SUMMARY
At the end of 2013, China reported a countrywide outbreak of measles. From January to May 2014, we investigated the clinical and immunological features of the cases of the outbreak admitted to our hospital. In this study, all 112 inpatients with clinically diagnosed measles were recruited from the 302 Military Hospital of China. The virus was isolated from throat swabs from these patients, and cytokine profiles were examined. By detecting the measles virus of 30 of the 112 patients, we found that this measles outbreak was of the H1 genotype, which is the major strain in China. The rates of complications, specifically pneumonia and liver injury, differed significantly in patients aged <8 months, 8 months to 18 years, and >18 years: pneumonia was more common in children, while liver injury was more common in adults. Pneumonia was a significant independent risk factor affecting measles duration. Compared to healthy subjects, measles patients had fewer CD4+IL-17+, CD4+IFN-γ+, and CD8+IFN-γ+ cells in both the acute and recovery phases. In contrast, measles patients in the acute phase had more CD8+IL-22+ cells than those in recovery or healthy subjects. We recommend that future studies focus on the age-related distribution of pneumonia and liver injury as measles-related complications as well as the association between immunological markers and measles prognosis.
Key words: Cytokine, H1 genotype, liver injury, measles, pneumonia
INTRODUCTION
Measles, also known as morbilli, rubeola, or red measles, is a highly contagious acute respiratory infection caused by the measles virus. Although most patients are cured within 3 weeks, some may develop complications such as pneumonia, enteritis, encephalitis, and liver injury [1, 2]. Complications from measles can occur in almost every organ system, and the most common causes of death are pneumonia, croup, and encephalitis [3]. With the implementation of the global measles eradication programme, the incidence of measles and related mortality has declined significantly in recent years. However, the situation in many developing countries remains challenging, particularly where endemic measles strains persist. Measles continues to cause morbidity and death in children worldwide. In 2014, there were 114 900 measles deaths globally – about 314 deaths every day or 13 deaths every hour (http://www.who.int/mediacentre/factsheets/fs286/en/). In China, 596 391 cases of measles and 368 measles-related deaths were reported from 2005 to 2013 [4]. From 1978, China began to carry out measles vaccination for 8-month-old infants. Starting in 1986, a second dose of measles vaccine was administered to 7-year-old children. From 2004, the implementation of the second dose of measles vaccine was administered to infants aged 18–24 months.
Although measles vaccines have been introduced, this disease may occur even in vaccinated individuals, and indigenous measles strains continue to circulate in China. In fact, a resurgence of measles occurred in Beijing at the end of 2013. To achieve the end of the measles epidemic, it is very important to characterize the clinical and immunological features of measles. Here, we report a clinical and immunological analysis of 112 cases of measles at the 302 Military Hospital of China, which is the largest hospital in China specializing in infectious disease and the centre for measles treatment in the Beijing district.
METHODS
Patients
Patients with a clinical diagnosis of measles at the 302 Military Hospital of China between January and May 2014 were prospectively recruited. Diagnosis was conducted in accordance with the WS 296-2008 guidelines (measles diagnostic criteria by Centers for Disease Control of China) according to typical clinical manifestations, such as fever, rash, upper respiratory catarrh, conjunctivitis, and oral Koplik spots with epidemiological features. Written informed consent was obtained from all patients. Patient consent was obtained for their blood and swab samples to be used for immunological and virological analyses.
