Abstract
Aim:
Evaluate cerebrovascular autoregulation (CAR) using near-infrared spectroscopy (NIRS) after pediatric cardiac arrest and determine if deviations from CAR-derived optimal mean arterial pressure (MAPopt) are associated with outcomes.
Methods:
CAR was quantified by a moving, linear correlation between time-synchronized mean arterial pressure (MAP) and regional cerebral oxygenation, called cerebral oximetry index (COx). MAPopt was calculated using a multi-window weighted algorithm. We calculated burden (magnitude and duration) of MAP less than 5 mmHg below MAPopt (MAPopt - 5), as the area between MAP and MAPopt - 5 curves using numerical integration and normalized as percentage of monitoring duration. Unfavorable outcome was defined as death or pediatric cerebral performance category (PCPC) at hospital discharge ≥3 with ≥1 change from baseline. Univariate logistic regression tested association between burden of MAP less than MAPopt - 5 and outcome.
Results:
Thirty-four children (median age 2.9 [IQR 1.5,13.4] years) were evaluated. Median COx in the first 24 h post-cardiac arrest was 0.06 [0,0.20]; patients spent 27% [19,43] of monitored time with COx ≥ 0.3. Patients with an unfavorable outcome (n = 24) had a greater difference between MAP and MAPopt - 5 (13 [11,19] vs. 9 [8,10] mmHg, p = 0.01) and spent more time with MAP below MAPopt - 5 (38% [26,61] vs. 24% [14,28], p = 0.03). Patients with unfavorable outcome had a higher burden of MAP less than MAPopt - 5 than patients with favorable outcome in the first 24 h post-arrest (187 [107,316] vs. 62 [43,102] mmHg × Min/Hr; OR 4.93 [95% CI 1.16–51.78]).
Conclusions:
Greater burden of MAP below NIRS-derived MAPopt - 5 during the first 24 h after cardiac arrest was associated with unfavorable outcomes.
Keywords: Cardiac arrest, Cerebral autoregulation, Cerebrovascular autoregulation, Hypoxic ischemic brain injury, NIRS, Pediatrics
Introduction
Among 20,000 children with cardiac arrests each year in the United States, most do not survive to hospital discharge and many survivors sustain new or worsened neurologic disability.1–6 The goal of post-cardiac arrest care is to reduce secondary brain injury and improve neurologic outcomes.7,8 Hypotension after cardiac arrest can cause cerebral hypoperfusion and is associated with worse outcomes.9–13 Thus, the American Heart Association (AHA) recommends maintaining a systolic blood pressure greater than the 5th percentile for age after cardiac arrest14; however, this approach does not account for variation in individual patients’ cerebrovascular autoregulatory status that impacts cerebral perfusion.
Cerebrovascular autoregulation (CAR) maintains cerebral blood flow over a range of arterial blood pressures (ABP).15–17 After cardiac arrest, CAR is impaired, which can lead to reduced cerebral perfusion and secondary brain injury.7,8,18–20 CAR integrity can be quantified by the correlation between mean arterial pressure (MAP) and a surrogate of cerebral blood flow; a positive correlation reflects loss of CAR integrity. The cerebral oximetry index (COx) is the correlation of regional cerebral tissue oximetry (StO2) derived from near infrared spectroscopy (NIRS) and MAP. The MAP at which a patient’s CAR is most intact can be derived using COx, so-called optimal MAP (MAPopt).21,22 To date, small adult and pediatric post-cardiac arrest studies have demonstrated associations between impaired CAR, CAR-derived MAPopt, and patient outcomes.22–28 However, it is unknown if deviations from CAR-derived MAPopt are associated with outcomes after pediatric cardiac arrest and how CAR-derived MAPopt compares to pediatric blood pressure targets.
The primary objective of this study was to determine the association of deviations from CAR-derived MAPopt during the first 72 h after cardiac arrest using NIRS-derived StO2 with outcomes. Secondary objectives were to determine the association of the severity of CAR impairment with outcomes and the age-based percentiles that correspond with CAR-derived MAPopt. We hypothesized that patients with unfavorable outcomes would have a larger magnitude and duration of MAP deviation below CAR-derived MAPopt than patients with favorable outcomes.
Methods
Study design
This was a retrospective analysis of prospectively collected data on patients ≤18 years old who received ≥1 min of cardiopulmonary resuscitation (CPR) for an in- or out-of-hospital cardiac arrest between November 2018 and March 2021 and received post-cardiac arrest care in the Children’s Hospital of Philadelphia pediatric intensive care unit (PICU). Eligible patients had an invasive arterial catheter and either unilateral or bilateral cerebral NIRS connected to our integrative multimodality neuromonitoring device (Moberg Research, Ambler, PA, USA). Patients were excluded if they received extracorporeal support, had unrepaired cyanotic congenital heart disease, had concomitant severe acute brain injury due to traumatic brain injury or ruptured vascular malformation, or had formal limitations of care at time of eligibility. Additional exclusion criteria were time between ROSC and initiation of multimodal recording >24 h or recording duration less than 6 h.
Post-cardiac arrest care was determined by the clinical team, guided by an institutional pathway.29 The clinical team was blinded to CAR and MAPopt data. Prospectively defined clinical data were abstracted via medical record review and included patient demographics, cardiac arrest characteristics, and details regarding post-cardiac arrest care. Research coordinators abstracting data were blinded to the primary outcome. The study was approved by the Children’s Hospital of Philadelphia Institutional Review Board with a waiver of consent.
Clinical outcomes
Clinical outcome was based on Pediatric Cerebral Performance Category (PCPC) scores, a 6-point scale of global neurologic function: (1) normal; (2) mild disability; (3) moderate disability; (4) severe disability; (5) coma or vegetative state; (6) death.30–32 It was assigned by trained nurse raters for preadmission baseline and at hospital discharge via medical record review and discussions with medical providers. Discrepancies were resolved by consensus of an independent internal review committee. Unfavorable outcome was defined as death or a change in PCPC ≥ 1 from pre-admission that resulted in PCPC score of 3, 4, or 5 at hospital discharge or 30 days post-cardiac arrest, whichever came first. Favorable outcome was defined as PCPC of 1 or 2 at hospital discharge or 30 days post-cardiac arrest, whichever came first, or no change in PCPC from baseline to hospital discharge or 30 days post-cardiac arrest.
