloned NCs could redifferentiate from dedifferentiated states |
| Adult goat (AC/NC) (n = 2) | Following expansion with FGF2 (5 ng/mL), cells pre-labeled with GFP were seeded onto collagen I/III membrane and grown in chondrogenic medium for 2 weeks followed by 4-week implantation to repair autologous cartilage defects | NCs could be reprogrammed to steadily express HOX genes characteristic of ACs once implanted into articular cartilage defects | |
| [64] | Adult goat (AC/NC: 3.6–7.6 yo) (n = 6) | GFP pre-labeled cells seeded onto collagen I/III membrane were chondrogenically induced for 1 week and implanted for 3 or 6 months to repair autologous cartilage defects | Implantation with NC based tissue constructs yielded typical structure of articular cartilage and better integration with adjacent cartilage and less subchondral bone |
| [8] | Adult human (NC: 18–55 yo) (n = 10) | 2-week expansion in the presence of autologous serum (5%), FGF2 (5 ng/mL) and TGFβ1 (1 ng/mL) and seeded on collagen I/III membrane and grown in DM-III for 2 weeks followed by 24-month implantation to repair cartilage defects | Clinical scores improved without adverse reactions. Radiology data suggested variable degrees of defect fillings. The repair tissue approached the composition of native cartilage |
AA ascorbic acid, AA2P l-ascorbic acid 2-phosphate, AC articular chondrocyte, DM-I differentiation medium I (0.1 mM AA, 10 µg/mL insulin, and 10 ng/mL TGFβ3), DM-II differentiation medium II (ITS + 1, 0.1 mM AA2P, 1.25 mg/mL human serum albumin, 10–7 M dexamethasone, and 10 ng/mL TGFβ1), DM-III differentiation medium III (5% autologous serum, 10 µg/mL insulin, and 0.1 mM AA2P), ECM extracellular matrix, FGF2 basic fibroblast growth factor, GAG glycosaminoglycan, GFP green fluorescence protein, IL-1β interleukin 1β, NC nasal chondrocyte, PDGFBB platelet-derived growth factor BB, PGA polyglycolic acid, SZP superficial zone protein, TGFβ transforming growth factor beta
In 2002, Kafienah et al. compared the chondrogenic capacity of articular chondrocytes and nasal chondrocytes in adult bovine and human [58]. Human articular and nasal cartilage was acquired from patients with femoral neck fractures and patients with nasal septum correction, respectively. Isolated chondrocytes were expanded and incubated on PGA (polyglycolic acid) scaffolds in differentiation medium for 40 days. The outcome showed that the fraction of type II collagen and GAGs of the matrix produced by bovine nasal chondrocytes was higher than that of articular chondrocytes. As for human samples, nasal chondrocytes also had greater potential for proliferation and chondrogenesis. When premature tissue constructs from 40-day culture were transplanted into nude mice subcutaneously, engineered cartilage from nasal chondrocytes survived and grew during the 6-week implantation while that from articular chondrocytes failed to survive.
In 2008, Candrian et al. assessed the reaction of human nasal and articular chondrocytes from five individuals to various mechanical forces resembling joint loading following 14-day growth in differentiation medium in porous polymeric scaffold [59]. The results showed that human nasal chondrocytes were responsive by up-regulating molecules typical for joint lubrication. Thus, the authors found it was feasible to use nasal chondrocytes for articular cartilage repair.
In 2009, Vinatier et al. investigated the use of nasal chondrocytes within a fibrin sealant for repair of cartilage defects [60]. In the experiment, adult human and rabbit nasal chondrocytes were demonstrated to express type II collagen and aggrecan prior to transplantation. Human nasal chondrocytes within a fibrin sealant were implanted subcutaneously into nude mice and a new cartilage-like tissue was formed 3 weeks after implantation. Rabbit nasal chondrocytes within a fibrin sealant were implanted to repair autologous osteochondral defects and a hyaline-like repair tissue was generated 6 weeks after implantation. Therefore, the conclusion is that fibrin sealant combined with autologous nasal chondrocytes appears to be a hopeful approach to treat articular cartilage defects.
In 2012, Scotti et al. tested how human nasal chondrocytes reacted when exposed to an injured joint environment resulting from interleukin-1 beta and low oxygen [61]. Donor matched human articular and nasal chondrocytes were grown ex vitro and then incubated in pellets or collagen-based scaffolds. The evaluation revealed that, under conditions imitating the articular environment postoperatively, nasal chondrocytes maintained a similar or even better cartilaginous tissue forming capacity than articular chondrocytes. These findings indicate a step forward in nasal chondrocyte-based cartilage tissue engineering.
In 2013, El Sayed et al. analyzed chondrogenic potential of nasal chondrocytes compared with articular chondrocytes isolated from 3–6-month porcine and adult human donors [62]. In monolayer culture, they found significantly higher expression of COL2A1 and SOX9 genes in articular chondrocytes compared with nasal chondrocytes in porcine donors. Similar results were also found in human chondrocytes; in addition, articular chondrocytes had remarkably greater gene expression level of PRG4 (proteoglycan 4) despite having a lower expression of COL9A1 and no significant differences in COL1A1. At the protein level in porcine chondrocytes, type II collagen deposition was more noticeable in articular chondrocytes, while type I collagen and lubricin showed nearly similar amounts in articular and nasal chondrocytes. After being grown in a three-dimensional PGA scaffold, porcine nasal chondrocytes showed a high capacity for producing hyaline-like ECM rich in proteoglycan, and comparable amounts of type II collagen production and type II/I collagen ratio compared with articular chondrocytes. Nasal chondrocytes monocultured in PGA or co-cultured with articular chondrocytes presented a similarly high chondrogenic potential. The outcomes shed light on the opportunity to use nasal chondrocytes for articular defect treatment.
