Summary
Wnt1 inducible protein-1 signaling pathway (WISP-1) is a relatively new adipokine involved in many cellular processes, including epithelial mucosa healing. The aim of the study was to compare circulating levels of WISP-1 and other selected adipokines [adiponectin, resistin and retinol-binding protein 4 (RBP-4)] in children with inflammatory bowel disease (IBD) with healthy controls and to investigate possible differences between Crohn’s disease patients. (CD) or ulcerative colitis (UC). The study was performed as a case-control study. In addition to adipokines, anthropometric, lipid parameters, markers of inflammation or disease activity were evaluated in all participants. Compared to healthy controls (n=20), significantly lower levels of adiponectin and higher levels of resistin and WISP-1 were found in patients with IBD (n=58). Elevation of WISP-1 was detected only in the CD group (n=31). There were no differences in RBP-4 levels between the groups. Adiponectin, WISP-1 and RBP-4 were independently associated with body mass index only, resistin levels were associated with C-reactive protein levels and leukocyte counts. Adverse adipokines production reflects presence of dysfunctional fat tissue in IBD patients. Higher levels of WISP-1 in CD compared to patients with UC may indicate a specific role for mesenteric adipose tissue in WISP-1 production.
Keywords: Inflammatory bowel disease, Wnt1 inducible signaling pathway protein-1, Adiponectin, Resistin, Retinol-binding protein 4
Introduction
Inflammatory bowel diseases (IBDs) include Crohn’s disease (CD) and ulcerative colitis (UC). Pediatric IBD is considered more severe, extensive, and it is associated with more frequent complications [1]. The etiopathogenesis of IBD results from imbalance between genetic predisposition, environmental factors (infections, diet, smoking, drugs, stress and socioeconomic status), and the gut microbiome [2,3,4]. Adipose tissue is an endocrine and immune organ that significantly contributes to inflammatory processes. Visceral fat, especially mesenteric adipose tissue, appears to be involved in the pathogenesis of IBD, especially in CD patients. Mesenteric fat serves as a barrier to inflammation and controls immune responses to translocation of intestinal bacteria. At the same time, mesenteric adipose tissue may be a major source of cytokines and adipokines responsible for the inflammatory processes associated with IBD [5]. Adipokines are a group of mediators primarily released by adipocytes that modulate various metabolic functions in adipose tissue, liver, brain, muscle, pancreas, and the immune system. Regulatory immune function has been identified in many of them. The role of several adipokines in mesenteric adipose tissue as well as in intestinal inflammation has recently been investigated [6,7].
Adiponectin exerts anti-inflammatory effects through the modulation of signaling pathways and may therefore play a critical role in IBD severity and its treatment [8]. The results of many studies suggest that adiponectin can promote the anti-inflammatory response through the modulation of phagocytosis or suppression of pro-inflammatory cytokines, such as tumor necrosing factor alpha (TNF-α) and interleukin (IL)-6, by suppressing nuclear factor-kappa B (NF-κB) signaling [9]. Adiponectin also increases the secretion of anti-inflammatory cytokines such as IL-10 and IL-1 receptor antagonist by human monocytes, macrophages, and dendritic cells [6]. However, some studies have revealed that the role of adiponectin in intestinal inflammatory disease may be pro-proliferative and pro-inflammatory through the activation of some kinases and NF-κB signaling in colonic epithelial cells [10]. Leptin has strong pro-inflammatory effects by synergizing with TNF-α, thereby activating macrophages and forming reactive oxygen species in neutrophils [6]. Inflamed colonic epithelial cells express and release leptin into the intestinal lumen. In response to luminal leptin, the model intestinal epithelium critically activates NF-κB, a key signaling system for pro-inflammatory stimuli; luminal leptin can cause neutrophil infiltration into the colonic epithelium [10,11]. Resistin is also associated with inflammatory processes because its expression in adipose tissue is induced by pro-inflammatory cytokines (IL-1, IL-6 and TNF-α) via the NF-κB signaling pathway [12,13]. Anti-inflammatory therapy in patients with IBD significantly reduce serum levels of resistin [14]. There are reports that serum levels of retinol-binding protein 4 (RBP-4), another adipokine linked to the metabolic syndrome, has been reported to be elevated in IBD patients [15] and inversely correlated with disease activity [16]. Many other adipokines, such as chemerin, visfatin, vaspin, apelin, omentin-1, or meteorin-like, may be also involved in IBD pathogenesis [5].
Wnt1-inducible signaling pathway protein 1 (WISP-1) is a member of the secreted extracellular matrix–associated proteins of the CCN family (originally abbreviated from the first three members CYR61/CCN1, CTGF/CCN2, and NOV/CCN3 and then officially renamed as cellular communication network factors) [17]. WISP-1 participates in many cellular processes, including proliferation, differentiation, apoptosis and adhesion. It plays an important role in diverse pathophysiological processes, such as embryonic development, inflammation, mucosal healing, injury repairs and cancers [18]. Growing evidence links the WISP-1 to the regulation of adipogenesis and low-grade inflammation. Circulating WISP-1 levels were increased in obese persons and are directly related to adiposity [19]. Recently, WISP-1 was validated as a novel adipokine. Human adipocyte differentiation was associated with increased its expression and secretion. Stimulation of human macrophages with WISP-1 led to a proinflammatory response [20].
Based on these facts we hypothesize, that circulating levels of WISP-1 could differ between children with IBD and healthy controls and could be related to clinical or laboratory markers of disease activity. Therefore, the aim of this study was to compare WISP-1 levels and other selected adipokines (adiponectin, resistin, and RBP-4) in children with IBD to healthy controls and also investigate possible differences between CD and UC patients.
