Abstract
Angiogenesis involves the formation of new blood vessels from preexisting ones, and it is an essential step during cutaneous wound healing, which supports cells at the wound site with nutrition and oxygen. Impaired angiogenesis in the wound tissues results in delayed wound closure and healing. Among the regulators of angiogenesis, the role of catecholamines (epinephrine, norepinephrine, and dopamine) is of interest due to their diverse roles in the process of wound healing. While both norepinephrine and epinephrine mostly inhibit the angiogenic process in cutaneous wounds, dopamine, the other member of the catecholamine family, has interesting and contradictory roles in the regulation of angiogenesis in the wound beds, depending on the type of dopamine receptor involved. The stimulation of dopamine D2 receptors negatively regulates the angiogenic process in normal dermal wounds and thereby delays healing, whereas the stimulation of dopamine D1 receptors promotes angiogenesis and expedites healing in diabetic wounds. Importantly, catecholamines also play important roles in other pathological conditions, and specific agonists and antagonists of catecholamines are available for the treatment of some disorders. Therefore, such drugs may be utilized for the management of angiogenesis to promote the healing of dermal wounds. This review provides a broad overview of the angiogenic process during cutaneous wound healing and the regulatory roles played by catecholamines during the process.
Keywords: Catecholamines, Angiogenesis, Skin, Wound
INTRODUCTION
Cutaneous wound healing (tissue repair and regeneration) is an essential physiological process required for the restoration of the integrity of skin after any trauma (acute injury, surgery, or other factors that cause the breakdown of intact skin) to maintain physiological homeostasis.1,2 It is a highly orchestrated complex process involving large numbers of cell types and factors. Classically, the process involves four phases: hemostasis, inflammation, proliferation, and remodeling. Successful wound healing relies upon rapid hemostasis to limit blood loss, appropriate inflammation, differentiation, proliferation, and successful migration of mesenchymal cells to the wound area, sufficient neovessel formation or angiogenesis, prompt re-growth of epithelial tissue over the wound area, as well as proper collagen synthesis and alignment in the regenerating tissue.3,4All these processes must occur in the right sequence at a specific time and for an appropriate duration.4,5 Any variation in the processes can result in impaired tissue repair and deficient healing of the wound, resulting in the development of chronic wounds. Non- healing wounds present a challenging clinical problem—they constitute a significant source of morbidity as well as substantial societal and economic burden.6, 7
Among all the processes, neovascularization or angiogenesis, which is the formation of new blood vessels from the preexisting vasculature, represents an essential component of optimal wound healing due to its primary impact from the beginning of the process (after skin injury) until the end of the remodeling phase.8,9 In wound healing, new capillaries first appear in the wound bed 3–5 days after injury along with granulation, and the creation of a provisional matrix is initiated. The new capillaries rapidly grow into the wound and produce an abundant network of new blood vessels to support cells at the wound site with nutrition and oxygen. Inadequate ability to reestablish functional blood supply to the injury site can cause chronic non-healing wounds.10 Several endogenous growth factors and hormones11 regulate the process of angiogenesis in cutaneous wound healing. Therefore, identifying and validating these endogenous factors that regulate angiogenesis in the wound bed is of immense interest for the development of potential therapeutic targets.12,13
Catecholamines (CA) have definitive roles in the regulation of angiogenesis in diseases;14–24 however, their roles in wound healing are highly underappreciated. This review provides the readers with a broad overview of the roles played by the three different CA in the angiogenic process during cutaneous wound healing. Furthermore, it discusses the potential mechanisms through which they influence the wound physiology.
ANGIOGENESIS AND WOUND HEALING
The normal vasculature is in a state of quiescence, which is maintained by a balance between basal levels of pro-angiogenic factors, such as vascular endothelial growth factor-A (VEGF-A) and fibroblast growth factor, and anti-angiogenic factors, such as angiopoietin1 (Ang1) and pigment epithelium-derived factor.10,25–27 An injury disrupts this homeostasis, leading to a low oxygen tension/hypoxic state, which is an important activator of the endothelial cells (EC).28 In the initial stages of wound healing, leukocytes and neutrophils are recruited to the site of injury.29 Thereafter in the proliferative stage, macrophages, neovessels, and loose connective tissue form the granulation tissue.30,31Active angiogenesis is the hallmark of the proliferative stage and involves the growth of neovessels that are characterized by their immaturity, permeability, and redundancy.8–10,32 Angiogenic response in this phase takes place along with fibroblast migration, proliferation, and collagen synthesis. After an injury has occurred, the microvascular EC lining the inner surface of blood vessels is activated by hypoxia, which activates hypoxia-inducible factor- 1 (HIF-1) and consequently stimulates the process of angiogenesis by upregulating target pro-angiogenic genes such as VEGF-A.26,33 Pro-angiogenic factors are also released by monocytes, platelets, and fibroblasts during angiogenesis, and VEGF-A is the most prominent cytokine that regulates this process. In addition to promoting angiogenesis, VEGF-A also increases vascular permeability and contributes to wound edema.33,34 Other pro-angiogenic factors associated with wounds include fibroblast growth factor −2, platelet-derived growth factor (PDGF), and members of the transforming growth factor beta (TGF-β) family.34–37 The activated EC then severs their interactions with the neighboring EC, degrades the basement membrane, and digests their surrounding extracellular matrix (ECM) components by secreting matrix metalloproteinases (MMP).38 Activated EC along with other cells, like fibroblasts, platelets, smooth muscle cells, and monocytes/macrophages, secrete important proangiogenic cytokines such as VEGF-A, PDGF, and TGF-β. These further help the EC to invade and migrate through the ECM, proliferate, and reestablish cell-cell contacts, thereby forming new capillaries.38 In wound healing, the neovessels grow into the wound at a rapid rate, forming a network of newly formed blood vessels that exceed the numbers of vessels in the normal skin by many folds. However, the new vessels formed are highly disorganized and poorly perfused.34,39Once immature granulation tissue and microvasculature fill up the wound bed, resolution factors trigger vascular maturation and remodeling.39 During this phase, the new capillaries are remodeled along with the connective tissue and epithelium; they gain normal vascular permeability, blood flow, and are pruned to exhibit normal vascular branching.39 Pericytes, a type of mural cells, help in this wound maturation phase by stabilizing the microvascular capillaries and the vascular basement membrane.40, 41 At the end of this phase, the granulation tissue is remodeled due to the reabsorption of excess blood vessels and the disappearance of fibroblasts, and ultimately, a scar composed primarily of dense collagen along with some intermittent, widely scattered fibrocytes and blood vessels are formed.34,39The role of angiogenesis in the different phases of wound healing are represented in Figure 1.
