Abstract
Cardiovascular diseases (CVD) remain the leading cause of death in developed and developing societies and aging is the primary risk factor for CVD. Much of the increased risk of CVD in midlife/older adults (i.e., adults aged 50 years and older) is due to increases in blood pressure, vascular endothelial dysfunction and stiffening of the large elastic arteries. Aerobic exercise training is an effective lifestyle intervention to improve CV function and decrease CVD risk with aging. However, <40% of midlife/older adults meet guidelines for aerobic exercise, due to time availability-related barriers and other obstacles to adherence. Therefore, there is a need for new lifestyle interventions that not only improve CV function with aging but also promote adherence. High-resistance inspiratory muscle strength training (IMST) is an emerging, time-efficient (5 minutes/day) lifestyle intervention. Early research suggests high-resistance IMST may promote adherence, lower blood pressure and potentially improve vascular endothelial function. However, additional investigation will be required to more definitively establish high-resistance IMST as a healthy lifestyle intervention for CV aging. This review will summarize the current evidence on high-resistance IMST for improving CV function with aging and will identify key research gaps and future directions.
Keywords: blood pressure, endothelial function, arterial stiffness, aerobic exercise
1. Introduction
Aging is the primary risk factor for cardiovascular diseases (CVD), the leading cause of death in the United States and other developed and developing countries (Virani et al., 2021). The number of older adults is expected to increase significantly in the coming years, predicting a large increase in the prevalence of CVD-related morbidity and mortality without effective interventions (Heidenreich et al., 2011).
The increased risk for CVD with aging is due, in part, to age-related changes in CV function (Figure 1), including increases in blood pressure (BP), vascular endothelial dysfunction and stiffening of the large elastic arteries (Lakatta and Levy, 2003). The age-related increase in BP is primarily driven by elevations in systolic BP (SBP) above normal, healthy levels (i.e., ≥120 mmHg), as diastolic BP (DBP) tends to plateau around age 50 years and then decrease at older ages (Lakatta and Levy, 2003). Vascular endothelial dysfunction is apparent as the impaired ability of the vascular endothelium to regulate vascular tone due to reductions in bioavailability of the important vasodilatory molecule, nitric oxide (NO) (Seals et al., 2011). Stiffening of the large elastic arteries, e.g., the aorta and carotid arteries, is characterized by degradation of elastin fibers, increased collagen deposition and increased collagen cross-linking due to formation of advanced glycation end products (Zieman et al., 2005).
Figure 1.
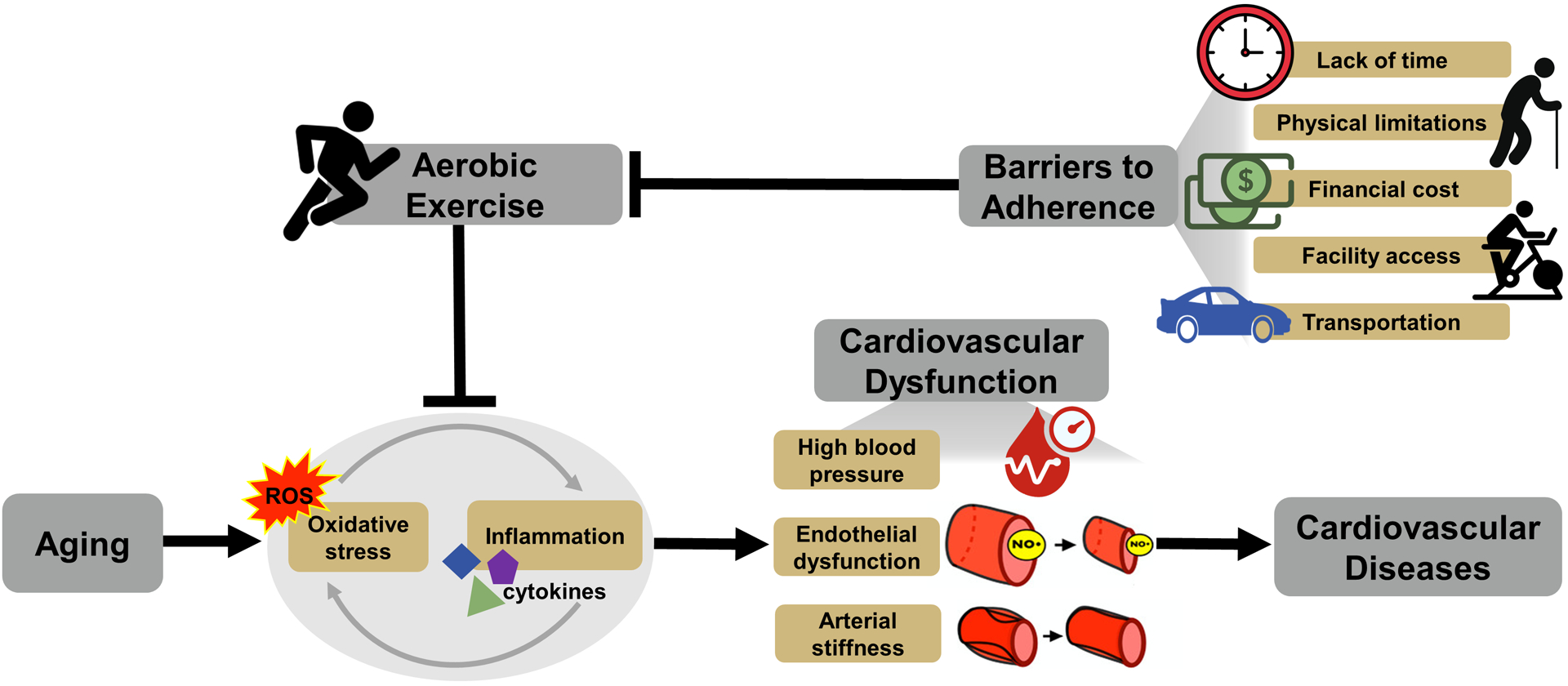
Aging is associated with excessive reactive oxygen species (ROS)-induced oxidative stress and excessive pro-inflammatory cytokines-associated chronic low-grade inflammation (lower left). These cellular processes evoke cardiovascular dysfunction, including high blood pressure, vascular endothelial dysfunction and stiffening of the large elastic arteries. Cardiovascular dysfunction contributes to an increased risk of cardiovascular diseases with aging (lower right). Aerobic exercise is an effective lifestyle strategy for reducing oxidative stress and inflammation, improving cardiovascular function, and reducing cardiovascular disease risk (top left). Despite this fact, barriers to adherence, including lack of time, physical limitations, financial cost, facility access and transportation can limit adherence to aerobic exercise, particularly in adults aged 50 years and older (top right).
Primary mechanisms involved in CV dysfunction with aging include increased oxidative stress and chronic low-grade inflammation (Craighead et al., 2020; Donato et al., 2008, 2007) (Figure 1). Increased oxidative stress with aging is mediated primarily by excessive production of reactive oxygen species (ROS) derived from multiple sources. There is an increase in ROS production from mitochondria as well as increases in the expression and activity of pro-oxidant enzymes, primarily nicotinamide adenine dinucleotide phosphate (NADPH) oxidase, with no change in, or a decrease in the expression of, antioxidant enzymes, such as manganese superoxide dismutase (MnSOD) (Donato et al., 2007; Pierce et al., 2011a). Increased ROS production from these and other sources can cause uncoupling of endothelial NO synthase (eNOS) (Förstermann and Sessa, 2012), which, in turn, produces less NO and more superoxide (Luo et al., 2014). Superoxide reacts directly with NO to form peroxynitrite, further reducing overall NO bioavailability (Pacher et al., 2007). In addition, aging is associated with chronic activation of the pro-inflammatory master regulator, nuclear factor κB (NFκB) and increased production of pro-inflammatory molecules, including interleukin-6, tumor necrosis factor-α, and C-reactive protein (CRP) (Donato et al., 2008; Lesniewski et al., 2011; Seals, 2014).
Collectively, these findings suggest strategies that improve CV function by decreasing oxidative stress and chronic low-grade inflammation would be effective for decreasing age-associated CVD. Conventional aerobic exercise training is a particularly effective lifestyle strategy for improving CV function and decreasing CVD risk with aging (Seals, 2014). To improve CV function, aerobic exercise must be performed regularly (Craighead et al., 2019; Seals, 2014; Seals et al., 2018). However, less than 40% of midlife and older adults, i.e., adults aged 50 years and older, adhere to aerobic exercise guidelines (Schoenborn and Stommel, 2011; Troiano et al., 2008). Thus, there is a need for alternative forms of physical training that both improve CV function and promote high rates of adherence in midlife/older adults.
The purpose of this review is to discuss novel, high-resistance inspiratory muscle strength training (IMST), a time-efficient form of physical training that may improve CV function with aging and promote excellent rates of adherence. We will start by briefly summarizing the effects of aerobic exercise for improving CV function with aging. We then will provide a short overview of the clinical and research history of IMST and what has been learned from investigations using low-resistance IMST paradigms for improving CV function. The bulk of this review will then focus on summarizing current knowledge regarding high-resistance IMST for improving CV function with aging, while also identifying knowledge gaps and future directions for research on this highly promising, emerging healthy lifestyle strategy.
2. Conventional aerobic exercise and cardiovascular function
Aerobic exercise training is the most well-known and well-studied healthy lifestyle intervention for improving CV function with aging (Craighead et al., 2019; Seals, 2014; Seals et al., 2018) (Figure 1). In midlife/older adults, aerobic exercise lowers casual (resting) SBP by 2–8 mmHg on average, with the largest reductions apparent in those with the highest baseline SBP (i.e., stage 2 hypertension, baseline SBP ≥140 mmHg) (Whelton et al., 2018, 2002). Aerobic exercise also lowers casual DBP by 2–3 mmHg (Whelton et al., 2018). The reductions in BP observed with aerobic exercise are associated with improvements in arterial function, including consistent increases in vascular endothelial function and, in some cases, reductions in large elastic artery stiffness (Guimarães et al., 2010; Pedralli et al., 2020; Vaitkevicius et al., 1993).
Aerobic exercise consistently improves vascular endothelial function in midlife/older men. In cross-sectional comparisons, endurance-trained midlife/older men have greater endothelial function compared to their untrained peers (DeSouza et al., 2000; Eskurza et al., 2004), measured either as brachial artery flow-mediated dilation (FMDBA), a well-established measure of conduit artery endothelial function that is primarily mediated by NO (Green et al., 2014), or the increase in forearm blood flow in response to brachial artery infusion of the endothelium-dependent dilator acetylcholine, the gold-standard measure of in vivo microvascular endothelial function (Seals et al., 2011). Consistent with such cross-sectional group comparisons, aerobic exercise training improves endothelial function in previously sedentary older men (DeSouza et al., 2000; Pierce et al., 2011b).
The improvements in endothelial function with aerobic exercise training in midlife/older men are largely mediated by reductions in oxidative stress. ROS (primarily superoxide)-mediated suppression of endothelial function can be measured in vivo as the change in FMDBA following acute infusion of a supratherapeutic dose of the potent antioxidant, vitamin C, compared to saline-infusion control. In sedentary older men, vitamin C infusion improves FMDBA (Eskurza et al., 2004), indicative of tonic superoxide-mediated suppression of endothelial function in this group. However, vitamin C infusion does not affect FMDBA in endurance-trained older men (Eskurza et al., 2004), suggesting a reduction in vascular oxidative stress with aerobic exercise training. Other important markers of oxidative stress also decrease with aerobic exercise. In endothelial cells sampled via endovascular biopsy from endurance-trained midlife/older men, abundance of nitrotyrosine, a marker of oxidative stress, and expression of NADPH oxidase are lower compared with older sedentary men and young adult controls (Pierce et al., 2011a). Plasma concentrations of oxidized-low density lipoprotein, a circulating marker of oxidative stress, also are reduced in endurance-trained midlife/older adults (Santos-Parker et al., 2017). Evidence from lifelong studies in male mice suggest reduced mitochondrial ROS production also is involved in suppressing oxidative stress in response to aerobic exercise training (Gioscia-Ryan et al., 2021). Other evidence suggests that pro-inflammatory markers including NFκB, tumor necrosis factor-α, and CRP also may be lower in endurance-trained older adults or following aerobic exercise training, suggesting reductions in chronic low-grade inflammation are also involved in the CV benefits of aerobic exercise (Gano et al., 2011; Santos-Parker et al., 2017).
