SUMMARY
Infection with Shiga toxin-producing Escherichia coli (STEC) by serotypes other than O157 (non-O157) have been increasingly reported in the United States. This increase in reporting is primarily due to the improvements in diagnostic tests. We analysed 1497 STEC cases reported in Michigan from 2001 to 2012. A significant increase in the number of non-O157 STEC cases was observed over time, and similar incidence rates were observed for O157 and non-O157 STEC cases in certain time periods. The odds of hospitalization was two times higher in O157 STEC cases relative to non-O157 STEC cases when adjusted for age and gender, suggesting that O157 STEC causes more severe clinical outcomes in all age groups. The use of population-based surveillance to better define trends and associations with disease severity are critical to enhance our understanding of STEC infections and improve upon current prevention and control efforts.
Key words: Epidemiology, Shiga-like toxin-producing E. coli
INTRODUCTION
Shiga toxin-producing Escherichia coli (STEC) represent subsets of E. coli strains that are capable of producing one or more Shiga toxins. STEC infections, mostly acquired from consuming contaminated food or water, are associated with outbreaks and sporadic cases of diarrhoea, haemorrhagic colitis (HC), and haemolytic uraemic syndrome (HUS) [1, 2]. Among individuals with HC, 3–20% of cases develop life-threatening complications associated with HUS [3–5], a leading cause of acute renal failure in young children worldwide [3, 6, 7]. In the USA, STEC is a major public health concern and contributes to an estimated 265 000 illnesses per year [8].
While STEC strains belonging to the O157 serogroup are more common in outbreaks and patients with severe clinical symptoms [9], infections caused by non-O157 STEC have been increasingly reported [9–12]. Indeed, it was recently estimated that 168 698 non-O157 infections and 96 534 O157 infections occur each year [8]. In the years between 2000 and 2010, the Foodborne Diseases Active Surveillance Network (FoodNet) noted an increase in the incidence of non-O157 STEC from 0·12 to 0·95/100 000 [11]. In addition, six serogroups (O26, O103, O111, O121, O45, O145) were reported to cause 83% of the 2006 non-O157 STEC infections identified; 65 additional serogroups were associated with the remainder of the non-O157 STEC infections detected during this 10-year period [11]. Although these additional serotypes contribute to the non-O157 STEC disease burden, they are not monitored routinely [13] and thus are more difficult to detect in both food products and human clinical samples.
The increasing incidence of non-O157 STEC infections was suggested to be correlated with the widespread use of more sensitive, culture-independent detection methods that target the Shiga toxin [14]. Notably, the number of laboratories utilizing culture-independent detection methods was not reported to be correlated with O157 STEC infections. Unlike non-O157 STEC, the incidence of O157 STEC has decreased from 2·17/100 000 in 2000 to 0·95/100 000 in 2010 across the 10 FoodNet sites [11]. Significant variation in disease frequencies, however, was observed across sites for both O157 and non-O157 infections. Little is known about those factors that contribute to disease variation in different geographical locations, particularly for non-O157 STEC.
Several previous studies have reported associations between specific demographic characteristics and infection with non-O157 STEC. For example, the 10 FoodNet sites identified a greater number of females to be infected with both O157 and non-O157 STEC, although the age distribution did not differ [11]. A study of the New Mexico population, however, detected a higher frequency of non-O157 STEC in children aged <5 years [12]. These data suggest that risk factors may also vary by geographical location. Consequently, large-scale population-based surveillance studies are important to identify factors associated with STEC infections as well as markers for more severe infections.
Through this study, we sought to examine the epidemiology of STEC cases reported in Michigan over a 12-year period. Trends associated with STEC cases were examined per year, as were the demographic and clinical data associated with each case. Because disease prevention plans can be improved by understanding which host characteristics are important for STEC infections, it is imperative to monitor disease trends over time, particularly in distinct geographical locations that are not part of the FoodNet surveillance system.
METHODS
Data source and management
The STEC case information was retrieved from the Michigan Disease Surveillance System (MDSS), a web-based surveillance system maintained by the Michigan Department of Health and Human Services (MDHHS) formerly known as the Michigan Department of Community Health. All STEC infections are reportable, and a STEC isolate, broth, and/or stool sample are required to be submitted to the MDHHS for isolation, confirmation and genotyping. In MDSS, STEC cases were designated under the reportable conditions ‘Escherichia coli O157:H7’, ‘Shiga toxin E. coli, unspecified’, ‘Shiga toxin E. coli, non-O157’, and ‘Shiga toxin-producing E. coli (STEC)’. Beginning in 2010, these terms were consolidated into one category, ‘STEC’. The case inclusion criteria included (1) ‘confirmed’ cases; (2) ‘completed’ investigations, and (3) report dates between 1 January 2001 and 31 December 2012. All protocols were approved by the Institutional Review Boards at Michigan State University (IRB no. 10–736SM) and the MDHHS (842-PHALAB).
The distribution of serotypes and serogroups, which included only the O-antigen type, was assessed. Serotypes that were detected in eight or more cases were examined for epidemiological associations. For the serogroup analysis, the predominant groups were collapsed into individual categories to increase the sample size and identify associations by serogroup. O157 infections, for instance, were collapsed into one category and included O157:H7, O157:NM (non-motile), and O157:H unknown cases. The O45:H unknown and O45:H2 cases were collapsed into one O45 serogroup category and the O103:H11, O103:H2, O103:H25, and O103:H unknown cases were classified as O103. O26 strains included O26:H11, O26:NM, and O26:H unknown, while O111 included O111:H8, O111:NM, and O111:H unknown. The O145 and O121 serogroups included O145:NM, O145:H7 and O145:H unknown, and O121:H19, O121:H7, O121:H9, and O121:H unknown, respectively. Cases (n = 26) reporting co-infection with more than one STEC serotype or another pathogen were omitted from the analysis. Season was categorized based on the report date, which was used to represent the date of the infection, while some variables such as race, ethnicity, travel and food history, and animal contacts could not be examined due to a high frequency of missing data.