Neuromonitoring signal acquisition and processing
Decisions to place an arterial catheter or NIRS device (Nonin SenSmart (Nonin Medical, Inc., Plymouth, MN, USA) or Edwards Foresight (Edwards Lifesciences Corporation, Irvine, CA, USA)) were made by the clinical team.29 An integrative bedside multimodality neuromonitoring device (Moberg Research, Ambler, PA, USA) was deployed by nurses per provider request and device availability. The device facilitated time synchronization of ABP and StO2 signals.
Artifacts were computationally removed using a semi-automated sliding-window median filter with variable window length to account for differences in amount of artifact. Given the homogeneous nature of post-cardiac arrest hypoxic-ischemic brain injury, right and left cerebral StO2 values were averaged together when both were available. Data obtained from the first 72 h after ROSC were divided into post-cardiac arrest Day 1 (0–24 h), Day 2 (24–48 h), and Day 3 (48–72 h).
Cerebrovascular autoregulation and MAPopt determination
CAR was assessed using NIRS-based cerebral oximetry index (COx), calculated as a Pearson correlation coefficient between 10-s averaged values of MAP and corresponding NIRS-measured StO2 over a 5-min window.33,34 COx values ranged from −1 to +1; negative or near-zero COx values, which result from MAP and StO2 being either negatively correlated or not correlated, respectively, indicated intact CAR. In contrast, impaired CAR was indicated by positive COx values due to MAP and StO2 being positively correlated. By moving the 5-min window by 1 min and repeating the correlation (80% overlap of data), COx can be analyzed as a continuous variable.35 A COx ≥ 0.3 was a priori defined as impaired CAR.36
To determine each patient’s MAPopt over time, we used a multi-window approach.37 COx was calculated using 3, 5, 10, 20, 30, 60, 90, and 120-min windows. Using windows of data from the prior 1–24 h (i.e., 1, 2, 4, 6, 8, 12, and 24 h), for each COx window, we plotted COx values versus corresponding MAP in 5 mmHg bins (e.g., 50–55 mgHg). We applied the Fisher transform to binned COx values to avoid ceiling effects.21 We then applied an automated curve fitting algorithm to fit a second-order polynomial representing a convex parabola.21,24,37–40 The nadir of the fitted curve (i.e., MAP where COx was most negative) represented MAPopt. This process generated up to 51 COx versus MAP parabolic curves that were combined using a weighted average to determine MAPopt. Curves with a better convex parabolic fit (i.e., greater adjusted r2 value) and those with more negative COx values at the nadir were given greater weight to generate MAPopt.37,38,41,42 This process was repeated to calculate an updated MAPopt every minute.
We also determined lower and upper limits of autoregulation (LLA and ULA, respectively) over time for each patient. The LLA is the MAP at which CBF decreases with decreasing MAP. Similarly, ULA is the MAP at which CBF increases with increasing MAP. We defined LLA and ULA as MAPs where COx-MAP parabolic curves crossed a COx value of 0.3.38
Data analyses
We compared each patient’s MAP to their CAR-derived MAPopt ± 5 mmHg to account for anticipated clinical variations in MAP and to make the results more clinically applicable.24,26 For each subject and for each post-cardiac arrest day, we calculated mean magnitude of difference between MAP and MAPopt - 5 mmHg during times when MAP < MAPopt - 5 mmHg. Analogously, we calculated mean magnitude of the difference between MAP and MAPopt + 5 mmHg during the times when MAP > MAPopt + 5 mmHg. We further calculated the percent of time MAP was 1) below MAPopt - 5 mmHg, 2) within MAPopt ± 5 mmHg, and 3) above MAPopt + 5 mmHg, for Days 1, 2 and 3 post-cardiac arrest. Using numerical integration, the burden (combination of magnitude and duration; mmHg × min/hour) of MAP below MAPopt - 5 was defined as the normalized area between MAP and MAPopt - 5 waveforms during the times when MAP < MAPopt - 5 (Fig. 1). This burden was computed for each post-cardiac arrest day and normalized by the amount of time concomitant MAP and MAPopt waveforms were available. Analogous burdens of MAP above MAPopt + 5 (i.e., normalized area between MAP and MAPopt + 5 waveforms during the times when MAP > MAPopt + 5) were also computed for each post-cardiac arrest day.