In 2014, Pelttari et al. investigated the phenotypic differences between nasal and articular chondrocytes pre- or post-culture [63]. According to the literature, in embryo and stem cell systems, MSCs originated from the neural crest can be differentiated from MDCs through a HOX-negative configuration that is related to enhanced tissue regeneration ability. In this experiment, the findings showed that adult human nasal chondrocytes lacked specific HOX gene expression, such as HOXC4 and HOXD8, and could be continuously inverted from differentiated to dedifferentiated states, maintaining the capacity to generate cartilage ex vitro and in vivo. In goats, nasal chondrocytes could also be adapted to stable expression of HOX genes specific to articular chondrocytes upon injection into articular cartilage defects for direct contribution to cartilage repair.
In 2016, Mumme et al. carried out an experiment to compare the outcomes of nasal and articular chondrocytes for articular defect repair in the adult goat [64]. The results showed that nasal chondrocytes formed characteristic structures of articular cartilage, including flattened cells on the surface, round cells populated the middle zone, and column-like clusters in the deep layers; the implanted tissues integrated efficiently with the adjacent host tissue. The articular chondrocyte group had a significantly increased subchondral bone area that was a signal for osteoarthritis, which was not detected in nasal chondrocyte grafts. Thus, the findings reinforced the possibility of using nasal chondrocyte engineered tissue for articular cartilage repair. Similar results have been tested in human nasal chondrocytes, as evidenced by a well-developed cartilage layer and firm integration to the subchondral layer; newly reconstructed tissue was successfully grafted in an inflammatory joint environment [65].
Finally, in 2016, the first human trial to repair articular defects showed compelling preliminary results. In the study, ten cases with symptomatic traumatic cartilage defects (2–6 cm2) on the distal end of the femur were repaired using chondrocytes isolated from nasal septum cartilage [8]. After 24-month follow-up, both primary clinical outcomes and MRI (magnetic resonance imaging)-based estimation were satisfactory. No side effects were recorded. Clinical scores for pain relief, joint mobility, and life quality were markedly improved. Radiological evaluation suggested variable degrees of lesion repair and development of new tissue with similar composition to native cartilage, showing that cartilaginous implants using autologous nasal chondrocytes can be used clinically for articular cartilage lesion repair in the knee.
Based on the abovementioned experiments comparing articular and nasal chondrocytes, nasal chondrocytes have a better proliferation and chondrogenic capacity than articular chondrocytes ex vitro and in vivo. In addition, nasal chondrocyte generated hyaline cartilage has a superior ability to integrate with surrounding tissue when implanted to repair cartilage defects. Furthermore, nasal chondrocytes also can be used for temporomandibular joint regeneration and provide an autologous cell origin for intervertebral disk repair [66, 67].
Stem cell sources for articular repair
Despite having been used clinically with satisfactory results, both articular and nasal chondrocytes have limited cell amounts for cartilage regeneration in any specific donor. Therefore, stem cell-based therapies hold the potential to become an ideal therapeutic approach for cartilage defects. According to a review from Lo Monaco and coworkers in 2018 [68], this approach is currently in its preclinical phase. In most cases, cells are cultured under stimuli for differentiation toward chondrogenic tissue followed by implantation into cartilage defects with biological constructs [4, 69]. Many hurdles, such as cell heterogeneity, differentiation protocols, and aging and serial passaging, have to be addressed [70–73]. In this section, the current status using mesoderm-derived stem cells and NCSCs is concisely summarized for articular cartilage repair.
Mesoderm-derived stem cells
As a multipotent population of progenitors derived from mesoderm, MSCs are capable of differentiation toward musculoskeletal tissues. Bornes et al. summarized the current progress of MSC-based treatments and underlying barriers for cartilage engineering [2]. MSCs are spindle-shaped cells with the capacity for self-renewal and quick proliferation; they exist in many tissues, such as bone marrow, synovium, blood, adipose, and periosteum [74, 75]. Much attention has been paid to determining the perfect source for the ancestry of MSCs. Given no consensus on cell origin, expansion approach, culture method, and scaffold composition, it is difficult to determine which group of stem cells is the best for treating cartilage defects despite increasing evidence supporting synovium-derived MSCs having superior chondrogenic capacity compared to other sources of MSCs [75–77].
Neural crest-derived stem cells
As summarized by a recent review [78], NCSCs could be isolated from embryonic, fetal, and adult tissues, including but not limited to bone marrow, dental pulp, hair follicles, and oral mucosa [79, 80]. Interestingly, some tissues, such as bone marrow and dental tissue, possess stem cells with dual origins, including MSCs and NCSCs at the same time [32, 81]. Xu et al. showed that around 90% of gingival stem cells are originated from cranial neural crest cells and 10% from the mesoderm using a gene tracing technique in mice [82]. In the study, compared with mesoderm MSCs, NCSCs showed an increased capacity to differentiate into neural cells and chondrocytes.
Dental stem cells are the most investigated NCSCs. Multipotent stem cells have been reported in teeth and their surrounding tissues and have similar characteristics with bone marrow derived MSCs (BMSCs) [83, 84]. Although much literature has shown that NCSCs have chondrogenic potential [85], few investigators compared chondrogenic capacities among different populations of NCSCs, or between NCSCs and BMSCs. Moreover, to the best of our knowledge, there has been no publication on NCSC-based therapies for articular cartilage defects.
Moshaverinia et al. investigated the chondrogenic capacity of human dental stem cells compared with BMSCs [86]. The results showed that gingival MSCs (GMSCs) and periodontal ligament stem cells (PDLSCs) expressed MSC surface markers (CD73, CD105, CD146, and CD166) without hematopoietic lineage markers (CD34 and CD45). After 4 weeks of chondrogenic induction in vitro, PDLSCs showed higher expression of SOX9 and COL2A1 genes than BMSCs and GMSCs. No significant difference was observed between GMSCs and BMSCs. After 8 weeks of implantation into nude mice subcutaneously with alginate hydrogel, PDLSCs revealed more chondrogenic differentiation than GMSCs and BMSCs.
