Abstract
Metabolites once considered solely in catabolism or anabolism turn out to have key regulatory functions. Among these, the citric acid cycle intermediate succinate stands out due to its multiple roles in disparate pathways, the dramatic concentration changes and its selective cell release. Here we propose that succinate has evolved as a signaling modality because its concentration reflects the coenzyme Q (CoQ) pool redox state, a central redox couple confined to the mitochondrial inner membrane. This connection is of general importance because CoQ redox state integrates three bioenergetic parameters: mitochondrial electron supply, oxygen tension and ATP demand. Succinate, by equilibrating with the CoQ pool, enables the status of this central bioenergetic parameter to be communicated from mitochondria to the rest of the cell, into the circulation and to other cells. The logic of this form of regulation explains many emerging roles of succinate in biology, and suggests future research questions.
The emerging regulatory roles of metabolites has transformed fields spanning cancer, immunology and metabolic disease1. Among these, the citric acid cycle (CAC) metabolite succinate is notable for the diverse range of functions it regulates, encompassing signaling within and between cells and tissues. Here we outline the many ways in which succinate can act as a regulatory molecule. In parallel, we develop a model to account for the bioenergetic logic of how succinate accumulation engenders a wide range of context-dependent adaptations. In particular, we propose that succinate is uniquely positioned to act as a mobile sensor of electron supply into, and demand by, mitochondrial oxidative phosphorylation. Once accumulated, succinate elicits a range of downstream adaptations through distinct, context-dependent effector pathways. Thus, succinate acts as a ubiquitous sentinel of cellular bioenergetics, capable of integrating major metabolic parameters to control local and systemic adaptation through distinct effector mechanisms.
In addition to being a constituent of the CAC cycle, succinate is now recognized to play a role in a broad range of physiological and pathophysiological settings2,3. Before considering how succinate signals in these diverse contexts, we first propose how its signaling roles can be rationalized in the context of mitochondrial bioenergetics. The functions of mitochondria are now well established as extending far beyond ATP production, with the outputs of the organelle regulating a myriad of cellular and physiological processes4. This mode of biological adaptation is rooted in the principle that cells must respond quickly and locally to distinct metabolic perturbations to facilitate appropriate and context-dependent biological outputs. Central to metabolism is mitochondrial oxidative phosphorylation, which passes electrons derived from carbohydrates and fats onto oxygen to drive phosphorylation of ADP to ATP, thereby maintaining the disequilibrium of this central energetic couple (that is, a high ATP to ADP ratio), which is essential to carry out work in the cell5. Because of its general importance for cellular function, multiple sensors exist to maintain the disequilibrium of the ATP/ADP couple, such as the O2-sensing HIF/PHD pathway6 and AMPK activation7. Similarly, an array of nutrient-sensing pathways responds to changes in the substrates that fuel oxidative phosphorylation8.
The coenzyme Q pool and mitochondrial succinate
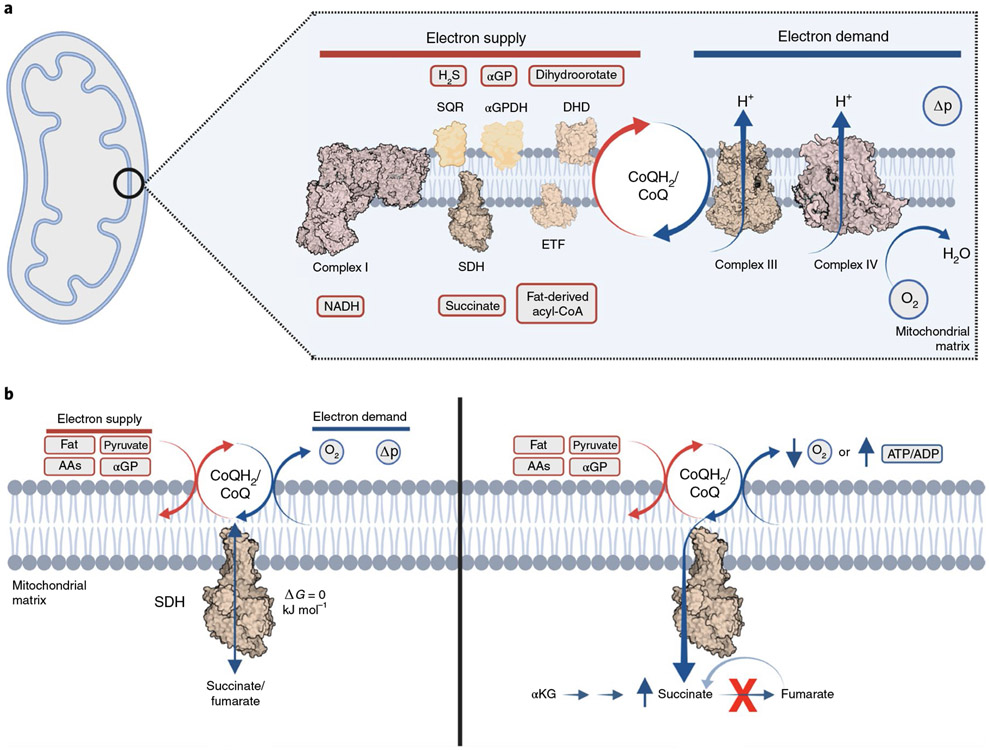
At the heart of mitochondrial function is the CoQ pool (Fig. 1). Within the mitochondrial inner membrane CoQ is an essential component of oxidative phosphorylation. Consisting of a hydrophobic isoprenoid chain and a redox-active benzoquinone, CoQ is predominantly restricted to this lipid bilayer where it shuttles electrons between membrane-bound dehydrogenases and mitochondrial complex III9-11. CoQ is reduced to CoQH2 by several dehydrogenases that contribute reducing equivalents derived from glucose, fats, amino acids and several other sources12. However, its only route to oxidation by O2 is through complex III. Critically, this makes CoQ the first common reservoir for electrons that contribute to oxidative phosphorylation, irrespective of source13. Thus, the redox state of the CoQ pool (CoQH2/CoQ) is a dynamic manifestation of a relatively small number of central bioenergetic parameters. On the supply side, CoQH2/CoQ depends on electron input from upstream dehydrogenases. On the demand side, CoQH2/CoQ depends on CoQH2 oxidation by complex III14. This electron transfer through complexes III/IV is tightly coupled to proton translocation across the mitochondrial inner membrane and thus depends on cellular ATP demand. This is communicated by the magnitude of the protonmotive force across the mitochondrial inner membrane (Δp) through the activity of mitochondrial ATP synthesis15. In addition, CoQ status is also affected by oxygen availability13. On this basis, the CoQH2/CoQ ratio integrates three mitochondrial bioenergetic parameters: aggregate electron supply, oxygen tension and the Δp. In this way, CoQH2/CoQ is sensitive to imbalances in supply and demand that are determined by these factors (Fig. 1a).