Flow cytometry
For comparison of cytokine levels, 30 measles patients who agreed to donate an additional blood sample for immunological analysis and 30 sex- and age-matched healthy individuals without any infectious disease were enrolled. Blood was collected in the acute (2–3 days after maculopapular eruption) and recovery (3–4 days after fever subsided) phases. APC-Cy7-conjugated anti-CD3, FITC-conjugated anti-IFN-γ, and PerCP- conjugated anti-CD8 were purchased from BD Biosciences (USA); phycoerythrin (PE)-conjugated anti-IL-22 and APC-conjugated anti-IL-17A, from eBioscience (USA); and PE-Cy7-conjugated anti-CD56 from Biolegend (USA). For intracellular cytokine staining, fresh heparinized peripheral blood (200 µl) was stimulated using PMA (50 ng/ml; Sigma, USA) and ionomycin (1 µg/ml, Sigma) in 800 µl RPMI 1640 medium supplemented with 10% fetal bovine serum, followed by incubation with 10 µg/ml Brefeldin A (Sigma) for 6 h. After surface markers were stained, the cells were washed, lysed, fixed, permeabilized utilizing a commercially available kit (eBioscience), stained with the corresponding intracellular antibodies, and analysed by multicolour flow cytometry using FACSAria and FlowJo software (Tristar, USA).
Virus antibody detection and RNA isolation and sequencing
Blood samples were collected from all patients for measles antibody detection. The measles IgM was measured by enzyme-linked immunosorbent assay using the anti-measles virus ELISA (IgM) (EUROIMMUN Medical Laboratory Diagnostics Stock Company, Germany).
Throat swabs were collected from the 30 patients in the acute phase of infection for virus isolation and sequencing. Viral RNA was extracted from these samples using a DP315-R kit (Tiangen, China). Nested reverse transcription–polymerase chain reaction (RT–PCR) was then performed using a TaKaRa One-Step RNA PCR kit and TransStartFastPfu DNA Polymerase (AP221; TransGen Biotech Co. Ltd, China). The product of the second round of PCR with the target band was sequenced using the MZ-R primer (Shenggong, China). The reference strain used to determine the primer locations was from GenBank (sequence FJ416068, Changchun, China). Five microlitres of PCR product was resolved via electrophoresis in 1% agarose gel. The results were compared to the measles virus sequence on the NCBI website using BLAST analysis. The PCR primers are shown in Table 1.
Table 1.
Primers used for nested PCR amplification
| Direction | Sequence (5′3′) | Site of the primers | Product size (bp) | |
|---|---|---|---|---|
| First round | Forward | AGAAAATGGTTGGATGTGGTGAG | 744–766 | 1201 |
| Reverse | GCTCCTGTCCTGGGTTGTCTGAT | 1923–1945 | ||
| Second round | Forward | GCTATGCCATGGGAGTAGGAGTGG | 1108–1131 | 593 |
| Reverse | CCTCGGCCTCTCGCACCTAGT | 1681–1701 |
Statistical analysis
Data were deposited in EpiData software (http://www.epidata.dk/) via double entry. They were analysed using SAS software (version 9.1.3; SAS Institute Inc., USA). For single-factor one-way analysis of variance, we chose the standard alpha (α) = 0·05 criterion. The disease course was defined as the period from the start of fever to cure and discharge. Cox proportional hazards analysis was used to analyse disease course on the basis of different baseline covariates, e.g. sex, age, alanine aminotransferase (ALT) levels, highest body temperature, white blood cell (WBC) count, and complications. The α value was set at 0·1 (two-sided test). P < 0·05 was considered statistically significant.
Ethical statement
The Ethics Committee of our hospital approved this prospective study.
RESULTS
General information regarding the 2014 measles outbreak
During the 2014 measles outbreak in Beijing, 112 measles patients were admitted to our hospital (54 male). The median age was 27·5 years (range 3 months to 78 years). Of these, 13 (11·61%) were aged <8 months, 14 (12·5%) were aged between 8 months and 18 years, and 85 (75·89%) were aged >18 years; the median ages in these groups were 0·58, 2·5, and 31 years, respectively. Hospitalization duration ranged from 1 to 11 days (mean 4·66 ± 1·86 days). Maculopapular eruption time ranged from within 24 h to 9 days (mean 3·07 ± 1·53 days) after fever development. Body temperatures ranged from 38·3 °C to 42 °C (mean 39·52 ± 0·63 °C), and the duration of fever ranged between 2 and 14 days (mean 7·33 ± 2·26 days). Last, 88 (78·57%) of the 112 patients had oral Koplik spots (Table 2).
