SUMMARY
In July 2013, a Belgian couple were admitted to hospital because of pneumonia. Medical history revealed contact with birds. Eleven days earlier, they had purchased a lovebird in a pet shop in The Netherlands. The bird became ill, with respiratory symptoms. The couple's daughter who accompanied them to the pet shop, reported similar symptoms, but was travelling abroad. On the suspicion of psittacosis, pharyngeal swabs from the couple were taken and sent to the Belgian reference laboratory for psittacosis. Culture and nested polymerase chain reaction (PCR) tests were positive for the presence of Chlamydia psittaci, and ompA genotyping indicated genotype A in both patients. The patients were treated with doxycycline and the daughter started quinolone therapy; all three recovered promptly. Psittacosis is a notifiable disease in Belgium and therefore local healthcare authorities were informed. They contacted their Dutch colleagues, who visited the pet shop. Seven pooled faecal samples were taken and analysed using PCR by the Dutch national reference laboratory for notifiable animal diseases for the presence of Chlamydia psittaci. Four (57%) samples tested positive, genotyping revealed genotype A. Enquiring about exposure to pet birds is essential when patients present with pneumonia. Reporting to health authorities, even across borders, is warranted to prevent further spread.
Key words: Chlamydia psittaci, outbreak, psittacosis, zoonoses
INTRODUCTION
Psittacosis is a zoonotic disease caused by Chlamydia psittaci, an obligate intracellular Gram- negative bacterium. Transmission of C. psittaci usually originates from close contact with infected birds, mostly in the context of poultry industry and contact with Psittaciformes such as parrots, cockatoos, parakeets and lories [1]. Humans become infected by inhalation of contaminated aerosols from dried faeces, nasal, ocular or respiratory secretions from a diseased bird or asymptomatic carrier. Therefore handling the plumage and tissues of infected birds, cleaning cages and, in rare cases, mouth-to-beak contact or biting represent a zoonotic risk. In addition, activities such as gardening and mowing or trimming lawns without a grass catcher, have been associated with human psittacosis [2, 3]. Because of the aerogenic transmission, a short contact period with a bird or its excrement can be enough for an infection. The incubation period is usually 5–14 days, although periods up to 1 month have been reported and the disease can vary from unapparent [4] to fatal in untreated patients [5]. C. psittaci mainly causes a respiratory infection in humans and clinical symptoms are highly variable, including abrupt onset of fever (up to 40·5 °C), rigors, headache, myalgia, malaise, cough usually non-productive, and atypical pneumonia [1]. Human-to-human transmission of psittacosis is possible but it is believed to be rare [6, 7]. Although recently a multiple human-to-human transmission has been reported in Sweden, where two family members, one hospital roommate and seven hospital workers became ill after nursing a patient with a severe case of psittacosis [8]. In most countries, psittacosis is a notifiable disease and must be reported within 48 h. In Flanders, the northern part of Belgium with a population of 6 million inhabitants, an average of 2–4 cases are notified yearly. However, there is a gross underestimation of the current number of infections as not all infections cause pneumonia and therefore often remain unnoticed. Moreover, microbiological testing is not often performed for milder respiratory infections, and even if done, standard testing does not include serology and nucleic acid testing for psittacosis. Interestingly, publications of psittacosis cases have increased since the implementation of nucleic acid amplification techniques. The current paper describes the management of a cluster outbreak of psittacosis, linked to the purchase of a lovebird (Agapornis roseicollis).
