SUMMARY
Slow wave sleep (SWS) in the northern fur seal (Callorhinus ursinus) is characterized by a highly expressed interhemispheric electroencephalogram (EEG) asymmetry, called ʻunihemisphericʼ or ʻasymmetricalʼ SWS. The aim of this study was to examine the regional differences in slow wave activity (SWA; power in the range of 1.2–4.0 Hz) within one hemisphere and differences in the degree of interhemispheric EEG asymmetry within this species. Three seals were implanted with 10 EEG electrodes, positioned bilaterally (five in each hemisphere) over the frontal, occipital and parietal cortex. The expression of interhemispheric SWA asymmetry between symmetrical monopolar recordings was estimated based on the asymmetry index [AI = (L)R) ⁄ (L+R), where L and R are the power in the left and right hemispheres, respectively]. Our findings indicate an anterior–posterior gradient in SWA during asymmetrical SWS in fur seals, which is opposite to that described for other mammals, including humans, with a larger SWA recorded in the parietal and occipital cortex. Interhemispheric EEG asymmetry in fur seals was recorded across the entire dorsal cerebral cortex, including sensory (visual and somatosensory), motor and associative (parietal or suprasylvian) cortical areas. The expression of asymmetry was greatest in occipital–lateral and parietal derivations and smallest in frontal–medial derivations. Regardless of regional differences in SWA, the majority (90%) of SWS episodes with interhemispheric EEG asymmetry meet the criteria for ʻunihemispheric SWSʼ (one hemisphere is asleep while the other is awake). The remaining episodes can be described as episodes of bilateral SWS with a local activation in one cerebral hemisphere.
Keywords: interhemispheric EEG asymmetry, local sleep, slow wave activity, unihemispheric sleep, northern fur seal
INTRODUCTION
Electroencephalogram (EEG) slow wave activity (SWA) is considered to be a measure of sleep pressure and intensity (Borbély, 1994). EEG power in the low-frequency range (<4 Hz) shows a frontal–medial predominance in human non-rapid eye movement (NREM) sleep [termed slow wave sleep (SWS) in animals], which is enhanced by sleep deprivation (Marzano et al., 2010; Werth et al., 1997). Frequency-specific EEG differences have been also described in rodents with a larger EEG power in the frontal derivations compared to the occipital derivations. This gradient is enhanced further during rebound following sleep deprivation (mouse: Huber et al., 2000; rat: Schwierin et al., 1999; Vyazovskiy et al., 2002). The anterior predominance of EEG slow waves during high sleep demands may reflect the increased vulnerability of frontal cortex functions to sleep loss (Horne, 1993).
SWS in dolphins and fur seals is characterized by a striking EEG asymmetry, called unihemispheric ʻSWSʼ (USWS) or ʻasymmetrical SWSʼ (Lyamin et al., 2008a,b,c; Mukhametov et al., 1977). Interhemispheric EEG asymmetry has also been recorded in some avian species (Fuchs et al., 2009; Rattenborg et al., 2000), but the expression of the asymmetry is smaller and the episodes are shorter than in marine mammals. To date, almost all EEG recordings in dolphins and seals have been performed using a bipolar technique, with both active electrodes positioned above the cortex (usually in frontal and occipital regions) and only occasionally employing a monopolar technique, with a reference electrode positioned in the nasal bones (Lyamin et al., 2008a,b,c; Mukhametov et al., 1997). The topographic aspect of EEG asymmetry has never been examined in detail in any marine mammal. The fur seal is a unique animal, showing all known types of sleep (bilateral and asymmetrical SWS as well as REM sleep) and with the degree of slow wave EEG asymmetry comparable to that of dolphins. Therefore, the aim of this study was to examine the regional differences (1) in SWA (power in the range of 1.2–4.0 Hz) within one cerebral hemisphere and (2) in the expression of SWA asymmetry in the fur seal.
MATERIALS AND METHODS
Subjects
Data were collected from three captive male fur seals (Callorhinus ursinus; 22–25 kg, aged 2–3 years) at the Utrish Marine Station of the Severtsov Institute of Ecology and Evolution (Black Sea, Russia). The study was approved by the Severtsov Institute Committee for Bioethics.
Design and procedure
Surgery and EEG electrode localization
Under general anaesthesia, fur seals were implanted with five pairs of stainless steel screws (1 mm in diameter) for EEG recording in symmetrical frontal, parietal and occipital cortical areas of the right and left hemispheres (Fig. 1). All EEG electrodes were epidural. A reference electrode was implanted into the nasal bone. The electrodes of the frontal group (a total of 12 electrodes for the three seals combined) were located in the vicinity of the motor and somatosensory cerebral cortex (Supin et al., 2001). Relative to S. cruciatus and S. postcruciatus, they could be divided into two subgroups (medial and lateral; labelled fm and fl, respectively, in Fig. 1). The occipital group of electrodes (10 electrodes for the three seals combined) was located above the visual cortex and could be divided into two subgroups: medial (four electrodes in seals 1 and 3, located 6–10 mm lateral to the midline) and lateral (six electrodes in all seals, located along the posterior section of S. lateralis; labelled om and ol, respectively, in Fig. 1). The parietal group of electrodes was located in the vicinity of S. suprasylvian in the area bordering the visual, auditory and somatosensory cortices (labelled p in Fig. 1). Surgical procedures and post-surgical animal care have been described in detail in our previous publications (e.g. Lyamin et al., 2008a,b).
Figure 1.