Table 2.
Clinical characteristics of enrolled subjects
| Subjects, n | 112 |
| Age, years (median, quartile) | |
| Total | 27·5 (19·2–35·0) |
| <8 months | 0·58 (0·42–0·67) |
| 8 months to 18 years | 2·5 (0·88–6·50) |
| >18 years | 31·0 (19·3–35·0) |
| Sex, n (M/F) | 54/58 |
| Age group, n | |
| <8 months | 13 |
| 8 months to 18 years | 14 |
| >18 years | 85 |
| Serum anti-measles virus antibody, n | |
| Positive | 101 |
| Negative | 11 |
| WBC count, ×109/l | 6·79 ± 5·90 |
| Neutrophil count, ×109/l | 4·20 ± 3·43 |
| Lymphocyte count, ×109/l | 2·86 ± 2·08 |
| Platelet count, ×109/l | 186·09 ± 93·51 |
| Complications, n | |
| Pneumonia | 37 |
| Liver injury | 56 |
| Enteritis | 28 |
| Fever duration, days | 7·33 ± 2·25 |
| Number of hospital days | 4·66 ± 1·86 |
WBC, White blood cell
Biochemical indicators
Among the 112 measles patients, 27 showed a lowered WBC count, while 12 showed an increased count during the acute phase. One hundred and one patients tested positive for IgM rubeola antibodies, but there was no significant difference regarding sex (P = 0·2832). Abnormal liver function as measured by ALT is defined as ALT >40 U/l, ALT between 40 and 79 U/l indicates mild injury, 80–400 U/l indicates moderate injury, and ALT >400 U/l means severe injury. We showed that 56 patients had liver injury as a complication, as evidenced by increased ALT levels. Further, the liver injury rates differed significantly in the three age groups (P = 0·0011).
Sequencing
The virus RNA was detected by PCR from throat swab samples of 30 of the 112 patients; 26 (86·7%) of these 30 patients tested positive. The PCR products formed a ~593-bp band, which was the expected size of the amplification products. The 450-bp sequence at the C-terminal of the nucleoprotein (N) gene of the measles virus was then sequenced and compared with 24 known measles virus genotypes. All the measles virus isolates from the outbreak cases were of the H1 genotype with a base coincidence rate of ⩾98·2% and showed the highest homology with the Jiangxi strain, which is the current epidemic strain of the measles virus in China.
Pneumonia
Thirty-seven patients developed measles-associated pneumonia (33·04%, Table 3). The percentage of measles patients who developed pneumonia did not differ significantly regarding sex (P = 0·9485) but it did differ significantly in the three age groups (P < 0·001).
Table 3.
Multivariate analysis of risk factors for pneumonia
| Pneumonia | No pneumonia | t | χ2 | P | |
|---|---|---|---|---|---|
| Sex, M/F | 18/19 | 36/39 | – | 0·004 | 0·554 |
| Age, years | 19·51 ± 19·25 | 28·71 ± 12·89 | −2·632 | – | 0·011 |
| Age group, n | |||||
| <8 months | 10 | 3 | – | 12·805 | 0·001 |
| 8 months to 18 years | 8 | 6 | – | 4·203 | 0·043 |
| >18 years | 19 | 66 | – | 18·189 | 0·000 |
| Fever duration, days | 8·27 ± 2·19 | 6·87 ± 2·16 | 3·220 | – | 0·002 |
| WBC count, 109/l | 9·930 ± 9·125 | 5·251 ± 2·099 | 3·079 | – | 0·004 |
| Neutrophil count, % | 60·38 ± 19·84 | 65·75 ± 19·05 | −1·384 | – | 0·169 |
| ALT level range, n | |||||
| <40 | 24 | 33 | – | 4·316 | 0·030 |
| 40–79 | 5 | 18 | – | 1·670 | 0·148 |
| 80–400 | 8 | 22 | – | 0·751 | 0·264 |
| >400 | 0 | 2 | – | 1·005 | 0·446 |
| Koplik spots positive | 29/8 | 59/16 | – | 0·001 | 0·577 |
| Measles antibody positive | 37/0 | 64/11 | – | 6·018 | 0·009 |
| Enteritis, n | 9/28 | 19/56 | – | 0·013 | 0·551 |
WBC, White blood cell; ALT, alanine aminotransferase.