Fig. 1 –
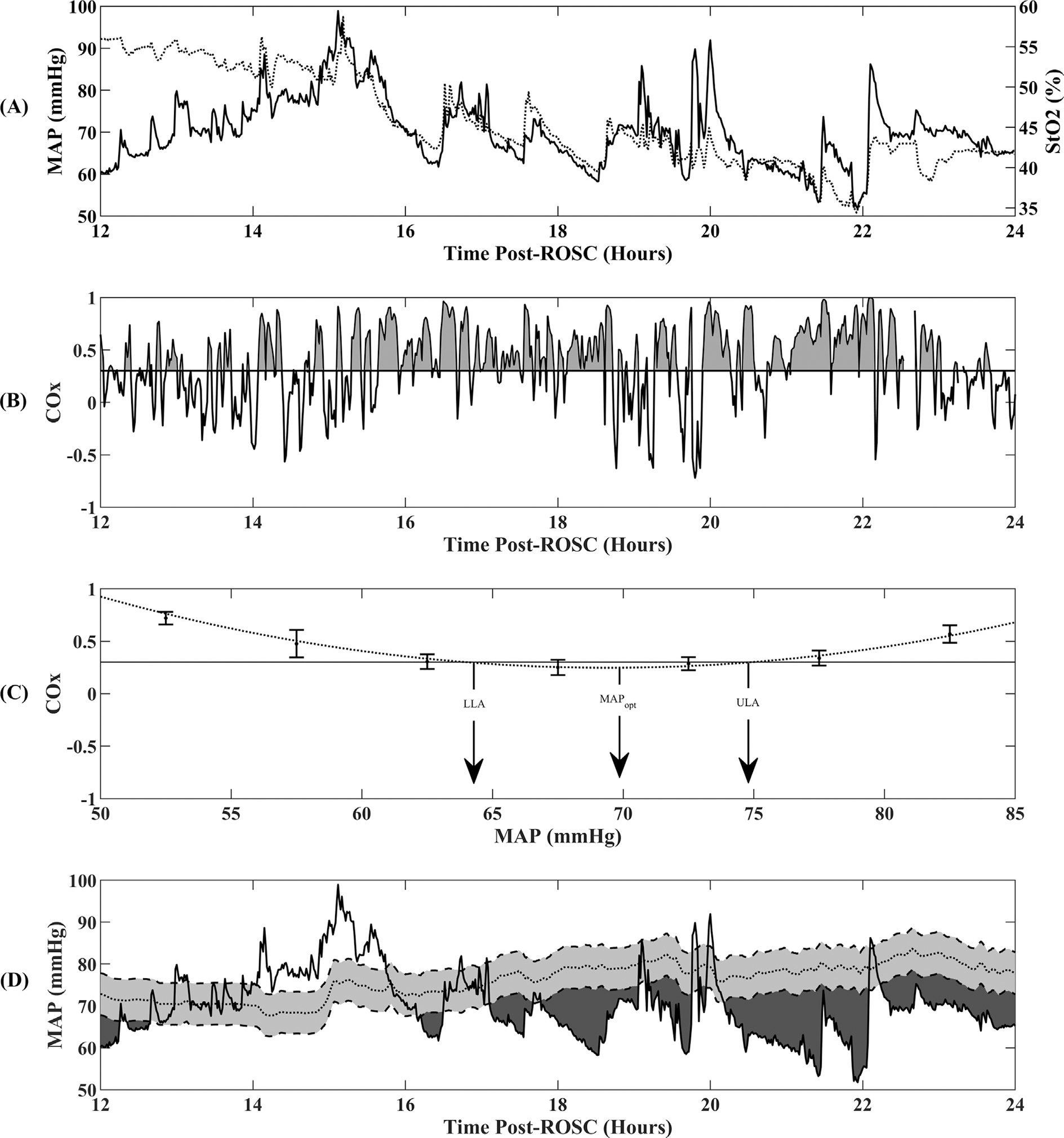
Physiologic data over 12 h of recording from a representative patient. The four rows depict (A) mean arterial pressure (MAP, solid line) and regional cerebral tissue oximetry (StO2, dotted line) derived from NIRS; (B) cerebral oximetry index (COx), the gray shaded region on the COx curve represents the burden of impaired CAR which was defined as the area below the COx waveform and COx threshold of 0.3, and normalized as a percentage of the monitoring duration; (C) representative COx versus binned MAP parabolic curve demonstrating MAPopt at the nadir of the curve and the lower and upper limits of autoregulation (LLA and ULA) where parabolic curve crosses 0.3; and (D) MAP (solid line), MAPopt (dotted line), gray shaded region is ±5 mmHg of MAPopt, black region is burden of MAP less than MAPopt - 5.
In a secondary analysis, we calculated the burden of impaired CAR (i.e., the normalized area between the COx waveform and the COx threshold of 0.3 during the times when Cox > 0.3 (Fig. 1)), for each post-cardiac arrest day.43,44 We additionally investigated brain oxygenation in relation to MAP by computing the mean StO2 for each patient when MAP was 1) less than MAPopt - 5, 2) within MAPopt ± 5, and 3) greater than MAPopt + 5 for each post-cardiac arrest day. Finally, using published normative values from critically ill children,45 we determined age-based MAP percentile equivalent for MAP, MAPopt, LLA, and ULA, for each post-cardiac arrest day.
Statistical analysis
Descriptive statistics are reported as median and interquartile ranges (IQR) for continuous variables and frequencies with percentages for categorical variables. Chi-squared or Fisher exact tests were used to test associations between categorical variables and clinical outcome and Wilcoxon rank-sum was used to compare differences in continuous variables between outcome groups. All statistical tests were two-sided, and p < 0.05 was considered to indicate significance. The primary exposure was the burden of MAP less than MAPopt - 5. Univariate logistic regression model tested the association between the normalized burden of MAP less than MAPopt - 5 and outcome. Analyses were performed using GraphPad Prism (v5.03, GraphPad Software Inc., La Jolla, CA, USA), IBM SPSS Statistics (v26.0, IBM Corp., Armonk, NY, USA) or MATLAB (vR2018a, The Mathworks, Inc., Natick, MA, USA).
3D visualization of the burden of MAP below MAPopt
A customized 3D visualization technique depicted the association between magnitude and duration of MAP below MAPopt and outcome.46 Using minute-by-minute MAP and MAPopt data, for each pair of a given magnitude (MAP below MAPopt by 0 to 30 mmHg) and for a given duration (0–40 min), the ratio between number of patients with an unfavorable outcome and total number of patients who experienced at least one such episode was recorded. This ratio, indicating probability of an unfavorable outcome, was displayed on a 3D color-coded contour plot.
Results
Forty-six patients met inclusion criteria. Twelve patients were excluded due > 24 h from ROSC to data recording (n = 3), inadequate recording duration (n = 4), ECMO (n = 2), or concomitant non-hypoxic-ischemic severe acute brain injury (n = 3). Data from 34 patients were analyzed. The median age was 2.9 [IQR 1.5, 13.4] years and 71% (n = 24) were male. Thirty-eight percent had no past medical history and 59% had baseline PCPC of 1. Seventy-one percent (n = 24) of patients had unfavorable outcomes; 63% (15/24) did not survive to hospital discharge. Demographic and cardiac arrest characteristics are summarized in Table 1.
Table 1 –
Patient demographics, cardiac arrest characteristics by outcomes.