FIGURE 1:
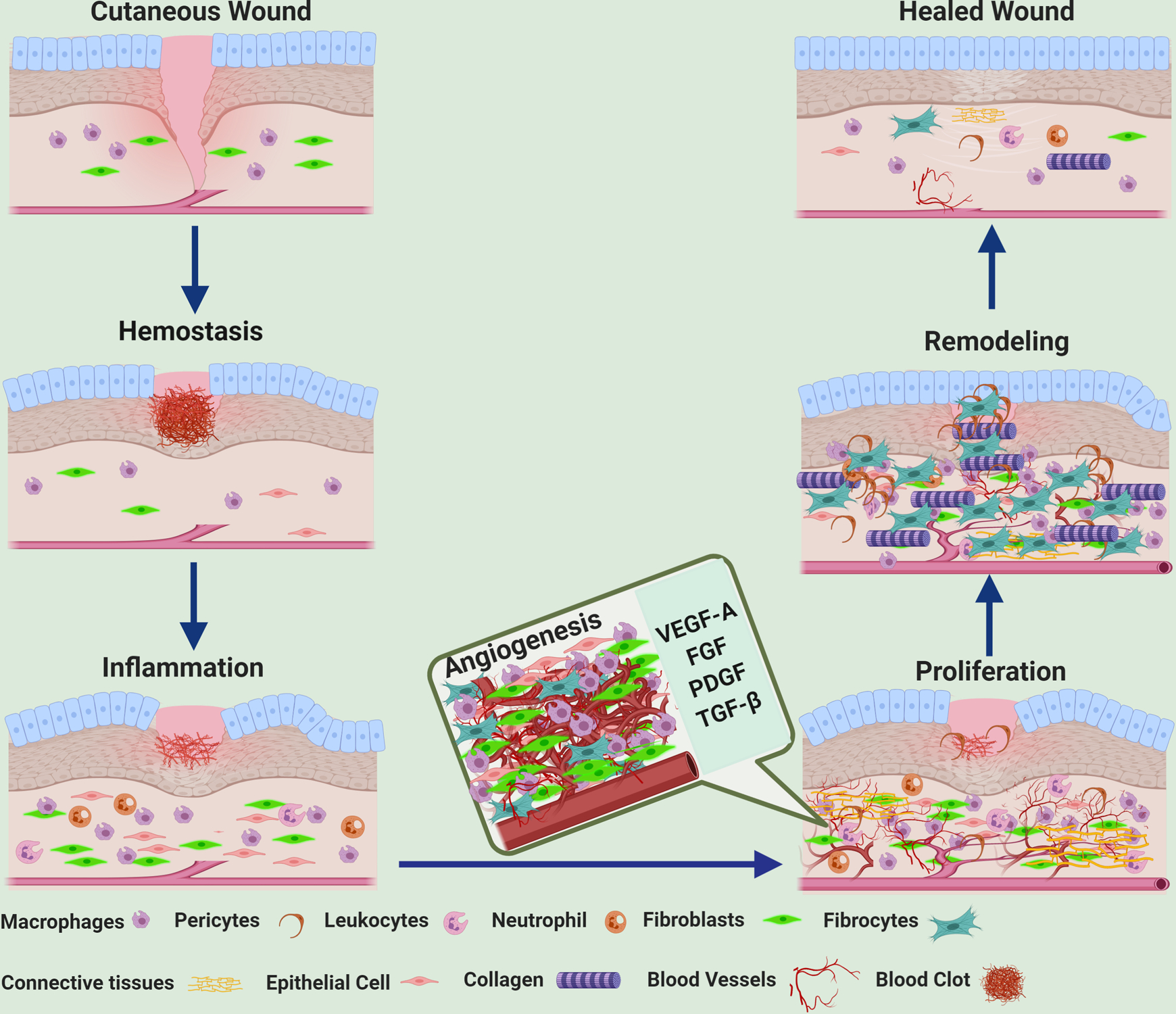
Schematic diagram showing that angiogenesis is an important step during the proliferative phase of dermal wound healing (Created with BioRender.com). VEGF-A = Vascular Endothelial Growth Factor-A, FGF = fibroblast growth factor, PDGF = platelet-derived growth factor, TGFβ = transforming growth factor beta
DIABETIC WOUNDS AND ANGIOGENESIS
Diabetic wound healing and angiogenesis present a global challenge clinically as well as in research settings. As evident in both clinical and experimental diabetes, the disease affects all stages of wound healing, resulting in an impaired healing capacity.39,42,43 Disruptions in hemostasis and inflammation, matrix deposition, and angiogenesis in the wound bed are associated with non-healing cutaneous diabetic wounds. Furthermore, cutaneous wounds in diabetics show a dysfunctional inflammatory response, microbial invasion, epithelial breakdown, and impaired immune function along with an abnormal expression of chemokines. The underlying factor associated with inadequate wound healing in diabetes is impaired vascular response.42
During normal wound healing, angiogenesis depends upon an intricate balance between pro- and anti-angiogenic factors, which maintains the balance between vessel growth promotion, vessel maturation, and quiescence.4,8,10 However, the scenario changes during diabetes, where a decrease in the pro-angiogenic stimulus is noted.39,42 In diabetes, macrophages, one of the major sources of VEGF-A and other pro-angiogenic mediators, as well as fibroblasts undergo phenotypic changes and have altered functions.44 The levels of VEGF-A are deficient in experimental and clinical diabetic wounds. Besides, diabetes also alters the levels of anti-angiogenic factors, which leads to reduced angiogenesis and delayed wound healing.39, 43
Furthermore, vascular integrity is disturbed in diabetes as a result of elevated levels of systemic glucose, which adversely affects EC and makes them dysfunctional; consequently, this causes several microvascular and macrovascular complications.39,45 Thus, wound healing under diabetic conditions exhibits decreased vascularity and capillary density. These processes, in turn, disrupt tissue regeneration and restoration of a functional vascular system, which leads to delayed wound closure.39,43 The angiopoietin 1/angiopoietin 2/TEK tyrosine kinase (Ang1/Ang2/Tie2) pathway also plays a role in the defective angiogenic process seen in diabetic wounds. The ratio of Ang1 to Ang2 is decreased in diabetes, which results in the disruption of vessel maturation.42, 46, 47
CATECHOLAMINES IN THE SKIN
Dopamine (DA), norepinephrine (NE)/noradrenaline, and epinephrine (E)/adrenaline are the naturally occurring CA derived from amino acid tyrosine.48,49 They play important roles as neurotransmitters in the brain or sympathetic nervous system, or as neurohormones produced by the adrenal medulla.48,49 Neurons, as well as non-neuronal cells of different tissues, including the skin50–52, produce DA, NE, and E.