OUTBREAK DESCRIPTION
Patient 1
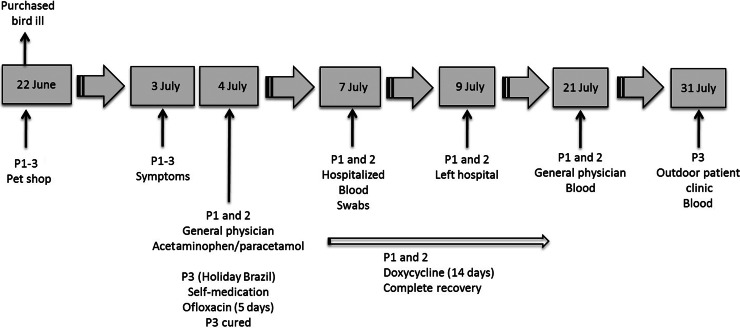
On 7 July 2013, a 54-year-old woman was admitted to the hospital because of pneumonia. A summary of the clinical events of the patient is presented in Figure 1. Four days before admission (3 July), she developed fever, muscle pain, headache, and a dry cough. The next day, she was seen by her general physician who prescribed acetaminophen/paracetamol. Because of clinical deterioration (fever augmented up to 39·5–40 °C, difficulties in breathing) she was referred to the emergency department. Medical history revealed that 11 days (22 June) before the onset of symptoms the patient had visited a pet shop together with her husband (patient 2) and daughter (patient 3) where they purchased a lovebird.
Fig. 1.
Summary of all clinical events for the purchased lovebird and the three patients (P1–P3).
Laboratory analysis showed an elevated C-reactive protein level of 214 mg/l. A chest X-ray (Fig. 2a) showed extensive right-sided para- and infra-hilar infiltrates. A presumptive diagnosis of C. psittaci pneumonia was made and doxycycline therapy (200 mg qid) was initiated. Upon admission, blood for C. psittaci IgG and IgM serology was sampled. In addition, a pharyngeal swab in Chlamydia transport medium and a pharyngeal swab in DNA stabilization buffer were taken and sent to the Belgian reference laboratory in Ghent. The patient responded promptly to doxycycline therapy and was sent home from hospital 2 days after admission (9 July). She continued taking doxycyline for 14 days and after treatment, a convalescent serum sample was taken.
Fig. 2.
Chest X-rays showing bilateral patchy infiltrates of (a) patient 1 and (b) patient 2.
Patient 2
The 53-year-old husband of patient 1 developed similar symptoms on the same day as his wife and the next day he accompanied her to the general physician. A summary of the clinical events of the patient is presented in Figure 1. As his wife, he also failed to respond well to the acetaminophen/paracetamol treatment and was admitted to hospital on the same day as patient 1. His chest X-ray showed extensive pneumonic infiltration in the left lower lobe (Fig. 2b). A pharyngeal swab for culture and DNA analysis was taken upon hospitalization. Blood for serology was taken on admission and 14 days later. Patient 2 was also treated with 200 mg doxycycline qid for 14 days, and had a prompt and uneventful recovery.
Patient 3
A 26-year-old woman, the daughter of patients 1 and 2 became ill on 3 July, during her summer holiday in Brazil. A summary of the clinical events of the patient is presented in Figure 1. She suffered from fever and neck pain. She started to take quinolones (400 mg ofloxacin qid for 5 days), which she had been prescribed for self-treatment in case of travellers' diarrhoea. Four weeks after onset of symptoms, on returning to Belgium, she was seen in our outpatient clinic. Clinical examination and laboratory tests were normal and blood for C. psittaci serology was sampled.
Veterinary investigation
The bird was 8 weeks of age at the time the patients purchased it. A summary of the clinical events of the bird is presented in Figure 1. Patient 2 had been hand feeding the bird, which was freshly weaned from its mother. Feeding was unsuccessful and the bird already started to show respiratory symptoms on the day of the purchase. The patients described it as ‘a cold’. A Dutch veterinarian, specializing in bird diseases, clinically examined the bird. The veterinarian told the patients that the bird had pneumonia and prescribed Doxoral grains (doxycycline). The bird was recovering from its illness, but died 6 days after purchase because of an unfortunate accident (the bird flew against a window) and the husband immediately buried the bird in the garden. The patients were not exposed to the blood or viscera of the dead bird.
