Abstract
BACKGROUND AND AIMS:
Professional societies recommend abdominal ultrasound (US) with or without alpha fetoprotein (AFP) for hepatocellular cancer (HCC) surveillance; however, there are several emerging surveillance modalities, including abbreviated MRI and blood-based biomarker panels. Most studies have focused on provider perspectives for surveillance logistics, but few have assessed patient preferences. We aimed to measure preferences among patients with cirrhosis regarding HCC surveillance modalities.
METHODS:
We conducted a choice-based conjoint survey to patients with cirrhosis at four institutions. Participants were provided 15 scenarios in which they were asked to choose surveillance modalities based on five test attributes: benefits, i.e. sensitivity for early HCC (range: 35–95%), physical harm, i.e. false positives requiring additional testing (range: 10–40%), financial harm, i.e. out-of-pocket costs (range: $10–100), test logistics and convenience, i.e. duration of testing (range: 10–60 min). Hierarchical Bayes discrete choice conjoint analysis was used to derive attribute importance, and preference shares were determined by simulation.
RESULTS:
In total 91% (182/199) of approached patients consented to participate in the study and 98% (n=179) successfully completed the survey. Surveillance benefits (importance: 51.3%, 95%CI: 49.0–53.4%) were valued more than risk of physical harm (importance: 7.6%, 95%CI 7.0–8.2%), financial harm (importance: 15.2%, 95%CI 14.0–16.3%), convenience (importance: 9.3%, 95% CI 8.5–10.1%) and test logistics (importance: 16.7%, 95%CI 15.4–18.1%). Based on simulations including all possible tests, patients preferred abbreviated MRI (29.0%), MRI (23.3%), or novel blood-based biomarkers (20.9%) to ultrasound alone (3.4%) or with AFP (8.8%).
CONCLUSIONS:
Patients with cirrhosis prioritize early HCC detection over potential surveillance-related harms or inconvenience.
Keywords: Harms, HCC, Screening, Sensitivity
Cirrhosis is the primary risk factor for hepatocellular carcinoma (HCC), the fastest growing cause of cancer-related mortality in the United States.1,2 HCC surveillance is associated with reduced mortality in patients with cirrhosis by improving early stage detection.3–5 Therefore, professional societies recommend HCC surveillance using abdominal ultrasound (US) with or without alpha-fetoprotein (AFP) in at-risk patients, including those with cirrhosis.6,7 However, several studies highlight limitations of the current approach including suboptimal sensitivity for early HCC detection and risk of false positives, leading to potential physical, financial, and psychological harms.8–10 Fortunately, there are several emerging surveillance modalities including abbreviated magnetic resonance imaging (MRI) as well as blood-based biomarker panels with promising early phase II results.11,12
The choice between HCC surveillance methods can be complex, as it is influenced by several factors, including potential benefits (early-stage detection), risks (eg, financial and physical harm from false positives), and convenience (eg, time commitment and exam logistics). Further, there are trade-offs that must be considered. For example, a high sensitivity for early HCC may carry a higher risk of false positives, or a method with high sensitivity and specificity may result in high out-of-pocket costs for patients. Finally, patient values and preferences vary, which can play a role in their adherence to recommended surveillance strategies; therefore, optimal test choice may differ between patients. As new modalities for surveillance are validated, this decision will grow more complicated, particularly without direct comparative data. The aim of this study was to measure patient preferences for HCC surveillance attributes in an era of emerging surveillance modalities. We prospectively surveyed patients at risk for HCC from 4 centers to directly elicit their preferences for characteristics of HCC surveillance methods.
Materials and Methods
We conducted a prospective discrete choice-based conjoint survey that was approved by the institutional review boards at each of the 4 participating institutions. Informed consent was obtained from all participants. Discrete choice–based conjoint surveys and analysis are commonly used in marketing research to evaluate individual preferences about complex purchasing decisions. However, in this context, it can also be used to evaluate patient preferences for complex decisions based on test attribute, such as choice of HCC surveillance modalities. Patients choose between a series of hypothetical HCC surveillance methods differing in benefits, risks, and convenience. The patient choices allow derivation of implied attribute preferences and permit competitive preference share simulations comparing individual surveillance methods.
