Summary
Diabetic nephropathy, included in diabetic kidney disease (DKD), is the primary disease leading to end-stage renal disease (ESRD) or dialysis treatment, accounting for more than 40 % of all patients with ESRD or receiving dialysis. Developing new therapeutics to prevent the transition to ESRD or dialysis treatment requires an understanding of the pathophysiology of DKD and an appropriate animal model for drug efficacy studies. In this study, we investigated the pathophysiology of diabetic kidney disease with type 2 diabetes in uninephrectomized db/db mice. In addition, the nephrectomized db/db mice from 10 weeks to 42 weeks were used to assess the efficacy of long-term administration of the angiotensin-II–receptor antagonist losartan. The blood and urinary biochemical parameters and the blood pressure which is a main pharmacological endpoint of the losartan therapy, were periodically measured. And at the end, histopathological analysis was performed. Uninephrectomized db/db mice clearly developed obesity and hyperglycemia from young age. Furthermore, they showed renal pathophysiological changes, such as increased urinary albumin-creatinine ratio (UACR) (the peak value 3104±986 in 40-week-old mice), glomerular hypertrophy and increased fibrotic areas in the tubulointerstitial tubules. The blood pressure in the losartan group was significantly low compared to the normotensive Vehicle group. However, as expected, Losartan suppressed the increase in UACR (829±500) indicating the medication was sufficient, but the histopathological abnormalities including tubular interstitial fibrosis did not improve. These results suggest that the uninephrectomized db/db mice are useful as an animal model of the severe DKD indicated by the comparison of the efficacy of losartan in this model with the efficacy of losartan in clinical practice.
Keywords: Diabetic kidney disease, db/db mice, Tubular interstitial fibrosis
Introduction
The incidence and prevalence of chronic kidney disease (CKD) are increasing worldwide [1]. According to the US Renal Data System, about 30 million Americans were afflicted by CKD in 2017, a prevalence of almost 15 % [2–4]. In Japan, approximately 13 % of the adult population, 13.3 million people are living with CKD [5]. The number of patients on dialysis due to end-stage renal disease (ESRD) is increasing every year, creating it a major economic burden in healthcare [6].
CKD is defined as any condition that causes abnormalities of kidney structure or function for more than 3 months with notable implications for patient health [7,8]. Regardless of initial etiology, the common pathological features of CKD are fibrosis, tubular atrophy, and interstitial inflammation. Kidney dysfunction has been shown to be more correlated with tubular interstitial damage than glomerular damage, often associated with the loss of peritubular capillaries, and it has been reported that hypoxia and other factors are the final common mechanism of transition from CKD to ESRD [9,10].
Diabetic nephropathy, included in diabetic kidney disease (DKD), is the primary disease leading to ESRD or dialysis treatment, accounting for more than 40 % of all patients with ESRD or receiving dialysis [5,11]. Lifestyle-related diseases and metabolic syndrome are intimately linked to the development of CKD. Reports from epidemiological studies in Japan have shown that there is an increase in the cumulative incidence and relative risk of CKD in patients with metabolic syndrome [12]. Renal damage due to obesity also involves insulin resistance; consequently insulin resistance increase the likelihood of proteinuria [13,14]. In addition, as renal function declines, insulin resistance rises, creating a vicious cycle.
Inhibiting the transition to ESRD would seem to be an important treatment strategy for CKD, but there are not any drugs preventing effectively the CKD from development to ESRD. In drug development, it is important to select appropriate animal models to estimate the efficacy in humans. In addition, it is necessary to determine the relevance of the various models to human pathology using those animal models to evaluate the efficacy of a drug.
Various models of renal injury have been described, including chemical-induced models such as the ones induced by Adriamycin [15] and Cisplatin [16] and surgical models such as the ones induced by 5/6 nephrectomy [17] and Unilateral Ureter Obstruction (UUO) [18]. However, in these animal models, disease progression begins with an artificial insult causing a single cellular injury, which is different from the complex pathogenesis of DKD in humans and is not accompanied by the background of systemic metabolic abnormalities, such as obesity and insulin resistance, normally linked to CKD progression. These models are useful for evaluating limited aspects of the mechanism. On the other hand, when evaluating drugs for CKD, which has complex characteristics, there may be gaps between drug efficacy estimated in existing animal models and drug efficacy estimated in humans.
The db/db mouse is a type 2 diabetes model with a mutation in the leptin receptor gene [19] and individuals have a background of overeating, obesity, and elevated levels of blood glucose, urine glucose, and urine protein from a young age. Furthermore, the renal damage worsens with age and the common pathological features of CKD such as tubulointerstitial fibrosis can develop. Therefore, db/db mice may be an useful animal model of CKD. Here, we removed one kidney of these db/db mice putting additional stress on the other remaining kidney to determine whether these animals could be used as a pathological model of severe DKD. It is also important that drugs used in clinical practice in humans is comparable to the effects detected in mice. In this study, we investigated the pharmacological effects of repeated administration with losartan in uninephrectomized db/db mice.