METHODS
Laboratory investigation of human samples
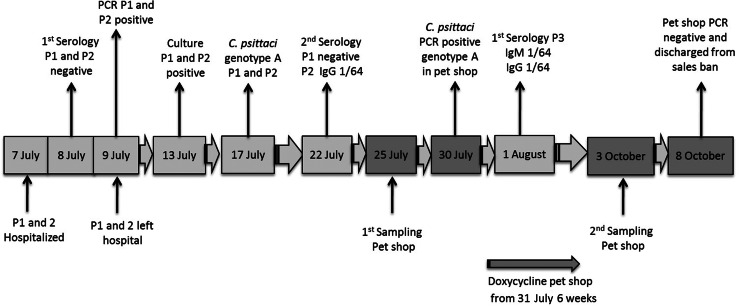
A summary of the laboratory diagnoses can be found in Figure 3. The blood samples taken on admission and at convalescence of patients 1 and 2 were examined for the presence of C. psittaci IgM and IgG antibodies using an indirect immunofluorescence test (Vircell, Spain). The test kit contains slides coated with C. pneumoniae (CM-1), C. trachomatis (434 LGV type II) and C. psittaci (6BC). The latter is an ompA genotype A strain. Patient 3 was only tested serologically (Vircell) after returning home from her holiday.
Fig. 3.
Summary of all C. psittaci diagnostic results. P, Patient.
Pharyngeal swabs of patients 1 and 2 were collected in transport medium and were examined by cell culture using Buffalo Green Monkey (BGM) cells as described by Vanrompay et al. [9]. Cultured C. psittaci were identified by the IMAGEN™ direct immunofluorescence assay (Oxoid, UK) [10]. A second set of pharyngeal swabs collected in DNA stabilization buffer was examined by nested polymerase chain reaction (PCR). The latter amplifies the C. psittaci outer membrane protein A (ompA) gene [11]. C. psittaci isolates were further characterized by the C. psittaci ompA genotype-specific real-time PCR allowing the detection of C. psittaci genotypes A–F and genotype E/B The shop staff were asked about their health status for the period of March up to 8 October [12].
Laboratory investigation of bird samples
A summary of the laboratory diagnoses can be found in Figure 3. Faecal bird samples were collected at the pet shop by a veterinarian of the Dutch Food and Consumer Product Safety Authority (NVWA). Faecal samples were tested by the Dutch reference laboratory for notifiable animal diseases, for the presence of C. psittaci using an in-house-developed ompA-based PCR. Positive samples were subsequently typed by use of a DNA genotyping microarray (C. psitt SeroGenoTye AS-4 kit, Alere Technologies GmbH, Germany) [13].
RESULTS
Laboratory investigation of human samples
For patients 1 and 2, serological blood tests on admission to hospital were negative for psittacosis. Nevertheless, psittacosis was suspected based on exposure to birds in the medical history, the clinical picture, and the coincidence of pneumonia in two relatives. Examination of the pharyngeal swabs by cell culture and PCR indeed revealed psittacosis, caused by C. psittaci genotype A in both patients. The blood samples, taken after recovery, were serologically negative for C. psittaci in patient 1, positive (IgG titre of 1/64) for patient 2 and positive (IgM and IgG titre of 1/64) for patient 3.
Laboratory investigation of bird samples collected at the pet shop
Four (57%) out of seven pooled faecal samples of the first sampling round were positive, including the sample taken from the cage of the purchased lovebird, and were confirmed as genotype A on 20 August 2013. The 12 pooled faecal samples taken after treatment tested negative for C. psittaci by PCR.
Control measures
Patient 1 described the pet shop (Northern Brabant, The Netherlands) as being quite dirty and crowded with birdcages. It was a dry windy summer day, and the three patients had been standing outside in the dust between the cages for at least 45 min. Since C. psittaci is a notifiable disease in Belgium, the local healthcare authorities, the Flemish Agency for Care and Health, were informed. On 10 July 2013, the Flemish Agency for Care and Health contacted their Dutch counterparts, the NVWA. The pet shop was visited on 25 July 2013 by a NVWA veterinarian who took seven fresh faecal samples from the floor of seven cages present in the shop. The samples were sent to the reference laboratory for notifiable animal diseases in The Netherlands. At the time of the visit a total of 156 lovebirds and four parakeets were present and one bird was anorexic and ill. Because of poor hygiene, the shop owner was strongly recommended to clean and disinfect the pet shop. Four (57%) faecal samples tested positive for C. psittaci. Subsequently, all birds in the pet shop were treated with doxycycline for 6 weeks and a sales ban was implemented from the start of treatment. No new birds were allowed to come in during the sales ban. Further source-finding by the NVWA revealed no other sources of psittacosis.
