Abstract
INTRODUCTION:
There are limited direct data on severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) long-term immune responses and reinfection. This study aimed to evaluate the rate, risk factors, and severity of COVID-19 reinfection.
METHODS:
This retrospective cohort study included five hospitals across Saudi Arabia. All subjects who were presented or admitted with positive SARS-CoV-2 real-time polymerase chain reaction (RT-PCR) tests were evaluated between March 2020 and August 2021. Reinfection was defined as a patient who was infected followed by clinical recovery, and later became infected again 90 days post first infection. The infection was confirmed with a positive SARS-CoV-2 (RT-PCR). Four hundred and seventeen recovered cases but with no reinfection were included as a control.
RESULTS:
A total of 35,288 RT-PCR-confirmed COVID-19 patients were observed between March 2020 and August 2021. Based on the case definition, (0.37%) 132 patients had COVID-19 reinfection. The mean age in the reinfected cases was 40.95 ± 19.48 (range 1–87 years); Females were 50.76%. Body mass index was 27.65 ± 6.65 kg/m2; diabetes and hypertension were the most common comorbidities. The first infection showed mild symptoms in 91 (68.94%) patients; and when compared to the control group, comorbidities, severity of infection, and laboratory investigations were not statistically different. Hospitalization at the first infection was higher, but not statistically different when compared to the control group (P = 0.093).
CONCLUSION:
COVID-19 reinfection is rare and does not carry a higher risk of severe disease. Further studies are required, especially with the continuously newly emerging variants, with the unpredictable risk of reinfection.
Keywords: Coronavirus disease of 2019, pandemic, real-time polymerase chain reaction, reinfection, severe acute respiratory syndrome coronavirus 2
Two years since, the COVID-19 was declared a pandemic by the World Health Organization in 2020,[1] the cases have been rising, with more than 370 million confirmed cases worldwide with 5.6 million deaths as of January 30, 2022.[2] The severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) persists in body fluids, such as sputum or feces weeks after the infection,[3] and viral antigens may persist in the gut for about 4 months.[4] Case reports of COVID-19 reinfection have been reported across different countries.[5,6,7,8] The possible reinfections reported in England as of January 2, 2022 were 106,297.[9] The median reported time between infection and reinfection was 200 days.[10] Studies have reported recovered patients can still be tested positive.[11,12,13] The mechanism of reinfection is not clear, with some cases having a less severe illness,[10] whereas others have reported having severe illness during reinfection.[10] A large multicenter study conducted in England had reported an 84% lower risk of reinfection after primary infection.[10] The SIREN study conducted in the UK reported symptomatic reinfections that were observed despite the vaccination.[14] The reinfection had been reported among vaccinated cases, which can be more severe.[15,16]
Once infected with COVID-19, the antibodies develop swiftly.[17,18] However, the antibody titers decline within 1–2 months after infection.[19,20] Prolonged viral shedding at low levels has been reported.[17] Whether it is a prolonged viral shedding or a true reinfection occurs remains unclear. Large observational studies addressing reinfection are scarce in the literature.
There are no large COVID-19 observational studies reported from the region. Therefore, the primary objective of this study was to evaluate the COVID-19 reinfection rate, clinical outcomes, and risk factors.
Methods
Study cohort
This retrospective cohort study included five hospitals across Saudi Arabia where COVID-19 patients were evaluated and treated. These hospitals, affiliated with the Ministry of National Guard, are in five regions across the country (Riyadh, Jeddah, Al-Ahsa, Dammam, and Medina). All subjects presented or admitted with positive SARS-CoV-2 real-time polymerase chain reaction (RT-PCR) tests between March 2020 and August 2021 were evaluated. Reinfection with COVID-19 was considered if a patient was infected and recovered but became infected again as diagnosed by positive SARS-CoV-2 RT-PCR test more than or equal to 90 days from the first infection. Cases with continued viral shedding and prolonged hospitalization were removed from the cohort. A negative RT-PCR was not required to confirm recovery rather than clinical improvement.