Data analysis
The annual age-adjusted incidence rates (case/100 000 population) of STEC cases were computed based on the population estimates of Michigan from the Bridged-Race Population Estimates 1990–2012 dataset [15]. The standard population was based on the USA 2000 standard population [16, 17]. χ2 tests were performed to assess the distribution of Shiga toxin gene profiles, serogroups and serotypes, and test the association with other variables. Kruskal–Wallis one-way analysis of variance (ANOVA) by ranks was employed to test the differences of age medians across serogroups. Statistical analyses were performed in SAS v. 9.3 (SAS Institute, USA); P values <0·05 were considered significant.
Hospitalization was used as an indicator for a severe clinical outcome associated with a STEC infection. The dependent variable was hospitalized STEC cases, and variables included serogroup (O157, non-O157), Shiga toxin genes (stx2 only, stx1 only, both stx1 and stx2), age group (≤10, 11–59, ≥60 years), gender (female, male), diarrhoea (bloody, non-bloody), and season. Univariate analysis was performed initially and biologically plausible independent variables with P < 0·25 were selected for inclusion in a multivariate model using logistic regression. The variable selection method followed the ‘purposeful selection’ steps described by Hosmer & Lemeshow [18]. A variable was kept in the model when the P value was <0·1 and removal of this variable caused the values of other variables' effect sizes to change >10%. The model goodness-of-fit was assessed by the Hosmer & Lemeshow test [18].
Geographical information system (GIS) mapping was conducted using ArcGIS v. 10.2.2 (ESRI, USA). The administrative shape files of Michigan were obtained from the CDC (http://wwwn.cdc.gov/epiinfo/html/shapefiles.htm), while the denominators used for incidence rates calculation per 100 000 persons were derived from annual population estimates from the Michigan Department of Technology, Management and Budget [19].
RESULTS
STEC serotype distribution and Shiga toxin (Stx) gene profiles
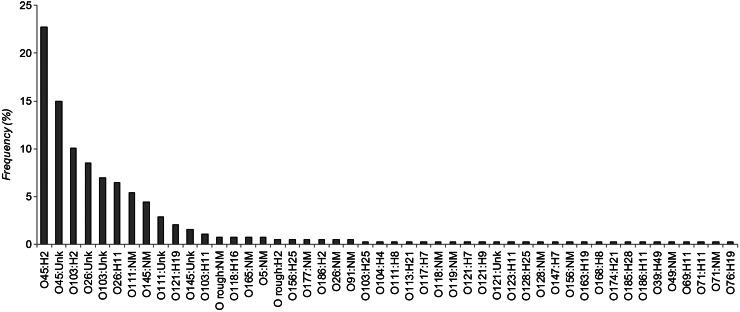
From 2001 to 2012, a total of 1497 confirmed STEC cases were reported in Michigan. Among these cases, 138 (9·2%) were associated with 29 different outbreaks and most (n = 108, 79·4%) of these outbreak-associated cases were infected with O157 STEC. Serotyping data were available for 85·6% (1282/1497) of all cases; 33 serogroups were reported representing 51 different serotypes. Among these 1282 cases, STEC O157:H7 (n = 823, 64·2%) was found in greatest frequency followed by O45:H2 (n = 88, 6·9%), O157:NM (n = 69, 5·4%), and O45:H unknown (n = 58, 4·5%). Three O157 isolates had an unknown H-type. Nine additional serotypes were reported in five or more cases (range 6–39 cases), while the remaining 38 serotypes were recovered from <0·3% of all 1282 individuals. Serotype frequencies were also examined in the 387 non-O157 STEC cases after excluding the 895 O157 cases (Fig. 1). Twelve serotypes predominated and were found in at least four (1%) non-O157 STEC cases, whereas the remaining 36 serotypes were reported for only 1–3 cases.
Fig. 1.
Distribution of non-O157 Shiga toxin-producing E. coli (STEC) serotypes recovered from Michigan cases, 2001–2012. Percentages are illustrated in order of decreasing frequency and represent the number of isolates with a given serotype out of 387 cases infected with non-O157 STEC.
Because the H-antigen type was missing in a high frequency of cases (n = 139), we also examined frequencies by serogroup. O157 was the most commonly reported serogroup among all 1282 STEC cases (n = 895, 69·8%) with data available; 387 isolates were classified as non-O157 STEC representing 32 serogroups. Overall, O45 was recovered from 11·4% (n = 146) of cases followed by O103 (n = 71, 5·5%), O26 (n = 60, 4·7%), O111 (n = 33, 2·6%), O145 (n = 23, 1·8%), and O121 (n = 11, 0·9%). The remaining 43 non-O157 STEC isolates comprised 26 additional serogroups.
The Shiga toxin gene profiles were available for 84·2% (1260/1497) of the cases and most had both stx1 and stx2 (n = 538, 42·7%) followed by stx1 only (n = 378, 30·0%) and stx2 only (n = 344, 27·3%). Although 237 cases with stx data lacked serogroup information, the overall frequency was similar after omitting these cases from the stratified analysis. Indeed, many of the cases with missing serogroup data (n = 51, 39·8%) were reportedly infected with STEC harbouring stx1 only, a profile more commonly found among the non-O157 STEC cases in this study. The 381 non-O157 STEC cases, for instance, were significantly more likely to have stx1 alone (n = 315, 82·7%) relative to O157 STEC (P < 0·001). The remaining 66 non-O157 STEC cases reported stx2 only (n = 42, 11·0%) or both stx1 and stx2 together (n = 24, 6·3%). Serotypes O145:NM (n = 14, 33·3%), O121:H19 (n = 8, 19·1%) and O145:H unknown (n = 6, 14·3%) predominated among the 42 cases with stx2 only. By contrast, the O157 cases were significantly more likely to report stx2 alone (n = 284, 35·9%) or in combination with stx1 (n = 496, 62·6%) than the non-O157 STEC cases (P < 0·0001). Only 12 (1·5%) O157 STEC cases had stx1-positive infections.