Fig. 1 ∣. Mitochondrial bioenergetics and the coenzyme Q (CoQ) pool.
a, An overview of the bioenergetic parameters that control CoQ/CoQH2 redox state. Aggregate electron supply from many sources in the cell dictate the rate of electron supply to CoQ. Rate of CoQH2 oxidation dictates the rate of electron loss from CoQH2. Rate of CoQH2 oxidation depends on cellular ATP demand, which is indirectly sensed via the Δp, as well as oxygen tension. αGP, α-glycerophosphate; αGPDH, α-glycerophosphate dehydrogenase; DHD, dihydroorotate dehydrogenase; ETF, electron transferring flavoprotein; SQR, sulfide:quinone oxidoreductase. Image created with BioRender.com. b, Summary of the effects of electron supply imbalance into and from the mitochondrial CoQ pool on mitochondrial succinate. Elevated aggregate supply and/or decreased demand are sufficient to drive selective accumulation of succinate via the ΔG = 0 reaction mediated by SDH. AAs, amino acids.
The above analysis demonstrates that the redox state of the CoQ pool—that is, the CoQH2/CoQ ratio—is a central bioenergetic parameter that changes rapidly and reversibly in response to changes in mitochondrial function. However, the important bioenergetic information on cellular and organismal energy homeostasis given by the CoQH2/CoQ ratio is confined to the mitochondrial inner membrane and cannot be directly communicated to the cytosol due to the extreme hydrophobicity of these molecules. This barrier can be overcome by the intimate connection between the CoQH2/CoQ redox state and that of the succinate/fumarate couple, which has a substantial impact on mitochondrial succinate abundance (Fig. 1b). This interaction is mediated by succinate dehydrogenase (SDH), which catalyzes succinate oxidation to fumarate, thereby reducing CoQ to CoQH2. The midpoint potential of both succinate/fumarate and CoQH2/CoQ at physiological pH is close to 0 mV (ref. 5). Moreover, the SDH reaction does not involve proton translocation across the mitochondrial inner membrane16. Therefore, SDH-mediated oxidation of succinate is specifically and reversibly sensitive to the CoQH2/CoQ ratio. As such, bioenergetic parameters that increase CoQH2/CoQ can drive a substantial increase in succinate abundance. This is observed in isolated mitochondria, cells and tissues, whereby hypoxia, inhibition of complexes III/IV or elevated electron supply is sufficient to drive reduction of CoQH2/CoQ and subsequent succinate accumulation17-22. Along similar lines, elevation of Δp inhibits oxidation of succinate, while Δp dissipation oxidizes CoQH2/CoQ17 and stimulates mitochondrial succinate oxidation23. While succinate oxidation is highly sensitive to CoQH2/CoQ, an additional aspect of succinate metabolism is important for its accumulation in these settings. Unlike other CAC metabolites, alternative metabolic reactions do not exist for succinate, which is restricted to utilization by SDH18,20,23,24. Thus, succinate abundance is a sentinel for aggregate electron supply into, and demand from, the mitochondrial CoQ pool. In this context, it is logical that selective accumulation of succinate is observed in a wide range of cellular and physiological contexts wherein acute perturbations in supply or demand drive imbalances to the CoQ pool. This raises the question of how succinate, once accumulated, can relay information from the CoQH2/CoQ redox status to the rest of the cell, and beyond.
How succinate accumulation can elicit a range of context-dependent adaptations is attributable to a series of parameters that determine the fate of accumulated succinate. We classify these two main branches of succinate-dependent adaptation into one that depends on its oxidation by SDH; and the other that depends on its export from mitochondria and the cell. We first consider the oxidative pathway.
Oxidative biological regulation by succinate
A major consequence of succinate accumulation arises from its capacity to contribute reducing power to the mitochondrial respiratory chain, and in doing so to regulate the production of superoxide25. Several oxidoreductases are capable of superoxide production under conditions that depend directly on succinate redox pressure. The most well studied is mitochondrial complex I, which can produce large amounts of superoxide in response to elevated succinate26-28. Complex I contains a flavin mononucleotide (FMN) cofactor that accepts electrons from NADH and passes them through a chain of seven iron–sulfur centers to CoQ29. A major site of complex I superoxide production is from the reaction of O2 with the fully reduced FMN30, although the CoQ binding site has also been proposed to contribute31. In isolated mitochondria, cellular models and living tissue, complex I superoxide production is driven by elevated succinate concentrations. Succinate-dependent superoxide production by complex I is highly sensitive to both Δp and the redox pressure of the CoQ pool26,32-35. Elevated succinate exerts reducing pressure on complex I through the CoQ pool and controls superoxide production only through the conformationally active form of the complex36. The rate of complex I superoxide production due to elevated succinate additionally depends on a high Δp and relatively high oxygen tension26,32-35. These two parameters indicate that the oxidative branch of succinate regulation is most relevant under conditions where succinate accumulation occurs in the context of high Δp, as opposed to hypoxia.
In addition to complex I, other mitochondrial oxidoreductases proximal to CoQ could plausibly engage in superoxide production. Depending on the nature of redox pressures on these centers, these sites may be subject to additional bioenergetic parameters to drive substantial superoxide production31. Since the topology of many superoxide-producing redox centers are distinct, this could additionally afford a mode of spatially regulated superoxide production, for example, in the mitochondria matrix versus the mitochondrial intermembrane space. The implications of this are discussed below.
While elevated succinate produces substantial superoxide from complex I under the conditions described above, the steady-state levels of superoxide in mitochondria are very low. This phenomenon is attributable to the ubiquitous presence of manganese superoxide dismutase (MnSOD), which rapidly dismutates superoxide produced in the mitochondrial matrix to hydrogen peroxide (k ≈ 2 × 109 M−1 s−1)25,37. As such, in mitochondria, cells and tissues where succinate is poised to produce superoxide, this manifests in elevated levels of H2O2. Indeed, accumulation of succinate has emerged as a metabolic control point for the regulated production of mitochondrial superoxide, which could facilitate communication within cells and tissues through the generation of H2O238. Steady-state H2O2 levels are additionally regulated by peroxiredoxins and glutathione peroxidases39. As such, H2O2 levels are subject to upstream regulation by controlled production pathways, consumption pathways and diffusion rates from the site of production. H2O2 is now widely accepted to modify protein function by reversible covalent modification of cysteine residues40. Indeed, protein cysteine oxidation by H2O2 can be fast and reversible, and is responsible for modulation of a wide range of protein functions and protein localization, either directly or via redox relays41,42. Thus, succinate-dependent superoxide production is linked into a well-established signaling modality (Fig. 2). More generally, redox modification of protein cysteines plays a central role in a vast array of tissue-specific regulatory processes43. Considering this, tools for modification of complex I-linked superoxide have been developed recently44, and will prove useful in understanding the expanding list of biological processes regulated by succinate-linked superoxide. Below, we consider some of the cellular and physiologic adaptations that depend on succinate-driven superoxide production.
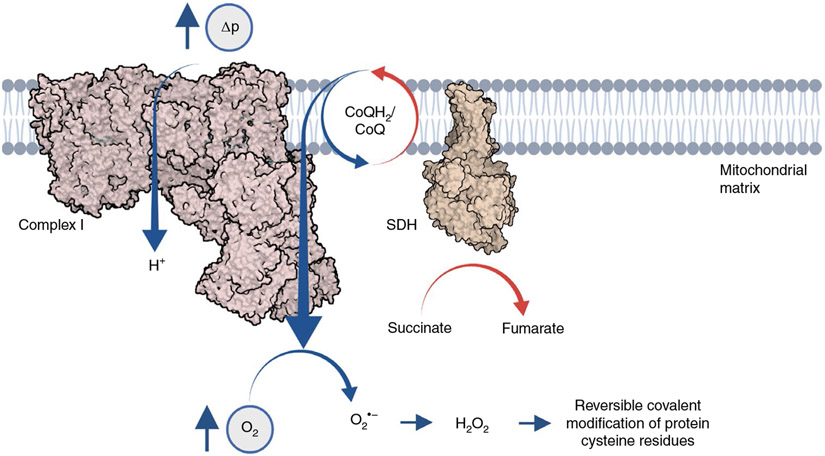
Fig. 2 ∣. Succinate controls mitochondrial superoxide production through mitochondrial complex I.