Multivariate analyses
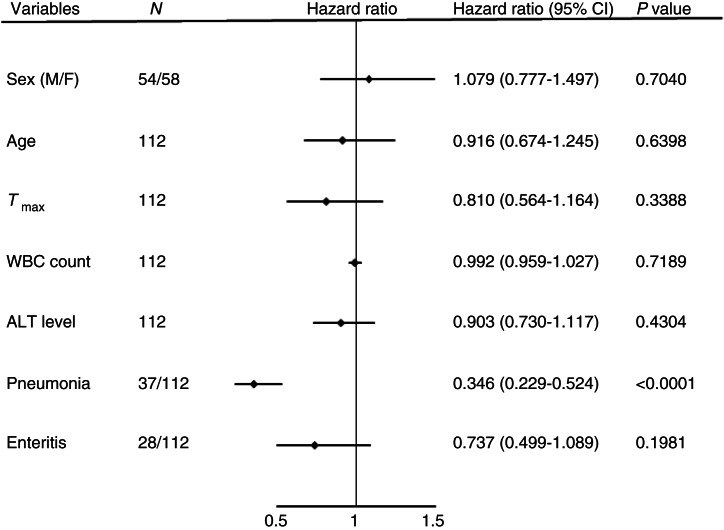
A multiple linear regression model was then applied to identify variables that were significant in independently predicting the disease course (Fig. 1), and pneumonia was found to be one such factor (P < 0·0001).
Fig. 1.
Cox analysis of independent risk factors related to measles infection. The disease course of measles was defined as the period from the start of fever to cure and discharge. Cox proportional hazards analysis was used to calculate the disease course on the basis of different baseline covariates, namely, sex, age, alanine aminotransferase (ALT) level, highest body temperature (Tmax), white blood cell (WBC) count, and complications (pneumonia and enteritis); α was set at 0·1 (two-sided test). P < 0·05 was considered statistically significant.
Immunological characteristics of measles patients
Immunological features were compared between 30 of the 112 measles patients and 30 age- and sex-matched healthy subjects, who were selected as the control group (Table 4). The percentage of CD3+ T cells was significantly lower in the acute phase of measles patients than in the recovery phase of measles patients and controls (P < 0·05). The measles patients had significantly fewer CD4+IL-17+ cells (P < 0·01), CD4+IFN-γ+ cells (Fig. 2), and CD8+IFN-γ+ cells than healthy subjects (P < 0·05). However, they had more CD8+IL-22+cells in the acute phase than in the recovery phase and had more of these cells than control subjects (P < 0·05).
Table 4.