Importantly, aerobic exercise training does not consistently improve vascular endothelial function in estrogen-deficient postmenopausal women (Seals et al., 2019), i.e., postmenopausal women not taking hormone replacement therapy, which represents the great majority (>90%) of postmenopausal women in the United States (Connelly et al., 2000). Cross-sectionally, endurance-trained estrogen-deficient postmenopausal women do not have greater endothelial function than their sedentary peers (Santos-Parker et al., 2017), whereas results from intervention studies fail to show consistent changes in endothelial function following aerobic exercise training in these women (Moreau et al., 2013; Pierce et al., 2011b). Evidence suggests this is due to reductions in estrogen, as formerly estrogen-deficient postmenopausal women treated with exogenous estrogen exhibit improvements in endothelial function following aerobic exercise training (Moreau et al., 2013). Mechanistically, this appears linked to an inability for aerobic exercise to decrease oxidative stress in postmenopausal women without the presence of estrogen. Indeed, acute supratherapeutic treatment with the antioxidant vitamin C increases FMDBA in both sedentary and endurance-trained estrogen-deficient postmenopausal women as well as in estrogen-deficient postmenopausal women following an aerobic exercise intervention but does not affect FMDBA in estrogen-treated postmenopausal women after chronic aerobic exercise training (Moreau et al., 2013).
Regular aerobic exercise also is associated with lower large elastic artery stiffness compared with the sedentary state in some groups of midlife/older men and women, although the literature is somewhat inconsistent and any observed benefits seem to be dependent on adequate exercise intervention duration and exercise bout frequency (Pierce, 2018, 2017). Endurance-trained midlife/older men and women have lower arterial stiffness, measured via carotid-femoral pulse wave velocity (CFPWV), the gold-standard clinical measure of aortic stiffness (Laurent et al., 2006; Vlachopoulos et al., 2010), or as carotid artery compliance, compared to their sedentary peers (Moreau et al., 2003; Tanaka et al., 2000, 1998). In addition, aerobic exercise training can decrease carotid artery compliance in previously sedentary midlife/older adults (Matsubara et al., 2014; Tanahashi et al., 2014; Tanaka et al., 2000). Data from animal models suggest that benefits of aerobic exercise for improving arterial stiffness are, at least in part, mediated by reductions in oxidative stress. In fact, reductions in carotid artery stiffness following 10–14 weeks of voluntary wheel running in mice are linked to reduced superoxide-mediated suppression of collagen deposition (Fleenor et al., 2010). Similarly, lifelong aerobic exercise in mice prevents the age-related increases in aortic whole-cell and mitochondrial-specific superoxide production observed in sedentary older mice and which is linked to stiffening of the aorta (Gioscia-Ryan et al., 2021).
Current aerobic exercise guidelines recommend at least 150 minutes of moderate intensity or 75 minutes of vigorous intensity aerobic exercise per week (Piercy et al., 2018). However, depending on whether adherence is measured through accelerometry or self-report, as few as 5%– 40% of midlife/older adults meet these guidelines (Schoenborn and Stommel, 2011; Troiano et al., 2008). The most common reported barrier to adherence to aerobic exercise guidelines is lack of time (El Ansari and Lovell, 2009; Kelly et al., 2016; Stutts, 2002). Other common barriers include physical limitations, financial cost, facility access and transportation barriers (Babakus and Thompson, 2012; Kelly et al., 2016; Siddiqi et al., 2011; Yarwood et al., 2005) (Figure 1). These barriers tend to be even more pronounced, and aerobic exercise adherence even worse, in midlife/older adults from racial and ethnic minorities or with lower levels of education and socioeconomic status, due to a lack of social support and less guidance from healthcare providers (Allen and Morey, 2010; Rivera-Torres et al., 2019). Therefore, there is a need for alternative lifestyle interventions that improve CV function in midlife/older adults and are accessible to all demographics while overcoming common barriers to adherence associated with conventional aerobic exercise training.
3. Inspiratory muscle strength training
IMST is a form of physical training that utilizes only the diaphragm and accessory respiratory muscles, particularly the sternocleidomastoid and intercostal muscles, to perform repeated inhalations against resistance, whereas expiration is unimpeded. IMST can be performed on a variety of devices that provide resistance to inhalation in slightly different ways. “Constant resistance” devices provide a consistent level of inspiratory resistance, most often by creating a narrowed airway for the user to breathe through. Pressure-threshold devices allow inspiration only once a target pressure has been reached, usually through the opening of a valve; inhalation then ceases once inspiratory pressure drops below the target pressure. Finally, tapered loading devices require a target inspiratory pressure to be reached to start inhalation, but the resistance to inspiration gradually decreases throughout the maneuver, allowing for inspiration to continue at higher lung volumes when force production begins to drop. The above types of devices can all be used to design IMST interventions with various training session duration, frequency and intensity. There also is hyperpnea-based inspiratory training, which involves training based on increasing lung volumes and ventilation, rather than increasing inspiratory resistance. Hyperpnea-based paradigms are very different from resistance-based protocols and will not be the focus of this review.
Interest in IMST began in earnest in the 1970’s as an ergogenic aid for improving athletic performance and investigation of the efficacy of IMST for improving athletic performance has continued through today (Chang et al., 2021; de Sousa et al., 2020; Leith and Bradley, 1976). Some evidence suggests that IMST may improve performance in endurance athletes by mitigating the respiratory muscle metaboreflex that occurs as respiratory muscles become fatigued, usually resulting in a redistribution of blood flow from the locomotor to respiratory muscles (Callegaro et al., 2011; Witt et al., 2007). In this way, IMST may help maintain blood flow to the working locomotor muscles.
Research on IMST quickly spread from use as a performance-enhancing protocol in athletic populations to a potential therapy in groups with respiratory failure, such as patients with chronic obstructive pulmonary disease or those needing to be weaned off ventilators (Beaumont et al., 2018; Elkins and Dentice, 2015). IMST was also explored as a potential treatment for groups with limited ability to perform full-body exercise, including patients with heart failure, muscular dystrophy or spinal cord injury (Sheel et al., 2008; Smart et al., 2013; Wanke et al., 1994).
The commercialization of the above-described devices eventually made IMST available to the general public. Indeed, the wide availability of affordable IMST devices may reduce several common barriers to adherence associated with conventional aerobic exercise training. Although devices costing >US$500 are available, other IMST devices can be purchased for a one-time expense of US$50-$100. This cost is less than many monthly gym memberships or the price of more expensive exercise equipment, overcoming some of the cost-related barriers to access. IMST devices can be used at home and do not require nearby exercise facilities or a built environment conducive to exercise, surmounting the requirement for access to specialized facilities. Finally, IMST reduces travel-related barriers as at-home training removes the need to commute to specialized facilities. The training devices are also small and portable, so they can easily be taken along during travel. Overall, IMST has multiple potential advantages that may overcome some of the common barriers to adherence reported for other healthy lifestyle strategies in midlife/older adults from multiple demographics.
3.1. Low-resistance IMST
Until recently, IMST has been performed against a relatively low inspiratory resistance of approximately 30% of the maximal inspiratory pressure (PIMAX) an individual can produce, a respiratory pressure roughly equivalent to that generated during maximal intensity aerobic exercise in fit individuals (Craighead et al., 2019). Such low-resistance protocols require individual training sessions of 30 minutes or more. When multiple training sessions are performed per week, the overall time commitment required for low-resistance IMST is similar to that required to meet aerobic exercise guidelines (i.e., approximately 150 minutes per week). Therefore, low-resistance IMST does not address the crucial time-availability barrier to adherence posed by conventional aerobic exercise training.
Despite being time-intensive, investigations of low-resistance IMST provide an initial foundation of evidence from which to assess the potential benefits of higher-resistance IMST on CV function with aging and age-related chronic disorders. For example, 30–60 minutes of low-resistance IMST (18–20 cmH2O, <30% PIMAX for most generally healthy adults) per day for 8 weeks has been reported by Jones and colleagues (Jones et al., 2010; Sangthong et al., 2016) to lower casual SBP by 13–18 mmHg in adults with hypertension, whereas casual DBP remained unchanged (Sangthong et al., 2016) or was reduced by 7 mmHg (Jones et al., 2010). These findings are promising and if confirmed by other laboratories would support the antihypertensive efficacy of this form of IMST. On the other hand, 2 studies involving patients with heart failure, 30 minutes per day, 7 days per week, of low-resistance IMST (30% PIMAX) reported no changes in casual BP after 4 weeks (Chiappa et al., 2008) or 12 weeks (Mello et al., 2012). In addition, no changes in casual BP were observed in patients with chronic obstructive pulmonary disease after 12 weeks of low-resistance IMST consisting of 3 days per week, 30 minutes per day at a resistance equivalent to 30% PIMAX (Cutrim et al., 2019). Differences in the baseline BP of the subjects, and the intensity, duration and frequency of the IMST paradigms used in these investigations all may have contributed to the variable reductions in casual BP reported. Additionally, underlying health status, including the presence of cardiopulmonary dysfunction, may influence efficacy as low-resistance IMST lowered BP in adults with hypertension but not patients with heart failure or chronic obstructive pulmonary disease. Overall, although select studies have reported significant BP-reducing responses to low-resistance IMST, other studies have found no such effects. Therefore, the efficacy of low-resistance IMST for lowering casual BP in midlife and older healthy adults and patients with chronic diseases remains to be established.
Twenty-four-hour BP measured via ambulatory monitoring is an additional independent CVD risk factor (Clement et al., 2003; Dolan et al., 2005; Mancia, 2007). A study in midlife/older adults with hypertension demonstrated that low-resistance IMST, consisting of 30 minutes of training at 30% PIMAX per day for 8 weeks, lowered 24-hour SBP by 8 mmHg and 24-hour DBP by 6 mmHg (Ferreira et al., 2013).
We are aware of one study that assessed the effects of low-resistance IMST on directly measured sympathetic nervous system activity using microneurography in midlife/older adults with heart failure. In these patients muscle sympathetic nerve activity (MSNA) burst frequency and incidence were reduced following 12 weeks of low-resistance IMST at 30% PIMAX performed in 3, 10-minute intervals each day (e.g., 30 minutes per day) (Mello et al., 2012). Additional studies have investigated the effects of low-resistance IMST on heart rate variability, an indirect and controversial measure of cardiac sympathetic activity. In healthy midlife/older adults and patient populations, low-resistance IMST has been reported to change indexes of heart rate variability purported to reflect increases in cardiac parasympathetic nerve activity and reductions in cardiac sympathetic nerve activity (de Abreu et al., 2017; Ferreira et al., 2013).
The effects of low-resistance IMST on vascular endothelial function and arterial stiffness have not been thoroughly investigated. However, given the promising albeit inconsistent results of low-resistance IMST for lowering BP in select studies, interests have turned towards adapting IMST to overcome the critical time-availability related barrier to adherence.