| Patient demographics/Cardiac arrest characteristics | All Patients (n = 34) | Favorable Outcome (n = 10) | Unfavorable Outcome (n = 24) | P-value |
|---|---|---|---|---|
|
| ||||
| Age (years), median’ (IQR) | 2.9 [1.5, 13.4] | 11.7 [2.7, 14.6] | 2.7 [0.7, 9.2] | 0.11 |
| Gender (male), N (%) | 24 (71) | 9 (90) | 15 (63) | 0.22 |
| Preexisting conditions | ||||
| None | 13 (38) | 6 (60) | 7 (29) | 0.09 |
| Premature infant | 10 (29) | 2 (20) | 8 (33) | 0.68 |
| Congenital heart disease | 6 (18) | 1 (10) | 5 (21) | 0.64 |
| Congenital malformation | 4 (12) | 0 (0) | 4 (17) | 0.30 |
| Cancer/BMT | 3 (9) | 1 (10) | 2 (8) | 1.00 |
| Respiratory disorder/CLD | 10 (29) | 1 (10) | 9 (38) | 0.22 |
| Renal insufficiency | 4 (12) | 1 (10) | 3 (13) | 1.00 |
| Neurological disorder | 5 (15) | 0 (0) | 5 (21) | 0.29 |
| Solid organ transplant | 1 (3) | 0 (0) | 1 (4) | 1.00 |
| Baseline PCPC | ||||
| PCPC 1 | 20 (59) | 8 (80) | 12 (50) | 0.47 |
| PCPC 2 | 4 (12) | 0 (0) | 4 (17) | |
| PCPC 3 | 6 (18) | 1 (10) | 5 (21) | |
| PCPC 4 | 4 (12) | 1 (10) | 3 (13) | |
| Arrest Etiology | 0.19 | |||
| Respiratory failure | 12 (35) | 3 (30) | 9 (38) | |
| Arrhythmia | 3 (9) | 3 (30) | 0 (0) | |
| Hypotension/shock/sepsis | 3 (9) | 1 (10) | 2 (8) | |
| Ingestion/toxic | 1 (3) | 0 (0) | 1 (4) | |
| Metabolic/electrolyte | 2 (6) | 1 (10) | 1 (4) | |
| Seizures | 1 (3) | 0 (0) | 1 (4) | |
| Drowning | 3 (9) | 0 (0) | 3 (13) | |
| Unknown | 9 (26) | 2 (20) | 7 (29) | |
| Out-of-hospital cardiac arrest | 22 (65) | 5 (50) | 17 (71) | 0.27 |
| CPR duration (min), (n = 27) | 12 [6,32] | 6 [5,10] | 28 [9, 36] | 0.01 |
| Number of Epi doses | 0.09 | |||
| 0–2 | 19 (56) | 9 (90) | 10 (42) | |
| 3–5 | 4 (12) | 0 (0) | 4 (17) | |
| >5 | 7 (21) | 1 (10) | 6 (25) | |
| Unknown | 4 (12) | 0 () | 4 (17) | |
| Initial Rhythm | ||||
| PEA/Asystole | 17 (50) | 3 (30) | 14 (58) | 0.06 |
| Bradycardia | 4 (12) | 2 (20) | 2 (8) | |
| V-tachycardia/fibrillation | 3 (9) | 3 (30) | 0 (0) | |
| Not documented/Unknown | 10 (29) | 2 (20) | 8 (33) | |
| Initial pH, median, (IQR) | 6.98 [6.81, 7.19] | 7.18 [7.05, 7.28] | 6.87 [6.75, 7.09] | 0.004 |
| Initial Lactate, median’ (IQR) | 7.3 [4.8, 11.3] | 6.0 [4.9, 7.3] | 8.0 [4.7, 12.7] | 0.14 |
| Temperature target | 0.07 | |||
| 33.0 °C | 4 (12) | 3 (30) | 1 (4) | |
| 36.0 °C | 30 (88) | 7 (70) | 23 (96) | |
| PCPC (hospital discharge) | <0.001 | |||
| PCPC 1 | 3 (9) | 3 (30) | 0 (0) | |
| PCPC 2 | 5 (15) | 5 (50) | 0 (0) | |
| PCPC 3 | 5 (15) | 1 (10) | 4 (17) | |
| PCPC 4 | 5 (15) | 1 (10) | 4 (17) | |
| PCPC 5 | 2 (6) | 0 (0) | 2 (8) | |
| PCPC 6 | 14 (41) | 0 (0) | 14 (58) | |
| PCPC Change (Discharge - Baseline) | <0.001 | |||
| 0 | 5 (15) | 5 (50) | 0 (0) | |
| 1 | 7 (21) | 5 (50) | 2 (8) | |
| 2 | 9 (27) | 0 (0) | 9 (38) | |
| 3 | 4 (12) | 0 (0) | 4 (17) | |
| 4 | 2 (6) | 0 (0) | 2 (8) | |
| 5 | 7 (21) | 0 (0) | 7 (29) | |
The median time between ROSC and initiation of data recording was 3 [2,7] h. A median of 52 [33, 63] h of concomitant MAP and StO2 data were recorded in the first 72 h post-cardiac arrest.
Association between deviations from MAPopt and outcome
We were able to calculate MAPopt in 94% [86%, 97%] of recorded time. On post-cardiac arrest Day 1, the magnitude of difference between MAP and MAPopt - 5 and percent of time MAP was less than MAPopt - 5 were greater for patients with unfavorable compared to favorable outcomes (Table 2). Burden of MAP less than MAPopt - 5 was greater for patients with unfavorable versus favorable outcomes (187 mmHg*Min/Hr [107, 316] vs. 62 mmHg*Min/Hr [43, 102], p = 0.01). Odds of unfavorable outcomes were 4.9 times higher for each standard deviation increase in burden of MAP less than MAPopt - 5 (OR 4.93 [95% CI 1.16 to 51.78]). Fig. 2 demonstrates the impact of magnitude and duration of MAP less than MAPopt - 5 on probability of unfavorable outcome.
Table 2 –
Burden of MAP deviation from MAPopt, index of impaired CAR, and StO2 on Day 1 after cardiac arrest.
| Cerebral physiology parameter Median [IQR] |
Favorable (n = 10) | Unfavorable (n = 24) | p-value |
|---|---|---|---|
|
| |||
| MAP < MAPopt−5 | |||
| Magnitude MAP below MAPopt − 5 (mmHg) | 9 [8, 10] | 13 [11, 17] | 0.01 |
| Percent time MAP < MAPopt−5 (%) | 24 [14, 28] | 38 [26, 61] | 0.03 |
| Burden MAP < MAPopt−5 (mmHg*Min/Hr) | 62 [43, 102] | 187 [107, 318] | 0.01 |
| MAP within ± 5 of MAPopt | |||
| Percent time MAP within ± 5 of MAPopt (%) | 36 [28, 40] | 31 [17, 43] | 0.30 |
| MAP > MAPopt + 5 | |||
| Magnitude MAP above MAPopt + 5 (mmHg) | 12 [10, 12] | 12 [9, 15] | 0.83 |