Study Population
We conducted a prospective observational discrete choice conjoint survey at the University of Michigan, University of Chicago, University of Texas Southwestern Medical Center, and Parkland Health and Hospital System from April 2019 to March 2020. The institutions are members of the North American Liver Cancer Consortium and were chosen to support a broad range of sociodemographic characteristics. We included patients seen in outpatient hepatology clinics eligible for HCC surveillance per American Association of Liver Disease guidelines, including cirrhosis of all etiologies and patients with chronic hepatitis B. We included patients with Child-Pugh A and B liver function and patients with Child-Pugh C cirrhosis listed for liver transplantation. We excluded patients with a history of HCC, liver transplantation, non–English-speaking patients, and those with uncontrolled hepatic encephalopathy or cognitive impairment.13
Conjoint Survey Development and Administration
Our discrete choice-based conjoint survey (Supplementary Figure 1) (Sawtooth Software, Provo, UT)14 used partial profile design to provide respondents with 15 paired choice sets with 3 options. The 3 options in each choice were 2 unique hypothetical HCC surveillance methods and an option to choose neither of the 2 surveillance methods. Each hypothetical surveillance method used the same 5 attributes set at varying levels: sensitivity for early HCC detection (range, 35%– 90%),11,12,15–18 risk of physical harm (ie, false positive results requiring additional tests [range: 10%– 40%]),11,12,15–18 financial harm (ie, out-of-pocket costs [range: $5-$100]), convenience (exam duration [range: 10–30 minutes]),12 and exam logistics (flat in small enclosed tube that makes loud noises, lay flat in open space, roll in different positions while a technician uses a probe over your liver, single blood draw). Out-of-pocket costs were derived from the Medicare Fee Schedule based on median deductible (Supplementary Table 1).19 For the novel biomarker panel and abbreviated MRI, early phase validation data were used to estimate all parameters.20–22 Participants either selected which of the 2 hypothetical surveillance scenarios with varying levels of the attributes they would prefer, or they indicated that they would chose not to be tested if those were the only 2 options available. The content of the survey was vetted by 5 volunteer patient advocates reviewing the survey for patient-centered language. The content of the survey was vetted by 3 hepatologists with expertise in HCC surveillance to ensure appropriate supporting literature for the conjoint analysis. The survey underwent precognitive testing for content validity and readability by 5 patients with cirrhosis at each site undergoing HCC surveillance.
The survey software considered the active ratings of the respondents to generate a personalized choice set that maximized analyzability of their respondents. The experiment had a near-orthogonal design with level balance and minimal attribute level overlap. Details of the administered surveys are provided in Supplementary Table 2.
The survey was administered by trained research assistants at each site. Participant demographics (age, sex, race/ethnicity) and clinical history (liver disease etiology and severity) were extracted from the electronic medical record.
Statistical Design
Hierarchical Bayesian modeling and a Markov chain Monte Carlo algorithm were used to estimate part-worth utilities (and their 95% confidence intervals [CIs]) for each attribute level.23–25 A total of 50,000 posterior simulation iterations were used. These part-worth utilities are an interval measure of patient preference for levels within an attribute, somewhat analogous to a beta coefficient from a logistic regression. The model estimates a unique set of part-worth utilities for each patient, which captures each patient’s unique weight that they place on the different attributes and levels while deciding on a preferred test. We will provide the individual patient-level part-worth utilities as Supplementary Material; results presentation will focus on average part-worth utilities.
Owing to dummy coding in the design matrix, part-worth utility values are scaled to an arbitrary additive constant within an attribute. For example, in a logistic regression, we can directly compare beta coefficients from different categorical variables because they are all on the same scale. However, here, while we can compare part-worth utilities for levels within an attribute (eg, sensitivity 90% vs sensitivity 80%), we cannot directly compare part-worth utilities across attributes (eg, sensitivity 90% vs cost $50). Attribute importance is the estimated average relative importance participants placed on that attribute when making HCC surveillance modality selection decisions. For each participant, attribute importance was calculated as the range of their part-worth utilities for that attribute, divided by the sum of the ranges for all attributes multiplied by 100 (ie, ).The average attribute importance was reported as a percentage and a 95% CI. All attribute importances sum to 100%.