3.2. High-resistance IMST
High-resistance IMST is a novel variation on the traditional low-resistance IMST paradigm. The most prominent high-resistance IMST protocol to emerge thus far consists of 30 inhalations per session at a resistance of 75% PIMAX, with one session performed per day and 5–7 sessions performed per week (Figure 2) (Craighead et al., 2021; DeLucia et al., 2018; Ramos-Barrera et al., 2020; Vranish and Bailey, 2016, 2015). Importantly, and unlike aerobic exercise or low-resistance IMST, this high-resistance IMST protocol requires a daily time commitment of only approximately 5 minutes, amounting to just a 30-minute total weekly time commitment if performed regularly. Thus, high-resistance IMST is a highly time-efficient lifestyle strategy which may promote greater rates of adherence than time-intensive protocols. Indeed, results from small, clinic-based trials using the above 5 minute per day high-resistance IMST protocol have observed excellent rates of adherence, with greater than 90% of prescribed training sessions performed (DeLucia et al., 2018; Vranish and Bailey, 2016, 2015).
Figure 2.
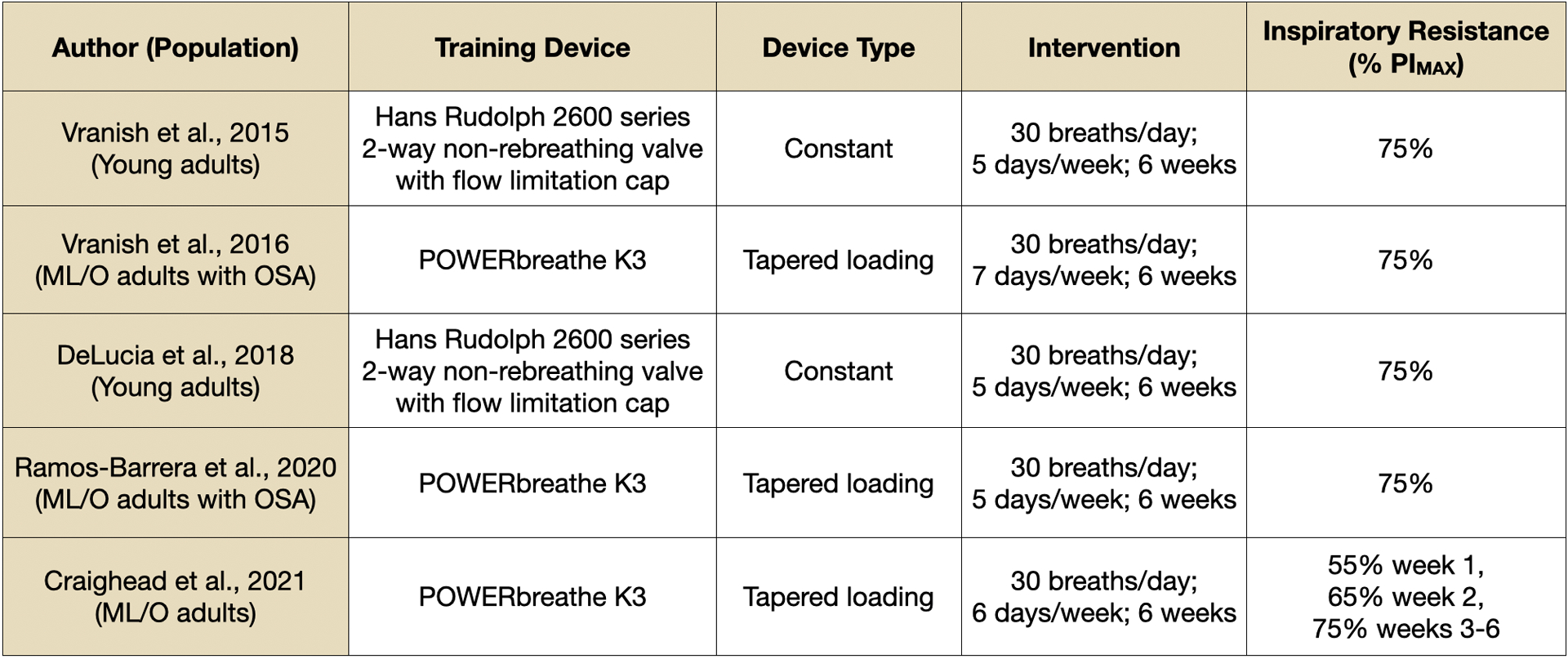
Training device description and intervention designs of published high-resistance inspiratory muscle strength training studies. Device type indicates whether the same absolute level of resistance is applied across an entire inspiration (constant) or whether absolute resistance declines during inspiration with decreasing inspiratory pressure production (tapered loading). Inspiratory resistance describes the protocol training intensity as a relative percentage of subjects’ maximal inspiratory pressure production. ML/O = midlife/older. OSA = obstructive sleep apnea. PIMAX = maximal inspiratory pressure.
Here, we will summarize what is currently known about the efficacy of high-resistance IMST for improving CV function with aging. While the results from intervention studies using high-resistance IMST will be the focus, findings from select studies investigating the CV effects of low-resistance IMST interventions or following acute bouts of IMST will be discussed where appropriate.
3.3. High-resistance IMST for lowering blood pressure
Bailey and colleagues were the first to investigate the effects of a high-resistance IMST intervention on casual BP. In the first study of its kind, Vranish and Bailey established that 6 weeks of high-resistance IMST, consisting of 30 breaths/day at 75% PIMAX performed 5 days per week, lowered casual SBP by 10 mmHg and casual DBP by 6 mmHg in a cohort of young healthy adults with normal baseline BP (Vranish and Bailey, 2015). This novel study also included a group training with large excursions in lung volume but against minimal resistance, and a group training at a high resistance but with a negligible change in lung volume (i.e., isometric inspiratory contraction). This innovative design established the necessity of training against a high resistance per se, irrespective of changes in lung volume, as equivalent reductions in BP were observed in the group performing isometric inspiratory contractions, but no changes were seen in the group inhaling against minimal resistance (Vranish and Bailey, 2015).
Next, using a similar 6-week high-resistance IMST protocol, Bailey and colleagues observed reductions in casual SBP of 12 mmHg and casual DBP by 5 mmHg in a group of midlife/older adults with obstructive sleep apnea (Vranish and Bailey, 2016), demonstrating potential efficacy of this intervention for lowering BP with aging in patients with this disorder. These findings have since been replicated in additional groups of young adults (DeLucia et al., 2018) and midlife/older adults with obstructive sleep apnea (Ramos-Barrera et al., 2020).
Our laboratory recently adopted the protocol developed by Bailey et al. to investigate whether the BP-lowering effects of high-resistance IMST could be conferred to otherwise healthy midlife/older adults with above-normal initial SBP, i.e., ≥120 mmHg at baseline (Craighead et al., 2021). Our study was a double-blind, sham-controlled parallel design pilot study of 36 participants randomized to 6 weeks of high-resistance IMST, consisting of 30 breaths per day at 75% PIMAX, performed 6 days per week, or low-resistance sham training at 15% PIMAX at the same frequency of breaths and duration of treatment. At the end of the intervention, casual SBP and DBP were reduced by 9 mmHg and 2 mmHg, respectively, in the group performing high-resistance IMST, with no change in the sham control group (Craighead et al., 2021).
On average, the BP reductions observed in the 5 high-resistance IMST studies performed across Dr. Bailey’s laboratory and our own are clinically meaningful. Reductions in SBP of 10 mmHg and DBP of 5 mmHg, approximately the average changes observed in these studies (Figure 3), are associated with a 30–40% lower risk of death from CVD (Lewington et al., 2002). Further supporting the clinical significance are the moderate to large effect sizes of high-resistance IMST on SBP across these 5 interventions (Figure 3). Thus, high-resistance IMST may decrease CVD risk through lowering BP. Of note, reductions in BP of this magnitude are equal to, or greater than, those induced by other common healthy lifestyle interventions, such as aerobic exercise (Cornelissen and Smart, 2013; Whelton et al., 2002), weight loss (Neter et al., 2003), eating a healthy DASH-style diet (Appel et al., 1997) and dietary sodium restriction (Aburto et al., 2013; He et al., 2013).
Figure 3.
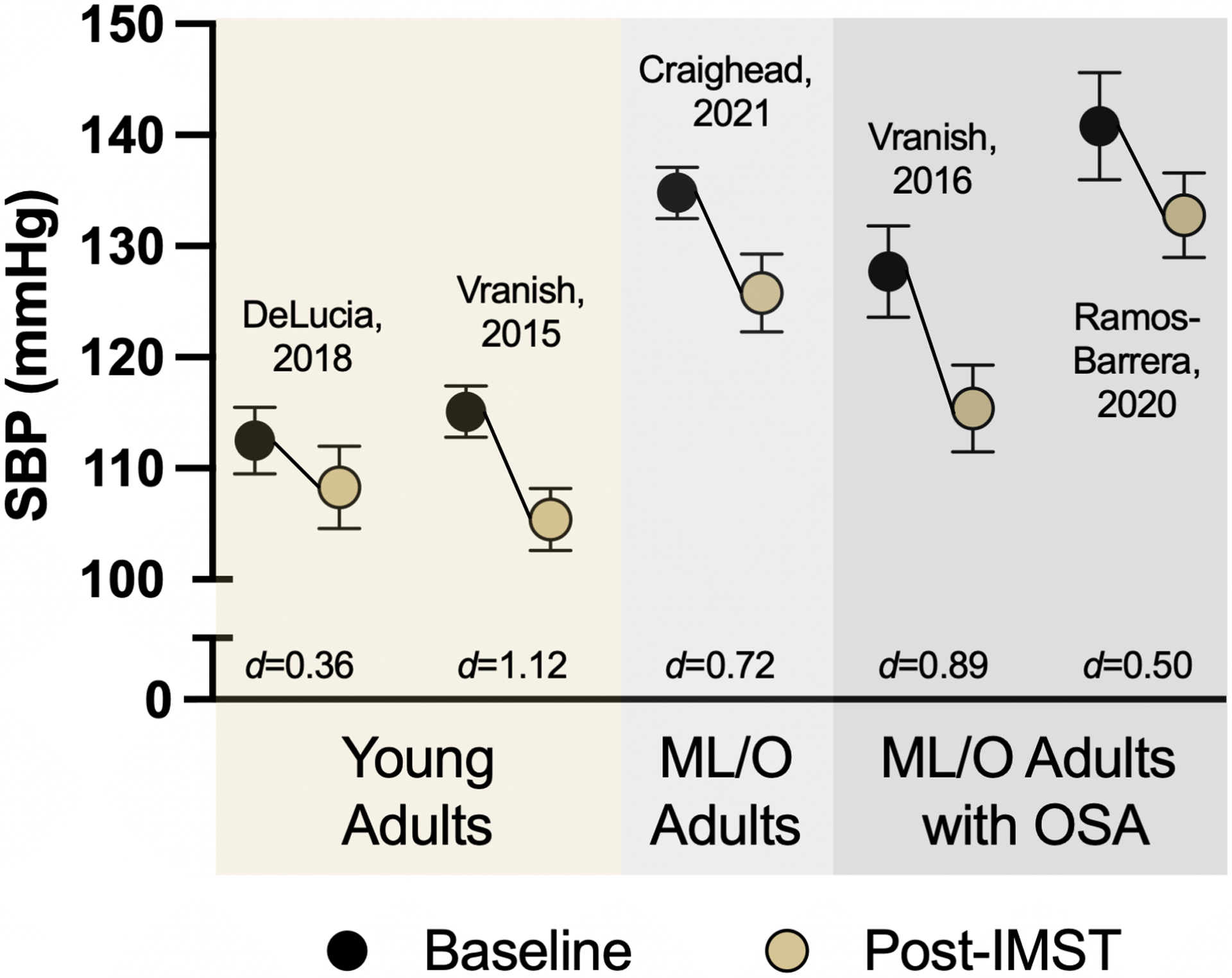
Current evidence of high-resistance inspiratory muscle strength training (IMST) for reducing casual (resting) systolic blood pressure (SBP) in young adults (age 18–30 years), midlife/older (ML/O) adults (age 50–79 years) with above-normal SBP but without concomitant disease, and ML/O adults with obstructive sleep apnea (OSA). Black circles indicate SBP at baseline; gold circles indicate SBP after 6 weeks of high-resistance IMST performed at 75% maximal inspiratory pressure. d indicates Cohen’s d effect size where d>0.2 indicates a small effect, d>0.5 indicates a medium effect and d>0.8 indicates a large effect (Cohen, 1988). Data are Mean ± SEM.