Accumulated succinate exerts redox pressure on the mitochondrial CoQ pool. Under conditions of elevated Δp (low cellular ATP demand) and normoxia, redox pressure on the CoQ pool poises electrons onto the terminal flavin of complex I to facilitate single-electron reduction of oxygen to generate superoxide. Mitochondrial superoxide is rapidly dismutated to hydrogen peroxide, which acts as a metabolic signal through reversible covalent modification of protein cysteine residues.
Macrophage production of pro-inflammatory cytokines
Macrophages exposed to pro-inflammatory stimulation by the Gram-negative bacterial product lipopolysaccharide respond by rapidly increasing both glucose and glutamine uptake and catabolism24. The substantial increase in glycolytic ATP production, combined with increase in flux of reducing equivalents from both glucose- and glutamine-derived carbons into mitochondria, results in a relative decrease in the contribution of oxidative phosphorylation to cellular ATP, concomitant with elevated substrate supply. This supply/demand imbalance manifests in the selective accumulation of succinate, which plays a critical role in elevating transcription of a range of pro-inflammatory genes, and production of the pro-inflammatory cytokine Il-1β24. Moreover, the selective accumulation of succinate in the presence of high Δp due to elevated cellular ATP/ADP, and normoxia, drives succinate-dependent superoxide45. Remarkably, succinate-driven superoxide is essential for the macrophage pro-inflammatory response, demonstrating a direct connection between bioenergetic sensing by succinate and a central immune cell effector process46. It is worth noting the inherent logic in succinate oxidation acting as an upstream signal in the pro-inflammatory response. By utilizing succinate as an overarching sensor of elevated substrate supply in the presence of maintained oxidative capacity (high Δp and high O2), the sensing system therefore inherently takes into account these bioenergetic parameters to elicit an effector response in tune with the metabolic state of the cell.
Thermogenic respiration by brown and beige adipocytes
Brown and beige adipocytes possess unique metabolic proteins that facilitate the oxidation of fuels in futile cycles, which can regulate systemic metabolism and counteract obesity. These cells are predominantly quiescent under basal conditions and require activation by peripheral signals for thermogenic respiration to occur47,48. In this context, selective accumulation of the mitochondrial metabolite succinate acts as a potent mode of activation in brown and beige fat23. The new-found thermogenic activity of succinate in these cells depends on two factors. First, brown adipocytes selectively accumulate succinate to very high concentrations, when compared to other CAC metabolites, as well as to succinate in other tissues under basal conditions. This accumulation is coupled with succinate oxidation by mitochondrial SDH, which drives superoxide/H2O2 production. Production of H2O2, and consequent oxidation of protein cysteine thiols, is required for the acute thermogenic effects of succinate accumulation23. Along similar lines, studies from many laboratories have shown that superoxide/H2O2 production plays a major role in controlling activation of thermogenesis in brown and beige adipose tissues49-51. Robust activation of adipocyte thermogenesis in vivo results from multiple different genetic manipulations that elevate superoxide/H2O2 levels in adipose tissue52-56. Similarly, stimulation of adipose tissue thermogenesis by physiologic interventions (for example, cold exposure) requires endogenously produced superoxide51,57,58. Using proteomic methodologies, it has been shown that one important functional site that is modified by thermogenic H2O2 is cysteine-253 on the thermogenic effector protein UCP1 (refs. 23,59,60). In addition, metabolic proteins involved in fatty acid oxidation and glucose/glycogen oxidation have been identified as H2O2 targets in thermogenic fat61, suggesting redox control of these processes may be relevant in acute regulation of thermogenesis.
Oxygen sensing in the carotid body
Peripheral cardiorespiratory regulation by the carotid body is regulated by O2-sensitive K+ channels. Recent work suggests that chemoreceptor cells in the carotid body utilize succinate-driven superoxide production through complex I to regulate the activity of these channels, while genetic and pharmacologic manipulation of succinate-driven superoxide production compromise the systemic hyperventilatory response in mice62. The relevant protein targets that are modified by succinate-driven superoxide production in this highly specialized adaptive process in these cells remain to be defined.
Mitochondrial superoxide production and organismal lifespan
The importance of superoxide/H2O2 dysregulation in age-dependent disease and tissue dysfunction has been appreciated for nearly three-quarters of a century63. This link was initially considered through the lens of irreversible macromolecular damage by reactive oxygen species (ROS) during aging64. However, in the last decade this model has fallen out of favor as numerous lines of evidence now demonstrate that indiscriminate damage by ROS is unlikely to fully explain their role in aging42,65. Studies across model organisms and humans have shown that pharmacological depletion of ROS using a range of antioxidant modalities have either no effect, or negative effects on lifespan, healthspan or pathologies associated with age65. Intriguingly, genetic manipulation of complex I-mediated superoxide production, through reverse electron transfer (the mode supported by high Δp and succinate), have demonstrated a role for this process in protection against age-related decline in flies. Specifically, genetic tools to manipulate the redox state of the mitochondrial CoQ pool to elevate mitochondrial superoxide are sufficient to extend lifespan and protect against markers of brain aging and pathogenesis in a range of organisms66-69. These findings argue that elevation of complex I-driven ROS could have distinct regulatory roles relevant to protection against age-related decline of cellular function. In this context, recent work tracking the redox proteome of mouse tissues with age demonstrated that the protein cysteines oxidized in young tissues are fundamentally remodeled with age61. Such findings indicate that cellular redox networks remodel with age, perhaps reflecting an alteration in the upstream metabolic pathways that control superoxide/H2O2 production. Delineating those protein targets of H2O2 associated with longevity and protection from age-related decline, and whether succinate accumulation controls modification of these targets, will be an interesting area of future research.
Succinate signaling to the cytosol and nucleus
In addition to its role ‘within’ the mitochondrion, succinate directly regulates biological activities beyond its organelle of origin. It has been known for decades that isolated mitochondria rapidly transport succinate across the inner membrane, via the dicarboxylate carrier—and that this pathway has high capacity (Fig. 3). Most mammalian cells express SLC25A10, the mitochondrial dicarboxylate carrier that mediates electroneutral export of succinate across the mitochondrial inner membrane, effectively equilibrating mitochondrial and cytosolic pools70. Thus, once succinate builds up in mitochondria it will very rapidly equilibrate with the cytosol and nucleus. While this fact did not attract much attention, it should have raised the question as to ‘why’ the mitochondrial inner membrane can so rapidly transport a metabolite that was both generated and consumed within the mitochondrial matrix with no known role in the cytosol or nucleus.
