Abstract
GABAergic interneurons are highly diverse and their synaptic outputs express various forms of plasticity. Compelling evidence indicates that activity-dependent changes of inhibitory synaptic transmission play a significant role in regulating neural circuits critically involved in learning and memory and circuit refinement. Here, we provide an updated overview of inhibitory synaptic plasticity with a focus on the hippocampus and neocortex. To illustrate the diversity of inhibitory interneurons, we discuss the case of two highly divergent interneuron types, parvalbumin-expressing basket cells and neurogliaform cells, which support unique roles on circuit dynamics. We also present recent progress on the molecular mechanisms underlying long-term, activity-dependent plasticity of fast inhibitory transmission. Lastly, we discuss the role of inhibitory synaptic plasticity in neuronal circuits’ function. The emerging picture is that inhibitory synaptic transmission in the CNS is extremely diverse, undergoes various mechanistically distinct forms of plasticity, and contributes to a much more refined computational role than initially thought. Both the remarkable diversity of inhibitory interneurons and the various forms of plasticity expressed by GABAergic synapses provide an amazingly rich inhibitory repertoire that is central to a variety of complex neural circuit functions, including memory.
Keywords: synaptic inhibition, synaptic plasticity, neural circuits, hippocampus, neocortex, memory
Introduction
Synaptic plasticity is a fundamental phenomenon that refers to the ability of synapses to change their efficacy as a result of transient neuronal activity. It is believed to represent a major cellular event sculpting circuit dynamics and underlying learning and memory (Takeuchi et al., 2014). Studies in animal models (e.g., rodents) established molecular mechanisms and neuronal circuits involved in this phenomenon (Luscher et al., 2000; Herring & Nicoll, 2016). Most of the information on synaptic plasticity was initially obtained from excitatory synapses, but many experimental and computational studies more recently established that inhibitory synaptic plasticity (ISP) is also a major player in neuronal circuit mechanisms underlying memory processes (Hennequin et al., 2017) as well as maintenance of circuit excitability (Nelson & Turrigiano, 2008). Identifying cellular and molecular mechanisms underlying ISP is important for several reasons: (i) ISP is expected to critically control the computation of cortical pyramidal neurons (Kullmann et al., 2012; Hennequin et al., 2017), (ii) it may promote stability of network activity following the formation of excitatory engrams (Barron et al., 2017), which are defined as ensembles of neurons involved in storing and recalling memory, (iii) it is likely to be involved in brain disease, e.g., synaptic remodeling after stroke – (Berger et al., 2019), or may be exploited for therapeutic intervention (Di Lazzaro et al., 2018).
This review article focuses on GABAergic inhibitory synapses and ISP as a central contributor to fundamental circuit functions. We first provide a brief account of GABAergic neuron diversity in the cerebral cortex and hippocampus, and discuss how molecular properties at specific GABAergic synapses may confer distinct capacity for plasticity. Next, we review key molecular mechanisms underlying different forms of ISP, and speculate on how this diversity in mechanisms for ISP may support the wide palette of sophisticated contribution of inhibitory synapses to circuit computations. Finally, we discuss emerging hypotheses about the role of ISP. Thus, we bring together recent experimental and theoretical studies, and discuss how GABAergic neuron diversity and ISP heterogeneity contribute to neural circuit computations and complex brain functions such as learning and memory.
GABAergic neuron diversity
Cortical GABAergic neurons are diverse in their developmental origin, gene expression, morphology, function and connectivity (Gupta et al., 2000; Klausberger & Somogyi, 2008; Tremblay et al., 2016; Pelkey et al., 2017; Fishell & Kepecs, 2019; Huang & Paul, 2019). This diversity is probably even more pronounced in the human cerebral cortex, where GABAergic cell types without apparent homology with rodent cortical cell types have been found (Boldog et al., 2018). Combined information of dendritic and axonal patterns, molecular markers and functional activities of neurons is useful to determine cell types. Consistent with this, multiparametric methods have been endorsed to classify GABAergic neurons (Petilla Interneuron Nomenclature et al., 2008; DeFelipe et al., 2013). GABAergic cells are eminently target specific, selectively innervating subcellular domains of postsynaptic cells. For example, axo-axonic interneurons make synapses exclusively on the axon initial segment of cortical pyramidal cells (Somogyi et al., 1983); basket cells (BCs) target preferentially the somata and proximal dendrites of postsynaptic neurons (Thomson et al., 1996; Tamas et al., 1997), Martinotti and neurogliaform cells (NGFCs) target the dendrites of postsynaptic cells (Tamas et al., 2003; Wang et al., 2004), some neocortical interneurons preferentially target other interneurons (Pfeffer et al., 2013). Functional specialization of inhibitory neurons provides subtle regulation of cortical networks. For example, cortical NGFCs provide feed-forward inhibition of distal dendrites of postsynaptic pyramidal neurons (Tamas et al., 2003) and also elicit presynaptic inhibition of transmitter release (Olah et al., 2009). A division of labor amongst interneuron types in governing network activity is well known in the hippocampus and cortex (Kawaguchi & Kubota, 1997; Klausberger & Somogyi, 2008; Tremblay et al., 2016). Recent advances in high-throughput single-cell transcriptomics (scRNAseq) provided a new quantitative genetic framework to elucidate GABAergic neuron diversity (Poulin et al., 2016; Shekhar et al., 2016; Paul et al., 2017; Que et al., 2019). Given this diversity, it is possible that different GABAergic neuron types display different forms of ISPs, an idea that for now is supported by a limited amount of experimental data and will need further work (Horn & Nicoll, 2018; Schulz et al., 2018).
Interneurons synapse specialization: molecular diversity of synapses from BC and NGFC
Here we will compare two GABAergic neuron types that are placed at the extremes of the GABAergic neuron diversity spectrum, namely, parvalbumin expressing (PV+) BCs and NGFCs of neorcortex and hippocampus. We will summarize molecular differences at the inhibitory synapses established by NGFCs and PV+ BCs, and discuss hypotheses about how these properties may endow synapses from these neuron types with distinct capacity for plasticity.
PV+ BCs mediate fast, phasic inhibition (Hefft & Jonas, 2005), in contrast NGFCs evokes volume transmission leading to slow inhibition (Capogna and Pearce, 2011). Information regarding synaptic-associated molecules expressed by PV BC+ and NGFCs is relatively abundant. By discussing their similarities and differences we can provide testable hypotheses about how molecular specificity may inform on interneuron-type specific signaling pathways underlying ISP. PV+ interneurons are estimated to be numerous (e.g, about 14% of CA1 hippocampal interneurons - (Bezaire & Soltesz, 2013) and elicit robust inhibitory responses in targeted principal neurons (Hu & Jonas, 2014), thus, they are likely to represent a significant group of interneuron mediating ISP in experimental conditions in which the presynaptic neuron is not identified (Vogels et al., 2013). However, certain experimental settings allow for the specific identification of the inhibitory connection. For example, long-term potentiation (LTP) of cortical PV+ BC-mediated synaptic inhibition is elicited by visual deprivation (Maffei et al., 2006). Spike timing dependent plasticity (STDP) has also been observed at this type of connection: short delay between spiking of a cortical PV+ BC and a principal neuron elicits long-term depression (LTD), whereas longer delays evoke LTP (Holmgren & Zilberter, 2001). More recently, cortical PV+ interneuron-mediated inhibition shows STDP that contributes to auditory map remodeling (Vickers et al., 2018). In contrast to PV+ interneurons, much less is known on ISP mediated by NGFCs. In the hippocampus, this interneuron type displays marked synaptic depression evoked by a train of presynaptic stimuli at theta frequency which has a recovery time constant of about 10 minutes (Karayannis et al., 2010). Furthermore, the injection into a postsynaptic NGFC in vitro of a firing pattern recorded in a NGFC in vivo displays shorter-term retrograde synaptic depression lasting about 1 minute (Li et al., 2014a). As for long-term ISP, the molecular properties we discuss below suggest that synapses established by NGFCs have the machinery for changing their synaptic efficacy. In support of this possibility, recent findings have implicated this interneuron subtype in memory processes in the cortex (Abs et al., 2018).
Salient features of hippocampal PV+ BCs and NGFCs are illustrated in Figure 1. Morphological properties of PV+ BCs and NGFCs are notably different. PV+ BCs have multiple dendrites that often cross layers (Gulyas et al., 1999; Tukker et al., 2013), suggesting heterogeneous inputs. In contrast, the dendrites of NGFCs are compact and arranged in a stellate fashion around the soma (Vida et al., 1998), pointing to inputs from a more restricted set of afferent pathways. The axon of PV+ BC shows extensive arborization, and generates a divergent inhibitory output restricted to the perisomatic domain of postsynaptic targets (Sik et al., 1995). The axon of NGFC is also extensive but it branches profusely and usually targets exclusively the dendritic domain of postsynaptic neurons in the hippocampus (Vida et al., 1998; Price et al., 2005), as in the neocortex (Tamas et al., 2003). Molecular features of PV+ BCs and NGFCs are also quite different. A subpopulation of BCs expresses PV with an average concentration of 10 μM at the soma in hippocampal dentate granule cell layer. Because PV is a slow calcium buffer, it affects the time course of intracellular calcium transients in terminals after an action potential, and hence regulate short-term synaptic plasticity favoring synaptic depression (Eggermann & Jonas, 2011). In contrast, both neocortical and hippocampal NGFCs express an array of marker proteins, including α-actinin2, neuropeptide Y, neuronal nitric oxide synthase (nNOS), the transcription factor COUP-TFII and the extracellular matrix protein reelin (Price et al., 2005; Fuentealba et al., 2008; Olah et al., 2009; Fuentealba et al., 2010; Szabadics et al., 2010). The presence of nNOS supports the idea that NGFCs synapses are plastic and this plasticity may have physiological significance (Makara et al., 2007). When hippocampal NGFCs generate a theta rhythm-associated activity their synapses display inhibitory short-term synaptic plasticity onto pyramidal neurons. This phenomenon, called “firing-induced suppression of inhibition” (FSI) requires backpropagation of action potentials, postsynaptic calcium influx through L-type calcium channels, nNOS activity and NO retrograde release, and activation of NO-sensitive guanylyl cyclase receptors at presynaptic terminals (Li et al., 2014a). FSI indirectly increases the amplitude of excitatory postsynaptic potentials (EPSPs), and may enhance spatial and temporal summation of excitatory inputs to NGFCs, thereby regulating their inhibition of pyramidal cells (Li et al., 2014a). Neocortical PV+ BCs also show NO-dependent short-term plasticity, but its time scale is markedly different from the one exhibited by hippocampal NGFCs. Somatic depolarization of pyramidal cells in layer 5 triggers Ca2+-dependent retrograde release of NO which diffuses to PV+ BC axon terminals and elicits a persistent increase of GABA release (Lourenco et al., 2014).
Figure 1. Interneuron diversity: neurogliaform and parvalbumin expressing basket cells.
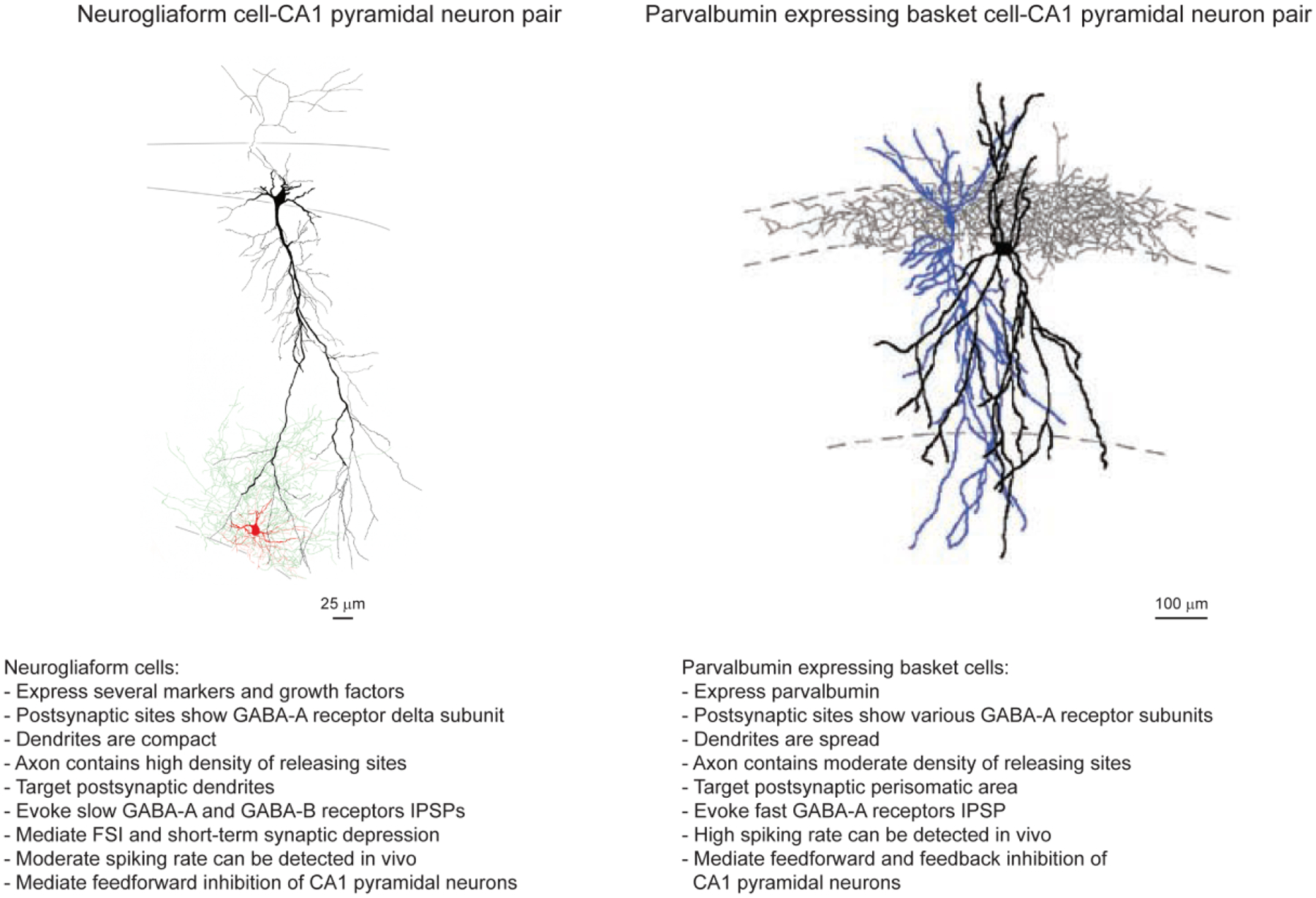
Left. Reconstruction of a neurogliaform cell (NGFC, soma and dendrites, red; axon, green) and a CA1 pyramidal neuron (soma and dendrites, black) recorded from a rat in vitro. Salient features detected in NGFCs are listed below the image. The picture is taken from Price et al, J. Neurosci., 28(27):6974–6982, 2008(Price et al., 2008). Right. Reconstruction of a parvalbumin expressing basket cell (BC, soma and dendrites, black; axon, gray) and a CA1 pyramidal neuron (soma and dendrites, blue) recorded from a rat in vitro. Key features observed in BCs are listed below the image. The picture is taken from Foldy et al, Nature Neurosci.,13(9):1047–1049, 2010 (Foldy et al., 2010).
