SUMMARY
Dengue has become a global problem in past few decades, with half of the world's population at risk of infection. For some countries of Asia and Latin America, severe dengue is a major cause of serious illness and even death in children. Pakistan has been reported as a hyperendemic area for dengue infection. Our study aimed to find seroprevalence of past dengue infection in asymptomatic children of Lahore with no previous history of dengue infection. A total of 400 samples were collected from children aged 1–12 years in Lahore using random sampling. The inclusion criteria were children aged 1–12 years, who had no previous symptoms of dengue fever during their lives. Children with known immunodeficiency status or fever at the time of recruitment were excluded from the study. Commercially available ELISA kits were used to determine the IgG status in sera of children. The data obtained was entered and analysed using SPSS v. 20.0. The overall prevalence of asymptomatic dengue infection was found to be 25%. There was no statistically significant difference between prevalence of infection in male and female children. There was, however, a strong relationship between increasing age of the child and number of cases with infection, with low incidence in children aged ⩽5 years.
Key words: Asymptomatic, children, dengue, school aged, seroprevalence
INTRODUCTION
Dengue virus (DENV) is an arthropod-borne, single-stranded RNA virus (genus Flavivirus, family Flaviviridae). It is comprised of four closely related but antigenically distinct serotypes, DENV-1, -2, -3, and -4 which are widespread in humans, and in recent years has become a major international public health concern [1]. A new genetically distinct serotype of DENV was isolated while sequencing samples collected in a 2007 Malaysian outbreak. The virus was found to be phylogenetically different and was classified as dengue serotype 5 [2]. DENV-5 is a sylvatic serotype, and so far there has been only one outbreak of this serotype reported in humans. Whereas DENV-1–4 serotypes are endemic in a number of tropical and subtropical countries, there is no evidence of sustained epidemics by sylvatic DENV in the past. Dengue outbreaks are a leading cause of illness, hospital admissions and death in children in various regions of Asia and Latin America [3]. Presently no specific therapies or vaccines are available for treatment or prevention of DENV transmission [1, 4].
Outbreaks have been recorded in all countries of the Indian subcontinent including India, Pakistan, Maldives and Sri Lanka. These outbreaks included thousands of reported and unreported cases along with causalities caused by dengue [5]. During the past few decades DENV-2 and DENV-3 have been predominant in most of the outbreaks, with either one or both serotypes isolated in each major outbreak [5, 6].
Pakistan first reported an outbreak of dengue fever in 1994. DENV is now endemic in Pakistan, circulating throughout the year with a peak incidence in the post-monsoon period [3, 7]. According to a report of the Punjab Health Department, the total number of dengue patients in Punjab from January to November 2011 reached 21 275, of which more than 17 454 were diagnosed in Lahore alone [1, 8]. The total figure of reported deaths due to dengue fever during this period was 335 in Punjab and 289 in Lahore [9].
The WHO has classified dengue infection into levels of severity of the disease. The classification is between patients with severe disease and those with non-severe disease. Patients with non-severe dengue are further classified into those with warning signs and those without any warning signs or asymptomatic dengue. Both patients with warning signs and asymptomatic dengue infection can develop severe disease [10].
Studies from populations where dengue is endemic suggest that 14–87% of cases are asymptomatic or subclinical [11, 12]. Asymptomatic dengue infection is defined as a DENV infection that results in no clinical manifestations or an illness that is mild and is not associated with a visit to a healthcare provider. Asymptomatic dengue infection, because of its nature, is not detected by surveillance programmes as most programmes use visits to a healthcare provider or hospitalization as an indicator of dengue illness. Most patients recover from this asymptomatic disease unnoticed; however, some cases may progress to severe disease characterized by plasma leakage with or without haemorrhage.
The asymptomatic seropositive population where a previous episode of dengue goes undetected is at risk for developing haemorrhagic dengue instead of classic dengue. This is significant because in most other prospective studies, all patients who were hospitalized for dengue suffered secondary dengue infections [13, 14]. Although infection with a given serotype confers lifelong protection against that serotype, DENV serotypes are not cross-protective, and re-infection with a second serotype has been linked to development of the more severe haemorrhagic form of dengue referred to as dengue haemorrhagic fever, characterized by plasma leakage with or without haemorrhage [15, 16].