Variables of interest
The data were collected from patients’ medical records using electronic health records (BESTCare 2.0). The hospitalization was defined as COVID-19 related hospital admission at the time of diagnosis. A similar group of COVID-19 patients with no documented RT-PCR or clinical history of reinfection was used as a control. The subjects were followed up from the time of admission to the time of death or discharge. The collected data included age, gender, body mass index (BMI), and comorbidities that are diabetes mellitus, hypertension, asthma, chronic obstructive pulmonary disease, history of cancer, ischemic heart disease, COVID-19 symptoms, and laboratory tests: (C-reactive protein (CRP), erythrocytes sedimentation rate (ESR), ferritin, lactate dehydrogenase (LDH), lymphocytes, neutrophils, procalcitonin). The length of hospital stay and length of intensive care unit (ICU) stay were also collected. COVID-19 severity was defined as “asymptomatic” (subjects with no reported symptoms and no hospitalization), “mild” (subjects who had reported symptoms but were not hospitalized), and “moderate–severe” (symptoms along with hospitalization or admission to ICU).
Statistical analysis
All the variables were summarized and reported with descriptive statistics. Age, gender, past medical history, family history, and comorbidities were reported as frequency and percentage. The variables were compared between groups, using a Chi-square test for independence, Fisher exact test, or Wilcoxon test as applicable. Risk factors of reinfection were identified using logistic regression analysis. Results were reported as odds ratio, 95% confidence intervals, and P value. Statistical significance was declared at P < 0.05. Statistical analyses were performed using SAS version 9.4 (SAS Institute, Cary, NC, USA).
Ethical aproval
The study was approved by King Abdullah International Medical Research Center, Institutional Review Board, number NRC21R/445/10. Written consent was waived for the retrospective nature of the data.
Results
A total of 35,288 RT-PCR-confirmed COVID-19 patients were observed between March 2020 and August 2021. Based on the case definition, (0.37%) 132 patients had COVID-19 reinfection. The matched control group included 417 subjects with similar age and gender.
Cohorts characteristics
The median days between infection and reinfection were 222 (90–462). The overall mean age was 40.80 ± 19.62 (range 1–87). Children were 4.5% and females were 50.76% among reinfected cases. BMI was 27.65 ± 6.65 kg/m2; Diabetes and hypertension were the most common comorbidities. The first infections were mild in 91 (68.94%) patients. When compared to the control group, BMI and comorbidities were not statistically different between reinfected cases and control.
The symptoms at first infections were not statistically different between cases and controls [Figure 1a]. However, within the reinfected cases, symptoms were more prominent at the first infection as compared to the reinfection presentation: fever (7.3% vs. 5.8%), cough (8.0% vs. 6.1%), shortness of breath (5.6% vs. 3.2%), flu-like symptoms (11.6% vs. 7%), and vomiting/diarrhea (3.0% vs. 2.5%) [Figure 1b].
Figure 1.
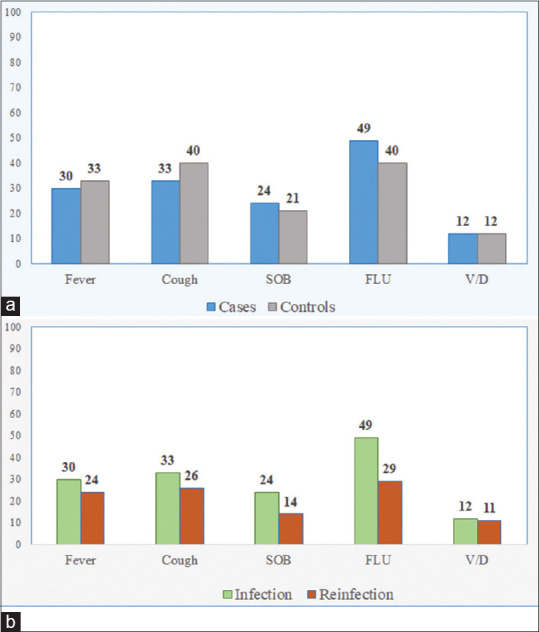
(a) Coronavirus disease 2019 symptoms in cases versus control. (b) coronavirus disease 2019 symptoms within cases (infection vs. reinfection)
Laboratory data
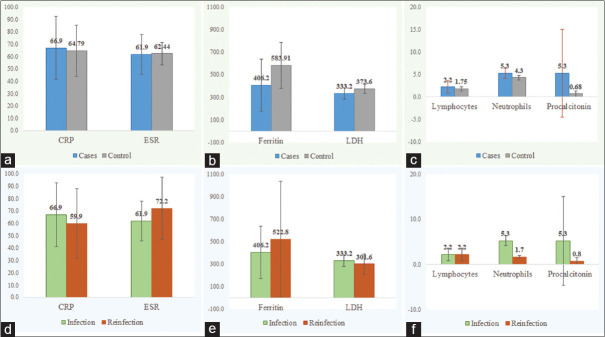
CRP, neutrophils, and procalcitonin were higher in cases when comparing first infections to the reinfections [Figure 2d-f]. However, laboratory parameters, including CRP, ESR, ferritin, LDH, lymphocytes, neutrophils, and procalcitonin, were not statistically different between cases and controls [Figure 2a-c] and infection versus reinfection [Figure 2d-f].