Lymphocyte subpopulations in measles patients and healthy subjects (%)
| Group | Mean difference | |||||
|---|---|---|---|---|---|---|
| I | J | I – J† | s.e. | P | 95% CI | |
| CD3/Lym | AP | RP | −11·24000* | 4·47090 | 0·016 | −19·2626 to –20·2626 |
| AP | HC | −10·24000* | 4·47090 | 0·027 | −10·0226 to –2·2174 | |
| HC | RP | −1·00000 | 4·47090 | 0·824 | −15·4726 to –1·2174 | |
| CD4/CD3 | AP | RP | −7·26667 | 4·06619 | 0·081 | −15·6126 to 8·0226 |
| AP | HC | −7·40667 | 4·06619 | 0·076 | −8·0659 to 0·9392 | |
| HC | RP | 0·14000 | 4·06619 | 0·973 | −0·7018 to 0·7992 | |
| CD8+/CD3+ | AP | RP | 7·45333 | 4·04102 | 0·072 | −0·8018 to 8·3459 |
| AP | HC | 7·35333 | 4·04102 | 0·076 | −8·0551 to 15·6084 | |
| HC | RP | 0·10000 | 4·04102 | 0·980 | −0·4338 to 15·5084 | |
| CD4+IL-17+/CD4+ | AP | RP | 0·56160 | 0·49322 | 0·261 | −2·8954 to 8·2551 |
| AP | HC | −1·90007* | 0·49322 | 0·000 | 1·4663 to 1·5570 | |
| HC | RP | 2·46167* | 0·49322 | 0·000 | −8·1819 to –0·9047 | |
| CD4+IFN-γ+/CD4+ | AP | RP | −1·22580 | 3·44688 | 0·724 | −19·6828 to 3·4570 |
| AP | HC | −12·72667* | 3·44688 | 0·001 | 4·5448 to 5·7303 | |
| HC | RP | 11·50087* | 3·44688 | 0·002 | −7·3239 to –5·7706 | |
| CD4+IL-22+/CD4+ | AP | RP | −0·18073 | 0·27071 | 0·508 | −0·8367 to 0·4752 |
| AP | HC | −0·53433 | 0·27071 | 0·0552 | −1·7236 to –0·4117 | |
| HC | RP | 0·35360 | 0·27071 | 0·199 | 0·2310 to 1·5429 | |
| CD8+IFN-γ+/CD8+ | AP | RP | 3·26520 | 4·47413 | 0·470 | −22·8625 to 18·4570 |
| AP | HC | −12·27333* | 4·47413 | 0·009 | 6·2827 to 11·1877 | |
| HC | RP | 15·53853* | 4·47413 | 0·001 | 0·0717 to –4·3509 | |
| CD8+IL-22+/CD8+ | AP | RP | 0·28893* | 0·12125 | 0·022 | 0·2976 to 24·7943 |
| AP | HC | 0·54147* | 0·12125 | 0·000 | −0·4697 to 0·5595 | |
| HC | RP | −0·25253* | 0·12125 | 0·043 | −0·8367 to 0·7853 | |
s.e., Standard error; CI, confidence interval; AP, acute phase; RP, recovery phase; HC, healthy control.
† Mean difference between the I and J groups.
*P < 0·05.
Fig. 2.
Measles patients had significantly fewer IL-17+ cells, IFN-γ+ cells, and IL-22+ cells than healthy subjects. (a) Freshly isolated peripheral blood mononucleocytes were gated from total peripheral leukocytes on the basis of their forward and side scatter, and CD4+ T cells were identified as CD3+CD8− T cells. IL-17+, IFN-γ+, and IL-22+ cells were gated from the CD4+ T cells. (b) Statistical analysis of distribution of the total population of CD4+ T cells, Th17 cells (IL-17+CD4+ T cells), Th1 cells (IFN-γ+CD4+ T cells), and Th22 cells (IL-22+CD4+ T cells) in patients and healthy controls. AP, Acute phase; RP, recovery phase; HC, healthy control.
DISCUSSION
In the present study, 112 measles patients were clinically evaluated, and 101 tested positive for measles antibody. However, the typical clinical manifestations of measles were also observed in the 11 remaining patients who tested negative for the antibody. We speculate that the IgM negative response in some measles patients may due to the sensitivity of the detection kit and the low titre of the measles antibody. Our clinical findings are in agreement with those observed in a previous study [5].
Moreover, the complications noted in the present study were also similar to those reported previously [6], i.e. liver injury (50%), pneumonia/bronchopneumonia (33·04%), and enteritis (25·0%). The significant differences in liver injury in the three age groups in the present study indicated that this complication correlated positively with age. All measles patients enrolled in this study had no alcoholism-, infection- or drug-related liver diseases. Although the exact underlying mechanism(s) remain unclear, the use of antipyretics in treating measles infection may be a possible cause that requires further investigation. In a study by Ackerman et al. [7], 56% of 118 measles patients had various degrees of liver damage, and the incidence was substantially higher in patients using acetaminophen (a well-known medication that may lead to liver damage) as an antipyretic than in those using metamizole. Further, liver protective treatment with compound glycyrrhizin injections reversed the liver damage, and severe complications such as liver failure did not occur.