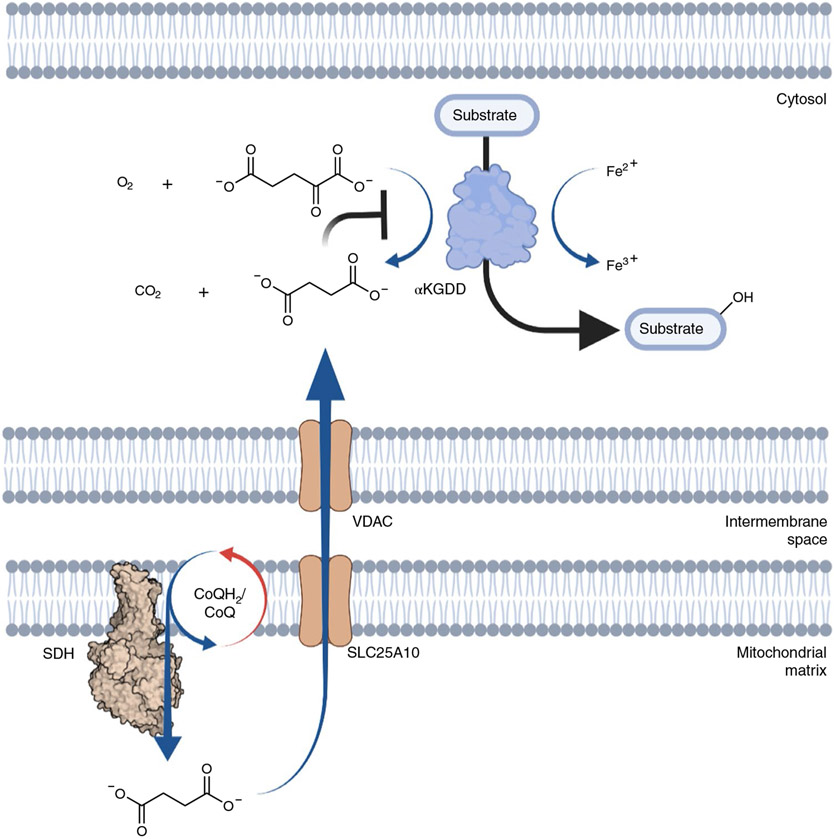
Fig. 3 ∣. Accumulated mitochondrial succinate regulates cellular α-KG-dependent dioxygenases.
The substrate of the α-KG-dependent dioxygenase (αKGDD) reaction becomes hydroxylated in a reaction utilizing three co-substrates: divalent iron (Fe2+), α-KG and molecular oxygen (O2). During catalysis, α-KG becomes decarboxylated to succinate and CO2. Accumulated succinate inhibits this reaction.
The above question has now been answered with the finding that succinate can also regulate cellular adaptation through inhibition of (α-ketoglutarate) αKG-dependent dioxygenases, a large family of over 60 enzymes with broad roles ranging from oxygen sensing, fatty acid catabolism and regulation of the epigenome71. All enzymes in this family utilize αKG and oxygen as substrates to introduce an oxygen atom into a target, generating succinate and CO2 as side products (Fig. 3). The first evidence of this form of regulation was shown in the case of SDH mutant cancers that manifest chronically elevated intracellular succinate72. In these cancers, genetic lesions of SDH subunits are sufficient to drive inhibition of HIF-1α prolyl hydroxylases, which elevates HIF-1α-dependent genetic programs and highly vascularized tumors. Since these discoveries, intracellular αKG/succinate ratios have been shown to affect numerous αKG-dependent dioxygenase regulated pathways71. While most of these effects have been characterized in cancers where mutations drive chronic succinate elevation, it is likely that physiologic elevation of succinate through the mechanisms described above would also impact many of these processes. Indeed, the inhibitory values of succinate for αKG-dependent dioxygenases vary widely, and for many are within the concentration range achieved as a response to physiologically elevated CoQH2/CoQ71. Moreover, it has been shown that mouse embryonic stem cells under hypoxic conditions can inhibit αKG-dependent dioxygenase dependent processes45,73. Because of the wide range of cellular processes regulated by αKG-dependent dioxygenases, a clear priority for future study is to examine how physiologic responses that drive elevated CoQH2/CoQ and succinate accumulation go on to regulate these targets.
Succinate as a systemic metabolic signal
We have described how succinate accumulation can lead to the generation of superoxide at complex I, and that it has effects outside mitochondria via αKG-dependent dioxygenases. Thus, the metabolic and regulatory roles of accumulated succinate have hitherto been considered through the lens of intracellular biology, since the plasma membrane of most mammalian cells is thought to be impermeable to most dicarboxylates, including succinate23,74-76. However, long-standing observations indicate that extracellular and circulating succinate exists across a range of concentrations, which alter in response to specific perturbations. Moreover, in 2004, a seminal study ‘de-orphaned’ the GPR91 G-protein coupled receptor to show that its natural ligand was extracellular succinate. GPR91, now referred to as succinate receptor 1 (SUCNR1), faces the extracellular environment and there responds to succinate with a half-maximum effective concentration (EC50) (28–56 μM, and ~99% responses are achieved at 200 μM) that is within the higher range of succinate concentrations reported for extracellular fluids.
It is noteworthy that SUNCR1 is widely and heterogeneously expressed in cell types throughout the body. The most well-studied SUCNR1-expressing cells are monocytes and macrophages, but, in addition, many metabolic tissues possess resident cells that express SUCNR1. For example, skeletal muscle satellite, endothelial and stromal cells express SUCNR1. In the liver, stellate and Kuppfer cells express SUCNR1, while in the brain, neural stem cells express this receptor. A common theme emerging in the study of SUCNR1 is that highly specialized tissue-resident cells express the receptor. A reasonable interpretation of this phenomenon is that these cell populations are programmed to respond to paracrine signaling in the form of succinate secretion in response to the local bioenergetic environment.
So, with the identification of SUCNR1, a physiological target for the substantial succinate levels in extracellular fluids emerged. The initial rationalization of the role for SUCNR1 was that it was a sensor for local cellular damage, based on the assumption that succinate would only be released from cells following nonspecific rupture. However, even the first descriptions of succinate elevation in the circulation suggested selective mechanisms of succinate release. Indeed, the original studies of succinate in the blood, by Hochachka and Taegtmeyer, demonstrated transient and reversible succinate increases during acute perturbations, such as exercise, tissue hypoxia and during prolonged periods underwater in diving mammals77-79. More recently, many studies of ischemic and hypoxic tissues have shown increases in secreted succinate that correlate with the degree of hypoxia and occur without overt cell damage18,80. Moreover, cells and tissues exposed to physiological hypoxia, or in hypoxic niches, accumulate and release succinate. A striking example is the retina, which exists in a hypoxic niche in the eye, and releases substantial amounts of succinate into extracellular fluids. Elegant metabolic tracing studies have shown that retinal cells release succinate into the local extracellular environment, which is subsequently taken up and utilized by retinal pigment epithelium and choroid cells, which exist in a region of the eye with elevated oxygen saturation81. In this way the reducing power of accumulated succinate, which cannot be oxidized in the hypoxic retina, is transmitted to the relatively oxygen-rich oxidative cells in the eye. Another example is exercising muscle, whereby vigorous muscle contraction is shown to promote selective release of succinate during exercise, which rapidly renormalizes upon rest19. Other physiologic states that result in chronically elevated succinate release include hypoxic tumors82, as well as tissues exposed to elevate lipid deposition, relatively low vascularization and local hypoxia83,84.