Distinct synaptic organization for PV+ BC and NGFC
The synaptic organization of PV BCs and NGFCs is markedly different. A single neocortical NGFC axon has a release site density comparable to that of five or six BC axons (Olah et al., 2009), suggesting that GABA released from NGFC axons can reach synaptic but also non-synaptic receptors. Moreover, axon boutons of NGFCs are often (Olah et al., 2009), but not always (Tamas et al., 2003; Price et al., 2008; Fuentealba et al., 2010), found 1–5 μm away from target dendrites, a surprisingly long distance compared to the 10–20 nm typically detected at conventional PV+ BC synapses (Tukker et al., 2013). The release of GABA from neocortical NGFCs can also inhibit the release of glutamate or GABA from axon terminals located remotely from NGFC release sites (Olah et al., 2009). Therefore, it has been suggested that NGFCs can mediate volume transmission (Olah et al., 2009) wherein a widespread, prolonged, low-level GABA transient is produced by a dense array of NGFC release sites (Capogna & Pearce, 2011). Furthermore, at the postsynaptic level, data obtained with high resolution replica immunogold labeling indicate that all CA1 pyramidal cell somatic inhibitory synapses contain the α1, α2, β1, β2, β3 and γ2 subunits along with the adhesion molecule neuroligin-2 (NL-2) (Kerti-Szigeti & Nusser, 2016), whereas neocortical NGFCs synapses show also high level of GABAA receptors GABAARs) containing δ subunits (Olah et al., 2009). The presence of the δ subunits in GABAA receptors at synapses is unique to NGFCs, as receptors containing this subunit are typically located extrasynaptically (Farrant & Nusser, 2005; Belelli et al., 2009). More recently, hippocampal NO-synthase expressing NGFCs have been found to activate postsynaptic α5-GABAA receptors which strongly contribute (50–80%) to the inhibitory synaptic conductance (Schulz et al., 2018). Importantly, the inhibition of dendritic NMDA spikes via α5-GABAA receptors-containing synapses provides a mechanistic basis for the powerful control of NMDA receptor-dependent burst firing and synaptic plasticity in CA1 pyramidal cells by dendrite-targeting interneurons (Schulz et al., 2018). Conversely, α5-GABAA receptors supply a negligible contribution to perisomatic inhibition elicited by fast-spiking PV interneurons (Schulz et al., 2018). Whether synaptic junctions formed by PV+ BCs or NGFCs also differ in the organization of postsynaptic molecular components, such as scaffolding proteins (e.g., gephyrin, collybistin), adhesion proteins (e.g., NL-2), kinases (e.g., PKC, ERK1/ERK2) and proteases (e.g., calpain) for GABAAR phosphorylation/dephosphorylation, is currently not known. These molecular components are pivotal for localization, stability and regulation of baseline as well as plastic GABAergic signaling (Chiu et al., 2019).
A distinctive functional feature is that action potentials of PV+ BC have fast kinetics that evoke GABAAR-mediated inhibitory postsynaptic responses with a time constant of 5–10 ms (Cobb et al., 1995), whereas NGFCs have broader spikes that evoke slow postsynaptic inhibitory potentials (time constant can be > 30 ms) (Tamas et al., 2003). Upon repetitive stimulation, inhibitory responses evoked by both PV+ BCs and NGFCs show marked depression (Hefft & Jonas, 2005; Karayannis et al., 2010).
Overall, PV+ BCs synapses onto their target neurons have been convincingly shown to express pre- and postsynaptic molecular motifs apt to confer fast neurotransmission proper of this GABAergic neuron type. These motifs include the tight “nanodomain” coupling between Ca2+ channels and release sensors for exocytosis, promoting efficacy and temporal precision of neurotransmitter release, as well as shortening the synaptic delay (Bucurenciu et al., 2008). Furthermore, a subpopulation of PV+ interneurons in the neocortex and hippocampus uses the synaptotagmin 2 isoform as a release sensor protein for neurotransmitter release (Kerr et al., 2008), in contrast to the most common synaptotagmin 1 expressed in principal cells (Geppert et al., 1994). The synaptotagmin 2 isoform has the fastest Ca2+ binding kinetics among synaptotagmin family proteins, a feature that is likely to contribute to fast neurotransmission. Conversely, direct measurements of presynaptic Ca2+ transients in hippocampal NGFCs reveal slow decaying Ca2+ transients at axonal boutons (Price et al., 2008), consistent with the slow kinetics of neurotransmission elicited by this neuron type.
The spiking patterns of PV+ BCs and NGFCs detected in vitro and in vivo are also quite characteristic. PV+ BCs show remarkable high action potential frequency (> 10 Hz) (Sik et al., 1995), whereas hippocampal NGFCs fire maximally up to 10 Hz during theta network oscillations in vivo (Fuentealba et al., 2010) under basal conditions without applying any stimulation. Neocortical and hippocampal NGFCs also display a so-called barrage firing (Sheffield et al., 2011; Suzuki et al., 2014; Chittajallu et al., 2020), a persistent spiking activity occurring up to several minutes after cells’ stimulation, which can reach frequencies up to 130 Hz. Because of prominent frequency-dependent synaptic depression, it is still unclear how much inhibition is provided to postsynaptic targets during NGFC barrage firing.
At the circuit level, a major difference between PV+ BCs and NGFCs is that PV+ BCs mediate both feedforward (FF) and feedback (FB) inhibition (Hu et al., 2010), whereas NGFCs only mediate FF inhibition (Capogna & Pearce, 2011). This has been well characterized in hippocampus. CA1 afferent glutamatergic axons (e.g. from entorhinal cortex or Schaffer collaterals) elicit FF inhibition via parallel activation of CA1 pyramidal cells, PV+ BCs and NGFCs. CA1 pyramidal cells driven by the same glutamatergic axons in turn activate PV+ BCs, but not NGFCs, recruiting circuit-specific FB inhibition. The speed of both FF and FB inhibition elicited by PV+ BCs is high; the latency of disynaptic inhibition under physiological conditions is less than 2 ms (Miles, 1990). Hippocampal NGFCs evoke much slower FF inhibition onto CA1 pyramidal neurons and influence the temporal integration of incoming excitatory signals on a longer time scale (Price et al., 2008). Hippocampal PV+ BCs powerfully inhibit spiking of postsynaptic neurons due to the perisomatic location of its GABAergic synapses, but also elicit rebound spiking after postsynaptic hyperpolarization that promote network synchronization activity in vitro (Cobb et al., 1995). Rebound spiking has also been observed in vivo (Adhikari et al., 2012). Neocortical NGFCs inhibit the dendrites of postsynaptic neurons and reduce dendritic Ca2+ signals in vitro (Perez-Garci et al., 2006) and in vivo (Abs et al., 2018). This neuron type presynaptically inhibits the release of glutamate from nearby excitatory terminal via a GABAB receptor mediated mechanism (Olah et al., 2009). In addition, PV+ BCs have specific functions in microcircuits. FF inhibition by CA1 hippocampal PV+ BCs sharpens the window for temporal summation of EPSPs and action potential initiation in principal neurons (Pouille & Scanziani, 2001), and broadens the dynamic range of activity in principal neuron ensembles (Pouille et al., 2009). Conversely, lateral FB inhibition promotes a “winner-takes-all” mechanism, enforcing spiking in principal cells with the strongest input, and inhibiting activity in the weaker principal cells (de Almeida et al., 2009). PV+ BCs could also contribute to other circuit functions such as sparsification of activity (Pernia-Andrade & Jonas, 2014), pattern separation (Leutgeb et al., 2007), and grid-to-place code conversion (de Almeida et al., 2009). For a review on the role of PV+ BC in these events see also Hu et al, Science 2010 (Hu et al., 2010). Hippocampal PV+ BCs usually enhance their spiking activity during network oscillations (Klausberger & Somogyi, 2008). Experimental manipulation of PV+ interneuron activity showed that these neurons modulate several phenomena such as the shape of place fields and the phase precession in CA1 pyramidal neurons (Royer et al., 2012), the gain of sensory responses (Lee et al., 2012), the regulation of learning (Donato et al., 2013). Much less is known on the functional role of NGFCs. Hippocampal NGFCs preferentially spike phased-locked to theta network oscillations (Fuentealba et al., 2010). In addition, activity of NGFCs is increased during fear memory retrieval in an auditory associative fear learning test. This effect is specific to NGFC, as the activity of another group of dendrite-targeting interneurons, those expressing SST, remains unaltered (Abs et al., 2018). Based on the distinct molecular and structural features, it is conceivable that synapses established by PV+ BCs and NGFCs onto principal neurons engage distinct mechanisms of ISP, which can mediate long-term redistribution of synaptic inhibition impinging on different compartments of pyramidal neurons. Recent experimental evidence appears to support this prediction (Horn & Nicoll, 2018).
Molecular mechanisms of Inhibitory Synaptic Plasticity
In addition to the molecular heterogeneity of inhibitory neurons and their synapses, there is also remarkable diversity in the mechanisms underlying ISP (for more extensive reviews, see (Gaiarsa et al., 2002; Castillo et al., 2011; Luscher et al., 2011; Kullmann et al., 2012; Maffei et al., 2017; Chiu et al., 2019). Although such diversity imposes an additional challenge to the study of ISP, some mechanistic principles have been identified. As for excitatory synaptic plasticity, ISP can be expressed presynaptically as changes in GABA release, or postsynaptically as changes in GABA receptor number or function (Figure 2). In this section, we summarize recent advances and emerging mechanisms on long-term, activity-dependent strengthening and weakening of fast inhibitory transmission mediated by GABAARs –i.e. I-LTP and I-LTD, respectively. While we will focus on data from synapses of the rodent neocortex and hippocampus, other brain areas sharing similar mechanisms of ISP will be cited in order to highlight generalizability.
Figure 2. Molecular mechanisms underlying activity-dependent forms of ISP.
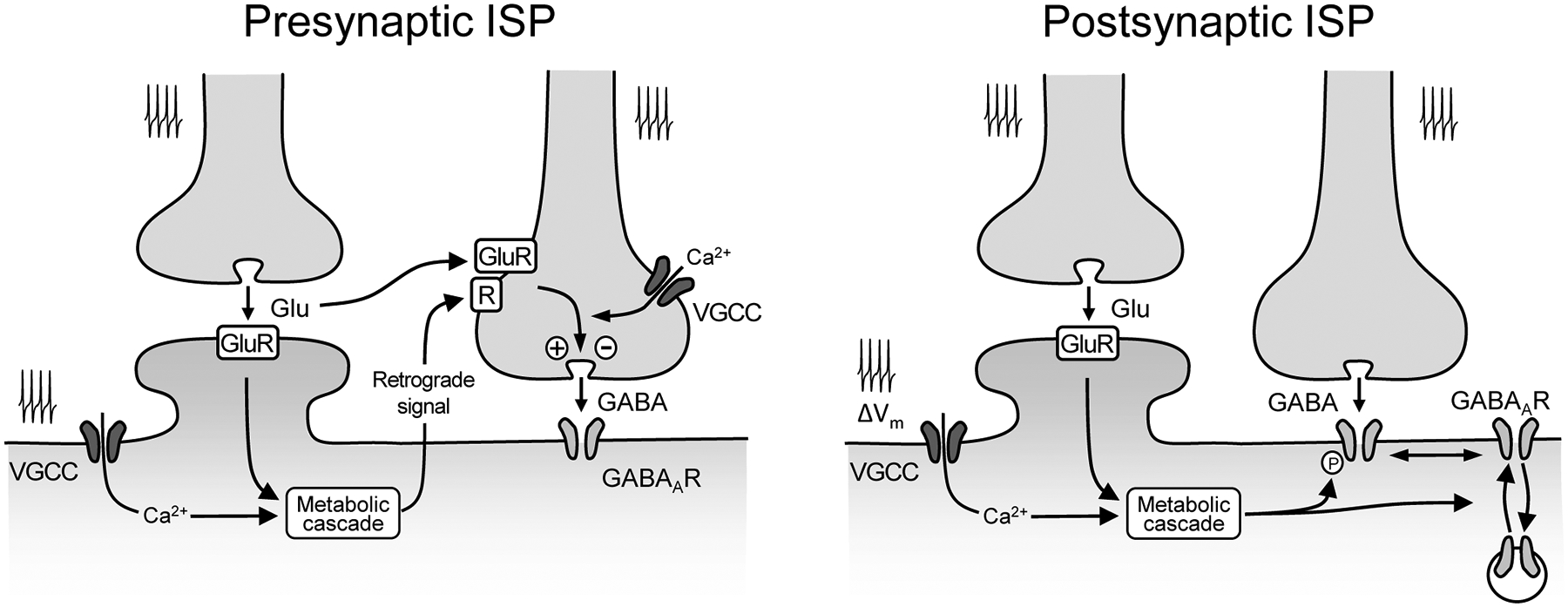
Left. Presynaptic ISP commonly involves activation of postsynaptic glutamate receptors (GluR), e.g. NMDAR and mGluR-I, and mobilization of retrograde signals that induce long-lasting changes in GABA release, by targeting a presynaptic receptor (R). Glutamate released from neighboring terminals, by activating glutamate receptors (e.g. NMDARs) on GABAergic terminals, can also induce presynaptic ISP. Coincident presynaptic and postsynaptic activity, via voltage-gated calcium channels (VGCC), can contribute to the mobilization of retrograde signals and/or modulation of downstream signaling in the GABAergic terminal. Right. Postsynaptic ISP can be induced by coordinated presynaptic and postsynaptic activity, including subthreshold changes in membrane potential (ΔVm), and GluR (NMDAR or mGluR-I) activation. Such activities set in motion a metabolic cascade of events that results in exo/endocytosis, lateral diffusion and phosphorylation/dephosphorylation of GABAARs
Presynaptic forms of ISP
Presynaptic I-LTP and I-LTD are widely expressed throughout the brain (Castillo et al., 2011; Castillo, 2012). Here, GABAergic terminals integrate diverse signals to induce long-lasting changes in GABA release by a (poorly understood) mechanism that may involve changes in the release machinery, presynaptic Ca2+ influx, as well as presynaptic structural changes (Castillo, 2012; Yang & Calakos, 2013; Atwood et al., 2014; Monday et al., 2018). Induction is commonly mediated by retrograde signals mobilized following transient, repetitive activation of nearby excitatory synapses – in a form of heterosynaptic plasticity – or repetitive firing of the postsynaptic neuron. Presynaptic Ca2+ elevations, via voltage-gated calcium channels (VGCCs) or presynaptic NMDA receptors (pre-NMDARs), activate metabolic cascades that may play a permissive, modulatory or instructive role in the induction of presynaptic ISP (Castillo, 2012; Monday et al., 2018). Diverse chemical messengers act as retrograde signals (Regehr et al., 2009), and a number of these messengers mediate ISP. Chief among them are endocannabinoids (eCBs), brain-derived neurotrophic factor (BDNF) and nitric oxide (NO). In some cases, ISP can be induced by presynaptic signals alone –i.e. in the absence of retrograde signals (Castillo et al., 2011).