Methods
Study design, IBD diagnosis, and disease activity index
The study was undertaken as a case-control study of children with diagnosed IBD and healthy controls in accordance with the principles of the Declaration of Helsinki for experiments involving humans. It was reviewed and approved by the Ethics Committee of the Faculty of Medicine and University Hospital Olomouc (Approval number 136/90); informed consent was obtained from all participants and their parents. All IBD patients underwent endoscopy (esophago-gastroduodenoscopy and ileocolonoscopy) with histological examination and imaging of the small bowel. The diagnosis of IBD was established for all patients according to the ESPGHAN Revised Porto Criteria for the diagnosis of IBD in children and adolescents [21]. Patients with ambiguous endoscopic and histological diagnoses were excluded. The control group consisted of sex- and age-matched healthy children. These children were free from any inter-current disease, trauma or chronic inflammation. Participants were asked about their medical history. According their weight and height body mass index (BMI = weight(kg) / height(m)2) was calculated.
The Pediatric Crohn’s Disease Activity Index (PCDAI) was used to evaluate CD in children [22]. The Index includes clinical and laboratory assessments (abdominal pain, general well-being, number of stools per day, hematocrit, erythrocyte sedimentation rate, serum albumin level, weight, height velocity, abdominal examination, perianal disease, extraintestinal manifestation). The PCDAI score ranges from 0 to 100. A score of ≤10 is consistent with remission, 11–30 indicates mild disease, and ≥30 means moderate to severe disease. The Pediatric Ulcerative Colitis Activity Index (PUCAI) was used for the evaluation of disease activity (abdominal pain, rectal bleeding, stool consistency, number of stools per 24 h, nocturnal stools and activity level) in children suffering from UC [20]. The PUCAI score ranges from 0 to 85; a score of <10 denotes remission, 10–34 mild disease, 35–64 moderate disease and 65–85 severe disease.
Laboratory analyses
Venous blood samples were drawn in the morning after a 12-h fasting. Concentrations of adipokines were measured in the sample aliquots stored at –80 °C, no longer than 6 months.
Adiponectin was determined by ELISA immunochemical kit: Human Adiponectin ELISA (Biovendor Laboratory Medicine Inc., Brno, Czech Republic), according to the manufacturer’s instructions. The antibodies used in this kit are specific for human adiponectin. Assay sensitivity was 26 ng/ml, precision CV was 4.9 % (intra-assay) and 6.7 % (inter-assay). WISP-1 was measured by immunochemical kit: Human WISP1 ELISA kit (Biovendor Laboratory Medicine Inc., Brno, Czech Republic), according to the manufacturer’s instructions. The antibodies used in this ELISA are specific for human WISP-1. Assay sensitivity was 50 pg/ml. Coefficients of variation were below 10.0 % (intra-assay), below 11.9 % (inter-assay), respectively. Resistin was obtained by ELISA immunochemical kit Human Resistin ELISA (Biovendor Laboratory Medicine Inc., Brno, Czech Republic) according to the manufacturer’s instructions. The antibodies used in this ELISA are specific for human Resistin. Assay sensitivity was 0.012 ng/ml, precision CV was 5.9 % (intra-assay) and 7.6 % (inter-assay). The quantitative determination of RBP4 was performed using ELISA immunochemical kit Human RBP4 (High Sensitivity) ELISA (Biovendor Laboratory Medicine Inc., Brno, Czech Republic) according to the manufacturer’s instructions. The antibodies used in this ELISA are specific for human RBP4. Assay sensitivity was 380 pg/ml, precision CV was 2.7 % (intra-assay) and 5.0 % (inter-assay).
Total cholesterol (TC), TG and HDL-C were determined enzymatically on Cobas 8000 system. Low density lipoprotein cholesterol (LDL-C) was calculated using Friedewald formula (LDL-C = TC - TG*0.4537 - HDL-C for TG < 4.5 mmol/l). Glucose was determined using the GOD-PAP method (Roche, Diagnostics GmbH, Mannheim, Germany). Blood counts were measured with an automated analyzer. C-reactive protein (CRP) was assessed using an immunoturbidimetric method (CRPL3; Roche Diagnostics GmbH, Mannheim, Germany) on an automatic analyzer Cobas 8000, Roche. Interleukin 6 was determined by immunochemical method (IL-6; Roche Diagnostics GmbH, Mannheim, Germany) on an automatic analyzer Cobas 8000, Roche. Ferritin was determined by chemiluminescent immunoassay (Architect Ferritin Reagent Kit, Abbott Laboratories, Diagnostics Division, Chicago, USA) on an automatic analyzer Architect i2000SR, Abbott. The quantitative determination of calprotectin in the stool was performed using a lateral flow immunoassay (Quantum Blue Calprotectin; Bühlmann Laboratories AG, Schönenbuch, Switzerland).
Statistical analyses
Values were expressed as median and interquartile ranges. Differences in variables between groups were analyzed by the Mann-Whitney U test. Spearman correlation analyzes the tested correlations between parameters in all groups. Multivariate regression analyzes were used to test the independent association between dependent and independent variables (abnormally distributed variables were logarithmically transformed prior to analysis). Probability values of p <0.05 were considered statistically significant.
Results
Basic characteristics and provided therapy of investigated groups
Fifty-eight children with IBD [age = 15 (13–15) years, number of boys = 32, number of girls = 26)] and twenty healthy controls of the same sex and age [age = 15 (12–16), number of boys = 11, number of girls = 9)] were included in this study. Compared to healthy controls, patients with IBD had significantly higher levels of CRP, IL-6 and F-CPT; and lower levels of ferritin, total cholesterol, LDL-C and HDL-C. There were no significant differences between the baseline characteristics within the CD and UC groups. Table 1 shows the basic clinical and laboratory characteristics of the participants.