Incidence rates and seasonal and geographical variation
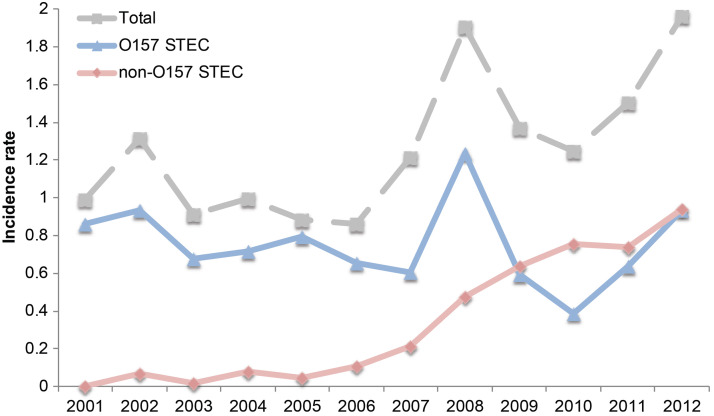
Significant differences in the frequency of STEC infections were observed over time (Fig. 2). The greatest number of STEC cases was reported in 2008 (n = 186) as well as 2012 (n = 189), while the lowest number was reported in 2006 (n = 88). Overall, the age-adjusted STEC incidence rate remained relatively constant before 2007, ranging from 0·88/100 000 in 2006 to 1·32/100 000 in 2002. For O157 STEC, the age-adjusted incidence rates ranged from 0·37/100 000 in 2010 to 1·23/100 000 in 2008 with the most recent rate of 0·9/100 000 in 2012. A notable peak was observed for all STEC cases (1·91/100 000) in 2008 as well as the O157 STEC cases alone. This peak may have been partly attributable to a high frequency of outbreak-associated O157 STEC cases (24·3%, 46/189) reported in 2008. A high frequency of outbreak-associated cases (28·6%, 54/189) was also detected in 2012. Although no non-O157 STEC cases were reported in 2001, there has been an increasing trend in the incidence rate of non-O157 STEC over time. Importantly, the rate (0·89/100 000) of non-O157 STEC in 2012 was similar to the rate of O157 STEC in the same year. A significant increase in the number of non-O157 outbreaks was also observed over time (Mantel–Haenszel χ2 P = 0·02). In 2010 and 2012, 12/12 (100%) and 15/54 (27·8%) outbreak-associated cases were caused by non-O157 STEC, respectively. All 12 of the outbreak-associated non-O157 cases reported in 2010 were infected with O145:NM, whereas the 2012 outbreaks were attributable to multiple serotypes including O45:H unknown (n = 3), O26: H unknown (n = 10), and O145:H unknown (n = 2).
Fig. 2.
Age-adjusted incidence rates of Shiga toxin-producing E. coli (STEC) cases in Michigan, 2001–2012. The incidence of all STEC cases (grey line) is compared to all non-O157 (pink line) and O157 (blue line) STEC over time.
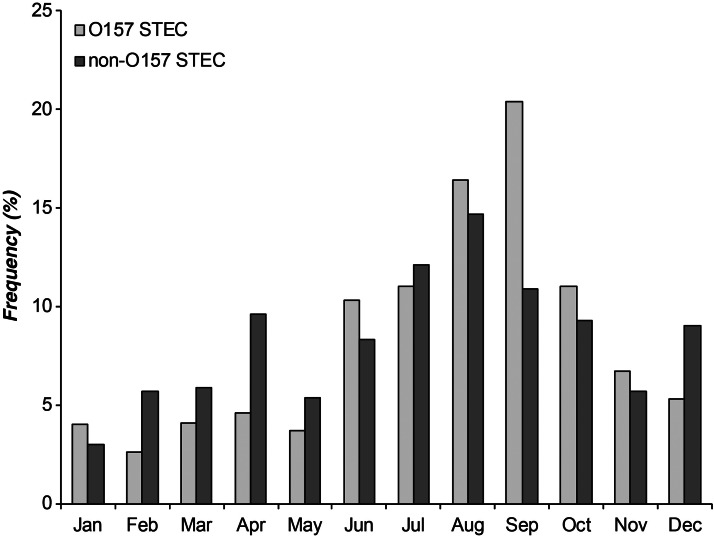
In addition to the observed variation in frequencies over time, the distribution of cases also varied by season and by serogroup (Fig. 3). Overall, most STEC infections were reported in summer (n = 558, 37·3%) and autumn (n = 497, 33·2%), and the frequencies were significantly greater than those reported in winter and spring (n = 442, 29·5%) (P < 0·0001). Among O157 STEC cases from all years, a peak in the number of cases (20·5%, 183/895) occurred in September. The peak in non-O157 STEC cases (14·1%, 57/387) was observed in August. Regardless of this peak, non-O157 STEC infections were significantly more likely to occur during the spring and winter months (December–May) relative to STEC O157 [odds ratio (OR) 2·0, 95% confidence interval (CI) 1·53–2·55, P < 0·0001), which predominated in summer and autumn (June–November). No differences in the frequency of specific non-O157 serotypes or serogroups were observed by season, although the stx profile distribution varied. Specifically, stx2-positive STEC isolates caused 74·1% of the 896 infections that occurred in summer and autumn, whereas isolates containing only stx1 were significantly more common in winter and spring (40·1%) relative to summer and autumn (25·9%).
Fig. 3.
Frequency of Shiga toxin-producing E. coli (STEC) cases by month of report in Michigan, 2001–2012.