The best-characterized form of presynaptic ISP is probably the eCB-mediated I-LTD. In many brain regions, repetitive activation of glutamatergic inputs triggers eCB mobilization from the postsynaptic cell to the presynaptic terminal, where they bind to Gi/o-coupled type 1 cannabinoid receptors (CB1) to suppress GABA release in a long-term manner (Castillo et al., 2012). Typically, eCB-mediated I-LTD is triggered by postsynaptic activation of group I metabotropic glutamate receptors (mGluR-I), leading to the production of diacylglyercol (DAG) by phospholipase C (PLC). Diacylglycerol lipase (DGL) converts DAG to the major eCB 2-AG, which is released from the postsynaptic cell and travels back across the synapse to activate presynaptic CB1 receptors (Heifets & Castillo, 2009; Kano et al., 2009). eCB-mediated I-LTD has been reported in several areas of the rodent brain, including the hippocampus (Chevaleyre & Castillo, 2003), amygdala (Marsicano et al., 2002; Azad et al., 2004), dorsal striatum (Adermark et al., 2009), hypothalamus (Crosby et al., 2011), and visual cortex (Jiang et al., 2010; Sun et al., 2015). In the hippocampus and basolateral amygdala (Freund et al., 2003; Vereczki et al., 2016), CCK+ (regular-spiking), but not in PV+ (fast-spiking) interneurons, selectively express CB1 receptors (Freund et al., 2003). However, this dichotomy is less clear in other brain areas (Younts & Castillo, 2014). Theta-burst firing of CA1 pyramidal neurons for a few minutes, by raising intracellular calcium and mobilizing eCBs, is sufficient to induce hippocampal I-LTD at both somatic and dendritic inhibitory synapses (Younts et al., 2013).
BDNF/TrkB-mediated I-LTP is induced by activity-dependent release of BDNF from either axon terminals or dendrites (Edelmann et al., 2014), and is typically observed in immature circuits (Castillo et al., 2011). There is good evidence that dendritically released BDNF mediates I-LTP by activating presynaptic TrkB receptors in the hippocampus (Gubellini et al., 2005; Sivakumaran et al., 2009) and visual cortex (Inagaki et al., 2008). This ISP is initiated by intracellular Ca2+ rise via NMDARs or VGCCs, or calcium release from intracellular stores. As in eCB-mediated I-LTD (Heifets et al., 2008), input-specificity of BDNF-mediated I-LTP may derive from a requirement for coincident presynaptic interneuron activity to enhance TrkB signaling (Liu et al., 2007; Edelmann et al., 2014). The effects of BDNF/TrkB signaling on GABAergic transmission and plasticity are particularly complex (Lu et al., 2014), given that BDNF can increase the number of GABAergic terminals (Marty et al., 2000; Hong et al., 2008; Jiao et al., 2011), but can also modulate the expression of the chloride transporter KCC2 (see below), and the surface expression, localization and function of GABAARs.
NO-mediated I-LTP of GABA release has been reported in neocortex and other brain areas. In layer 5 pyramidal neurons of the somatosensory cortex, postsynaptic Ca2+ rise following repetitive firing activates nitric oxide synthase (NOS) and mobilizes NO to induce presynaptic I-LTP (Lourenco et al., 2014). NO readily permeates through the membrane and presumably stimulates presynaptic guanylate cyclase (GC), thereby augmenting cGMP levels to enhance GABA release via an unknown mechanism. The NO-dependent increase in GABA release is selective for perisomatic inhibition from PV+ but not SST+ interneurons (Lourenco et al., 2014) indicating cell-type specificity for this form of ISP. In several other brain areas (Castillo et al., 2011), NO-mediated I-LTP is also induced by repetitive activation of glutamatergic inputs and NMDAR-mediated postsynaptic Ca2+ rise, which activates NOS. However, evidence for this heterosynaptic mechanism of ISP at hippocampal and neocortical synapses is lacking.
Retrograde signaling is a highly regulated process (Iremonger et al., 2013), allowing for multiple points of modulation and cross-talk with several signaling systems. For example, theta-burst stimulation in layer 2/3 neurons of somatosensory cortex induces I-LTD that requires BDNF-TrkB signaling, but not mGluR-I activation (Zhao et al., 2015). Activation of presynaptic, Gi/o-coupled type 2 dopamine receptors (D2R) act synergistically with CB1 downstream signaling to induce I-LTD in the prefrontal cortex (Chiu et al., 2010), suggesting additional layers of modulatory complexity in presynaptic terminals. Lastly, in CA1 pyramidal neurons, STDP-induced I-LTD requires coactivation of (presumably presynaptic) Gi/o-coupled M2-type muscarinic acetylcholine receptors and eCB signaling (Ahumada et al., 2013).
ISP can be induced homosynaptically in the absence of retrograde signaling (Castillo, 2012). This is the case of fast-spiking interneurons in layer 2/3 of the mouse visual cortex where repetitive activity of these neurons can induce presynaptic I-LTP that likely relies on presynaptic calcium influx via P/Q type calcium channels (Sarihi et al., 2012). Adding to the diversity of presynaptic mechanisms for ISP, there is evidence that neuromodulatory systems can also induce ISP. A good example can be found at PV+ to CA2 pyramidal cell synapses, where activation of presynaptic Gi/o-coupled delta-opioid receptors, likely acting on the release machinery, induces a form of presynaptic I-LTD reminiscent of eCB-dependent I-LTD (Piskorowski & Chevaleyre, 2013). Other presynaptic metabotropic receptors (Atwood et al., 2014) may also contribute to the induction of ISP.
Postsynaptic forms of ISP
In many cases activity-dependent ISP relies on postsynaptic modifications such as changes in the properties or number of GABAARs expressed at the synapse (Luscher et al., 2011; Vithlani et al., 2011). Here, we briefly summarize the induction and expression mechanisms underlying this form of plasticity. Postsynaptic ISP can be induced by repetitive firing of the postsynaptic neuron, coordinated activity of the GABAergic interneuron and the postsynaptic neuron, or heterosynaptically by repetitive activation of nearby glutamatergic synapses (Gaiarsa et al., 2002; Castillo et al., 2011; Lourenco et al., 2014; Chiu et al., 2019). A common theme is that Ca2+ rise either via VGCCs or NMDARs sets in motion a metabolic cascade of events that leads to changes in GABAAR function or number. In layer 5 pyramidal neurons of rat visual cortex, repetitive firing from a depolarized membrane potential, induces I-LTD at somatic inhibitory synapses, whereas cell firing during slow membrane voltage oscillations induces I-LTP. Interestingly, while I-LTD is mediated by L-type calcium channels, I-LTP is mediated by R-type calcium channels, suggesting that the relative amount of calcium entry through these channels determines the polarity of this ISP (Kurotani et al., 2008). As mentioned above, timing of pre and postsynaptic activity is also important for the polarity of ISP (Holmgren & Zilberter, 2001), although the relationship between timing and sign of plasticity differs from that reported for excitatory synapses (Bi & Poo, 2001). Pairing of presynaptic burst activity and subthreshold postsynaptic depolarization also induces I-LTP at PV+ and layer 4 pyramidal neuron synapses in the developing visual cortex (Maffei et al., 2006; Wang & Maffei, 2014). This plasticity is occluded by visual deprivation, a manipulation that potentiates inhibitory transmission, suggesting that I-LTP can occur in vivo.
Glutamatergic activity can induce heterosynaptic, postsynaptic ISP by activating ionotropic (typically NMDARs) or mGluRs. (Ouardouz & Sastry, 2000). This form of postsynaptic I-LTP has recently been reported at dendritic synapses between SST+ interneurons and layer 2/3 pyramidal neurons of the mouse medial prefrontal cortex (Chiu et al., 2018). I-LTD induction in the CA1 area of the hippocampus relies on a similar mechanism (Lu et al., 2000). Earlier studies (Aizenman et al., 1998) documented a form of NMDAR-dependent I-LTP requiring postsynaptic calcium rise in DCN neurons induced by high frequency stimulation of glutamatergic inputs.
The expression mechanisms of postsynaptic ISP are diverse. Increases or decreases in channel function can occur as a result of GABAAR phosphorylation by multiple kinases, including, CaMKII, PKC, PKA and Src (Vithlani et al., 2011). GABAARs are further controlled by constitutive cycling, regulating insertion and removal, as well as lateral diffusion at the synaptic membrane surface (Luscher et al., 2011; Vithlani et al., 2011; Maynard & Triller, 2019; Pizzarelli et al., 2019; Tomita, 2019). The number of GABAARs at the cell surface is regulated by endocytosis and exocytosis. Evidence that postsynaptic I-LTP is due to GABAAR insertion in the plasma membrane comes from the observation that intracellular loading of botulinum toxin or tetanus toxin, which blocks vesicular exocytosis and insertion of new receptors into the plasma membrane, reduces this potentiation in layer 5 pyramidal neurons in the rat visual cortex (Kurotani et al., 2008). The activation of NMDARs by either exogenous agonists or endogenous glutamate potentiates GABAergic synapses from SST+, but not PV+, interneurons in pyramidal neurons of layer 2/3 of prefrontal cortex (Chiu et al., 2018). Furthermore, intracellular cellular loading of a peptide that interferes with endocytosis, not only blocks I-LTD but uncovers I-LTP in layer 5 pyramidal neurons (Kurotani et al., 2008).
The molecular mechanisms involved in postsynaptic GABAAR plasticity have been largely investigated in heterologous systems and cultured neurons where the circuit architecture is not preserved and where “plasticity” is commonly induced using various chemical methods (Vithlani et al., 2011; Petrini et al., 2014; Maynard & Triller, 2019; Pizzarelli et al., 2019; Tomita, 2019). While informative, these studies may not capture the nuances of synaptic inhibition and activity-dependent ISP (i.e. I-LTP, I-LTD) in vivo, including the remarkably diverse interneuron subtypes, and the unique postsynaptic sub-cellular compartments (e.g. soma, axon, proximal and remote dendrites) (Chiu et al., 2019). While new molecular players continue to be discovered (Uezu et al., 2016; Martenson et al., 2017) and the nanoscale molecular structure of the GABAergic postsynaptic density is better understood (Pennacchietti et al., 2017; Crosby et al., 2019), more work is required to elucidate the molecular basis underlying GABAAR regulation in postsynaptic ISP, especially in preparations where the circuit architecture is preserved. This includes the precise role of scaffolding and auxiliary proteins in receptor trafficking, and the postsynaptic molecular determinants of ISP heterogeneity.
Postsynaptic GABAergic plasticity can occur independently of direct receptor regulation. In hippocampal neurons, coincident pre- and postsynaptic activity alters the activity of the K+/Cl− co- transporter KCC2, resulting in long-lasting changes in the reversal potential of GABAergic synaptic currents (Woodin et al., 2003). In this case, plasticity is induced by the activation of postsynaptic VGCCs. This mechanism should affect all inhibitory synapses, in a neuron type-independent manner. This plastic change in GABAergic synaptic strength, which is dependent on coincident pre- and postsynaptic spiking, should set the level of inhibition in accordance to the temporal pattern of postsynaptic excitation (Woodin et al., 2003). ISP induced by regulation of the reversal potential of GABA currents has powerful effects on the ability of pyramidal neurons to generate action potentials (Saraga et al., 2008), providing powerful control over activity propagation in neural circuits.
Inhibitory plasticity and circuit function
A growing body of evidence highlighted the contribution of inhibitory neurons to a variety of neural circuit functions (Maffei, 2017). Different populations of GABAergic neurons are activated during behaviors (Kepecs & Fishell, 2014). However, very little is currently known about the function of GABAergic ISP.
Several circuit computations are thought to depend on inhibitory circuits (Kepecs & Fishell, 2014). Experimental results and computational/theoretical approaches strongly suggest that GABAergic circuits are engaged in establishing the flow of signals in cortical circuits. A prominent example is lateral inhibition, a process by which the flow of signals is directed by dampening the activation of neighboring neurons (Kayser & Miller, 2002). GABAergic inhibition is also thought to contribute to adjusting the excitability of principal neurons through subtractive or divisive normalization (Pouille et al., 2009; Silver, 2010; Mejias et al., 2014; Seybold et al., 2015; Bhatia et al., 2019): the modulation of neurons input/output function that determines the sensitivity of neurons responsiveness to small changes in incoming input (gain). Differences in tuning of responses to stimuli between principal neurons, relatively narrowly tuned to incoming inputs, and GABAergic inhibitory neurons, mostly broadly tuned (but see (Moore & Wehr, 2013)), contribute to adjusting the magnitude of principal neurons responsiveness to incoming activity (Cardin et al., 2007).
Experimental work showed that somatodendritic targeting inhibitory neurons are primarily involved in this process (Cardin et al., 2008; Pouille et al., 2013; Keller et al., 2018), though a contribution of perisomatic targeting neurons to gain modulation has also been reported (Atallah et al., 2012; Lourenco et al., 2020). These apparently opposing findings raise the possibility that distinct inhibitory neuron groups could contribute to the same computational function through different mechanisms, and that the recruitment of specific neuron groups or mechanisms may depend on local circuit connectivity or network state (Mejias & Longtin, 2014).
At the network level, inhibitory neurons are thought to contribute to network synchronization and to the generation of oscillatory patterns of activity (Vierling-Claassen et al., 2010; Avoli, 2019). The connectivity of neural circuits and the distinct firing properties of different neuron groups (e.g. fast spiking GABAergic neurons versus regular spiking principal cells) can shape network oscillations by the alternation of neurons activation (Cardin et al., 2009; Drexel et al., 2017; Panthi & Leitch, 2019). As specific oscillatory rhythms are associated with distinct cognitive functions, alterations in the coupling between excitatory and inhibitory neurons can results in changes in networks states (Francavilla et al., 2018; Pala & Petersen, 2018).