Methods
Animals and chemicals
Male BKS.Cg-+Leprdb/+Leprdb/Jcl (db/db) and BKS.Cg-m+/+Leprdb/Jcl (db/m) were purchased from CLEA Japan, Inc. (Tokyo, Japan). All animal protocols complied with the Laboratory Guidelines for Animal Experimentation of Japan Tobacco. Animals were housed in a controlled room (temperature 23±3 °C, humidity 55±15 %, 12 h lighting cycle) and allowed free access to diet and water.
At 7 weeks of age uninephrectomy (defined as 1K mice) was performed through a 1-cm flank incision as previously described in db/m and db/db mice [20]. The kidneys in other mice were left intact (defined as 2K mice).
db/m group (n=6 from each group), 2K db/db (n=5) and 1K db/db (n=4) a normal powdered diet (CE-2) (Oriental Yeast; Tokyo, Japan) was fed, and the losartan group (n=4) was fed CE-2 supplemented with 0.04 % (w/w) losartan potassium (LKT Laboratories Inc.; St. Paul, MN, USA) from the age of 10 weeks.
Body mass and biochemical parameters
Body mass was monitored once every four weeks and the other parameters were measured once every eight weeks until the animals were 42 weeks of age. Blood samples were collected from the subclavian vein using a heparin-treated syringe. To collect urine samples, mice were housed in metabolic cages. Glucose and urea nitrogen concentration (BUN) levels were measured using commercial kits (Roche Diagnostics, Basel, Switzerland) and an automatic analyzer (Hitachi 7180; Hitachi, Tokyo, Japan). Plasma and urinary creatinine concentrations were measured by a High-Performance Liquid Chromatography (HPLC) system (Waters Corporation; Milford, MA, USA). The urinary albumin concentration was measured using the LBIS Mouse Albumin ELISA kit (AKRAL-121, FUJIFILM Wako Shibayagi, Gunma, Japan) according to the manufacturer’s instructions. Urinary albumin-creatinine ratio (UACR) was calculated as [urinary albumin (μg/ml)] divided by [urinary creatinine (mg/dl)]. Creatinine clearance (Ccr, ml/min) and body mass corrected creatinine clearance (Ccr BM, ml/min/kg) were calculated from plasma creatinine concentration (Pcr, mg/dl), urine creatinine concentration (Ucr, mg/dl), urine volume (UV, ml/24 hr), and body mass (BM, g) using the following equation:
Blood pressure and heart rate
Systolic blood pressure (SBP) and heart rate (HR) were measured in mice at 36 weeks of age using a non-invasive automatic blood pressure measurement device (BP-98A, Softron Co., Ltd., Tokyo, Japan).
Morphological analysis
At 42 weeks of age, all mice were sacrificed under anesthesia and both kidneys were dissected for subsequent analysis. The kidneys were weighed, fixed with 4 % paraformaldehyde, embedded in paraffin, sectioned, stained with hematoxylin and eosin (HE), Schiff periodate (PAS), and Sirius red (SR), and examined using a light microscope to visualize pathological change and evaluate the Sirius red-stained fibrotic areas. We photographed three representative fields of the cortex, avoiding the glomeruli, examined the fibrotic area in each field at ×200 magnification, and quantified the fibrotic area in each field using the image processing integration software inForm (PerkinElmer, Inc., Waltham, MA, USA). The fibrotic rate was calculated by dividing the area stained with Sirius red by the total area. The mean value of fibrotic rate in the three fields was calculated as the overall fibrosis rate.
Statistical analysis
All results are expressed as mean±standard deviation. Statistical analyses of differences between groups were performed by a Student t-test or Aspin-Welch t-test. All statistical analyses were performed using Statlight 2000 (Yukms Corp., Kawasaki, Japan) statistical software. Differences were accepted as significant at p<0.05.
Results
Parameters of type 2 diabetes in db/db mice
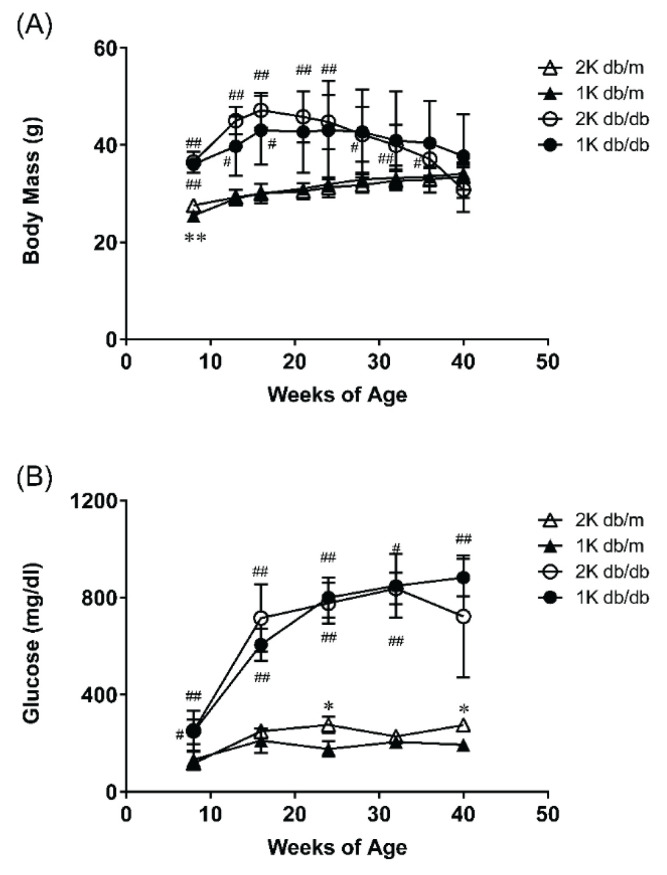
db/db mice demonstrated heavier body mass and higher blood glucose compared to db/m mice (Fig. 1). Due to the effect of the operation, the body mass gain was moderate in 1K db/db mice comparable to the 2K db/db mice. The body mass of 2K db/db peaked at 16 weeks and decreased since then. At 42 weeks, the body mass of 2K db/db were lighter than 2K db/m. Blood glucose was not affected by uninephrectomy throughout the whole period, blood glucose in db/db mice was higher than that in db/m mice (Fig. 1B).