Demographic data were summarized with descriptive statistics. Patient-specific attribute importances were modeled using linear regression. The covariates used for analysis included age, location, sex, race, ethnicity, education, health insurance, employment status, household income, commute time, patient perception of lifetime cancer risk, patient perception of cancer curability with early stage, and patient perception of the chance of detecting cancer at an early stage with surveillance. The mean attribute importance difference was calculated between each covariate subgroup.
The part-worth utility values for a combination of HCC surveillance method properties were combined to build a multiproduct competitive model using the randomized first choice method to estimate share of preference. Patient-specific share of preference was calculated as the antilog of the total product utility (based on patient-specific part-worth utilities). Results for each product and none (ie, “chose not to be tested”) are rescaled to sum to 100%. Simulation results were completed in order to complete a sensitivity analysis of preference shares for sensitivity of early HCC detection, false positive rate, out-of-pocket expenses, involvement during the exam, and time commitment. A reference test was made assuming 35% HCC detection sensitivity, 40% false positive rate, $100 out-of-pocket cost, requirement of lying flat in loud tube, and 60-minute test duration. Simulations were performed changing 1 attribute level at a time against the no surveillance option. The outcome was the percentage of patients that would prefer to get HCC surveillance under each test parameter.
Market simulations were performed to compare existing surveillance methods, abbreviated MRI, and novel biomarker panel surveillance methods using the patient-level part-worth utilities derived from our study. The surveillance methods and associated parameters are listed in Supplementary Table 3. Although 7 HCC surveillance methods were formally analyzed in our patient-centered preference share simulations, the simulator derived from our data (Supplementary Material) permits the user to input any combination of attribute levels based on available HCC surveillance methods and to update the analysis as novel surveillance methods become available.
Statistical analysis was performed using SAS software (version 9.4; SAS Institute, Cary, NC). For primary endpoints, P < .05 was considered significant. For secondary endpoints (ie, when assessing differences in attribute importance), P < .01 was considered significant to account for multiple comparisons.
Results
Patient Characteristics
Of the 199 patients approached to participate, 179 (90.0%) completed the survey (Supplementary Figure 1). Details of the study population are provided in Tables 1 and 2. The most common etiologies of liver disease were nonalcoholic fatty liver disease (26%), chronic hepatitis C (21%), and alcohol-related liver disease (18%). The majority were Child-Pugh class A (76%) with a mean Model for End-Stage Liver Disease score of 11. The cohort was diverse in terms race-ethnicity, educational attainment, and socioeconomic status.
Table 1.
Participant Characteristics
| Characteristic | Respondents (n = 179) |
|---|---|
|
| |
| Age, y | 64 (54–69) |
| Location | |
| University of Michigan | 54 (30) |
| University of Texas Southwestern Medical Center | 44 (25) |
| Parkland | 26 (14) |
| University of Chicago | 55 (31) |
| Sex | |
| Female | 80 (45) |
| Male | 99 (55) |
| Race | |
| White | 107 (60) |
| Hispanic or Latino | 29 (16) |
| Black or African American | 30 (17) |
| Asian/Pacific Islander | 10(6) |
| Other | 3(2) |
| Ethnicity | |
| Non-Hispanic | 146 (82) |
| Hispanic | 33 (18) |
| Education | |
| Less than high school | 24 (13) |
| High school graduate | 50 (28) |
| Trade/technical/vocational training | 11 (6) |
| Associate degree | 17(9) |
| College graduate | 44 (25) |
| More than college | 25 (14) |
| Prefer not to answer | 8(4) |
| Health insurance | |
| None | 6 (3) |
| Self-insured | 2 (1) |
| Employer based plan | 46 (26) |
| Medicaid | 25 (14) |
| Medicare | 91 (51) |
| Other | 9 (5) |
| Employment status | |
| Full time employed | 42 (23) |
| Part-time employed | 9(5) |
| Disability | 35 (20) |
| Unemployed | 15(8) |
| Retired | 74 (41) |
| Prefer not to answer | 4(2) |
| Household income | |
| <$25,000 | 19(11) |
| $25,000-$49,999 | 23 (13) |
| $50,000–$99,999 | 27(17) |
| >$100,000-$149,999 | 17(9) |
| Prefer not to answer | 93 (52) |
| Commute time to hospital from home | |
| 0–10 min | 12(7) |
| 11–30 min | 55 (31) |
| 31–60 min | 69 (39) |
| 61–120 min | 27 (15) |
| >120 min | 15(8) |
| Prefer not to answer | 1 (1) |
NOTE. Values are median (interquartile range) or n (%).