Our IMST trial was the first study on high-resistance IMST to include a follow-up period. In our study, BP was reassessed after 6 weeks of cessation from IMST. We observed that roughly 75% of the reductions in casual SBP observed at the end of IMST were maintained following this 6-week layoff, indicating high-resistance IMST has potentially long-lasting effects on SBP (Craighead et al., 2021). This contrasts with what is usually observed following aerobic exercise training, where BP typically returns to pre-intervention levels soon after the cessation of regular exercise (Mora-Rodriguez et al., 2014; Nolan et al., 2018). This may be because much of the reduction in BP associated with aerobic exercise is due to post-exercise hypotension (Halliwill, 2001), necessitating that aerobic exercise be performed regularly to maintain reductions in BP. In comparison, high-resistance IMST does not induce hypotension after a single bout (Ferreira et al., 2021). In fact, it was recently shown that BP remains slightly elevated for a short period of time in young adults following a single session of high-resistance IMST (DeLucia et al., 2021). These differing acute effects on BP between aerobic exercise and IMST suggest potentially different mechanisms mediating BP reductions in these two interventions.
Two of the above studies assessed high-resistance IMST for improving 24-hour BP. Bailey and colleagues found no changes in 24-hour SBP or DBP, but did observe a 12 mmHg reduction in nighttime SBP following 6 weeks of high-resistance IMST in midlife/older adults with obstructive sleep apnea (Ramos-Barrera et al., 2020). That a circadian-dependent effect was observed in this patient group with nighttime sleep disturbances is not unexpected. We observed a 4 mmHg reduction in 24-hour SBP, such that 24-hour SBP was significantly lower compared to the sham control group at the end of the intervention, with no change in 24-hour DBP (Craighead et al., 2021).
Overall, findings from this initial set of small trials suggest that high-resistance IMST is a time-efficient intervention for lowering casual SBP (Figure 3) and DBP in adults, including midlife/older adults who are generally healthy and those with obstructive sleep disorders. High-resistance IMST may also be effective for improving 24-hour SBP and inducing long-lasting reductions in SBP. Importantly, the changes in BP with high-resistance IMST are similar to those observed following time-intensive aerobic exercise (Whelton et al., 2002) and low-resistance IMST (Ferreira et al., 2013; Jones et al., 2010; Sangthong et al., 2016), but accomplished with a daily time commitment of only approximately 5 minutes.
3.4. High-resistance IMST for improving arterial function
Presently there is very limited information regarding the potential of high-resistance IMST for improving arterial function. Our recent study was the first to assess the efficacy of high-resistance IMST for improving vascular endothelial function in healthy midlife/older adults. We observed an approximately 45% increase in FMDBA following 6 weeks of high-resistance IMST, with no change in the sham control group (Craighead et al., 2021). When expressed as a percent change in brachial artery diameter, we observed a 2.5 Δ% increase in FMDBA units following IMST (Craighead et al., 2021). This improvement is potentially clinically meaningful as a 1 Δ% increase in FMDBA units is associated with an 8–13% lower risk of CVD (Green et al., 2011; Inaba et al., 2010; Matsuzawa et al., 2015; Ras et al., 2013; Xu et al., 2014). Importantly, we observed improvements in FMDBA in both the men and estrogen-deficient postmenopausal women enrolled in our study (Craighead et al., 2021). As such, our findings indicate that high-resistance IMST may be a promising intervention for improving vascular endothelial function in both estrogen-deficient postmenopausal women, a group in which regular aerobic exercise training does not consistently improve endothelial function (Seals et al., 2019), and midlife/older men.
A study using isocapnic hyperventilation, a different form of respiratory muscle training, assessed changes in endothelial function via FMDBA in a group of young, healthy women. No change in FMDBA was observed in these women after 8 weeks of training 3 days per week, 15–30 minutes per day (Bisconti et al., 2018). However, FMDBA in these women was very high (>10 Δ%) at baseline and therefore unlikely to improve. Lastly, a study in patients with heart failure assessed relatively high-resistance IMST, consisting of training at 60% PIMAX until exhaustion, 3 days per week, for 10 weeks, on reactive hyperemia, an indirect, non-specific measurement of endothelial function measured with venous occlusion plethysmography, and found no effect (Laoutaris et al., 2008).
The heterogeneity of IMST protocols employed, and the populations examined, make it difficult to draw conclusions on the promise of high-resistance IMST for improving vascular endothelial function at this point. However, our pilot study in midlife/older adults with initial above-normal BP demonstrated substantial increases in FMDBA, the gold-standard measure of in vivo conduit artery endothelial function in humans (Kobayashi et al., 2004), following 6 weeks of high-resistance IMST, strongly suggesting the potential for efficacy in this group.
The potential efficacy of high-resistance IMST on arterial stiffness has only been assessed in 2 studies to date. In our recent trial, we found no change in large elastic artery stiffness, measured as either CFPWV or carotid artery compliance, after 6 weeks of IMST. A very different hyperpnea-based form of respiratory training found no change in CFPWV after 4 weeks of training in young adults (Beltrami et al., 2020). Importantly, both of these interventions were short in duration. As lifestyle interventions generally take at least 3 months to reduce arterial stiffness (Pierce, 2017), longer intervention durations may be needed to better elucidate the effects of high-resistance IMST on this important expression of vascular function.
3.5. Mechanisms of action
There is still much to learn about the mechanisms through which high-resistance IMST improves CV function in midlife/older adults. However, early results indicate high-resistance IMST may reduce oxidative stress and inflammation, two primary mechanisms behind CV aging. High-resistance IMST may also act on the sympathetic nervous system in select subgroups.
Oxidative Stress.
Our laboratory has utilized an innovative ex vivo cell culture model whereby human umbilical vein endothelial cells (HUVECs) are exposed to serum collected from subjects pre- and post-IMST or sham control training to assess the effects of factors circulating in the serum on endothelial cell function. In our midlife/older cohort, serum collected from subjects who performed 6 weeks of high-resistance IMST induced greater acetylcholine-induced production of NO in HUVECs compared to serum sampled at baseline or before or after sham-training (Craighead et al., 2021). This increase in NO bioavailability was associated with increased activity of the NO-producing enzyme, eNOS, as well as decreased basal ROS production from HUVECs treated with serum sampled post-IMST versus baseline (Craighead et al., 2021). These results suggest that the improvement in NO-mediated endothelial function (FMDBA) observed following high-resistance IMST is likely mediated by a reduction in NO-scavenging ROS and increased NO production from eNOS, leading to an overall increase in NO bioavailability.
Other studies have investigated the potential for low- and moderate-resistance IMST to induce changes in circulating markers of oxidative stress and endothelial function. In patients on hemodialysis, 8 weeks of low-resistance IMST (≤20 cmH2O) performed 3 times per week did not change plasma concentrations of malondialdehyde (Campos et al., 2018), a marker of oxidative stress. However, plasma concentrations of the endothelium-derived vasoconstrictor, endothelin-1, were reduced in these patients (Campos et al., 2018), which may have reflected improved endothelial function. In a study in healthy older adults who performed moderate-resistance IMST, consisting of 2, 30-breath sessions at 50% PIMAX per day for 8 weeks, oxidative stress was measured as the change in DNA damage to peripheral blood mononuclear cells (Mills et al., 2015). This marker of oxidative stress did not change following IMST in these healthy older adults (Mills et al., 2015); however, peripheral blood mononuclear cell oxidative stress may not be directly indicative of vascular endothelial oxidative stress per se. High-resistance IMST may decrease endothelial oxidative stress, but given the differences in IMST protocols, study populations and oxidative stress markers, the role of oxidative stress in mediating the CV benefits of high-resistance IMST remains to be confirmed by additional investigation.
Inflammation.
In our high-resistance IMST intervention, plasma concentrations of CRP, an important marker of systemic inflammation, decreased in the IMST group but remained unchanged in the sham control group (Craighead et al., 2021). This suggests high-resistance IMST may improve CV function by reducing systemic inflammation. Additionally, plasma tumor necrosis factor receptor 2 and CRP were reduced in patients on hemodialysis following 8 to 10 weeks of IMST at 50% PIMAX, further suggesting IMST may decrease systemic inflammation (Figueiredo et al., 2018; Pellizzaro et al., 2013). However, plasma interleukin-6 and tumor necrosis factor α, two other circulating markers of inflammation, remained unchanged following 6 weeks of IMST in our study (Craighead et al., 2021). Additionally, plasma concentrations of inflammatory cytokines did not change following 8 weeks of IMST consisting of 2, 30-breath sessions at 50% PIMAX per day in healthy older adults (Mills et al., 2015). Plasma inflammatory cytokines were also unchanged following an acute bout of high-resistance IMST in patients with obstructive sleep apnea (Ferreira et al., 2021). As with oxidative stress, the diverse IMST protocols and populations examined make it difficult to draw conclusions on the efficacy of high-resistance IMST for decreasing systemic inflammation with aging. The above studies suggest that suppression of chronic low-grade inflammation may contribute to improved CV function with certain IMST paradigms or subject groups. However, because the overall effects of IMST on circulating markers of inflammation has been inconsistent, this question requires further investigation.
Sympathetic Nervous System Activity.
There are inconsistent reports that high-resistance IMST may modulate the activity of the sympathetic nervous system. We observed no change in plasma concentrations of epinephrine and norepinephrine, markers of sympathoadrenal activity, after 6 weeks of high-resistance IMST in midlife/older adults with above-normal SBP (Craighead et al., 2021). However, Bailey and colleagues have observed reductions in plasma catecholamines and MSNA in midlife/older adults with obstructive sleep apnea after high-resistance IMST intervention (Ramos-Barrera et al., 2020; Vranish and Bailey, 2016). Of note, obstructive sleep apnea is independently associated with elevated sympathetic activity (Narkiewicz and Somers, 2003). Accordingly, the baseline plasma norepinephrine concentrations in the patients with obstructive sleep apnea enrolled in the studies by Bailey and colleagues were approximate 2-fold greater than those observed in our laboratory’s cohort of generally healthy midlife/older adults.
In young men and women, high-resistance IMST acutely decreases MSNA, with the magnitude and duration of the reduction in sympathetic activity greater in young women than young men (DeLucia et al., 2021). However, whether these sex differences are apparent following chronic high-resistance IMST in midlife/older adults is unknown. Collectively, these data suggest chronic high-resistance IMST may influence sympathetic activity only in select groups, such as patients with conditions associated with elevated sympathetic activity.
4. Research gaps and future directions
The results of the human trials summarized above suggest high-resistance IMST is a promising lifestyle intervention for improving CV function, particularly BP and endothelial function, while promoting adherence to the intervention in midlife/older adults. However, many research gaps remain to further translate high-resistance IMST as an established intervention for improving CV health (Figure 4).
Figure 4.