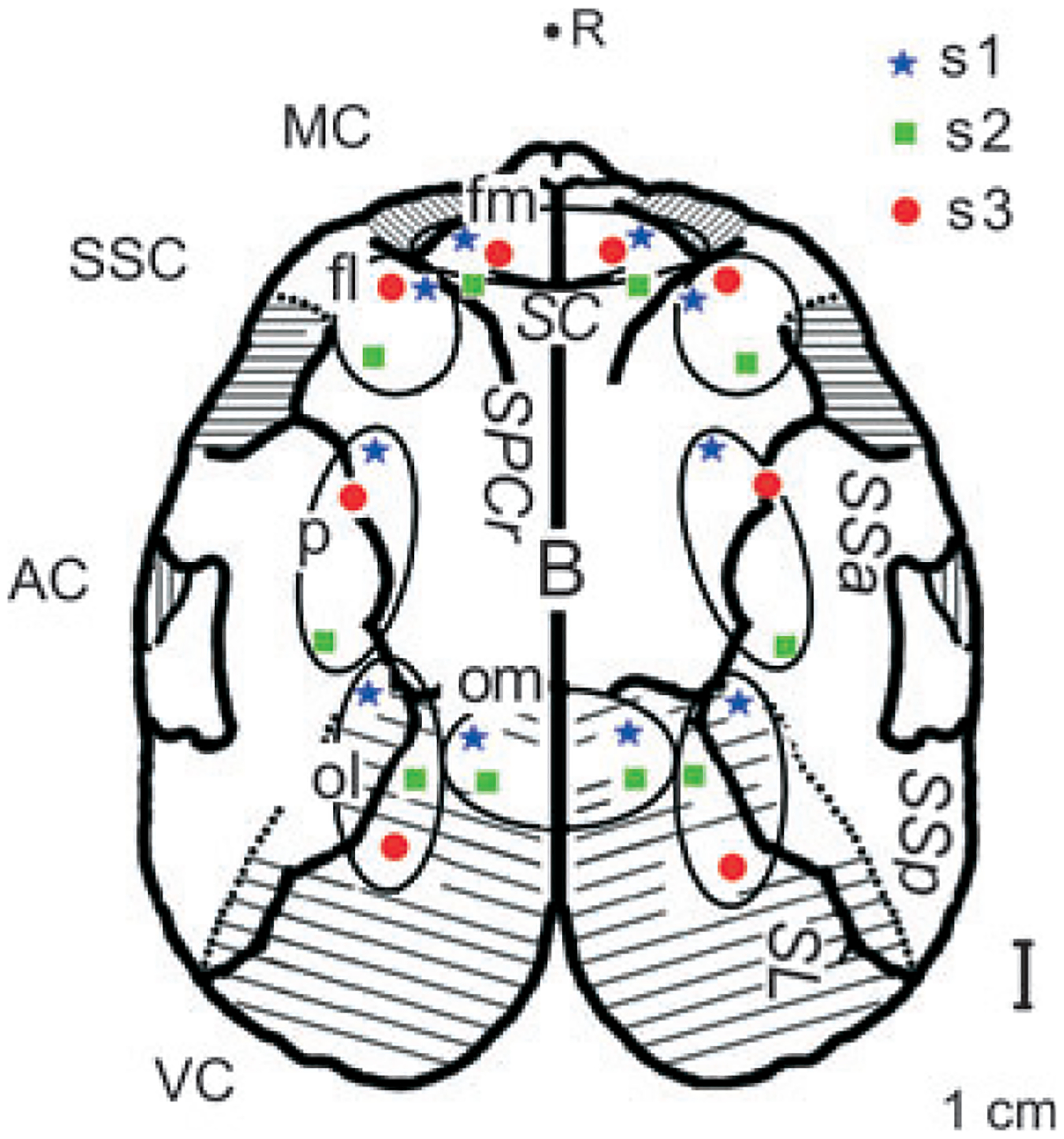
Localization of EEG electrodes relative to the first-order sulci (SL, s. lateralis; SSa, s. suprasylvian anterior; SSp, s. suprasylvian posterior; SPCr, s. postcruciatus, SC, s. cruciatus) and bregma (B) in fur seals (s1, s2 and s3) as indicated by color markers. VC, AC, SSC, MC – visual, auditory, somatosensory and motor cortex, respectively. Circled areas mark the frontal (fm and fl – frontal-medial and lateral, respectively), parietal (p) and occipital (om and ol – occipital-medial and lateral, respectively) groups of EEG electrodes (based on Supin et al. 2001). R – position of the reference electrodes relative to the seal brain.
Polygraphic recording
Recordings started at 17:00 and finished after 09:00 hours. The seals were connected to a recording cable and placed into a 0.8 × 0.8 × 1.0 m experimental chamber positioned in a sound-attenuated and light-controlled empty indoor pool. All animals were adapted to the experimental conditions. They were fed fish at 18:00 hours and sprayed with water for 15 min after feeding and then left undisturbed for the remainder of the night. Lights were turned off between 20:00 and 08:00 hours. During this period the seals were monitored via infrared cameras. The room temperature during recording was maintained at 18–20 °C.
EEG from all derivations was recorded relative to a reference electrode using a conventional cable technique and a multi-channel polygraph (Medicom 19 ⁄ 26, Taganrog, Russia; bandwidth 0.3–30.0 Hz) and stored on a hard disk (sampling rate 250 Hz). Neck electromyography (EMG) was also recorded simultaneously. The data were analysed offline using Spike 2 software (Cambridge Medical Design, UK).
Data analysis
For each seal the data collected during the third recording night were used for the analysis. EEG slow wave power (SWA) was computed in the frequency range of 1.2–4.0 Hz in 5-s epochs. In two seals (1 and 2) SWA was computed in five symmetrical derivations of the left and right hemispheres. In seal 3, due to contamination of the EEG with artefacts in the left occipital–medial derivation, SWA was computed in four symmetrical recordings. Epochs with artefacts were excluded from the analysis. SWA was averaged whenever at least three of the four consecutive 5-s epochs were artefact-free. The asymmetry index (AI) of SWA (a measure of the expression of interhemispheric EEG asymmetry) was evaluated in each monopolar derivation for each 20-s epoch, as follows: AI = (L)R) ⁄ (L+R), where L and R are standardized spectral power in the left and right hemispheres, respectively (described in Lyamin et al., 2008a,b).
During our previous studies we noted that SWA activity in fur seals in occipital–lateral derivations was usually greater than in frontal derivations. Therefore, we first scored SWS based on the EEG recorded from symmetrical occipital–lateral derivations. To allow between-derivation and between-animal comparisons, SWA in each 20-s epoch was standardized to the average SWA in the same derivation during high-voltage bilateral SWS (BSWS). Such an episode was selected as a 4–5-min period (or three periods in seal 2; Table 1) of SWS, with the greatest average SWA in both hemispheres and minimal SWA asymmetry (based on the AI). Each of these SWS episodes was followed by REM sleep. For comparison, in quiet wakefulness SWA varied between 4 and 14% of that in BSWS (on average 6–12% in different deviations and seals when calculated for 1-min episodes of uninterrupted quiet waking).
Table 1.
Characteristics of episodes of slow wave sleep (SWS) in the fur seal based on the electroencephalogram (EEG) recorded in occipital–lateral derivations
| Slow wave activity (SWA; % in BSWS) | |||||
|---|---|---|---|---|---|
| Seal | Episodes | SWS episode duration (min) | SWA-L | SWA-R | Asymmetry index (AI) of SWA |
| 1 | RASWS (n = 8) | 11.4 ± 2.6 (4.8, 24.8) | 16 ± 2 (11, 23) | 85 ± 3 (73, 100) | −0.68 ± 0.02 (−0.76, −0.57) |
| LASWS (n = 4) | 9.5 ± 3.0 (5.3, 18.5) | 91 ± 10 (64, 109) | 21 ± 4 (11, 27) | +0.60 ± 0.02 (+0.54, +0.65) | |
| BSWS (n = 1) | 4.7 | 100 | 100 | −0.01 | |
| 2 | RASWS (n = 5) | 4.0 ± 0.6 (2.4, 5.4) | 22 ± 5 (11, 41) | 63 ± 7 (47, 83) | −0.49 ± 0.04 (−0.60, −0.41) |
| LASWS (n = 5) | 7.1 ± 1.3 (2.3, 10.7) | 82 ± 9 (56, 100) | 23 ± 9 (5, 43) | +0.54 ± 0.10 (+0.31, +0.83) | |
| BSWS* (n = 3) | 4.0 ± 0.3 (3.7, 10.7) | 100 ± 1 (100, 101) | 100 ± 0 | 0.00 ± 0.01 (−0.02, +0.02) | |
| 3 | RASWS (n = 6) | 5.4 ± 1.5 (2.0, 12.0) | 37 ± 7 (13, 60) | 133 ± 15 (75, 175) | −0.54 ± 0.07 (−0.74, −0.31) |
| LASWS (n = 1) | 6.3 | 90 | 49 | +0.32 | |
| BSWS (n = 1) | 4.0 | 100 | 100 | −0.07 | |
RASWS, right asymmetrical SWS; LASWS, left asymmetrical SWS; BSWS, high-voltage bilateral SWS.