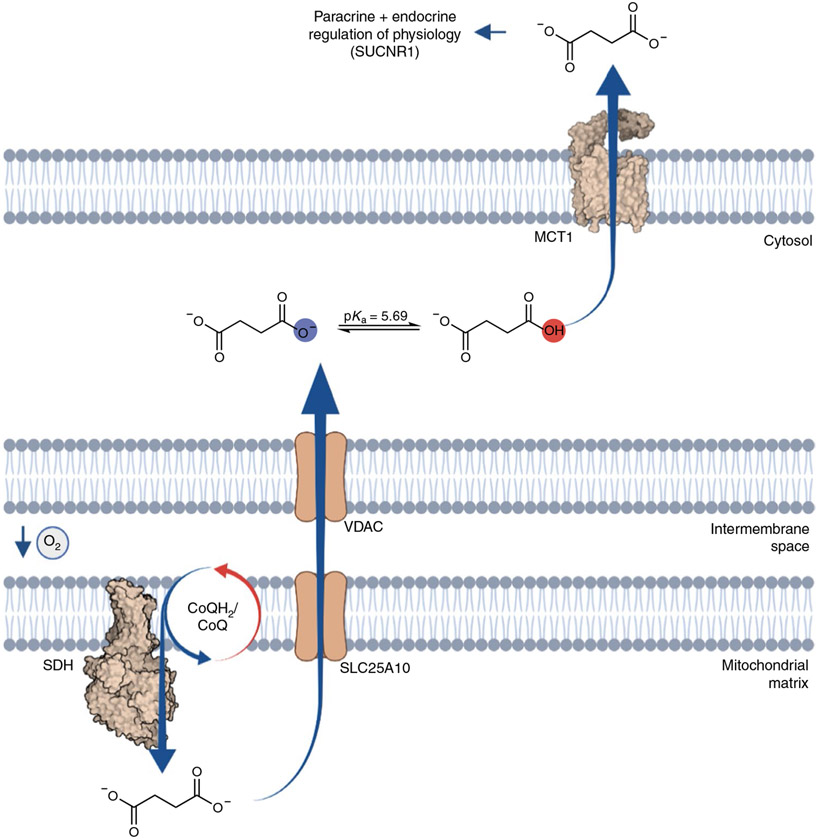
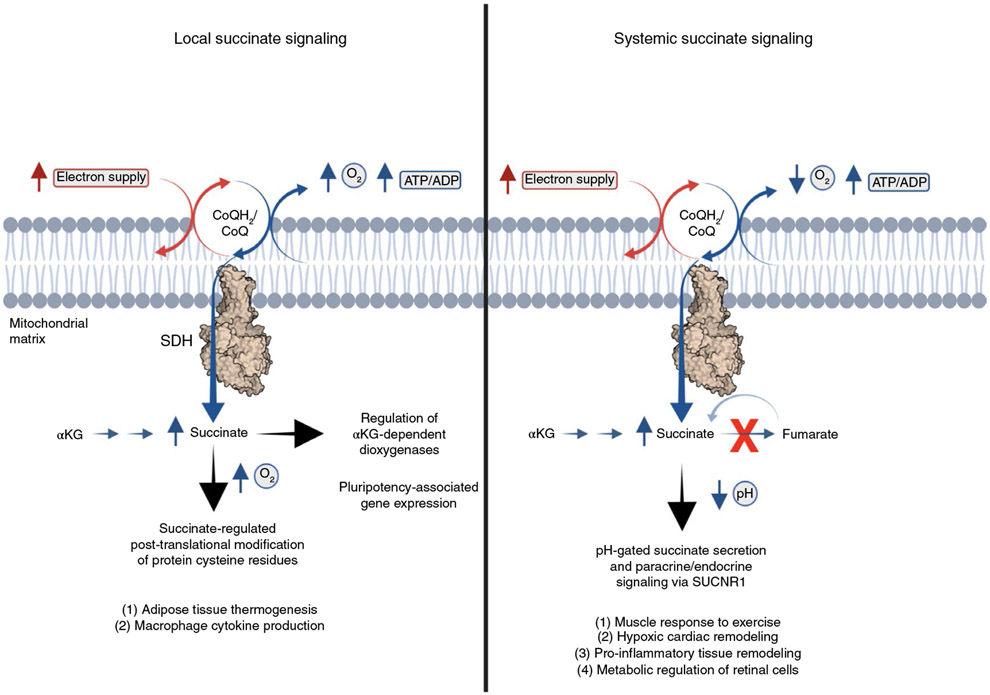
The common metabolic parameters shared by the many tissue environments that promote succinate release are relative supply/imbalance into the mitochondrial CoQ pool, which, in the above cases, is driven by the relative decrease in demand driven by low local mitochondrial O2 tension. But, how is succinate subsequently secreted by cells that accumulate succinate? The plasma membrane of most mammalian cells is impermeable to dicarboxylates, so succinate accumulation alone is not sufficient to promote release. However, a physiochemical feature of succinate, which distinguishes it from other cellular dicarboxylates, is its unusually high monocarboxylic pKa of 5.69. Unlike other cellular dicarboxylates, this renders a substantial proportion (7–16%) of succinate as protonated within the pH range 6.4–6.8, which is readily achieved upon cellular hypoxia, or due to increased glycolytic demand, as is typically observed in tumors or during acute physiological perturbations, such as muscle exercise.
Transient transformation of succinate to a monocarboxylate is of substantial interest because most cells express plasma membrane monocarboxylate transporters (MCTs), including MCT1 and MCT4. Indeed, a role has been proposed for MCT1 and pH gradients in succinate release during heart attack, and MCT1-expressing oocytes can facilitate pH transients upon external succinate addition at pH 6.0 (ref. 85). Moreover, it has been shown that recombinant MCT1 facilitates succinate transport that is strongly pH dependent over the range where succinate exists as a monocarboxylate19. These findings indicate proton-linked succinate transport by MCT1 and also demonstrate pH dependence. In mice, genetic depletion and pharmacological inhibition of MCT1 is sufficient to inhibit succinate release upon reperfusion of the ischemic heart80. Along similar lines, it has been shown that in exercising muscle, and hypoxic muscle cells, release of succinate requires MCT1 (ref. 19). Also, it has recently been reported that retinal cells, which accumulate succinate in a hypoxic niche, facilitate succinate export via MCT1 (ref. 86). Taken together, transient protonation and MCT1-mediated secretion of succinate is at least one mechanism through which intracellular succinate is released into the extracellular environment (Fig. 4). In addition to the bioenergetic parameters that regulate intracellular levels of succinate, an additional parameter of cellular pH is coded into succinate sensing through the chemical properties inherent to the molecule itself.
Fig. 4 ∣. pH-gated succinate secretion regulates systemic physiology.
Succinate accumulation within mitochondria is equilibrated and trapped within the cell. However, under conditions of cellular acidification, achieved under states of high glycolytic flux or hypoxia, a proportion of the succinate pool is transformed into a monocarboxylate, which renders it amenable to secretion through MCT1. Upon pH-gated secretion, succinate exerts a broad range of context- and cell-type-specific paracrine and endocrine responses via its cognate G-protein coupled receptor SUCNR1.