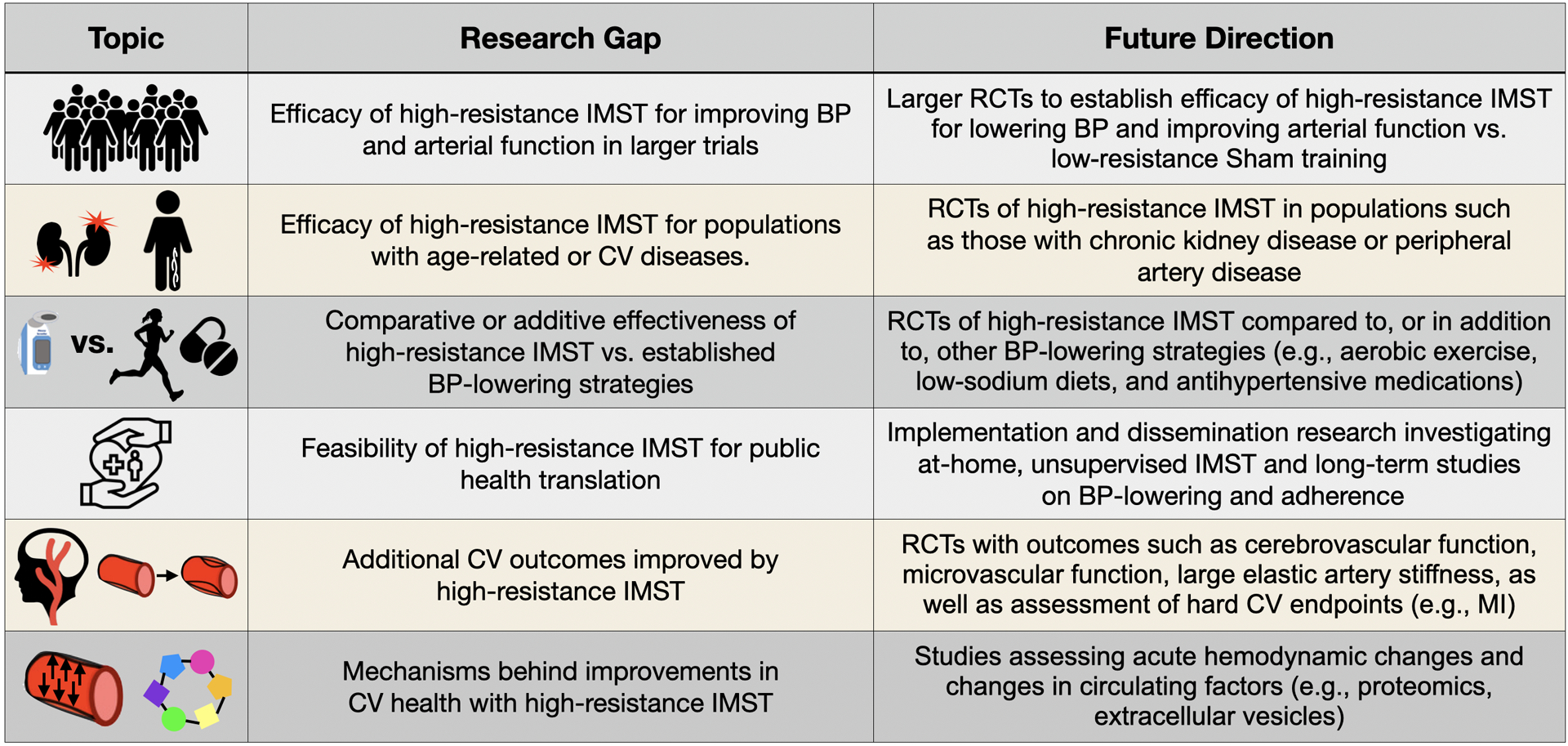
Current gaps in knowledge and future research directions for investigating the potential cardiovascular benefits of high-resistance inspiratory muscle strength training (IMST). BP = blood pressure. RCT = randomized controlled trial. CV = cardiovascular. MI = myocardial infarction.
Current trials are limited to small, laboratory-based, short-term study designs. The next step is to establish the efficacy of longer durations of high-resistance IMST for improving BP and arterial function in larger clinical trials. Such trials could examine different treatment protocols, such as once versus twice per day training, and extended intervention periods (e.g., 3–12 months), to determine the optimal “dose” of high-resistance IMST for evoking favorable CV adaptations. In addition, these trials should include serial examination of the long-lasting effects of IMST, such as measuring BP every 2 weeks after cessation of the intervention for a 2 to 3-month period. The latter type of investigation will provide important insight into the clinically relevant question of whether IMST needs to be performed chronically or only intermittently to produce healthy CV benefits.
Future trials should also seek to establish the efficacy of high-resistance IMST in other at-risk populations. CV dysfunction underlies many other age-related diseases, such as chronic kidney disease and Alzheimer’s disease and related dementias (Hamrahian and Falkner, 2017; SPRINT MIND Investigators for the SPRINT Research Group et al., 2019; Stakos et al., 2020); thus, future trials should determine whether IMST can improve outcomes in these and other patient groups with age-related chronic disorders. For example, high-resistance IMST also may benefit groups with mobility limitations, such as overweight or obese adults or patients with peripheral artery disease. Moreover, emerging evidence suggests inflammation and oxidative stress associated with COVID-19 may induce prolonged CV dysfunction (Chung et al., 2021; Magadum and Kishore, 2020).
After establishing the efficacy of high-resistance IMST, the next step would be to demonstrate relative effectiveness compared to established treatments, such as pharmacotherapy, aerobic exercise, weight loss and dietary sodium restriction. Given it is time-efficient and has limited side effects, high-resistance IMST also should be investigated as an additive therapy to established interventions. Then, if the relative or additive effectiveness of high-resistance IMST is established, the following step would be to initiate implementation and dissemination research to determine the translational potential of high-resistance IMST for use in public health settings. Such research should utilize intervention study designs without researcher instruction or supervision in order to establish real-world adherence and effectiveness. Additionally, studies pairing high-resistance IMST with novel vehicles for dissemination, such as gerotechnology or mHealth approaches, will be key for determining the best way to disseminate IMST for widespread use in the community.
Although early data strongly suggest that high-resistance IMST can lower casual BP, and data regarding the efficacy for improving vascular endothelial function is promising, more research on these and other measures of CV function and health are needed to fully understand the potential CV benefits of high-resistance IMST. We did not observe changes in arterial stiffness, an important marker of arterial function and independent CVD risk factor, in our 6-week pilot study. However, as noted above, arterial stiffness often takes at least 3 months to change with lifestyle therapies; therefore, arterial stiffness should be assessed in longer duration studies of high-resistance IMST. Cerebrovascular dysfunction can contribute to cognitive decline, vascular dementia and Alzheimer’s disease (Cortes-Canteli and Iadecola, 2020; Walker et al., 2017; Yang et al., 2017). Whether the vascular benefits of high-resistance IMST extend to the cerebrovasculature is currently unknown but should be examined.
A greater understanding of the mechanisms by which high-resistance IMST improves CV function may provide additional insight as to potential diseases and disorders that could be effectively treated with high-resistance IMST. Further investigation of the acute hemodynamic changes with high-resistance IMST would provide insight on the potential stimulus for improvements in CV function. As described above, information regarding the effects of high-resistance IMST on oxidative stress, inflammation and sympathetic nervous system activity are mixed. Longer, well-controlled clinical trials with enough participants for meaningful subgroup analyses should be performed to more thoroughly examine these and other putative mechanisms of IMST in the setting of CV aging. Data from our pilot clinical trial suggest changes in factors circulating in the blood are in part responsible for improvements in CV function. At this point, the identities of these altered circulating factors remain relatively unknown. Finally, aerobic exercise is known to positively influence multiple hallmarks of aging (Gioscia-Ryan et al., 2016; LaRocca et al., 2010; López-Otín et al., 2013; Rossman et al., 2017); however, it is unknown if high-resistance IMST, an alternative form of physical training, can influence these fundamental aging processes.
5. Conclusions
The number of midlife/older adults is increasing, predicting an increase in CVD morbidity and mortality without appropriate intervention. At the same time, adherence to guidelines for aerobic exercise, an effective healthy lifestyle intervention for decreasing CVD risk, remains poor among several subgroups of the midlife/older adult population. Therefore, novel lifestyle interventions that are efficacious for improving CV health, while also promoting high rates of adherence, are needed. Time-efficient, high-resistance IMST is a novel intervention that may lower BP and promote adherence, while also holding promise for improving other components of CV health, particularly vascular endothelial function. Early results on high-resistance IMST are promising, supporting continued research with the goal of translating high-resistance IMST for improving public health.
Funding
This work was supported by NIH R01AG071506. DHC is supported by NIH K01HL153326, KAF is supported by NIH F31HL154782
Footnotes
Declarations of interest
None
References
- Aburto NJ, Ziolkovska A, Hooper L, Elliott P, Cappuccio FP, Meerpohl JJ, 2013. Effect of lower sodium intake on health: systematic review and meta-analyses. BMJ 346, f1326. 10.1136/bmj.f1326 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Allen K, Morey MC, 2010. Physical Activity and Adherence, in: Bosworth H (Ed.), Improving Patient Treatment Adherence. Springer; New York, New York, NY, pp. 9–38. 10.1007/978-1-4419-5866-2_2 [DOI] [Google Scholar]
- Appel LJ, Moore TJ, Obarzanek E, Vollmer WM, Svetkey LP, Sacks FM, Bray GA, Vogt TM, Cutler JA, Windhauser MM, Lin PH, Karanja N, 1997. A clinical trial of the effects of dietary patterns on blood pressure. DASH Collaborative Research Group. N. Engl. J. Med 336, 1117–1124. 10.1056/NEJM199704173361601 [DOI] [PubMed] [Google Scholar]
- Babakus WS, Thompson JL, 2012. Physical activity among South Asian women: a systematic, mixed-methods review. Int. J. Behav. Nutr. Phys. Act 9, 150. 10.1186/1479-5868-9-150 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beaumont M, Forget P, Couturaud F, Reychler G, 2018. Effects of inspiratory muscle training in COPD patients: A systematic review and meta-analysis. Clin. Respir. J 12, 2178–2188. 10.1111/crj.12905 [DOI] [PubMed] [Google Scholar]
- Beltrami FG, Mzee D, Spengler CM, 2020. No Evidence That Hyperpnea-Based Respiratory Muscle Training Affects Indexes of Cardiovascular Health in Young Healthy Adults. Front. Physiol 11, 530218. 10.3389/fphys.2020.530218 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bisconti AV, Devoto M, Venturelli M, Bryner R, Olfert IM, Chantler PD, Esposito F, 2018. Respiratory muscle training positively affects vasomotor response in young healthy women. PloS One 13, e0203347. 10.1371/journal.pone.0203347 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Callegaro CC, Ribeiro JP, Tan CO, Taylor JA, 2011. Attenuated inspiratory muscle metaboreflex in endurance-trained individuals. Respir. Physiol. Neurobiol 177, 24–29. 10.1016/j.resp.2011.03.001 [DOI] [PubMed] [Google Scholar]
- Campos NG, Marizeiro DF, Florêncio ACL, Silva ÍC, Meneses GC, Bezerra GF, Martins AMC, Libório AB, 2018. Effects of respiratory muscle training on endothelium and oxidative stress biomarkers in hemodialysis patients: A randomized clinical trial. Respir. Med 134, 103–109. 10.1016/j.rmed.2017.12.005 [DOI] [PubMed] [Google Scholar]
- Chang Y-C, Chang H-Y, Ho C-C, Lee P-F, Chou Y-C, Tsai M-W, Chou L-W, 2021. Effects of 4-Week Inspiratory Muscle Training on Sport Performance in College 800-Meter Track Runners. Med. Kaunas Lith 57. 10.3390/medicina57010072 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chiappa GR, Roseguini BT, Vieira PJC, Alves CN, Tavares A, Winkelmann ER, Ferlin EL, Stein R, Ribeiro JP, 2008. Inspiratory muscle training improves blood flow to resting and exercising limbs in patients with chronic heart failure. J. Am. Coll. Cardiol 51, 1663–1671. 10.1016/j.jacc.2007.12.045 [DOI] [PubMed] [Google Scholar]
- Chung MK, Zidar DA, Bristow MR, Cameron SJ, Chan T, Harding CV, Kwon DH, Singh T, Tilton JC, Tsai EJ, Tucker NR, Barnard J, Loscalzo J, 2021. COVID-19 and Cardiovascular Disease: From Bench to Bedside. Circ. Res 128, 1214–1236. 10.1161/CIRCRESAHA.121.317997 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clement DL, De Buyzere ML, De Bacquer DA, de Leeuw PW, Duprez DA, Fagard RH, Gheeraert PJ, Missault LH, Braun JJ, Six RO, Van Der Niepen P, O’Brien E, Office versus Ambulatory Pressure Study Investigators, 2003. Prognostic value of ambulatory blood-pressure recordings in patients with treated hypertension. N. Engl. J. Med 348, 2407–2415. 10.1056/NEJMoa022273 [DOI] [PubMed] [Google Scholar]