On 3 October 2013, the pet shop was revisited. A total of 148 birds were still present in the shop as 12 had died in the meantime. Twelve pooled faecal samples were taken from the floor of 12 cages and analysed by PCR. All samples were negative and the pet shop was discharged from the sales ban. Afterwards, no more cases of psittacosis were reported that could be linked to the pet shop.
DISCUSSION AND CONCLUSION
The first description of a psittacosis outbreak dates from 1879 by Jacob Ritter, linking the disease to pet parrots and finches. With, on average 2–4 cases yearly, psittacosis is a disease that is only occasionally notified in Flanders. Most notified cases are sporadic. Clustering does occur but outbreaks of psittacosis are very rare in Flanders. In 1994 an outbreak with two serologically confirmed cases and six with atypical pneumonia was described in a group of customhouse officers after an incident involving illegal importation of pet birds [14]. In 1985 a large outbreak with 123 cases, 98 suspicious and 25 serologically confirmed, occurred after a pet bird show [15]. C. psittaci is divided into outer membrane gene A (ompA) genotypes which differ in their virulence for birds [16] and probably also for humans, as most reported cases of human psittacosis are caused by the highly virulent genotype A.
In the present case report, three persons became ill after purchasing a lovebird in a pet shop. C. psittaci-positive birds were present in the pet shop, while the purchased bird, although suffering from pneumonia, was not examined for C. psittaci. Thus, C. psittaci transmission could have occurred at the pet shop and/or while handling the diseased purchased lovebird. The latter had probably less impact on transmission, as the daughter who did not live at the same address as her parents, also became ill.
Laboratory diagnosis of psittacosis
Blood samples from patients 1 and 2 were taken in hospital 11 days after they visited the pet shop and before treatment was started. Nevertheless, serology was negative for both patients. The convalescent sera, taken 14 days after the first sera, were negative for patient 1, but positive for patient 2. Serum of patient 3, taken on 31 July, 5 weeks after exposure, tested positive. Thus, the cluster outbreak was not detected and could not be proven (three- to fourfold rise in antibody titre) by serology only. There is inadequate scientific justification for making clinical decisions about patient management on the basis of C. psittaci serology, as serology at presentation of symptoms is neither sensitive nor specific enough [17–19]. Given that much better methods are available for specific and sensitive detection of a current C. psittaci infection, clinicians are strongly recommended to focus their efforts on seeking direct evidence of a C. psittaci infection wherever possible, using the best locally available test, preferably one using nucleic acid amplification. In the past decade, diagnostic C. psittaci PCR assays have been developed and introduced into the clinical setting. In Belgium and The Netherlands this has aided the diagnostic process for suspected psittacosis cases [20–23].
Public health implications and control measures
A history of exposure to pet birds is essential for identification of psittacosis in patients with pneumonia. Molecular diagnosis is preferred above serology. One of the major advantages of a PCR approach over serological testing is the demonstration of C. psittaci DNA in clinical samples. These samples are therefore suitable for further genotyping assays and for tracing the origin of the infection, which is crucial for preventing further dissemination of the infection. In fact, management of community-acquired pneumonia (CAP) requires sampling prior to treatment. However, (a) early serology is often not followed up with a second sample so serological diagnosis is incomplete and (b) PCR of respiratory samples for psittacosis is not performed as a standard investigation (2011 guidance on CAP from The Netherlands does not recommend PCR routinely nor suggest serology for psittacosis).