Figure 2.
(a-c) Laboratory parameters cases versus control. (d-f) Laboratory parameters within cases (infection vs. reinfection)
Coronavirus disease 2019 outcomes
The COVID-19 severity (mild, moderate, and severe) was not different between cases and controls [Table 1]. Hospitalization was higher in cases after the first infection (26.5% vs. 19.6%), but not statistically different when compared to the control group (P = 0.093); and hospitalization in first infections and reinfections among the cases was not different (26% vs. 27%) [Table 2].
Table 1.
Characteristics of the study groups
| Variables | Overall (n=549) | Cases (n=132) | Controls (n=417) | P |
|---|---|---|---|---|
| Age, mean±SD | 40.80±19.62 | 40.95±19.48 | 40.76±19.69 | 0.884^ |
| Age, n (%) | ||||
| Children | 31 (5.65) | 6 (4.55) | 25 (6.0) | 0.989* |
| 15-25 years | 87 (15.85) | 22 (16.67) | 65 (15.59) | |
| 26-35 years | 137 (24.95) | 34 (25.76) | 103 (24.70) | |
| 36-45 years | 113 (20.58) | 26 (19.70) | 87 (20.86) | |
| 46-59 years | 54 (9.84) | 13 (9.85) | 41 (9.83) | |
| ≥60 years | 127 (23.13) | 31 (23.48) | 96 (23.02) | |
| Gender, n (%) | ||||
| Female | 293 (53.37) | 67 (50.76) | 226 (54.20) | 0.490* |
| Male | 256 (46.63) | 65 (49.24) | 191 (45.80) | |
| BMI, mean±SD | 28.35±8.02 | 27.65±6.65 | 28.58±8.43 | 0.423^ |
| Comorbidities, n (%) | ||||
| Diabetes mellitus type II | 126 (22.95) | 37 (28.03) | 89 (21.34) | 0.111* |
| Hypertension | 126 (22.95) | 38 (28.79) | 88 (21.10) | 0.067* |
| COPD | 4 (0.73) | 1 (0.76) | 3 (0.72) | 1.000** |
| Asthma | 30 (5.59) | 7 (5.47) | 23 (5.62) | 0.947* |
| Ischemic heart disease | 26 (4.74) | 10 (7.58) | 16 (3.84) | 0.078* |
| History of cancer | 16 (2.91) | 7 (5.30) | 9 (2.16) | 0.074** |
| Type of cancer | ||||
| Lymphoma | 4 (26.67) | 3 (42.86) | 1 (12.50) | - |
| Breast cancer | 3 (20) | 0 | 3 (37.5) | |
| Thyroid cancer | 2 (13.33) | 1 (14.29) | 1 (12.50) | |
| Colorectal cancer | 1 (6.67) | 1 (14.29) | 0 | |
| Nasopharyngeal cancer | 1 (6.67) | 1 (14.29) | 0 | |
| Pancreatic cancer | 1 (6.67) | 0 | 1 (12.5) | |
| Hepatocellular cancer | 1 (6.67) | 0 | 1 (12.5) | |
| Number of comorbidities | ||||
| None | 357 (65.03) | 84 (63.64) | 273 (65.47) | 0.700* |
| Yes | 192 (34.97) | 48 (36.36) | 144 (34.53) | |
| COVID-19 severity | ||||
| Asymptomatic | 153 (27.87) | 37 (28.03) | 116 (27.82) | 0.862* |
| Mild | 375 (68.31) | 91 (68.94) | 284 (68.11) | |
| Moderate-severe | 21 (3.83) | 4 (3.03) | 17 (4.08) |
*Chi-square test, **Fisher’s exact test, ^Wilcoxon rank-sum test. Column percentages are reported. SD=Standard deviation, BMI=Body mass index, COPD=Chronic obstructive pulmonary disease
Table 2.