Age is an important variable in the outcome of dengue infection. In Pakistan the median age of dengue patients has decreased during 2003–2007 leading to a progressive increase in the proportion of children affected with dengue [17]. No children were affected in 2003 and 2004 but the number of children testing positive for DENV IgM increased markedly by 2·6%, 10·9%, and 13·9% in 2005, 2006 and 2007, respectively [17]. The observed shift in median age may explain the acquisition of immunity due to earlier symptomatic/asymptomatic infections [18]. Since the risk of death in a child during a second dengue infection is nearly 15 times higher than in adults, probably due to increased microvascular permeability, a secondary dengue infection in seropositive children must be prevented in order to avoid deaths [19, 20].
METHODOLOGY
Study design
The study design utilized a cross-sectional survey.
Study population
The study population was comprised of children aged between 1 and 12 years, resident in Lahore, Pakistan for the last 5 years.
Sample size
Sample size was estimated at the 5% level of significance with 80% power of test for an expected frequency of 36·9 ± 7% of asymptomatic children for dengue [21]. An estimated sample size of 400 was calculated for Lahore. For the selection of 400 children about 200 households were required on the basis of 2·33 children aged 1–12 years per household.
Sampling technique
A two-stage random sampling technique was adopted using the following plan. The city is divided into 19 towns and 146 Union Councils (UC) for administrative purposes. For sample collection in this study, Lahore was divided into four regions by assuming a cross between two geographical landmarks, Canal road and Ferozepur road. In stage 1, five UCs from each of the four regions were selected at random. The UCs of Lahore randomly selected were: region I (Gulberg town), region IV (Iqbal town), region III (Aziz Bhatti town and Wahga town), and region II (Ravi town and Data Gunj Baksh town). In stage 2, 20 children were randomly selected from each UC.
Inclusion/exclusion criteria
Inclusion criteria: children aged 1–12 years, without a history of dengue fever at any point in their life. Exclusion criteria: children with known immunodeficiency, or fever (auxiliary temperature ⩾37·5 °C) at the time of recruitment.
Data collection
After ethical clearance from the Institutional Review Board of Shaikh Zayed Medical Complex Lahore (IRB no. 1275), a minimum of 20 children aged 1–12 years, fulfilling inclusion criteria, were selected from each UC. In the areas where the target of 20 enrolled children from a UC was not achieved, the number of households was extended in the adjacent area until the sample size was obtained. Informed consent was obtained from parent/legal guardian of each child. Demography and history of study participants was collected through a questionnaire.
Collection of blood samples
From each enrolled child, 5 ml of venous blood was drawn; of which 2 ml was transferred into a plain glass tube. Serum was separated by centrifugation at 2683 g for 10 min and stored at −20 °C in labelled vials until further analysis for DENV IgG antibodies.
DENV IgG antibodies were determined in blood samples of children from Lahore in order to screen asymptomatic dengue infection in apparently healthy children with no clinical history of dengue fever. For the determination of dengue IgG a DENV IgG ELISA (Calbiotech, USA) was used. The cut-off was calculated by multiplying the mean absorbance of the calibrator with the calibrator factor. Further, the antibody index for positive and negative control was calculated from the absorbance of each control and cut-off value of the assay batch. All values <0·9 were considered negative for DENV, 0·9–1·1 were considered as borderline-positive and >1·1 were positive for IgG antibody to DENV. Analysis of DENV IgG was performed in five batches where each batch included 80 samples.
Ethical standards
The authors assert that all procedure contributing to this work comply with the ethical standards of the relevant national and institutional committees on human experimentation and with the Helsiniki Declaration of 1975, as revised in 2008.
RESULTS
Age and gender distribution of children
A total of 191 households were visited for the collection of blood samples to determine DENV IgG. Out of 191 households 61 were selected from region I, 45 from region II, 40 from region III and 45 from region IV.
Table 1 shows the distribution and number of children according to age and gender selected from different regions of Lahore. A total of 413 samples from children aged 1–12 years were collected. After data entry and cleaning, a sample of 400 children was processed for analysis. Data of 13 children was not included due to incomplete information (Table 1).
Table 1.
