SUMMARY
This report describes an increased number of cases of Chikungunya virus (CHIKV) infection imported in northern Italy (Emilia-Romagna region) during the period May–September 2014, indicating that the recent spread of CHIKV and its establishment in the Caribbean and in central America, resulted in a high number of imported cases in Europe, thus representing a threat to public health. From May to September 2014, 14 imported cases of CHIKV infection were diagnosed; the patients were returning to Italy from Dominican Republic (n = 6), Haiti (n = 3), Guadeloupe (n = 2), Martinique (n = 1), Puerto Rico (n = 1) and Venezuela (n = 1). Phylogenetic analysis performed on the envelope protein (E1) gene sequences, obtained from plasma samples from two patients, indicated that the virus strain belongs to the Caribbean clade of the Asian genotype currently circulating in the Caribbean and Americas. The rise in the number of imported cases of CHIKV infection should increase healthcare professionals' awareness of the epidemiological situation and clinical presentation of CHIKV infection in order to enhance surveillance and early diagnosis in the forthcoming season of vector activity in Europe and North America.
Key words: Arbovirus, Chikungunya virus, epidemiology, Italy, phylogeny
Chikungunya virus (CHIKV) is a single-stranded, positive-sense RNA virus belonging to the Alphavirus genus of the Togaviridae family. CHIKV is transmitted by the bite of Aedes mosquitoes, primarily Aedes aegypti and Aedes albopictus. Clinical signs of CHIKV infection are high fever, myalgia, skin rash and arthralgia, which may persist for weeks.
CHIKV is endemic in parts of Africa, South-East Asia and in the Indian subcontinent. First evidence of CHIKV infections in the Western Hemisphere was reported in December 2013 in the Caribbean island of St Martin [1], spreading thereafter to other Caribbean islands and to Central, South and North America (http://www.cdc.gov/chikungunya/geo/index.html) [2]. The vector involved in the CHIKV outbreak in the Caribbean is A. aegypti [3] and the virus strain circulating belongs to the Asian genotype [1, 4]. The high density of the vector together with increasing number of potential viraemic patients returning from endemic countries highlights the potential risk of local CHIKV outbreaks in Europe [5].
Since its introduction to the Caribbean in 2013 [1] the number of imported cases of CHIKV infection in Europe has increased, as recently reported in Spain [6] and France [7]. After the outbreak of autochthonous CHIKV infection that occurred in the Emilia-Romagna region in 2007 (with 217 confirmed cases) [8], only sporadic imported cases have been reported in Italy (two cases in 2011, five cases in 2012, three cases in 2013). While the number of cases of CHIKV imported into Emilia-Romagna region, Northern Italy, was very low between 2011 and 2013 (one case in 2011, 0 cases in 2012, one case in 2013), the number has increased significantly over the course of 2014.
In Italy, a surveillance system for arboviral diseases, defined annually by the National Ministry of Health, is implemented and allows detection of imported cases of CHIKV throughout the year, with more attention during the period of vector activity. Following the activities of the integrated surveillance plan for arboviruses, 14 patients were diagnosed with CHIKV infection in Emilia-Romagna region from May to September 2014. All the 14 patients had history of recent travel to the Dominican Republic (n = 6), Haiti (n = 3), Guadeloupe (n = 2), Martinique (n = 1), Puerto Rico (n = 1) and Venezuela (n = 1). All patients had symptoms compatible with CHIKV infection, including fever (>38·5 °C), arthralgia, headache, and myalgia. The median age of CHIKV cases was 46 (range 8–68) years; CHIKV infection was diagnosed in a median time of 7 days from disease onset (range 1–26 days) and was supported by virological and serological means.
All patients were tested for both dengue (DENV) and CHIKV infection. Serum samples were tested for the presence of IgM and IgG antibodies specific for DENV and CHIKV, respectively, by indirect immunofluorescence assay (Euroimmun AG, Germany); for the presence of DENV non-structural protein (NS1) (Platelia Dengue NS1 AG kit, Bio-Rad Laboratories, USA) and for the presence of CHIKV and DENV RNA in plasma and/or serum samples by using real-time RT–PCR [9, 10]. All the patients tested negative for DENV infection.
CHIKV RNA was identified in the plasma and/or serum samples of seven patients; in six patients the diagnosis was based on the presence of CHIKV-specific IgM and IgG in serum samples and one patient was only IgM positive and thus considered a probable case. A summary of epidemiological, clinical and laboratory findings from imported cases is reported in Table 1.
Table 1.