Average for three episodes of BSWS. The data are presented as mean ± standard error of the mean with minimal and maximal values given in parentheses.
All episodes of SWS were scored visually and subdivided into left asymmetrical SWS (LASWS; SWA in the left hemisphere is greater than in the right hemisphere), right asymmetrical SWS (RASWS; SWA in the right hemisphere is greater than in the left hemisphere) or BSWS (SWA in the right and left hemispheres are comparable) based on the criteria described in previous publications (Lapierre et al., 2007; Lyamin et al., 2008a,b,c). Only episodes of RASWS and LASWS with an average absolute AI > 0.3 were selected for further analysis. The beginning of episodes of LASWS and RASWS was determined as the time of the first 20-s epoch in which SWA exceeded 10% of that seen in BSWS in either hemisphere. The end of SWS episodes was easily marked by behavioural awakening or the transition into REM sleep.
Differences in AI between symmetrical derivations and in SWA within one-hemisphere derivations were tested with one-way repeated-measures analysis of variance (anova) followed by a paired Tukeyʼs test. All other comparisons were conducted using either a t-test or Mann–Whitney U-test with SigmaPlot software.
RESULTS
Characteristics of episodes of asymmetrical SWS
Seals slept while lying on their sides or bellies. During BSWS both eyes were usually closed. During asymmetrical SWS, one eye could open briefly and this eye was always contralateral to the hemisphere with a low-voltage SWA, as reported in our previous studies (Lyamin et al., 2004). However, these episodes were rare under conditions of complete darkness.
Eight episodes of RASWS and four episodes of LASWS were available in seal 1, five episodes of RASWS and five episodes of LASWS in seal 2, and six episodes of RASWS and only one episode of LASWS in seal 3 (Table 1). All episodes were characterized by a substantial difference in SWA between the left and right hemispheres in occipital–lateral derivations (on average greater than a threefold difference; t-test; P < 0.01 for all comparisons). In two seals (1 and 2) the average SWA in the hemisphere with a greater SWA (the right hemisphere during RASWS and left during LASWS) was smaller than in the same hemisphere during BSWS. In seal 3, SWA activity in the right hemisphere during five of six episodes of RASWS was greater than in the same hemisphere in BSWS. A representative example of an episode of LASWS, RASWS and BSWS is shown in Fig. 2.
Figure 2.
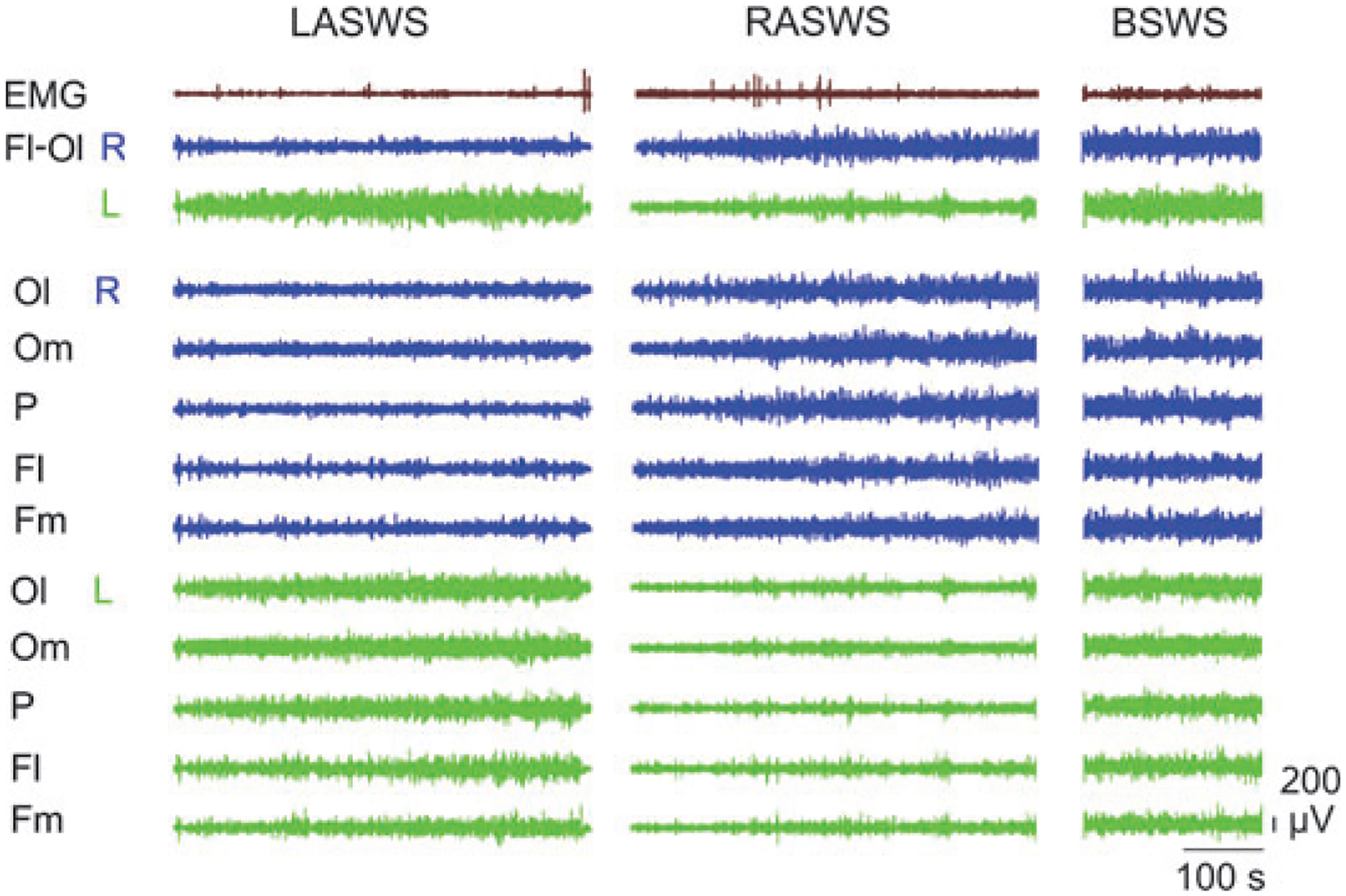
Cortical EEG of the left and right hemispheres and neck electromyogram (EMG) during SWS in a fur seal. LASWS and RASWS – episodes of left (L) and right (R) asymmetrical SWS. BSWS – an episode of bilateral SWS. Fl-Ol – bipolar recording from frontal-lateral and occipital-lateral derivations. Ol, Om, P, Fl and Fm mark monopolar recording from occipital-lateral, occipital-medial, parietal, frontal-lateral and frontal-medial derivations, respectively.