Physiological regulation by secreted succinate
SUCNR1 agonism as a consequence of elevated extracellular succinate can be viewed as a mode of paracrine or endocrine regulation in response to pH-gated succinate secretion. In this framework, the parameters of electron supply/demand by oxidative phosphorylation, as well as local intracellular pH, are integrated to communicate mitochondrial and nonmitochondrial cellular energetics to the peripheral environment. This type of regulation is expected to be relevant in the context of physiological adaptation that is initiated by altered local energetics in one cell type, but that also requires coordinated paracrine or endocrine responses. In fact, pH-gated succinate secretion and consequent succinate-SUCNR1 signaling was recently described to mediate components of the muscle response to acute exercise in mice and humans19.
Interestingly, in contrast to physiologic models that initiate transient succinate secretion, for example, during exercise or temporary tissue hypoxia, several pathogenic states are associated with chronically elevated succinate levels in local extracellular fluids and the circulation. These chronic pathologies include obesity, diabetes and hypertension, as well as physiologic states associated with chronic inflammation. In many cases, inflammation and fibrosis associated with these pathologies are at least in part attributable to chronic SUCNR1 agonism of tissue-resident immune cell populations. The contrast in physiologic consequences between transient elevation of extracellular succinate and chronically elevated extracellular succinate clearly indicate that temporal and cell-type-specific responses dictate the consequences of succinate-SUCNR1 signaling. A common feature of pathologies involving chronically elevated extracellular succinate are source tissues that exhibit metabolic features of persistent hypoxia and/or reliance on glycolytic metabolism. It would be reasonable to suppose that the local intracellular pH of these tissues should support elevated pH-gated succinate secretion. Perhaps the same SUCNR1 paracrine and endocrine circuits that respond to transient elevations in tissue remodeling drive maladaptive tissue inflammation and fibrosis under conditions of chronic succinate exposure.
On the other hand, it is important to note that transient elevations in circulating succinate, such as those initiated by exercise and transient tissue hypoxia, rapidly re-normalize in healthy humans and animal models. This implies that secreted succinate is readily re-absorbed by sequestering cells that maintain low baseline levels of extracellular succinate. Among these, kidney cells are well established to exchange succinate with the blood through the selective expression of the plasma membrane succinate transporter SLC13A3 (ref. 87). As such, these cells are likely to play a key role in maintaining low baseline levels of circulating succinate. In addition, thermogenic adipocytes have recently been shown to have a particular avidity for sequestering circulating succinate23,83, and are capable of utilizing circulating succinate as a substrate to initiate thermogenic respiration via succinate-dependent ROS production (as described in ‘Thermogenic respiration by brown and beige adipocytes’). Interestingly, modulating brown and beige fat content can affect capacity for extracellular succinate handling, although the transport mechanism involved in this process remains to be defined. Nonetheless, these findings suggest that cells with succinate-sequestering capacity can impinge on the temporal effects of released succinate signaling through SUCNR1.
Conclusions and future directions
Here we propose that the ubiquitous signaling role of succinate arises from its intimate connection to the mitochondrial CoQ/CoQH2 redox state, a central bioenergetic parameter in eukaryotic cells. This unusual feature of succinate metabolism enables its accumulation to drive local control over cellular adaptation through superoxide production by mitochondrial complex I as a redox signal, and through regulation of αKG-dependent dioxygenases. Moreover, succinate additionally acts as a mobile sentinel for the redox state of the CoQ pools and the cytosolic pH, capable of modulating systemic physiology via SUCNR1 signaling.
In this way, we consider succinate a sentinel for electron supply and demand imbalance in the CoQ pool and oxidative phosphorylation. Through the above pathways, succinate integrates bioenergetic parameters of oxygen tension, substrate supply, ATP utilization and intracellular pH, to elicit highly context-dependent adaptation (Fig. 5).
Fig. 5 ∣. Logic of succinate as a local and systemic bioenergetic sensor.
Summary of how succinate integrates bioenergetic parameters of oxygen tension, substrate supply, ATP utilization and intracellular pH, to elicit highly context-dependent local and systemic adaptation.
In the coming years, key questions to address include assessing the robustness of this model and determining the extent to which it captures the roles of succinate as an intracellular, autocrine, paracrine and endocrine signal. Further questions are to understand the basis for the divergent outcomes of acute versus chronic succinate-SUCNR1 signaling, which presumably are defined by the nature of the responding SUCNR1-expressing cell type(s) and the temporal component of the succinate signal. In the context of local succinate regulation, defining the protein targets of succinate-mediated redox signals will be critical to understanding the specific effector mechanisms that respond to locally elevated succinate. Understanding these questions will likely open up new therapeutic possibilities for the range of physiological processes that are modulated by accumulated succinate.
Footnotes
Competing interests
The authors declare no competing interests.
References
- 1.Martínez-Reyes I & Chandel NS Mitochondrial TCA cycle metabolites control physiology and disease. Nat. Commun 11, 102 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Murphy MP & O’Neill LAJ Krebs cycle reimagined: the emerging roles of succinate and itaconate as signal transducers. Cell 174, 780–784 (2018). [DOI] [PubMed] [Google Scholar]
- 3.Winther S, Trauelsen M & Schwartz TW Protective succinate-SUCNR1 metabolic stress signaling gone bad. Cell Metab. 33, 1276–1278 (2021). [DOI] [PubMed] [Google Scholar]
- 4.Chandel NS Evolution of mitochondria as signaling organelles. Cell Metab. 22, 204–206 (2015). [DOI] [PubMed] [Google Scholar]
- 5.Nicholls DG & Ferguson SJ Bioenergetics 3rd edn, 31–55 (Academic Press, 2003). [Google Scholar]
- 6.Majmundar AJ, Wong WJ & Simon MC Hypoxia-inducible factors and the response to hypoxic stress. Mol. Cell 40, 294–309 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Mihaylova MM & Shaw RJ The AMPK signalling pathway coordinates cell growth, autophagy and metabolism. Nat. Cell Biol 13, 1016–1023 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Efeyan A, Comb WC & Sabatini DM Nutrient-sensing mechanisms and pathways. Nature 517, 302–310 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Stefely JA & Pagliarini DJ Biochemistry of mitochondrial coenzyme Q biosynthesis. Trends Biochem. Sci 42, 824–843 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Lester RL, Crane FL & Hatefi Y Coenzyme Q: a new group of quinones. J. Am. Chem. Soc 80, 4751–4752 (1958). [Google Scholar]
- 11.Rebstock AS, Mongin F, Trecourt F & Queguiner G Metallation of pyridines and quinolines in the presence of a remote carboxylate group. New syntheses of heterocyclic quinones. Org. Biomol. Chem 2, 291–295 (2004). [DOI] [PubMed] [Google Scholar]
- 12.Wang Y & Hekimi S Understanding ubiquinone. Trends Cell Biol. 26, 367–378 (2016). [DOI] [PubMed] [Google Scholar]
- 13.Rich PR & Maréchal A The mitochondrial respiratory chain. Essays Biochem. 47, 1–23 (2010). [DOI] [PubMed] [Google Scholar]
- 14.Mitchell P Protonmotive redox mechanism of the cytochrome b-c1 complex in the respiratory chain: protonmotive ubiquinone cycle. FEBS Lett. 56, 1–6 (1975). [DOI] [PubMed] [Google Scholar]
- 15.Walker JE The ATP synthase: the understood, the uncertain and the unknown. Biochem. Soc. Trans 41, 1–16 (2013). [DOI] [PubMed] [Google Scholar]
- 16.Sun F et al. Crystal structure of mitochondrial respiratory membrane protein complex II. Cell 121, 1043–1057 (2005). [DOI] [PubMed] [Google Scholar]
- 17.Burger N et al. A sensitive mass spectrometric assay for mitochondrial CoQ pool redox state in vivo. Free Radic. Biol. Med 147, 37–47 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Zhang J et al. Accumulation of succinate in cardiac ischemia primarily occurs via canonical krebs cycle activity. Cell Rep. 23, 2617–2628 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Reddy A et al. pH-gated succinate secretion regulates muscle remodeling in response to exercise. Cell 183, 62–75.e17 (2020). This paper demonstrated protonation-dependent secretion of succinate via MCT1.