Outcomes data
| Variables | Overall (n=549) | Cases (n=132) | Controls (n=417) | P |
|---|---|---|---|---|
| Hospital admission after first infection, n (%) | 117 (21.31) | 35 (26.52) | 82 (19.66) | 0.093* |
| ICU admission after first infection, n (%) | 25 (4.55) | 4 (3.03) | 21 (5.04) | 0.335* |
| Hospital admission after second infection, n (%) | - | 36 (27.48) | NA | - |
| ICU admission after second infection, n (%) | - | 4 (11.1) | NA | - |
*Chi-square test, NA=Not applicable
Risk factors of reinfection
Comorbidities, symptoms at first infection, hospital admission, and ICU admission during the first infection were explored as risk factors for reinfection. The subjects who had hospital admission during the first infection were 1.6 times more likely to have reinfection (P = 0.047) [Table 3]. Other variables did not show any risk for COVID-19 reinfection.
Table 3.
Predictors of COVID-19 reinfection
| Risk Factors | OR | 95% CI (lower limit–upper limit) | P |
|---|---|---|---|
| Comorbidity (yes vs. none) | 1.121 | 0.726-1.730 | 0.606 |
| Had symptoms at first infection (yes vs. no) | 1.007 | 0.653-1.554 | 0.974 |
| Hospital admission during first infection (yes vs. no) | 1.682 | 1.007-2.811 | 0.047 |
| ICU admission during first infection (yes vs. no) | 0.369 | 0.116-1.175 | 0.091 |
*Probabilistic model is based on probability of having the reinfection. OR=Odds ratio, CI=Confidence interval, ICU=Intensive care unit
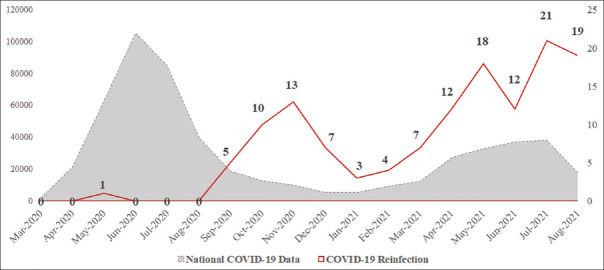
When observed overtime, the COVID-19 reinfections presented mainly after the first pandemic wave in Saudi Arabia. It also showed some direct proportional relationship to the increase in the COVID-19 cases during the national second wave of COVID-19 [Figure 3].
Figure 3.
Coronavirus disease 2019 reinfection overtime, from March 2020 to August 2021. The primary axis is national coronavirus disease 2019 data. The red line represents reinfection numbers in the current study
Discussion
The presented study reports COVID-19 reinfection among a large cohort in the Saudi population, with a low rate of reinfection (0.37%). The reinfection had been reported across several countries.[8,10,21,22,23] The reinfection rate is consistent with the overall surge in the COVID-19 infection. Recent reports showed that COVID-19 reinfection in England was reported as 4.0%; however, the number increased to 10% following a spike in total cases.[24] It has also been observed that reinfection rates were higher after the onset of new variants.[16] Our study did not include cases after August 2021, and a higher incidence might be observed with the spread of Omicron (B.1.1.529) variant. Overall, the reinfection rate in our study is lower than what is reported by studies conducted in the western region.[15,25]
A study has suggested defining reinfection as having a positive retesting more than 83 days after the first positive test.[26] National Institute of Health has recommended 90 days between both episodes as a diagnostic criterion for reinfection.[27] Such a long period would provide some certainty toward considering a new infection if backed with the supporting clinical picture. There are inconsistencies in defining COVID-19 reinfection in the literature; it is also unclear whether it is due to prolonged viral shedding or reactivation of less evident infections. The current study defines reinfection as any RT-PCR-positive case beyond 90 days from the clinical recovery of the first COVID-19 infection. Continuous shedding of SARS-CoV-2 is a possibility and cannot be totally excluded without advance testing, that is, metagenomic sequencing that would rule out any traces of viral replication.
