Summary
Glucagon-like-peptide 2 (GLP-2) is an endogenous enteroendocrine physiological trophic peptide. Glepaglutide is a novel long-acting GLP-2 analog under development for the treatment of patients with Short Bowel Syndrome (SBS). The objective of this work was to compare the small intestinal trophic effects in both genders following short (1 week) versus long-term (26–39 weeks) GLP-2 treatment in Wistar rats and Beagle dogs. Following both short- and long-term treatment with glepaglutide, a significant dose-dependent intestinotrophic effect was seen in both genders and species. At all doses increased length and weight of the small intestine as well as macroscopic thickening and villous hypertrophy were noted in all segments of the small intestine, without any differences between genders. The findings were still present following a 6-week recovery period, indicating long-acting intestinotrophic effects of glepaglutide. These studies demonstrate that a long-acting GLP-2 analog (glepaglutide) has a fast onset and long duration of intestinotrophic action with similar profile in both genders and species (rat and dog).
Keywords: Epithelia, Glepaglutide, GLP-2 receptor, Intestine. Mucosa, Receptor agonist, Short bowel syndrome
Glucagon-like-peptide 2 (GLP-2) is a 33-amino-acid endogenous peptide formed from the cleavage of pro-glucagon in enteroendocrine L cells of the intestines. Under normal conditions, GLP-2 is secreted in response to nutrient intake but is rapidly degraded by the enzyme dipeptidyl peptidase IV. The activation of the GLP-2 receptor with GLP-2 induces growth of primarily the intestinal epithelium via proliferation in the crypts, and inhibition of apoptosis of the villi. Pre-clinical animal experiments have shown that the intestinotrophic effect of GLP-2 is not gender or strain specific. Furthermore, no clinically relevant gender differences have been observed in clinical studies with GLP-2 (1–9).
Glepaglutide is a novel long-acting GLP-2 analog (39-amino acid peptide) currently in phase 3 clinical trials for the treatment of patients with Short Bowel Syndrome (SBS). Nonclinical pharmacology studies in rodents (mice and rats) have shown that glepaglutide is a potent and selective GLP-2 receptor agonist, producing a dose dependent intestinotrophic effect on small intestinal mass. For glepaglutide, it is unknown whether this intestinotrophic effect declines over long-time treatment and whether they are gender and/or species dependent (small versus large species).
The objective of this study was to compare the small intestinal trophic effects following short- and long-term GLP-2 treatment (glepaglutide) in both genders of Wistar rats and Beagle dogs.
All studies were conducted in accordance with the applicable sections of the United Kingdom Animals (Scientific Procedures) Act 1986, Amendment Regulations 2012 (the Act) or the Danish Animal Welfare Act (2014).
Short-term treatment
Wistar rats (7 males/group) were dosed subcutaneously (SC) with vehicle, 0.1, 0.25, 1, 4 and 10 mg/kg glepaglutide once daily (QD) for 7 days. The dose levels were selected in order to investigate a broad dose range to determine no, minimal and maximal effects. The weight of the small intestine was measured at necropsy as indicators of intestinotrophic effects.
Long-term treatment
Wistar rats (15 animals/sex/group) and Beagle dogs (4 animals/sex/group) received QD SC doses of vehicle or increasing doses of glepaglutide for 26 and 39 weeks, respectively. Sub-groups of the vehicle and high dose groups in the rat (8 animals/sex/group) and the dog (2 animals/sex/group) study were allowed a 6-week recovery period. The highest dose levels were selected based on the maximum tolerable dose seen in previous studies with glepaglutide (ICH M3(R2)).
During the studies, blood samples were collected for determination of the pharmacokinetic profile, body weight was recorded, and the food consumption measured. In addition to other standard toxicology parameters measured during the studies (data not presented), the length and the weight of the small intestine were measured at necropsy as indicators of intestinotrophic effects and the small intestine was evaluated histologically.
Intestinotrophic effects
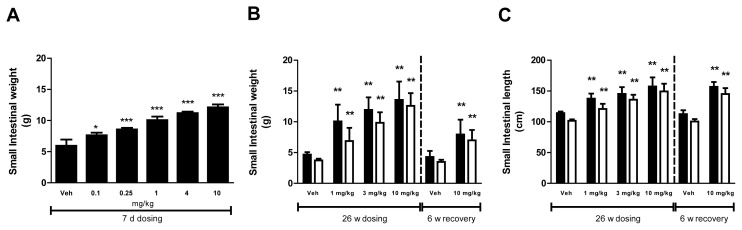
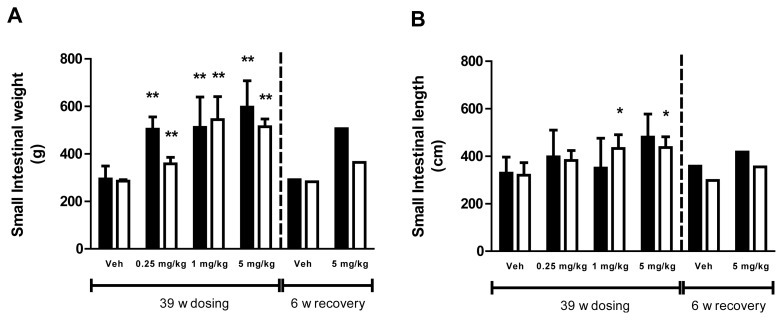
In rats, short-term treatment with glepaglutide caused dose-dependent increases in the weight of the small intestine of all dose groups (Fig. 1A). Long-term treatment with glepaglutide caused dose-dependent increases in the weight of the small intestine of all dose groups. The weight of the small intestine was still increased following the recovery period (Fig. 1B). In addition, the length of the small intestine was statistically increased at all doses and this effect was also still present following the recovery period (Fig. 1C). Increased length of the small intestine was also seen following long-term exposure in dogs, which was significant at the mid and the high dose in females. The weight of the small intestine was increased at all dose levels. Increases in both length and weight were still present after recovery; however, no statistical analysis was performed due to the low number of animals in each group (Fig. 2A,B). No differences between genders were seen for both weight and length measurements.