Table 2.
Participant Liver Disease and Cancer Risk Perception characteristics
| Characteristic | Distribution |
|---|---|
|
| |
| Etiology of liver disease | |
| Chronic hepatitis B | 14 (8) |
| Chronic hepatitis C | 37 (21)) |
| Alcoholic liver disease | 33 (18) |
| Nonalcoholic steatohepatitis | 47 (26) |
| Cryptogenic cirrhosis | 7 (4) |
| Other (includes cardiac) | 41 (25) |
| Child-Pugh class | |
| A | 136 (76) |
| B | 29 (16) |
| C | 12 (7) |
| Missing | 2 (1) |
| MELD score | |
| 1–10 | 118 (66) |
| 11–20 | 42 (24) |
| 21–30 | 15 (8) |
| Missing | 3 (2) |
| Patient perception of lifetime cancer risk | |
| Very likely | 15 (8) |
| Likely | 48 (27) |
| Neutral | 89 (50) |
| Unlikely | 20 (11) |
| Very unlikely | 7 (4) |
| Patient perception of cancer curability with early stage | |
| Very likely | 26 (15) |
| Likely | 82 (46) |
| Neutral | 49 (27) |
| Unlikely | 13 (7) |
| Very unlikely | 9 (5) |
| Patient perception of the chance of detecting cancer at an early stage with surveillance | |
| All of the time | 21 (12) |
| Most of the time | 109 (61) |
| Some of the time | 39 (22) |
| Rarely | 10 (6) |
NOTE. Values are n (%).
MELD, Model for End-Stage Liver Disease.
Over one-third (35%) of patients reported being likely or very likely to develop HCC, while 50% reported they were neither likely nor unlikely to develop HCC in their lifetime. Patients appeared to believe HCC surveillance was effective at facilitating early tumor detection and curative treatment. Approximately three-fourths (73%) of patients felt that surveillance was likely or very likely to detect HCC at an early stage, and 61% felt that HCC was likely or very likely to be amenable to curative treatment if detected at an early stage.