Induced neural crest cells (iNCCs) have also been investigated in several studies. Umeda et al. successfully generated chondrogenic ectomesenchymal cells expressing SOX9 from the neural crest-like progeny of human pluripotent stem cells (PSCs), which were expandable and maintained chondrogenic potential for up to 16 passages [87]. Therefore, the induced cells hold the potential for regenerative cartilage therapy. In another study, MSC-like cells originated from induced PSCs via neural crest cells (iPSC-NCCs) were tested for osteochondral repair [88]. These iPSC-NCC-derived MSC-like cells (iNCMSCs) displayed osteochondral differentiation potential in vitro that was comparable to human BMSCs. After 2-month implantation into rat osteochondral defects without any pre-induction for specific differentiation lineages, however, these cells failed to repair the defects. Considering the good chondrogenic capacity in vitro, iNCMSCs may become a new source for cartilage regeneration under optimization [89].
Progenitor cells of cartilage
Cartilage-derived stem/progenitor cells (CSPCs), released from a variety of cartilage sources, are considered to have great chondrogenic capacity, such as those from articular and nasal cartilage.
In 2001, Hayes et al. studied the growth mechanisms of articular cartilage and hypothesized that the superficial zone contained a progenitor/stem cell population, which contributed to the appositional growth of the cartilage [90]. Later, they studied the distribution of Notch molecules in the process of cartilage development. The results implied complex Notch signaling interactions taking part in the development of articular cartilage [91]. Moreover, a group of progenitor cells located in the superficial zone of articular cartilage was isolated via their different adhesion to fibronectin. These cells possess high affinity for fibronectin and express the cell fate selector gene NOTCH1. Inhibited Notch signaling can abolish their colony forming capacity, which can be rescued by activation of the Notch signal. Meaningfully, the identification of this group of cells with progenitor-like properties will promote our knowledge in cartilage development and provide promising solutions to articular cartilage regeneration [92].
Shafiee et al. separated a group of progenitor cells from human nasal cartilage and found that these pluripotent cells could express mesoectodermal stem cell markers [93]. These cells had lots of similarities with MSCs, expressing CD90, CD105, CD106, CD166, and HLA (human leukocyte antigen)-ABC, and lacking CD34, CD45, and HLA-DR. Nasal septal progenitors strikingly expressed CD133 (Prominin-1). These progenitors showed a rapid proliferation in vitro and represented a greater potential of chondrogenesis. They also could live longer and exhibited multipotent properties with high differentiation capacity toward other lineages such as chondrocytes, osteocytes, and neural-like cells. In addition, this population could be harmlessly expanded ex vitro without risk of chromosomal abnormality.
Jessop et al. performed a systematic review to evaluate and compare the separation, characterization, and chondrogenic capacity of CSPCs from different sources [69]. Seventy-five percent of the included studies showed CSPCs have differentiation capacity in three lineages despite the heterogeneity in isolation protocols. The expression profiles of cell surface markers were consistent but nonspecific, expressing MSC markers including CD90, CD105, CD44, CD166, CD73, and CD29, and no hematopoietic markers including CD34 and CD45. Moreover, CSPCs have been applied to repair cartilage in animal experiments, in which they showed equal chondrogenic capacity and were better than MSCs from other tissue sources. The authors concluded that further study is necessary to promote the clinical application of CSPCs for cartilage regeneration.
Conclusion and perspective
Despite differences, some groups of NCDCs show many similarities with MDCs, and present better chondrogenic capacity, which has been shown in vitro and in vivo, not only for animal models, but also for humans. For example, nasal chondrocytes have similar composition and structure as articular chondrocytes but possess greater cell density and a larger ratio of collagen to GAG and a relatively conservative genotype. These characteristics might contribute to their greater chondrogenic ability compared with articular chondrocytes. At the stem cell level, NCSCs have comparable or even better chondrogenic capacity than mesoderm-derived stem cells in vitro; however, neither has been used successfully for human articular cartilage defect repair. Many issues should be addressed in the future; for example, where are the interzone cells from? Is there a possibility they are also derived from the neural crest? What is the difference in cartilage regeneration in human NCSCs and mesoderm-derived stem cells? In any case, NCDCs have drawn the attention of researchers and surgeons in the orthopaedic field and may develop into an ideal cell origin for articular cartilage regeneration.
Acknowledgements
We thank Suzanne Danley for editing the manuscript. This work was supported by Research Grants from the Musculoskeletal Transplant Foundation (MTF) and the National Institutes of Health (1R01AR067747) to M.P., China Scholarship Council (201909370041) and National Natural Science Foundation of China (81501844) to T.Y.L., and Health Commission of Sichuan Province (18PJ008), Science & Technology Department of Sichuan Province (2019YFS0267) and National Natural Science Foundation of China (81601889) to S.C.
Compliance with ethical standards
Conflict of interest
The authors declare no conflict of interest.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Song Chen is the co-first author.