| Percent time MAP > MAPopt + 5 (%) | 36 [27, 44] | 21 [13, 38] | 0.07 |
| Burden MAP > MAPopt + 5 (mmHg*Min/Hr) | 272 [198, 306] | 207 [92, 295] | 0.14 |
| COx | |||
| COx | 0.06 [−0.01, 0.20] | 0.08 [−0.00, 0.20] | 0.92 |
| Percent time COx ≥ 0.3 (%) | 25 [19, 42] | 32 [19, 44] | 0.78 |
| Percent COx burden ≥ 0.3 (%) | 8 [4, 16] | 10 [5, 16] | 0.66 |
| StO2 | |||
| StO2 (%) | 76 [70, 81] | 70 [59, 78] | 0.07 |
| StO2 when MAP < MAPopt−5 (%) | 76 [73, 80] | 70 [58, 78] | 0.10 |
| StO2 when MAP within ± 5 MAPopt (%) | 76 [69, 83] | 71 [59, 79] | 0.11 |
| StO2 when MAP > MAPopt + 5 (%) | 77 [71, 81] | 69 [60, 78] | 0.07 |
Fig. 2 –
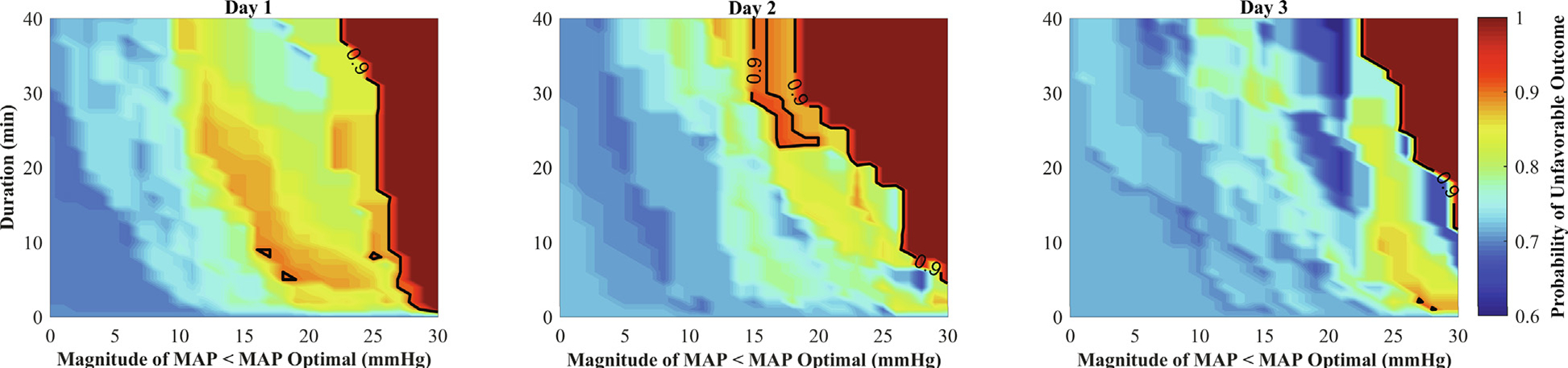
Association between magnitude and duration of MAP less than MAPopt on the probability of an unfavorable outcome for Days 1, 2, and 3 after cardiac arrest. Color scale represents the probability of an unfavorable outcome. Dark blue indicates low probability of an unfavorable outcome and dark red indicates a high probability of an unfavorable outcome.
There were no differences between patients with favorable and unfavorable outcomes for magnitude, duration, or burden of MAP less than MAPopt - 5 on Days 2 or 3 post-cardiac arrest (Supplementary Table 1). Odds of unfavorable outcomes were not increased based on burden of MAP less than MAPopt - 5 for Days 2 (OR 1.60 [0.72 to 4.59]) or 3 (OR 1.21 [0.58 to 3.08]) post-cardiac arrest. Magnitude, duration, and burden of MAP within MAPopt ± 5 or greater than MAPopt + 5 were not significantly different between patients with favorable or unfavorable outcomes on any post-cardiac arrest day (Supplementary Table 1).
There were no differences in StO2 between patients with unfavorable or favorable outcomes on any day after arrest (Table 2 and Supplementary Table 1).
CAR impairment
COx was ≥ 0.3 28% [20%, 35%] of time. Median COx, percent time COx ≥ 0.3, and percent burden COx ≥ 0.3 on post-cardiac arrest Days 1, 2, or 3 did not differ between favorable and unfavorable outcome groups (Table 2 and Supplemental Table 1).
Comparing MAPopt, LLA and ULA to age-based MAP percentiles
MAPopt for post-cardiac arrest Days 1, 2, and 3 was equivalent to the 77th [62, 88] percentile of MAP for age. The LLA and ULA were equivalent to the 22nd [13,37] and 98th [94, 99] percentiles of MAP for age. The range between LLA and ULA was 38 mmHg [29, 44], and did not differ between favorable and unfavorable outcome groups (38 mmHg [27, 43] vs 39 mmHg [29, 44], p = 0.696). There were no differences in percentiles of MAPopt, LLA and ULA for age between favorable and unfavorable outcome groups on any post-cardiac arrest day (Table 3).
Table 3 –
Age-based blood pressure percentiles of MAP, MAPopt, LLA and ULA by outcome.
| Age-based percentile of MAP Median [IQR] |
Favorable* (n = 10) | Unfavorable (n = 24) | p-value |
|---|---|---|---|
|
| |||
| Age-based percentile of MAP | |||
| Day 1 | 77th [49, 85] | 64th [48, 93] | 0.84 |
| Day 2 | 63rd [36, 91] | 70th [51, 82] | 0.80 |
| Day 3 | 66th [36, 86] | 77th [61, 85] | 0.49 |
| Age-based percentile of MAPopt | |||
| Day 1 | 68th [56, 80] | 83rd [58, 94] | 0.26 |
| Day 2 | 66th [59, 77] | 69th [59, 83] | 0.56 |
| Day 3 | 69th [43, 88] | 82nd [65, 93] | 0.23 |
| Age-based percentile of LLA | |||
| Day 1 | 24th [6, 34] | 27th [19, 46] | 0.20 |
| Day 2 | 8th [3, 31] | 23rd [16, 32] | 0.15 |
| Day 3 | 7th [3, 29] | 23rd [8, 47] | 0.13 |
| Age-based percentile of ULA | |||
| Day 1 | 94th [91, 98] | 98th [90, 99] | 0.28 |
| Day 2 | 96th [93, 99] | 95th [92, 99] | 0.86 |
| Day 3 | 98th [92, 99] | 99th [94, 99] | 0.32 |
Day 2: n = 9 for favorable patients and 23 for unfavorable patients.
Day 3: n = 8 for favorable patients and 20 for unfavorable patients.
Discussion
In this single center study of CAR following pediatric cardiac arrest, patients whose MAP was more than 5 mmHg below their CAR-derived MAPopt in the first 24 h after cardiac arrest were more likely to have unfavorable outcomes. Both magnitude and duration of MAP deviation from MAPopt were associated with unfavorable outcomes, however the severity of CAR impairment, as measured by COx, was not. Notably, CAR-derived MAPopt was equivalent to the 77th percentile for age and the difference between the lower and upper limits of CAR was 38 mmHg, suggesting that after cardiac arrest the MAP range of intact CAR is substantially narrowed and that blood pressures higher than age-based means may be required to maintain adequate cerebral perfusion.