- Cohen J, 1988. Statistical power analysis for the behavioral sciences, 2nd ed. Erlbaum, Hillsdale NJ. [Google Scholar]
- Connelly MT, Richardson M, Platt R, 2000. Prevalence and duration of postmenopausal hormone replacement therapy use in a managed care organization, 1990–1995. J. Gen. Intern. Med 15, 542–550. 10.1046/j.1525-1497.2000.03499.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cornelissen VA, Smart NA, 2013. Exercise training for blood pressure: a systematic review and meta-analysis. J. Am. Heart Assoc 2, e004473. 10.1161/JAHA.112.004473 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cortes-Canteli M, Iadecola C, 2020. Alzheimer’s Disease and Vascular Aging: JACC Focus Seminar. J. Am. Coll. Cardiol 75, 942–951. 10.1016/j.jacc.2019.10.062 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Craighead DH, Freeberg KA, Seals DR, 2020. Vascular Endothelial Function in Midlife/Older Adults Classified According to 2017 American College of Cardiology/American Heart Association Blood Pressure Guidelines. J. Am. Heart Assoc 9, e016625. 10.1161/JAHA.120.016625 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Craighead Daniel H, Freeberg KA, Seals DR, 2019. The protective role of regular aerobic exercise on vascular function with aging. Curr. Opin. Physiol 10, 55–63. 10.1016/j.cophys.2019.04.005 [DOI] [Google Scholar]
- Craighead DH, Heinbockel TC, Freeberg KA, Rossmas MJ, Jackman RA, Jankowski LR, Hamilton MN, Ziemba BP, Reisz JA, D’Alessandro A, Brewster LM, DeSouza CA, You Z, Chonchol M, Bailey EF, Seals DR, 2021. Time-efficient inspiratory muscle strength training lowers blood pressure and improves endothelial function, NO bioavailablity and oxidative stress in midlife/older adults with above-normal blood pressure. J. Am. Heart Assoc 10, e020980. 10.1161/JAHA.121.020980 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Craighead Daniel H., Heinbockel TC, Hamilton MN, Bailey EF, MacDonald MJ, Gibala MJ, Seals DR, 2019. Time-efficient physical training for enhancing cardiovascular function in midlife and older adults: promise and current research gaps. J. Appl. Physiol. Bethesda Md 1985 127, 1427–1440. 10.1152/japplphysiol.00381.2019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cutrim ALC, Duarte AAM, Silva-Filho AC, Dias CJ, Urtado CB, Ribeiro RM, Rigatto K, Rodrigues B, Dibai-Filho AV, Mostarda CT, 2019. Inspiratory muscle training improves autonomic modulation and exercise tolerance in chronic obstructive pulmonary disease subjects: A randomized-controlled trial. Respir. Physiol. Neurobiol 263, 31–37. 10.1016/j.resp.2019.03.003 [DOI] [PubMed] [Google Scholar]
- de Abreu RM, Rehder-Santos P, Minatel V, Dos Santos GL, Catai AM, 2017. Effects of inspiratory muscle training on cardiovascular autonomic control: A systematic review. Auton. Neurosci. Basic Clin 208, 29–35. 10.1016/j.autneu.2017.09.002 [DOI] [PubMed] [Google Scholar]
- de Sousa MM, Pimentel MDS, Sobreira I. de A., Barros R. de J., Borghi-Silva A, Mazzoli-Rocha F, 2020. Inspiratory Muscle Training Improves Aerobic Capacity in Amateur Indoor Football Players. Int. J. Sports Med 10.1055/a-1255-3256 [DOI] [PubMed] [Google Scholar]
- DeLucia CM, De Asis RM, Bailey EF, 2018. Daily inspiratory muscle training lowers blood pressure and vascular resistance in healthy men and women. Exp. Physiol 103, 201–211. 10.1113/EP086641 [DOI] [PubMed] [Google Scholar]
- DeLucia CM, DeBonis DR, Schwyhart SM, Bailey EF, 2021. Acute cardiovascular responses to a single bout of high intensity inspiratory muscle strength training in healthy young adults. J. Appl. Physiol. Bethesda Md 1985. 10.1152/japplphysiol.01015.2020 [DOI] [PubMed] [Google Scholar]
- DeSouza CA, Shapiro LF, Clevenger CM, Dinenno FA, Monahan KD, Tanaka H, Seals DR, 2000. Regular aerobic exercise prevents and restores age-related declines in endothelium-dependent vasodilation in healthy men. Circulation 102, 1351–1357. 10.1161/01.cir.102.12.1351 [DOI] [PubMed] [Google Scholar]
- Dolan E, Stanton A, Thijs L, Hinedi K, Atkins N, McClory S, Den Hond E, McCormack P, Staessen JA, O’Brien E, 2005. Superiority of ambulatory over clinic blood pressure measurement in predicting mortality: the Dublin outcome study. Hypertens. Dallas Tex 1979 46, 156–161. 10.1161/01.HYP.0000170138.56903.7a [DOI] [PubMed] [Google Scholar]
- Donato AJ, Black AD, Jablonski KL, Gano LB, Seals DR, 2008. Aging is Associated with Greater Nuclear NFκB, Reduced IκBα and Increased Expression of Proinflammatory Cytokines in Vascular Endothelial Cells of Healthy Humans. Aging Cell 7, 805–812. 10.1111/j.1474-9726.2008.00438.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Donato AJ, Eskurza I, Silver AE, Levy AS, Pierce GL, Gates PE, Seals DR, 2007. Direct evidence of endothelial oxidative stress with aging in humans: relation to impaired endothelium-dependent dilation and upregulation of nuclear factor-kappaB. Circ. Res 100, 1659–1666. 10.1161/01.RES.0000269183.13937.e8 [DOI] [PubMed] [Google Scholar]
- El Ansari W, Lovell G, 2009. Barriers to exercise in younger and older non-exercising adult women: a cross sectional study in London, United Kingdom. Int. J. Environ. Res. Public. Health 6, 1443–1455. 10.3390/ijerph6041443 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elkins M, Dentice R, 2015. Inspiratory muscle training facilitates weaning from mechanical ventilation among patients in the intensive care unit: a systematic review. J. Physiother 61, 125–134. 10.1016/j.jphys.2015.05.016 [DOI] [PubMed] [Google Scholar]
- Eskurza I, Monahan KD, Robinson JA, Seals DR, 2004. Effect of acute and chronic ascorbic acid on flow-mediated dilatation with sedentary and physically active human ageing. J. Physiol 556, 315–324. 10.1113/jphysiol.2003.057042 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferreira JB, Plentz RDM, Stein C, Casali KR, Arena R, Lago PD, 2013. Inspiratory muscle training reduces blood pressure and sympathetic activity in hypertensive patients: a randomized controlled trial. Int. J. Cardiol 166, 61–67. 10.1016/j.ijcard.2011.09.069 [DOI] [PubMed] [Google Scholar]
- Ferreira STBP, do Socorro Brasileiro-Santos M, Teixeira JB, da Silva Rabello MC, de Lorena VMB, Farah BQ, Silva TNS, de Lima AMJ, 2021. Clinical safety and hemodynamic, cardiac autonomic and inflammatory responses to a single session of inspiratory muscle training in obstructive sleep apnea. Sleep Breath. Schlaf Atm 10.1007/s11325-021-02364-6 [DOI] [PubMed] [Google Scholar]
- Figueiredo PHS, Lima MMO, Costa HS, Martins JB, Flecha OD, Gonçalves PF, Alves FL, Rodrigues VGB, Maciel EHB, Mendonça VA, Lacerda ACR, Vieira ÉLM, Teixeira AL, de Paula F, Balthazar CH, 2018. Effects of the inspiratory muscle training and aerobic training on respiratory and functional parameters, inflammatory biomarkers, redox status and quality of life in hemodialysis patients: A randomized clinical trial. PloS One 13, e0200727. 10.1371/journal.pone.0200727 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fleenor BS, Marshall KD, Durrant JR, Lesniewski LA, Seals DR, 2010. Arterial stiffening with ageing is associated with transforming growth factor-β1-related changes in adventitial collagen: reversal by aerobic exercise. J. Physiol 588, 3971–3982. 10.1113/jphysiol.2010.194753 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Förstermann U, Sessa WC, 2012. Nitric oxide synthases: regulation and function. Eur. Heart J 33, 829–837, 837a–837d. 10.1093/eurheartj/ehr304 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gano LB, Donato AJ, Pierce GL, Pasha HM, Magerko KA, Roeca C, Seals DR, 2011. Increased proinflammatory and oxidant gene expression in circulating mononuclear cells in older adults: amelioration by habitual exercise. Physiol. Genomics 43, 895–902. 10.1152/physiolgenomics.00204.2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gioscia-Ryan RA, Battson ML, Cuevas LM, Zigler MC, Sindler AL, Seals DR, 2016. Voluntary aerobic exercise increases arterial resilience and mitochondrial health with aging in mice. Aging 8, 2897–2914. 10.18632/aging.101099 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gioscia-Ryan RA, Clayton ZS, Zigler MC, Richey JJ, Cuevas LM, Rossman MJ, Battson ML, Ziemba BP, Hutton DA, VanDongen NS, Seals DR, 2021. Lifelong voluntary aerobic exercise prevents age- and Western diet- induced vascular dysfunction, mitochondrial oxidative stress and inflammation in mice. J. Physiol 599, 911–925. 10.1113/JP280607 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Green DJ, Dawson EA, Groenewoud HMM, Jones H, Thijssen DHJ, 2014. Is flow-mediated dilation nitric oxide mediated?: A meta-analysis. Hypertens. Dallas Tex 1979 63, 376–382. 10.1161/HYPERTENSIONAHA.113.02044 [DOI] [PubMed] [Google Scholar]
- Green DJ, Jones H, Thijssen D, Cable NT, Atkinson G, 2011. Flow-mediated dilation and cardiovascular event prediction: does nitric oxide matter? Hypertens. Dallas Tex 1979 57, 363–369. 10.1161/HYPERTENSIONAHA.110.167015 [DOI] [PubMed] [Google Scholar]
- Guimarães GV, Ciolac EG, Carvalho VO, D’Avila VM, Bortolotto LA, Bocchi EA, 2010. Effects of continuous vs. interval exercise training on blood pressure and arterial stiffness in treated hypertension. Hypertens. Res. Off. J. Jpn. Soc. Hypertens 33, 627–632. 10.1038/hr.2010.42 [DOI] [PubMed] [Google Scholar]
- Halliwill JR, 2001. Mechanisms and clinical implications of post-exercise hypotension in humans. Exerc. Sport Sci. Rev 29, 65–70. 10.1097/00003677-200104000-00005 [DOI] [PubMed] [Google Scholar]
- Hamrahian SM, Falkner B, 2017. Hypertension in Chronic Kidney Disease. Adv. Exp. Med. Biol 956, 307–325. 10.1007/5584_2016_84 [DOI] [PubMed] [Google Scholar]
- He FJ, Li J, Macgregor GA, 2013. Effect of longer term modest salt reduction on blood pressure: Cochrane systematic review and meta-analysis of randomised trials. BMJ 346, f1325. 10.1136/bmj.f1325 [DOI] [PubMed] [Google Scholar]