The theoretical framework established to better investigate these functions tends to consider inhibition as a uniform system that is broadly connected and primarily recurrent (van Vreeswijk & Sompolinsky, 1996; Vogels & Abbott, 2009; Kanashiro et al., 2017; Huang et al., 2019). This approach is supported by reports of widespread connectivity of different groups of inhibitory neurons (Fino & Yuste, 2011; Fino et al., 2013) suggesting that GABAergic inhibition may act by modulating circuits broadly without specificity. It is also supported by studies showing GABAergic neurons broad tuning to incoming stimuli (Cardin et al., 2007; Li et al., 2015a; Li et al., 2015b; Hayashi et al., 2018; Li et al., 2019), a property that renders them not particularly sensitive to the granular features of an input.
However, there is evidence that the tuning of inhibitory neurons to incoming stimuli is not always broader than that of principal cells (Moore & Wehr, 2013; Camillo et al., 2018; Khan et al., 2018), and can be modified by experience (Dorrn et al., 2010; Cai et al., 2018). While it is reliably found that inhibitory neurons connect broadly to principal neurons in cortical circuits, specificity can be achieved through selective targeting of neuronal compartments by distinct groups of neurons with distinct molecular identities (Somogyi et al., 1983; Thomson et al., 1996; Tamas et al., 2003; Wang et al., 2004; Chamberland & Topolnik, 2012; Paul et al., 2017), or by activity-dependent changes in inhibitory synapses efficacy that depend on the level of activity of the postsynaptic neuron (Wang & Maffei, 2014; Chiu et al., 2018; Lourenco et al., 2020). Furthermore, inhibitory synapses can change their efficacy in response to shifts in circuit excitability (Maffei et al., 2004; Maffei et al., 2006), a diverse array of patterns of activity (Woodin et al., 2003; Chevaleyre et al., 2007; Younts et al., 2013; Petrini et al., 2014; D’Amour J & Froemke, 2015), can display distinct mechanisms depending on the identity of the presynaptic neuron (Chiu et al., 2018) and the level of activity of the postsynaptic neuron (Vogels et al., 2013; Wang & Maffei, 2014) supporting the interpretation that inhibition and ISP contribute to sculpting circuit activity and function in a more refined fashion than previously anticipated.
These experimental findings, together with novel approaches to neural network models that incorporate feedforward inhibition (Troyer et al., 1998; Miller, 2003; Miska et al., 2018), differential effects of distinct population of inhibitory neurons (Vierling-Claassen et al., 2010; Hertag & Sprekeler, 2019; Wilmes & Clopath, 2019) and plasticity rules for inhibitory synapses (Vogels et al., 2011; Wilmes & Clopath, 2019), provide new insights into the contributions of inhibition and ISP to circuit function.
Inhibition and ISP as mechanisms to stabilize circuit excitability
One of the better characterized roles of changes in efficacy of inhibitory synapses is that of stabilizer (or homeostatic regulator) of circuit excitability (Turrigiano et al., 1998). Experimental work showed that the efficacy of GABAergic inhibitory synapses can change in response to network activity in a compensatory fashion: acting to preserve excitatory neurons excitability in the absence of circuit activity (Kilman et al., 2002). A similar effect was observed in response to manipulation of sensory drive in rodents, whereby loss of visual drive induced two distinct forms of plasticity of GABAergic synapses at soma-targeting and dendrite-targeting inhibitory neurons (Maffei et al., 2004). The idea that ISP can participate in the maintenance of circuit excitability was further confirmed in a variety of different cortical circuits and its implications have been further explored experimentally (House et al., 2011; Campanac et al., 2013; Graupner & Reyes, 2013; Xue et al., 2014; Froemke, 2015) and with theoretical/computational approaches (van Vreeswijk & Sompolinsky, 1996; Vogels & Abbott, 2009; Lo et al., 2015; Rubin et al., 2017).
The hypothesis emerging from this work is that balanced excitation and inhibition is crucial for neural circuit function and that ISP plays an important role in this balancing process. The displacement from balance is thought to be needed for processing incoming inputs, and subsequent induction of ISP would bring the system back to a stable set point (Nelson & Turrigiano, 2008). Under these premises, changes in inhibitory synaptic efficacy are induced to adjust inhibitory tone and oppose shifts in network activity (Vogels et al., 2011). Overall, this principle is conceptually quite similar to the proposed role for homeostatic plasticity (Turrigiano & Nelson, 2000). Nevertheless, several neural network models implement a stabilizing form of ISP by adapting Hebbian-like rules (Vogels et al., 2013), and approach that has the advantage of modulating the induction of ISP depending on the level of circuit activity, as well as on the timing of pre- and postsynaptic activity (Vogels et al., 2013; Wang & Maffei, 2014). Models including ISP provide a substantial advancement from models that do not include it, as they do not require as much parameter adjustment, and can recapitulate a variety of experimental results. However, even these models are not fully capable of reflecting the complex, self-generating dynamics of a neural network and are not yet capable to account for the diversity of forms of ISP of which spike timing-dependent forms are just a subset (Chevaleyre et al., 2007; Kullmann et al., 2012; Wang & Maffei, 2014).
GABAergic inhibitory synaptic plasticity, learning and memory
The initial reports that GABAergic synapses are plastic, suggested that ISP contributes to cortical circuit refinement (Komatsu & Iwakiri, 1993; Maffei et al., 2004; Maffei et al., 2006) and possibly learning (Kano, 1995; Buzsaki, 1997; Nugent et al., 2007). Since these pioneering studies, experimental work demonstrated that changes in inhibitory synaptic efficacy are associated with adaptation of olfactory responses in drosophila (Das et al., 2011), with changes in sensory experience (Maffei et al., 2004; Maffei et al., 2006; House et al., 2011; D’Amour J & Froemke, 2015; Cai et al., 2018), fear conditioning (Letzkus et al., 2011; Xu et al., 2014; Lucas et al., 2016) and exposure to drugs of abuse (Nugent et al., 2007; Dacher & Nugent, 2011). The specific mechanisms by which ISP contributes to learning and memory are only beginning to being unraveled. Experimental work is currently focused on determining the mechanisms of ISP (Maffei, 2011; Kullmann et al., 2012; Younts & Castillo, 2014; Chiu et al., 2019) and the role of ISP in information transfer (Lourenco et al., 2020). Computational work has only recently begun to introduce ISP in network models (Vogels et al., 2011; Mongillo et al., 2018) and theoretical ideas are being developed regarding its functional role (Barron et al., 2017; Hennequin et al., 2017; Sprekeler, 2017; Mongillo et al., 2018).
The efficacy of inhibitory synapses is sensitive to changes in sensory inputs (Maffei et al., 2004; Maffei et al., 2006; Dorrn et al., 2010; Takesian et al., 2012; Kotak et al., 2013; Gainey et al., 2016). This is especially prominent during windows of heightened sensitivity to changes in sensory drive (Hensch, 2005b; Sanes & Kotak, 2011; Li et al., 2014b; Cai et al., 2018). A model tested the possibility that gradient-like changes in inhibitory tone may contribute to the experience-dependent development of ocular dominance in rodent visual cortex (Toyoizumi et al., 2013). This idea was based on results showing that the postnatal maturation of inhibitory synaptic drive is essential for regulating onset and duration of the critical period for visual cortical plasticity (Hensch, 2005a). While this effect is thought to depend on the maturation of PV+ inhibitory neurons (Katagiri et al., 2007), inhibitory tone could also potentially be modulated by changes in synaptic transmission from different inhibitory neuron types, including NGFCs which signal through both synaptic and volume transmission (Olah et al., 2009)
Recent theoretical work suggests that ISP also plays a central role in learning and memory (Vogels et al., 2011; Wilmes & Clopath, 2019). A current working hypothesis is that ISP is induced to rebalance the network following plastic changes at excitatory synapses (Vogels et al., 2011; Sprekeler, 2017). Potentiation of glutamatergic synapses is associated with memory formation and the emergence of memory engrams (Fig.3A) (Ryan et al., 2015; Tonegawa et al., 2015; Bocchio et al., 2017). Nevertheless, the formation of multiple memory engrams that can partially overlap, may saturate the dynamics range of neural circuits, leading to saturation of storage capacity (Rashid et al., 2016; Mongillo et al., 2017). Recent experimental work proposed that ISP may act to protect stored memories by contributing to inhibitory engrams that rebalance circuit activity (Fig. 3B) (Koolschijn et al., 2019). The definition of inhibitory engram is currently ambiguous: it is not specified whether it is a counter-engram that depends on ISP at inhibitory synapses onto the principal neurons participating in the memory engram, or a group of coactivated inhibitory neurons counterbalancing the excitability of the engram circuit, but not directly connected to the engram neurons. In network models, the inhibitory engram mediated by ISP would stabilize network excitability while preserving relative differences at excitatory connections (Abbott & Nelson, 2000; Renart et al., 2003). This would preserve memories and maintain the dynamic range of circuit excitability so that it is permissive to the formation and storage of new memories. Conceptually, the role inhibitory engrams is an upgraded version of the well-established idea that the primary role of inhibition is to keep circuit excitability in check. In this case ISP would be induced to prevent overactivation of a subcircuit of excitatory neurons (Barron et al., 2017). Instead of providing widespread control on excitability, ISP would act in a more restricted fashion.
Figure 3. Inhibitory plasticity as a mechanism for circuit reconfiguration.
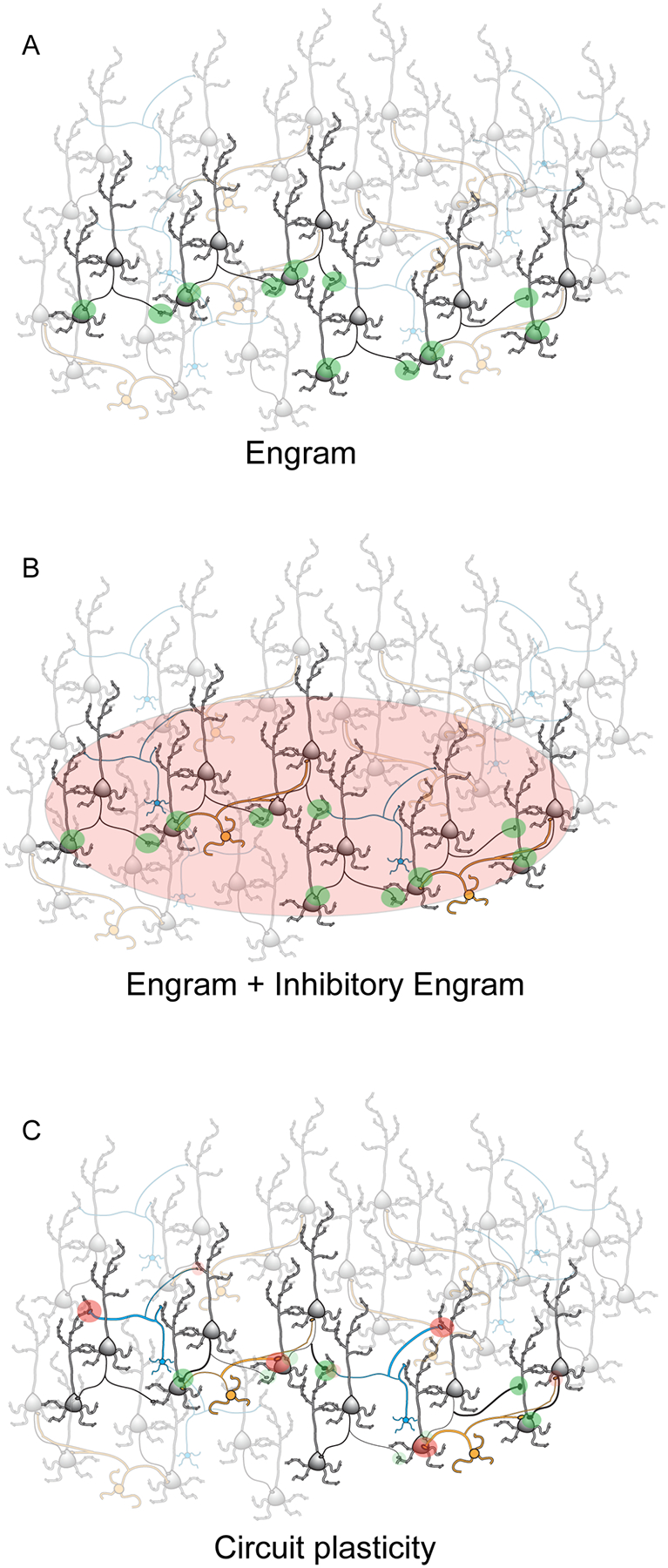
Simplified diagram of how inhibitory plasticity can increase the dynamic range of neural circuits. Black and grey: pyramidal neurons; yellow: soma targeting inhibitory neurons; blue: dendrite targeting inhibitory neurons. A. Plasticity at excitatory synapses is thought to facilitate the activation of memory storage units (engrams). Green shaded areas and thick lines indicate the site of LTP at excitatory connections. The darker neurons highlight an example engram, while the surrounding faded neurons are part of the circuit but do not participate in the excitatory engram. B. In this diagram, the excitatory engram shown in A is paired with an inhibitory engram triggered by ISP. As the inhibitory engram is not defined to be connection-specific (Hennequin et al., 2017), the area of influence of the inhibitory engram is indicated by the red shaded area. Greed shaded areas and thick lines indicate LTP at excitatory synapses. Neurons not participating in either the excitatory or the inhibitory engrams are shown in lighter colors. C. The diagram shows how connection-specific LTP and LTD at excitatory and inhibitory synapses can expand the dynamic range of the circuit, facilitating the accommodation of multiple engrams. Large green shaded areas and thick black lines indicate LTP at excitatory synapses; small green shaded areas and thin black lines indicate LTD at excitatory synapses. Large red shaded areas and thick yellow and blue lines indicate LTP at inhibitory synapses; small red shaded areas and think yellow and blue lines indicate LTD at inhibitory synapses. In this diagram both excitatory and inhibitory plasticity are connection-specific. Cooperative interactions between excitatory and inhibitory plasticity are taken into account to indicate the site of LTP and LTD at both populations of synaptic connections (see (Wang & Maffei, 2014; Hennequin et al., 2017)).