CA are the principal autonomic neurotransmitters in the skin, as 25–50 % of sympathetic nerve terminals target skin effectors, innervate blood vessels, sweat glands, and hair follicles, and emerge as nerve fibers in the dermal and epidermal layers of the skin.53 The skin is also capable of synthesizing CA, their degrading enzymes, and high-affinity receptors, which have been identified in nerve fibers, keratinocytes, and melanocytes.50
NE and E act through α (α1 and α2) and β (β1 and β2) adrenergic receptors in target cells.12,50 These receptors are part of the classic seven-transmembrane G-protein-coupled receptor (GPCR) family. α1 (α1a, α1b, and α1d) adrenoceptors (AR) couple to phospholipase C via Gqα, leading to the formation of diacylglycerol and inositol trisphosphate and increased intracellular calcium level, while α2 (α2a, α2b, and α2c) AR couple to Giα, causing the downregulation of adenylate cyclase and consequently the inhibition of intracellular cyclic AMP (cAMP).50 β-AR activates adenylate cyclase to increase intracellular cAMP via Gsα.50 α- and β-AR are present on epidermal and dermal cells and are also expressed in dermal blood vessels,54 while β2- and α1-AR are expressed mostly on keratinocytes.50,52,55
DA acts on specific receptors present in target cells belonging to the GPCR family, which are divided into D1 (D1 and D5) and D2 (D2, D3, and D4) classes of receptors. Activated D1 class of receptors increases Gs-dependent intracellular cAMP, whereas the D2 class activates Gi proteins and inhibits intracellular cAMP. In addition, D2-like receptors via Gβγ subunits can inhibit N- and L-type calcium channels leading to the activation of G protein-regulated inwardly rectifying potassium channels.50,56 Keratinocytes express D2-like receptors; D4 receptors are mostly found in the uppermost part of the epidermis, whereas D2 receptors occur in the basal layer of the epidermis.50
CA plays vital roles in cutaneous homeostasis and inflammation.50,57 Furthermore, it plays diverse roles in cutaneous wound healing by either positively or negatively affecting the process, which in turn either speeds up or delays wound closure.
CATECHOLAMINES, ANGIOGENESIS AND CUTANEOUS WOUNDS
Epinephrine and norepinephrine
The roles of E and NE in the process of wound healing have been demonstrated by studies that have mainly shown the effect of stress on the healing of wounds, as both acute and chronic stress enhance circulating and tissue CA in the body due to the activation of the sympathoadrenal system.57,58 The results of these studies indicate that stress has adverse effects on the cutaneous wound restoration process and that the process of wound healing is impaired in stressed animals due to the high levels of CA, which leads to the activation of AR.57,58 Both E and NE act through AR,48,49 and hence, in this review, we will discuss the roles of NE and E along with AR modulation.
In general, it has been shown that the blockade or inhibition of β2-AR promoted angiogenesis in wound bed by increasing proangiogenic growth factors and therefore expedited the healing of wounds, which confirmed the deleterious effect of these receptors on the overall wound-healing process.59,60,61 However, β2-AR is expressed in all types of cells in the skin-like epithelial cells, macrophages, and EC that take part in the wound repair process; therefore, their roles in the wound-healing process are multifaceted.62,63 In particular, β2-AR activation in EC suppressed the angiogenic processes in the wound bed by inhibiting factors such as collagen III deposition, myofibroblasts density, and re-epithelialization, and by prolonging the overall healing time, which indicates that β2-AR blockade may improve angiogenesis and expedite the healing of these wounds.63 A study using murine excisional skin wound models showed that the stimulation of β-AR in EC caused delayed wound healing through the inhibition of EC functions such as mobilization, proliferation, and tube formation capabilities of dermal EC, which are crucial for the process of angiogenesis.62 The study demonstrated that the activation of β-AR resulted in the inhibition of secretion of proangiogenic growth factors, which included fibroblast growth factor 2 from human dermal microvascular endothelial cells (HDMEC) and VEGF from keratinocytes. The findings further showed that in isolated HDMEC, the stimulation of β-AR caused impaired angiogenic response via a cAMP-dependent but PKA-independent pathway.62
Although high levels of systemic CA in chronically stressed mice have been implicated with impaired and delayed wound healing via activation of β-AR, interestingly, a significant increase in the number of blood vessels has been reported in these stressed mice compared to non-stressed control animals. This increase in the blood vessels has been attributed to stress or CA induced vasoconstriction, which resulted in increased tissue hypoxia, i.e., reduced blood flow and oxygen availability to the skin and other extremities64,65 and therefore more angiogenesis in the wound bed. The infusion of exogenous E (level achieved in acute stress) also reduced blood perfusion in tissues, an essential part of tissue regeneration and healing.66 Treatment with propranolol, a β adrenergic blocker, abrogated the systemic effects of CA by activating MMP‐2 and MMP‐9 and increasing collagen turnover, along with less vascularized and more organized collagenous granulation tissue.59,61
NE, like E in most of the cases, was shown to affect the overall wound-healing process negatively.67,68 The role of NE in wound bed has been demonstrated in studies that either use pharmacological blockade of α or β receptors or use global knockout (of these receptors) mice to study the effect of these receptors on the overall wound-healing process. NE-depleted mice showed increased wound angiogenesis69 and skin wounds were more vascularized in β-AR antagonist-treated rats.70 However, the effect of NE on isolated EC is contradictory and mostly context-dependent.69 Different studies have indicated that NE could directly stimulate proliferation and hypertrophy of large vessel-derived EC as well as promote apoptotic cell death in cardiac-derived EC.71,72
In addition to β-AR, the other AR, that is, α1/α2 AR, also play substantial roles in wound angiogenesis and wound restoration processes. α2A/α2C receptor-knockout animals showed a high level of CA that is commonly observed in stressed individuals and animals suffering from chronic inflammatory conditions.68,73 The deletion of the α2A/α2C‐AR significantly promoted cutaneous wound healing and wound closure by increasing angiogenesis, TGF‐β 1/2/3 expression, keratinocyte migration, collagen deposition, and myofibroblastic differentiation.68
In summary, the role of E and NE in wound angiogenesis needs more in-depth investigation. From available reports till date it is difficult to single out the effects of these two CA on angiogenesis during wound healing due to the presence of AR not only on EC but also on other cell types such as keratinocytes, fibroblasts, macrophages that are present in wound beds and take part in the wound healing response. The main effects of AR activation and inhibition on the angiogenic process of wound healing have been summarized in Table 1.