Distribution of study children according to age and gender in different regions of Lahore
| Gender distribution of children | |||
|---|---|---|---|
| Age (years) | Male n (%) | Female n (%) | Total n (%) |
| Region I | |||
| ⩽5·0 | 12 (17·9) | 10 (22·2) | 22 (19·6) |
| 5·1–9·0 | 27 (40·3) | 19 (42·2) | 46 (41·1) |
| 9·1–12·0 | 28 (41·8) | 16 (35·6) | 44 (39·3) |
| Region total | 67 (100) | 45 (100) | 112 (100) |
| Region II | |||
| ⩽5·0 | 16 (26·2) | 14 (35·0) | 30 (29·7) |
| 5·1–9·0 | 22 (36·1) | 18 (45·0) | 40 (39·6) |
| 9·1–12·0 | 23 (37·7) | 8 (20·0) | 31 (30·7) |
| Region total | 61 (100) | 40 (100) | 101 (100) |
| Region III | |||
| ⩽5·0 | 21 (42·0) | 15 (30·6) | 36 (36·4) |
| 5·1–9·0 | 18 (36·0) | 23 (46·9) | 41 (41·4) |
| 9·1–12·0 | 11 (22·0) | 11 (22·4) | 22 (22·2) |
| Region total | 50 (100) | 49 (100) | 99 (100) |
| Region IV | |||
| ⩽5·0 | 15 (23·8) | 10 (40·0) | 25 (28·4) |
| 5·1–9·0 | 23 (36·5) | 10 (40·0) | 33 (37·5) |
| 9·1–12·0 | 25 (39·7) | 5 (20·0) | 30 (34·1) |
| Region total | 63 (100) | 25 (100) | 88 (100) |
| Subtotal | |||
| ⩽5·0 | 64 (26·6) | 49 (30·8) | 113 (28·3) |
| 5·1–9·0 | 90 (37·3) | 70 (44·0) | 160 (40·0) |
| 9·1–12·0 | 87 (36·1) | 40 (25·2) | 127 (31·8) |
| Grand total | 241 (100) | 159 (100) | 400 (100) |
Prevalence of asymptomatic dengue in Lahore
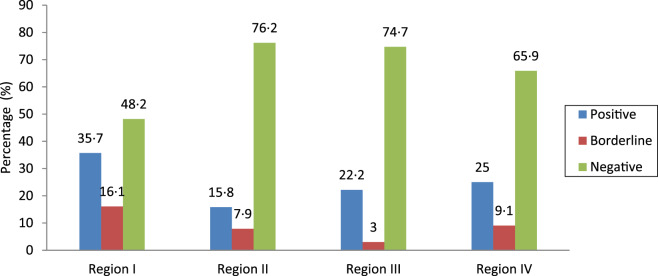
The overall prevalence of positive DENV IgG cases, i.e. asymptomatic dengue infection in children aged 1–12 years residing in Lahore, was 25%. The prevalences of positive, borderline-positive and negative cases found in different regions of Lahore are given in Figure 1. It was determined that the highest prevalence of asymptomatic dengue infection (DENV IgG-positive cases) was in region I (35·7%) with lowest in region II (15·8%) (Fig. 1).
Fig. 1.
Prevalence of dengue IgG-positive cases in asymptomatic children in different regions in Lahore.
DENV IgG status according to gender
DENV IgG status of children (positive, borderline-positive, negative), with respect to gender is described in Table 2. It was noted that most of the DENV IgG-positive cases, i.e. asymptomatic dengue, were males (26·1%). the same trend was also observed in borderline-positive cases. But the difference between male and female positive cases was not statistically significant (P = 0·157), showing that asymptomatic dengue infection was not associated with male gender (Table 2).
Table 2.