The reported reinfection was more severe in the literature,[28] in contrast with our results. The symptoms were less in frequency during reinfection. Reinfection was more likely reported in adults, women, and immunocompromised patients.[15] Previous reports noted that high reinfection rate is found in females.[15,29,30] The reinfection was lowest in children as reported by other studies.[15,29] One possible explanation of low reinfection rates in children is that children immune system was shown to react in a more focused mechanism by eliciting anti-spike IgG more than anti-nucleocapsid IgG, which is found in adults. This may lead to more focused response on the spike, providing protection to viral entry through the spike protein.[31] On this regard, our study did not investigate the level of immune responses of the subjects; therefore, the role of immune responses or the search for protective level of immunity is not discussed and would remain a possible risk factor for reinfection.
A study had reported a higher prevalence of comorbidities in patients with reinfection.[32] However, we did not find a difference in comorbidities between the control group and patients with reinfection. In the current study, the hospitalization in first infections and reinfections among the cases was similar, which indicates that the severity of reinfection cannot be established as less or more than the first infection. In addition, our findings showed that CRPs and neutrophils were higher at the first infection than the reinfection (but not higher than controls). This may indicate lower inflammation in the second infection; therefore, the severity of reinfection might be individual-related and would require further studies, including inflammation and immune responses. Nevertheless, patients with hospitalization during the first infection were predicted to be more likely to have reinfection, which is in agreement with previous studies.[33,34]
Although our cohort had extensive clinical data, COVID-19 vaccination status could not be obtained. This study describes a large portion of Saudi population across different regions; although it might not change the main conclusion, nationwide data would provide a concise description and conclusions. The presence of electronic medical records delivered high-quality data.
Conclusion
As many viral infections can protect from repeated infections, COVID-19 reinfection can occur, taking a pattern similar to the common human coronaviruses. Our study showed that it is not very often that COVID-19 reinfections occur. There are no apparent risk factors of reinfection, and second infections can be as severe as the first. Further work is needed to better understand reinfections with COVID-19 to advise public health policymakers. This is urgently required when there is a community spread of new variants of concern, such as Omicron or other virus variants.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
References
- 1.WHO. WHO Director-General’s Opening Remarks at the Media Briefing on COVID19. 2020 [Google Scholar]
- 2.WHO. Weekly Epidemiological Update on COVID-19 – February 01, 2022. [Last accessed on 2022 Feb 02]. Available from: https://www.who.int/publications/m/ item/weekly-epidemiological-update-on-covid-19---1-februa ry-2022 .