Table 1.
Basic clinical and laboratory characteristics in individual groups
| IBD group (n=58) | CD group (n=31) | UC group (n=27) | healthy controls (n=20) | |
|---|---|---|---|---|
| Age (years) | 15 (13–15) | 15 (13–16) | 15 (13–15) | 15 (12–16) |
| BMI (kg/m 2 ) | 20.3 (17.2–22.5) | 20.3 (17.3–24.0) | 20.0 (17.0–22.1) | 19.1 (16.1–21.5) |
| CRP (mg/l) | 1.1 (0.5–4.2)*** | 1.1 (0.5–8.4)*** | 0.8 (0.5–2.0)** | 0.5 (0.5–0.7) |
| IL-6 (ng/l) | 2.3 (1.4–4.6)*** | 2.4 (1.4–4.1)*** | 1.9 (1.4–4.7)** | 1.4 (1.4–1.6) |
| F-CPT (μg/g) | 419 (145–1071)*** | 532 (111–1636)*** | 403 (183–723)*** | 46 (35–74) |
| Ferritin (μg/l) | 14.0 (7.5–33.6)* | 14.0 (7.1–32.4)* | 16.5 (7.8–36.8)* | 29.0 (15.4–39.0) |
| Hemoglobin (g/l) | 137 (125–147) | 139 (128–148) | 135 (121–146) | 131 (129–136) |
| Thrombocytes (×10 9 /l) | 318 (272–371) | 317 (283–366) | 319 (235–373) | 293 (247–326) |
| Leucocytes (×10 9 /l) | 7.2 (5.4–9.4) | 7.5 (6.1–9.0) | 6.6 (4.8–10.4) | 6.5 (5.6–7.8) |
| TC (mmol/l) | 3.5 (3.0–3.9)** | 3.5 (3.1–3.9)** | 3.6 (3.0–4.2)* | 4.1 (3.7–4.7) |
| LDL-C (mmol/l) | 1.7 (1.4–2.1)** | 1.8 (1.5–2.1)* | 1.7 (1.3–2.2)* | 2.2 (1.9–2.6) |
| HDL-C (mmol/l) | 1.3 (1.0–1.5)* | 1.2 (1.0–1.4)* | 1.4 (1.1–1.5) | 1.5 (1.2–1.7) |
| TG (mmol/l) | 0.9 (0.7–1.4) | 1.0 (0.7–1.4) | 0.9 (0.6–1.4) | 1.1 (0.7–1.6) |
| FG (mmol/l) | 4.9 (4.6–5.1) | 4.8 (4.5–5.1) | 5.0 (4.8–5.2) | 4.8 (4.7–5.0) |
IBD = inflammatory bowel disease; CD = Crohn’s disease; UC = ulcerative colitis; BMI = body mass index; CRP = C-reactive protein; IL-6 = interleukin-6; F-CPT = fecal calprotectin; TC = total cholesterol; LDL-C = LDL-cholesterol; HDL-C = HDL-cholesterol; TG = triglycerides; FG = fasting glycemia. Values are expressed as median and interquartile range (25–75 percentile). Significant differences of patients group versus controls:
p<0.05;
p<0.01;
p<0.001 according to Mann-Whitney U test.
Induction therapy in the CD group (n=31) included: exclusive enteral nutrition (84 %), corticosteroids (9.7 %) or Crohn’s Disease Exclusion Diet (6.3 %). Maintenance treatment consisted of azathioprine (32 %), biologic therapy plus azathioprine (64.5 %), azathioprine plus mesalamine (3.5 %). Patients with UC (n=27) were treated with mesalamine (44.3 %), corticosteroids (52 %) or corticosteroids plus infliximab (3.7 %) in the induction phase. Their maintenance treatment included mesalamine alone (22.6 %), azathioprine alone (7.4 %), azathioprine plus mesalamine (37 %), azathioprine + biologic therapy (33 %). The majority of IBD patients were in remission (mean PUCAI in the UC group was 6.9, mean PCDAI in the CD group was 6.0).
Adipokines in individual groups, their relation to metabolic parameters
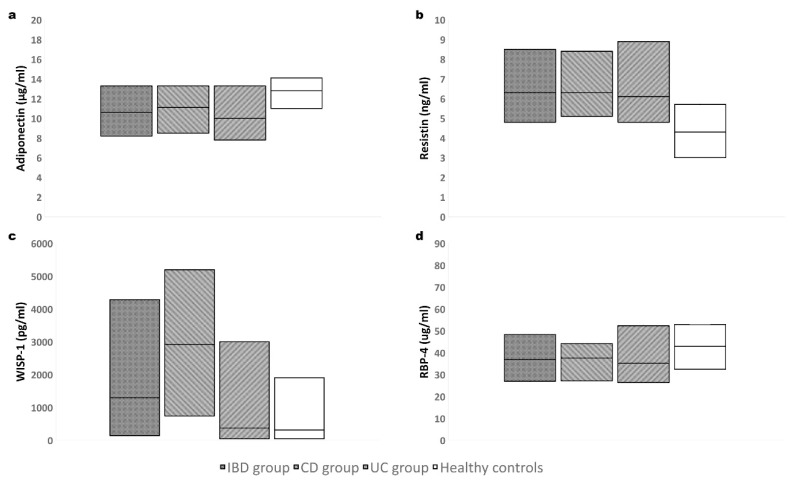
Levels of adiponectin were significantly decreased, and levels of resistin were significantly increased in IBD children (both in CD and UC patients) compared to healthy controls. WISP-1 levels were also significantly increased in IBD children, namely in CD group (WISP-1 was higher in this group compared to UC group). UC patients did not differ in WISP-1 levels compared to healthy children. There were no significant differences between levels of RBP-4 within the groups – see Table 2 and Fig. 1.