Fig. 1.
(A) Body mass was monitored once every four weeks and (B) blood glucose levels were measured once every eight weeks from study start to study termination. Data represent means ±standard deviations (n=3–6). # p<0.05, ## p<0.01; significantly different from db/m group. * p<0.05; significantly different from 2K group. 2K db/m = intact db/m mice, 1K db/m = uninephrectomized db/m mice, 2K db/db = intact db/db mice, 1K db/db = uninephrectomized db/db mice
Renal function parameters of type 2 diabetes in db/db mice
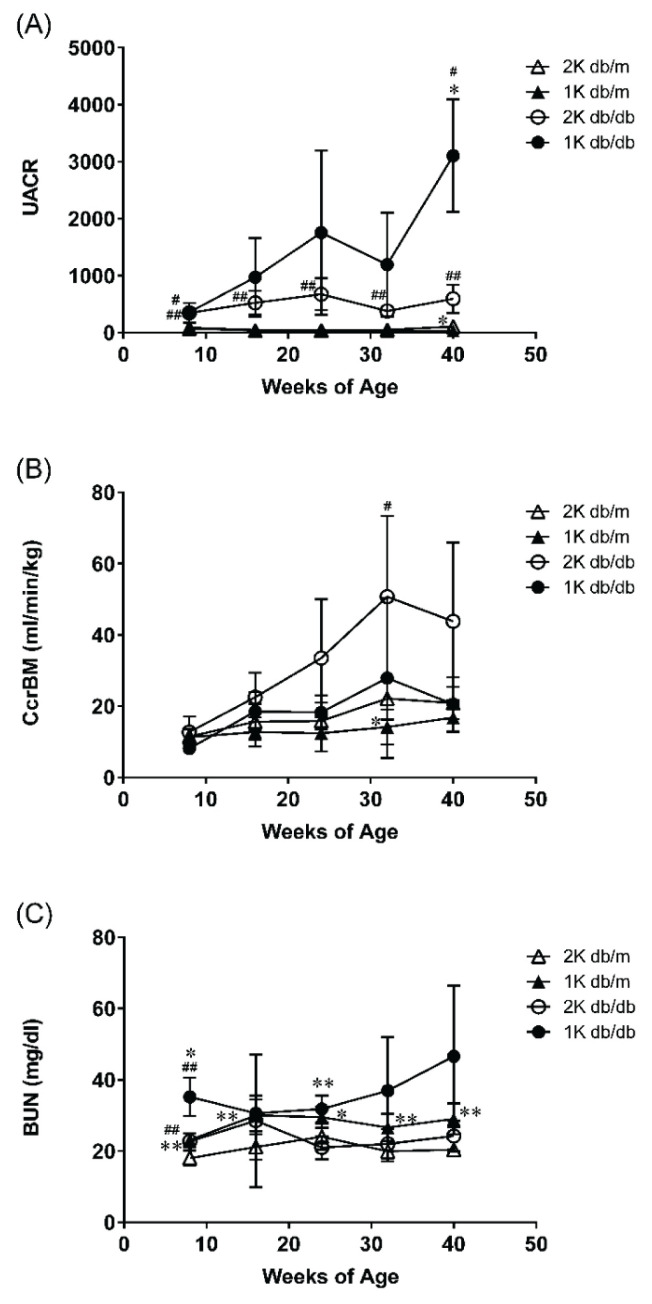
In db/db mice compared with db/m mice, UACR increased significantly from 8 weeks of age (Fig. 2A). The UACR of 1K db/db mice tend to increase from 16 weeks (9 weeks post-operatively) 2 times higher than that of 2K db/db mice and at 40 weeks significant increase was seen.
Fig. 2.
(A) Urinary albumin-creatinine ratio (UACR), (B) Creatinine clearance by body mass corrected (Ccr BM) and (C) blood urea nitrogen concentration (BUN) were measured once every eight weeks from study start to study termination. Data represent mean ±standard deviation (n=3–6). # p<0.05, ## p<0.01; significantly different from db/m group. * p<0.05, ** p<0.01; significantly different from 2K group.
As for Ccr (Fig. 2B), the state of hyperfiltration continued after 16 weeks of age in db/db mice. In 1K db/db mice compared with 2K db/db mice, there was a declining trend in Ccr from 32 weeks of age.