- Heidenreich PA, Trogdon JG, Khavjou OA, Butler J, Dracup K, Ezekowitz MD, Finkelstein EA, Hong Y, Johnston SC, Khera A, Lloyd-Jones DM, Nelson SA, Nichol G, Orenstein D, Wilson PWF, Woo YJ, American Heart Association Advocacy Coordinating Committee, Stroke Council, Council on Cardiovascular Radiology and Intervention, Council on Clinical Cardiology, Council on Epidemiology and Prevention, Council on Arteriosclerosis, Thrombosis and Vascular Biology, Council on Cardiopulmonary, Critical Care, Perioperative and Resuscitation, Council on Cardiovascular Nursing, Council on the Kidney in Cardiovascular Disease, Council on Cardiovascular Surgery and Anesthesia, and Interdisciplinary Council on Quality of Care and Outcomes Research, 2011. Forecasting the future of cardiovascular disease in the United States: a policy statement from the American Heart Association. Circulation 123, 933–944. 10.1161/CIR.0b013e31820a55f5 [DOI] [PubMed] [Google Scholar]
- Inaba Y, Chen JA, Bergmann SR, 2010. Prediction of future cardiovascular outcomes by flow-mediated vasodilatation of brachial artery: a meta-analysis. Int. J. Cardiovasc. Imaging 26, 631–640. 10.1007/s10554-010-9616-1 [DOI] [PubMed] [Google Scholar]
- Jones CU, Sangthong B, Pachirat O, 2010. An inspiratory load enhances the antihypertensive effects of home-based training with slow deep breathing: a randomised trial. J. Physiother 56, 179–186. 10.1016/s1836-9553(10)70023-0 [DOI] [PubMed] [Google Scholar]
- Kelly S, Martin S, Kuhn I, Cowan A, Brayne C, Lafortune L, 2016. Barriers and Facilitators to the Uptake and Maintenance of Healthy Behaviours by People at Mid-Life: A Rapid Systematic Review. PloS One 11, e0145074. 10.1371/journal.pone.0145074 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kobayashi K, Akishita M, Yu W, Hashimoto M, Ohni M, Toba K, 2004. Interrelationship between non-invasive measurements of atherosclerosis: flow-mediated dilation of brachial artery, carotid intima-media thickness and pulse wave velocity. Atherosclerosis 173, 13–18. 10.1016/j.atherosclerosis.2003.10.013 [DOI] [PubMed] [Google Scholar]
- Lakatta EG, Levy D, 2003. Arterial and Cardiac Aging: Major Shareholders in Cardiovascular Disease Enterprises: Part I: Aging Arteries: A “Set Up” for Vascular Disease. Circulation 107, 139–146. 10.1161/01.CIR.0000048892.83521.58 [DOI] [PubMed] [Google Scholar]
- Laoutaris ID, Dritsas A, Brown MD, Manginas A, Kallistratos MS, Chaidaroglou A, Degiannis D, Alivizatos PA, Cokkinos DV, 2008. Effects of inspiratory muscle training on autonomic activity, endothelial vasodilator function, and N-terminal pro-brain natriuretic peptide levels in chronic heart failure. J. Cardiopulm. Rehabil. Prev 28, 99–106. 10.1097/01.HCR.0000314203.09676.b9 [DOI] [PubMed] [Google Scholar]
- LaRocca TJ, Seals DR, Pierce GL, 2010. Leukocyte telomere length is preserved with aging in endurance exercise-trained adults and related to maximal aerobic capacity. Mech. Ageing Dev 131, 165–167. 10.1016/j.mad.2009.12.009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laurent S, Cockcroft J, Van Bortel L, Boutouyrie P, Giannattasio C, Hayoz D, Pannier B, Vlachopoulos C, Wilkinson I, Struijker-Boudier H, European Network for Non-invasive Investigation of Large Arteries, 2006. Expert consensus document on arterial stiffness: methodological issues and clinical applications. Eur. Heart J 27, 2588–2605. 10.1093/eurheartj/ehl254 [DOI] [PubMed] [Google Scholar]
- Leith DE, Bradley M, 1976. Ventilatory muscle strength and endurance training. J. Appl. Physiol 41, 508–516. 10.1152/jappl.1976.41.4.508 [DOI] [PubMed] [Google Scholar]
- Lesniewski LA, Durrant JR, Connell ML, Folian BJ, Donato AJ, Seals DR, 2011. Salicylate treatment improves age-associated vascular endothelial dysfunction: potential role of nuclear factor kappaB and forkhead Box O phosphorylation. J. Gerontol. A. Biol. Sci. Med. Sci 66, 409–418. 10.1093/gerona/glq233 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lewington S, Clarke R, Qizilbash N, Peto R, Collins R, Prospective Studies Collaboration, 2002. Age-specific relevance of usual blood pressure to vascular mortality: a meta-analysis of individual data for one million adults in 61 prospective studies. Lancet Lond. Engl 360, 1903–1913. 10.1016/s0140-6736(02)11911-8 [DOI] [PubMed] [Google Scholar]
- López-Otín C, Blasco MA, Partridge L, Serrano M, Kroemer G, 2013. The hallmarks of aging. Cell 153, 1194–1217. 10.1016/j.cell.2013.05.039 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luo S, Lei H, Qin H, Xia Y, 2014. Molecular mechanisms of endothelial NO synthase uncoupling. Curr. Pharm. Des 20, 3548–3553. 10.2174/13816128113196660746 [DOI] [PubMed] [Google Scholar]
- Magadum A, Kishore R, 2020. Cardiovascular Manifestations of COVID-19 Infection. Cells 9. 10.3390/cells9112508 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mancia G, 2007. Blood pressure reduction and cardiovascular outcomes: past, present, and future. Am. J. Cardiol 100, 3J–9J. 10.1016/j.amjcard.2007.05.008 [DOI] [PubMed] [Google Scholar]
- Matsubara T, Miyaki A, Akazawa N, Choi Y, Ra S-G, Tanahashi K, Kumagai H, Oikawa S, Maeda S, 2014. Aerobic exercise training increases plasma Klotho levels and reduces arterial stiffness in postmenopausal women. Am. J. Physiol. Heart Circ. Physiol 306, H348–355. 10.1152/ajpheart.00429.2013 [DOI] [PubMed] [Google Scholar]
- Matsuzawa Y, Kwon T-G, Lennon RJ, Lerman LO, Lerman A, 2015. Prognostic Value of Flow-Mediated Vasodilation in Brachial Artery and Fingertip Artery for Cardiovascular Events: A Systematic Review and Meta-Analysis. J. Am. Heart Assoc 4. 10.1161/JAHA.115.002270 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mello PR, Guerra GM, Borile S, Rondon MU, Alves MJ, Negrão CE, Dal Lago P, Mostarda C, Irigoyen MC, Consolim-Colombo FM, 2012. Inspiratory muscle training reduces sympathetic nervous activity and improves inspiratory muscle weakness and quality of life in patients with chronic heart failure: a clinical trial. J. Cardiopulm. Rehabil. Prev 32, 255–261. 10.1097/HCR.0b013e31825828da [DOI] [PubMed] [Google Scholar]
- Mills DE, Johnson MA, Barnett YA, Smith WHT, Sharpe GR, 2015. The effects of inspiratory muscle training in older adults. Med. Sci. Sports Exerc 47, 691–697. 10.1249/MSS.0000000000000474 [DOI] [PubMed] [Google Scholar]
- Mora-Rodriguez R, Ortega JF, Hamouti N, Fernandez-Elias VE, Cañete Garcia-Prieto J, Guadalupe-Grau A, Saborido A, Martin-Garcia M, Guio de Prada V, Ara I, Martinez-Vizcaino V, 2014. Time-course effects of aerobic interval training and detraining in patients with metabolic syndrome. Nutr. Metab. Cardiovasc. Dis 24, 792–798. 10.1016/j.numecd.2014.01.011 [DOI] [PubMed] [Google Scholar]
- Moreau KL, Donato AJ, Seals DR, DeSouza CA, Tanaka H, 2003. Regular exercise, hormone replacement therapy and the age-related decline in carotid arterial compliance in healthy women. Cardiovasc. Res 57, 861–868. 10.1016/s0008-6363(02)00777-0 [DOI] [PubMed] [Google Scholar]
- Moreau KL, Stauffer BL, Kohrt WM, Seals DR, 2013. Essential role of estrogen for improvements in vascular endothelial function with endurance exercise in postmenopausal women. J. Clin. Endocrinol. Metab 98, 4507–4515. 10.1210/jc.2013-2183 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Narkiewicz K, Somers VK, 2003. Sympathetic nerve activity in obstructive sleep apnoea. Acta Physiol. Scand 177, 385–390. 10.1046/j.1365-201X.2003.01091.x [DOI] [PubMed] [Google Scholar]
- Neter JE, Stam BE, Kok FJ, Grobbee DE, Geleijnse JM, 2003. Influence of weight reduction on blood pressure: a meta-analysis of randomized controlled trials. Hypertens. Dallas Tex 1979 42, 878–884. 10.1161/01.HYP.0000094221.86888.AE [DOI] [PubMed] [Google Scholar]
- Nolan PB, Keeling SM, Robitaille CA, Buchanan CA, Dalleck LC, 2018. The Effect of Detraining after a Period of Training on Cardiometabolic Health in Previously Sedentary Individuals. Int. J. Environ. Res. Public. Health 15. 10.3390/ijerph15102303 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pacher P, Beckman JS, Liaudet L, 2007. Nitric oxide and peroxynitrite in health and disease. Physiol. Rev 87, 315–424. 10.1152/physrev.00029.2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pedralli ML, Marschner RA, Kollet DP, Neto SG, Eibel B, Tanaka H, Lehnen AM, 2020. Different exercise training modalities produce similar endothelial function improvements in individuals with prehypertension or hypertension: a randomized clinical trial Exercise, endothelium and blood pressure. Sci. Rep 10, 7628. 10.1038/s41598-020-64365-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pellizzaro CO, Thomé FS, Veronese FV, 2013. Effect of Peripheral and Respiratory Muscle Training on the Functional Capacity of Hemodialysis Patients. Ren. Fail 35, 189–197. 10.3109/0886022X.2012.745727 [DOI] [PubMed] [Google Scholar]
- Pierce GL, 2018. Initiating life-long aerobic exercise 4–5 days per week before or near age 50 years: is this the “holy-grail” of preventing age-related central artery stiffness? J. Physiol 596, 2635–2636. 10.1113/JP276253 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pierce GL, 2017. Aortic Stiffness in Aging and Hypertension: Prevention and Treatment with Habitual Aerobic Exercise. Curr. Hypertens. Rep 19, 90. 10.1007/s11906-017-0788-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pierce GL, Donato AJ, LaRocca TJ, Eskurza I, Silver AE, Seals DR, 2011a. Habitually exercising older men do not demonstrate age-associated vascular endothelial oxidative stress. Aging Cell 10, 1032–1037. 10.1111/j.1474-9726.2011.00748.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pierce GL, Eskurza I, Walker AE, Fay TN, Seals DR, 2011b. Sex-specific effects of habitual aerobic exercise on brachial artery flow-mediated dilation in middle-aged and older adults. Clin. Sci. Lond. Engl 1979 120, 13–23. 10.1042/CS20100174 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Piercy KL, Troiano RP, Ballard RM, Carlson SA, Fulton JE, Galuska DA, George SM, Olson RD, 2018. The Physical Activity Guidelines for Americans. JAMA 320, 2020–2028. 10.1001/jama.2018.14854 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramos-Barrera GE, DeLucia CM, Bailey EF, 2020. Inspiratory muscle strength training lowers blood pressure and sympathetic activity in older adults with OSA: a randomized controlled pilot trial. J. Appl. Physiol. Bethesda Md 1985 129, 449–458. 10.1152/japplphysiol.00024.2020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ras RT, Streppel MT, Draijer R, Zock PL, 2013. Flow-mediated dilation and cardiovascular risk prediction: a systematic review with meta-analysis. Int. J. Cardiol 168, 344–351. 10.1016/j.ijcard.2012.09.047 [DOI] [PubMed] [Google Scholar]