- 20.Chouchani ET et al. Ischaemic accumulation of succinate controls reperfusion injury through mitochondrial ROS. Nature 515, 431–435 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kumar R et al. A redox cycle with complex II prioritizes sulfide quinone oxidoreductase dependent H2S oxidation. J. Biol. Chem 298, 101435 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Spinelli JB et al. Fumarate is a terminal electron acceptor in the mammalian electron transport chain. Science 374, 1227–1237 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Mills EL et al. Accumulation of succinate controls activation of adipose tissue thermogenesis. Nature 560, 102–106 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Tannahill GM et al. Succinate is an inflammatory signal that induces IL-1β through HIF-1α. Nature 496, 238–242 (2013). This paper defined succinate accumulation as an upstream metabolite regulator of inflammatory cytokine production.
- 25.Murphy MP How mitochondria produce reactive oxygen species. Biochem. J 417, 1–13 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Hinkle PC, Butow RA, Racker E & Chance B Partial resolution of the enzymes catalyzing oxidative phosphorylation. XV. Reverse electron transfer in the flavin-cytochrome b region of the respiratory chain of beef heart submitochondrial particles. J. Biol. Chem 242, 5169–5173 (1967). [PubMed] [Google Scholar]
- 27.Kussmaul L & Hirst J The mechanism of superoxide production by NADH:ubiquinone oxidoreductase (complex I) from bovine heart mitochondria. Proc. Natl Acad. Sci. USA 103, 7607–7612 (2006). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Hirst J, King MS & Pryde KR The production of reactive oxygen species by complex I. Biochem. Soc. Trans 36, 976–980 (2008). This paper illustrated the mechanism of superoxide production by mitochondrial complex I at the FMN site.
- 29.Agip A-NA, Blaza JN, Fedor JG & Hirst J Mammalian respiratory complex I through the lens of cryo-EM. Annu. Rev. Biophys 48, 165–184 (2019). [DOI] [PubMed] [Google Scholar]
- 30.Pryde KR & Hirst J Superoxide is produced by the reduced flavin in mitochondrial complex I: a single, unified mechanism that applies during both forward and reverse electron transfer. J. Biol. Chem 286, 18056–18065 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Goncalves RL, Quinlan CL, Perevoshchikova IV, Hey-Mogensen M & Brand MD Sites of superoxide and hydrogen peroxide production by muscle mitochondria assessed ex vivo under conditions mimicking rest and exercise. J. Biol. Chem 290, 209–227 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Chance B & Hollunger G The interaction of energy and electron transfer reactions in mitochondria. I. General properties and nature of the products of succinate-linked reduction of pyridine nucleotide. J. Biol. Chem 236, 1534–1543 (1961). [PubMed] [Google Scholar]
- 33.Krishnamoorthy G & Hinkle PC Studies on the electron transfer pathway, topography of iron-sulfur centers, and site of coupling in NADH-Q oxidoreductase. J. Biol. Chem 263, 17566–17575 (1988). [PubMed] [Google Scholar]
- 34.Cino M & Del Maestro RF Generation of hydrogen peroxide by brain mitochondria: the effect of reoxygenation following postdecapitative ischemia. Arch. Biochem. Biophys 269, 623–638 (1989). [DOI] [PubMed] [Google Scholar]
- 35.Lambert AJ & Brand MD Inhibitors of the quinone-binding site allow rapid superoxide production from mitochondrial NADH:ubiquinone oxidoreductase (complex I). J. Biol. Chem 279, 39414–39420 (2004). [DOI] [PubMed] [Google Scholar]
- 36.Yin Z et al. Structural basis for a complex I mutation that blocks pathological ROS production. Nat. Commun 12, 707 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Tyler DD Polarographic assay and intracellular distribution of superoxide dismutase in rat liver. Biochemical J. 147, 493–504 (1975). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Sena LA & Chandel NS Physiological roles of mitochondrial reactive oxygen species. Mol. Cell 48, 158–167 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Cox AG, Winterbourn CC & Hampton MB Mitochondrial peroxiredoxin involvement in antioxidant defence and redox signalling. Biochem. J 425, 313–325 (2009). [DOI] [PubMed] [Google Scholar]
- 40.Reczek CR & Chandel NS ROS-dependent signal transduction. Curr. Opin. Cell Biol 33, 8–13 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Holmstrom KM & Finkel T Cellular mechanisms and physiological consequences of redox-dependent signalling. Nat. Rev. Mol. Cell Biol 15, 411–421 (2014). [DOI] [PubMed] [Google Scholar]
- 42.Murphy MP et al. Unraveling the biological roles of reactive oxygen species. Cell Metab. 13, 361–366 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Lennicke C & Cochemé HM Redox metabolism: ROS as specific molecular regulators of cell signaling and function. Mol. Cell 81, 3691–3707 (2021). [DOI] [PubMed] [Google Scholar]
- 44.Brand MD et al. Suppressors of superoxide-H2O2 production at site IQ of mitochondrial complex I protect against stem cell hyperplasia and ischemia-reperfusion injury. Cell Metab. 24, 582–592 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Laukka T et al. Fumarate and succinate regulate expression of hypoxia-inducible genes via TET enzymes. J. Biol. Chem 291, 4256–4265 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Mills EL et al. Succinate dehydrogenase supports metabolic repurposing of mitochondria to drive inflammatory macrophages. Cell 167, 457–470.e13 (2016). This paper defined succinate accumulation as an upstream metabolite regulator of inflammatory cytokine production via mitochondrial ROS production.