BUN showed little change throughout the whole period (Fig. 2C). However, 1K db/db mice showed an increasing trend between 32 and 40 weeks of age (2K db/m 20.4±1.7 mg/dl, 1K db/m 29.1±4.3 mg/dl, 2K db/db 24.3±4.3 mg/dl, 1K db/db 46.6±19.8 mg/dl at 40 weeks of age).
Kidney mass at 42 weeks of age was heavier in db/db mice than in db/m mice, and even greater after uninephrectomy (Table 1).
Table 1.
Kidney mass in db/m and db/db mice
| 2K db/m | 1K db/m | 2K db/db | 1K db/db | |
|---|---|---|---|---|
| Body mass (g) | 33.5±2.8 | 34.1±1.8 | 30.8±4.6 | 39.3±9.9 |
| Kidney mass (right) (mg) | 286±19 | 455±41** | 382±36## | 518±174 |
| Kidney mass (right) (mg/100 g body mass) | 866±83 | 1335±107** | 1235±177## | 1326±372 |
Body mass and kidney mass in db/m and db/db mice were measured at 42 weeks of age. Each value is a mean ± standard deviation (n=3–6).
p<0.01; significantly different from db/m group.
p<0.01; significantly different from 2K group.
Kidney pathology of type 2 diabetes in db/db mice
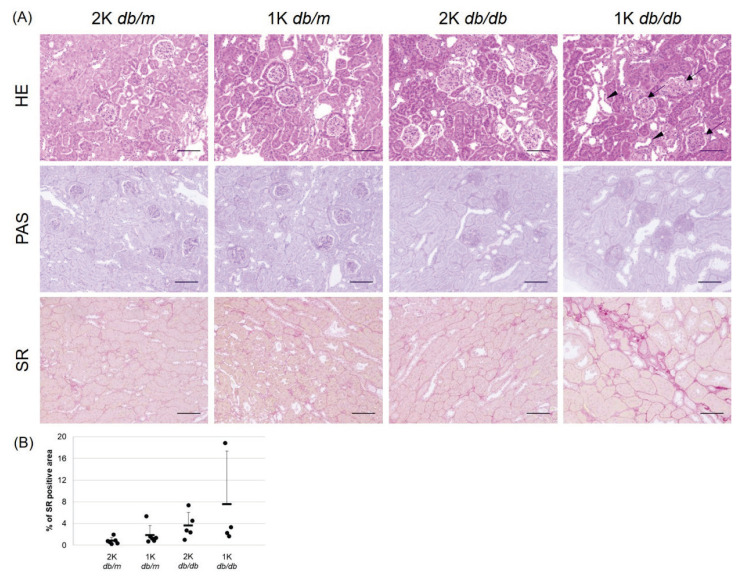
The histopathological changes of the kidney were examined by HE, PAS, and SR staining in four groups of 42-week-old db/m mice and db/db mice is shown in Fig. 3A. There were no changes suggestive of renal impairment in both the 2K and 1K groups of db/m mice. In db/db mice, an increase in glomerular size, an increase in mesangial area, and development of tubular lesions including tubular dilatation were observed and tend to be more severe in 1K mice than 2K mice. In addition, glomerular sclerosis, degeneration, and regeneration of tubular epithelial cells were also increased in 1K (data not shown).
Fig. 3.
(A) Kidney histopathology at 42 weeks of age examined by staining with hematoxylin and eosin (HE), Schiff periodate (PAS), and Sirius Red (SR) in four mouse groups. Bar = 100 μm. Uninephrectomy was associated with a shift toward increasing glomerular size (arrows), an increase in mesangial area, and tubular damage including dilatation (arrowheads) in 1K db/db mice. (B) The data (percent of Sirius Red positive areas) are shown as individual points as well as means ±standard deviations.
Fibrosis was quantified using the area stained with Sirius Red as an index, and the results of positive area evaluation of Sirius Red staining are shown in Fig. 3B. The Sirius Red positive area tended to be larger in db/db mice than in db/m mice and expanded more in 1K mice than in 2K mice (2K db/m 0.8±0.6 %, 1K db/m 1.9±1.8 %, 2K db/db 3.6±2.5 %, 1K db/db 7.6±9.8 %).
Effect of losartan on type 2 diabetes in uninephrectomised db/db mice
Blood pressure, the main pharmacological endpoint of losartan, and heart rate were assessed (Table 2). In the 2K db/db group, blood pressure was significantly lower than in the 2K db/m group and was unaffected by removing one kidney. In 1K db/db mice, losartan (compared with vehicle) significantly lowered systolic blood pressure. Heart rate was lower in db/db mice compared with db/m mice, but was unaffected by uninephrectomy and losartan treatment.
Table 2.