Table 1:
Effects of AR activation and inhibition on the angiogenic process of wound healing
| Receptor activation/ inhibition | Effect on angiogenesis and wound healing | Reference |
|---|---|---|
| β2-AR activation | inhibition of mobilization, proliferation, and tube formation capabilities of dermal EC; delayed wound healing | 62 |
| β2-AR activation | inhibition of secretion of fibroblast growth factor 2 from human dermal microvascular endothelial cells (HDMEC), and VEGF from keratinocytes; delayed wound healing | 62 |
| β–AR inhibition | stimulates MMP‐2 and MMP‐9 and promotes collagen turnover and less vascularized and more organized collagenous granulation tissue; faster wound healing | 59 |
| α1/α2 AR deletion | increases angiogenesis, TGF‐β 1/2/3 expression, keratinocyte migration, collagen deposition, and myofibroblastic differentiation; faster wound healing | 68 |
Dopamine
In addition to its essential role in the central nervous system (CNS), DA also plays important regulatory roles in different peripheral organs, including the skin.50,56,74,75 The nerve endings present in the skin synthesize DA, and keratinocytes, the most abundant cell type in the epidermis, and also express enzymes that synthesize and metabolize DA.50 Different cell types in the skin, such as the keratinocytes, also express different DA receptors.50 Our group has reported that EC express D2 receptors and dermal fibroblasts express D1 receptors.76,77 Differential expression of the D2 and D4 receptors has been demonstrated in the different epidermal layers50,74 of the skin. D2 receptors on the epidermal keratinocytes play a critical role in cutaneous barrier homeostasis.74
The regulatory role of DA in VEGF-A-mediated angiogenesis, via its D2 receptors present in the EC and endothelial progenitor cells, was reported by our laboratory and confirmed by other research groups.16,18, 20,78 In the context of wound healing, we have identified that DA plays either a negative or a positive role depending on whether the wound is a normal cutaneous wound or a diabetic wound.76,77 Our group has reported that DA D1 receptor activation in dermal fibroblasts could promote angiogenesis and shorten healing time both in type 1 (streptozotocin-induced) and type 2 (db/db) diabetic mice. DA D1 receptor activation stimulated the production of VEGF-A by the cells through the protein kinase A pathway and the phosphorylation of cAMP response element-binding protein.77
In contrast, reports from our laboratory have also indicated that DA can negatively regulate the angiogenic process in normal dermal wounds via the D2 receptors. DA through its D2 receptors downregulated VEGF induced expressions of homeobox transcription factor (HoxD3) and its target genes α5 and β1 integrins,76 which play critical roles in wound angiogenesis and thereby slowed down the healing process. Treatment with specific D2 receptor antagonist, eticlopride, could significantly accelerate the process of healing by inducing angiogenesis through the upregulation of HoxD3 and its target, α5β1 integrin.76 Our studies have thus demonstrated that DA plays opposing roles in normal and diabetic cutaneous wounds, which involves the differential activation of D1 or D2 receptors.76,77
Several studies have indicated that angiogenesis can also be induced by mesenchymal stem cells (MSCs) that differentiate into EC and promote neoangiogenesis.79–82 During the process of wound healing, circulating MSCs migrate to the wound site by chemotaxis and subsequently get incorporated into neovessels and are transdifferentiated into EC.83,84 Furthermore, these incorporating MSCs release different proangiogenic factors, such as VEGF-A, which further promote neovessel formation and healing.82,85,86 A report from our group indicated that the concentration of DA in the synaptic clefts negatively regulated the mobilization of MSCs into the wound sites. DA acting through its D2 receptors present on MSCs inhibited VEGF-A-induced phosphorylation of Akt, which in turn suppressed the polymerization dynamics of the actin cytoskeleton. This decreased actin polymerization subsequently inhibited the migration of MSCs and inhibited angiogenesis.86 DA mediated regulation of angiogenesis during cutaneous wound healing has been summarized in Figure 2.
FIGURE 2:
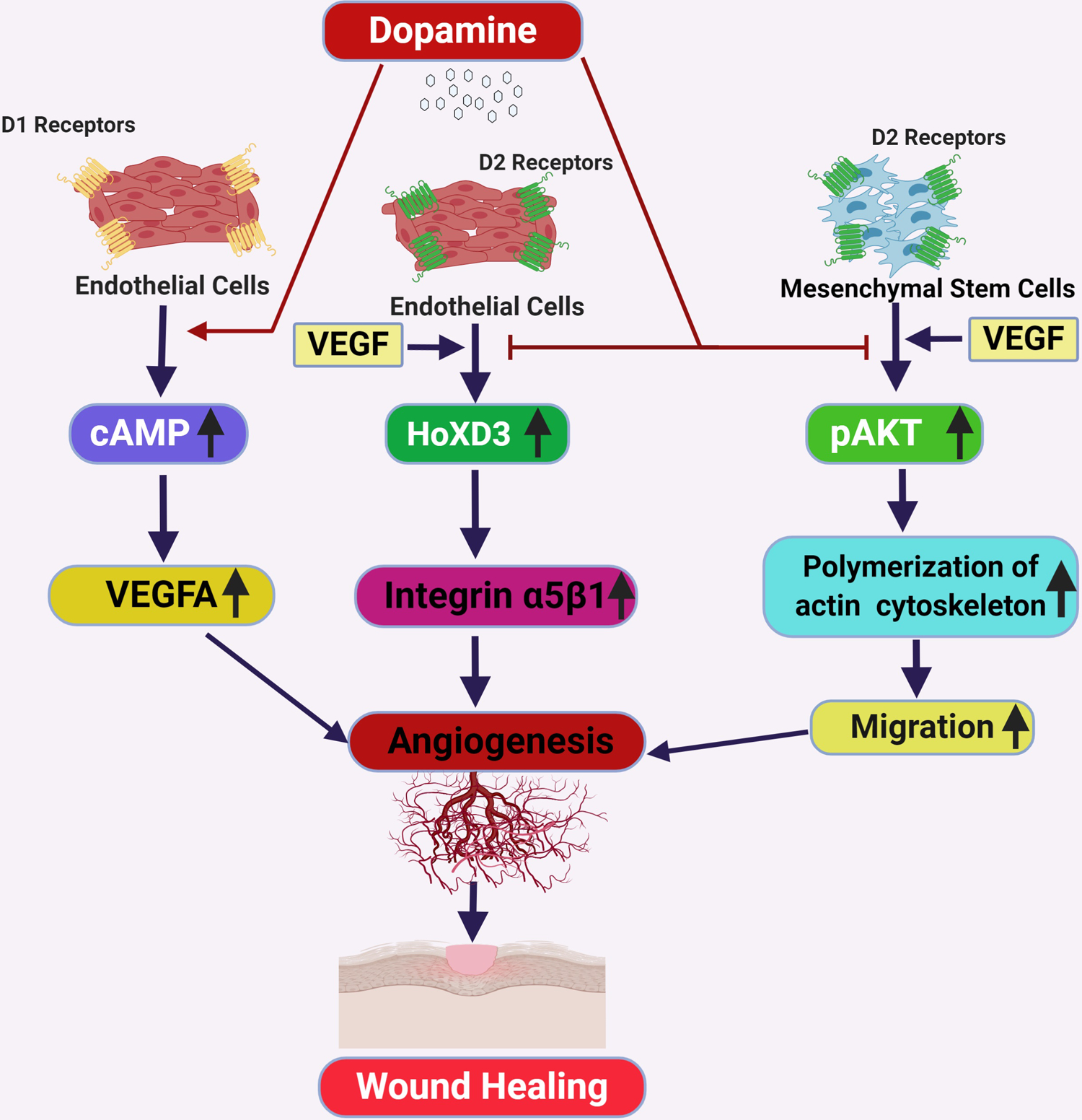
Schematic diagram of dopamine mediated regulation of angiogenesis during wound healing (Created with BioRender.com). VEGF= Vascular Endothelial Growth Factor, pAKT= phopho-AKT, cAMP=cyclic adenosine 3′,5′-monophosphate, HoXD3=Homeobox D3, D1 receptor= Dopamine D1 receptor, D2 receptor = Dopamine D2 receptor
CONCLUSION AND FUTURE DIRECTIONS
Few reports have suggested that wound closure can proceed normally even with decreased angiogenesis, which may occur with antiangiogenic agents or blockade of signaling pathways,34,87,88 implying that angiogenesis might not solely be responsible for the regulation of the process. However, several other reports have suggested that angiogenesis plays critical roles in the cutaneous wound healing process by supporting cellular proliferation, migration, and other metabolic activities. Therefore, approaches that can promote angiogenesis during wound healing may be considered as possible therapeutic strategies to improve wound healing. CA neurotransmitters or neurohormones are important regulators of physiological angiogenesis.14–17,19, 22–24,75–78 NE and E inhibit the process of angiogenesis and thereby retard the healing process.59–63 On the contrary, DA inhibits angiogenesis during post-ischemic healing by suppressing angiotensin receptor type 1 expression in EC and either promotes or inhibits cutaneous wound angiogenesis, depending on whether the wound is diabetic or normal, through the activation of DA D1 or D2 receptors, respectively.14–21,75–77
Furthermore, one of the early events in granulation tissue formation during wound healing is angiogenesis.8,9 To date, there are only a few studies that have looked into the role of CA in angiogenesis during wound healing. Therefore, it is necessary to conduct such studies to fill in the significant gaps in the knowledge about the exact roles of CA in wound healing. These studies might help to identify new therapeutic approaches to manage this critical step of the wound healing response. Lastly, it is of utmost importance that the mechanism of cutaneous wound healing is well-understood to develop strategies to overcome impaired wound healing. Results from clinical trials have confirmed that DA D2 receptor agonists can inhibit VEGF-A-mediated angiogenesis in diseases.78 Besides, both β blockers and DA agonists are used clinically for different disorders, and they are considered to have established safety profiles.78,89 Therefore, clinical trials may be conducted with these compounds towards the development of therapeutic agents for cutaneous wounds.