The association between magnitude and duration of MAP deviation below CAR-derived MAPopt and outcomes implies that cerebral hypoperfusion occurs when MAP is below MAPopt and this critical reduction in cerebral blood flow contributes to secondary brain injury and unfavorable outcomes. Interestingly, COx, an index of CAR, was not different between patients with favorable and unfavorable outcomes and the burden of impaired CAR was also not different. Thus, impaired CAR may predispose patients to brain injury, but both duration and magnitude of MAP deviation below each patient’s individual MAPopt is a more substantial contributor to secondary brain injury.
Our results build upon findings from adults23,24,26,47 and children25 that have examined CAR using NIRS after cardiac arrest and found varying associations between impaired CAR, deviation of MAP from MAPopt, and outcomes.22 Lee and colleagues found that a greater area under the MAPopt curve, similar to the computed burden in the current study, on day 2 after cardiac arrest was associated with children receiving tracheostomy or gastrostomy tubes but did not find an association with changes in PCPC scores.25 Ameloot et al. demonstrated percentage time spent below MAPopt was negatively associated with survival.23 A recent multicenter adult study using a similar multi-window approach to our study failed to find differences in CAR metrics and deviation from MAPopt between outcome groups.24
The AHA guidelines recommend maintaining a systolic blood pressure greater than the 5th percentile for age during post-cardiac arrest care.14 This threshold was based on observational studies that demonstrated worse outcomes, primarily survival, when systolic hypotension (below 5th percentile for age and sex) was present following cardiac arrest.7,14 In our study, CAR-derived MAPopt was approximately the 75th percentile for age. In adults, targeting a higher MAP (80–100 mmHg versus 65–75 mmHg) improved cerebral oxygenation, but not biomarkers of brain injury, hypoxic-ischemic injury on MRI, or neurologic outcomes.48,49 Our data suggest that active titration of blood pressure to cerebral hemodynamic parameters like MAPopt or LLA is a potentially promising approach to improve outcomes rather than simply avoiding age-based 5th percentile blood pressures. This approach is the subject of an ongoing feasibility study in adults with traumatic brain injury.41
This study has several limitations. Although cerebral NIRS is attractive because of non-invasiveness and feasibility, the NIRS signal can be influenced by sensor placement, ambient light, and scalp blood flow, which would tend to skew our results to the null. Nevertheless, NIRS-derived CAR parameters were associated with outcomes. Due to small sample size, we were unable to control for cardiac arrest characteristics associated with outcomes (e.g., arrest location, arrest duration, initial rhythm). Due to small sample size, there was the potential for not finding significant differences where they may exist (type II errors). Similarly, with multiple comparisons in a small sample there was the potential for type I error. Future studies of larger cohorts of patients are necessary to adequately address these limitations. As in previous studies, duration of CPR was associated with outcomes.7 Because CAR impairment after cardiac arrest presumably reflects severity of brain injury, it is thus in the causal pathway from injury severity to outcome. Future larger studies are needed to evaluate the association of pre-, intra- and post-cardiac arrest features on CAR impairment. We used a COx threshold of 0.3 to define impaired CAR, consistent with adult post-cardiac arrest studies,24,26 although pediatric trials are needed to more clearly define thresholds of impaired CAR that are associated with outcomes. All patients had invasive arterial catheters, cerebral NIRS, and multimodality neuromonitoring. Therefore, these data may not be generalizable to less severely injured patients without such intensive post-cardiac arrest monitoring. While we attempted to enroll consecutive patients, data collection was limited by technical considerations or clinical circumstances. Some patients did not have data collection through Day 3 after cardiac arrest, mainly due to clinical decisions to remove the arterial catheter or stop NIRS monitoring.
Conclusions
A greater burden of MAP below NIRS-derived MAPopt - 5 was associated with unfavorable outcomes in children within the first 24 h after cardiac arrest. Further research is needed to determine whether active titration of blood pressure to cerebral hemodynamic parameters like MAPopt can limit secondary brain injury and improve outcomes after pediatric cardiac arrest.
Supplementary Material
Acknowledgements
We thank Kathryn Graham for assistance with database management and medical chart review. We appreciate the support of the nurses in the CHOP PICU who were instrumental in timely deployment of our neuromonitoring system.
Funding
Funding provided by the Department of Anesthesiology and Critical Care Medicine at the Children’s Hospital of Philadelphia.
Footnotes
CRediT authorship contribution statement
Matthew Kirschen: Conceptualization, Methodology, Validation, Formal analysis, Investigation, Data curation, Project administration, Writing–original draft. Tanmay Majmudar: Methodology, Software, Formal analysis, Investigation, Writing – review & editing, Visualization. Forrest Beaulieu: Investigation, Data curation, Writing – review & editing. Ryan Burnett: Investigation, Data curation, Writing – review & editing. Mohammed Shaik: Methodology, Software, Formal analysis, Investigation, Writing – review & editing. Ryan W. Morgan: Conceptualization, Methodology, Validation, Formal analysis, Writing – review & editing. Wesley Baker: Supervision, Methodology, Writing – review & editing. Tiffany Ko: Methodology, Investigation, Writing – review & editing. Ramani Balu: Conceptualization, Methodology, Investigation, Writing – review & editing. Kenya Agarwal: Investigation, Writing – review & editing. Kristen Lourie: Investigation, Writing – review & editing. Robert Sutton: Supervision, Methodology, Investigation, Writing – review & editing. Todd Kilbaugh: Supervision, Conceptualization, Methodology, Investigation, Writing – review & editing. Ramon Diaz-Arrastia: Supervision, Conceptualization, Methodology, Writing – review & editing. Robert Berg: Supervision, Conceptualization, Methodology, Investigation, Resources, Writing – review & editing, Funding acquisition. Alexis Topjian: Supervision, Conceptualization, Methodology, Formal analysis, Investigation, Resources, Writing – review & editing.
Conflicts of interest
Dr. Morgan is supported by a grant from NIH NHLBI (K23HL148541). Dr. Sutton is supported by NIH grants to the institution. Dr. Topjian is the Chair of the AHA Emergency Cardiovascular Care Committee Pediatric Guidelines Writing Group. The remaining authors have disclosed that they do not have any conflicts of interest.
Appendix A. Supplementary material
Supplementary data to this article can be found online at https://doi.org/10.1016/j.resuscitation.2021.09.023.