Dengue IgG status in asymptomatic children according to gender
| Dengue IgG status | Male n (%) | Female n (%) | χ2 | P value |
|---|---|---|---|---|
| Positive | 63 (26·1) | 37 (23·3) | 3·70 | 0·157 |
| Negative | 151 (62·6) | 112 (70·4) | ||
| Borderline | 27 (11·3) | 10 (6·3) | ||
| Total | 241 (100) | 159 (100) |
DENV IgG status according to age
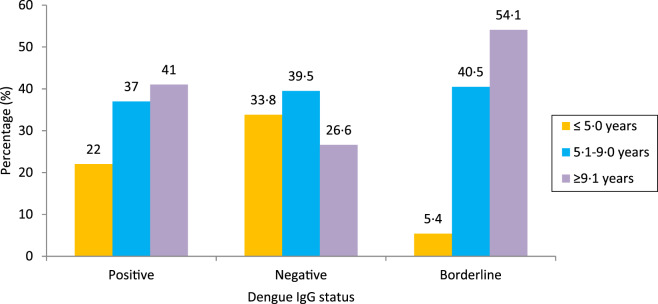
DENV IgG status according to the age of enrolled children is shown in Figure 2. An increase was seen in number of DENV IgG-positive cases with an increase in age. The prevalence of DENV IgG-positive or asymptomatic dengue infection was lowest (22·0%) in children aged ⩽5 years, which increased with an increase in age, i.e. 37·0% in children aged 5·1–9·0 years and 41·0% in children aged 9·1–12·0 years. It was observed that increasing age was significantly associated with positive asymptomatic dengue infection (χ2 = 20·018, P = 0·000 <0·05) (Fig. 2).
Fig. 2.
Dengue IgG status in asymptomatic children with respect to their age (χ2 = 20·018, P = 0·000 < 0·05).
DISCUSSION
Pakistan has been reported as a hyperendemic area for dengue infection similar to various other countries in Asia including Malaysia, Taiwan, and India [22]. The U.S. Center for Infectious Disease Control Unit and the WHO consider DENV infection as a threat for India, Pakistan and Brazil [23]. The serious outcome of disease is more frequent in children and young adults. DENV causes asymptomatic infection much more frequently than symptomatic infection. A true burden of infection due to DENV can only be estimated when the prevalence due to both asymptomatic and symptomatic dengue infections is recorded, which becomes difficult due to unreported and misdiagnosed cases [24, 25].
There has been much effort applied to the estimation of symptomatic dengue infection or dengue fever with signs, and dengue haemorrhagic fever in retrospective as well as prospective studies in Pakistan. However little is known about the burden of disease caused by asymptomatic infection due to lack of screening for dengue infection. The present study aimed to estimate prevalence of asymptomatic dengue infection in school-aged children in Lahore. For this purpose, Lahore was divided into four regions.
It was observed that highest prevalence of asymptomatic dengue infection was in region I followed by regions IV and III, while lowest prevalence recorded in region II. The demography of Lahore city shows a marked difference in socioeconomic status and lifestyle of people residing in these four regions. The four regions vary considerably in terms of urbanization and green belt areas, with region I consisting of large green belts and increased urbanization, and region II lacking the most in green belt areas. The difference could be accounted for by lack of host sites for mosquitoes in region II compared to region I. In another study it was reported that certain demographic and societal changes are thought to be associated with the reappearance of lethal dengue infection over the past 50 years [26, 27]. The factors responsible for endemics in such areas are rapid population growth, urbanization, lack of vector control, climate variability, green belts, rainfall, and increased travel (especially air travel) to endemic areas [28]. Due to the above-mentioned factors, there is an increase in the reportable cases of dengue infection in different countries with more severe forms of the disease [29].
In the present study the overall seroprevalence of asymptomatic dengue infection was measured as 25% in children aged 1–12 years, residing in the city of Lahore, Pakistan. A similar study conducted in rural areas of Rawalpindi district showed the seroprevalence of dengue viral infection to be 13·5% in healthy adults [30]. Our study showed a higher prevalence compared to the Rawalpindi study, this may be due the fact that dengue viral infection is considered to be present mostly in urban and semi-urban areas [31]. Our results were consistent with the findings of another study, in which the seroprevalence of DENV antibodies in asymptomatic Costa Rican children was determined during 2002–2003. It was seen that a significant number of Costa Rican children were infected with asymptomatic dengue infection, showing a prevalence of 36·9% [21].
In children in whom previous dengue infection went unnoticed due to lack of symptoms, the threat of dengue haemorrhagic fever or dengue shock syndrome can increase dangerously. The present study has emphasized the imminent threat of future cases of dengue haemorrhagic fever and dengue shock syndrome in these seropositive cases.
It was observed that increase in number of DENV IgG cases, increases with the age of the child. The prevalence of DENV IgG or asymptomatic dengue infection was lowest (22·0%) in children aged ⩽5 years. One plausible explanation could be children begin formal schooling at this age. There is an increase in prevalence of infected children further with an increase in age; i.e. 37·0% in children aged 5·1–9·0 years and 41·0% in children aged 9·1–12·0 years.