Table 2.
Adipokines in individual groups
| IBD group (n=75) | CD group (n=44) | UC group (n=31) | healthy controls (n=20) | |
|---|---|---|---|---|
| Adiponectin (μg/ml) | 10.6 (8.2–13.3)* | 11.1 (8.5–13.3)* | 10.0 (7.8–13.3)* | 12.8 (11.0–14.1) |
| Resistin (ng/ml) | 6.3 (4.8–8.5)** | 6.3 (5.1–8.4)* | 6.1 (4.8–8.9)* | 4.3 (3.0–5.7) |
| WISP-1 (pg/ml) | 1296 (146–4286)** | 2917 (740–5198)**# | 373 (49–3004) | 311 (38–1905) |
| RBP-4 (μg/ml) | 37.0 (27.0–48.4) | 37.6 (27.2–44.2) | 35.2 (26.4–52.4) | 42.9 (32.5–52.9) |
IBD = inflammatory bowel disease; CD = Crohn’s disease; UC = ulcerative colitis; WISP-1 = Wnt1-inducible signaling pathway protein-1; RBP-4 = retinol-binding protein-4. Values are expressed as median and interquartile range (25–75 percentile). Significant differences of patients group versus controls:
p<0.05,
p<0.01,
p<0.001; or CD versus UC groups:
p<0.05 according to Mann-Whitney U test.
Fig. 1.
Adipokines levels in individual groups: a) adiponectin; b) resistin; c) Wnt1-inducible signaling pathway protein-1; d) retinol-binding protein-4. Boxes represent a median and interquartile range.
Adiponectin significantly (p <0.05) positively correlated with HDL-C (rho = 0.23); negatively with BMI (rho = −0.37), CRP (rho = −0.28), IL-6 (rho = −0.24) and F-CPT (rho = −0.31). Resistin correlated positively with CRP (rho = 0.52), IL-6 (rho = 0.47), F-CPT (rho = 0.52), platelet count (rho = 0.36), leukocyte count (rho = 0.46) as PUCAI −1 (rho = 0.43); negatively correlates with HDL-C (rho = −0.22). There was an inverse correlation between adiponectin and resistin (rho = −0.35). WISP-1 positively correlated with BMI (rho = 0.26); negative with ferritin (rho = −0.25) and HDL-C (rho = −0.23). RBP-4 correlated only positively with total cholesterol (rho = 0.28), TG (rho = 0.28) and BMI (rho = 0.30).
Table 3 shows a multivariate logistic regression analysis of independent factors affecting adiponectin, resistin, WISP-1 and RBP-4 as dependent variables. Adiponectin, WISP-1 and RBP-4 were independently associated with BMI only, resistin was independently associated with CRP and leukocyte count.
Table 3.
Multivariate logistic regression analysis of independent factors affecting adipokines as dependent variables
| adiponectin | resistin | WISP-1 | RBP-4 | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
|
| ||||||||||||
| B | Std. Error | Sig. | B | Std. Error | Sig. | B | Std. Error | Sig. | B | Std. Error | Sig. | |
| BMI | −0.37 | 0.11 | 0.001 | - | - | - | 0.26 | 0.11 | 0.020 | 0.28 | 0.11 | 0.011 |
| CRP | −0.11 | 0.13 | 0.399 | 0.37 | 0.12 | 0.003 | - | - | - | - | - | - |
| IL-6 | −0.07 | 0.13 | 0.576 | −0.05 | 0.11 | 0.676 | - | - | - | - | - | - |
| F-CPT | −0.19 | 0.12 | 0.111 | 0.18 | 0.11 | 0.104 | - | - | - | - | - | - |
| Ferritin | - | - | - | - | - | - | −0.21 | 0.11 | 0.052 | - | - | - |
| Thrombocytes | - | - | - | 0.07 | 0.10 | 0.515 | - | - | - | - | - | - |
| Leucocytes | - | - | - | 0.27 | 0.10 | 0.013 | - | - | - | - | - | - |
| TC | - | - | - | - | - | - | - | - | - | 0.11 | 0.13 | 0.422 |
| HDL-C | 0.14 | 0.11 | 0.205 | −0.13 | 0.09 | 0.222 | −0.20 | 0.11 | 0.071 | - | - | - |
| TG | - | - | - | - | - | - | - | - | - | 0.17 | 0.14 | 0.203 |
WISP-1 = Wnt1-inducible signaling pathway protein-1; RBP-4 = retinol-binding protein-4; BMI = body mass index; CRP = C-reactive protein; IL-6 = interleukin-6; F-CPT = fecal calprotectin; TC = total cholesterol; HDL-C = HDL-cholesterol; TG = triglycerides
Discussion
Children with IBD had significantly lower levels of adiponectin, and higher levels of resistin and of WISP-1 compared to heathy controls. WISP-1 elevation was detected only in CD group. There were no differences in RBP-4 levels within groups.