- 3.Xu Y, Li X, Zhu B, Liang H, Fang C, Gong Y, et al. Characteristics of pediatric SARS-CoV-2 infection and potential evidence for persistent fecal viral shedding. Nat Med. 2020;26:502–5. doi: 10.1038/s41591-020-0817-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Gaebler C, Wang Z, Lorenzi JC, Muecksch F, Finkin S, Tokuyama M, et al. Evolution of antibody immunity to SARS-CoV-2. Nature. 2021 Mar;591(7851):639–644. doi: 10.1038/s41586-021-03207-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Sharma R, Sardar S, Mohammad Arshad A, Ata F, Zara S, Munir W. A patient with asymptomatic SARS-CoV-2 infection who presented 86 days later with COVID-19 pneumonia possibly due to reinfection with SARS-CoV-2. Am J Case Rep. 2020;21:e927154. doi: 10.12659/AJCR.927154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.West J, Everden S, Nikitas N. A case of COVID-19 reinfection in the UK. Clin Med (Lond) 2021;21:e52–3. doi: 10.7861/clinmed.2020-0912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Jain A, Kaur J, Rai AK, Pandey AK. Anosmia: A clinical indicator of COVID-19 reinfection. Ear Nose Throat J. 2021;100:180S–1S. doi: 10.1177/0145561320978169. [DOI] [PubMed] [Google Scholar]
- 8.Bonifácio LP, Pereira AP, Araújo DC, Balbão VD, Fonseca BA, Passos AD, et al. Are SARS-CoV-2 reinfection and COVID-19 recurrence possible.A case report from Brazil? Rev Soc Bras Med Trop. 2020;53:e20200619. doi: 10.1590/0037-8682-0619-2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Weekly National Influenza and COVID-19 Surveillance Report. [Last accessed on 2022 Jan 19]. Available from: https://assets.publishing.service.gov.uk/ government/uploads/system/uploads/attachment_data/ file/1046284/weekly-flu-and-covid-19-report-week-2-2022. pdf .
- 10.Parry J. COVID-19: Hong Kong scientists report first confirmed case of reinfection. BMJ. 2020;370:m3340. doi: 10.1136/bmj.m3340. [DOI] [PubMed] [Google Scholar]
- 11.Qu YM, Kang EM, Cong HY. Positive result of Sars-Cov-2 in sputum from a cured patient with COVID-19. Travel Med Infect Dis. 2020;34:101619. doi: 10.1016/j.tmaid.2020.101619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Yuan B, Liu HQ, Yang ZR, Chen YX, Liu ZY, Zhang K, et al. Recurrence of positive SARS-CoV-2 viral RNA in recovered COVID-19 patients during medical isolation observation. Sci Rep. 2020;10:11887. doi: 10.1038/s41598-020-68782-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Lan L, Xu D, Ye G, Xia C, Wang S, Li Y, et al. Positive RT-PCR test results in patients recovered from COVID-19. JAMA. 2020;323:1502–3. doi: 10.1001/jama.2020.2783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hall VJ, Foulkes S, Charlett A, Atti A, Monk EJ, Simmons R, et al. SARS-CoV-2 infection rates of antibody-positive compared with antibody-negative health-care workers in England: A large, multicentre, prospective cohort study (SIREN) Lancet. 2021;397:1459–69. doi: 10.1016/S0140-6736(21)00675-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Slezak J, Bruxvoort K, Fischer H, Broder B, Ackerson B, Tartof S. Rate and severity of suspected SARS-Cov-2 reinfection in a cohort of PCR-positive COVID-19 patients. Clin Microbiol Infect. 2021;27:1860.e7–10. doi: 10.1016/j.cmi.2021.07.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Shastri J, Parikh S, Aggarwal V, Agrawal S, Chatterjee N, Shah R, et al. Severe SARS-CoV-2 breakthrough reinfection with delta variant after recovery from breakthrough infection by alpha variant in a fully vaccinated health worker. Front Med (Lausanne) 2021;8:737007. doi: 10.3389/fmed.2021.737007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.To KK, Tsang OT, Leung WS, Tam AR, Wu TC, Lung DC, et al. Temporal profiles of viral load in posterior oropharyngeal saliva samples and serum antibody responses during infection by SARS-CoV-2: An observational cohort study. Lancet Infect Dis. 2020;20:565–74. doi: 10.1016/S1473-3099(20)30196-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Liu L, To KK, Chan KH, Wong YC, Zhou R, Kwan KY, et al. High neutralizing antibody titer in intensive care unit patients with COVID-19. Emerg Microbes Infect. 2020;9:1664–70. doi: 10.1080/22221751.2020.1791738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Robbiani DF, Gaebler C, Muecksch F, Lorenzi JC, Wang Z, Cho A, et al. Convergent antibody responses to SARS-CoV-2 in convalescent individuals. Nature. 2020;584:437–42. doi: 10.1038/s41586-020-2456-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Long QX, Tang XJ, Shi QL, Li Q, Deng HJ, Yuan J, et al. Clinical and immunological assessment of asymptomatic SARS-CoV-2 infections. Nat Med. 2020;26:1200–4. doi: 10.1038/s41591-020-0965-6. [DOI] [PubMed] [Google Scholar]
- 21.Chen D, Xu W, Lei Z, Huang Z, Liu J, Gao Z, et al. Recurrence of positive SARS-CoV-2 RNA in COVID-19: A case report. Int J Infect Dis. 2020;93:297–9. doi: 10.1016/j.ijid.2020.03.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kang YJ, Joo H. South Korea's COVID-19 infection status: From the perspective of reconfirmation after complete recovery. J Pure Appl Microbiol. 2020;14:1073–5. [Google Scholar]
- 23.Selvaraj V, Herman K, Dapaah-Afriyie K. Severe, symptomatic reinfection in a patient with COVID-19. R I Med J (2013) 2020;103:24–6. [PubMed] [Google Scholar]
- 24.GOV.UK. COVID-19 Daily Dashboard Amended to Include Reinfections. [Last accessed on 2022 Feb 03]. Available from: https://www.gov.uk/ government/news/covid-19-daily-dashboard-amended-toinclude- reinfections .