Importance of Surveillance Attributes
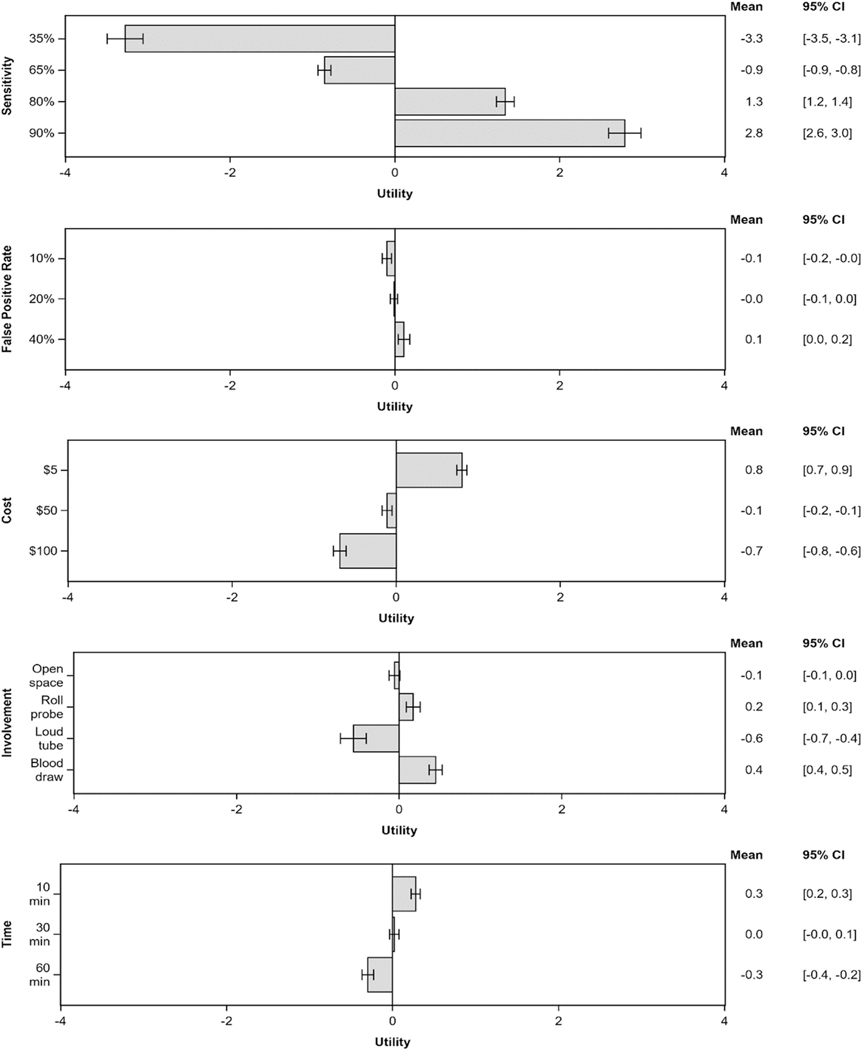
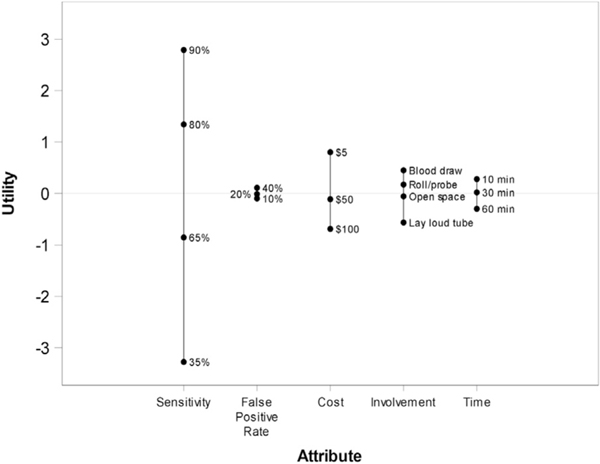
Respondents’ values for each HCC surveillance attribute are included in Figures 1 and 2, with pairwise comparisons provided in Supplementary Table 4. Patients considered sensitivity for early HCC detection to be the most important attribute (Table 3). Attributes are listed as follows in descending order of importance (%): (1) sensitivity for early HCC detection (51.2%; 95% CI, 49.0%–53.4%), (2) test logistics (16.7%; 95% CI, 15.4%– 18.1%), (3) risk of financial harms (15.2%; 95% CI, 14.0%–16.3%), (4) convenience (9.3%; 95% CI, 8.5%– 10.1%), and (5) risk of physical harms (7.6%; 95% CI, 7.0%–8.2%).
Figure 1.
Tornado plots of average part-worth utilities and 95% CIs for all attribute levels.
Figure 2.
Thermometer plot of range of part-worth utilities for each attribute. A wider range indicates higher importance. Each attribute is centered at 0.
Table 3.