A little is known about the possible role of WISP-1 in IBD pathogenesis. Zang et al. demonstrated increased WISP-1 epithelial expression in colonic biopsies and lamina propria mononuclear cells of IBD patients [24]. This expression was induced by TNF-α in healthy controls and downregulated in colonic biopsies from IBD patients who had achieved clinical remission with infliximab. The authors presume WISP1 may contributes to the proinflammatory cascades in the gut in IBD patients. There is evidence that Wnt signaling pathway is highly interconnected with numerous other inflammatory cascades (NF-κB, mitogen activated protein kinase, protein kinase B, or signal transducer and activator of transcription signaling pathways) [25]. WISP-1 induced epithelial cell proliferation is mediated by IL-10 and interpheres to an important phase of intestinal mucosal healing [26]. WISP-1 is a marker of systemic and adipose tissue inflammation [19], thus it may also represent pro-inflammatory mediator by which adipose tissue participates on IBD pathogenesis. In this study, we did not observe an association between WIPS-1 and markers of inflammation or disease activity, possibly because most patients with IBD were in remission. WISP-1 correlated positively with BMI, negatively with ferritin and HDL-C. Because ferritin levels were significantly decreased in IBD patients compared to healthy controls, they served as marker of low iron storage. Only BMI remained an independent predictor of WISP-1 levels in the multivariate regression analysis. According some results circulating WISP-1 levels may be used as a suitable biomarker of obesity and BMI has been identified as a strong predictor for WISP-1 levels [20,27]. The difference between children with CD and UC in this study may indicate a specific role for visceral adipose tissue (VAT) in WISP-1 production. Uko et al. demonstrated pediatric CD patients had a higher adjusted VAT volumes than age- and BMI-matched controls [28]. Higher VAT was associated with fistulizing or fibrostenotic disease, higher disease activity, and shorter intervals from diagnosis to surgery. Therefore, we could assume that the intestinal process in patients with CD, where intestinal inflammation penetrates the surrounding adipose tissue along the mesentery [5], is associated with activation of the Wnt signaling pathway leading to increased WISP-1 expression more than in patients with UC. These findings support differences in dysfunctional adiposity in IBD. Accumulation of mesenteric fat in form of creeping/wrapping fat around the bowel wall infiltrated by inflammatory macrophages and T-lymphocytes is typical for CD, especially for its ileal forms. While in case of UC, there is visceral adiposity with accumulation of fat mainly localized in omental region [13].
Serum adiponectin levels were found decreased [15,29,30], increased [31,32], or unchanged [33,34,35] in different studies focusing on IBD patients. The discrepancy in these results in part could be explained by small data cohorts used in some of the studies, inadequate controls, and/or different treatment status of the patients. Controversial reports support the concept that adiponectin plays an important role in maintaining intestinal homeostasis, but its exact action in bowel inflammation remains unclear [5]. Decreased adiponectin levels negatively correlated with markers of inflammation (CRP, IL-6) and F-CPT in our study. These findings are compatible with results suggesting adiponectin can promote the anti-inflammatory response via decreased production of pro-inflammatory cytokines, such as TNF-α and IL-6, by suppressing NF-κB signaling [9]. Negative association between adiponectin and F-CTP was also detected in patients with diverticulosis and pointed on contribution of VAT to intestinal inflammation not only in IBD patients [36]. The fact that BMI was the only independent predictor of adiponectin in this study again underlines the role of adipose tissue in its production.
Resistin was inversely associated with adiponectin, and positively correlated with markers of inflammation, diseases activity (only in UC patients) and F-CPT in children with IBD. CRP levels and leukocyte counts were identified as independent predictors of resistin levels. Resistin used to be associated with inflammatory processes because its expression in adipose tissue is induced by pro-inflammatory cytokines (IL-1β, IL-6, and TNF-α) via the NF-κB signaling pathway [12,13]. Several studies have demonstrated circulating resistin levels increased in IBD patients [31,33] and anti-inflammatory therapy IBD significantly reduced its levels [14]. Resistin was an independent predictor of disease activity in patients with CD according to one study [37]. However, there was no apparent differences in serum resistin between IBD and other diseases characterized by chronic inflammation, which may indicate it is a non-specific marker of inflammation [13]. Resistin negatively correlated with HDL-C in this study. Similar findings were observed in healthy individuals [38], in patients with metabolic syndrome [39], type 2 diabetes, and individuals with acute inflammatory disease [40]. Some data suggest that cholesterol downregulates resistin expression in human white adipose tissue [41].
There are different reports of RBP-4 levels in IBD patients. One study reported increased serum levels of RBP-4 in adult patients [15]. On the contrary, no difference in the RBP-4 levels between pediatric IBD patients and healthy controls as well as between active and remission state of the disease was noticed in another study. Although, RBP-4 negatively correlated with IBD activity of children that maybe indicating a protective anti-inflammatory mechanism of action in addition to transport of vitamin A [16]. In this presented study, there was no associations between RBP-4 and disease activity or markers of inflammation, maybe due to most of investigated children was in IBD remission. Levels of RBP-4 correlated with BMI, total cholesterol, and TG. These findings probably reflect RBP-4 contribution to a plasma lipoprotein profile. According to some authors RBP-4 could directly interfere with the metabolism of triglyceride-rich apoB-containing lipoproteins [42,43].
There are some limitations of the presented study. In addition to the relatively small size of patient groups (although similar for pediatric cohorts) one of the limitations is a fact that most of IBD patients was successfully treated and was in disease remission. This could influence the determination of the relation between examined adipokines and disease activity. Despite this, significant differences in adipokine levels indicate the persistence of dysfunctional adipose tissue and its association with chronic inflammation in children with IBD. Due to the cross-sectional design, the study could only demonstrate significant correlations, but not causality or the potential effect of treatment. This may be a suitable target for prospective studies examining the longitudinal changes of these adipokines in patients with IBD, including testing different intervention approaches.
Conclusion
Significantly lower levels of adiponectin and higher levels of resistin and WISP-1 in children with IBD reflect the persistence of dysfunctional adipose tissue and its potential contribution to chronic inflammation. In addition, higher WISP-1 levels in CD patients compared to the UC group may indicate a specific role for visceral adipose tissue in WISP-1 production and may reflect activation of the Wnt signaling pathway leading to increased WISP-1 epithelial expression. Further studies are needed to elucidate the role of these adipokines in the pathogenesis of IBD.