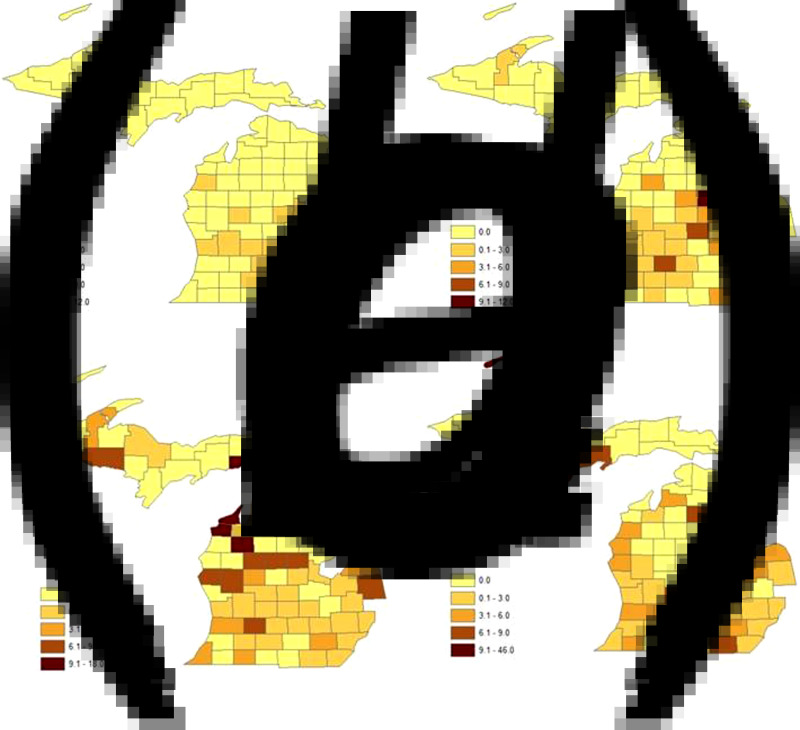
With respect to geographical location, we also observed a trend of increasing frequency of non-O157 STEC cases from southern to northern Michigan over time (Fig. 4). No trends were observed for O157 STEC cases. Between 2001 and 2006, non-O157 STEC infections had not been reported in most of the counties located in northern Michigan. However, between 2007 and 2008, several new northern Michigan counties including one located in the Upper Peninsula, reported cases of non-O157 STEC infection. At this time, the emergence of non-O157 STEC in the Upper Peninsula was attributable to serogroups O103, O121 and O45. Non-O157 STEC became widespread throughout the state of Michigan in 2009 and 2010, and by 2012, only 13 counties had failed to report a case.
Fig. 4.
County-level maps representing the two-year mean isolation rates of non-O157 STEC per 100 000 individuals. A total of 602 non-O157 STEC cases were recovered in Michigan from (a) 2001–2006, (b) 2007 and 2008, (c) 2009 and 2010, and (d) 2011 and 2012.
Demographic characteristics and association with clinical outcomes
Examination of the demographic data identified 790 (53·5%) female cases out of the total 1478 cases with data available. Overall, the proportion of women affected was significantly greater than the proportion of men (P = 0·008). The gender distribution did not vary across serotypes or serogroups (Table 1), although women were slightly more likely to have O157 vs. non-O157 infections (P = 0·09). The median age for all STEC cases was 22 years (range 1 day to 102 years). The proportion of cases (62·8%) aged between 11 and 59 years was significantly greater than any other age groups (P < 0·0001). A total of 290 (22·7%) cases were children aged <10 years, while 174 (14·4%) adults aged ≥60 years were also affected. It is noteworthy that 71 cases were aged <2 years. Most (n = 31, 55·4%) of these cases with serotyping data available (n = 56) were infected with STEC O157:H7, although serotypes O rough:H2 (n = 1), O103:H2 (n = 4), O111:NM (n = 2), O118:H16 (n = 1), O157:NM (n = 4), O186:H11 (n = 1), O45:H2 (n = 3), O5:NM (n = 1), and O76:H19 (n = 1) were also found. Serogroups O26, O103, O157 with unknown H-types were identified in the remaining seven babies. Only three cases aged <2 years were associated with outbreaks.
Table 1.
Demographic characteristics of 1282 patients with STEC infections by serogroup
| Total no. of cases (%)† | O157 (n = 895) (69·8%) |
O45 (n = 146) (11·4%) |
O103 (n = 71) (5·7%) |
O26 (n = 60) (4·7%) |
O111 (n = 33) (2·6%) |
O145 (n = 23) (1·8%) |
O121 (n = 11) (0·9%) |
Others* (n = 43) (3·6%) |
|
|---|---|---|---|---|---|---|---|---|---|
| Age (years) | |||||||||
| Median (range) | 22 (0·003–102) | 22 (0·003–102) | 23 (0·9–91) | 20 (0·6–81) | 24 (1·1–83) | 17 (0·9–66) | 22 (10–78) | 21 (5–56) | 30 (0·3–85) |
| <5 | 153 (12·0) | 107 (12·0‡) | 13 (9·0) | 12 (16·9) | 6 (10·0) | 9 (27·3) | 0 (0·0) | 0 (0·0) | 6 (14·0) |
| 5–10 | 137 (10·7) | 100 (11·2) | 14 (9·7) | 5 (7·0) | 5 (8·3) | 4 (12·1) | 1 (4·4) | 3 (27·3) | 5 (11·6) |
| 11–59 | 812 (63·6) | 558 (62·7) | 100 (69·0) | 45 (63·4) | 43 (71·7) | 17 (51·5) | 18 (78·3) | 7 (72·7) | 23 (53·5) |
| ≥60 | 174 (13·6) | 125 (14·1) | 18 (12·4) | 9 (12·7) | 6 (10·0) | 3 (9·1) | 4 (17·4) | 0 (0·0) | 9 (20·9) |
| Gender | |||||||||
| Male | 688 (46·6) | 419 (47·3) | 60 (41·4) | 33 (46·5) | 21 (35·6) | 15 (48·4) | 13 (56·5) | 3 (27·3) | 16 (38·1) |
| Female | 790 (53·5) | 466 (52·7) | 85 (58·6) | 38 (53·5) | 38 (64·4) | 16 (51·6) | 10 (43·5) | 8 (72·7) | 26 (61·9) |
The age was missing for six cases and gender was missing from four leaving different denominators for each variable.
* Others included all other non-O157 serogroups.
† Row percentages represent the % of the total no. of cases with serogrouping data available (n = 1282).
‡ Column percentages within each variable represent the % of the total no. of cases within each serogroup.