References
- 1.Knutsen G, Drogset JO, Engebretsen L, Grontvedt T, Ludvigsen TC, Loken S, Solheim E, Strand T, Johansen O. A randomized multicenter trial comparing autologous chondrocyte implantation with microfracture: long-term follow-up at 14 to 15 years. J Bone Jt Surg Am. 2016;98(16):1332–1339. doi: 10.2106/JBJS.15.01208. [DOI] [PubMed] [Google Scholar]
- 2.Bornes TD, Adesida AB, Jomha NM. Mesenchymal stem cells in the treatment of traumatic articular cartilage defects: a comprehensive review. Arthritis Res Ther. 2014;16(5):432. doi: 10.1186/s13075-014-0432-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Jones MH, Williams AM. Osteochondritis dissecans of the knee: a practical guide for surgeons. Bone Jt J. 2016;98-B(6):723–729. doi: 10.1302/0301-620X.98B6.36816. [DOI] [PubMed] [Google Scholar]
- 4.Makris EA, Gomoll AH, Malizos KN, Hu JC, Athanasiou KA. Repair and tissue engineering techniques for articular cartilage. Nat Rev Rheumatol. 2015;11(1):21–34. doi: 10.1038/nrrheum.2014.157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Di Matteo B, Vandenbulcke F, Vitale ND, Iacono F, Ashmore K, Marcacci M, Kon E. Minimally manipulated mesenchymal stem cells for the treatment of knee osteoarthritis: a systematic review of clinical evidence. Stem Cells Int. 2019;2019:1735242. doi: 10.1155/2019/1735242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Brittberg M, Lindahl A, Nilsson A, Ohlsson C, Isaksson O, Peterson L. Treatment of deep cartilage defects in the knee with autologous chondrocyte transplantation. N Engl J Med. 1994;331(14):889–895. doi: 10.1056/NEJM199410063311401. [DOI] [PubMed] [Google Scholar]
- 7.Pelttari K, Mumme M, Barbero A, Martin I. Nasal chondrocytes as a neural crest-derived cell source for regenerative medicine. Curr Opin Biotechnol. 2017;47:1–6. doi: 10.1016/j.copbio.2017.05.007. [DOI] [PubMed] [Google Scholar]
- 8.Mumme M, Barbero A, Miot S, Wixmerten A, Feliciano S, Wolf F, Asnaghi AM, Baumhoer D, Bieri O, Kretzschmar M, Pagenstert G, Haug M, Schaefer DJ, Martin I, Jakob M. Nasal chondrocyte-based engineered autologous cartilage tissue for repair of articular cartilage defects: an observational first-in-human trial. Lancet (Lond, Engl) 2016;388(10055):1985–1994. doi: 10.1016/s0140-6736(16)31658-0. [DOI] [PubMed] [Google Scholar]
- 9.Olsen BR, Reginato AM, Wang W. Bone development. Annu Rev Cell Dev Biol. 2000;16:191–220. doi: 10.1146/annurev.cellbio.16.1.191. [DOI] [PubMed] [Google Scholar]
- 10.Fraggetta F, Magro G, Vasquez E. Primitive neuroectodermal tumour of the uterus with focal cartilaginous differentiation. Histopathology. 1997;30(5):483–485. doi: 10.1046/j.1365-2559.1997.00525.x. [DOI] [PubMed] [Google Scholar]
- 11.Rosenquist GC. Epiblast origin and early migration of neural crest cells in the chick embryo. Dev Biol. 1981;87(2):201–211. doi: 10.1016/0012-1606(81)90143-3. [DOI] [PubMed] [Google Scholar]
- 12.Solnica-Krezel L, Sepich DS. Gastrulation: making and shaping germ layers. Annu Rev Cell Dev Biol. 2012;28:687–717. doi: 10.1146/annurev-cellbio-092910-154043. [DOI] [PubMed] [Google Scholar]
- 13.Copp AJ, Greene ND, Murdoch JN. The genetic basis of mammalian neurulation. Nat Rev Genet. 2003;4(10):784–793. doi: 10.1038/nrg1181. [DOI] [PubMed] [Google Scholar]
- 14.Duband JL. Diversity in the molecular and cellular strategies of epithelium-to-mesenchyme transitions: Insights from the neural crest. Cell Adh Migr. 2010;4(3):458–482. doi: 10.4161/cam.4.3.12501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ferretti E, Hadjantonakis AK. Mesoderm specification and diversification: from single cells to emergent tissues. Curr Opin Cell Biol. 2019;61:110–116. doi: 10.1016/j.ceb.2019.07.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Cohn MJ, Tickle C. Limbs: a model for pattern formation within the vertebrate body plan. Trends Genet. 1996;12(7):253–257. doi: 10.1016/0168-9525(96)10030-5. [DOI] [PubMed] [Google Scholar]
- 17.Brent AE, Tabin CJ. Developmental regulation of somite derivatives: muscle, cartilage and tendon. Curr Opin Genet Dev. 2002;12(5):548–557. doi: 10.1016/S0959-437X(02)00339-8. [DOI] [PubMed] [Google Scholar]
- 18.Tickle C. How the embryo makes a limb: determination, polarity and identity. J Anat. 2015;227(4):418–430. doi: 10.1111/joa.12361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Cohn MJ, Patel K, Krumlauf R, Wilkinson DG, Clarke JD, Tickle C. Hox9 genes and vertebrate limb specification. Nature. 1997;387(6628):97–101. doi: 10.1038/387097a0. [DOI] [PubMed] [Google Scholar]
- 20.Decker RS, Koyama E, Pacifici M. Genesis and morphogenesis of limb synovial joints and articular cartilage. Matrix Biol. 2014;39:5–10. doi: 10.1016/j.matbio.2014.08.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Chijimatsu R, Saito T. Mechanisms of synovial joint and articular cartilage development. Cellular and molecular life sciences: CMLS; 2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Nalin AM, Greenlee TK, Jr, Sandell LJ. Collagen gene expression during development of avian synovial joints: transient expression of types II and XI collagen genes in the joint capsule. Dev Dyn Off Publ Am Assoc Anat. 1995;203(3):352–362. doi: 10.1002/aja.1002030307. [DOI] [PubMed] [Google Scholar]