Aedes aegypti is a biting mosquito that has become highly adapted domestically and feeds during daylight hours. The mosquito dwells indoors in dark and cold places, and often attacks the back or lower limbs, usually from under desks and chairs [32]. As children grow older and reach school age, they are exposed to the vector for a longer period of time due to school and outdoor play. Even though cases of dengue have been reported in coastal regions in children aged <6 years, the incidence shows a tendency to rise after this age [21].
In Pakistan the majority of the population attend government-funded public schools. Such schools are mostly neglected in terms of maintenance and hygiene and hence provide an ideal setting for dengue mosquitos. Potential sources of dengue mosquitoes are thus easy to find in public school buildings and their surroundings. This puts the majority of school-aged children in the country at a greater risk of being exposed to dengue mosquitoes. Our results show high prevalence of asymptomatic dengue infection in the region. If left untreated in the future this infected population could lead to a major public health crisis in the form of severe disease. Such high prevalence of the infection also suggests the necessity of precautionary measures for protection from infection by decreasing exposure to the vector. Such measures include concentrating mosquito control programmes in areas where children spend more time, covering the body with protective clothing during day time, and keeping the environment clean and dry.
CONCLUSION
Prevalence of asymptomatic dengue infection was substantially higher in children residing in Lahore, Pakistan. The present study has emphasized the likely threat of dengue outbreaks in children of this region at any time in the near future. Secondary dengue in seropositive children must be prevented in order to avoid deaths. Precautionary measures should be targeted to prevent these children from developing severe disease in future because the risk of morbidity and mortality is higher in this group. More extensive serological surveys and selective surveillance to assess the real extent of disease burden due to DENV infection are needed to advise appropriate measures for its prevention and control.
ACKNOWLEDGEMENTS
This material is based on the work supported by the Pakistan Medical and Research Council. The authors thank and acknowledge the hard work and dedication of staff of the National Health Research Complex, Lahore.
This work was supported by the Pakistan Medical and Research Council, grant no. 4-23-8/12/MCS/Dengue/RDC/NHRC/651. The grant was approved for multicenter project with total value of PKR 2 928 957 for 12 months. A sum of PKR 987 505 was allocated to the National Health Research Complex, Lahore under the supervision of principal author S. Mohsin.
DECLARATION OF INTEREST
None.
REFERENCES
- 1.WHO Media Centre (http://www.who.int/mediacentre/factsheets/fs117/en/). Accessed 15 February 2015.
- 2.Science Mag (www.sciencemag.org/news/2013/10/first-new-dengue-virus-50-years). Accessed 25 January 2016.
- 3.Rigau JG, et al. Dengue and dengue hemorrhagic fever. Lancet 1998; 352: 971–977. [DOI] [PubMed] [Google Scholar]
- 4.Mathew A, Rothman AL. Understanding the contribution of cellular immunity to dengue disease pathogenesis. Immunological Reviews 2008; 225: 300–313. [DOI] [PubMed] [Google Scholar]
- 5.Raheel U, et al. Dengue fever in the Indian subcontinent: an overview. Journal of Infection in Developing Countries 2011; 5: 239–247. [DOI] [PubMed] [Google Scholar]
- 6.Fatima Z, et al. Serotype and genotype analysis of dengue virus by sequencing followed by phylogenetic analysis using samples from three mini outbreaks – 2007–2009 in Pakistan. BMC Microbiology 2011; 11: 200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.WHO. Fact sheet No. 117 (http://www.who.int/mediacentre/factsheets/fs117/en/). Accessed 14 December 2011.
- 8.Morrison AC, et al. Defining challenges and proposing solutions for control of the virus vector Aedes aegypti. PLoS Medicine 2008; 5: e68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Tropical Medical Bureau (http://www.tmb.ie/exodus/news.asp?title=289Dengue-cases-in-Pakistan-show-encouraging-declineandid=184659). Accessed 13 December 2011.