- 25.Hansen CH, Michlmayr D, Gubbels SM, Mølbak K, Ethelberg S. Assessment of protection against reinfection with SARS-CoV-2 among 4 million PCR-tested individuals in Denmark in 2020: A population-level observational study. Lancet. 2021;397:1204–12. doi: 10.1016/S0140-6736(21)00575-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Falahi S, Kenarkoohi A. COVID-19 reinfection: Prolonged shedding or true reinfection? New Microbes New Infect. 2020;38:100812. doi: 10.1016/j.nmni.2020.100812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Arteaga-Livias K, Panduro-Correa V, Pinzas-Acosta K, Perez-Abad L, Pecho-Silva S, Espinoza-Sánchez F, et al. COVID-19 reinfection.A suspected case in a Peruvian patient? Travel Med Infect Dis. 2021;39:101947. doi: 10.1016/j.tmaid.2020.101947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Larson D, Brodniak SL, Voegtly LJ, Cer RZ, Glang LA, Malagon FJ, et al. A case of early reinfection with severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) Clin Infect Dis. 2021;73:e2827–8. doi: 10.1093/cid/ciaa1436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Zhang W, Davis BD, Chen SS, Sincuir Martinez JM, Plummer JT, Vail E. Emergence of a novel SARS-CoV-2 variant in Southern California. JAMA. 2021;325:1324–6. doi: 10.1001/jama.2021.1612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Nau C, Bruxvoort K, Navarro RA, Chevez SG, Hogan TA, Ironside KR, et al. COVID-19 inequities across multiple racial and ethnic groups: Results from an integrated health care organization. Ann Intern Med. 2021;174:1183–6. doi: 10.7326/M20-8283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Weisberg SP, Connors TJ, Zhu Y, Baldwin MR, Lin WH, Wontakal S, et al. Distinct antibody responses to SARS-CoV-2 in children and adults across the COVID-19 clinical spectrum. Nat Immunol. 2021;22:25–31. doi: 10.1038/s41590-020-00826-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.SeyedAlinaghi S, Oliaei S, Kianzad S, Afsahi AM, MohsseniPour M, Barzegary A, et al. Reinfection risk of novel coronavirus (COVID-19): A systematic review of current evidence. World J Virol. 2020;9:79–90. doi: 10.5501/wjv.v9.i5.79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Cevik M, Tate M, Lloyd O, Maraolo AE, Schafers J, Ho A. SARS-CoV-2, SARS-CoV, and MERS-CoV viral load dynamics, duration of viral shedding, and infectiousness: A systematic review and meta-analysis. Lancet Microbe. 2021;2:e13–22. doi: 10.1016/S2666-5247(20)30172-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Munker D, Osterman A, Stubbe H, Muenchhoff M, Veit T, Weinberger T, et al. Dynamics of SARS-CoV-2 shedding in the respiratory tract depends on the severity of disease in COVID-19 patients. Eur Respir J. 2021;58:2002724. doi: 10.1183/13993003.02724-2020. [DOI] [PMC free article] [PubMed] [Google Scholar]