Among all cases, the lowest median age was 17 years (range 0·92–66) for O111 STEC. The highest median age was 30 years (range 0·33–85) among cases infected with non-O157 STEC representing serogroups other than the six most common types. Although the age medians did not differ significantly across serogroups (P = 0·18), children aged <10 years were significantly more likely to have a STEC O111 infection compared to all other non-O157 serogroups (OR 2·6, 95% CI 1·25–5·54, P = 0·009) as well as O157 cases (OR 2·1, 95% CI 1·01–4·39, P = 0·03). In the 13 children with O111 infections, serotype O111:NM was detected most frequently (n = 7, 53·8%) followed by O111:H unknown (n = 5, 38·5%) and O111:H8 (n = 1, 7·7%); none of these cases were associated with an outbreak. By contrast, a significantly greater proportion of cases with STEC O103 (P = 0·02), O145 (P = 0·007), O26 (P = 0·001), and O157 (P < 0·001) infections were aged 11–59 years. The 45 O103 isolates infecting these individuals were further classified as serotypes O103:H2 (n = 24), O103:H11 (n = 3), O103:H25 (n = 1) and O103:H unknown (n = 17), while the 18 O145 isolates were O145:NM (n = 15) and O145:H unknown (n = 3).
Clinical outcomes also varied across patients and two of the cases with O157:H7 STEC infections died within the time of the epidemiologic investigation. The most common symptom was abdominal pain, which was reported in 1069 (80·3%) cases, followed by bloody diarrhoea (n = 956, 71·8%), and non-bloody diarrhoea (n = 330, 24·8%); the denominator varied for several symptom variables because of missing data. A total of 590 of these cases reported both bloody and non-bloody diarrhoea, while 46 (3·5%) cases reported no history of diarrhoea. In addition, 456 (45·3%) cases were hospitalized for one to 41 days with a 5-day average duration.
Variation in clinical outcomes was observed across serogroups (Table 2). HUS was reported in 26 (2·2%) of the 1334 cases with data available; 22 (84·6%) of these HUS cases were infected with O157 STEC (P < 0·0001). The proportion of cases reporting bloody diarrhoea ranged from 38·5% for non-O157 STEC representing serogroups other than the six most common types to 86·4% in O145 STEC cases (P < 0·0001). Indeed, the O145 STEC cases were significantly more likely to report bloody diarrhoea vs. diarrhoea when compared to all other non-O157 STEC cases combined (OR 4·2, 95% CI 1·22–14·48, Fisher's exact P = 0·01). Fifteen of the 19 O145 cases reporting a history of bloody diarrhoea were classified as serotype O145:NM and four were O145:H unknown; most (n = 16, 84·2%) of the O145 isolates were positive for stx2 only. A similar association with bloody diarrhoea was observed for cases infected with O45 STEC (OR 1·7, 95% CI 1·07–2·64, P = 0·02), although 50 of these cases had stx1-positive O45:H2 infections and 38 had stx1-positive O45:NM infections. When comparing all non-O157 and O157 STEC cases, however, the number of O157 STEC cases reporting bloody diarrhoea was significantly greater (OR 2·9, 95% CI 2·16–3·77, P < 0·0001). In addition, cases with non-O157 STEC representing other serotypes were significantly less likely to cause bloody diarrhoea (OR 0·3, 95% CI 0·17–0·68, P = 0·002) relative to cases with non-O157 STEC belonging to the predominant six or unknown serogroups.
Table 2.
Clinical outcomes among cases infected with STEC serogroups in Michigan, 2001–2012
| Serogroup | HUS (N = 1334) n (%)* |
Any bloody diarrhoea (N = 1332) n (%)* |
Non-bloody diarrhoea only (N = 1332) n (%)* |
Hospitalization (N = 1349) n (%)* |
|---|---|---|---|---|
| O157 | 22 (2·6) | 668 (82·2) | 145 (17·8) | 432 (51·1) |
| O45 | 0 (0·0) | 94 (69·1) | 42 (30·9) | 60 (43·2) |
| O103 | 1 (1·5) | 31 (50·0) | 31 (50·0) | 10 (14·9) |
| O26 | 0 (0·0) | 34 (63·0) | 20 (37·0) | 13 (22·4) |
| O111 | 0 (0·0) | 19 (63·3) | 11 (36·7) | 12 (40·0) |
| O145 | 0 (0·0) | 19 (86·4) | 3 (13·6) | 7 (30·4) |
| O121 | 0 (0·0) | 6 (60·0) | 4 (40·0) | 5 (45·5) |
| Others | 1 (2·4) | 15 (38·5) | 24 (61·5) | 12 (29·3) |
| Unknown | 2 (1·5) | 70 (58·3) | 50 (41·7) | 60 (44·4) |
| Total† | 26 (2·2) | 956 (71·8) | 330 (24·8) | 611 (45·3) |
The denominator varied for each of the variable/serotype combinations due to missing data.
* Percentages represent the % of the total number of cases for each serogroup.
† Totals represent the total number and % of cases with each outcome including those cases missing serogroup data.
To identify predictors of more severe disease, as indicated by hospitalization status, we examined 1349 STEC cases with complete data available; 287 cases were excluded from the analysis due to missing data for the outcome variable (hospitalization status) and/or covariates. The greatest univariate effect sizes were observed for STEC serogroup, stx profile, diarrhoea presentation and age (Table 3). Although the proportion of cases who were hospitalized varied across serogroups, with a range of 14·9% for O103 STEC to 51·1% for O157 STEC cases (P < 0·0001), the odds of hospitalization was 1·9 times higher in O157 STEC vs. non-O157 STEC cases. Hospitalization frequency was also 1·5 and 3·3 times higher in cases aged 11–59 and ≥60 years, respectively, compared to cases aged <10 years. In addition, stx2-positive STEC infections significantly enhanced the odds of hospitalization relative to STEC with other stx profiles in the univariate analysis as did the bloody diarrhoea vs. non-bloody diarrhoea.
Table 3.