- 23.Hartmann C, Tabin CJ. Wnt-14 plays a pivotal role in inducing synovial joint formation in the developing appendicular skeleton. Cell. 2001;104(3):341–351. doi: 10.1016/s0092-8674(01)00222-7. [DOI] [PubMed] [Google Scholar]
- 24.Settle SH, Jr, Rountree RB, Sinha A, Thacker A, Higgins K, Kingsley DM. Multiple joint and skeletal patterning defects caused by single and double mutations in the mouse Gdf6 and Gdf5 genes. Dev Biol. 2003;254(1):116–130. doi: 10.1016/s0012-1606(02)00022-2. [DOI] [PubMed] [Google Scholar]
- 25.Pacifici M, Koyama E, Shibukawa Y, Wu C, Tamamura Y, Enomoto-Iwamoto M, Iwamoto M. Cellular and molecular mechanisms of synovial joint and articular cartilage formation. Ann NY Acad Sci. 2006;1068:74–86. doi: 10.1196/annals.1346.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Storm EE, Kingsley DM. Joint patterning defects caused by single and double mutations in members of the bone morphogenetic protein (BMP) family. Dev (Camb Engl) 1996;122(12):3969–3979. doi: 10.1242/dev.122.12.3969. [DOI] [PubMed] [Google Scholar]
- 27.Rountree RB, Schoor M, Chen H, Marks ME, Harley V, Mishina Y, Kingsley DM. BMP receptor signaling is required for postnatal maintenance of articular cartilage. PLoS Biol. 2004;2(11):e355. doi: 10.1371/journal.pbio.0020355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Spagnoli A, O’Rear L, Chandler RL, Granero-Molto F, Mortlock DP, Gorska AE, Weis JA, Longobardi L, Chytil A, Shimer K, Moses HL. TGF-beta signaling is essential for joint morphogenesis. J Cell Biol. 2007;177(6):1105–1117. doi: 10.1083/jcb.200611031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Li T, Longobardi L, Myers TJ, Temple JD, Chandler RL, Ozkan H, Contaldo C, Spagnoli A. Joint TGF-beta type II receptor-expressing cells: ontogeny and characterization as joint progenitors. Stem Cells Dev. 2013;22(9):1342–1359. doi: 10.1089/scd.2012.0207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Selleck MA, Bronner-Fraser M. Origins of the avian neural crest: the role of neural plate-epidermal interactions. Dev (Camb, Engl) 1995;121(2):525–538. doi: 10.1242/dev.121.2.525. [DOI] [PubMed] [Google Scholar]
- 31.Kleber M, Lee HY, Wurdak H, Buchstaller J, Riccomagno MM, Ittner LM, Suter U, Epstein DJ, Sommer L. Neural crest stem cell maintenance by combinatorial Wnt and BMP signaling. J Cell Biol. 2005;169(2):309–320. doi: 10.1083/jcb.200411095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Glejzer A, Laudet E, Leprince P, Hennuy B, Poulet C, Shakhova O, Sommer L, Rogister B, Wislet-Gendebien S. Wnt1 and BMP2: two factors recruiting multipotent neural crest progenitors isolated from adult bone marrow. Cell Mol Life Sci CMLS. 2011;68(12):2101–2114. doi: 10.1007/s00018-010-0558-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Basch ML, Bronner-Fraser M, Garcia-Castro MI. Specification of the neural crest occurs during gastrulation and requires Pax7. Nature. 2006;441(7090):218–222. doi: 10.1038/nature04684. [DOI] [PubMed] [Google Scholar]
- 34.Hall BK. The neural crest as a fourth germ layer and vertebrates as quadroblastic not triploblastic. Evol Dev. 2000;2(1):3–5. doi: 10.1046/j.1525-142x.2000.00032.x. [DOI] [PubMed] [Google Scholar]
- 35.Bronner ME, Simoes-Costa M. The neural crest migrating into the twenty-first century. Curr Top Dev Biol. 2016;116:115–134. doi: 10.1016/bs.ctdb.2015.12.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Abzhanov A, Tzahor E, Lassar AB, Tabin CJ. Dissimilar regulation of cell differentiation in mesencephalic (cranial) and sacral (trunk) neural crest cells in vitro. Dev (Camb, Engl) 2003;130(19):4567–4579. doi: 10.1242/dev.00673. [DOI] [PubMed] [Google Scholar]
- 37.Soldatov R, Kaucka M, Kastriti ME, Petersen J, Chontorotzea T, Englmaier L, Akkuratova N, Yang Y, Haring M, Dyachuk V, Bock C, Farlik M, Piacentino ML, Boismoreau F, Hilscher MM, Yokota C, Qian X, Nilsson M, Bronner ME, Croci L, Hsiao WY, Guertin DA, Brunet JF, Consalez GG, Ernfors P, Fried K, Kharchenko PV, Adameyko I. Spatiotemporal structure of cell fate decisions in murine neural crest. Science. 2019 doi: 10.1126/science.aas9536. [DOI] [PubMed] [Google Scholar]
- 38.Xie M, Kamenev D, Kaucka M, Kastriti ME, Zhou B, Artemov AV, Storer M, Fried K, Adameyko I, Dyachuk V, Chagin AS. Schwann cell precursors contribute to skeletal formation during embryonic development in mice and zebrafish. Proc Natl Acad Sci USA. 2019;116(30):15068–15073. doi: 10.1073/pnas.1900038116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Lo L, Dormand EL, Anderson DJ. Late-emigrating neural crest cells in the roof plate are restricted to a sensory fate by GDF7. Proc Natl Acad Sci USA. 2005;102(20):7192–7197. doi: 10.1073/pnas.0502581102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Liem KF, Jr, Tremml G, Jessell TM. A role for the roof plate and its resident TGFbeta-related proteins in neuronal patterning in the dorsal spinal cord. Cell. 1997;91(1):127–138. doi: 10.1016/s0092-8674(01)80015-5. [DOI] [PubMed] [Google Scholar]