In the present study, we found that younger patients had a higher chance of developing pneumonia and that pneumonia was an independent risk factor that influenced the disease course. Severe respiratory complications have previously been reported to be significant independent risk factors for mortality in children with measles [8]. Pathological studies of children who died with acute measles found multinucleated giant cells typical of measles virus infection throughout the respiratory and gastrointestinal tracts and in most lymphoid tissues [3]. Therefore, lung imaging is highly recommended for children with respiratory symptoms, to check for possible pulmonary inflammatory lesions and to administer timely and proper treatment. Further, in rural areas or districts with poor nutritional conditions and hygiene, children with measles need extra care, to prevent pneumonia.
Measles virus infection is known to cause severe immunosuppression, which contributes to many related complications [9]. The immunosuppressive state can last from several weeks to several months [10], and the pathogenicity of measles is related to the immune status of the infected individual. It has been shown that measles leads to a decline in CD4 lymphocytes, dysfunction of cellular immune response, and reduced proliferation of lymphocytes [11]. In the present study, we found that the number of CD3+ cells was significantly reduced in the acute phase of measles virus infection but returned to normal level after recovery, and the percentages of CD4+ and CD8+ cells, which produce IFN-γ, were reduced during the disease course. These results are consistent with those of a previous study showing that measles changes the T helper 1 and T helper 2 cytokine balance, thereby enhancing T helper 2-mediated responses [11]. Another study showed that children with acute measles infection had considerably impaired cellular immune function [12]. Further, in adult patients, the percentages of CD3+ and CD4+ T cells were found to be significantly reduced [13]. We also found that the percentages of CD4+ cells that produce IL-17 were also decreased during acute measles infection. Cytokine IL-17 is crucial to the innate and adaptive arms of the immune system. IL-17A was initially reported to be mainly expressed by activated CD4+ T cells, which fight against pathogen invasion at different phases and locations of infection [14]. Given the important role of IFN-γ in combating viral and bacterial infections and that of IL-17 in mediating both innate and cellular immune responses, the reduction in the number of IL-7-producing CD4+ cells may be responsible for the immunosuppression observed in measles infection and be associated with the complications observed during the disease course.
In the acute phase of measles, the percentage of CD8+ T cells that produced IL-22 was higher than that in recovering patients or control subjects (P < 0·05) in the present study. IL-22 modulates tissue responses during inflammation and promotes antimicrobial immunity and tissue repair at barrier surfaces by binding to the IL-22R receptor. This cytokine can trigger pro-inflammatory and antimicrobial responses to clear pathogens. Recently, IL-22 was found to be essential for lung epithelial repair after influenza [15]. It was initially thought to be produced by CD4+ T cells, but recent studies have shown that CD8+ T cells in psoriatic lesions are an important source of IL-22 [16, 17]. Thus, the increased proliferation of CD8+ T cells that produce IL-22 during acute infection suggests that these cells play an important role in the control of measles infection. Additionally, they may also contribute to tissue repair during recovery.
This study has some limitations. We identified a high prevalence of pneumonia in children with measles and liver damage in adults. However, we were unable to determine the causes underlying this age-related distribution of complications. Further, immunological analysis was not conducted for all patients since blood samples were not available. Therefore, we were not able to investigate the association between immunological markers and disease prognosis. In addition, the patients enrolled in this study are those that were admitted to our hospital, whose illness may be more serious, and are not representative of all measles cases.
In conclusion, the measles virus in our study was the H1 genotype, the dominant epidemic strain in China, suggesting it remains controllable using the current measles vaccines. The clinical manifestations are the same as those of typical measles. However, diagnosis and treatment should pay attention to complications such as paediatric pneumonia and adult liver injury. Measles infection alters the host immune response with decreased IFN-γ and IL-17 production, which may lead to immunosuppresseed status. A better understanding of the immunological profiles of measles infection may further our understanding of its pathogenesis.