FUNDING
This work was supported by the grant from the National Institutes of Health, USA (R01DK098045 to S.Basu).
ABBREVIATIONS
- CA
catecholamines
- VEGF-A
vascular endothelial growth factor-A
- Ang1
angiopoietin1
- EC
endothelial cells
- HIF-1
hypoxia-inducible factor-1
- PDGF
platelet-derived growth factor
- TGF-β
transforming growth factor beta
- ECM
extracellular matrix
- MMP
metalloproteinases
- Ang2
angiopoietin 2
- Tie2
TEK tyrosinekinase
- DA
Dopamine
- Norepinephrine
norepinephrine
- E
epinephrine
- GPCR
G-protein-coupled receptor
- AR
adrenoceptors
- cAMP
cyclic AMP
Footnotes
CONFLICT OF INTEREST STATEMENTS
The authors declare that they have no conflict of interests.
REFERENCES
- 1.Shaw TJ, Martin P Wound repair at a glance. J Cell Sci. 2009;122:3209–3213. doi: 10.1242/jcs.031187 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Sorg H, Tilkorn DJ, Hager S, Hauser J, Mirastschijski U. Skin Wound Healing: An Update on the Current Knowledge and Concepts. Eur Surg Res. 2017;58:81–94. doi: 10.1159/000454919 [DOI] [PubMed] [Google Scholar]
- 3.Gosain A, DiPietro LA. Aging and wound healing. World J Surg. 2004;28:321–326. doi: 10.1007/s00268-003-7397-6 [DOI] [PubMed] [Google Scholar]
- 4.Guo S, Dipietro LA. Factors affecting wound healing. J Dent Res. 2010;89:219–229. doi: 10.1177/0022034509359125 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Mathieu D, Linke J-C, Wattel F. Non-healing wounds. In: Handbook on hyperbaric medicine, Mathieu DE, editor. Netherlands: Springer, 2006, 401–427 [Google Scholar]
- 6.Sen CK, Gordillo GM, Roy S, et al. Human skin wounds: a major and snowballing threat to public health and the economy. Wound Repair Regen. 2009;17:763–771. doi: 10.1111/j.1524-475X.2009.00543.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Menke NB, Ward KR, Witten TM, Bonchev DG, Diegelmann RF. Impaired wound healing. Clin Dermatol. 2007;25:19–25. doi: 10.1016/j.clindermatol.2006.12.005 [DOI] [PubMed] [Google Scholar]
- 8.Eming SA, Brachvogel B, Odorisio T, Koch M. Regulation of angiogenesis: wound healing as a model. Prog Histochem Cytochem. 2007;42:115–170. doi: 10.1016/j.proghi.2007.06.001 [DOI] [PubMed] [Google Scholar]
- 9.Gurtner G, Werner S, Barrandon Y et al. Wound repair and regeneration. Nature. 2008;453: 314–321. 10.1038/nature07039 [DOI] [PubMed] [Google Scholar]
- 10.Tonnesen MG, Feng X, Clark RA. Angiogenesis in wound healing. J Investig Dermatol Symp Proc. 2000;5:40–46. doi: 10.1046/j.1087-0024.2000.00014.x [DOI] [PubMed] [Google Scholar]
- 11.Werner S, Grose R. Regulation of wound healing by growth factors and cytokines. Physiol Rev. 2003;83:835–870. doi: 10.1152/physrev.2003.83.3.835 [DOI] [PubMed] [Google Scholar]
- 12.Yamakawa S, Hayashida K. Advances in surgical applications of growth factors for wound healing. Burn Trauma. 2019; 7:10. 10.1186/s41038-019-0148-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Öhnstedt E, Tomenius HL, Vågesjö E, Phillipson M. The discovery and development of topical medicines for wound healing, Expert Opinion on Drug Discovery. 2019;14:485–497, DOI: 10.1080/17460441.2019.1588879 [DOI] [PubMed] [Google Scholar]
- 14.Chakroborty D, Sarkar C, Basu B, Dasgupta PS, Basu S. Catecholamines regulate tumor angiogenesis. Cancer Res. 2009;69:3727–3730. doi: 10.1158/0008-5472.CAN-08-4289 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Sarkar C, Chakroborty D, Basu S. Neurotransmitters as regulators of tumor angiogenesis and immunity: the role of catecholamines. J Neuroimmune Pharmacol. 2013;8:7–14. doi: 10.1007/s11481-012-9395-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Basu S, Nagy JA, Pal S, et al. The neurotransmitter dopamine inhibits angiogenesis induced by vascular permeability factor/vascular endothelial growth factor. Nat Med. 2001;7:569–574. doi: 10.1038/87895 [DOI] [PubMed] [Google Scholar]
- 17.Basu S, Sarkar C, Chakroborty D, et al. Ablation of peripheral dopaminergic nerves stimulates malignant tumor growth by inducing vascular permeability factor/vascular endothelial growth factor-mediated angiogenesis. Cancer Res. 2004;64:5551–5555. doi: 10.1158/0008-5472.CAN-04-1600 [DOI] [PubMed] [Google Scholar]
- 18.Sarkar C, Chakroborty D, Mitra RB, Banerjee S, Dasgupta PS, Basu S. Dopamine in vivo inhibits VEGF-induced phosphorylation of VEGFR-2, MAPK, and focal adhesion kinase in endothelial cells. Am J Physiol Heart Circ Physiol. 2004;287:H1554–H1560. doi: 10.1152/ajpheart.00272.2004 [DOI] [PubMed] [Google Scholar]
- 19.Chakroborty D, Sarkar C, Mitra RB, Banerjee S, Dasgupta PS, Basu S. Depleted dopamine in gastric cancer tissues: dopamine treatment retards growth of gastric cancer by inhibiting angiogenesis. Clin Cancer Res. 2004;10:4349–4356. doi: 10.1158/1078-0432.CCR-04-0059 [DOI] [PubMed] [Google Scholar]
- 20.Chakroborty D, Chowdhury UR, Sarkar C, Baral R, Dasgupta PS, Basu S. Dopamine regulates endothelial progenitor cell mobilization from mouse bone marrow in tumor vascularization. J Clin Invest. 2008;118:1380–1389. doi: 10.1172/JCI33125 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Chakroborty D, Sarkar C, Yu H, et al. Dopamine stabilizes tumor blood vessels by up-regulating angiopoietin 1 expression in pericytes and Kruppel-like factor-2 expression in tumor endothelial cells. Proc Natl Acad Sci USA. 2011;108:20730–20735. doi: 10.1073/pnas.1108696108 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Yang EV, Kim SJ, Donovan EL, et al. Norepinephrine up-regulates VEGF, IL-8, and IL-6 expression in human melanoma tumor cell lines: implications for stress-related enhancement of tumor progression. Brain Behav Immun. 2009;23:267–275. doi: 10.1016/j.bbi.2008.10.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Tilan J, Kitlinska J. Sympathetic Neurotransmitters and Tumor Angiogenesis-Link between Stress and Cancer Progression. J Oncol. 2010;2010:539706. doi: 10.1155/2010/539706 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Thaker PH, Han LY, Kamat AA, et al. Chronic stress promotes tumor growth and angiogenesis in a mouse model of ovarian carcinoma. Nat Med. 2006;12:939–944. doi: 10.1038/nm1447 [DOI] [PubMed] [Google Scholar]