Blood pressure, biochemical tests of urine and blood and histology result in db/m and db/db mice
| 2K db/m | 1K db/m | 2K db/db | 1K db/db Vehicle | 1K db/db Losartan | |
|---|---|---|---|---|---|
| Systolic blood pressure (mmHg) | 134±6 | 130±8 | 118±13# | 125±9 | 90±12†† |
| Heart rate (bpm) | 648±61 | 656±37 | 572±53 | 582±53 | 575±59 |
| UACR | 107±67 | 34±7* | 594±247# | 3104±986* | 829±500† |
| Ccr BM (ml/min/kg) | 20.9±4.6 | 16.9±4.2 | 43.8±22.2 | 20.6±7.6 | 27.7±17.1 |
| BUN (mg/dl) | 20.4±1.7 | 29.1±4.3** | 24.3±4.3 | 46.6±19.8 | 32.6±1.8 |
| Sirius red positive area (%) | 0.82±0.62 | 1.88±1.75 | 3.63±2.45 | 6.54±8.24 | 5.15±1.08 |
Systolic blood pressure and heart rate were measured at 36 weeks of age. Biochemical tests of urine and blood were measured at 40 weeks of age. The Sirius red positive area assessed the fibrous area of the kidney at 42 weeks of age. Each value is represented as mean ± standard deviation (n=3–6).
p<0.05; significantly different from db/m group.
p<0.05,
p<0.01; significantly different from 2K group.
p<0.05,
p<0.01; significantly different from Vehicle group.
UACR, urinary albumin-creatinine ratio; Ccr BM, creatinine clearance body mass corrected; BUN, blood urea nitrogen.
On the renal function endpoint, losartan significantly reduced the UACR, but not Ccr in 40-week-old 1K db/db mice, and the pathology of both glomerular and tubular specimens was similar to that in the vehicle group (data not shown).
The tubulointerstitial fibrosis observed in the losartan group was similar to that observed in the vehicle group. The quantification of the fibrosis area showed that fibrosis tended to decrease with losartan administration, but was not significantly different between the groups (vehicle 7.6±9.8%, losartan 5.2±1.1%).
Discussion
Clinically, DKD is a kidney disease caused by diabetes mellitus and is characterized by increased urinary protein excretion. Moreover, in severe DKD, glomerular filtration rate is decreased and pathological fibrosis of tubulointerstitium is also observed [10]. The db/db mice with a diabetic background, and the removal of one of their kidneys resulted in changes similar to those seen in human DKD, such as increased urinary protein excretion, a decreased creatinine clearance, an indicator of glomerular filtration rate, and fibrosis of tubular interstitium. Thus, the uninephrectomized db/db mice may mimic the condition of severe DKD.
In this study, we focused on interstitial fibrosis and renal biomarkers using creatinine clearance and BUN to evaluate the severity of renal disease. The presence of renal tubulointerstitial fibrosis in a final common pathway [10], which is commonly observed regardless of the primary disease, and considered to be an indicator of the severity of the disease.
Previously, we looked for increased interstitial fibrosis and decreased creatinine clearance in db/db intact mice up to 42 weeks of age but found no evidence of either change (data not shown). Therefore, unilateral nephrectomy was performed to further exacerbate the renal damage, and the pathological changes over time were evaluated.
In the present study, urinary albumin excretion and UACR increased with age in 2K db/db mice compared to 2K db/m mice, suggesting the development of renal lesions. Histopathologically, glomerular hypertrophy, an increase in mesangial matrix, and development of tubular lesions including dilatation were observed. However, no tubulointerstitial fibrosis nor a decrease in Ccr, an index of glomerular filtration function, was found as in previous studies. In addition, the mass of 2K db/db mice was lighter than 2K db/m mice at 42 weeks. We consider that mass reduction of 2K db/db mice is due to a decrease of glucose utilization by insulin deficiency. Dalbøge et al. [21] reported that plasma insulin and C-peptide concentrations increased up to 10 weeks, whereafter they declined until 34 weeks of age. They hypothesized that this due to a low beta cell proliferation and as a result, in their 2K db/db mice developed glucose intolerance and their body mass significantly decreased. Remarkably, similar to Dalbøge et al.’s observations, in one of our 2K db/db mice also became obese at the start, but the mass rapidly declined after 30 weeks.
On the other hand, in 1K db/db mice, urinary albumin excretion and UACR were further increased beyond their levels compared to 2K db/db mice. As the creatinine clearance findings show, total renal glomerular filtration rate is decreased by uninephrectomy. Therefore, it is thought that albumin excretion and UACR was decreased in 1K db/m compared to 2K db/m. On the other hand, db/db mice were hyperglycemic and reached a hyperfiltration state to excrete glucose [22]. It is considered that the enhanced glucose excretion mechanism associated with hyperglycemia exceeding the reduction in filtration rate due to nephrectomy. The pathological findings in 1K db/db showed not only a tendency toward aggravation of the above glomerular damage, but also an expansion of Sirius Red-stained tubulointerstitial area, suggesting accelerated fibrosis of the tubules. Accelerated renal lesion development in unilaterally nephrectomized mice suggest that unilateral nephrectomy accelerated the development of renal lesions, as in previous studies [20]. Furthermore, it is considered that we were able to evaluate the severe pathology by extending the observation period where the level of Ccr and BUN changed. There was a downward trend in Ccr after 32 weeks of age, suggesting a decline in renal function due to a decrease in glomerular filtration rate. Creatinine is used as a tracer because creatinine is not absorbed from the tubules and secreted creatinine accounts for only about 10–20 % of urinary creatinine excretion in humans. In mice, on the other hand, about 35–50 % of creatinine excreted is secreted from the tubules [23]. Therefore, the amount of creatinine in the urine of mice is more compared to the amount of creatinine in the urine of humans. In other words, Ccr is considered less likely to decrease in mice than in humans. However, the fact that Ccr levels tended to decrease in mice suggest that the renal function of both glomeruli and tubules had deteriorated considerably. BUN levels also changed after 32 weeks of age, as well as in Ccr levels. In db/db mice where polyuria develops due to diabetes, increased excretion lowers BUN, making it difficult to observe an increase in BUN due to decreased renal function. Nevertheless, an increase of BUN was observed at the same time as Ccr decreased [24]. These results suggest that in addition to pathological changes in the kidney, the reabsorption mechanism is beginning to malfunction. The pathological features of CKD, such as fibrosis, tubular atrophy, and interstitial inflammation, were observed regardless of the initial etiology, and suggested that the disease was similar to severe DKD.