Fig. 1.
Intestinotrophic effects of glepaglutide in male (■) and female (□) Wistar rats. (A) Effects of short-term treatment with glepaglutide on small intestinal weight in male rats (g), (B) Effects of long-term treatment with glepaglutide on small intestinal weight (g) in male and female rats, and (C) Effects of long-term treatment with glepaglutide on small intestinal length (cm) in male and female rats. Data are presented as mean + SD. Statistical analysis were one-way ANOVA followed by Dunnett’s Multiple Comparison tests (A), and one-way ANOVA followed by Shirley’s test for male and female weight and female length during dosing, one-way ANCOVA followed by Williams’ test for male length during dosing. One-way ANCOVA followed by t-test for all during recovery (B and C).
Fig. 2.
Intestinotrophic effects of glepaglutide in male (■) and female (□) Beagle dogs. (A) Effects of long-term treatment with glepaglutide on small intestinal weight (g) in male and female dogs, and (B) Effects of long-term treatment with glepaglutide on small intestinal length (cm) in male and female dogs. Data are presented as mean + SD. Statistical analysis was one-way ANCOVA followed by Williams’ test during dosing. No statistical analysis on recovery data.
Pathology
At all dose levels in both species, macroscopic thickening of the duodenum, jejunum and ileum was present. Histologically, glepaglutide produced a dose-related increase in mucosal hyperplasia of the duodenum, jejunum and ileum, still present at end of recovery period.
Toxicokinetics
At steady state, the pharmacokinetic profiles of glepaglutide within the dosing interval were relatively constant in both rats and dogs, and therefore an accurate half-life could not be determined. At the end of the recovery period, glepaglutide could still be measured in the plasma of 2 out of 4 dogs.
Bodyweight and food consumption
Following long-term treatment, the food consumption in rats was slightly low in the mid and high dose group. However, no differences in body weight were observed. In dogs, there was a lower weight gain in females given the high dose after long term treatment. However, no differences in food consumption were seen.
Following short-term treatment with glepaglutide, a significant dose-dependent intestine-trophic effect was seen in rats. This response was similar to findings after 26 and 39 weeks of dosing in rats and dogs, respectively. At all doses, increased length and weight of the small intestine as well as macroscopic thickening and villous hypertrophy were noted in all segments of the small intestine, with no differences between genders. These findings were still present following a 6-week recovery period, indicating long-acting intestinotrophic effects of glepaglutide.
These studies demonstrate that short and long-term treatment with long-acting GLP-2 analog (glepaglutide) has a fast onset and long duration of action (intestinal trophic effects) with similar profile in small and larger mammalian species and in both genders. The prolonged action can be explained by the protracted pharmacokinetic profile of glepaglutide observed in the long-term studies. Clinical trials in humans are underway to validate this concept.
Acknowledgements
The authors would like to thank Dr Wayne Russell for his contribution to the short term studies and for the visual presentation of data.
Footnotes
Conflict of Interest
There is no conflict of interest.
References
- 1.Benjamin MA, McKay DM, Yang PC, Cameron H, Perdue MH. Glucagon-like peptide-2 enhances intestinal epithelial barrier function of both transcellular and paracellular pathways in the mouse. Gut. 2000;47(1):112–119. doi: 10.1136/gut.47.1.112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Drucker DJ, Yusta B. Physiology and pharmacology of the entero-endocrine hormone glucagon-like peptide-2. Annu Rev Physiol. 2014;76:561–583. doi: 10.1146/annurev-physiol-021113-170317. [DOI] [PubMed] [Google Scholar]
- 3.Hartmann B, Harr MB, Jeppesen PB, Wojdemann M, Deacon CF, Mortensen PB, Holst JJ. In vivo and in vitro degradation of glucagon-like peptide-2 in humans. J Clin Endocrinol Metab. 2000;85:2884–2888. doi: 10.1210/jcem.85.8.6717. [DOI] [PubMed] [Google Scholar]
- 4.ICH M3 (R2) Non-clinical safety studies for the conduct of human clinical trials for pharmaceuticals
- 5.NPS Pharmaceuticals. GATTEX (teduglutide [rDNA origin]): Highlights of prescribing information. 2012. [Google Scholar]
- 6.Wallis K, Walters JR, Forbes A. Review article: glucagon-like peptide 2--current applications and future directions. Aliment Pharmacol Ther. 2007;25:365–72. doi: 10.1111/j.1365-2036.2006.03193.x. [DOI] [PubMed] [Google Scholar]
- 7.Warner BW. GLP-2 as therapy for the short-bowel syndrome. Gastroenterology. 2001;120(4):1041–1043. doi: 10.1053/gast.2001.22560. [DOI] [PubMed] [Google Scholar]
- 8.Sinclair E, Drucker DJ. Proglucagon-derived peptides: mechanisms of action and therapeutic potential. Physiology. 2005;20:357–365. doi: 10.1152/physiol.00030.2005. [DOI] [PubMed] [Google Scholar]
- 9.Tavares W, Drucker DJ, Brubaker PL. Enzymatic- and renal-dependent catabolism of the intestinotropic hormone glucagon-like peptide-2 in rats. Am J Physiol Endocrinol Metab. 2000;278:E134–E139. doi: 10.1152/ajpendo.2000.278.1.E134. [DOI] [PubMed] [Google Scholar]