- 10.Almas A, Parkash O, Akhter J. Clinical factors associated with mortality in dengue infection at a tertiary care center. Southeast Asian Journal of Tropical Medicine and Public Health 2010; 41: 333–340. [PubMed] [Google Scholar]
- 11.Burke DS, et al. A prospective study of dengue infections in Bangkok. American Journal of Tropical Medicine and Hygiene 1988; 38: 172–180. [DOI] [PubMed] [Google Scholar]
- 12.McBride W. The 1993 dengue 2 epidemic in Charters Towers, North Queensland: clinical features and public health impact. Epidemiology and Infection 1998; 121: 151–156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Sangkawibha N, et al. Risk factors in dengue shock syndrome: a prospective 304 epidemiological study in Rayong, Thailand. American Journal of Epidemiology 1984; 20: 653–659. [DOI] [PubMed] [Google Scholar]
- 14.Winter PE, et al. An insular outbreak of dengue hemorrhagic fever Epidemiological observations. American Journal of Tropical Medicine and Hygiene 1968; 17: 561–573. [DOI] [PubMed] [Google Scholar]
- 15.Rothman AL, Ennis FA. Immunopathogenesis of dengue hemorrhagic fever. Virology 1999; 257: 1–6. [DOI] [PubMed] [Google Scholar]
- 16.Henchal E, et al. Detection of dengue virus RNA using nucleic acid hybridization. Journal of Virological Methods 1987; 15: 187–200. [DOI] [PubMed] [Google Scholar]
- 17.Khan E, et al. Demographic and Clinical Features of Dengue Fever in Pakistan from 2003–2007: A retrospective cross-sectional study. PLoS ONE 2010; 5: e12505. Published online: 13 September 2010. doi: 10.1371/journal.pone.0012505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Chan YC, et al. Dengue hemorrhagic fever outbreak in Karachi, Pakistan. Transaction of the Royal Society of Tropical Medicine and Hygiene 1995; 89: 619–620. [DOI] [PubMed] [Google Scholar]
- 19.Halstead SB. Dengue. Current Opinion in Infectious Diseases 2002; 15: 471–476. [DOI] [PubMed] [Google Scholar]
- 20.Herrera-Basto E, et al. First report outbreak of classical dengue fever at 1,700 meters above sea level in Guerrero State, Mexico, Jun 1988. American Journal of Tropical Medicine and Hygiene 1992; 46: 649–653. [DOI] [PubMed] [Google Scholar]
- 21.Iturrino-Monge R, et al. Seroprevalence of dengue virus antibodies in asymptomatic Costa Rican children, 2002–2003: a pilot study. Pan American Journal of Public Health 2006; 20: 39–43. [DOI] [PubMed] [Google Scholar]
- 22.Gubler DJ. Dengue and dengue hemorrhagic fever in the Americas. Puerto Rico Health Sciences Journal 1987; 6: 107–111. [PubMed] [Google Scholar]
- 23.New US. Army mosquito control technology licensed for deployment against dengue (http://www.medicalnewstoday.com/articles/13006/php). Accessed 13 November 2011.
- 24.Halstead SB, Rigau-Perez JG, Gubler DJ. Is there an unapparent dengue explosion?. Lancet 2009; 353: 1100–1101. [DOI] [PubMed] [Google Scholar]
- 25.Hayes EB, Gubler DJ. Dengue and dengue hemorrhagic fever. Pediatric Infectious Disease Journal 1992; 11: 311–317. [DOI] [PubMed] [Google Scholar]
- 26.Ahmed A. Diagnosing Dengue Fever. Infectious Diseases Journal Pakistan 2005; 14: 129–132. [Google Scholar]
- 27.Lanciotti RS, et al. Rapid detection and typing of dengue viruses from clinical samples by using reverse transcriptase-polymerase chain reaction. Journal of Clinical Microbiology 1992; 30: 545–551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Medscape Clinical Reference (http://emedicine.medscape.com/article/215840-overview). Accessed 15 May 2009.
- 29.Gubler DJ. Dengue and dengue hemorrhagic fever. Clinical Microbiology Reviews 1998; 11: 480–496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Zafar H, et al. Seroprevalence of dengue viral infection in healthy population residing in rural areas of district Rawalpindi. International Journal of Pathology 2010; 8: 13–15. [Google Scholar]
- 31.Gubler DJ. The global resurgence of arboviral diseases. Transactions of the Royal Society of Tropical Medicine and Hygiene. 1996; 90: 449–451. [DOI] [PubMed] [Google Scholar]
- 32.Dengue virus net.com (http://www.denguevirusnet.com/aedes-aegypti.html). Accessed 23 January 2016.