The AI of SWA ranged between −0.07 and +0.02 in episodes of BSWS, between −0.31 and −0.76 in episodes of RASWS and between +0.37 and +0.83 in episodes of LASWS (Table 1). Neither duration of RASWS and LASWS episodes nor the average SWA in the hemisphere with a greater power (right hemisphere during RASWS and left during LASWS) differed in seals 1 and 2, which displayed asymmetrical SWS in both hemispheres (t-test, P > 0.05 for all comparisons). Absolute AI of SWA in the two hemispheres were within the same range both in RASWS and LASWS (0.31–0.76 and 0.37–0.80, respectively) and the average values did not differ (t-test; P > 0.05 for all comparisons).
To summarize, these data indicate that (1) episodes of asymmetrical SWS in the fur seal selected based, on the average AI, are characterized by a substantial difference in the SWA between occipital–lateral derivations of the two hemispheres; and (2) there is no evidence for predominance of the right or left hemispheres in the expression of SWA in these derivations in the fur seal.
Regional differences in SWA during asymmetrical SWS
One way-repeated-measures anova revealed consistent regional differences in SWA during ASWS in the hemisphere with greater power in all three fur seals (seal 1: F(7,28) = 4.82, P = 0.004 for RASWS and F(3,12) = 5.56, P = 0.018 for LASWS; seal 2: F(4,16) = 5.25, P = 0.007 for RASWS and F(4,16) = 3.75, P = 0.025 for LASWS; seal 3: F(5,15) = 6.79, P = 0.04 for RASWS). In two seals (1 and 2), SWA in the frontal–medial derivations was the smallest (36–65% of SWA in BSWS in the corresponding derivations) and SWA in the parietal derivations was the greatest (67–100% of SWA in BSWS; post-hoc Tukeyʼs test, P < 0.05; in both seals for RASWS and in seal 1 for LASWS; Fig. 3, SWA1). SWA in the occipital–lateral derivations (63–91% of SWA in BSWS) did not differ from the high level of SWA in the parietal derivations (67–100%). At the same time, SWA in the occipital–medial and frontal–lateral derivations were comparable except for one case (LASWS in seal 1). In seal 3, only RASWS was recorded. As in the other two animals, SWA in seal 3 was the smallest in the frontal–medial derivations (on average 61% of SWS in BSWS) and the greatest in the occipital–lateral derivations (on average 133%; P < 0.05). SWA in the frontal–lateral derivations was greater than in frontal–medial derivations (113 and 61% of SWA in BSWS, respectively; P < 0.05). Therefore, these data indicate clearly an anterior–posterior gradient in SWA in the hemisphere with an overall greater power during asymmetrical SWS with a larger SWA recorded in the parietal and occipital cortex.
Figure 3.
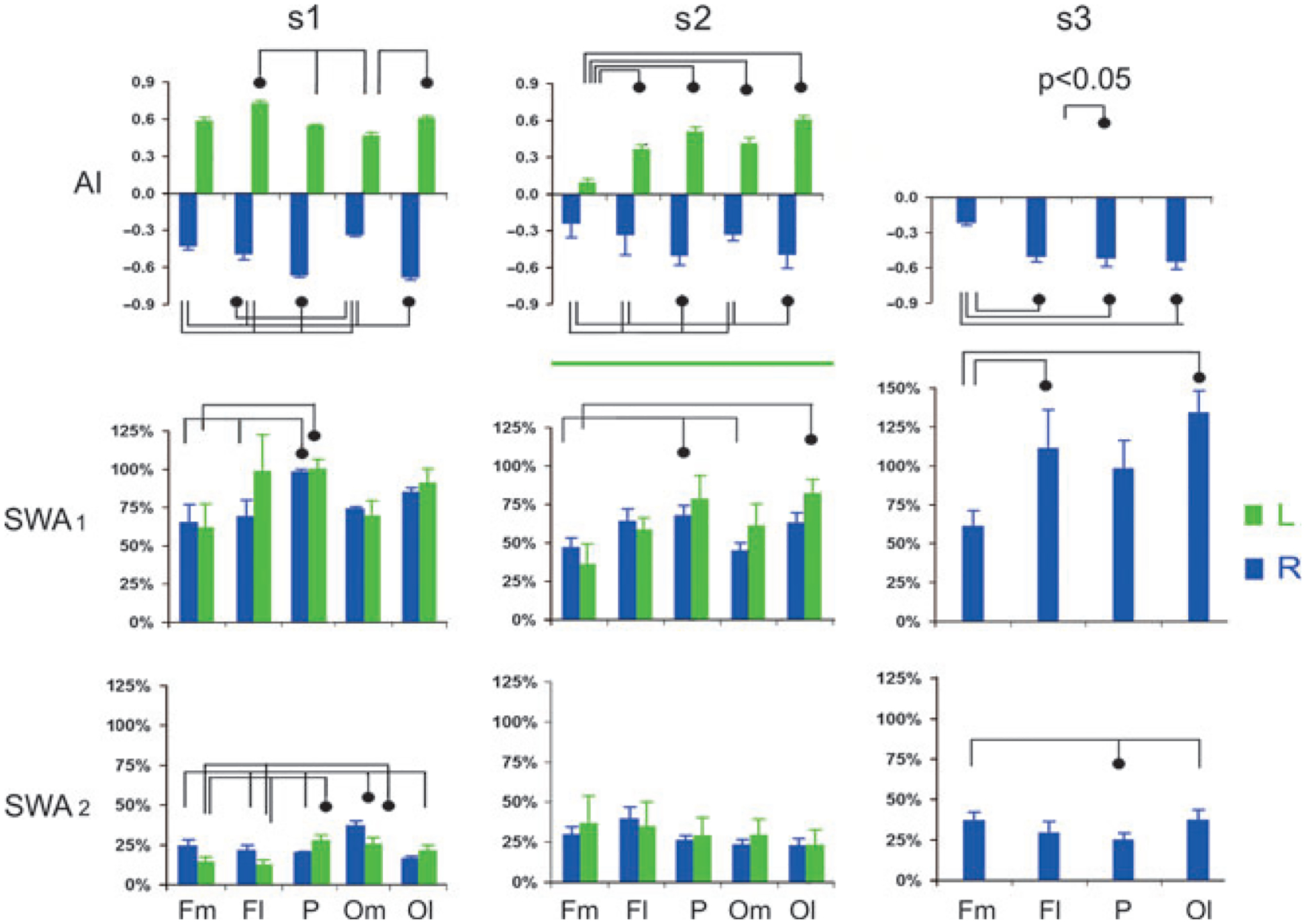
Power and asymmetry index of slow wave activity (SWA, 1.2–4.0 hz) during episodes of asymmetrical SWS in 3 fur seals. SWA 1 and SWA 2 – average power + SER in the hemisphere with a higher voltage and low voltage EEG activity, respectively, shown as percent of SWA power in the corresponding derivations in bilateral SWS (described in Methods). L and R – left and right hemisphere, respectively. AI – asymmetry index (+ SER). Fm, Fl, P, Om and Ol mark monopolar recording from frontal-lateral, frontal-medial, parietal, occipital-medial and occipital-lateral derivations, respectively. P < 0.05 - Tukey paired test following one way repeated measures anova.