A caveat of the inhibitory engram idea stems from its reliance of a temporal sequence in which the induction of excitatory plasticity precedes changes in inhibitory synaptic efficacy (Hennequin et al., 2017): plasticity at excitatory synapses is induced during learning, shifting the balance between excitation and inhibition, then ISP is induced to restore balance (Vogels et al., 2011). Experimental evidence in favor of such a sequence of events has been reported in some cortical circuits (Kuhlman et al., 2013). Nevertheless, changes in inhibition can be induced before or together with excitatory plasticity at recurrent synapses (Wang & Maffei, 2014; Gainey et al., 2018). Firing rates of inhibitory neurons recover more rapidly than those of excitatory neurons following brief sensory deprivation (Hengen et al., 2013), indicating that the temporal sequence suggested by the inhibitory engram hypothesis is not generally applicable. Furthermore, the baseline ratio of excitatory and inhibitory charge onto cortical pyramidal neurons is shifted toward inhibition (Tatti et al., 2017), suggesting that disinhibition may be needed for excitatory plasticity to be induced. Finally, the ratio of excitation and inhibition is circuit and experience-dependent (Tatti et al., 2017; Bridi et al., 2019), adding complexity to how ISP may affect learning. These data are consistent with theoretical work suggesting that sparse cortical activity depends on inhibition being dominant, and with experimental results indicating that disinhibition of cortical circuits is permissive for learning (Froemke et al., 2007; Letzkus et al., 2011; Kuhlman et al., 2013). This alternative framework suggests that depression of inhibitory synaptic transmission facilitates the induction of excitatory plasticity correlated with the formation of associations between conditioned and unconditioned stimuli. In support of this idea, a study demonstrated that excitatory and inhibitory plasticity cooperatively interact in an anticorrelated fashion, with potentiation of inhibition facilitating depression of convergent excitatory synapses, and depression of GABAergic synapses enabling potentiation of glutamatergic input onto the same postsynaptic target (Wang & Maffei, 2014). As this crosstalk is connection-specific and relies on postsynaptic activity (Maffei et al., 2006; Wang & Maffei, 2014), it has sufficient resolution to modulate single cortical connections instead of acting as a broad balancing factor.
Finally, it is unclear whether long term ISP would be necessary if its role is to restore the balance between excitation and inhibition. Homeostatic synaptic plasticity can be a slow process effectively restoring stability over the course of several hours to a few days (Kilman et al., 2002; Lambo & Turrigiano, 2013; Whitt et al., 2014; Glazewski et al., 2017). Such time course is compatible with the idea that changes in inhibitory synaptic transmission restore circuit excitability to a balanced state. However, ISP in the form of I-LTP or I-LTD is typically induced rapidly, is long-lasting (Woodin et al., 2003; Chevaleyre et al., 2007; Nugent et al., 2007; Wang & Maffei, 2014), and recruits complex post translational mechanisms that can lead to structural and functional changes in inhibitory connectivity (Tyagarajan et al., 2011; Petrini et al., 2014; Ghosh et al., 2015). These effects are comparable to what is typically reported for long term plasticity at excitatory synapses (Herring & Nicoll, 2016).
It has been proposed that ISP can act as a form of metaplasticity (Chevaleyre & Castillo, 2004; Xu et al., 2014), a set of changes that alter the state of a principal neuron modulating its responsiveness to incoming activity (Abraham, 2008). This hypothesis is compatible with the experimental results discussed above, but does not fully explain the duration of the changes in synaptic strength, unless we consider ISP as a direct contributor to memory storage. Indeed, a long lasting state change in a neuron, whether excitatory or inhibitory, may serve as memory signature shaping how this neuron (or circuit) will be activated by future stimuli. Instead of contributing to rebalance, or protect, the memory engram activated by excitatory plasticity, long lasting changes in GABAergic synapses and their plasticity would expand the dynamic range of circuit plasticity, thus being a central component of the memory trace (Fig 3C).
Conclusions
Inhibitory synaptic transmission is extremely diverse and undergoes various mechanistically distinct forms of activity-dependent plasticity. Such diversity endows neural circuits with a broad repertoire for inhibitory control of neural circuits. By sampling inputs widely, as GABAergic neurons are often less narrowly tuned than their excitatory counterpart, and connecting broadly to many excitatory neurons, inhibitory circuits have the capacity of influencing cortical computations quite significantly. Their plasticity - which can result in changes in GABA release, or in synapse-specific or connection-specific changes in GABAergic responses - the diversity of neuron types, postsynaptic target regions and cellular mechanisms available to change synaptic efficacy, make them fundamental contributors to complex and sophisticated processes including learning, memory and other cognitive functions.
Acknowledgements:
Supported by the Danish National Research Foundation Center of Excellence PROMEMO to MC, NIH grants R01DA017392, R01NS113600 and R01MH116673 to PEC, NIH-NIDCD awards DC013770, DC015234 to AM.
Abbreviations:
- 2-AG
2-arachidonoylglycerol
- BC
basket cell
- BDNF
brain-derived neurotrophic factor
- CB1
type 1 cannabinoid receptors
- CCK
cholecystokinin
- DAG
diacylglyercol
- DGL
diacylglycerol lipase
- D2R
type 2 dopamine receptors
- eCB
endocannabinoid
- EPSP
excitatory postsynaptic potential
- ERK
extracellular signal-regulated kinase
- FB
feedback
- FF
feedforward
- FSI
Firing Induced Suppression of Inhibition
- GABA
gamma amino butyric acid
- GC
guanylate cyclase
- ISP
inhibitory synaptic plasticity
- KCC2
potassium chloride co-transporter
- LTD
long-term depression
- LTP
long-term potentiation
- mGluR-I
group I metabotropic glutamate receptors
- NGFC
neurogliaform cell
- NL-2
neuroligin-2
- NMDA
N-methyl-D-aspartate
- nNOS
neuronal nitric oxide synthas
- NO
nitric oxide
- PLC
phospholipase C
- PV
parvalbumin
- PKC
protein kinase C
- scRNAseq
single cell RNA sequence
- STDP
Spike timing dependent plasticity
- TrkB
tropomysin related kinase B
- VGCC
voltage-gated calcium channel
Footnotes
Publisher's Disclaimer: This article has been accepted for publication and undergone full peer review but has not been through the copyediting, typesetting, pagination and proofreading process, which may lead to differences between this version and the Version of Record. Please cite this article as doi: 10.1111/ejn.14907
Disclosures: None.
References
- Abbott LF & Nelson SB (2000) Synaptic plasticity: taming the beast. Nat Neurosci, 3 Suppl, 1178–1183. [DOI] [PubMed] [Google Scholar]
- Abraham WC (2008) Metaplasticity: tuning synapses and networks for plasticity. Nat Rev Neurosci, 9, 387. [DOI] [PubMed] [Google Scholar]
- Abs E, Poorthuis RB, Apelblat D, Muhammad K, Pardi MB, Enke L, Kushinsky D, Pu DL, Eizinger MF, Conzelmann KK, Spiegel I & Letzkus JJ (2018) Learning-Related Plasticity in Dendrite-Targeting Layer 1 Interneurons. Neuron, 100, 684–699 e686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Adermark L, Talani G & Lovinger DM (2009) Endocannabinoid-dependent plasticity at GABAergic and glutamatergic synapses in the striatum is regulated by synaptic activity. Eur J Neurosci, 29, 32–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Adhikari MH, Quilichini PP, Roy D, Jirsa V & Bernard C (2012) Brain state dependent postinhibitory rebound in entorhinal cortex interneurons. J Neurosci, 32, 6501–6510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ahumada J, Fernandez de Sevilla D, Couve A, Buno W & Fuenzalida M (2013) Long-term depression of inhibitory synaptic transmission induced by spike-timing dependent plasticity requires coactivation of endocannabinoid and muscarinic receptors. Hippocampus, 23, 1439–1452. [DOI] [PubMed] [Google Scholar]
- Aizenman CD, Manis PB & Linden DJ (1998) Polarity of long-term synaptic gain change is related to postsynaptic spike firing at a cerebellar inhibitory synapse. Neuron, 21, 827–835. [DOI] [PubMed] [Google Scholar]
- Atallah BV, Bruns W, Carandini M & Scanziani M (2012) Parvalbumin-expressing interneurons linearly transform cortical responses to visual stimuli. Neuron, 73, 159–170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Atwood BK, Lovinger DM & Mathur BN (2014) Presynaptic long-term depression mediated by Gi/o-coupled receptors. Trends Neurosci, 37, 663–673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Avoli M (2019) Inhibition, oscillations and focal seizures: An overview inspired by some historical notes. Neurobiol Dis, 130, 104478. [DOI] [PubMed] [Google Scholar]
- Azad SC, Monory K, Marsicano G, Cravatt BF, Lutz B, Zieglgansberger W & Rammes G (2004) Circuitry for associative plasticity in the amygdala involves endocannabinoid signaling. J Neurosci, 24, 9953–9961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barron HC, Vogels TP, Behrens TE & Ramaswami M (2017) Inhibitory engrams in perception and memory. Proc Natl Acad Sci U S A, 114, 6666–6674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Belelli D, Harrison NL, Maguire J, Macdonald RL, Walker MC & Cope DW (2009) Extrasynaptic GABAA receptors: form, pharmacology, and function. J Neurosci, 29, 12757–12763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berger D, Varriale E, van Kessenich LM, Herrmann HJ & de Arcangelis L (2019) Three cooperative mechanisms required for recovery after brain damage. Sci Rep, 9, 15858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bezaire MJ & Soltesz I (2013) Quantitative assessment of CA1 local circuits: knowledge base for interneuron-pyramidal cell connectivity. Hippocampus, 23, 751–785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bhatia A, Moza S & Bhalla US (2019) Precise excitation-inhibition balance controls gain and timing in the hippocampus. Elife, 8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bi G & Poo M (2001) Synaptic modification by correlated activity: Hebb’s postulate revisited. Annu Rev Neurosci, 24, 139–166. [DOI] [PubMed] [Google Scholar]
- Bocchio M, Nabavi S & Capogna M (2017) Synaptic Plasticity, Engrams, and Network Oscillations in Amygdala Circuits for Storage and Retrieval of Emotional Memories. Neuron, 94, 731–743. [DOI] [PubMed] [Google Scholar]
- Boldog E, Bakken TE, Hodge RD, Novotny M, Aevermann BD, Baka J, Borde S, Close JL, Diez-Fuertes F, Ding SL, Farago N, Kocsis AK, Kovacs B, Maltzer Z, McCorrison JM, Miller JA, Molnar G, Olah G, Ozsvar A, Rozsa M, Shehata SI, Smith KA, Sunkin SM, Tran DN, Venepally P, Wall A, Puskas LG, Barzo P, Steemers FJ, Schork NJ, Scheuermann RH, Lasken RS, Lein ES & Tamas G (2018) Transcriptomic and morphophysiological evidence for a specialized human cortical GABAergic cell type. Nat Neurosci, 21, 1185–1195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bridi MCD, Zong FJ, Min X, Luo N, Tran T, Qiu J, Severin D, Zhang XT, Wang G, Zhu ZJ, He KW & Kirkwood A (2019) Daily Oscillation of the Excitation-Inhibition Balance in Visual Cortical Circuits. Neuron, 105, 621–629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bucurenciu I, Kulik A, Schwaller B, Frotscher M & Jonas P (2008) Nanodomain coupling between Ca2+ channels and Ca2+ sensors promotes fast and efficient transmitter release at a cortical GABAergic synapse. Neuron, 57, 536–545. [DOI] [PubMed] [Google Scholar]
- Buzsaki G (1997) Functions for interneuronal nets in the hippocampus. Can J Physiol Pharmacol, 75, 508–515. [PubMed] [Google Scholar]
- Cai D, Han R, Liu M, Xie F, You L, Zheng Y, Zhao L, Yao J, Wang Y, Yue Y, Schreiner CE & Yuan K (2018) A Critical Role of Inhibition in Temporal Processing Maturation in the Primary Auditory Cortex. Cereb Cortex, 28, 1610–1624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Camillo D, Ahmadlou M, Saiepour MH, Yasaminshirazi M, Levelt CN & Heimel JA (2018) Visual Processing by Calretinin Expressing Inhibitory Neurons in Mouse Primary Visual Cortex. Sci Rep, 8, 12355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Campanac E, Gasselin C, Baude A, Rama S, Ankri N & Debanne D (2013) Enhanced intrinsic excitability in basket cells maintains excitatory-inhibitory balance in hippocampal circuits. Neuron, 77, 712–722. [DOI] [PubMed] [Google Scholar]
- Capogna M & Pearce RA (2011) GABA A, slow: causes and consequences. Trends Neurosci, 34, 101–112. [DOI] [PubMed] [Google Scholar]
- Cardin JA, Carlen M, Meletis K, Knoblich U, Zhang F, Deisseroth K, Tsai LH & Moore CI (2009) Driving fast-spiking cells induces gamma rhythm and controls sensory responses. Nature, 459, 663–667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cardin JA, Palmer LA & Contreras D (2007) Stimulus feature selectivity in excitatory and inhibitory neurons in primary visual cortex. J Neurosci, 27, 10333–10344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cardin JA, Palmer LA & Contreras D (2008) Cellular mechanisms underlying stimulus-dependent gain modulation in primary visual cortex neurons in vivo. Neuron, 59, 150–160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Castillo PE (2012) Presynaptic LTP and LTD of excitatory and inhibitory synapses. Cold Spring Harb Perspect Biol, 4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Castillo PE, Chiu CQ & Carroll RC (2011) Long-term plasticity at inhibitory synapses. Curr Opin Neurobiol, 21, 328–338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Castillo PE, Younts TJ, Chavez AE & Hashimotodani Y (2012) Endocannabinoid signaling and synaptic function. Neuron, 76, 70–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chamberland S & Topolnik L (2012) Inhibitory control of hippocampal inhibitory neurons. Front Neurosci, 6, 165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chevaleyre V & Castillo PE (2003) Heterosynaptic LTD of hippocampal GABAergic synapses: a novel role of endocannabinoids in regulating excitability. Neuron, 38, 461–472. [DOI] [PubMed] [Google Scholar]
- Chevaleyre V & Castillo PE (2004) Endocannabinoid-mediated metaplasticity in the hippocampus. Neuron, 43, 871–881. [DOI] [PubMed] [Google Scholar]