Clinical, epidemiological and diagnostic features of cases of CHIKV infection in patients returning from the Caribbean and Americas to the Emilia-Romagna region, Italy, May–September 2014.
| Case no. | Sex | Age, yr | Country visited | Period of stay | Clinical symptoms | Laboratory findings | Time to diagnosis (days) |
|---|---|---|---|---|---|---|---|
| 1 | F | 42 | Guadeloupe | 25 Apr.–4 May | Fever, arthralgia, myalgia, rash, headache | CHIKV RNA+; IgM+; IgG- | 7 |
| 2 | M | 50 | Guadeloupe | 25 Apr.–4 May | Fever, arthralgia, myalgia, asthenia, headache | CHIKV RNA-; IgM+; IgG+ | 7 |
| 3 | F | 53 | Haiti | Apr.–14 May | Fever, arthralgia, rash, asthenia | CHIKV RNA-; IgM+; IgG+ | 13 |
| 4 | F | 35 | Haiti | 7 May–24 May | Fever, arthralgia, rash, asthenia, myalgia, headache, retro-orbital pain | CHIKV RNA-; IgM+; IgG+ | 9 |
| 5 | F | 54 | Dominican Rep. | 28 Apr.–30 May | Fever, arthralgia, rash, asthenia, myalgia, retro-orbital pain | CHIKV RNA-; IgM+; IgG+ | n.a. |
| 6 | M | 68 | Haiti | 26 May–5 June | Fever, arthralgia, rash, asthenia, myalgia, | CHIKV RNA+; IgM+; IgG+ | 9 |
| 7 | M | 62 | Dominican Rep. | 25 May–8 June | Fever, arthralgia, asthenia | CHIKV RNA+; IgM-; IgG- | 2 |
| 8 | M | 67 | Dominican Rep. | 28 May–10 June | Fever, arthralgia, myalgia | CHIKV RNA+; IgM-; IgG- | 4 |
| 9 | F | 8 | Dominican Rep. | 7 June–6 July | Fever, rash, headache | CHIKV RNA+; IgM-; IgG- | 1 |
| 10 | M | 32 | Dominican Rep. | 17 June–27 June | Fever, arthralgia, myalgia, headache, asthenia | CHIKV RNA-; IgM+; IgG+ | n.a. |
| 11 | F | 40 | Dominican Rep. | 3 July–31 July | Fever, rash, retro-orbital pain, headache, arthralgia | CHIKV RNA-; IgM+; IgG+ | 26 |
| 12 | M | 34 | Martinique | 4 Aug.–17 Aug. | Fever, rash, asthenia, headache, retro-orbital pain | CHIKV RNA-; IgM+; IgG- | 6 |
| 13 | M | 60 | Puerto Rico | n.a.–18 Sept. | Fever, arthralgia, rash, myalgia | CHIKV RNA+; IgM-; IgG- | n.a. |
| 14 | M | 21 | Venezuela | 11 Apr –18 Sept. | Fever, arthralgia, rash, asthenia, myalgia, headache | CHIKV RNA+; IgM-; IgG- | 4 |
n.a., Not available.
Nucleotide sequences (1518 nt) of the envelope protein (E1) gene of CHIKV were obtained from plasma samples from two patients (case nos. 7 and 9) returning to Italy from the Dominican Republic. Overlapping amplicons were amplified and bi-directionally sequenced. Primers used for sequencing were [11]: 19 F: 5′-AGTTGTGTCAGTGGCCTCGTTC-3′ and 19 R: 5′-TAAAGGACGCGGAGCTTAGCTG-3′; 20 F: 5′-ACAAAACCGTCATCCCGTCTC-3′ and 20 R: 5′-TGACTATGTGGTCCTTCGGAGG-3′; 21 F: 5′-CAGCAAGAAAGGCAAGTGTGC-3′ and 21 R: 5′-TACGTCCCTGTGGGTTCG-3′. The sequences were deposited in GenBank under accession numbers KP252141 and KP252142. Multiple alignments of the two new sequences of the E1 region, along with the corresponding sequences from 36 other CHIKV strains available in GenBank and the European Virus Archive (EVA), were generated by Clustal W software (http://www2.ebi.ac.uk/clustalw).
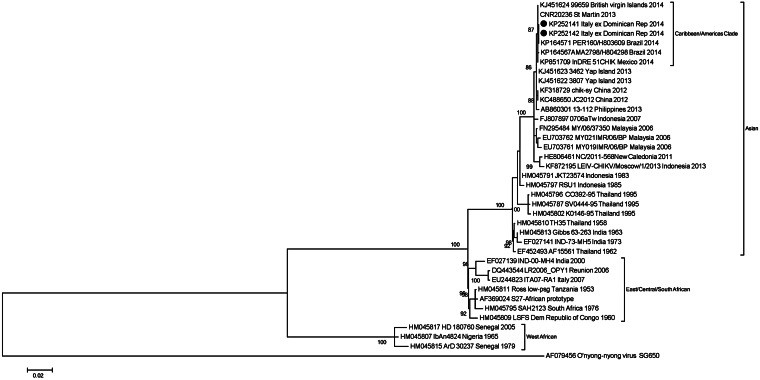
Phylogenetic analysis of the E1 sequences (Fig. 1) confirmed that the two strains belonged to the Asian genotype, currently circulating in the Caribbean and Americas [1, 4, 6] and are closely related to a strain identified in St Martin in 2013 (CNR20235) and in the British Virgin Islands in 2014 (99659-KJ451624) with 0·1% nucleotide distance.
Fig. 1.