REFERENCES
- 1.Benjamin EJ, Virani SS, Callaway CW, et al. Heart disease and stroke statistics-2018 update: a report from the American Heart Association. Circulation 2018;137:e67–e492. [DOI] [PubMed] [Google Scholar]
- 2.Holmberg MJ, Ross CE, Fitzmaurice GJ, et al. Annual incidence of adult and pediatric in-hospital cardiac arrest in the United States. Circulation 2019;12. [PMC free article] [PubMed] [Google Scholar]
- 3.Slomine BS, Silverstein FS, Christensen JR, et al. Neuropsychological outcomes of children 1 year after pediatric cardiac arrest: secondary analysis of 2 randomized clinical trials. JAMA Neurol 2018;75:1502. 10.1001/jamaneurol.2018.2628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Slomine BS, Silverstein FS, Christensen JR, et al. Neurobehavioral outcomes in children after out-of-hospital cardiac arrest. Pediatrics 2016;137:e20153412. 10.1542/peds.2015-3412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Morgan RW, Kirschen MP, Kilbaugh TJ, Sutton RM, Topjian AA. Pediatric In-Hospital Cardiac Arrest and Cardiopulmonary Resuscitation in the United States: A Review. JAMA Pediatr 2021;175:293. 10.1001/jamapediatrics.2020.5039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Holmberg MJ, Wiberg S, Ross CE, et al. Trends in survival after pediatric in-hospital cardiac arrest in the United States. Circulation 2019;140:1398–408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Topjian AA, de Caen A, Wainwright MS, et al. Pediatric post-cardiac arrest care: a scientific statement from the American Heart Association. Circulation 2019;140. 10.1161/CIR.0000000000000697. [DOI] [PubMed] [Google Scholar]
- 8.Neumar RW, Nolan JP, Adrie C, et al. Post-cardiac arrest syndrome: epidemiology, pathophysiology, treatment, and prognostication. A consensus statement from the International Liaison Committee on Resuscitation (American Heart Association, Australian and New Zealand Council on Resuscitation, European Resuscitation Council, Heart and Stroke Foundation of Canada, InterAmerican Heart Foundation, Resuscitation Council of Asia, and the Resuscitation Council of Southern Africa); the American Heart Association Emergency Cardiovascular Care Committee; the Council on Cardiovascular Surgery and Anesthesia; the Council on Cardiopulmonary, Perioperative, and Critical Care; the Council on Clinical Cardiology; and the Stroke Council. Circulation 2008;118:2452–83. [DOI] [PubMed] [Google Scholar]
- 9.Topjian AA, French B, Sutton RM, et al. Early postresuscitation hypotension is associated with increased mortality following pediatric cardiac arrest. Crit Care Med 2014;42:1518–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Conlon TW, Falkensammer CB, Hammond RS, Nadkarni VM, Berg RA, Topjian AA. Association of left ventricular systolic function and vasopressor support with survival following pediatric out-of-hospital cardiac arrest. Pediatr Crit Care Med 2015;16:146–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Laverriere EK, Polansky M, French B, Nadkarni VM, Berg RA, Topjian AA. Association of Duration of Hypotension With Survival After Pediatric Cardiac Arrest. Pediatr Crit Care Med 2020;21:143–9. [DOI] [PubMed] [Google Scholar]
- 12.Topjian AA, Telford R, Holubkov R, Nadkarni VM, Berg RA, Dean JM, Moler FW. Association of Early Postresuscitation Hypotension With Survival to Discharge After Targeted Temperature Management for Pediatric Out-of-Hospital Cardiac Arrest: Secondary Analysis of a Randomized Clinical Trial. JAMA Pediatr 2018;172:143. 10.1001/jamapediatrics.2017.4043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Topjian AA, Telford R, Holubkov R, et al. The association of early post-resuscitation hypotension with discharge survival following targeted temperature management for pediatric in-hospital cardiac arrest. Resuscitation 2019;141:24–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Topjian AA, Raymond TT, Atkins D, et al. Part 4: Pediatric Basic and Advanced Life Support: 2020 American Heart Association Guidelines for Cardiopulmonary Resuscitation and Emergency Cardiovascular Care. Circulation 2020;142:S469–523. [DOI] [PubMed] [Google Scholar]
- 15.Carrera E, Lee LK, Giannopoulos S, Marshall RS. Cerebrovascular reactivity and cerebral autoregulation in normal subjects. J Neurol Sci 2009;285:191–4. [DOI] [PubMed] [Google Scholar]
- 16.Tasker RC. Intracranial pressure and cerebrovascular autoregulation in pediatric critical illness. Semin Pediatr Neurol 2014;21:255–62. [DOI] [PubMed] [Google Scholar]
- 17.Lassen NA. Autoregulation of cerebral blood flow. Circ Res 1964;15:201–4. [PubMed] [Google Scholar]
- 18.Sundgreen C, Larsen FS, Herzog TM, Knudsen GM, Boesgaard S, Aldershvile J. Autoregulation of cerebral blood flow in patients resuscitated from cardiac arrest. Stroke 2001;32:128–32. [DOI] [PubMed] [Google Scholar]
- 19.Sekhon MS, Ainslie PN, Griesdale DE. Clinical pathophysiology of hypoxic ischemic brain injury after cardiac arrest: a “two-hit” model. Crit Care 2017;21:90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Nishizawa H, Kudoh I. Cerebral autoregulation is impaired in patients resuscitated after cardiac arrest. Acta Anaesthesiol Scand 1996;40:1149–53. [DOI] [PubMed] [Google Scholar]
- 21.Aries MJ, Czosnyka M, Budohoski KP, et al. Continuous determination of optimal cerebral perfusion pressure in traumatic brain injury. Crit Care Med 2012;40:2456–63. [DOI] [PubMed] [Google Scholar]
- 22.Rikhraj KJK, Wood MD, Hoiland RL, Thiara S, Griesdale DEG, Sekhon MS. Determining optimal mean arterial pressure after cardiac arrest: a systematic review. Neurocrit Care 2021;34:621–34. [DOI] [PubMed] [Google Scholar]
- 23.Ameloot K, Genbrugge C, Meex I, et al. An observational near-infrared spectroscopy study on cerebral autoregulation in post-cardiac arrest patients: time to drop ‘one-size-fits-all’ hemodynamic targets? Resuscitation 2015;90:121–6. [DOI] [PubMed] [Google Scholar]