Univariate analysis of characteristics associated with hospitalization in STEC cases in Michigan
| Characteristic | Number with characteristic | Number (%) hospitalized | OR (95% CI) | P value* |
|---|---|---|---|---|
| STEC serogroup | ||||
| O157 | 845 | 432 (51·1) | 2·2 (1·70–2·84) | <0·0001 |
| Non-O157 | 369 | 119 (32·3) | 1·0 | – |
| Shiga toxin profile | ||||
| stx1 | 358 | 117 (32·7) | 1·0 | – |
| stx2 | 331 | 163 (49·2) | 2·0 (1·47–2·72) | <0·0001 |
| stx1, stx2 | 508 | 255 (50·2) | 2·1 (1·57–2·75) | <0·0001 |
| Clinical symptoms | ||||
| Diarrhoea only | 328 | 87 (26·5) | 1·0 | – |
| Bloody diarrhoea | 954 | 489 (51·3) | 2·9 (2·21–3·84) | <0·0001 |
| Age group (years) | ||||
| ≤10 | 305 | 107 (35·1) | 1·0 | – |
| 11–59 | 839 | 373 (44·5) | 1·5 (1·13–1·94) | 0·004 |
| ≥60 | 197 | 126 (64·0) | 3·3 (2·26–4·77) | <0·0001 |
| Gender | ||||
| Female | 714 | 328 (45·9) | 1·1 (0·86–1·32) | 0·58 |
| Male | 617 | 274 (44·4) | 1·0 | – |
| Season | ||||
| Winter and spring | 395 | 180 (45·6) | 1·0 | 0·90 |
| Summer and autumn | 954 | 431 (45·2) | 1·0 (0·80–1·29) | – |
OR, Odds ratio; CI, confidence interval.
* Likelihood ratio χ2 test.
For the multivariate analysis, all covariates except gender and season met the initial screening significance level of P < 0·25 in the univariate analysis, and were included in the final model (Table 4). Gender was included to limit confounding effects and the model fit was acceptable (P = 0·97). The strongest association was observed for age as cases ≥60 years were 3·8 times more likely to be hospitalized than cases aged <10 years while controlling for serogroup and gender. A similar association was identified for cases aged 11–59 years. In addition, individuals infected with O157 STEC were twice as likely to be hospitalized relative to non-O157 STEC cases, while those presenting with bloody vs. non-bloody diarrhoea were three times more likely. Neither gender nor stx profile were significantly associated with hospitalization in the multivariate analysis. When the model was limited to 716 O157 STEC cases with complete data, only individuals aged ≥60 years (OR 3·9, 95% CI 2·22–6·71, P < 0·0001) and with bloody diarrhoea (OR 3·3, 95% CI 2·10–5·05, P < 0·0001) were significantly more likely to be hospitalized. Limiting to the 342 non-O157 STEC cases resulted in significant associations for patients aged 11–59 (OR 2·7, 95% CI 1·29–5·80, P = 0·009) and ≥60 (OR 5·3, 95% CI 2·12–13·29, P = 0·0004) years, and bloody diarrhoea (OR 2·5, 95% CI 1·47–4·33, P = 0·0008).
Table 4.
Multivariate analysis of characteristics associated with hospitalization in STEC cases from Michigan
| Characteristic | Adjusted OR (95% CI) | P value* |
|---|---|---|
| STEC serogroup | ||
| Non-O157 | 1·0 | – |
| O157 | 2·1 (1·22–3·56) | 0·009 |
| stx profile | ||
| stx1 only | 1·0 | – |
| stx2 only | 1·1 (0·61–1·89) | 0·82 |
| stx1, stx2 | 1·0 (0·53–1·71) | 0·87 |
| Symptoms | ||
| Diarrhoea only | 1·0 | – |
| Bloody diarrhoea | 3·0 (2·15–4·24) | <0·0001 |
| Age group (years) | ||
| ≤10 | 1·0 | – |
| 11–59 | 1·5 (1·06–2·00) | 0·02 |
| ≥60 | 3·8 (2·42–6·03) | <0·0001 |
| Gender | ||
| Male | 1·0 | – |
| Female | 1·2 (0·94–1·59) | 0·13 |
OR, Odds ratio; CI, confidence interval.
† Wald χ2 test.
DISCUSSION
An examination of STEC cases from Michigan demonstrated significant variation in the age-adjusted incidence rates over the 12-year time period. Overall, there was an increasing trend in incidence, which was attributable to an increase in non-O157 STEC. Importantly, no difference in the 2012 age-adjusted incidence rates was detected for non-O157 (0·89/100 000) and O157 STEC (0·9/100 000). These data demonstrate that non-O157 STEC is equally as important as O157 STEC in terms of causing enteric infections in Michigan. Similar increases in non-O157 STEC were reported in other studies [11, 20, 21], including the most recent FoodNet report [13]. Specifically, the incidence of non-O157 STEC increased significantly in 2014 relative to 2011–2013. On the other hand, the O157 STEC incidence decreased by 32% in 2014 relative to the period between 2006 and 2008 [13]. A similar decrease in O157 STEC was not observed in Michigan between 2001 and 2012, which may be due in part to the high frequency of O157 outbreaks within the last five years of the study. Among the 108 O157 outbreak-associated cases, 100 (93%) were reported in 2008–2012; these 100 cases comprised 27% of the total number of O157 STEC cases (n = 367) during that 5-year period.
The increasing number of non-O157 STEC cases in Michigan is likely linked to the adoption of improved diagnostic tests, which is consistent with findings from FoodNet [8, 11, 13]. Between 2001 and 2005, Michigan clinical laboratories participated in a sentinel surveillance system to evaluate an enzyme immunoassay targeting the Stx [21]. An increased number of non-O157 STEC were recovered as a result of those efforts. Statewide recommendations were then issued to evaluate all stool samples from acute, community-acquired diarrhoea cases according to CDC guidelines [22]. Consequently, the incidence of non-O157 STEC infections increased steadily between 2006 and 2012, and cases were identified in regions that were previously unaffected, particularly in the Upper Peninsula. Indeed, a similar trend occurred for infections caused by O157 STEC, which were considered a ‘rare serotype’ in the original 1983 report [23] but is now widespread throughout Michigan (data not shown) and the United States. For non-O157 STEC, it is difficult to determine if the more remote locations were slower to adopt new laboratory practices, which could contribute to the geographic distribution of cases observed. Other factors that impact geographic variation could include temperature and climate, pathogen density in the environment or reservoir hosts, herd immunity, and food distribution and consumption practices. The higher frequencies of stx1-positive infections in winter/spring and stx2-positive infections in summer/autumn suggest that the Stx-bacteriophage distribution also varies and may impact disease frequencies. Indeed, it is possible that solar radiation, temperature or other phage-inactivating factors present in the warmer months [24] may have a greater impact on the reservoir Stx1 phage population, although additional studies are needed to address this hypothesis. Now that the majority of clinical laboratories in Michigan follow the new CDC recommendations [14], future studies will also be needed to determine whether non-O157 STEC incidence continues to increase and exceed O157 STEC frequencies.