Phylogenetic analysis of the envelope E1 gene sequences (1518 nt) from two cases of CHIKV infection. Maximum-likelihood tree was inferred with the GRT + G model with 1000 bootstrap replicates using MEGA 5 software (bootstrap values ⩾80% are shown). CHIKV strains sequenced in this study (GenBank accession nos.: KP252141 and KP252142) are indicated by a black circle (•). The tree was rooted by using the O'nyong-nyong virus as the outgroup virus. Scale bar indicates nucleotide substitution per site.
The recent spread of CHIKV, and its establishment in the Caribbean and the Americas, accounts for the increased number of imported case of CHIKV in Europe as already documented in Spain [6], mainland France [7], and now in Italy. The recent rapid risk assessment from the European Centre for Disease Prevention and Control (ECDC) established that the CHIKV epidemic in the Americas represents a threat to public health in Europe, constituting a source of introduction of CHIKV in the Northern Hemisphere, overlapping with the presence of competent vectors in Europe [12], even if the Asian genotype strains have been considered constrained in their ability to adapt to A. albopictus, which is the dominant mosquitoes species in Europe [13]. Nevertheless, European A. albopictus was shown to experimentally transmit Caribbean CHIKV strains belonging to the Asian genotype [14] but colder temperatures may reduce transmission efficacy by European A. albopictus; these data might explain the lack of autochthonous transmission in Europe despite the high number of imported cases from the Caribbean and Americas. The transmission of CHIKV is dependent on mosquito species, environmental conditions such as temperature and viral genotype [14] and in this regard, sequencing data and virus genotype definition of imported cases will provide additional information to assess more accurately the risk of CHIKV transmission in Europe by combining data on mosquitoes and viral genetic characteristics, in addition to tracking the possible evolution of the virus during an epidemic. Healthcare professionals should become increasingly aware of the clinical presentation of CHIKV and the diagnostics and should consider CHIKV infection in patients returning from the Caribbean region and the Americas in order to enhance surveillance for early identification of imported cases.
ACKNOWLEDGEMENTS
This study was supported by ‘Fondi Finalizzati LABP3’ from Regione Emilia-Romagna.
DECLARATION OF INTEREST
None.
REFERENCES
- 1.Leparc-Goffart I, et al. Chikungunya in the Americas. Lancet 2014; 383: 514. [DOI] [PubMed] [Google Scholar]
- 2.Pan American Health Organization (PAHO). Number of reported cases of chikungunya fever in the Americas, by country or territory with autochthonous transmission 2013–2015 (to week noted). Epidemiological week/EW 15. Washington, D.C.: World Health Organization Regional Office for the Americas, 2015. (http://www.paho.org/hq/index.php?option=com_topics&view=article&id=343&Itemid=40931).
- 3.Weaver SC. Arrival of chikungunya virus in the new world: prospects for spread and impact on public health. PLoS Neglected Tropical Diseases 2014; 8: e2921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Gallian P, et al. Prospective detection of chikungunya virus in blood donors, Caribbean 2014. Blood 2014; 123: 3679–3681. [DOI] [PubMed] [Google Scholar]
- 5.Van Bortel W, et al. Chikungunya outbreak in the Caribbean region, December 2013 to March 2014, and the significance for Europe. Eurosurveillance 2014; 19(13). [DOI] [PubMed] [Google Scholar]
- 6.Requena-Mendez A, et al. Cases of chikungunya virus infection in travellers returning to Spain from Haiti or Dominican Republic, April–June 2014. Eurosurveillance 2014; 19(28). [DOI] [PubMed] [Google Scholar]
- 7.Paty M, et al. Large number of imported chikungunya cases in mainland France, 2014: a challenge for surveillance and response. Eurosurveillance 2014; 19(28). [DOI] [PubMed] [Google Scholar]
- 8.Angelini R, et al. Chikungunya in north-eastern Italy: a summing up of the outbreak. Eurosurveillance 2007; 12(11): E071122·2. [DOI] [PubMed] [Google Scholar]
- 9.Kong YY, et al. Rapid detection, serotyping and quantitation of dengue viruses by TaqMan real-time one-step RT-PCR. Journal of Virological Methods 2006; 138: 123–130. [DOI] [PubMed] [Google Scholar]
- 10.Edwards CJ, et al. Molecular diagnosis and analysis of Chikungunya virus. Journal of Clinical Virology 2007; 39: 271–275. [DOI] [PubMed] [Google Scholar]
- 11.Sam IC, et al. Genotypic and phenotypic characterization of Chikungunya virus of different genotypes from Malaysia. PLoS ONE 2012; 7(11). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Noel H, Rizzo C. Spread of chikungunya from the Caribbean to mainland Central and South America: a greater risk of spillover in Europe? Eurosurveillance 2014; 19(28). [DOI] [PubMed] [Google Scholar]
- 13.Morrison TE. Reemergence of chikungunya virus. Journal of Virology 2014; 88: 11644–11647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Vega-Rúa A, et al. Chikungunya virus transmission potential by local Aedes mosquitoes in the Americas and Europe. PLoS Neglected Tropical Diseases 2015; 9(5). [DOI] [PMC free article] [PubMed] [Google Scholar]