ACKNOWLEDGEMENTS
This work was supported by grants from the National Natural Science Foundation of China (81301432) and the State Key Laboratory Proteomics (SKLP-O201410) and Beijing Natural Science Foundation (7152141). We thank Jingfeng Bi for his excellent technical assistance in statistical analysis, and we are grateful to Shengdong Luo for assistance in virus cultures and PCR detection. We especially acknowledge Weiwei Chen and Wen Xu for their consultation and advice about this study.
DECLARATION OF INTEREST
None
REFERENCES
- 1.World Healt Organization. Measles (http://www.who.int/mediacentre/factsheets/fs286/en/).
- 2.Wolfson LJ, et al. Estimates of measles case fatality ratios: a comprehensive review of community-based studies. International Journal of Epidemiology 2009; 38: 192–205. [DOI] [PubMed] [Google Scholar]
- 3.Perry RT, Halsey NA. The clinical significance of measles: a review. Journal of Infectious Diseases 2004; 189 (Suppl. 1): S4–16. [DOI] [PubMed] [Google Scholar]
- 4.Ma C, et al. Monitoring progress towards the elimination of measles in China: an analysis of measles surveillance data. Bulletin of the World Health Organization 2014; 92: 340–347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Loukides S, et al. Bacterial pneumonia as a suprainfection in young adults with measles. European Respiratory Journal 1999; 13: 356–360. [DOI] [PubMed] [Google Scholar]
- 6.Caseris M, et al. French 2010–2011 measles outbreak in adults: report from a Parisian teaching hospital. Clinical Microbiology and Infection 2014; 20: O242–244. [DOI] [PubMed] [Google Scholar]
- 7.Ackerman Z, et al. Hepatitis during measles in young adults: possible role of antipyretic drugs. Hepatology 1989; 10: 203–206. [DOI] [PubMed] [Google Scholar]
- 8.Fu HY, et al. Retrospective study of risk factors of mortality in patients with measles in a tertiary pediatric hospital. Chinese Journal of Infectious Diseases 2013; 31: 598–602. [Google Scholar]
- 9.Permar SR, Griffin DE, Letvin NL. Immune containment and consequences of measles virus infection in healthy and immunocompromised individuals. Clinical and Vaccine Immunology 2006; 13: 437–443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Koga R, et al. Measles virus-induced immunosuppression in SLAM knock-in mice. Journal of Virology 2010; 84: 5360–5367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Sun X, et al. Suppression of antigen-specific T cell proliferation by measles virus infection: role of a soluble factor in suppression. Virology 1998; 246: 24–33. [DOI] [PubMed] [Google Scholar]
- 12.Dagan R, et al. Cellular immunity and T-lymphocyte subsets in young children with acute measles. Journal of Medical Virology 1987; 22: 175–182. [DOI] [PubMed] [Google Scholar]
- 13.Lv QQ, Xu WF. Expression of T lymphocyte and regulatory T lymphocyte in patients with measles. Disease Surveillance 2011; 26: 516–518. [Google Scholar]
- 14.Jin W, Dong C. IL-17 cytokines in immunity and inflammation. Emerging Microbes & Infections 2013; 2: e60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Pociask DA, et al. IL-22 is essential for lung epithelial repair following influenza infection. American Journal of Pathology 2013; 182: 1286–1296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hijnen D, et al. CD8(+) T cells in the lesional skin of atopic dermatitis and psoriasis patients are an important source of IFN-gamma, IL-13, IL-17, and IL-22. Journal of Investigative Dermatology 2013; 133: 973–979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Res PC, et al. Overrepresentation of IL-17A and IL-22 producing CD8 T cells in lesional skin suggests their involvement in the pathogenesis of psoriasis. PLoS ONE 2010; 5: e14108. [DOI] [PMC free article] [PubMed] [Google Scholar]