Average Attribute Importance and Univariable Linear Regression Results Testing Differences in Mean Attribute Importance by Participant Characteristics
| Parameter | Sensitivity for Early HCC | Risk of Physical Harms | Risk of Financial Harms | Test Logistics | Convenience |
|---|---|---|---|---|---|
|
| |||||
| Mean (95% CI) | 51.2 (49.0 to 53.4) | 7.6 (7.0 to 8.2) | 15.2 (14.0,16.3) | 16.7 (15.4 to 18.1) | 9.3 (8.5 to 10.1) |
| Comparisons: Mean importance differences (99% CI) | |||||
| Parameter | Sensitivity | False Positive Rate | Cost | Involvement | Time |
| Age | P = .39 | P = .76 | P = .23 | P = .98 | P = .39 |
| Age: per 10 y | −0.7 (−2.9 to 1.5) | −0.1 (−0.7 to 0.6) | 0.6 (−0.6 to 1.7) | −0.0 (−1.4 to 1.4) | 0.3 (−0.5 to 1.1) |
| Location | P = .38 | p = .32 | p = .43 | p = .22 | p = .14 |
| Chicago vs UT Southwestern/Parkland | −3.2 (−10.1 to 3.7) | 0.4 (−1.5 to 2.3) | 1.3 (−2.4 to 4.9) | −0.3 (−4.5 to 4.0) | 1.8 (−0.7 to 4.3) |
| Michigan vs UT Southwestern/Parkland | −3.1 (−10.0 to 3.8) | 1.1 (−0.8 to 3.1) | −0.7 (−4.4 to 3.0) | 2.5 (−1.8 to 6.8) | 0.1 (−2.4 to 2.7) |
| Sex | p = .35 | p = .94 | p = .44 | p = .63 | p = .52 |
| Female vs Male | 2.1 (−3.7 to 7.8) | 0.0 (−1.6 to 1.7) | −0.9 (−4.0 to 2.1) | −0.7 (−4.2 to 2.9) | −0.5 (−2.6 to 1.6) |
| Race | p = .14 | p = .17 | p = .31 | p = .99 | p = .14 |
| Black vs White | −5.0 (−12.9 to 2.8) | 1.9 (−0.3 to 4.1) | 1.8 (−2.4 to 6.0) | 0.0 (−4.9 to 5.0) | 1.4 (−1.5 to 4.2) |
| Hispanic vs White | −5.6 (−13.6 to 2.3) | 0.0 (−2.2 to 2.2) | 2.7 (−1.6 to 6.9) | 0.7 (−4.3 to 5.7) | 2.2 (−0.7 to 5.2) |
| Other vs White | 1.0 (−10.1 to 12.2) | 0.5 (−2.7 to 3.6) | −0.7 (−6.7 to 5.3) | 0.2 (−6.8 to 7.2) | −0.9 (−5.0 to 3.2) |
| Ethnicity | p = .01a | p = .46 | p = .04 | p = .19 | p = .01a |
| Hispanic vs non-Hispanic | −7.5 (−14.8 to −0.3)b | −0.6 (−2.7 to 1.5) | 3.2 (−0.7 to 7.0) | 2.3 (−2.2 to 6.9) | 2.6 (−0.0 to 5.3) |
| Education | p = .05 | p = .18 | p = .54 | p = .76 | p = .002a |
| ≤ High school vs ≥ college | −4.6 (−10.4 to 1.3) | 0.9 (−0.8 to 2.5) | 0.7 (−2.4 to 3.9) | 0.4 (−3.2 to 4.0) | 2.5 (0.4 to 4.7)b |
| Health insurance | p = .07 | p = .87 | p = .06 | p = .70 | p = .06 |
| Medicaid vs employer | −8.7 (−18.1 to 0.6) | −0.6 (−3.3 to 2.1) | 4.0 (−1.0 to 8.9) | 2.5 (−3.4 to 8.4) | 2.9 (−0.5 to 6.3) |