We found no consistent regional differences between SWA in the hemisphere with a low-voltage EEG during asymmetrical SWS in fur seals. SWA in the hemisphere with a low-voltage EEG differed between derivations in two of three seals (Fig. 3, SWA2). In seal 1, SWA in posterior (parietal and occipital–medial in LASWS and parietal in RASWS) derivations was greater than in the frontal derivations (anova, F(7,28) = 20.89, P < 0.001 in RASWS and F(3,12) = 35.36, P < 0.001 in LASWS; post-hoc Tukeyʼs test, P < 0.05). Conversely, in seal 3 the power in the parietal derivations was the smallest compared to occipital–lateral and frontal–medial derivations (F(3,15) = 5.39, P = 0.01; post-hoc Tukeyʼs test, P < 0.05).
Regional differences in interhemispheric asymmetry of SWA
One-way repeated-measures anova revealed regional differences in the degree of interhemispheric asymmetry of SWA during asymmetrical SWS in all three seals (seal 1: F(7,28) = 42.69, P < 0.001 in RASWS and F(3,12) = 9.41, P = 0.001 in LASWS; seal 2: F(4,16) = 11.84, P < 0.001 in RASWS and F(4,16) = 7.15, P = 0.002 in LASWS; seal 3: F(5,15) = 10.39, P < 0.001 in RASWS). The average absolute AI in the parietal (which ranged between 0.38 and 0.66 in different episodes) and occipital (0.49–0.68) derivations was greater than in frontal–medial derivations (0.06–0.50) in all seals (Fig. 3, AI). The difference was found to be significant for all comparisons except for LASWS in seal 1 (post-hoc Tukeyʼs test, P < 0.05). In two seals (1 and 2) with electrodes in the occipital–medial position, the absolute AI in these derivations was smaller (0.33–0.46) than that in occipital–lateral (0.49–0.68; P < 0.05 in both seals in RASWS and in seal 1 in LASWS) and parietal (0.38–0.66; P < 0.05 in both seals in RASWS) derivations. In all three seals the AI in frontal–lateral derivations (0.33–0.70) was greater than in frontal–medial derivations (0.06–0.58; P < 0.05 in seal 2 in LASWS and seal 3 in RASWS). At the same time, the difference between AI in the frontal–lateral and other derivations was inconsistent.
Therefore, the expression of interhemispheric SWA asymmetry in the fur seal was greatest in the occipital–lateral and parietal derivations and smallest in the frontal–medial derivations, suggesting a posterio–anterior gradient for the degree of SWA asymmetry in the dorsal cortex of the fur seal. The degree of lateralization of SWA in medial derivations was also smaller than in the bordering, more lateral derivations, suggesting a medial–lateral gradient.
Characteristics of ASWS episodes
In this study we recorded three main types of asymmetrical SWS episodes in the fur seal based on the regional differences of SWA and the degree of asymmetry in two cerebral hemispheres (Fig. 4). These episodes accounted for 25 of 28 episodes (90% of all episodes) recorded in the three seals as follows:
Figure 4.
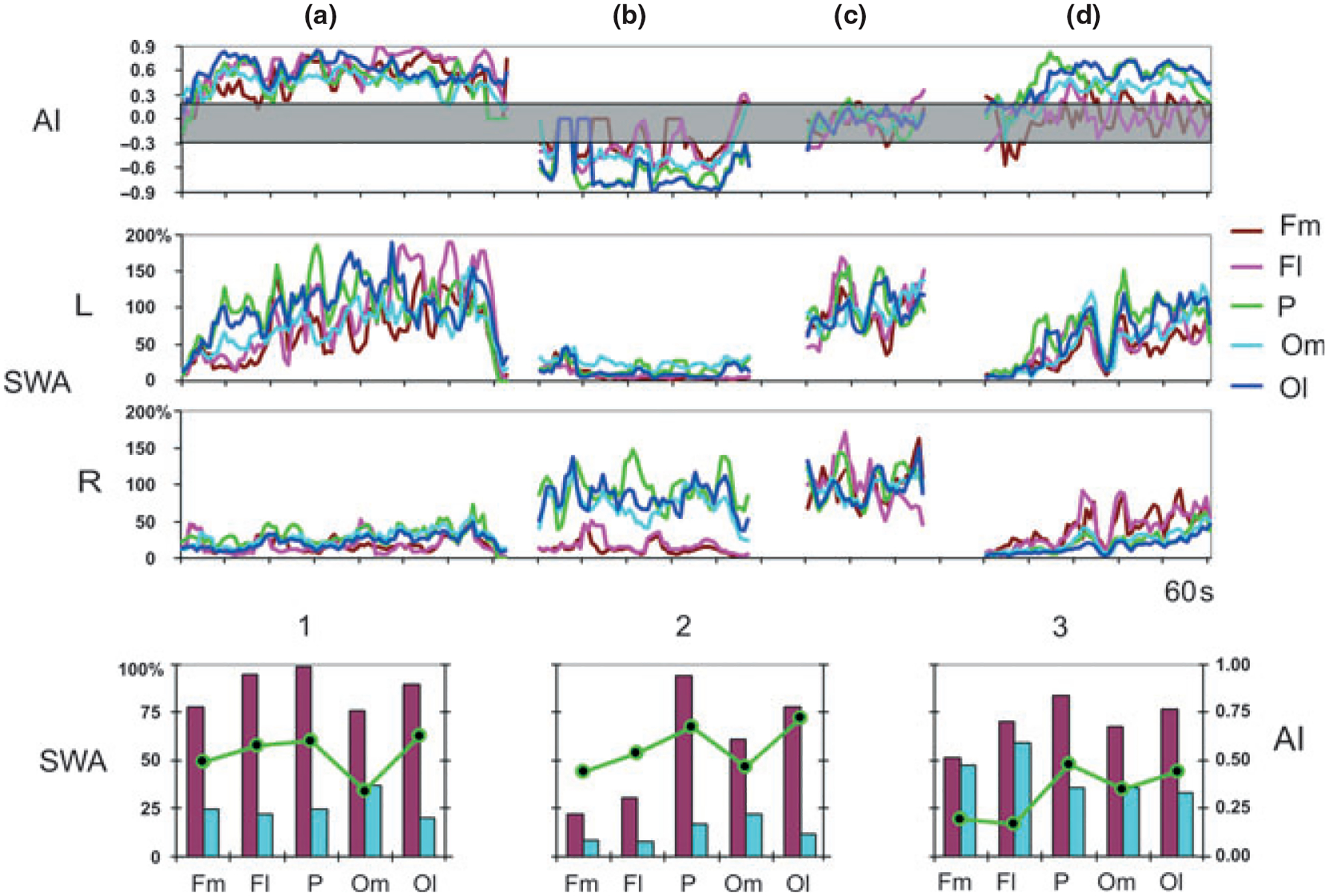
EEG spectral power and asymmetry index (AI) in symmetrical monopolar derivations during SWS in a fur seal. The first three row diagrams are representative examples showing normalized slow wave activity (SWA) power in the right and left hemispheres (R and L) and the AI of SWA calculated for consecutive 20-sec epochs in episodes of asymmetrical SWS (a,b, and d) and in an episode of BSWS (c). The three graphs in the bottom row show average SWA power and the average AI (± SER) during 3 main types (1–3) of episodes of asymmetrical SWS (described in Results). Black and gray bars – SWA in the hemisphere with higher voltage and lower voltage EEG, respectively. The lines connect circles marking values of the average AI for the corresponding deviations. Fm, Fl, P, Om and Ol - monopolar recording from frontal-medial, frontal-lateral, parietal, occipital- medial and occipital-lateral derivations, respectively. Episode a, b and d (first three rows of diagrams) are representative examples of type 1, 2 and 3 episodes, respectively. Note that in episode ʻaʼ SWA in all derivations of the left hemisphere was substantially greater in the right hemisphere. In episode ʻbʼ and ʻdʼ SWA in the occipital and parietal derivations of one hemisphere (right in ʻbʼ and left in ʻdʼ) was substantially greater than in the corresponding derivations of the opposite hemisphere. Both in episodes ʻbʼ and ʻdʼ the difference in SWA between the frontal derivations in two hemispheres was minimal. However, in episode “b” SWA in the frontal area was low (transitional between quiet waking and SWS) while in episode “d” SWA in the symmetrical frontal areas was large, comparable to that during high voltage BSWS.