- Chevaleyre V, Heifets BD, Kaeser PS, Sudhof TC & Castillo PE (2007) Endocannabinoid-mediated long-term plasticity requires cAMP/PKA signaling and RIM1alpha. Neuron, 54, 801–812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chittajallu R, Auville K, Mahadevan V, Lai M, Hunt S, Calvigioni D, Pelkey KA, Zaghloul KA & McBain CJ (2020) Activity-dependent tuning of intrinsic excitability in mouse and human neurogliaform cells. Elife, 9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chiu CQ, Barberis A & Higley MJ (2019) Preserving the balance: diverse forms of long-term GABAergic synaptic plasticity. Nat Rev Neurosci, 20, 272–281. [DOI] [PubMed] [Google Scholar]
- Chiu CQ, Martenson JS, Yamazaki M, Natsume R, Sakimura K, Tomita S, Tavalin SJ & Higley MJ (2018) Input-Specific NMDAR-Dependent Potentiation of Dendritic GABAergic Inhibition. Neuron, 97, 368–377 e363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chiu CQ, Puente N, Grandes P & Castillo PE (2010) Dopaminergic modulation of endocannabinoid-mediated plasticity at GABAergic synapses in the prefrontal cortex. J Neurosci, 30, 7236–7248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cobb SR, Buhl EH, Halasy K, Paulsen O & Somogyi P (1995) Synchronization of neuronal activity in hippocampus by individual GABAergic interneurons. Nature, 378, 75–78. [DOI] [PubMed] [Google Scholar]
- Crosby KC, Gookin SE, Garcia JD, Hahm KM, Dell’Acqua ML & Smith KR (2019) Nanoscale Subsynaptic Domains Underlie the Organization of the Inhibitory Synapse. Cell Rep, 26, 3284–3297 e3283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crosby KM, Inoue W, Pittman QJ & Bains JS (2011) Endocannabinoids gate state-dependent plasticity of synaptic inhibition in feeding circuits. Neuron, 71, 529–541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- D’Amour JA & Froemke RC (2015) Inhibitory and excitatory spike-timing-dependent plasticity in the auditory cortex. Neuron, 86, 514–528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dacher M & Nugent FS (2011) Morphine-induced modulation of LTD at GABAergic synapses in the ventral tegmental area. Neuropharmacology, 61, 1166–1171. [DOI] [PubMed] [Google Scholar]
- Das S, Sadanandappa MK, Dervan A, Larkin A, Lee JA, Sudhakaran IP, Priya R, Heidari R, Holohan EE, Pimentel A, Gandhi A, Ito K, Sanyal S, Wang JW, Rodrigues V & Ramaswami M (2011) Plasticity of local GABAergic interneurons drives olfactory habituation. Proc Natl Acad Sci U S A, 108, E646–654. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Almeida L, Idiart M & Lisman JE (2009) A second function of gamma frequency oscillations: an E%-max winner-take-all mechanism selects which cells fire. J Neurosci, 29, 7497–7503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DeFelipe J, Lopez-Cruz PL, Benavides-Piccione R, Bielza C, Larranaga P, Anderson S, Burkhalter A, Cauli B, Fairen A, Feldmeyer D, Fishell G, Fitzpatrick D, Freund TF, Gonzalez-Burgos G, Hestrin S, Hill S, Hof PR, Huang J, Jones EG, Kawaguchi Y, Kisvarday Z, Kubota Y, Lewis DA, Marin O, Markram H, McBain CJ, Meyer HS, Monyer H, Nelson SB, Rockland K, Rossier J, Rubenstein JL, Rudy B, Scanziani M, Shepherd GM, Sherwood CC, Staiger JF, Tamas G, Thomson A, Wang Y, Yuste R & Ascoli GA (2013) New insights into the classification and nomenclature of cortical GABAergic interneurons. Nat Rev Neurosci, 14, 202–216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Di Lazzaro V, Rothwell J & Capogna M (2018) Noninvasive Stimulation of the Human Brain: Activation of Multiple Cortical Circuits. Neuroscientist, 24, 246–260. [DOI] [PubMed] [Google Scholar]
- Donato F, Rompani SB & Caroni P (2013) Parvalbumin-expressing basket-cell network plasticity induced by experience regulates adult learning. Nature, 504, 272–276. [DOI] [PubMed] [Google Scholar]
- Dorrn AL, Yuan K, Barker AJ, Schreiner CE & Froemke RC (2010) Developmental sensory experience balances cortical excitation and inhibition. Nature, 465, 932–936. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Drexel M, Romanov RA, Wood J, Weger S, Heilbronn R, Wulff P, Tasan RO, Harkany T & Sperk G (2017) Selective Silencing of Hippocampal Parvalbumin Interneurons Induces Development of Recurrent Spontaneous Limbic Seizures in Mice. J Neurosci, 37, 8166–8179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Edelmann E, Lessmann V & Brigadski T (2014) Pre- and postsynaptic twists in BDNF secretion and action in synaptic plasticity. Neuropharmacology, 76 Pt C, 610–627. [DOI] [PubMed] [Google Scholar]
- Eggermann E & Jonas P (2011) How the ‘slow’ Ca(2+) buffer parvalbumin affects transmitter release in nanodomain-coupling regimes. Nat Neurosci, 15, 20–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Farrant M & Nusser Z (2005) Variations on an inhibitory theme: phasic and tonic activation of GABA(A) receptors. Nat Rev Neurosci, 6, 215–229. [DOI] [PubMed] [Google Scholar]
- Fino E, Packer AM & Yuste R (2013) The logic of inhibitory connectivity in the neocortex. Neuroscientist, 19, 228–237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fino E & Yuste R (2011) Dense inhibitory connectivity in neocortex. Neuron, 69, 1188–1203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fishell G & Kepecs A (2019) Interneuron Types as Attractors and Controllers. Annu Rev Neurosci. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Foldy C, Lee SH, Morgan RJ & Soltesz I (2010) Regulation of fast-spiking basket cell synapses by the chloride channel ClC-2. Nat Neurosci, 13, 1047–1049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Francavilla R, Villette V, Luo X, Chamberland S, Munoz-Pino E, Camire O, Wagner K, Kis V, Somogyi P & Topolnik L (2018) Connectivity and network state-dependent recruitment of long-range VIP-GABAergic neurons in the mouse hippocampus. Nat Commun, 9, 5043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Freund TF, Katona I & Piomelli D (2003) Role of endogenous cannabinoids in synaptic signaling. Physiol Rev, 83, 1017–1066. [DOI] [PubMed] [Google Scholar]
- Froemke RC (2015) Plasticity of cortical excitatory-inhibitory balance. Annu Rev Neurosci, 38, 195–219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Froemke RC, Merzenich MM & Schreiner CE (2007) A synaptic memory trace for cortical receptive field plasticity. Nature, 450, 425–429. [DOI] [PubMed] [Google Scholar]
- Fuentealba P, Begum R, Capogna M, Jinno S, Marton LF, Csicsvari J, Thomson A, Somogyi P & Klausberger T (2008) Ivy cells: a population of nitric-oxide-producing, slow-spiking GABAergic neurons and their involvement in hippocampal network activity. Neuron, 57, 917–929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fuentealba P, Klausberger T, Karayannis T, Suen WY, Huck J, Tomioka R, Rockland K, Capogna M, Studer M, Morales M & Somogyi P (2010) Expression of COUP-TFII nuclear receptor in restricted GABAergic neuronal populations in the adult rat hippocampus. J Neurosci, 30, 1595–1609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gaiarsa JL, Caillard O & Ben-Ari Y (2002) Long-term plasticity at GABAergic and glycinergic synapses: mechanisms and functional significance. Trends Neurosci, 25, 564–570. [DOI] [PubMed] [Google Scholar]
- Gainey MA, Aman JW & Feldman DE (2018) Rapid Disinhibition by Adjustment of PV Intrinsic Excitability during Whisker Map Plasticity in Mouse S1. J Neurosci, 38, 4749–4761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gainey MA, Wolfe R, Pourzia O & Feldman DE (2016) Whisker Deprivation Drives Two Phases of Inhibitory Synapse Weakening in Layer 4 of Rat Somatosensory Cortex. PLoS One, 11, e0148227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Geppert M, Goda Y, Hammer RE, Li C, Rosahl TW, Stevens CF & Sudhof TC (1994) Synaptotagmin I: a major Ca2+ sensor for transmitter release at a central synapse. Cell, 79, 717–727. [DOI] [PubMed] [Google Scholar]
- Ghosh S, Reuveni I, Lamprecht R & Barkai E (2015) Persistent CaMKII activation mediates learning-induced long-lasting enhancement of synaptic inhibition. J Neurosci, 35, 128–139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glazewski S, Greenhill S & Fox K (2017) Time-course and mechanisms of homeostatic plasticity in layers 2/3 and 5 of the barrel cortex. Philos Trans R Soc Lond B Biol Sci, 372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Graupner M & Reyes AD (2013) Synaptic input correlations leading to membrane potential decorrelation of spontaneous activity in cortex. J Neurosci, 33, 15075–15085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gubellini P, Ben-Ari Y & Gaiarsa JL (2005) Endogenous neurotrophins are required for the induction of GABAergic long-term potentiation in the neonatal rat hippocampus. J Neurosci, 25, 5796–5802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gulyas AI, Megias M, Emri Z & Freund TF (1999) Total number and ratio of excitatory and inhibitory synapses converging onto single interneurons of different types in the CA1 area of the rat hippocampus. J Neurosci, 19, 10082–10097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gupta A, Wang Y & Markram H (2000) Organizing principles for a diversity of GABAergic interneurons and synapses in the neocortex. Science, 287, 273–278. [DOI] [PubMed] [Google Scholar]
- Hayashi A, Yoshida T & Ohki K (2018) Cell Type Specific Representation of Vibro-tactile Stimuli in the Mouse Primary Somatosensory Cortex. Front Neural Circuits, 12, 109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hefft S & Jonas P (2005) Asynchronous GABA release generates long-lasting inhibition at a hippocampal interneuron-principal neuron synapse. Nat Neurosci, 8, 1319–1328. [DOI] [PubMed] [Google Scholar]
- Heifets BD & Castillo PE (2009) Endocannabinoid signaling and long-term synaptic plasticity. Annu Rev Physiol, 71, 283–306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heifets BD, Chevaleyre V & Castillo PE (2008) Interneuron activity controls endocannabinoid-mediated presynaptic plasticity through calcineurin. Proc Natl Acad Sci U S A, 105, 10250–10255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hengen KB, Lambo ME, Van Hooser SD, Katz DB & Turrigiano GG (2013) Firing rate homeostasis in visual cortex of freely behaving rodents. Neuron, 80, 335–342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hennequin G, Agnes EJ & Vogels TP (2017) Inhibitory Plasticity: Balance, Control, and Codependence. Annu Rev Neurosci, 40, 557–579. [DOI] [PubMed] [Google Scholar]
- Hensch TK (2005a) Critical period mechanisms in developing visual cortex. Curr Top Dev Biol, 69, 215–237. [DOI] [PubMed] [Google Scholar]
- Hensch TK (2005b) Critical period plasticity in local cortical circuits. Nat Rev Neurosci, 6, 877–888. [DOI] [PubMed] [Google Scholar]
- Herring BE & Nicoll RA (2016) Long-Term Potentiation: From CaMKII to AMPA Receptor Trafficking. Annu Rev Physiol, 78, 351–365. [DOI] [PubMed] [Google Scholar]
- Hertag L & Sprekeler H (2019) Amplifying the redistribution of somato-dendritic inhibition by the interplay of three interneuron types. PLoS Comput Biol, 15, e1006999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holmgren CD & Zilberter Y (2001) Coincident spiking activity induces long-term changes in inhibition of neocortical pyramidal cells. J Neurosci, 21, 8270–8277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hong EJ, McCord AE & Greenberg ME (2008) A biological function for the neuronal activity-dependent component of Bdnf transcription in the development of cortical inhibition. Neuron, 60, 610–624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Horn ME & Nicoll RA (2018) Somatostatin and parvalbumin inhibitory synapses onto hippocampal pyramidal neurons are regulated by distinct mechanisms. Proc Natl Acad Sci U S A, 115, 589–594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- House DR, Elstrott J, Koh E, Chung J & Feldman DE (2011) Parallel regulation of feedforward inhibition and excitation during whisker map plasticity. Neuron, 72, 819–831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hu H & Jonas P (2014) A supercritical density of Na(+) channels ensures fast signaling in GABAergic interneuron axons. Nat Neurosci, 17, 686–693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hu H, Martina M & Jonas P (2010) Dendritic mechanisms underlying rapid synaptic activation of fast-spiking hippocampal interneurons. Science, 327, 52–58. [DOI] [PubMed] [Google Scholar]
- Huang C, Ruff DA, Pyle R, Rosenbaum R, Cohen MR & Doiron B (2019) Circuit Models of Low-Dimensional Shared Variability in Cortical Networks. Neuron, 101, 337–348 e334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang ZJ & Paul A (2019) The diversity of GABAergic neurons and neural communication elements. Nat Rev Neurosci, 20, 563–572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Inagaki T, Begum T, Reza F, Horibe S, Inaba M, Yoshimura Y & Komatsu Y (2008) Brain-derived neurotrophic factor-mediated retrograde signaling required for the induction of long-term potentiation at inhibitory synapses of visual cortical pyramidal neurons. Neurosci Res, 61, 192–200. [DOI] [PubMed] [Google Scholar]
- Iremonger KJ, Wamsteeker Cusulin JI & Bains JS (2013) Changing the tune: plasticity and adaptation of retrograde signals. Trends Neurosci, 36, 471–479. [DOI] [PubMed] [Google Scholar]
- Jiang B, Huang S, de Pasquale R, Millman D, Song L, Lee HK, Tsumoto T & Kirkwood A (2010) The maturation of GABAergic transmission in visual cortex requires endocannabinoid-mediated LTD of inhibitory inputs during a critical period. Neuron, 66, 248–259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jiao Y, Zhang Z, Zhang C, Wang X, Sakata K, Lu B & Sun QQ (2011) A key mechanism underlying sensory experience-dependent maturation of neocortical GABAergic circuits in vivo. Proc Natl Acad Sci U S A, 108, 12131–12136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kanashiro T, Ocker GK, Cohen MR & Doiron B (2017) Attentional modulation of neuronal variability in circuit models of cortex. Elife, 6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kano M (1995) Plasticity of inhibitory synapses in the brain: a possible memory mechanism that has been overlooked. Neurosci Res, 21, 177–182. [DOI] [PubMed] [Google Scholar]
- Kano M, Ohno-Shosaku T, Hashimotodani Y, Uchigashima M & Watanabe M (2009) Endocannabinoid-mediated control of synaptic transmission. Physiol Rev, 89, 309–380. [DOI] [PubMed] [Google Scholar]
- Karayannis T, Elfant D, Huerta-Ocampo I, Teki S, Scott RS, Rusakov DA, Jones MV & Capogna M (2010) Slow GABA transient and receptor desensitization shape synaptic responses evoked by hippocampal neurogliaform cells. J Neurosci, 30, 9898–9909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Katagiri H, Fagiolini M & Hensch TK (2007) Optimization of somatic inhibition at critical period onset in mouse visual cortex. Neuron, 53, 805–812. [DOI] [PubMed] [Google Scholar]
- Kawaguchi Y & Kubota Y (1997) GABAergic cell subtypes and their synaptic connections in rat frontal cortex. Cereb Cortex, 7, 476–486. [DOI] [PubMed] [Google Scholar]