- 25.Demidova-Rice TN, Durham JT, Herman IM. Wound Healing Angiogenesis: Innovations and Challenges in Acute and Chronic Wound Healing. Adv Wound Care (New Rochelle). 2012;1:17–22. doi: 10.1089/wound.2011.0308 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Johnson KE, Wilgus TA. Vascular Endothelial Growth Factor and Angiogenesis in the Regulation of Cutaneous Wound Repair. Adv Wound Care (New Rochelle). 2014;3:647–661. doi: 10.1089/wound.2013.0517 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Michalczyk ER, Chen L, Fine D, et al. Pigment Epithelium-Derived Factor (PEDF) as a Regulator of Wound Angiogenesis. Sci Rep. 2018;8:11142. 10.1038/s41598-018-29465-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Tandara AA, Mustoe TA. Oxygen in wound healing--more than a nutrient. World J Surg. 2004;28:294–300. doi: 10.1007/s00268-003-7400-2 [DOI] [PubMed] [Google Scholar]
- 29.de Oliveira S, Rosowski EE, Huttenlocher A. Neutrophil migration in infection and wound repair: going forward in reverse. Nat Rev Immunol. 2016;16:378–391. doi: 10.1038/nri.2016.49 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Schultz GS, Chin GA, Moldawer L, et al. Principles of Wound Healing. In: Fitridge R, Thompson M, editors. Mechanisms of Vascular Disease: A Reference Book for Vascular Specialists [Internet]. Adelaide (AU): University of Adelaide Press; 2011. 23. Available from: https://www.ncbi.nlm.nih.gov/books/NBK534261/ [PubMed] [Google Scholar]
- 31.Koh TJ, DiPietro LA. Inflammation and wound healing: the role of the macrophage. Expert Rev Mol Med. 2011;13:e23. doi: 10.1017/S1462399411001943 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Demidova-Rice TN, Hamblin MR, Herman IM. Acute and impaired wound healing: pathophysiology and current methods for drug delivery, part 2: role of growth factors in normal and pathological wound healing: therapeutic potential and methods of delivery. Adv Skin Wound Care. 2012;25:349–370. doi: 10.1097/01.ASW.0000418541.31366.a3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Bao P, Kodra A, Tomic-Canic M, Golinko MS, Ehrlich HP, Brem H. The role of vascular endothelial growth factor in wound healing. J Surg Res. 2009;153:347–358. doi: 10.1016/j.jss.2008.04.023 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.DiPietro LA. Angiogenesis and wound repair: when enough is enough. J Leukoc Biol. 2016;100:979–984. doi: 10.1189/jlb.4MR0316-102R [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Nissen NN, Polverini PJ, Gamelli RL, DiPietro LA. Basic fibroblast growth factor mediates angiogenic activity in early surgical wounds. Surgery. 1996;119:457–465. doi: 10.1016/s0039-6060(96)80148-6 [DOI] [PubMed] [Google Scholar]
- 36.Grotendorst GR, Grotendorst CA, Gilman T. Production of growth factors (PDGF & TGF-beta) at the site of tissue repair. Prog Clin Biol Res. 1988;266:47–54 [PubMed] [Google Scholar]
- 37.Uutela M, Wirzenius M, Paavonen K, et al. PDGF-D induces macrophage recruitment, increased interstitial pressure, and blood vessel maturation during angiogenesis. Blood. 2004;104:3198–3204. doi: 10.1182/blood-2004-04-1485 [DOI] [PubMed] [Google Scholar]
- 38.Caley MP, Martins VL, O’Toole EA. Metalloproteinases and Wound Healing. Adv Wound Care (New Rochelle). 2015;4:225–234. doi: 10.1089/wound.2014.0581 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Okonkwo UA, Chen L, Ma D, et al. Compromised angiogenesis and vascular Integrity in impaired diabetic wound healing. PLoS One. 2020;15:e0231962. doi: 10.1371/journal.pone.0231962 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Dulmovits BM, Herman IM. Microvascular remodeling and wound healing: a role for pericytes. Int J Biochem Cell Biol. 2012;44:1800–1812. doi: 10.1016/j.biocel.2012.06.031 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Thomas H, Cowin AJ, Mills SJ. The Importance of Pericytes in Healing: Wounds and other Pathologies. Int J Mol Sci. 2017;18:1129. doi: 10.3390/ijms18061129 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Okonkwo UA, DiPietro LA. Diabetes and Wound Angiogenesis. Int J Mol Sci. 2017;18:1419. doi: 10.3390/ijms18071419 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Altavilla D, Saitta A, Cucinotta D, et al. Inhibition of lipid peroxidation restores impaired vascular endothelial growth factor expression and stimulates wound healing and angiogenesis in the genetically diabetic mouse. Diabetes. 2001;50:667–674. doi: 10.2337/diabetes.50.3.667 [DOI] [PubMed] [Google Scholar]
- 44.Mirza R, Koh TJ. Dysregulation of monocyte/macrophage phenotype in wounds of diabetic mice. Cytokine. 2011;56:256–264. doi: 10.1016/j.cyto.2011.06.016 [DOI] [PubMed] [Google Scholar]