Using this model, we evaluated the effect of losartan, which is clinically used for the treatment of hypertension and proteinuria in type 2 diabetic nephropathy. First, we examined the effect of losartan on blood pressure. The blood pressure was significantly lower in 2K db/db mice than in 2K db/m mice. Although no effect of unilateral nephrectomy on blood pressure was observed, losartan administration had the effect of adequately lowering blood pressure. Therefore, the exposure of losartan in this study was sufficient to show a drug effect. Blood pressure in 2K db/db was significantly reduced, compared with 2K db/m. The tendency of low blood pressure in db/db was confirmed in our previous study as well. Simonds et al. [25] reported blockade of leptin signaling reduces blood pressure in obese mice, although they did not report a clear hypotensive effect in db/db, suggesting that this may be a protective response to obesity hypertension. Next, we examined the effect of losartan on the kidneys. Compared to the vehicle group, the losartan group showed a significantly reduced UACR from the start of treatment to the end of the study. Creatinine clearance and BUN also showed tendency to delay the progression of the disease but without apparent pathological effect on either glomeruli or tubules. Fibrosis in the tubular interstitium was evaluated based on the positive area of Sirius red staining, but no significant decrease was observed. The effect of losartan on glomerular efflux was thought to be due to the reduction of tubular burden associated with the suppression of proteinuria in the early stages of CKD. Losartan may reduce the burden and inhibit disease progression by decreasing intraglomerular pressure and glomerular filtration rate in the early stages of CKD. One of the reasons for reducing the burden was thought to be the reduction of reactive oxygen species (ROS) by decreasing the amount of reabsorption in the proximal tubules. Sustained hyperglycemia, together with increased blood levels of fatty acids, induce oxidative and inflammatory stress, which is largely associated with a progressive renal failure in type 2 diabetes [26]. Fatty acids and other ligands bound to albumin are known to induce the production of ROS that impair proximal tubule function [27]. Enhanced oxidative stress may transform tubular epithelial cells into αSMA-positive myofibroblasts [28]. Another report that evaluated losartan’s effect on renal fibrosis progression in a non-clinical animal model (the UUO rat model) showed efficacy in inhibiting renal fibrosis and apoptosis via phosphorylation of STAT3 [29]. Losartan alleviates renal fibrosis and inhibits endothelial-mesenchymal transition under high-fat diet-induced hyperglycemic conditions [30]. However, both of these structural changes were only partially inhibited. In a clinical study, a follow-up study of the long-term effects of losartan on kidney disease in American Indians with type 2 diabetic diabetes, long-term risk of glomerular filtration rate (GFR) decline was not significantly different between persons randomized to early treatment with losartan and those randomized to placebo [31]. It was reported to have an inhibitory effect on the excretion of albuminuria but not on GFR, suggesting limited structural changes [32]. It is speculated that the lack of clinical efficacy of losartan is probably due to the lack of histological improvement. In this study, the suppression of albuminuria excretion by losartan medication from an early stage of DKD may have tended to decrease fibrosis in the tubulointerstitium, but there was no direct effect. In the uninephrectomized db/db mice used in this study, long-term treatment with losartan showed a suppression of albuminuria excretion but did not achieve sufficient histological effects in the kidney, indicated the efficacy of losartan in this model and thus the efficacy of losartan in clinical DKD patients. In the future, it will be essential to use this model to evaluate the pharmacological effects of SGLT2 inhibitors for CKD in clinical practice.
In summary, we found a trend toward increased tubulointerstitial fibrosis and decreased creatinine clearance in older 1K db/db mice. However, losartan treatment exibited only a partial effect in this model. In the future, it is expected that drugs that could directly improve glomerular filtration rate and inhibit tubulointerstitial fibrosis will be developed. For the development of such drugs, uninephrectomized db/db mice, as an animal model of severe DKD, may be efficient to evaluate the drug.
Footnotes
Conflict of Interest
M. Maekawa, T. Maekawa, Sasase, Takagi, Takeuchi, Kitamoto, Nakagawa, Toyoda and Konishi are employees of Japan Tobacco Inc.