- 41.Nakata K, Nagai T, Aruga J, Mikoshiba K. Xenopus Zic3, a primary regulator both in neural and neural crest development. Proc Natl Acad Sci USA. 1997;94(22):11980–11985. doi: 10.1073/pnas.94.22.11980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Simoes-Costa M, Bronner ME. Establishing neural crest identity: a gene regulatory recipe. Dev (Camb, Engl) 2015;142(2):242–257. doi: 10.1242/dev.105445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Cohen NP, Foster RJ, Mow VC. Composition and dynamics of articular cartilage: structure, function, and maintaining healthy state. J Orthop Sports Phys Ther. 1998;28(4):203–215. doi: 10.2519/jospt.1998.28.4.203. [DOI] [PubMed] [Google Scholar]
- 44.Buckwalter JA, Mankin HJ. Articular cartilage: tissue design and chondrocyte-matrix interactions. Instr Course Lect. 1998;47:477–486. [PubMed] [Google Scholar]
- 45.Alford JW, Cole BJ. Cartilage restoration, part 1: basic science, historical perspective, patient evaluation, and treatment options. Am J Sports Med. 2005;33(2):295–306. doi: 10.1177/0363546504273510. [DOI] [PubMed] [Google Scholar]
- 46.Mow VC, Ratcliffe A, Poole AR. Cartilage and diarthrodial joints as paradigms for hierarchical materials and structures. Biomaterials. 1992;13(2):67–97. doi: 10.1016/0142-9612(92)90001-5. [DOI] [PubMed] [Google Scholar]
- 47.Morrison EH, Ferguson MW, Bayliss MT, Archer CW. The development of articular cartilage: I. The spatial and temporal patterns of collagen types. J Anat. 1996;189:9–22. [PMC free article] [PubMed] [Google Scholar]
- 48.Homicz MR, McGowan KB, Lottman LM, Beh G, Sah RL, Watson D. A compositional analysis of human nasal septal cartilage. Arch Facial Plast Surg. 2003;5(1):53–58. doi: 10.1001/archfaci.5.1.53. [DOI] [PubMed] [Google Scholar]
- 49.Quinn TM, Hauselmann HJ, Shintani N, Hunziker EB. Cell and matrix morphology in articular cartilage from adult human knee and ankle joints suggests depth-associated adaptations to biomechanical and anatomical roles. Osteoarthr Cartil. 2013;21(12):1904–1912. doi: 10.1016/j.joca.2013.09.011. [DOI] [PubMed] [Google Scholar]
- 50.Stockwell RA. The interrelationship of cell density and cartilage thickness in mammalian articular cartilage. J Anat. 1971;109(Pt 3):411–421. [PMC free article] [PubMed] [Google Scholar]
- 51.Bortel EL, Langer M, Rack A, Forien J-B, Duda GN, Fratzl P, Zaslansky P. Combining coherent hard X-ray tomographies with phase retrieval to generate three-dimensional models of forming bone. Front Mater. 2017 doi: 10.3389/fmats.2017.00039. [DOI] [Google Scholar]
- 52.Akizuki S, Mow VC, Muller F, Pita JC, Howell DS, Manicourt DH. Tensile properties of human knee joint cartilage: I. Influence of ionic conditions, weight bearing, and fibrillation on the tensile modulus. J Orthop Res Off Publ Orthop Res Soc. 1986;4(4):379–392. doi: 10.1002/jor.1100040401. [DOI] [PubMed] [Google Scholar]
- 53.Neuman MK, Briggs KK, Masuda K, Sah RL, Watson D. A compositional analysis of cadaveric human nasal septal cartilage. Laryngoscope. 2013;123(9):2120–2124. doi: 10.1002/lary.23727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Carter DR, Beaupre GS, Wong M, Smith RL, Andriacchi TP, Schurman DJ. The mechanobiology of articular cartilage development and degeneration. Clin Orthop Relat Res. 2004 doi: 10.1097/01.blo.0000144970.05107.7e. [DOI] [PubMed] [Google Scholar]
- 55.Poole AR, Kojima T, Yasuda T, Mwale F, Kobayashi M, Laverty S. Composition and structure of articular cartilage: a template for tissue repair. Clin Orthop Relat Res. 2001 doi: 10.1097/00003086-200110001-00004. [DOI] [PubMed] [Google Scholar]
- 56.Venn MF. Variation of chemical composition with age in human femoral head cartilage. Ann Rheum Dis. 1978;37(2):168–174. doi: 10.1136/ard.37.2.168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Popko M, Bleys RL, De Groot JW, Huizing EH. Histological structure of the nasal cartilages and their perichondrial envelope. I. The septal and lobular cartilage. Rhinology. 2007;45(2):148–152. [PubMed] [Google Scholar]
- 58.Kafienah W, Jakob M, Demarteau O, Frazer A, Barker MD, Martin I, Hollander AP. Three-dimensional tissue engineering of hyaline cartilage: comparison of adult nasal and articular chondrocytes. Tissue Eng. 2002;8(5):817–826. doi: 10.1089/10763270260424178. [DOI] [PubMed] [Google Scholar]
- 59.Candrian C, Vonwil D, Barbero A, Bonacina E, Miot S, Farhadi J, Wirz D, Dickinson S, Hollander A, Jakob M, Li Z, Alini M, Heberer M, Martin I. Engineered cartilage generated by nasal chondrocytes is responsive to physical forces resembling joint loading. Arthritis Rheum. 2008;58(1):197–208. doi: 10.1002/art.23155. [DOI] [PubMed] [Google Scholar]