Among all of the non-O157 serogroups, O45 (11%) predominated in Michigan followed by O103 (6%), O26 (5%), O111 (3%), O145 (2%), and O121 (1%). In the FoodNet sites, serogroup O26 (26%) was most common followed by O103 (22%), O111 (19%), O121 (6%), O45 (5%), and O145 (4%) [11]. Significantly more O45 STEC were recovered from Michigan relative to the FoodNet sites (P < 0·0001), while significantly fewer O26 (P < 0·0001) and O111 (P < 0·0001) STEC were identified in Michigan. Differences in the serogroup distribution were also observed relative to Minnesota [25], another Midwestern location (P < 0·0001). Such differences in serogroup distributions may indicate a differential risk of exposures across sites or variation in the circulation of epidemic strains. Indeed, several previous studies have observed different epidemiologic associations with some STEC serogroups. The O111, O103 and O26 serogroups, for instance, were more commonly found among cases with a history of international travel [11, 12, 25, 26]. These associations clearly highlight the need to better understand serogroup-specific risk factors in different geographic locations. Although identifying risk factors for specific serotypes is also important, these efforts have been dampened in recent years because fewer laboratories are characterizing and reporting the H-antigen associated with each STEC isolate.
In the present study, we also found that patient demographics (age and gender) were similar between O157 STEC and non-O157 STEC cases, which is consistent with FoodNet data [11]. One exception was reported in New Mexico as non-O157 STEC infections were more common in children aged <5 years [12]. When specific serogroups were examined separately, additional differences were noted. Cases aged <10 years, for example, were more frequently infected with O111 STEC relative to all other non-O157 STEC serogroups and O157. The median age for the non-O157 STEC serogroups identified in Michigan (range 17–27 years) was also higher than the FoodNet cases (range 9–15 years) for all serogroups except O45 and O121 [11]. Although it is difficult to identify factors that contributed to these differences, it is likely that the number and source of outbreaks play a role as well as the molecular characteristics of the pathogens in circulation and age-associated behaviours.
Consistent with findings from Minnesota [25], Connecticut [25], and all FoodNet sites combined [11], we also observed an increased likelihood of hospitalization among cases infected with O157 vs. non-O157 STEC. Similarly, a German study reported a higher risk of hospitalization in O157 STEC cases compared to non-O157 STEC cases, except for patients infected with STEC O104:H4 [27]. In fact, the Stx-producing O104:H4 German outbreak strain resulted in the highest frequency of both HUS and death recorded for a single outbreak [28], and highlights the importance of pathogen characteristics. Using logistic regression, we observed that children aged <5 years were less likely to be hospitalized compared to individuals aged 11–59 years and ≥60 years. This finding is similar to findings from the previous Michigan study [21] and in Germany [27] and suggests that other factors (e.g. immune status and behaviours) associated with age may impact disease severity and risk of hospitalization. It is important to note that various age-specific risk factors have previously been identified to be important for STEC infections. For example, one study identified hamburger consumption to be associated with disease in children aged <12 years, while contact with ruminants was associated with disease in children aged <3 years [29]. Additional studies, however, are needed to determine whether age-associated severity of disease, as measured by hospitalization status in this study, is related to differential exposures or variation in host susceptibility.
STEC infections represent a significant public health concern. In Michigan, the number of non-O157 STEC cases has increased over time and has recently surpassed the number of O157 STEC cases. Despite this high frequency, the actual number of cases likely represents an underestimate of the incidence. For O157 STEC, for instance, the degree of under-reporting was estimated to be 20-fold [30]. A previous study also observed an association between non-O157 STEC and milder clinical symptoms [25], which may contribute to under-reporting as well given that sick individuals are less likely to seek medical attention [31]. Nonetheless, as detection methods and public health practices improve, the continuous review of population-based surveillance data is essential for monitoring disease trends over time and in different geographical locations. Studies that aim to characterize the STEC population in an effort to link pathogen characteristics with clinical outcomes are also critical to identify additional risk factors and markers for more severe infections.
ACKNOWLEDGEMENTS
Sincere thanks to the MDHHS for assisting with the collection of epidemiological and laboratory data as well as Dr Melinda Wilkins and Dr Paul Bartlett for feedback on statistical analyses.
Funding was provided by the National Institutes of Health Enterics Research Investigational Network (ERIN) Cooperative Research Center at Michigan State University (S.D.M., grant number U19AI090872) and the United States Department of Agriculture, National Institute of Food and Agriculture (S.D.M., grant number 2011–67005–30004).
DECLARATION OF INTEREST
None.