- 45.Cade WT. Diabetes-related microvascular and macrovascular diseases in the physical therapy setting. Phys Ther. 2008;88:1322–1335. doi: 10.2522/ptj.20080008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Isidori AM, Venneri MA, Fiore D. Angiopoietin-1 and Angiopoietin-2 in metabolic disorders: therapeutic strategies to restore the highs and lows of angiogenesis in diabetes. J Endocrinol Invest. 2016;39:1235–1246. doi: 10.1007/s40618-016-0502-0 [DOI] [PubMed] [Google Scholar]
- 47.Kämpfer H, Pfeilschifter J, Frank S. Expressional regulation of angiopoietin-1 and −2 and the tie-1 and −2 receptor tyrosine kinases during cutaneous wound healing: a comparative study of normal and impaired repair. Lab Invest. 2001;81(3):361–373. doi: 10.1038/labinvest.3780244 [DOI] [PubMed] [Google Scholar]
- 48.Ganong WF. Synaptic and junctional transmission. In: Ganong WF, editor. Review of medical physiology. New York: McGraw-Hill; 2005. p. 85–120 [Google Scholar]
- 49.Goldstein DS, Eisenhofer G, Kopin IJ. Sources and significance of plasma levels of catechols and their metabolites in humans. J Pharmacol Exp Ther. 2003;305:800–811. doi: 10.1124/jpet.103.049270 [DOI] [PubMed] [Google Scholar]
- 50.Slominski AT, Zmijewski MA, Skobowiat C, Zbytek B, Slominski RM, Steketee JD. Sensing the environment: regulation of local and global homeostasis by the skin’s neuroendocrine system. Adv Anat Embryol Cell Biol. 2012;212:v–115 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Kim LR, Whelpdale K, Zurowski M, Pomeranz B. Sympathetic denervation impairs epidermal healing in cutaneous wounds. Wound Repair Regen. 1998;6:194–201. doi: 10.1046/j.1524-475x.1998.60305.x [DOI] [PubMed] [Google Scholar]
- 52.Sivamani RK, Lam ST, Isseroff RR. Beta adrenergic receptors in keratinocytes. Dermatol Clin. 2007;25:643–653. doi: 10.1016/j.det.2007.06.012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Borrel V, Thomas P, Catovic C, et al. Acne and Stress: Impact of Catecholamines on Cutibacterium acnes. Front Med (Lausanne). 2019;6:155. doi: 10.3389/fmed.2019.00155 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Schallreuter KU, Wood JM, Lemke R, et al. Production of catecholamines in the human epidermis. Biochem Biophys Res Commun. 1992;189:72–78. doi: 10.1016/0006-291x(92)91527-w [DOI] [PubMed] [Google Scholar]
- 55.Steinkraus V, Steinfath M, Körner C, Mensing H. Binding of beta-adrenergic receptors in human skin. J Invest Dermatol. 1992;98:475–480. doi: 10.1111/1523-1747.ep12499860 [DOI] [PubMed] [Google Scholar]
- 56.Beaulieu JM, Gainetdinov RR. The physiology, signaling, and pharmacology of dopamine receptors. Pharmacol Rev. 2011;63:182–217. doi: 10.1124/pr.110.002642 [DOI] [PubMed] [Google Scholar]
- 57.Kim MH, Gorouhi F, Ramirez S, et al. Catecholamine stress alters neutrophil trafficking and impairs wound healing by β2-adrenergic receptor-mediated upregulation of IL-6. J Invest Dermatol. 2014;134:809–817. doi: 10.1038/jid.2013.415 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Sivamani RK, Pullar CE, Manabat-Hidalgo CG, et al. Stress-mediated increases in systemic and local epinephrine impair skin wound healing: potential new indication for beta blockers. PLoS Med. 2009;6:e12. doi: 10.1371/journal.pmed.1000012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Romana-Souza B, Porto LC, Monte-Alto-Costa A. Cutaneous wound healing of chronically stressed mice is improved through catecholamines blockade. Exp Dermatol. 2010;19:821–829. doi: 10.1111/j.1600-0625.2010.01113.x [DOI] [PubMed] [Google Scholar]
- 60.Pullar CE, Le Provost GS, O’Leary AP, Evans SE, Baier BS, Isseroff RR. β2AR antagonists and β2AR gene deletion both promote skin wound repair processes. J Invest Dermatol. 2012;132:2076–2084. doi: 10.1038/jid.2012.108 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Romana-Souza B, Nascimento AP, Monte-Alto-Costa A. Propranolol improves cutaneous wound healing in streptozotocin-induced diabetic rats. Eur J Pharmacol. 2009;611:77–84. doi: 10.1016/j.ejphar.2009.03.053 [DOI] [PubMed] [Google Scholar]
- 62.O’Leary AP, Fox JM, Pullar CE. Beta-Adrenoceptor Activation Reduces Both Dermal Microvascular Endothelial Cell Migration via a cAMP-Dependent Mechanism and Wound Angiogenesis. J Cell Physiol. 2015;230:356–365. doi: 10.1002/jcp.24716 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Pullar CE, Manabat-Hidalgo CG, Bolaji RS, Isseroff RR. beta-Adrenergic receptor modulation of wound repair. Pharmacol Res. 2008;58:158–164. doi: 10.1016/j.phrs.2008.07.012 [DOI] [PubMed] [Google Scholar]
- 64.Franz MG, Robson MC, Steed DL, et al. Guidelines to aid healing of acute wounds by decreasing impediments of healing. Wound Repair Regen. 2008;16:723–748. doi: 10.1111/j.1524-475X.2008.00427.x [DOI] [PubMed] [Google Scholar]
- 65.Romana-Souza B, Otranto M, Vieira AM, Filgueiras CC, Fierro IM, Monte-Alto-Costa A. Rotational stress-induced increase in epinephrine levels delays cutaneous wound healing in mice. Brain Behav Immun. 2010;24:427–437. doi: 10.1016/j.bbi.2009.11.012 [DOI] [PubMed] [Google Scholar]
- 66.Bryant R, Nix D. Acute & Chronic Wounds: Current Management Concepts Editors: Chapter 28: Perfusion and Oxygenation, Whitney Joanne D 5th edition, Elsevier, St. Louis, MO. 2016. [Google Scholar]