- 60.Vinatier C, Gauthier O, Masson M, Malard O, Moreau A, Fellah BH, Bilban M, Spaethe R, Daculsi G, Guicheux J. Nasal chondrocytes and fibrin sealant for cartilage tissue engineering. J Biomed Mater Res, Part A. 2009;89(1):176–185. doi: 10.1002/jbm.a.31988. [DOI] [PubMed] [Google Scholar]
- 61.Scotti C, Osmokrovic A, Wolf F, Miot S, Peretti GM, Barbero A, Martin I. Response of human engineered cartilage based on articular or nasal chondrocytes to interleukin-1beta and low oxygen. Tissue Eng Part A. 2012;18(3–4):362–372. doi: 10.1089/ten.TEA.2011.0234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.El Sayed K, Marzahn U, John T, Hoyer M, Zreiqat H, Witthuhn A, Kohl B, Haisch A, Schulze-Tanzil G. PGA-associated heterotopic chondrocyte cocultures: implications of nasoseptal and auricular chondrocytes in articular cartilage repair. J Tissue Eng Regen Med. 2013;7(1):61–72. doi: 10.1002/term.496. [DOI] [PubMed] [Google Scholar]
- 63.Pelttari K, Pippenger B, Mumme M, Feliciano S, Scotti C, Mainil-Varlet P, Procino A, von Rechenberg B, Schwamborn T, Jakob M, Cillo C, Barbero A, Martin I. Adult human neural crest-derived cells for articular cartilage repair. Sci Transl Med. 2014 doi: 10.1126/scitranslmed.3009688. [DOI] [PubMed] [Google Scholar]
- 64.Mumme M, Steinitz A, Nuss KM, Klein K, Feliciano S, Kronen P, Jakob M, von Rechenberg B, Martin I, Barbero A, Pelttari K. Regenerative potential of tissue-engineered nasal chondrocytes in goat articular cartilage defects. Tissue Eng Part A. 2016;22(21–22):1286–1295. doi: 10.1089/ten.TEA.2016.0159. [DOI] [PubMed] [Google Scholar]
- 65.Barandun M, Iselin LD, Santini F, Pansini M, Scotti C, Baumhoer D, Bieri O, Studler U, Wirz D, Haug M, Jakob M, Schaefer DJ, Martin I, Barbero A. Generation and characterization of osteochondral grafts with human nasal chondrocytes. J Orthop Res Off Publ Orthop Res Soc. 2015;33(8):1111–1119. doi: 10.1002/jor.22865. [DOI] [PubMed] [Google Scholar]
- 66.de Souza TR, Takamori ER, Menezes K, Carias RBV, Dutra CLM, de Freitas AM, Torraca TSS, Senegaglia AC, Rebelatto CLK, Daga DR, Brofman PRS, Borojevic R. Temporomandibular joint regeneration: proposal of a novel treatment for condylar resorption after orthognathic surgery using transplantation of autologous nasal septum chondrocytes, and the first human case report. Stem Cell Res Ther. 2018;9(1):94. doi: 10.1186/s13287-018-0806-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Vedicherla S, Buckley CT. In vitro extracellular matrix accumulation of nasal and articular chondrocytes for intervertebral disc repair. Tissue Cell. 2017;49(4):503–513. doi: 10.1016/j.tice.2017.05.002. [DOI] [PubMed] [Google Scholar]
- 68.Lo Monaco M, Merckx G, Ratajczak J, Gervois P, Hilkens P, Clegg P, Bronckaers A, Vandeweerd JM, Lambrichts I. Stem cells for cartilage repair: preclinical studies and insights in translational animal models and outcome measures. Stem Cells Int. 2018;2018:9079538. doi: 10.1155/2018/9079538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Jessop ZM, Manivannan S, Zhang Y, Thornton CA, Narayan R, Whitaker IS. Tissue specific stem/progenitor cells for cartilage tissue engineering: a systematic review of the literature. Appl Phys Rev. 2019;6(3):031301. doi: 10.1063/1.5050814. [DOI] [Google Scholar]
- 70.Kretlow JD, Jin YQ, Liu W, Zhang WJ, Hong TH, Zhou G, Baggett LS, Mikos AG, Cao Y. Donor age and cell passage affects differentiation potential of murine bone marrow-derived stem cells. BMC Cell Biol. 2008;9:60. doi: 10.1186/1471-2121-9-60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Lynch K, Pei M. Age associated communication between cells and matrix: a potential impact on stem cell-based tissue regeneration strategies. Organgenesis. 2014;10(3):289–298. doi: 10.4161/15476278.2014.970089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Li J, Pei M. Cell senescence: a challenge in cartilage engineering and regeneration. Tissue Eng Part B Rev. 2012;18(4):270–287. doi: 10.1089/ten.TEB.2011.0583. [DOI] [PubMed] [Google Scholar]
- 73.Tang X, Fan L, Pei M, Zeng L, Ge Z. Evolving concepts of chondrogenic differentiation: history, state-of-the-art and future perspectives. Eur Cells Mater. 2015;30:12–27. doi: 10.22203/eCM.v030a02. [DOI] [PubMed] [Google Scholar]
- 74.Pittenger MF, Mackay AM, Beck SC, Jaiswal RK, Douglas R, Mosca JD, Moorman MA, Simonetti DW, Craig S, Marshak DR. Multilineage potential of adult human mesenchymal stem cells. Science. 1999;284(5411):143–147. doi: 10.1126/science.284.5411.143. [DOI] [PubMed] [Google Scholar]
- 75.Sakaguchi Y, Sekiya I, Yagishita K, Muneta T. Comparison of human stem cells derived from various mesenchymal tissues: superiority of synovium as a cell source. Arthritis Rheum. 2005;52(8):2521–2529. doi: 10.1002/art.21212. [DOI] [PubMed] [Google Scholar]
- 76.Jones BA, Pei M. Synovium-derived stem cells: a tissue-specific stem cell for cartilage engineering and regeneration. Tissue Eng Part B Rev. 2012;18(4):301–311. doi: 10.1089/ten.TEB.2012.0002. [DOI] [PubMed] [Google Scholar]