1. In nine of 28 episodes (32% of all episodes) the average SWA in all derivations of the hemisphere with a greater power exceeded by 70% that in the corresponding derivations in BSWS, apparently indicating that the dorsal cortex of this hemisphere was in a state of high-voltage (ʻdeepʼ) SWS. The average SWA in all areas of the hemisphere with lower power was, on average, less than 25% of that in the corresponding derivations in BSWS, except for occipital–medial derivations, indicating that this portion of the hemisphere is in a state of transition between waking and SWS. The average absolute AI in all derivations was greater than 0.40 (i.e. exceeding the ʻ0.3 criteriaʼ set for asymmetrical SWS) except for the occipital–medial derivations (which, on average, is 0.35). All these episodes were recorded in seal 1, which was the most ʻasymmetricalʼ animal. These episodes meet the criteria of USWS (where one hemisphere is awake and the other is asleep) with a similar intensity of sleep in all areas of the ʻsleepingʼ hemisphere.
2. In 12 of 28 episodes (43%; these episodes were recorded in all three seals) the average SWA in the parietal and occipital areas of the hemisphere with a greater power exceeded 60% of that in BSWS, indicating that the posterior area of this hemisphere was in a state of high-voltage (ʻdeepʼ) SWS. At the same time SWA in the frontal cortex (only in medial or in both medial and lateral areas) did not exceed on average 30% of that in BSWS, thus indicating a low-voltage (ʻlightʼ) SWS in the anterior cortical area. The average SWA in the opposite hemisphere was smaller than 20% of that in BSWS, indicating waking or low-voltage SWS. The average AI in the frontal derivations was a little smaller than that in the parietal and occipital-lateral derivations, but in all dorsal cortical derivations met the criteria of asymmetrical SWS. These episodes also meet the definition of USWS with regional differences of SWA in the ʻsleepingʼ hemisphere.
3. In four of 28 episodes (14%; all were recorded in seal 3) the average SWA in one hemisphere exceeded by 60% of that during BSWS except for the medial–frontal derivations in which it was slightly smaller (50%), indicating that one hemisphere was in a state of ʻdeepʼ SWS. The average SWA in the other hemisphere was low in the parietal and occipital areas (25–30% of that in BSWS, indicative of a state transitional between waking and sleep). At the same time, SWA in the frontal area was very similar to that in the hemisphere with greater power, indicating that both frontal areas were in a state of high-voltage (ʻdeepʼ) SWS. The average AI was greater (meeting the criteria of asymmetrical SWS) in the parietal and occipital derivations, while in the frontal areas it was smaller than 0.3 (indicating BSWS). Therefore, this group of episodes is better described as (1) episodes with BSWS in the frontal cortex and asymmetrical SWS in the occipital–parietal area or (2) BSWS with unilateral activation of the occipital–parietal cortical area.
DISCUSSION
In our previous study we showed that AI can be used to measure quantitatively the overall expression of EEG asymmetry in the range of 1.2–16 Hz. The AI defines substantial differences in the degree and frequency range of the interhemispheric EEG asymmetry between bilaterally sleeping rats and unihemispherically sleeping dolphins, as well as in fur seals and some birds (Lyamin et al., 2008a). Much smaller state-related interhemispheric EEG asymme-tries have been reported for humans and some animal species. Use-dependent local changes and hemispheric dominance in the expression of different behaviours and major EEG rhythms may underlie these regional differences in SWA both within and between hemispheres producing the EEG asymmetry (e.g. Goldstein et al., 1972; Kattler et al., 1994; Krueger et al., 1999; Vyazovskiy et al., 2000, 2002).
The major finding of this study is that interhemispheric SWA in the fur seal is recorded across the entire dorsal cerebral cortex. The occipital (medial and lateral) electrodes in this study were located in the visual cortex. The frontal–lateral electrodes in all seals were located in the vicinity of the somatosensory cortex (Supin et al., 2001). The parietal electrodes were located in the vicinity of S. suprasylvian, which in mammals is the parietal associative cortex. The activity of neurones in this area has never been tested in the fur seal, as was performed for the visual and somatosensory cortex (Supin et al., 2001). However, the functional characteristics of neurones in this area (cortical fields 5 and 7) have been examined extensively in the cat and in primates. It is known that this part of cerebral cortex contributes primarily to the programming and executions of visually guided movements (e.g. Drew et al., 2008). Finally, the medial–frontal electrodes were located dorsal to S. postcruciatus, which in the fur seal is the motor cortex (Supin et al., 2001). Therefore, the interhemispheric asymmetry of SWA in the fur seal is a feature of sensory (visual and somatosensory), motor and associative (parietal or suprasylvian) cortical areas.
It is known that interhemispheric EEG asymmetry correlates with asymmetrical eye state in fur seals and cetaceans (Lyamin et al., 2004, 2008a,b,c). The duration of episodes of asymmetrical SWS can be extended and the degree of asymmetry can be enhanced by auditory stimuli (Lyamin et al., 2008b), so visual stimulation is not the only type of sensory stimulus which can cause unilateral cortical activation during SWS in the fur seal. We hypothesized that unilateral cortical activation during SWS (manifested in the EEG asymmetry) in the fur seal allows sensory processing and continuous vigilance (Lyamin et al., 2004). The data collected in this study indicate that SWA asymmetry in fur seals is expressed highly in the visual and parietal cortical areas and provides additional support for this hypothesis, specifically for the involvement of cortical areas of one brain hemisphere in the processing of the visual information during USWS in marine mammals.