Acknowledgements
This research was supported by MH CZ DRO (FNOl, 00098892) and IGA LF 2022 003. The authors thank RNDr. Milena Krskova for assistance with statistical analysis.
Footnotes
Conflict of Interest
There is no conflict of interest.
References
- 1.Bousvaros A. Use of immunomodulators and biologic therapies in children with inflammatory bowel disease. Expert Rev Clin Immunol. 2010;6:659–666. doi: 10.1586/eci.10.46. [DOI] [PubMed] [Google Scholar]
- 2.Gonçalves P, Magro F, Martel F. Metabolic inflammation in inflammatory bowel disease: crosstalk between adipose tissue and bowel. Inflamm Bowel Dis. 2015;21:453–467. doi: 10.1097/MIB.0000000000000209. [DOI] [PubMed] [Google Scholar]
- 3.Schwarz J, Vecka M, Stožický F, Pomahačová R, Staňková B, Tvrzická E, Kreslová M, Zahálková R, Sýkora J. The assessment of plasma fatty acid profiles in newly diagnosed treatment-naive paediatric Crohn’s disease. Physiol Res. 2021;70:799–808. doi: 10.33549/physiolres.934665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Lackeyram D, Young D, Kim CJ, Yang C, Archbold TL, Mine Y, Fan MZ. Interleukin-10 is differentially expressed in the small intestine and the colon experiencing chronic inflammation and ulcerative colitis induced by dextran sodium sulfate in young pigs. Physiol Res. 2017;66:147–162. doi: 10.33549/physiolres.933259. [DOI] [PubMed] [Google Scholar]
- 5.Karaskova E, Velganova-Veghova M, Geryk M, Foltenova H, Kucerova V, Karasek D. Role of Adipose Tissue in Inflammatory Bowel Disease. Int J Mol Sci. 2021;22:4226. doi: 10.3390/ijms22084226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Weidinger C, Ziegler JF, Letizia M, Schmidt F, Siegmund B. Adipokines and Their Role in Intestinal Inflammation. Front Immunol. 2018;9:1974. doi: 10.3389/fimmu.2018.01974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Wang HH, Luo WY, Lin M, Li XJ, Xiang GD, Triganti DS. Plasma asprosin, CCDC80 and ANGPTL4 levels are associated with metabolic and cardiovascular risk in patients with inflammatory bowel disease. Physiol Res. 2021;70:203–211. doi: 10.33549/physiolres.934547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Morshedzadeh N, Rahimlou M, Asadzadeh Aghdaei H, Shahrokh S, Reza Zali M, Mirmiran P. Association Between Adipokines Levels with Inflammatory Bowel Disease (IBD): Systematic Reviews. Dig Dis Sci. 2017;62:3280–3286. doi: 10.1007/s10620-017-4806-5. [DOI] [PubMed] [Google Scholar]
- 9.Wulster-Radcliffe MC, Ajuwon KM, Wang J, Christian JA, Spurlock ME. Adiponectin differentially regulates cytokines in porcine macrophages. Biochem Biophys Res Commun. 2004;316:924–929. doi: 10.1016/j.bbrc.2004.02.130. [DOI] [PubMed] [Google Scholar]
- 10.Singh UP, Singh NP, Guan H, Busbee B, Price RL, Taub DD, Mishra MK, Fayad R, Nagarkatti M, Nagarkatti PS. The emerging role of leptin antagonist as potential therapeutic option for inflammatory bowel disease. Int Rev Immunol. 2014;33:23–33. doi: 10.3109/08830185.2013.809071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Sitaraman S, Liu X, Charrier L, Gu LH, Ziegler TR, Gewirtz A, Merlin D. Colonic leptin: source of a novel proinflammatory cytokine involved in IBD. FASEB J. 2004;18:696–698. doi: 10.1096/fj.03-0422fje. [DOI] [PubMed] [Google Scholar]
- 12.Harper JW, Zisman TL. Interaction of obesity and inflammatory bowel disease. World J Gastroenterol. 2016;22:7868–7881. doi: 10.3748/wjg.v22.i35.7868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Eder P, Adler M, Dobrowolska A, Kamhieh-Milz J, Witowski J. The Role of Adipose Tissue in the Pathogenesis and Therapeutic Outcomes of Inflammatory Bowel Disease. Cells. 2019;8:628. doi: 10.3390/cells8060628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Karmiris K, Koutroubakis IE, Xidakis C, Polychronaki M, Kouroumalis EA. The effect of infliximab on circulating levels of leptin, adiponectin and resistin in patients with inflammatory bowel disease. Eur J Gastroenterol Hepatol. 2007;19:789–794. doi: 10.1097/MEG.0b013e3282202bca. [DOI] [PubMed] [Google Scholar]
- 15.Valentini L, Wirth EK, Schweizer U, Hengstermann S, Schaper L, Koernicke T, Dietz E, Norman K, Buning C, Winklhofer-Roob BM, Lochs H, Ockenga J. Circulating adipokines and the protective effects of hyperinsulinemia in inflammatory bowel disease. Nutrition. 2009;25:172–181. doi: 10.1016/j.nut.2008.07.020. [DOI] [PubMed] [Google Scholar]
- 16.Roma E, Krini M, Hantzi E, Sakka S, Panayiotou I, Margeli A, Papassotiriou I, Kanaka-Gantenbein C. Retinol Binding Protein 4 in children with Inflammatory Bowel Disease: a negative correlation with the disease activity. Hippokratia. 2012;16:360–365. [PMC free article] [PubMed] [Google Scholar]