References
- 1.Coresh J. Update on the Burden of CKD. J Am Soc Nephrol. 2017;28:1020–1022. doi: 10.1681/ASN.2016121374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Saran R, Robinson B, Abbott KC, Agodoa LYC, Bhave N, Bragg-Gresham J, Balkrishnan R, Dietrich X, Eckard A, Eggers PW, Gaipov A, Gillen D, Gipson D, Hailpern SM, Hall YN, Han Y, He K, Herman W, Heung M, et al. US Renal Data System 2017 Annual Data Report: Epidemiology of Kidney Disease in the United States. Am J Kidney Dis. 2018;71:A7. doi: 10.1053/j.ajkd.2018.01.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Saran R, Robinson B, Abbott KC, Agodoa LYC, Bragg-Gresham J, Balkrishnan R, Bhave N, Dietrich X, Ding Z, Eggers PW, Gaipov A, Gillen D, Gipson D, Gu H, Guro P, Haggerty D, Han Y, He K, Herman W, et al. US Renal Data System 2018 Annual Data Report: Epidemiology of Kidney Disease in the United States. Am J Kidney Dis. 2019;73:A7–A8. doi: 10.1053/j.ajkd.2019.01.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Andrade-Oliveira V, Foresto-Neto O, Watanabe IKM, Zatz R, Camara NOS. Inflammation in Renal Diseases: New and Old Players. Front Pharmacol. 2019;10:1192. doi: 10.3389/fphar.2019.01192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Japanese Society of Nephrology. Clinical Practice Guidebook for Diagnosis and Treatment of Chronic Kidney Disease 2012. Tokyo Igakusha; Tokyo: 2012. [Google Scholar]
- 6.Sato Y, Yanagita M. Functional heterogeneity of resident fibroblasts in the kidney. Proc Jpn Acad Ser B Phys Biol Sci. 2019;95:468–478. doi: 10.2183/pjab.95.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.National Kidney F. K/DOQI clinical practice guidelines for chronic kidney disease: evaluation, classification, and stratification. Am J Kidney Dis. 2002;39:S1–266. [PubMed] [Google Scholar]
- 8.Andrassy KM. Comments on ‘KDIGO 2012 Clinical Practice Guideline for the Evaluation and Management of Chronic Kidney Disease’. Kidney Int. 2013;84:622–623. doi: 10.1038/ki.2013.243. [DOI] [PubMed] [Google Scholar]
- 9.Bohle A, von Gise H, Mackensen-Haen S, Stark-Jakob B. The obliteration of the postglomerular capillaries and its influence upon the function of both glomeruli and tubuli. Functional interpretation of morphologic findings. Klin Wochenschr. 1981;59:1043–1051. doi: 10.1007/BF01747747. [DOI] [PubMed] [Google Scholar]
- 10.Yamaguchi J, Tanaka T, Nangaku M. Recent advances in understanding of chronic kidney disease. F1000Res. 2015:4. doi: 10.12688/f1000research.6970.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Nitta K, Abe M, Masakane I, Hanafusa N, Taniguchi M, Hasegawa T, Nakai S, Wada A, Hamano T, Hoshino J, Joki N, Goto S, Wakasugi M, Yamamoto K, Nakamoto H, Maeno K, Kawata T, Oyama C, Seino K, Sato T, Sato S, Ito M, Kazama J, Ueda A, Saito O, Ando T, Ogawa T, Kumagai H, Terawaki H, Ando R, Abe M, Kashiwagi T, Hamada C, Shibagaki Y, Hirawa N, Shimada H, Ishida Y, Yokoyama H, Miyazaki R, Fukasawa M, Kamijyo Y, Matsuoka T, Kato A, Mori N, Ito Y, Kasuga H, Koyabu S, Arimura T, Hashimoto T, Inaba M, Hayashi T, Yamakawa T, Nishi S, Fujimori A, Yoneda T, Negi S, Nakaoka A, Ito T, Sugiyama H, Masaki T, Nitta Y, Okada K, Yamanaka M, Kan M, Ota K, Tamura M, Mitsuiki K, Ikeda Y, Nishikido M, Miyata A, Tomo T, Fujimoto S, Nosaki T, Oshiro Y on behalf of the Japanese Society for Dialysis Therapy Renal Data Registry C. Annual dialysis data report 2018, JSDT Renal Data Registry: dialysis fluid quality, hemodialysis and hemodiafiltration, peritoneal dialysis, and diabetes. Renal Replacement Therapy. 2020;6:51. doi: 10.1186/s41100-020-00290-z. [DOI] [Google Scholar]
- 12.Ninomiya T, Kiyohara Y, Kubo M, Yonemoto K, Tanizaki Y, Doi Y, Hirakata H, Iida M. Metabolic syndrome and CKD in a general Japanese population: the Hisayama Study. Am J Kidney Dis. 2006;48:383–391. doi: 10.1053/j.ajkd.2006.06.003. [DOI] [PubMed] [Google Scholar]
- 13.Zhang X, Lerman LO. The metabolic syndrome and chronic kidney disease. Transl Res. 2017;183:14–25. doi: 10.1016/j.trsl.2016.12.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kim Y, Park CW. Mechanisms of Adiponectin Action: Implication of Adiponectin Receptor Agonism in Diabetic Kidney Disease. Int J Mol Sci. 2019;20:1782. doi: 10.3390/ijms20071782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Wang YM, Wang Y, Harris DCH, Alexander SI, Lee VWS. Adriamycin nephropathy in BALB/c mice. Curr Protoc Immunol. 2015;108:15.28.1–15.28.6. doi: 10.1002/0471142735.im1528s108. [DOI] [PubMed] [Google Scholar]