- Kayser AS & Miller KD (2002) Opponent inhibition: a developmental model of layer 4 of the neocortical circuit. Neuron, 33, 131–142. [DOI] [PubMed] [Google Scholar]
- Keller CH, Kaylegian K & Wehr M (2018) Gap encoding by parvalbumin-expressing interneurons in auditory cortex. J Neurophysiol, 120, 105–114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kepecs A & Fishell G (2014) Interneuron cell types are fit to function. Nature, 505, 318–326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kerr AM, Reisinger E & Jonas P (2008) Differential dependence of phasic transmitter release on synaptotagmin 1 at GABAergic and glutamatergic hippocampal synapses. Proc Natl Acad Sci U S A, 105, 15581–15586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kerti-Szigeti K & Nusser Z (2016) Similar GABAA receptor subunit composition in somatic and axon initial segment synapses of hippocampal pyramidal cells. Elife, 5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khan AG, Poort J, Chadwick A, Blot A, Sahani M, Mrsic-Flogel TD & Hofer SB (2018) Distinct learning-induced changes in stimulus selectivity and interactions of GABAergic interneuron classes in visual cortex. Nat Neurosci, 21, 851–859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kilman V, van Rossum MC & Turrigiano GG (2002) Activity deprivation reduces miniature IPSC amplitude by decreasing the number of postsynaptic GABA(A) receptors clustered at neocortical synapses. J Neurosci, 22, 1328–1337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klausberger T & Somogyi P (2008) Neuronal diversity and temporal dynamics: the unity of hippocampal circuit operations. Science, 321, 53–57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Komatsu Y & Iwakiri M (1993) Long-term modification of inhibitory synaptic transmission in developing visual cortex. Neuroreport, 4, 907–910. [DOI] [PubMed] [Google Scholar]
- Koolschijn RS, Emir UE, Pantelides AC, Nili H, Behrens TEJ & Barron HC (2019) The Hippocampus and Neocortical Inhibitory Engrams Protect against Memory Interference. Neuron, 101, 528–541 e526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kotak VC, Takesian AE, MacKenzie PC & Sanes DH (2013) Rescue of inhibitory synapse strength following developmental hearing loss. PLoS One, 8, e53438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuhlman SJ, Olivas ND, Tring E, Ikrar T, Xu X & Trachtenberg JT (2013) A disinhibitory microcircuit initiates critical-period plasticity in the visual cortex. Nature, 501, 543–546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kullmann DM, Moreau AW, Bakiri Y & Nicholson E (2012) Plasticity of inhibition. Neuron, 75, 951–962. [DOI] [PubMed] [Google Scholar]
- Kurotani T, Yamada K, Yoshimura Y, Crair MC & Komatsu Y (2008) State-dependent bidirectional modification of somatic inhibition in neocortical pyramidal cells. Neuron, 57, 905–916. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lambo ME & Turrigiano GG (2013) Synaptic and intrinsic homeostatic mechanisms cooperate to increase L2/3 pyramidal neuron excitability during a late phase of critical period plasticity. J Neurosci, 33, 8810–8819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee SH, Kwan AC, Zhang S, Phoumthipphavong V, Flannery JG, Masmanidis SC, Taniguchi H, Huang ZJ, Zhang F, Boyden ES, Deisseroth K & Dan Y (2012) Activation of specific interneurons improves V1 feature selectivity and visual perception. Nature, 488, 379–383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Letzkus JJ, Wolff SB, Meyer EM, Tovote P, Courtin J, Herry C & Luthi A (2011) A disinhibitory microcircuit for associative fear learning in the auditory cortex. Nature, 480, 331–335. [DOI] [PubMed] [Google Scholar]
- Leutgeb JK, Leutgeb S, Moser MB & Moser EI (2007) Pattern separation in the dentate gyrus and CA3 of the hippocampus. Science, 315, 961–966. [DOI] [PubMed] [Google Scholar]
- Li G, Stewart R, Canepari M & Capogna M (2014a) Firing of hippocampal neurogliaform cells induces suppression of synaptic inhibition. J Neurosci, 34, 1280–1292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li H, Liang F, Zhong W, Yan L, Mesik L, Xiao Z, Tao HW & Zhang LI (2019) Synaptic Mechanisms for Bandwidth Tuning in Awake Mouse Primary Auditory Cortex. Cereb Cortex, 29, 2998–3009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li L, Gainey MA, Goldbeck JE & Feldman DE (2014b) Rapid homeostasis by disinhibition during whisker map plasticity. Proc Natl Acad Sci U S A, 111, 1616–1621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li LY, Xiong XR, Ibrahim LA, Yuan W, Tao HW & Zhang LI (2015a) Differential Receptive Field Properties of Parvalbumin and Somatostatin Inhibitory Neurons in Mouse Auditory Cortex. Cereb Cortex, 25, 1782–1791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li YT, Liu BH, Chou XL, Zhang LI & Tao HW (2015b) Strengthening of Direction Selectivity by Broadly Tuned and Spatiotemporally Slightly Offset Inhibition in Mouse Visual Cortex. Cereb Cortex, 25, 2466–2477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu X, Grishanin RN, Tolwani RJ, Renteria RC, Xu B, Reichardt LF & Copenhagen DR (2007) Brain-derived neurotrophic factor and TrkB modulate visual experience-dependent refinement of neuronal pathways in retina. J Neurosci, 27, 7256–7267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lo CC, Wang CT & Wang XJ (2015) Speed-accuracy tradeoff by a control signal with balanced excitation and inhibition. J Neurophysiol, 114, 650–661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lourenco J, De Stasi AM, Deleuze C, Bigot M, Pazienti A, Aguirre A, Giugliano M, Ostojic S & Bacci A (2020) Modulation of Coordinated Activity across Cortical Layers by Plasticity of Inhibitory Synapses. Cell Rep, 30, 630–641 e635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lourenco J, Pacioni S, Rebola N, van Woerden GM, Marinelli S, DiGregorio D & Bacci A (2014) Non-associative potentiation of perisomatic inhibition alters the temporal coding of neocortical layer 5 pyramidal neurons. PLoS Biol, 12, e1001903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lu B, Nagappan G & Lu Y (2014) BDNF and synaptic plasticity, cognitive function, and dysfunction. Handb Exp Pharmacol, 220, 223–250. [DOI] [PubMed] [Google Scholar]
- Lu YM, Mansuy IM, Kandel ER & Roder J (2000) Calcineurin-mediated LTD of GABAergic inhibition underlies the increased excitability of CA1 neurons associated with LTP. Neuron, 26, 197–205. [DOI] [PubMed] [Google Scholar]
- Lucas EK, Jegarl AM, Morishita H & Clem RL (2016) Multimodal and Site-Specific Plasticity of Amygdala Parvalbumin Interneurons after Fear Learning. Neuron, 91, 629–643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luscher B, Fuchs T & Kilpatrick CL (2011) GABAA receptor trafficking-mediated plasticity of inhibitory synapses. Neuron, 70, 385–409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luscher C, Nicoll RA, Malenka RC & Muller D (2000) Synaptic plasticity and dynamic modulation of the postsynaptic membrane. Nat Neurosci, 3, 545–550. [DOI] [PubMed] [Google Scholar]
- Maffei A (2011) The many forms and functions of long term plasticity at GABAergic synapses. Neural Plast, 2011, 254724. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maffei A (2017) Fifty shades of inhibition. Curr Opin Neurobiol, 43, 43–47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maffei A, Charrier C, Caiati MD, Barberis A, Mahadevan V, Woodin MA & Tyagarajan SK (2017) Emerging Mechanisms Underlying Dynamics of GABAergic Synapses. J Neurosci, 37, 10792–10799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maffei A, Nataraj K, Nelson SB & Turrigiano GG (2006) Potentiation of cortical inhibition by visual deprivation. Nature, 443, 81–84. [DOI] [PubMed] [Google Scholar]
- Maffei A, Nelson SB & Turrigiano GG (2004) Selective reconfiguration of layer 4 visual cortical circuitry by visual deprivation. Nat Neurosci, 7, 1353–1359. [DOI] [PubMed] [Google Scholar]
- Makara JK, Katona I, Nyiri G, Nemeth B, Ledent C, Watanabe M, de Vente J, Freund TF & Hajos N (2007) Involvement of nitric oxide in depolarization-induced suppression of inhibition in hippocampal pyramidal cells during activation of cholinergic receptors. J Neurosci, 27, 10211–10222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marsicano G, Wotjak CT, Azad SC, Bisogno T, Rammes G, Cascio MG, Hermann H, Tang J, Hofmann C, Zieglgansberger W, Di Marzo V & Lutz B (2002) The endogenous cannabinoid system controls extinction of aversive memories. Nature, 418, 530–534. [DOI] [PubMed] [Google Scholar]
- Martenson JS, Yamasaki T, Chaudhury NH, Albrecht D & Tomita S (2017) Assembly rules for GABAA receptor complexes in the brain. Elife, 6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marty S, Wehrle R & Sotelo C (2000) Neuronal activity and brain-derived neurotrophic factor regulate the density of inhibitory synapses in organotypic slice cultures of postnatal hippocampus. J Neurosci, 20, 8087–8095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maynard SA & Triller A (2019) Inhibitory Receptor Diffusion Dynamics. Front Mol Neurosci, 12, 313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mejias JF & Longtin A (2014) Differential effects of excitatory and inhibitory heterogeneity on the gain and asynchronous state of sparse cortical networks. Front Comput Neurosci, 8, 107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mejias JF, Payeur A, Selin E, Maler L & Longtin A (2014) Subtractive, divisive and non-monotonic gain control in feedforward nets linearized by noise and delays. Front Comput Neurosci, 8, 19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miles R (1990) Synaptic excitation of inhibitory cells by single CA3 hippocampal pyramidal cells of the guinea-pig in vitro. J Physiol, 428, 61–77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller KD (2003) Understanding layer 4 of the cortical circuit: a model based on cat V1. Cereb Cortex, 13, 73–82. [DOI] [PubMed] [Google Scholar]
- Miska NJ, Richter LM, Cary BA, Gjorgjieva J & Turrigiano GG (2018) Sensory experience inversely regulates feedforward and feedback excitation-inhibition ratio in rodent visual cortex. Elife, 7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Monday HR, Younts TJ & Castillo PE (2018) Long-Term Plasticity of Neurotransmitter Release: Emerging Mechanisms and Contributions to Brain Function and Disease. Annu Rev Neurosci, 41, 299–322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mongillo G, Rumpel S & Loewenstein Y (2017) Intrinsic volatility of synaptic connections - a challenge to the synaptic trace theory of memory. Curr Opin Neurobiol, 46, 7–13. [DOI] [PubMed] [Google Scholar]
- Mongillo G, Rumpel S & Loewenstein Y (2018) Inhibitory connectivity defines the realm of excitatory plasticity. Nat Neurosci, 21, 1463–1470. [DOI] [PubMed] [Google Scholar]
- Moore AK & Wehr M (2013) Parvalbumin-expressing inhibitory interneurons in auditory cortex are well- tuned for frequency. J Neurosci, 33, 13713–13723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nelson SB & Turrigiano GG (2008) Strength through diversity. Neuron, 60, 477–482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nugent FS, Penick EC & Kauer JA (2007) Opioids block long-term potentiation of inhibitory synapses. Nature, 446, 1086–1090. [DOI] [PubMed] [Google Scholar]
- Olah S, Fule M, Komlosi G, Varga C, Baldi R, Barzo P & Tamas G (2009) Regulation of cortical microcircuits by unitary GABA-mediated volume transmission. Nature, 461, 1278–1281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ouardouz M & Sastry BR (2000) Mechanisms underlying LTP of inhibitory synaptic transmission in the deep cerebellar nuclei. J Neurophysiol, 84, 1414–1421. [DOI] [PubMed] [Google Scholar]
- Pala A & Petersen CC (2018) State-dependent cell-type-specific membrane potential dynamics and unitary synaptic inputs in awake mice. Elife, 7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Panthi S & Leitch B (2019) The impact of silencing feed-forward parvalbumin-expressing inhibitory interneurons in the cortico-thalamocortical network on seizure generation and behaviour. Neurobiol Dis, 132, 104610. [DOI] [PubMed] [Google Scholar]
- Paul A, Crow M, Raudales R, He M, Gillis J & Huang ZJ (2017) Transcriptional Architecture of Synaptic Communication Delineates GABAergic Neuron Identity. Cell, 171, 522–539 e520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pelkey KA, Chittajallu R, Craig MT, Tricoire L, Wester JC & McBain CJ (2017) Hippocampal GABAergic Inhibitory Interneurons. Physiol Rev, 97, 1619–1747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pennacchietti F, Vascon S, Nieus T, Rosillo C, Das S, Tyagarajan SK, Diaspro A, Del Bue A, Petrini EM, Barberis A & Cella Zanacchi F (2017) Nanoscale Molecular Reorganization of the Inhibitory Postsynaptic Density Is a Determinant of GABAergic Synaptic Potentiation. J Neurosci, 37, 1747–1756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perez-Garci E, Gassmann M, Bettler B & Larkum ME (2006) The GABAB1b isoform mediates long-lasting inhibition of dendritic Ca2+ spikes in layer 5 somatosensory pyramidal neurons. Neuron, 50, 603–616. [DOI] [PubMed] [Google Scholar]
- Pernia-Andrade AJ & Jonas P (2014) Theta-gamma-modulated synaptic currents in hippocampal granule cells in vivo define a mechanism for network oscillations. Neuron, 81, 140–152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petilla Interneuron Nomenclature G, Ascoli GA, Alonso-Nanclares L, Anderson SA, Barrionuevo G, Benavides-Piccione R, Burkhalter A, Buzsaki G, Cauli B, Defelipe J, Fairen A, Feldmeyer D, Fishell G, Fregnac Y, Freund TF, Gardner D, Gardner EP, Goldberg JH, Helmstaedter M, Hestrin S, Karube F, Kisvarday ZF, Lambolez B, Lewis DA, Marin O, Markram H, Munoz A, Packer A, Petersen CC, Rockland KS, Rossier J, Rudy B, Somogyi P, Staiger JF, Tamas G, Thomson AM, Toledo-Rodriguez M, Wang Y, West DC & Yuste R (2008) Petilla terminology: nomenclature of features of GABAergic interneurons of the cerebral cortex. Nat Rev Neurosci, 9, 557–568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petrini EM, Ravasenga T, Hausrat TJ, Iurilli G, Olcese U, Racine V, Sibarita JB, Jacob TC, Moss SJ, Benfenati F, Medini P, Kneussel M & Barberis A (2014) Synaptic recruitment of gephyrin regulates surface GABAA receptor dynamics for the expression of inhibitory LTP. Nat Commun, 5, 3921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pfeffer CK, Xue M, He M, Huang ZJ & Scanziani M (2013) Inhibition of inhibition in visual cortex: the logic of connections between molecularly distinct interneurons. Nat Neurosci, 16, 1068–1076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Piskorowski RA & Chevaleyre V (2013) Delta-opioid receptors mediate unique plasticity onto parvalbumin-expressing interneurons in area CA2 of the hippocampus. J Neurosci, 33, 14567–14578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pizzarelli R, Griguoli M, Zacchi P, Petrini EM, Barberis A, Cattaneo A & Cherubini E (2019) Tuning GABAergic Inhibition: Gephyrin Molecular Organization and Functions. Neuroscience. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pouille F, Marin-Burgin A, Adesnik H, Atallah BV & Scanziani M (2009) Input normalization by global feedforward inhibition expands cortical dynamic range. Nat Neurosci, 12, 1577–1585. [DOI] [PubMed] [Google Scholar]