- 77.Pizzute T, Lynch K, Pei M. Impact of tissue-specific stem cells on lineage-specific differentiation: a focus on the musculoskeletal system. Stem Cell Rev Rep. 2015;11(1):119–132. doi: 10.1007/s12015-014-9546-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Liu JA, Cheung M. Neural crest stem cells and their potential therapeutic applications. Dev Biol. 2016;419(2):199–216. doi: 10.1016/j.ydbio.2016.09.006. [DOI] [PubMed] [Google Scholar]
- 79.Pierdomenico L, Bonsi L, Calvitti M, Rondelli D, Arpinati M, Chirumbolo G, Becchetti E, Marchionni C, Alviano F, Fossati V, Staffolani N, Franchina M, Grossi A, Bagnara GP. Multipotent mesenchymal stem cells with immunosuppressive activity can be easily isolated from dental pulp. Transplantation. 2005;80(6):836–842. doi: 10.1097/01.tp.0000173794.72151.88. [DOI] [PubMed] [Google Scholar]
- 80.Gomez GA, Prasad MS, Wong M, Charney RM, Shelar PB, Sandhu N, Hackland JOS, Hernandez JC, Leung AW, Garcia-Castro MI. WNT/beta-CATENIN modulates the axial identity of ES derived human neural crest. Development (Camb, Engl) 2019 doi: 10.1242/dev.175604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Feng J, Mantesso A, De Bari C, Nishiyama A, Sharpe PT. Dual origin of mesenchymal stem cells contributing to organ growth and repair. Proc Natl Acad Sci USA. 2011;108(16):6503–6508. doi: 10.1073/pnas.1015449108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Xu X, Chen C, Akiyama K, Chai Y, Le AD, Wang Z, Shi S. Gingivae contain neural-crest- and mesoderm-derived mesenchymal stem cells. J Dent Res. 2013;92(9):825–832. doi: 10.1177/0022034513497961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Mayo V, Sawatari Y, Huang CY, Garcia-Godoy F. Neural crest-derived dental stem cells–where we are and where we are going. J Dent. 2014;42(9):1043–1051. doi: 10.1016/j.jdent.2014.04.007. [DOI] [PubMed] [Google Scholar]
- 84.Sharpe PT. Dental mesenchymal stem cells. Dev (Camb, Engl) 2016;143(13):2273–2280. doi: 10.1242/dev.134189. [DOI] [PubMed] [Google Scholar]
- 85.Ferre FC, Larjava H, Loison-Robert LS, Berbar T, Owen GR, Berdal A, Cherifi H, Gogly B, Hakkinen L, Fournier BP. Formation of cartilage and synovial tissue by human gingival stem cells. Stem Cells Dev. 2014;23(23):2895–2907. doi: 10.1089/scd.2013.0547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Moshaverinia A, Xu X, Chen C, Akiyama K, Snead ML, Shi S. Dental mesenchymal stem cells encapsulated in an alginate hydrogel co-delivery microencapsulation system for cartilage regeneration. Acta Biomater. 2013;9(12):9343–9350. doi: 10.1016/j.actbio.2013.07.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Umeda K, Oda H, Yan Q, Matthias N, Zhao J, Davis BR, Nakayama N. Long-term expandable SOX9+ chondrogenic ectomesenchymal cells from human pluripotent stem cells. Stem Cell Rep. 2015;4(4):712–726. doi: 10.1016/j.stemcr.2015.02.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Chijimatsu R, Ikeya M, Yasui Y, Ikeda Y, Ebina K, Moriguchi Y, Shimomura K, Hart DA, Hideki Y, Norimasa N. Characterization of mesenchymal stem cell-like cells derived from human IPSCs via neural crest development and their application for osteochondral repair. Stem Cells Int. 2017;2017:1960965. doi: 10.1155/2017/1960965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Li WJ, Jiao H, Walczak BE. Emerging opportunities for induced pluripotent stem cells in orthopaedics. J Orthop Translat. 2019;17:73–81. doi: 10.1016/j.jot.2019.03.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Hayes AJ, MacPherson S, Morrison H, Dowthwaite G, Archer CW. The development of articular cartilage: evidence for an appositional growth mechanism. Anat Embryol (Berl) 2001;203(6):469–479. doi: 10.1007/s004290100178. [DOI] [PubMed] [Google Scholar]
- 91.Hayes AJ, Dowthwaite GP, Webster SV, Archer CW. The distribution of Notch receptors and their ligands during articular cartilage development. J Anat. 2003;202(6):495–502. doi: 10.1046/j.1469-7580.2003.00185.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Dowthwaite GP, Bishop JC, Redman SN, Khan IM, Rooney P, Evans DJ, Haughton L, Bayram Z, Boyer S, Thomson B, Wolfe MS, Archer CW. The surface of articular cartilage contains a progenitor cell population. J Cell Sci. 2004;117(Pt 6):889–897. doi: 10.1242/jcs.00912. [DOI] [PubMed] [Google Scholar]
- 93.Shafiee A, Kabiri M, Ahmadbeigi N, Yazdani SO, Mojtahed M, Amanpour S, Soleimani M. Nasal septum-derived multipotent progenitors: a potent source for stem cell-based regenerative medicine. Stem Cells Dev. 2011;20(12):2077–2091. doi: 10.1089/scd.2010.0420. [DOI] [PubMed] [Google Scholar]
- 94.Williams ML, Solnica-Krezel L. Regulation of gastrulation movements by emergent cell and tissue interactions. Curr Opin Cell Biol. 2017;48:33–39. doi: 10.1016/j.ceb.2017.04.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Musumeci G, Castrogiovanni P, Trovato F, Di Giunta A, Loreto C, Castorina S. microscpic and macroscopic anatomical features in healthy and osteoarthritic kenn cartilage. OA Anatomy. 2013;1(3):30. doi: 10.13172/2052-7829-1-3-898. [DOI] [Google Scholar]