- 17.Perbal B, Tweedie S, Bruford E. The official unified nomenclature adopted by the HGNC calls for the use of the acronyms, CCN1–6, and discontinuation in the use of CYR61, CTGF, NOV and WISP 1–3 respectively. J Cell Commun Signal. 2018;12:625–629. doi: 10.1007/s12079-018-0491-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Feng M, Jia S. Dual effect of WISP-1 in diverse pathological processes. Chin J Cancer Res. 2016;28:553–560. doi: 10.21147/j.issn.1000-9604.2016.06.01. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Barchetta I, Cimini FA, Capoccia D, De Gioannis R, Porzia A, Mainiero F, Di Martino M, Bertoccini L, De Bernardinis M, Leonetti F, Baroni MG, Lenzi A, Cavallo MG. WISP1 Is a Marker of Systemic and Adipose Tissue Inflammation in Dysmetabolic Subjects With or Without Type 2 Diabetes. J Endocr Soc. 2017;1:660–670. doi: 10.1210/js.2017-00108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Murahovschi V, Pivovarova O, Ilkavets I, Dmitrieva RM, Döcke S, Keyhani-Nejad F, Gögebakan Ö, Osterhoff M, Kemper M, Hornemann S, Markova M, Klöting N, Stockmann M, Weickert MO, Lamounier-Zepter V, Neuhaus P, Konradi A, Dooley S, von Loeffelholz C, Blüher M, Pfeiffer AF, Rudovich N. WISP1 is a novel adipokine linked to inflammation in obesity. Diabetes. 2015;64:856–866. doi: 10.2337/db14-0444. [DOI] [PubMed] [Google Scholar]
- 21.Levine A, Koletzko S, Turner D, Escher JC, Cucchiara S, de Ridder L, Kolho KL, Veres G, Russell RK, Paerregaard A, Buderus S, Greer ML, Dias JA, Veereman-Wauters G, Lionetti P, Sladek M, Martin de Carpi J, Staiano A, Ruemmele FM, Wilson DC European Society of Pediatric Gastroenterology, Hepatology and Nutrition. ESPGHAN revised porto criteria for the diagnosis of inflammatory bowel disease in children and adolescents. J Pediatr Gastroenterol Nutr. 2014;58:795–806. doi: 10.1097/MPG.0000000000000239. [DOI] [PubMed] [Google Scholar]
- 22.Hyams J, Markowitz J, Otley A, Rosh J, Mack D, Bousvaros A, Kugathasan S, Pfefferkorn M, Tolia V, Evans J, Treem W, Wyllie R, Rothbaum R, del Rosario J, Katz A, Mezoff A, Oliva-Hemker M, Lerer T, Griffiths A Pediatric Inflammatory Bowel Disease Collaborative Research Group. Evaluation of the pediatric crohn disease activity index: a prospective multicenter experience. J Pediatr Gastroenterol Nutr. 2005;41:416–421. doi: 10.1097/01.mpg.0000183350.46795.42. [DOI] [PubMed] [Google Scholar]
- 23.Turner D, Hyams J, Markowitz J, Lerer T, Mack DR, Evans J, Pfefferkorn M, Rosh J, Kay M, Crandall W, Keljo D, Otley AR, Kugathasan S, Carvalho R, Oliva-Hemker M, Langton C, Mamula P, Bousvaros A, LeLeiko N, Griffiths AM Pediatric IBD Collaborative Research Group. Appraisal of the pediatric ulcerative colitis activity index (PUCAI) Inflamm Bowel Dis. 2009;15:1218–1223. doi: 10.1002/ibd.20867. [DOI] [PubMed] [Google Scholar]
- 24.Zhang Q, Zhang C, Li X, Yu Y, Liang K, Shan X, Zhao K, Niu Q, Tian Z. WISP1 Is increased in intestinal mucosa and contributes to inflammatory cascades in inflammatory bowel disease. Dis Markers. 2016;2016:3547096. doi: 10.1155/2016/3547096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Moparthi L, Koch S. Wnt signaling in intestinal inflammation. Differentiation. 2019;108:24–32. doi: 10.1016/j.diff.2019.01.002. [DOI] [PubMed] [Google Scholar]
- 26.Quiros M, Nishio H, Neumann PA, Siuda D, Brazil JC, Azcutia V, Hilgarth R, O’Leary MN, Garcia-Hernandez V, Leoni G, Feng M, Bernal G, Williams H, Dedhia PH, Gerner-Smidt C, Spence J, Parkos CA, Denning TL, Nusrat A. Macrophage-derived IL-10 mediates mucosal repair by epithelial WISP-1 signaling. J Clin Invest. 2017;127:3510–3520. doi: 10.1172/JCI90229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Tacke C, Aleksandrova K, Rehfeldt M, Murahovschi V, Markova M, Kemper M, Hornemann S, Kaiser U, Honig C, Gerbracht C, Kabisch S, Hörbelt T, Ouwens DM, Weickert MO, Boeing H, Pfeiffer AFH, Pivovarova O, Rudovich N. Assessment of circulating Wnt1 inducible signalling pathway protein 1 (WISP-1)/CCN4 as a novel biomarker of obesity. J Cell Commun Signal. 2018;12:539–548. doi: 10.1007/s12079-017-0427-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Uko V, Vortia E, Achkar JP, Karakas P, Fiocchi C, Worley S, Kay MH. Impact of abdominal visceral adipose tissue on disease outcome in pediatric Crohn's disease. Inflamm Bowel Dis. 2014;20:2286–2291. doi: 10.1097/MIB.0000000000000200. [DOI] [PubMed] [Google Scholar]