- 16.Perse M, Veceric-Haler Z. Cisplatin-Induced Rodent Model of Kidney Injury: Characteristics and Challenges. Biomed Res Int. 2018;2018:1462802. doi: 10.1155/2018/1462802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kujal P, Vernerova Z. [5/6 nephrectomy as an experimental model of chronic renal failure and adaptation to reduced nephron number] Cesk Fysiol. 2008;57:104–109. [PubMed] [Google Scholar]
- 18.Dendooven A, Ishola DA, Jr, Nguyen TQ, Van der Giezen DM, Kok RJ, Goldschmeding R, Joles JA. Oxidative stress in obstructive nephropathy. Int J Exp Pathol. 2011;92:202–210. doi: 10.1111/j.1365-2613.2010.00730.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Sharma K, McCue P, Dunn SR. Diabetic kidney disease in the db/db mouse. Am J Physiol Renal Physiol. 2003;284:F1138–1144. doi: 10.1152/ajprenal.00315.2002. [DOI] [PubMed] [Google Scholar]
- 20.Ninichuk V, Kulkarni O, Clauss S, Anders H. Tubular atrophy, interstitial fibrosis, and inflammation in type 2 diabetic db/db mice. An accelerated model of advanced diabetic nephropathy. Eur J Med Res. 2007;12:351–355. [PubMed] [Google Scholar]
- 21.Dalb⊘ge LS, Almholt DL, Neerup TS, Vassiliadis E, Vrang N, Pedersen L, Fosgerau K, Jelsing J. Characterisation of age-dependent beta cell dynamics in the male db/db mice. PLoS One. 2013;8:e82813. doi: 10.1371/journal.pone.0082813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Trevisan R, Dodesini AR. The Hyperfiltering Kidney in Diabetes. Nephron. 2017;136:277–280. doi: 10.1159/000448183. [DOI] [PubMed] [Google Scholar]
- 23.Breyer MD, Qi Z. Better nephrology for mice--and man. Kidney Int. 2010;77:487–489. doi: 10.1038/ki.2009.544. [DOI] [PubMed] [Google Scholar]
- 24.Barsanti JA. Urinary Disorders. In: Willard M, Tvedten H, editors. Small Animal Clinical Diagnosis by Laboratory Methods. Saunders; Philadelphia, PA, U.S.A: 2012. pp. 126–155. [Google Scholar]
- 25.Simonds SE, Pryor JT, Ravussin E, Greenway FL, Dileone R, Allen AM, Bassi J, Elmquist JK, Keogh JM, Henning E, Myers MG, Jr, Licinio J, Brown RD, Enriori PJ, O’Rahilly S, Sternson SM, Grove KL, Spanswick DC, Farooqi IS, Cowley MA. Leptin mediates the increase in blood pressure associated with obesity. Cell. 2014;159:1404–1416. doi: 10.1016/j.cell.2014.10.058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Hirsch GE, Heck TG. Inflammation, oxidative stress and altered heat shock response in type 2 diabetes: the basis for new pharmacological and non-pharmacological interventions. Arch Physiol Biochem. 2019:1–15. doi: 10.1080/13813455.2019.1687522. [DOI] [PubMed] [Google Scholar]
- 27.Long KR, Rbaibi Y, Gliozzi ML, Ren Q, Weisz OA. Differential kidney proximal tubule cell responses to protein overload by albumin and its ligands. Am J Physiol Renal Physiol. 2020;318:F851–F859. doi: 10.1152/ajprenal.00490.2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Sato Y, Yanagita M. [Current concept in renal fibrosis] Nihon Jinzo Gakkai Shi. 2015;57:1187–1192. [PubMed] [Google Scholar]
- 29.He P, Li D, Zhang B. Losartan attenuates renal interstitial fibrosis and tubular cell apoptosis in a rat model of obstructive nephropathy. Mol Med Rep. 2014;10:638–644. doi: 10.3892/mmr.2014.2304. [DOI] [PubMed] [Google Scholar]
- 30.Yao Y, Li Y, Zeng X, Ye Z, Li X, Zhang L. Losartan Alleviates Renal Fibrosis and Inhibits Endothelial-to-Mesenchymal Transition (EMT) Under High-Fat Diet-Induced Hyperglycemia. Front Pharmacol. 2018;9:1213. doi: 10.3389/fphar.2018.01213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Tanamas SK, Saulnier PJ, Fufaa GD, Wheelock KM, Weil EJ, Hanson RL, Knowler WC, Bennett PH, Nelson RG. Long-term Effect of Losartan on Kidney Disease in American Indians With Type 2 Diabetes: A Follow-up Analysis of a Randomized Clinical Trial. Diabetes Care. 2016;39:2004–2010. doi: 10.2337/dc16-0795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Looker HC, Mauer M, Saulnier PJ, Harder JL, Nair V, Boustany-Kari CM, Guarnieri P, Hill J, Esplin CA, Kretzler M, Nelson RG, Najafian B. Changes in Albuminuria But Not GFR are Associated with Early Changes in Kidney Structure in Type 2 Diabetes. J Am Soc Nephrol. 2019;30:1049–1059. doi: 10.1681/ASN.2018111166. [DOI] [PMC free article] [PubMed] [Google Scholar]