- 67.Gosain A, Muthu K, Gamelli RL, DiPietro LA. Norepinephrine suppresses wound macrophage phagocytic efficiency through alpha- and beta-adrenoreceptor dependent pathways. Surgery. 2007;142:170–179. doi: 10.1016/j.surg.2007.04.015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Romana-Souza B, Nascimento AP, Brum PC, Monte-Alto-Costa A. Deletion of the α2A/α2C-adrenoceptors accelerates cutaneous wound healing in mice. Int J Exp Pathol. 2014;95:330–341. doi: 10.1111/iep.12093 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Gosain A, Jones SB, Shankar R, Gamelli RL, DiPietro LA. Norepinephrine modulates the inflammatory and proliferative phases of wound healing. J Trauma. 2006;60:736–744. doi: 10.1097/01.ta.0000196802.91829.cc [DOI] [PubMed] [Google Scholar]
- 70.Zhang H, Cotecchia S, Thomas SA, Tanoue A, Tsujimoto G, Faber JE. Gene deletion of dopamine beta-hydroxylase and alpha1-adrenoceptors demonstrates involvement of catecholamines in vascular remodeling. Am J Physiol Heart Circ Physiol. 2004;287:H2106–H2114. doi: 10.1152/ajpheart.00290.2004 [DOI] [PubMed] [Google Scholar]
- 71.Fu YC, Chi CS, Yin SC, Hwang B, Chiu YT, Hsu SL. Norepinephrine induces apoptosis in neonatal rat endothelial cells via down-regulation of Bcl-2 and activation of beta-adrenergic and caspase-2 pathways. Cardiovasc Res. 2004;61:143–151. doi: 10.1016/j.cardiores.2003.10.014 [DOI] [PubMed] [Google Scholar]
- 72.Padgett DA, Marucha PT, Sheridan JF. Restraint stress slows cutaneous wound healing in mice. Brain Behav Immun. 1998;12:64–73. doi: 10.1006/brbi.1997.0512 [DOI] [PubMed] [Google Scholar]
- 73.Vaughn AR, Davis MJ, Sivamani RK, Isseroff RR. A Concise Review of the Conflicting Roles of Dopamine-1 versus Dopamine-2 Receptors in Wound Healing. Molecules. 2017;23:50. doi: 10.3390/molecules23010050 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Missale C, Nash SR, Robinson SW, Jaber M, Caron MG. Dopamine receptors: from structure to function. Physiol Rev. 1998;78:189–225. doi: 10.1152/physrev.1998.78.1.189 [DOI] [PubMed] [Google Scholar]
- 75.Sarkar C, Ganju RK, Pompili VJ, Chakroborty D. Enhanced peripheral dopamine impairs post-ischemic healing by suppressing angiotensin receptor type 1 expression in endothelial cells and inhibiting angiogenesis. Angiogenesis. 2017;20:97–107. doi: 10.1007/s10456-016-9531-8 [DOI] [PubMed] [Google Scholar]
- 76.Shome S, Rana T, Ganguly S, et al. Dopamine regulates angiogenesis in normal dermal wound tissues. PLoS One. 2011;6:e25215. doi: 10.1371/journal.pone.0025215 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Chakroborty D, Sarkar C, Lu K, Bhat M, Dasgupta PS, Basu S. Activation of Dopamine D1 Receptors in Dermal Fibroblasts Restores Vascular Endothelial Growth Factor-A Production by These Cells and Subsequent Angiogenesis in Diabetic Cutaneous Wound Tissues. Am J Pathol. 2016;186:2262–2270. doi: 10.1016/j.ajpath.2016.05.008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Alvarez C, Martí-Bonmatí L, Novella-Maestre E, et al. Dopamine agonist cabergoline reduces hemoconcentration and ascites in hyperstimulated women undergoing assisted reproduction. J Clin Endocrinol Metab. 2007;92:2931–2937. doi: 10.1210/jc.2007-0409 [DOI] [PubMed] [Google Scholar]
- 79.Wu Y, Chen L, Scott PG, Tredget EE. Mesenchymal stem cells enhance wound healing through differentiation and angiogenesis. Stem Cells. 2007;25:2648–2659. doi: 10.1634/stemcells.2007-0226 [DOI] [PubMed] [Google Scholar]
- 80.Sasaki M, Abe R, Fujita Y, Ando S, Inokuma D, et al. Mesenchymal stem cells are recruited into wounded skin and contribute to wound repair by transdifferentiation into multiple skin cell type. J Immunol. 2008;180:2581–2587. doi: 10.4049/jimmunol.180.4.2581. [DOI] [PubMed] [Google Scholar]
- 81.Kasper G, Dankert N, Tuischer J, Hoeft M, Gaber T. Mesenchymal stem cells regulate angiogenesis according to their mechanical environment. Stem Cells. 2007;25:903–910. doi: 10.1634/stemcells.2006-0432 [DOI] [PubMed] [Google Scholar]
- 82.Karp JM, Leng Teo GS. Mesenchymal stem cell homing: the devil is in the details. Cell Stem Cell. 2009;4:206–216. doi: 10.1016/j.stem.2009.02.001 [DOI] [PubMed] [Google Scholar]
- 83.He Q, Wan C, Li G. Concise review: multipotent mesenchymal stromal cells in blood. Stem Cells. 2007;25:69–77. doi: 10.1634/stemcells.2006-0335. [DOI] [PubMed] [Google Scholar]
- 84.Hocking AM, Gibran NS. Mesenchymal stem cells: paracrine signaling and differentiation during cutaneous wound repair. Exp Cell Res. 2010;316:2213–2219. doi: 10.1016/j.yexcr.2010.05.009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Kaigler D, Krebsbach PH, Polverini PJ, Mooney DJ. Role of vascular endothelial growth factor in bone marrow stromal cell modulation of endothelial cells. Tissue Eng. 2003;9:95–103. doi: 10.1089/107632703762687573 [DOI] [PubMed] [Google Scholar]
- 86.Shome S, Dasgupta PS, Basu S. Dopamine regulates mobilization of mesenchymal stem cells during wound angiogenesis. PLoS One. 2012;7:e31682. doi: 10.1371/journal.pone.0031682 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Berger AC, Feldman AL, Gnant MF, et al. The angiogenesis inhibitor, endostatin, does not affect murine cutaneous wound healing. J Surg Res. 2000;91:26–31. doi: 10.1006/jsre.2000.5890 [DOI] [PubMed] [Google Scholar]
- 88.Roman CD, Choy H, Nanney L, et al. Vascular endothelial growth factor-mediated angiogenesis inhibition and postoperative wound healing in rats. J Surg Res. 2002; 105:43–47. doi: 10.1006/jsre.2002.6444 [DOI] [PubMed] [Google Scholar]
- 89.Perron L, Bairati I, Harel F, Meyer F. Antihypertensive drug use and the risk of prostate cancer (Canada). Cancer Causes Control. 2004;15:535–541. doi: 10.1023/B:CACO.0000036152.58271.5e [DOI] [PubMed] [Google Scholar]