- Rivera-Torres S, Fahey TD, Rivera MA, 2019. Adherence to Exercise Programs in Older Adults: Informative Report. Gerontol. Geriatr. Med 5, 2333721418823604. 10.1177/2333721418823604 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rossman MJ, Kaplon RE, Hill SD, McNamara MN, Santos-Parker JR, Pierce GL, Seals DR, Donato AJ, 2017. Endothelial cell senescence with aging in healthy humans: prevention by habitual exercise and relation to vascular endothelial function. Am. J. Physiol. Heart Circ. Physiol 313, H890–H895. 10.1152/ajpheart.00416.2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sangthong B, Ubolsakka-Jones C, Pachirat O, Jones DA, 2016. Breathing Training for Older Patients with Controlled Isolated Systolic Hypertension. Med. Sci. Sports Exerc 48, 1641–1647. 10.1249/MSS.0000000000000967 [DOI] [PubMed] [Google Scholar]
- Santos-Parker JR, Strahler TR, Vorwald VM, Pierce GL, Seals DR, 2017. Habitual aerobic exercise does not protect against micro- or macrovascular endothelial dysfunction in healthy estrogen-deficient postmenopausal women. J. Appl. Physiol. Bethesda Md 1985 122, 11–19. 10.1152/japplphysiol.00732.2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schoenborn CA, Stommel M, 2011. Adherence to the 2008 adult physical activity guidelines and mortality risk. Am. J. Prev. Med 40, 514–521. 10.1016/j.amepre.2010.12.029 [DOI] [PubMed] [Google Scholar]
- Seals DR, 2014. Edward F. Adolph Distinguished Lecture: The remarkable anti-aging effects of aerobic exercise on systemic arteries. J. Appl. Physiol. Bethesda Md 1985 117, 425–439. 10.1152/japplphysiol.00362.2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seals DR, Brunt VE, Rossman MJ, 2018. Keynote lecture: strategies for optimal cardiovascular aging. Am. J. Physiol.-Heart Circ. Physiol 315, H183–H188. 10.1152/ajpheart.00734.2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seals DR, Jablonski KL, Donato AJ, 2011. Aging and vascular endothelial function in humans. Clin. Sci. Lond. Engl 1979 120, 357–375. 10.1042/CS20100476 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seals DR, Nagy EE, Moreau KL, 2019. Aerobic exercise training and vascular function with ageing in healthy men and women. J. Physiol 597, 4901–4914. 10.1113/JP277764 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sheel AW, Reid WD, Townson AF, Ayas NT, Konnyu KJ, Spinal Cord Rehabilitation Evidence Research Team, 2008. Effects of exercise training and inspiratory muscle training in spinal cord injury: a systematic review. J. Spinal Cord Med 31, 500–508. 10.1080/10790268.2008.11753645 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Siddiqi Z, Tiro JA, Shuval K, 2011. Understanding impediments and enablers to physical activity among African American adults: a systematic review of qualitative studies. Health Educ. Res 26, 1010–1024. 10.1093/her/cyr068 [DOI] [PubMed] [Google Scholar]
- Smart NA, Giallauria F, Dieberg G, 2013. Efficacy of inspiratory muscle training in chronic heart failure patients: a systematic review and meta-analysis. Int. J. Cardiol 167, 1502–1507. 10.1016/j.ijcard.2012.04.029 [DOI] [PubMed] [Google Scholar]
- SPRINT MIND Investigators for the SPRINT Research Group, Williamson JD, Pajewski NM, Auchus AP, Bryan RN, Chelune G, Cheung AK, Cleveland ML, Coker LH, Crowe MG, Cushman WC, Cutler JA, Davatzikos C, Desiderio L, Erus G, Fine LJ, Gaussoin SA, Harris D, Hsieh M-K, Johnson KC, Kimmel PL, Tamura MK, Launer LJ, Lerner AJ, Lewis CE, Martindale-Adams J, Moy CS, Nasrallah IM, Nichols LO, Oparil S, Ogrocki PK, Rahman M, Rapp SR, Reboussin DM, Rocco MV, Sachs BC, Sink KM, Still CH, Supiano MA, Snyder JK, Wadley VG, Walker J, Weiner DE, Whelton PK, Wilson VM, Woolard N, Wright JT, Wright CB, 2019. Effect of Intensive vs Standard Blood Pressure Control on Probable Dementia: A Randomized Clinical Trial. JAMA 321, 553–561. 10.1001/jama.2018.21442 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stakos DA, Stamatelopoulos K, Bampatsias D, Sachse M, Zormpas E, Vlachogiannis NI, Tual-Chalot S, Stellos K, 2020. The Alzheimer’s Disease Amyloid-Beta Hypothesis in Cardiovascular Aging and Disease: JACC Focus Seminar. J. Am. Coll. Cardiol 75, 952–967. 10.1016/j.jacc.2019.12.033 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stutts WC, 2002. Physical activity determinants in adults. Perceived benefits, barriers, and self efficacy. AAOHN J. Off. J. Am. Assoc. Occup. Health Nurses 50, 499–507. [PubMed] [Google Scholar]
- Tanahashi K, Akazawa N, Miyaki A, Choi Y, Ra S-G, Matsubara T, Kumagai H, Oikawa S, Maeda S, 2014. Aerobic exercise training decreases plasma asymmetric dimethylarginine concentrations with increase in arterial compliance in postmenopausal women. Am. J. Hypertens 27, 415–421. 10.1093/ajh/hpt217 [DOI] [PubMed] [Google Scholar]
- Tanaka H, DeSouza CA, Seals DR, 1998. Absence of age-related increase in central arterial stiffness in physically active women. Arterioscler. Thromb. Vasc. Biol 18, 127–132. 10.1161/01.atv.18.1.127 [DOI] [PubMed] [Google Scholar]
- Tanaka H, Dinenno FA, Monahan KD, Clevenger CM, DeSouza CA, Seals DR, 2000. Aging, habitual exercise, and dynamic arterial compliance. Circulation 102, 1270–1275. 10.1161/01.cir.102.11.1270 [DOI] [PubMed] [Google Scholar]
- Troiano RP, Berrigan D, Dodd KW, Mâsse LC, Tilert T, McDowell M, 2008. Physical activity in the United States measured by accelerometer. Med. Sci. Sports Exerc 40, 181–188. 10.1249/mss.0b013e31815a51b3 [DOI] [PubMed] [Google Scholar]
- Vaitkevicius PV, Fleg JL, Engel JH, O’Connor FC, Wright JG, Lakatta LE, Yin FC, Lakatta EG, 1993. Effects of age and aerobic capacity on arterial stiffness in healthy adults. Circulation 88, 1456–1462. 10.1161/01.cir.88.4.1456 [DOI] [PubMed] [Google Scholar]
- Virani SS, Alonso A, Aparicio HJ, Benjamin EJ, Bittencourt MS, Callaway CW, Carson AP, Chamberlain AM, Cheng S, Delling FN, Elkind MSV, Evenson KR, Ferguson JF, Gupta DK, Khan SS, Kissela BM, Knutson KL, Lee CD, Lewis TT, Liu J, Loop MS, Lutsey PL, Ma J, Mackey J, Martin SS, Matchar DB, Mussolino ME, Navaneethan SD, Perak AM, Roth GA, Samad Z, Satou GM, Schroeder EB, Shah SH, Shay CM, Stokes A, VanWagner LB, Wang N-Y, Tsao CW, American Heart Association Council on Epidemiology and Prevention Statistics Committee and Stroke Statistics Subcommittee, 2021. Heart Disease and Stroke Statistics-2021 Update: A Report From the American Heart Association. Circulation 143, e254–e743. 10.1161/CIR.0000000000000950 [DOI] [PubMed] [Google Scholar]
- Vlachopoulos C, Aznaouridis K, Stefanadis C, 2010. Prediction of cardiovascular events and all-cause mortality with arterial stiffness: a systematic review and meta-analysis. J. Am. Coll. Cardiol 55, 1318–1327. 10.1016/j.jacc.2009.10.061 [DOI] [PubMed] [Google Scholar]
- Vranish JR, Bailey EF, 2016. Inspiratory Muscle Training Improves Sleep and Mitigates Cardiovascular Dysfunction in Obstructive Sleep Apnea. Sleep 39, 1179–1185. 10.5665/sleep.5826 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vranish JR, Bailey EF, 2015. Daily respiratory training with large intrathoracic pressures, but not large lung volumes, lowers blood pressure in normotensive adults. Respir. Physiol. Neurobiol 216, 63–69. 10.1016/j.resp.2015.06.002 [DOI] [PubMed] [Google Scholar]
- Walker KA, Power MC, Gottesman RF, 2017. Defining the Relationship Between Hypertension, Cognitive Decline, and Dementia: a Review. Curr. Hypertens. Rep 19, 24. 10.1007/s11906-017-0724-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wanke T, Toifl K, Merkle M, Formanek D, Lahrmann H, Zwick H, 1994. Inspiratory muscle training in patients with Duchenne muscular dystrophy. Chest 105, 475–482. 10.1378/chest.105.2.475 [DOI] [PubMed] [Google Scholar]
- Whelton PK, Carey RM, Aronow WS, Casey DE, Collins KJ, Dennison Himmelfarb C, DePalma SM, Gidding S, Jamerson KA, Jones DW, MacLaughlin EJ, Muntner P, Ovbiagele B, Smith SC, Spencer CC, Stafford RS, Taler SJ, Thomas RJ, Williams KA, Williamson JD, Wright JT, 2018. 2017 ACC/AHA/AAPA/ABC/ACPM/AGS/APhA/ASH/ASPC/NMA/PCNA Guideline for the Prevention, Detection, Evaluation, and Management of High Blood Pressure in Adults: A Report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines. Hypertens. Dallas Tex 1979 71, e13–e115. 10.1161/HYP.0000000000000065 [DOI] [PubMed] [Google Scholar]
- Whelton SP, Chin A, Xin X, He J, 2002. Effect of aerobic exercise on blood pressure: a meta-analysis of randomized, controlled trials. Ann. Intern. Med 136, 493–503. 10.7326/0003-4819-136-7-200204020-00006 [DOI] [PubMed] [Google Scholar]
- Witt JD, Guenette JA, Rupert JL, McKenzie DC, Sheel AW, 2007. Inspiratory muscle training attenuates the human respiratory muscle metaboreflex. J. Physiol 584, 1019–1028. 10.1113/jphysiol.2007.140855 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu Y, Arora RC, Hiebert BM, Lerner B, Szwajcer A, McDonald K, Rigatto C, Komenda P, Sood MM, Tangri N, 2014. Non-invasive endothelial function testing and the risk of adverse outcomes: a systematic review and meta-analysis. Eur. Heart J. Cardiovasc. Imaging 15, 736–746. 10.1093/ehjci/jet256 [DOI] [PubMed] [Google Scholar]
- Yang T, Sun Y, Lu Z, Leak RK, Zhang F, 2017. The impact of cerebrovascular aging on vascular cognitive impairment and dementia. Ageing Res. Rev 34, 15–29. 10.1016/j.arr.2016.09.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yarwood J, Carryer J, Gagan MJ, 2005. Women maintaining physical activity at midlife: contextual complexities. Nurs. Prax. N. Z. Inc 21, 24–37. [PubMed] [Google Scholar]
- Zieman SJ, Melenovsky V, Kass DA, 2005. Mechanisms, pathophysiology, and therapy of arterial stiffness. Arterioscler. Thromb. Vasc. Biol 25, 932–943. 10.1161/01.ATV.0000160548.78317.29 [DOI] [PubMed] [Google Scholar]