- Pouille F & Scanziani M (2001) Enforcement of temporal fidelity in pyramidal cells by somatic feed-forward inhibition. Science, 293, 1159–1163. [DOI] [PubMed] [Google Scholar]
- Pouille F, Watkinson O, Scanziani M & Trevelyan AJ (2013) The contribution of synaptic location to inhibitory gain control in pyramidal cells. Physiol Rep, 1, e00067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Poulin JF, Tasic B, Hjerling-Leffler J, Trimarchi JM & Awatramani R (2016) Disentangling neural cell diversity using single-cell transcriptomics. Nat Neurosci, 19, 1131–1141. [DOI] [PubMed] [Google Scholar]
- Price CJ, Cauli B, Kovacs ER, Kulik A, Lambolez B, Shigemoto R & Capogna M (2005) Neurogliaform neurons form a novel inhibitory network in the hippocampal CA1 area. J Neurosci, 25, 6775–6786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Price CJ, Scott R, Rusakov DA & Capogna M (2008) GABA(B) receptor modulation of feedforward inhibition through hippocampal neurogliaform cells. J Neurosci, 28, 6974–6982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Que L, Winterer J & Foldy C (2019) Deep Survey of GABAergic Interneurons: Emerging Insights From Gene-Isoform Transcriptomics. Front Mol Neurosci, 12, 115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rashid AJ, Yan C, Mercaldo V, Hsiang HL, Park S, Cole CJ, De Cristofaro A, Yu J, Ramakrishnan C, Lee SY, Deisseroth K, Frankland PW & Josselyn SA (2016) Competition between engrams influences fear memory formation and recall. Science, 353, 383–387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Regehr WG, Carey MR & Best AR (2009) Activity-dependent regulation of synapses by retrograde messengers. Neuron, 63, 154–170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Renart A, Song P & Wang XJ (2003) Robust spatial working memory through homeostatic synaptic scaling in heterogeneous cortical networks. Neuron, 38, 473–485. [DOI] [PubMed] [Google Scholar]
- Royer S, Zemelman BV, Losonczy A, Kim J, Chance F, Magee JC & Buzsaki G (2012) Control of timing, rate and bursts of hippocampal place cells by dendritic and somatic inhibition. Nat Neurosci, 15, 769–775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rubin R, Abbott LF & Sompolinsky H (2017) Balanced excitation and inhibition are required for high-capacity, noise-robust neuronal selectivity. Proc Natl Acad Sci U S A, 114, E9366–E9375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ryan TJ, Roy DS, Pignatelli M, Arons A & Tonegawa S (2015) Memory. Engram cells retain memory under retrograde amnesia. Science, 348, 1007–1013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanes DH & Kotak VC (2011) Developmental plasticity of auditory cortical inhibitory synapses. Hear Res, 279, 140–148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saraga F, Balena T, Wolansky T, Dickson CT & Woodin MA (2008) Inhibitory synaptic plasticity regulates pyramidal neuron spiking in the rodent hippocampus. Neuroscience, 155, 64–75. [DOI] [PubMed] [Google Scholar]
- Sarihi A, Mirnajafi-Zadeh J, Jiang B, Sohya K, Safari MS, Arami MK, Yanagawa Y & Tsumoto T (2012) Cell type-specific, presynaptic LTP of inhibitory synapses on fast-spiking GABAergic neurons in the mouse visual cortex. J Neurosci, 32, 13189–13199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schulz JM, Knoflach F, Hernandez MC & Bischofberger J (2018) Dendrite-targeting interneurons control synaptic NMDA-receptor activation via nonlinear alpha5-GABAA receptors. Nat Commun, 9, 3576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seybold BA, Phillips EAK, Schreiner CE & Hasenstaub AR (2015) Inhibitory Actions Unified by Network Integration. Neuron, 87, 1181–1192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sheffield ME, Best TK, Mensh BD, Kath WL & Spruston N (2011) Slow integration leads to persistent action potential firing in distal axons of coupled interneurons. Nat Neurosci, 14, 200–207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shekhar K, Lapan SW, Whitney IE, Tran NM, Macosko EZ, Kowalczyk M, Adiconis X, Levin JZ, Nemesh J, Goldman M, McCarroll SA, Cepko CL, Regev A & Sanes JR (2016) Comprehensive Classification of Retinal Bipolar Neurons by Single-Cell Transcriptomics. Cell, 166, 1308–1323 e1330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sik A, Penttonen M, Ylinen A & Buzsaki G (1995) Hippocampal CA1 interneurons: an in vivo intracellular labeling study. J Neurosci, 15, 6651–6665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Silver RA (2010) Neuronal arithmetic. Nat Rev Neurosci, 11, 474–489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sivakumaran S, Mohajerani MH & Cherubini E (2009) At immature mossy-fiber-CA3 synapses, correlated presynaptic and postsynaptic activity persistently enhances GABA release and network excitability via BDNF and cAMP-dependent PKA. J Neurosci, 29, 2637–2647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Somogyi P, Nunzi MG, Gorio A & Smith AD (1983) A new type of specific interneuron in the monkey hippocampus forming synapses exclusively with the axon initial segments of pyramidal cells. Brain Res, 259, 137–142. [DOI] [PubMed] [Google Scholar]
- Sprekeler H (2017) Functional consequences of inhibitory plasticity: homeostasis, the excitation-inhibition balance and beyond. Curr Opin Neurobiol, 43, 198–203. [DOI] [PubMed] [Google Scholar]
- Sun W, Wang L, Li S, Tie X & Jiang B (2015) Layer-specific endocannabinoid-mediated long-term depression of GABAergic neurotransmission onto principal neurons in mouse visual cortex. Eur J Neurosci, 42, 1952–1965. [DOI] [PubMed] [Google Scholar]
- Suzuki N, Tang CS & Bekkers JM (2014) Persistent barrage firing in cortical interneurons can be induced in vivo and may be important for the suppression of epileptiform activity. Front Cell Neurosci, 8, 76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Szabadics J, Varga C, Brunner J, Chen K & Soltesz I (2010) Granule cells in the CA3 area. J Neurosci, 30, 8296–8307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takesian AE, Kotak VC & Sanes DH (2012) Age-dependent effect of hearing loss on cortical inhibitory synapse function. J Neurophysiol, 107, 937–947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takeuchi T, Duszkiewicz AJ & Morris RG (2014) The synaptic plasticity and memory hypothesis: encoding, storage and persistence. Philos Trans R Soc Lond B Biol Sci, 369, 20130288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tamas G, Buhl EH & Somogyi P (1997) Fast IPSPs elicited via multiple synaptic release sites by different types of GABAergic neurone in the cat visual cortex. J Physiol, 500 (Pt 3), 715–738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tamas G, Lorincz A, Simon A & Szabadics J (2003) Identified sources and targets of slow inhibition in the neocortex. Science, 299, 1902–1905. [DOI] [PubMed] [Google Scholar]
- Tatti R, Swanson OK, Lee MSE & Maffei A (2017) Layer-specific Developmental Changes in Excitation and Inhibition in Rat Primary Visual Cortex. eNeuro, 4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomson AM, West DC, Hahn J & Deuchars J (1996) Single axon IPSPs elicited in pyramidal cells by three classes of interneurones in slices of rat neocortex. J Physiol, 496 (Pt 1), 81–102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tomita S (2019) Molecular constituents and localization of the ionotropic GABA receptor complex in vivo. Curr Opin Neurobiol, 57, 81–86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tonegawa S, Pignatelli M, Roy DS & Ryan TJ (2015) Memory engram storage and retrieval. Curr Opin Neurobiol, 35, 101–109. [DOI] [PubMed] [Google Scholar]
- Toyoizumi T, Miyamoto H, Yazaki-Sugiyama Y, Atapour N, Hensch TK & Miller KD (2013) A theory of the transition to critical period plasticity: inhibition selectively suppresses spontaneous activity. Neuron, 80, 51–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tremblay R, Lee S & Rudy B (2016) GABAergic Interneurons in the Neocortex: From Cellular Properties to Circuits. Neuron, 91, 260–292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Troyer TW, Krukowski AE, Priebe NJ & Miller KD (1998) Contrast-invariant orientation tuning in cat visual cortex: thalamocortical input tuning and correlation-based intracortical connectivity. J Neurosci, 18, 5908–5927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tukker JJ, Lasztoczi B, Katona L, Roberts JD, Pissadaki EK, Dalezios Y, Marton L, Zhang L, Klausberger T & Somogyi P (2013) Distinct dendritic arborization and in vivo firing patterns of parvalbumin-expressing basket cells in the hippocampal area CA3. J Neurosci, 33, 6809–6825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Turrigiano GG, Leslie KR, Desai NS, Rutherford LC & Nelson SB (1998) Activity-dependent scaling of quantal amplitude in neocortical neurons. Nature, 391, 892–896. [DOI] [PubMed] [Google Scholar]
- Turrigiano GG & Nelson SB (2000) Hebb and homeostasis in neuronal plasticity. Curr Opin Neurobiol, 10, 358–364. [DOI] [PubMed] [Google Scholar]
- Tyagarajan SK, Ghosh H, Yevenes GE, Nikonenko I, Ebeling C, Schwerdel C, Sidler C, Zeilhofer HU, Gerrits B, Muller D & Fritschy JM (2011) Regulation of GABAergic synapse formation and plasticity by GSK3beta-dependent phosphorylation of gephyrin. Proc Natl Acad Sci U S A, 108, 379–384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Uezu A, Kanak DJ, Bradshaw TW, Soderblom EJ, Catavero CM, Burette AC, Weinberg RJ & Soderling SH (2016) Identification of an elaborate complex mediating postsynaptic inhibition. Science, 353, 1123–1129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Vreeswijk C & Sompolinsky H (1996) Chaos in neuronal networks with balanced excitatory and inhibitory activity. Science, 274, 1724–1726. [DOI] [PubMed] [Google Scholar]
- Vereczki VK, Veres JM, Muller K, Nagy GA, Racz B, Barsy B & Hajos N (2016) Synaptic Organization of Perisomatic GABAergic Inputs onto the Principal Cells of the Mouse Basolateral Amygdala. Front Neuroanat, 10, 20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vickers ED, Clark C, Osypenko D, Fratzl A, Kochubey O, Bettler B & Schneggenburger R (2018) Parvalbumin-Interneuron Output Synapses Show Spike-Timing-Dependent Plasticity that Contributes to Auditory Map Remodeling. Neuron, 99, 720–735 e726. [DOI] [PubMed] [Google Scholar]
- Vida I, Halasy K, Szinyei C, Somogyi P & Buhl EH (1998) Unitary IPSPs evoked by interneurons at the stratum radiatum-stratum lacunosum-moleculare border in the CA1 area of the rat hippocampus in vitro. J Physiol, 506 (Pt 3), 755–773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vierling-Claassen D, Cardin JA, Moore CI & Jones SR (2010) Computational modeling of distinct neocortical oscillations driven by cell-type selective optogenetic drive: separable resonant circuits controlled by low-threshold spiking and fast-spiking interneurons. Front Hum Neurosci, 4, 198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vithlani M, Terunuma M & Moss SJ (2011) The dynamic modulation of GABA(A) receptor trafficking and its role in regulating the plasticity of inhibitory synapses. Physiol Rev, 91, 1009–1022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vogels TP & Abbott LF (2009) Gating multiple signals through detailed balance of excitation and inhibition in spiking networks. Nat Neurosci, 12, 483–491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vogels TP, Froemke RC, Doyon N, Gilson M, Haas JS, Liu R, Maffei A, Miller P, Wierenga CJ, Woodin MA, Zenke F & Sprekeler H (2013) Inhibitory synaptic plasticity: spike timing-dependence and putative network function. Front Neural Circuits, 7, 119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vogels TP, Sprekeler H, Zenke F, Clopath C & Gerstner W (2011) Inhibitory plasticity balances excitation and inhibition in sensory pathways and memory networks. Science, 334, 1569–1573. [DOI] [PubMed] [Google Scholar]
- Wang L & Maffei A (2014) Inhibitory plasticity dictates the sign of plasticity at excitatory synapses. J Neurosci, 34, 1083–1093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Y, Toledo-Rodriguez M, Gupta A, Wu C, Silberberg G, Luo J & Markram H (2004) Anatomical, physiological and molecular properties of Martinotti cells in the somatosensory cortex of the juvenile rat. J Physiol, 561, 65–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Whitt JL, Petrus E & Lee HK (2014) Experience-dependent homeostatic synaptic plasticity in neocortex. Neuropharmacology, 78, 45–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilmes KA & Clopath C (2019) Inhibitory microcircuits for top-down plasticity of sensory representations. Nat Commun, 10, 5055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woodin MA, Ganguly K & Poo MM (2003) Coincident pre- and postsynaptic activity modifies GABAergic synapses by postsynaptic changes in Cl- transporter activity. Neuron, 39, 807–820. [DOI] [PubMed] [Google Scholar]
- Xu J, Antion MD, Nomura T, Kraniotis S, Zhu Y & Contractor A (2014) Hippocampal metaplasticity is required for the formation of temporal associative memories. J Neurosci, 34, 16762–16773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xue M, Atallah BV & Scanziani M (2014) Equalizing excitation-inhibition ratios across visual cortical neurons. Nature, 511, 596–600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang Y & Calakos N (2013) Presynaptic long-term plasticity. Front Synaptic Neurosci, 5, 8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Younts TJ & Castillo PE (2014) Endogenous cannabinoid signaling at inhibitory interneurons. Curr Opin Neurobiol, 26, 42–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Younts TJ, Chevaleyre V & Castillo PE (2013) CA1 pyramidal cell theta-burst firing triggers endocannabinoid-mediated long-term depression at both somatic and dendritic inhibitory synapses. J Neurosci, 33, 13743–13757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao L, Yeh ML & Levine ES (2015) Role for Endogenous BDNF in Endocannabinoid-Mediated Long-Term Depression at Neocortical Inhibitory Synapses. eNeuro, 2. [DOI] [PMC free article] [PubMed] [Google Scholar]