- 47.Cannon B & Nedergaard J Brown adipose tissue: function and physiological significance. Physiol. Rev 84, 277–359 (2004). [DOI] [PubMed] [Google Scholar]
- 48.Muzik O, Mangner TJ, Leonard WR, Kumar A & Granneman JG Sympathetic innervation of cold-activated brown and white fat in lean young adults. J. Nucl. Med 58, 799–806 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Lettieri Barbato D et al. Glutathione decrement drives thermogenic program in adipose cells. Sci. Rep 5, 13091 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Ro SH et al. Sestrin2 inhibits uncoupling protein 1 expression through suppressing reactive oxygen species. Proc. Natl Acad. Sci. USA 111, 7849–7854 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Chouchani ET et al. Mitochondrial ROS regulate thermogenic energy expenditure and sulfenylation of UCP1. Nature 532, 112–116 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Han YH et al. Adipocyte-specific deletion of manganese superoxide dismutase protects from diet-induced obesity through increased mitochondrial uncoupling and biogenesis. Diabetes 65, 2639–2651 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Schneider K et al. Increased energy expenditure, Ucp1 expression, and resistance to diet-induced obesity in mice lacking nuclear factor-erythroid-2-related transcription factor-2 (Nrf2). J. Biol. Chem 291, 7754–7766 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Lee SJ, Kim SH, Park KM, Lee JH & Park JW Increased obesity resistance and insulin sensitivity in mice lacking the isocitrate dehydrogenase 2 gene. Free Radic. Biol. Med 99, 179–188 (2016). [DOI] [PubMed] [Google Scholar]
- 55.Chouchani ET, Kazak L & Spiegelman BM Mitochondrial reactive oxygen species and adipose tissue thermogenesis: bridging physiology and mechanisms. J. Biol. Chem 292, 16810–16816 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Kazak L et al. UCP1 deficiency causes brown fat respiratory chain depletion and sensitizes mitochondria to calcium overload-induced dysfunction. Proc. Natl Acad. Sci. USA 114, 7981–7986 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Sanchez-Alavez M, Bortell N, Galmozzi A, Conti B & Marcondes MC Reactive oxygen species scavenger N-acetyl cysteine reduces methamphetamine-induced hyperthermia without affecting motor activity in mice. Temperature 1, 227–241 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Sanchez-Alavez M et al. ROS and sympathetically mediated mitochondria activation in brown adipose tissue contribute to methamphetamine-induced hyperthermia. Front. Endocrinol 4, 44 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Jedrychowski MP et al. Facultative protein selenation regulates redox sensitivity, adipose tissue thermogenesis, and obesity. Proc. Natl Acad. Sci. USA 117, 10789–10796 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Mills EL et al. Cysteine 253 of UCP1 regulates energy expenditure and sex-dependent adipose tissue inflammation. Cell Metab. 34, 140–157.e8 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Xiao H et al. A quantitative tissue-specific landscape of protein redox regulation during aging. Cell 180, 968–983.e24 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Fernández-Agüera MC et al. Oxygen sensing by arterial chemoreceptors depends on mitochondrial complex I signaling. Cell Metab. 22, 825–837 (2015). [DOI] [PubMed] [Google Scholar]
- 63.Harman D Aging: a theory based on free radical and radiation chemistry. J. Gerontol 11, 298–300 (1956). [DOI] [PubMed] [Google Scholar]
- 64.Lopez-Otin C, Blasco MA, Partridge L, Serrano M & Kroemer G The hallmarks of aging. Cell 153, 1194–1217 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Ristow M Unraveling the truth about antioxidants: mitohormesis explains ROS-induced health benefits. Nat. Med 20, 709–711 (2014). [DOI] [PubMed] [Google Scholar]
- 66.Schulz TJ et al. Glucose restriction extends Caenorhabditis elegans life span by inducing mitochondrial respiration and increasing oxidative stress. Cell Metab. 6, 280–293 (2007). [DOI] [PubMed] [Google Scholar]
- 67. Scialò F et al. Mitochondrial ROS produced via reverse electron transport extend animal lifespan. Cell Metab. 23, 725–734 (2016). This paper demonstrates a role for regulated production of mitochondrial ROS in organismal lifespan.
- 68.Yang W & Hekimi S A mitochondrial superoxide signal triggers increased longevity in Caenorhabditis elegans. PLoS Biol. 8, e1000556 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Owusu-Ansah E, Song W & Perrimon N Muscle mitohormesis promotes longevity via systemic repression of insulin signaling. Cell 155, 699–712 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Fiermonte G et al. The sequence, bacterial expression, and functional reconstitution of the rat mitochondrial dicarboxylate transporter cloned via distant homologs in yeast and Caenorhabditis elegans. J. Biol. Chem 273, 24754–24759 (1998). [DOI] [PubMed] [Google Scholar]
- 71.Losman J-A, Koivunen P & Kaelin WG 2-Oxoglutarate-dependent dioxygenases in cancer. Nat. Rev. Cancer 20, 710–726 (2020). [DOI] [PubMed] [Google Scholar]
- 72. Selak MA et al. Succinate links TCA cycle dysfunction to oncogenesis by inhibiting HIF-α prolyl hydroxylase. Cancer Cell 7, 77–85 (2005). This paper illustrated the importance of SDH mutations in cance pathogenesis via accumulated succinate.
- 73.Carey BW, Finley LW, Cross JR, Allis CD & Thompson CB Intracellular α-ketoglutarate maintains the pluripotency of embryonic stem cells. Nature 518, 413–416 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Hems R, Stubbs M & Krebs HA Restricted permeability of rat liver for glutamate and succinate. Biochem. J 107, 807–815 (1968). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Ehinger JK et al. Cell-permeable succinate prodrugs bypass mitochondrial complex I deficiency. Nat. Commun 7, 12317 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.MacDonald MJ, Fahien LA, Mertz RJ & Rana RS Effect of esters of succinic acid and other citric acid cycle intermediates on insulin release and inositol phosphate formation by pancreatic islets. Arch. Biochem. Biophys 269, 400–406 (1989). [DOI] [PubMed] [Google Scholar]
- 77.Hochachka PW & Dressendorfer RH Succinate accumulation in man during exercise. Eur. J. Appl. Physiol. Occup. Physiol 35, 235–242 (1976). [DOI] [PubMed] [Google Scholar]
- 78.Taegtmeyer H Metabolic responses to cardiac hypoxia. Increased production of succinate by rabbit papillary muscles. Circ. Res 43, 808–815 (1978). [DOI] [PubMed] [Google Scholar]
- 79.Hochachka PW, Owen TG, Allen JF & Whittow GC Multiple end products of anaerobiosis in diving vertebrates. Comp. Biochem. Physiol. B 50, 17–22 (1975). [DOI] [PubMed] [Google Scholar]
- 80.Prag HA et al. Mechanism of succinate efflux upon reperfusion of the ischaemic heart. Cardiovasc. Res 117, 1188–1201 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Bisbach CM et al. Succinate can shuttle reducing power from the hypoxic retina to the O2-rich pigment epithelium. Cell Rep. 31, 107606 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Wu J-Y et al. Cancer-derived succinate promotes macrophage polarization and cancer metastasis via succinate receptor. Mol. Cell 77, 213–227.e5 (2020). [DOI] [PubMed] [Google Scholar]
- 83.Mills EL et al. UCP1 governs liver extracellular succinate and inflammatory pathogenesis. Nat. Metab 3, 604–617 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.An YA et al. The mitochondrial dicarboxylate carrier prevents hepatic lipotoxicity by inhibiting white adipocyte lipolysis. J. Hepatol 75, 387–399 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Andrienko TN, Pasdois P, Pereira GC, Ovens MJ & Halestrap AP The role of succinate and ROS in reperfusion injury—a critical appraisal. J. Mol. Cell. Cardiol 110, 1–14 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Bisbach C, Hass D & Hurley J Monocarboxylate transporter 1 (MCT1) mediates succinate export in the retina. Preprint at bioRxiv 10.1101/2021.11.19.469314 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Pajor AM Sodium-coupled dicarboxylate and citrate transporters from the SLC13 family. Pflug. Arch 466, 119–130 (2014). [DOI] [PubMed] [Google Scholar]