Fur seals sleep in water floating on their sides, while holding the head above the water and paddling with one front flipper. When in this posture the vibrissae from only one side (ipsilateral to the paddling flipper) are directed to the water. It is reasonable to expect that both ipsilateral vibrissae and flipper movement supply the brain with ascending afferent information crucial for controlling the position of the head and adjusting the movement of the flipper. It is also known that the projection area of vibrissae to the contralateral somatosensory cortex in the fur seal is magnified disproportionally compared to other parts of the body (Supin et al., 2001). Previous bipolar recordings from the frontal–occipital cortical areas showed that the EEG in the fur seal is more desynchronized (activated) in the hemisphere that is contralateral to the paddling flipper (and the vibrissae directed to the water) when compared to the EEG in the ipsilateral hemisphere, which displays a higher-voltage SWA. Based on these data, we hypothesized that the unilateral cortical activation during SWS in the fur seal also allows motion and sleep while in water (Lyamin et al., 2008a,b,c). However, the degree of functional lateralization of the somatosensory and motor cortex has not been evaluated until this study. The data collected via monopolar recording revealed that SWA asymmetry in the fur seal is also expressed in the somatosensory cortex. The smallest lateralization of SWA was found in the motor cortex. This makes sense, because the animals were lying on the chamber floor without apparent movement. However, the meaning of functional lateralization of EEG in the somatosensory cortex (indicating unilateral activation) while the seal sleeps on the ground remains unclear. It could be due to generalized unilateral cortical activation spread from other areas, e.g. from the visual cortex, or whole hemisphere activation (Nir et al., 2011). It is interesting that SWA asymmetry in the occipital–medial cortex was smaller than in the lateral occipital cortex, tentatively suggesting a smaller functional lateralization of the primary visual cortex compared to the secondary visual cortex during SWA in the fur seal.
It remains to be determined whether the EEG asymmetry is present in other cortical regions (e.g. medial, temporal and basal) and ⁄ or subcortical areas. In dolphins EEG was recorded opportunistically simultaneously from the dorsal thalamic areas and two cerebral hemispheres (Mukhametov et al., 1997). In all cases the EEG changes in the ipsilateral cortex and thalamus were synchronous. Based on these observations, it was hypothesized that USWS in dolphins is not only a cortical phenomenon, but appears to involve at a minimum the entire cortical–thalamic system (Lyamin et al., 2008c). The asymmetry in the range of 12–16 Hz (spindles) and lateralized release of acetylcholine in the cortex during ASWS in the fur seal also suggest a functional asymmetry between thalamic and basal forebrain (cholinergic) areas, which promotes wakefulness and sleep and sends ascending projections to the cortex (Lapierre et al., 2007; Lyamin et al., 2008a).
Our data show that the majority (90%) of SWS episodes in the fur seal can be described as USWS. They meet the definition of USWS because, regardless of regional differences in the SWA power, the SWA in all derivations of one (ʻsleepingʼ) hemisphere is greater than that in the other (ʻwakingʼ) hemisphere. The third group of sleep episodes concerns episodes with BSWS in the frontal cortex and asymmetrical SWS in the occipital and parietal areas. These episodes should, rather, be described as episodes of BSWS with local activation (or lower-voltage sleep) in one cerebral hemisphere. In all these episodes local activation was recorded in the occipital–parietal hemisphere, the area involved in the processing of visual information. Behaviour-ally, these episodes may be episodes of sleep while motionless on the floor and briefly opening one eye.
The second finding of this study is the character of regional differences (topography) of SWA in the fur seal. We found that SWA in the fur seal in the occipital and parietal derivations was usually greater than in the frontal deviations. A frontal predominance of SWA is a characteristic feature of sleep in humans (Marzano et al., 2010; Werth et al., 1997). In the rat, EEG spectral power is usually measured in the frontal derivations, located in the vicinity of the motor and prefrontal cortex, and in occipital (parietal or posterior) sites, located in the vicinity of the barrel (somatosensory) cortex. SWA in the frontal derivations was larger than in the posterior derivations, particularly during recovery after sleep deprivation (e.g. Schwierin et al., 1999; Vyazovskiy et al., 2002). We are not aware of any detailed studies on the regional differences of EEG activity in the rat beyond the area of the motor and somatosensory cortex. However, based on visual inspection the EEG activity in the rat occipital area was reported to be smaller than that of the frontal area (Timo-Iaria et al., 1970). In the cat, spindles in the sensorimotor cortex are accompanied by high amplitude slow waves in the visual cortex (Lucas and Sterman, 1974), so the power of SWA in the occipital derivations is obviously greater than that in the frontal derivations. This would be in agreement with data collected in the fur seal.
There is a possibility that some factors could have an effect on the inverse gradient in SWA in the fur seal. Due to the frontal (nasal) position of a reference electrode and differential amplification of active screw electrodes referenced to it might be picking up some EEG activity, and this would be reducing SWA in anterior derivations to a greater degree than in posterior derivations. However, it seems not to be the key factor determining the inverse gradient of SWA in the fur seal, because SWA in the parietal derivation (more anterior) did not differ from SWA in the occipital–lateral (more posterior) derivations and SWA in the frontal–lateral derivation (more anterior) could be greater than in the occipital–medial (more posterior) derivation (Fig. 3). There is evidence on the highest synaptic density in the human frontal cortex (e.g. Ringli and Huber, 2011). No such studies have ever been conducted in marine mammals, as well as studies on the thickness of cortex in different cortical regions. The EEG electrodes were placed at the bottom of holes drilled through the skull, so skull thickness could not be responsible for the regional variations we see. Examining the effects of sleep deprivation on regional differences of SWA in the fur seal would also help understanding of the difference between terrestrial mammals and seals.
It has been shown that slow waves may occur locally in different cortical and subcortical areas in humans, monkeys and rats, as indicated by EEG slow waves and ⁄ or the pattern of neuronal discharge (Krueger et al., 1999; Nir et al., 2011; Pigarev et al., 1997; Vyazovskiy et al., 2011). According to this paradigm, sleep is viewed as a distributed process in the brain in which parts of the brain can be asleep while other parts are awake (e.g. Krueger and Tononi, 2011). Recent human and rodent studies (Nir et al., 2011; Vyazovskiy et al., 2011) have detailed an asynchrony in occurrence of the corresponding EEG slow waves (an indication of local sleep) in the cortex and subcortical areas. These data indicate that local sleep in humans and rodents is an issue of synchrony in the appearance of single slow waves or a characteristic pattern of neuronal discharge (over a time-frame of a few seconds or several slow wave events) in the corresponding sleeping cortical areas, while all these areas are displaying periods of SWA (over a time-frame of minutes or a SWS episode). In other words, even if cortical slow waves are generated locally by multiple generators (Murphy et al., 2009), all generators in the two brain hemispheres of humans and rodents are still in the same functional state—going ʻONʼ and ʻOFFʼ over the time-frame of seconds. If we use the same terminology during USWS in dolphins and fur seals, cortical EEG slow wave generators of the two cortical hemispheres appear to be in different functional states. We hypothesize that in the extreme case (dolphin USWS), those generators are going ʻONʼ and ʻOFFʼ in the sleeping hemisphere and are being largely ʻOFFʼ in the waking hemisphere over a period of longer than an hour.