REFERENCES
- 1.Nataro JP, Kaper JB. Diarrheagenic Escherichia coli. Clinical Microbiology Reviews 1998; 11: 142–201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Karmali MA, et al. The association between idiopathic hemolytic uremic syndrome and infection by verotoxin-producing Escherichia coli. Journal of Infectious Diseases 2004; 189: 556–563. [DOI] [PubMed] [Google Scholar]
- 3.Tarr PI, Gordon CA, Chandler WL. Shiga-toxin-producing Escherichia coli and haemolytic uraemic syndrome. Lancet 2005; 365: 1073–1086. [DOI] [PubMed] [Google Scholar]
- 4.Zoja C, Buelli S, Morigi M. Shiga toxin-associated hemolytic uremic syndrome: pathophysiology of endothelial dysfunction. Pediatric Nephrology 2010; 25: 2231–2240. [DOI] [PubMed] [Google Scholar]
- 5.Noris M, Remuzzi G. Hemolytic uremic syndrome. Journal of the American Society of Nephrology 2005; 16: 1035–1050. [DOI] [PubMed] [Google Scholar]
- 6.Karmali MA, Gannon V, Sargeant JM. Verocytotoxin-producing Escherichia coli (VTEC). Veterinary Microbiology 2010; 140: 360–370. [DOI] [PubMed] [Google Scholar]
- 7.Williams DM, et al. Acute kidney failure – a pediatric experience over 20 years. Archives of Pediatrics and Adolescent Medicine 2002; 156: 893–900. [DOI] [PubMed] [Google Scholar]
- 8.Scallan E, et al. Foodborne illness acquired in the United States – major pathogens. Emerging Infectious Diseases 2011; 17: 7–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Gyles CL. Shiga toxin-producing Escherichia coli: an overview. Journal of Animal Science 2007; 85: E45–E62. [DOI] [PubMed] [Google Scholar]
- 10.Bettelheim KA. The non-O157 Shiga-toxigenic (Verocytotoxigenic) Escherichia coli; under-rated pathogens. Critical Reviews in Microbiology 2007; 33: 67–87. [DOI] [PubMed] [Google Scholar]
- 11.Gould LH, et al. Increased recognition of non-O157 shiga toxin-producing Escherichia coli infections in the United States during 2000–2010: epidemiologic features and comparison with E. coli O157 infections. Foodborne Pathogens and Disease 2013; 10: 453–460. [DOI] [PubMed] [Google Scholar]
- 12.Lathrop S, Edge K, Bareta J. Shiga toxin-producing Escherichia coli, New Mexico, USA, 2004–2007. Emerging Infectious Diseases 2009; 15: 1289–1291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Crim SM, et al. Preliminary incidence and trends of infection with pathogens transmitted commonly through food – foodborne diseases active surveillance network, 10 U.S. sites, 2006–2014. Morbidity and Mortality Weekly Report 2015; 64: 495–499. [PMC free article] [PubMed] [Google Scholar]
- 14.Gould LH. Update: recommendations for diagnosis of shiga toxin-producing Escherichia coli infections by clinical laboratories. Clinical Microbiology Newsletter 2012; 34: 75–83. [PubMed] [Google Scholar]
- 15.United States Department of Health and Human Services. July 1st population estimates for 1990–2012 by Year, state, county, race (4 categories), ethnicity, sex and age (1-year or 5-year groups). (http://wonder.cdc.gov/bridged-race-v2012.html). Accessed in January 2014.
- 16.Anderson RN, Rosenberg HM. Age standardization of death rates: implementation of the year 2000 standard. National Vital Statistics System 1998; 43: 1–12. [PubMed] [Google Scholar]
- 17.Klein RJ, Schoenborn CA. Age adjustment using the 2000 projected U.S. population: United States Department of Health and Human Services, Centers for Disease Control and Prevention, National Center for Health Statistics; 2001. [PubMed]
- 18.Hosmer DW, Lemeshow S. Model-building Strategies and Methods for Logistic Regression, 2nd edn. Hoboken, NJ: John Wiley & Sons Inc., 2000. [Google Scholar]
- 19.Michigan Department of Technology Management and Budget. (http://www.michigan.gov/cgi/0,1607,7-158-54534---,00.html). Accessed March 2014.
- 20.Stigi KA, et al. Laboratory practices and incidence of non-O157 shiga toxin-producing Escherichia coli infections. Emerging Infectious Diseases 2012; 18: 477–479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Manning SD, et al. Surveillance for shiga toxin-producing Escherichia coli, Michigan, 2001–2005. Emerging Infectious Diseases 2007; 13: 318–321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Gould LH, et al. Recommendations for diagnosis of shiga toxin-producing Escherichia coli infections by clinical laboratories. Morbidity and Mortality Weekly Report Recommendations and reports 2009; 58: 1–14. [PubMed] [Google Scholar]
- 23.Riley LW, et al. Hemorrhagic colitis associated with a rare Escherichia coli serotype. New England Journal of Medicine 1983; 308: 681–685. [DOI] [PubMed] [Google Scholar]
- 24.Allue-Guardia A, Martinez-Castillo A, Muniesa M. Persistence of infectious Shiga toxin-encoding bacteriophages after disinfection treatments. Applied and Environmental Microbiology 2014; 80: 2142–2149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Hedican EB, et al. Characteristics of O157 versus non-O157 shiga toxin-producing Escherichia coli infections in Minnesota, 2000–2006. Clinical Infectious Diseases 2009; 49: 358–364. [DOI] [PubMed] [Google Scholar]
- 26.Hadler JL, et al. Ten-year trends and risk factors for non-O157 shiga toxin-producing Escherichia coli found through shiga toxin testing, Connecticut, 2000–2009. Clinical Infectious Diseases 2011; 53: 269–276. [DOI] [PubMed] [Google Scholar]
- 27.Preussel K, et al. Shiga toxin-producing Escherichia coli O157 is more likely to lead to hospitalization and death than non-O157 serogroups – except O104. PLoS ONE 2013; 8(11). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Frank C, et al. Epidemic profile of shiga-toxin-producing Escherichia coli O104:H4 outbreak in Germany. New England Journal of Medicine 2011; 365: 1771–1780. [DOI] [PubMed] [Google Scholar]
- 29.Voetsch AC, et al. Risk factors for sporadic shiga toxin-producing Escherichia coli O157 infections in FoodNet sites, 1999–2000. Epidemiology and Infection 2007; 135: 993–1000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Mead PS, et al. Food-related illness and death in the United States. Emerging Infectious Diseases 1999; 5: 607–625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Tauxe R. Real burden and potential risks from foodborne infections: the value of multi-jurisdictional collaborations. Trends in Food Science and Technology 2008; 19: S18–S25. [Google Scholar]