- 29.Rodrigues VS, Milanski M, Fagundes JJ, Torsoni AS, Ayrizono ML, Nunez CE, Dias CB, Meirelles LR, Dalal S, Coy CS, Velloso LA, Leal RF. Serum levels and mesenteric fat tissue expression of adiponectin and leptin in patients with Crohn's disease. Clin Exp Immunol. 2012;170:358–364. doi: 10.1111/j.1365-2249.2012.04660.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Kahraman R, Calhan T, Sahin A, Ozdil K, Caliskan Z, Bireller ES, Cakmakoglu B. Are adipocytokines inflammatory or metabolic mediators in patients with inflammatory bowel disease? Ther Clin Risk Manag. 2017;13:1295–1301. doi: 10.2147/TCRM.S140618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Karmiris K, Koutroubakis IE, Xidakis C, Polychronaki M, Voudouri T, Kouroumalis EA. Circulating levels of leptin, adiponectin, resistin, and ghrelin in inflammatory bowel disease. Inflamm Bowel Dis. 2006;12:100–105. doi: 10.1097/01.MIB.0000200345.38837.46. [DOI] [PubMed] [Google Scholar]
- 32.Weigert J, Obermeier F, Neumeier M, Wanninger J, Filarsky M, Bauer S, Aslanidis C, Rogler G, Ott C, Schäffler A, Schölmerich J, Buechler C. Circulating levels of chemerin and adiponectin are higher in ulcerative colitis and chemerin is elevated in Crohn's disease. Inflamm Bowel Dis. 2010;16:630–637. doi: 10.1002/ibd.21091. [DOI] [PubMed] [Google Scholar]
- 33.Waluga M, Hartleb M, Boryczka G, Kukla M, Zwirska-Korczala K. Serum adipokines in inflammatory bowel disease. World J Gastroenterol. 2014;20:6912–6917. doi: 10.3748/wjg.v20.i22.6912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Chouliaras G, Panayotou I, Margoni D, Mantzou E, Pervanidou P, Manios Y, Chrousos GP, Roma E. Circulating leptin and adiponectin and their relation to glucose metabolism in children with Crohn's disease and ulcerative colitis. Pediatr Res. 2013;74:420–426. doi: 10.1038/pr.2013.114. [DOI] [PubMed] [Google Scholar]
- 35.Ortega Moreno L, Sanz-Garcia A, Fernández de la Fuente MJ, Arroyo Solera R, Fernández-Tomé S, Marin AC, Mora-Gutierrez I, Fernández P, Baldan-Martin M, Chaparro M, Gisbert JP, Bernardo D. Serum adipokines as non-invasive biomarkers in Crohn's disease. Sci Rep. 2020;10:18027. doi: 10.1038/s41598-020-74999-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Murray KA, Hoad CL, Garratt J, Kaviani M, Marciani L, Smith JK, Siegmund B, Gowland PA, Humes DJ, Spiller RC. A pilot study of visceral fat and its association with adipokines, stool calprotectin and symptoms in patients with diverticulosis. PLoS One. 2019;14:e0216528. doi: 10.1371/journal.pone.0216528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Konrad A, Lehrke M, Schachinger V, Seibold F, Stark R, Ochsenkühn T, Parhofer KG, Göke B, Broedl UC. Resistin is an inflammatory marker of inflammatory bowel disease in humans. Eur J Gastroenterol Hepatol. 2007;19:1070–1074. doi: 10.1097/MEG.0b013e3282f16251. [DOI] [PubMed] [Google Scholar]
- 38.Chen CC, Li TC, Li CI, Liu CS, Wang HJ, Lin CC. Serum resistin level among healthy subjects: relationship to anthropometric and metabolic parameters. Metabolism. 2005;54:471–475. doi: 10.1016/j.metabol.2004.10.015. [DOI] [PubMed] [Google Scholar]
- 39.Norata GD, Ongari M, Garlaschelli K, Raselli S, Grigore L, Catapano AL. Plasma resistin levels correlate with determinants of the metabolic syndrome. Eur J Endocrinol. 2007;156:279–284. doi: 10.1530/eje.1.02338. [DOI] [PubMed] [Google Scholar]
- 40.Stejskal D, Adamovská S, Bartek J, Juráková R, Prosková J. Resistin - concentrations in persons with type 2 diabetes mellitus and in individuals with acute inflammatory disease. Biomed Pap Med Fac Univ Palacky Olomouc Czech Repub. 2003;147:63–69. doi: 10.5507/bp.2003.009. [DOI] [PubMed] [Google Scholar]
- 41.Jové M, Planavila A, Cabrero A, Novell F, Ros E, Zambón D, Laguna JC, Carrera MV. Reductions in plasma cholesterol levels after fenofibrate treatment are negatively correlated with resistin expression in human adipose tissue. Metabolism. 2003;52:351–355. doi: 10.1053/meta.2003.50055. [DOI] [PubMed] [Google Scholar]
- 42.Wessel H, Saeed A, Heegsma J, Connelly MA, Faber KN, Dullaart RPF. Plasma Levels of Retinol Binding Protein 4 Relate to Large VLDL and Small LDL Particles in Subjects with and without Type 2 Diabetes. J Clin Med. 2019;8:1792. doi: 10.3390/jcm8111792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Vergès B, Guiu B, Cercueil JP, Duvillard L, Robin I, Buffier P, Bouillet B, Aho S, Brindisi MC, Petit JM. Retinol-binding protein 4 is an independent factor associated with triglycerides and a determinant of very low-density lipoprotein-apolipoprotein B100 catabolism in type 2 diabetes mellitus. Arterioscler Thromb Vasc Biol. 2012;32:3050–3057. doi: 10.1161/ATVBAHA.112.255190. [DOI] [PubMed] [Google Scholar]