ACKNOWLEDGEMENTS
This study was supported by NSF (0919929), NIH (069640) and Utrish Dolphinarium Ltd. The authors are thankful to J. Lapierre for comments on this manuscript.
REFERENCES
- Borbély AA Sleep homeostasis and models of sleep regulation. In: Kryger MH, Roth T and Dement WC DC (Eds) Principles and Practice of Sleep Medicine, 2nd edn. W.B. Saunders, Philadelphia, 1994: 309–320. [Google Scholar]
- Drew T, Andujar JE, Lajoie K and Yakovenko S Cortical mechanisms involved in visuomotor coordination during precision walking. Brain Res. Rev, 2008, 1: 199–211. [DOI] [PubMed] [Google Scholar]
- Fuchs T, Maury D, Moore FR and Bingman VP Daytime micro-naps in a nocturnal migrant: an EEG analysis. Biol. Lett, 2009, 5: 77–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldstein L, Stoltzfus NW and Gardocki JF Changes in interhemispheric amplitude relationships in the EEG during sleep. Physiol. Behav, 1972, 8: 811–815. [DOI] [PubMed] [Google Scholar]
- Horne JA Human sleep, sleep loss and behaviour: implications for the prefrontal cortex and psychiatric disorder. Br. J. Psychiatry, 1993, 162: 413–419. [DOI] [PubMed] [Google Scholar]
- Huber R, Deboer T and Tobler I Topography of EEG dynamics after sleep deprivation in mice. J. Neurophysiol, 2000, 84: 1888–1893. [DOI] [PubMed] [Google Scholar]
- Kattler H, Dijk DJ and Borbély AA Effect of unilateral somatosensory stimulation prior to sleep on the sleep EEG in humans. J. Sleep Res, 1994, 3: 159–164. [DOI] [PubMed] [Google Scholar]
- Krueger JM and Tononi J Local-use dependent sleep; synthesis of the new paradigm. Curr. Top. Med. Chem, 2011, 11: 2490–2492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krueger JM, Obál F and Fang J Why we sleep: a theoretical view of sleep function. Sleep Med. Rev, 1999, 3: 119–129. [DOI] [PubMed] [Google Scholar]
- Lapierre JL, Kosenko PO, Lyamin OI, Kodama T, Mukhametov LM and Siegel JM Cortical acetylcholine release is lateralized during asymmetrical slow-wave sleep in northern fur seals. J. Neurosci, 2007, 27: 11999–12006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lucas EA and Sterman MB The polycyclic sleep–wake cycle in the cat: effects produced by sensorimotor rhythm conditioning. Exp. Neurol, 1974, 42: 347–368. [DOI] [PubMed] [Google Scholar]
- Lyamin OI, Mukhametov LM and Siegel JM Relationship between sleep and eye state in Cetaceans and Pinnipeds. Arch. Ital. Biol, 2004, 142: 557–568. [PMC free article] [PubMed] [Google Scholar]
- Lyamin OI, Kosenko PO, Lapierre JL, Mukhametov LM and Siegel JM Fur seals display a strong drive for bilateral slow. J. Neurosci, 2008a, 28: 12614–12621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lyamin OI, Lapierre JL, Kosenko PO, Mukhametov LM and Siegel JM Electroencephalogram asymmetry and spectral power during sleep in the northern fur seal. J. Sleep Res, 2008b, 17: 154–165. [DOI] [PubMed] [Google Scholar]
- Lyamin OI, Manger PR, Ridgway SH, Mukhametov LM and Siegel JM Cetacean sleep: an unusual form of mammalian sleep. Neurosci. Biobehav. Rev, 2008c, 32: 1451–1484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marzano C, Ferrara M, Curcio G and De Gennaro L The effects of sleep deprivation in humans: topographical electroencephalogram changes in non-rapid eye movement (NREM) sleep versus REM sleep. J. Sleep Res, 2010, 19: 260–268. [DOI] [PubMed] [Google Scholar]
- Mukhametov LM, Supin AY and Polyakova IG Interhemispheric asymmetry of the electroencephalographic sleep patterns in dolphins. Brain Res, 1977, 134: 581–584. [DOI] [PubMed] [Google Scholar]
- Mukhametov LM, Oleksenko AI and Polyakova IG The Black Sea bottlenose dolphin: the structure of sleep. In: Sokolov VE and Romanenko EV (Eds) The Black Sea Bottlenose Dolphin. Nauka, Moscow, 1997: 492–512 [in Russian]. [Google Scholar]
- Murphy M, Riedner BA, Huber R, Massimini M, Ferrarelli F and Tononi G Source modeling sleep slow waves. Proc. Natl Acad. Sci. USA, 2009, 106: 1608–1613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nir Y, Staba RJ, Andrillon T et al. Regional slow waves and spindles in human sleep. Neuron, 2011, 70: 153–169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pigarev IN, Nothdurft HC and Kastner S Evidence for asynchronous development of sleep in cortical areas. NeuroRe-port, 1997, 8: 2557–2560. [DOI] [PubMed] [Google Scholar]
- Rattenborg NC, Amlaner CJ and Lima SL Behavioral, neurophysiological and evolutionary perspectives on unihemispheric sleep. Neurosci. Biobehav. Rev, 2000, 24: 817–842. [DOI] [PubMed] [Google Scholar]
- Ringli M and Huber R Developmental aspects of sleep slow waves: linking sleep, brain maturation and behavior. Prog. Brain Res, 2011, 193: 63–82. [DOI] [PubMed] [Google Scholar]
- Schwierin B, Achermann P, Deboer T, Oleksenko A, Borbély AA and Tobler I Regional differences in the dynamics of the cortical EEG in the rat after sleep deprivation. Clin. Neurophysiol, 1999, 110: 869–875. [DOI] [PubMed] [Google Scholar]
- Supin AY, Popov VV and Mass AM The Sensory Physiology of Aquatic Mammals. Kluwer Academic, Boston, 2001. [Google Scholar]
- Timo-Iaria C, Negrao N, Schmidek WR, Hoshino K, De Menezes CEL and Da Rocha TL Phases and states of sleep in the rat. Physiol. Behav, 1970, 5: 1057–1062. [DOI] [PubMed] [Google Scholar]
- Vyazovskiy V, Borbély AA and Tobler I Unilateral vibrissae stimulation during waking induces interhemispheric EEG asymmetry during subsequent sleep in the rat. J. Sleep Res, 2000, 9: 367–371. [DOI] [PubMed] [Google Scholar]
- Vyazovskiy VV, Borbely AA and Tobler I Interhemispheric sleep EEG asymmetry in the rat is enhanced by sleep deprivation. J. Neurophysiol, 2002, 88: 2280–2286. [DOI] [PubMed] [Google Scholar]
- Vyazovskiy VV, Olcese U, Hanlon EC, Nir Y, Cirelli C and Tononi G Local sleep in awake rats. Nature, 2011, 472: 443–447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Werth E, Achermann P and Borbély AA Fronto-occipital EEG power gradients in human sleep. J. Sleep Res, 1997, 6: 102–112. [DOI] [PubMed] [Google Scholar]