The shop staff remained clinically healthy. They had been employed in the shop for 15 years. The staff had also been observed in slaughterhouses processing C. psittaci-positive chickens or turkeys [24]. The good health of the staff could be explained by the fact that the poultry workers were almost continuously exposed to C. psittaci and therefore might have natural immunity against disease.
Active case-finding was not done because of the difficulties in contacting the population at risk and their potential clinicians. Therefore, obligatory recording of the identity of at least the purchasers of Psittaciformes is advisable but, to the best of our knowledge, no country has implemented this obligation by law. Thus, it is simply not done.
Rapidly informing health authorities is warranted so that appropriate action can be taken. In our case, the health authorities of both countries were aware of the outbreak but no new psittacosis cases were notified to these official health authorities.
Cross-border cooperation
Source-finding in this case was complex as the human cases occurred in Belgium and the suspected bird was purchased in The Netherlands; however, the Belgian authorities contacted their Dutch counterparts. Finally, the authorities visited the pet shop where the suspected bird was purchased. Sampling of the pet shop revealed the presence of C. psittaci genotype A. This corresponded with the Belgian test results of the patients and helped to establish the link between the pet shop, the bird and the patients. Instructions regarding extra hygienic measures and treatment of the birds in the pet shop were issued in order to prevent new cases of psittacosis occurring. Observing the timeline of the events shows that it took about 4 weeks before the pet shop was visited. Ideally the time between diagnosing the patients and finding the source should be as short as possible. However, taking into account that two countries and several public health authorities were involved the delay was understandable.
Active case-finding was not performed in Belgium or The Netherlands, as psittacosis is a notifiable disease in both countries. The procedure is to inform the official health authorities as soon as possible (within 24 h after diagnosing psittacosis in a patient), which was indeed done on both sides of the border (GGD region South-East Brabant for The Netherlands and Flemish Agency for Care and Health for Belgium). However, it is wrong to assume that all psittacosis cases will be revealed by this method. Contacting local physicians and the respiratory physicians and microbiologists at the local hospitals might have added some additional cases. Once notified, official health authorities normally start to be on the alert for new cases, but during this cluster outbreak no additional reports on psittacosis were registered. Active case-finding would have been initiated had there been further cases of psittacosis in either area.
ACKNOWLEDGEMENTS
The authors thank the Public Health Service Brabant-Zuidoost (GGD Helmond) for their contribution to the cluster investigation in The Netherlands and the pet shop examination. A. Dumont (Ghent University) is acknowledged for technical assistance. The study was funded by the Federal Public Service of Health, Safety of the Food Chain and Environment (convention RF-11/6245 MINSPEC-PRO).
DECLARATION OF INTEREST
None.
REFERENCES
- 1.Beeckman DSA, Vanrompay DCG. Zoonotic Chlamydophila psittaci infections from a clinical perspective. Clinical Microbiology and Infection 2009; 15: 11–17. [DOI] [PubMed] [Google Scholar]
- 2.Telfer BL, et al. Probable psittacosis outbreak linked to wild birds. Emerging Infectious Diseases 2005; 11: 391–397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Williams J, et al. Community outbreak of psittacosis in a rural Australian town. Lancet 1998; 351: 1697–1699. [DOI] [PubMed] [Google Scholar]
- 4.Moroney JF, et al. Detection of chlamydiosis in a shipment of pet birds, leading to recognition of an outbreak of clinically mild psittacosis in humans. Clinical Infectious Diseases 1998; 26; 1425–1429. [DOI] [PubMed] [Google Scholar]