- 24.Griesdale DEG, Sekhon MS, Wood MD, et al. Near-infrared spectroscopy to assess cerebral autoregulation and optimal mean arterial pressure in patients with hypoxic-ischemic brain injury: a prospective multicenter feasibility study. Crit Care Explor 2020;2: e0217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Lee JK, Brady KM, Chung SE, et al. A pilot study of cerebrovascular reactivity autoregulation after pediatric cardiac arrest. Resuscitation 2014;85:1387–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Sekhon MS, Smielewski P, Bhate TD, et al. Using the relationship between brain tissue regional saturation of oxygen and mean arterial pressure to determine the optimal mean arterial pressure in patients following cardiac arrest: a pilot proof-of-concept study. Resuscitation 2016;106:120–5. [DOI] [PubMed] [Google Scholar]
- 27.Hoiland RL, Sekhon MS, Cardim D, et al. Lack of agreement between optimal mean arterial pressure determination using pressure reactivity index versus cerebral oximetry index in hypoxic ischemic brain injury after cardiac arrest. Resuscitation 2020;152:184–91. [DOI] [PubMed] [Google Scholar]
- 28.Burton VJ, Gerner G, Cristofalo E, et al. A pilot cohort study of cerebral autoregulation and 2-year neurodevelopmental outcomes in neonates with hypoxic-ischemic encephalopathy who received therapeutic hypothermia. BMC Neurol 2015;15. 10.1186/s12883-015-0464-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Fowler JC, Wolfe HA, Xiao R, et al. Deployment of a clinical pathway to improve postcardiac arrest care: a before-after study. Pediatr Crit Care Med 2020;21:e898–907. [DOI] [PubMed] [Google Scholar]
- 30.Fiser DH. Assessing the outcome of pediatric intensive care. J Pediatr 1992;121:68–74. [DOI] [PubMed] [Google Scholar]
- 31.Fiser DH, Long N, Roberson PK, Hefley G, Zolten K, Brodie-Fowler M. Relationship of pediatric overall performance category and pediatric cerebral performance category scores at pediatric intensive care unit discharge with outcome measures collected at hospital discharge and 1- and 6-month follow-up assessments. Crit Care Med 2000;28:2616–20. [DOI] [PubMed] [Google Scholar]
- 32.Topjian AA, Scholefield BR, Pinto NP, et al. P-COSCA (Pediatric Core Outcome Set for Cardiac Arrest) in Children: An Advisory Statement From the International Liaison Committee on Resuscitation. Circulation 2020;142:e246–61. [DOI] [PubMed] [Google Scholar]
- 33.Czosnyka M, Smielewski P, Kirkpatrick P, Laing RJ, Menon D, Pickard JD. Continuous assessment of the cerebral vasomotor reactivity in head injury. Neurosurgery 1997;41:11–7. discussion 7–9. [DOI] [PubMed] [Google Scholar]
- 34.Zeiler FA, Ercole A, Czosnyka M, et al. Continuous cerebrovascular reactivity monitoring in moderate/severe traumatic brain injury: a narrative review of advances in neurocritical care. Br J Anaesth 2020. [DOI] [PubMed] [Google Scholar]
- 35.Brady K, Joshi B, Zweifel C, et al. Real-time continuous monitoring of cerebral blood flow autoregulation using near-infrared spectroscopy in patients undergoing cardiopulmonary bypass. Stroke 2010;41:1951–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Brady KM, Lee JK, Kibler KK, et al. Continuous time-domain analysis of cerebrovascular autoregulation using near-infrared spectroscopy. Stroke 2007;38:2818–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Depreitere B, Gü iza F, Van den Berghe G, et al. Pressure autoregulation monitoring and cerebral perfusion pressure target recommendation in patients with severe traumatic brain injury based on minute-by-minute monitoring data. J Neurosurg 2014;120:1451–7. [DOI] [PubMed] [Google Scholar]
- 38.Donnelly J, Czosnyka M, Adams H, et al. Individualizing thresholds of cerebral perfusion pressure using estimated limits of autoregulation. Crit Care Med 2017;45:1464–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Kramer AH, Couillard PL, Zygun DA, Aries MJ, Gallagher CN. Continuous assessment of “optimal” cerebral perfusion pressure in traumatic brain injury: a cohort study of feasibility, reliability, and relation to outcome. Neurocrit Care 2019;30:51–61. [DOI] [PubMed] [Google Scholar]
- 40.Steiner LA, Czosnyka M, Piechnik SK, et al. Continuous monitoring of cerebrovascular pressure reactivity allows determination of optimal cerebral perfusion pressure in patients with traumatic brain injury. Crit Care Med 2002;30:733–8. [DOI] [PubMed] [Google Scholar]
- 41.Beqiri E, Smielewski P, Robba C, et al. Feasibility of individualised severe traumatic brain injury management using an automated assessment of optimal cerebral perfusion pressure: the COGiTATE phase II study protocol. BMJ Open 2019;9:e030727. 10.1136/bmjopen-2019-030727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Liu X, Maurits NM, Aries MJH, et al. Monitoring of Optimal Cerebral Perfusion Pressure in Traumatic Brain Injured Patients Using a Multi-Window Weighting Algorithm. J Neurotrauma 2017;34:3081–8. [DOI] [PubMed] [Google Scholar]
- 43.Ono M, Brady K, Easley RB, et al. Duration and magnitude of blood pressure below cerebral autoregulation threshold during cardiopulmonary bypass is associated with major morbidity and operative mortality. J Thorac Cardiovasc Surg 2014;147:483–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Kirschen MP, Morgan RW, Majmudar T, et al. The association between early impairment in cerebral autoregulation and outcome in a pediatric swine model of cardiac arrest. Resuscitation Plus 2020;4:100051. 10.1016/j.resplu.2020.100051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Roberts JS, Yanay O, Barry D. Age-based percentiles of measured mean arterial pressure in pediatric patients in a hospital setting. Pediatr Crit Care Med 2020. [DOI] [PubMed] [Google Scholar]
- 46.Guiza F, Depreitere B, Piper I, et al. Visualizing the pressure and time burden of intracranial hypertension in adult and paediatric traumatic brain injury. Intensive Care Med 2015;41:1067–76. [DOI] [PubMed] [Google Scholar]
- 47.Pham P, Bindra J, Chuan A, Jaeger M, Aneman A. Are changes in cerebrovascular autoregulation following cardiac arrest associated with neurological outcome? Results of a pilot study. Resuscitation 2015;96:192–8. [DOI] [PubMed] [Google Scholar]
- 48.Ameloot K, De Deyne C, Eertmans W, et al. Early goal-directed haemodynamic optimization of cerebral oxygenation in comatose survivors after cardiac arrest: the Neuroprotect post-cardiac arrest trial. Eur Heart J 2019;40:1804–14. [DOI] [PubMed] [Google Scholar]
- 49.Jakkula P, Pettila V, Skrifvars MB, et al. Targeting low-normal or high-normal mean arterial pressure after cardiac arrest and resuscitation: a randomised pilot trial. Intensive Care Med 2018;44:2091–101. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