- 5.Kovácová E, et al. A fatal case of psittacosis in Slovakia, January 2006. Eurosurveillance 2007; 12: pii = 3244. [DOI] [PubMed] [Google Scholar]
- 6.Hughes C, et al. Possible nosocomial transmission of psittacosis. Infection Control & Hospital Epidemiology 1997; 18: 165–168. [DOI] [PubMed] [Google Scholar]
- 7.Ito I, et al. Familial cases of psittacosis: possible person-to-person transmission. Journal of Internal Medicine 2002; 41: 580–583. [DOI] [PubMed] [Google Scholar]
- 8.Wallensten A, Fredlund H, Runehagen A. Multiple human-to-human transmission from a severe case of psittacosis, Sweden, January–February 2013. Eurosurveillance 2014; 19: pii = 20937. [DOI] [PubMed] [Google Scholar]
- 9.Vanrompay D, Ducatelle T, Haesebrouck F. Diagnosis of avian chlamydiosis: specificity of the modified Gimenez staining on smears and comparison of the sensitivity of isolation in eggs and three different cell cultures. Journal of Veterinary Medicine, Series B 1992; 39: 105–112. [DOI] [PubMed] [Google Scholar]
- 10.Vanrompay D, et al. Evalutation of five immunoassays for detection of Chlamydia psittaci in cloacal and conjunctival specimens from turkey. Journal of Clinical Microbiology 1994; 32: 1470–1474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Van Loock M, et al. Use of a nested PCR-enzyme immunoassay with an internal control to detect Chlamydophila psittaci in turkeys. BMC Infectious Diseases 2005; 5: 76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Geens T, et al. Development of a Chlamydophila psittaci species-specific and genotype- specific real-time PCR. Veterinary Research 2005; 36: 787–797. [DOI] [PubMed] [Google Scholar]
- 13.Sache K, et al. Genotyping of Chlamydophila psittaci using a new DNA microarray assay based on sequence analusis of ompA genes. BMC Micobiology 2008; 8: 63–75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.De Schrijver K. A psittacosis outbreak in Belgian customs officers. Eurosurveillance 1995; 0: pii = 173. [DOI] [PubMed] [Google Scholar]
- 15.De Schrijver K. Ornithosis outbreak after a bird exhibit [in Dutch]. Tijdschrift voor Geneeskunde 1987; 43: 501–505. [Google Scholar]
- 16.Vanrompay D, et al. Chlamydia psittaci in turkeys: pathogenesis of infections in avian serovars A, B and D. Veterinary Microbiology 1995; 47: 445–456. [DOI] [PubMed] [Google Scholar]
- 17.Sachse K, et al. Detection of Chlamydia suis from clinical specimens: comparison of PCR, antigen ELISA, and culture. Journal of Medical Methods 2003; 54: 233–238. [DOI] [PubMed] [Google Scholar]
- 18.Beeckman DSA, Vanrompay DCG. Zoonotic Chlamydophila psittaci infections from a clinical perspective. Clinical Microbiology and Infection 2009; 15: 11–17. [DOI] [PubMed] [Google Scholar]
- 19.Rohde G, et al. Chlamydial zoonoses. Deutsches Ärtzeblatt International 2010; 107: 174–180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Heddema ER, et al. Prevalence of Chlamydophila psittaci in fecal droppings from feral pigeons in Amsterdam, The Netherlands. Applied and Environmental Microbiology 2006; 72: 4423–4425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.van der Bruggen T, et al. Fast diagnostics of psittacosis with help from recently developed real-time PCR [in Dutch]. Nederlands Tijdschrift voor Geneeskunde 2008; 152: 1886–1888. [PubMed] [Google Scholar]
- 22.Verminnen K, et al. Evaluation of a Chlamydophila psittaci infection diagnostic platform for zoonotic risk assessment. Journal of Clinical Microbiology 2008; 45: 281–285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Heddema ER, et al. Typing of Chlamydia psittaci to monitor epidemiology of psittacosis and aid disease control in the Netherlands, 2008 to 2013. Eurosurveillance 2015; 20: pii = 21026. [DOI] [PubMed] [Google Scholar]
- 24.Dickx V, et al. Chlamydophilia psittaci zoonotic risk assessment in a chicken and turkey slaughterhouse. Journal of Clinical Microbiology 2010; 48: 3244–3250. [DOI] [PMC free article] [PubMed] [Google Scholar]