| Medicare vs employer | −5.3 (−12.1 to 1.6) | −0.8 (−2.8 to 1.1) | 3.4 (−0.2 to 7.0) | 0.6 (−3.7 to 4.9) | 2.1 (−0.4 to 4.6) |
| None vs employer | −8.7 (−25.0 to 7.7) | −0.4 (−5.1 to 4.3) | 6.6 (−2.2 to 15.3) | 3.7 (−6.6 to 14.0) | −1.2 (−7.2 to 4.8) |
| Other vs employer | −10.0 (−22.7 to 2.6) | −0.4 (−4.0 to 3.3) | 4.7 (−2.1 to 11.4) | 2.5 (−5.5 to 10.4) | 3.3 (−1.4 to 7.9) |
| Employment status | p = .47 | p = .60 | p = .56 | p = .66 | p = .76 |
| Disability vs full time | −3.8 (−12.5 to 5.0) | −1.1 (−3.5 to 1.4) | 2.8 (−1.9 to 7.5) | 0.7 (−4.7 to 6.2) | 1.3 (−1.9 to 4.6) |
| Part-time vs full time | 3.4 (−10.6 to 17.5) | −1.6 (−5.6 to 2.3) | 0.1 (−7.4 to 7.6) | −3.7 (−12.4 to 5.0) | 1.8 (−3.4 to 7.0) |
| Prefer not to answer vs full time | −0.3 (−20.4 to 19.7) | −3.4 (−9.0 to 2.3) | 3.1 (−7.5 to 13.8) | 1.9 (−10.6 to 14.3) | −1.3 (−8.7 to 6.0) |
| Retired vs full time | −1.6 (−9.0 to 5.8) | −1.0 (−3.1 to 1.1) | 1.5 (−2.5 to 5.4) | 0.4 (−4.2 to 5.0) | 0.7 (−2.0 to 3.4) |
| Unemployed vs full time | −7.4 (−18.9 to 4.1) | −1.0 (−4.2 to 2.2) | 3.6 (−2.5 to 9.7) | 3.1 (−4.1 to 10.2) | 1.8 (−2.5 to 6.0) |
| Household income | p = .01a | p = .39 | p = .07 | p = .20 | p = .03a |
| <$50,000 vs ≥$50,000 | −9.2 (−17.6 to −0.9)b | 0.8 (−1.5 to 3.1) | 3.2 (−1.4 to 7.9) | 2.7 (−2.7 to 8.1) | 2.5 (−0.4 to 5.5) |
| Income reported | p = .84 | p = .28 | p = .48 | p = .97 | p = .42 |
| Reported vs Not reported | −0.4 (−6.2 to 5.3) | 0.7 (−0.9 to 2.3) | −0.8 (−3.9 to 2.2) | −0.1 (−3.6 to 3.5) | 0.7 (−1.4 to 2.8) |
| Commute time | p = .28 | p = .68 | p = .10 | p = .29 | p = .04 |
| 0−10 min vs 31−60 min | 1.4 (−10.5 to 13.3) | 1.6 (−1.8 to 5.0) | −3.1 (−9.4 to 3.2) | 0.4 (−7.0 to 7.8) | −0.3 (−4.6 to 4.0) |
| 11−30 min vs 31−60 min | −2.6 (−9.5 to 4.2) | 1.1 (−0.8 to 3.1) | 2.1 (−1.5 to 5.7) | −1.2 (−5.4 to 3.1) | 0.6 (−1.9 to 3.1) |
| 121−180 min vs 31−60 min | 6.4 (−6.0 to 18.7) | −0.2 (−3.7 to 3.3) | −4.2 (−10.7 to 2.4) | 2.2 (−5.4 to 9.9) | −4.3 (−8.7 to 0.2) |
| 61−120 min vs 31−60 min | −5.6 (−14.2 to 3.1) | 1.2 (−1.2 to 3.7) | 2.0 (−2.6 to 6.5) | 0.4 (−5.0 to 5.7) | 2.0 (−1.1 to 5.1) |
| >181 min vs 31−60 min | 4.5 (−15.1 to 24.0) | 0.7 (−4.9 to 6.2) | 3.0 (−7.3 to 13.3) | −10.8 (−22.9 to 1.3) | 2.6 (−4.4 to 9.7) |
| Prefer not to answer vs 31−60 min | 7.9 (−30.4 to 46.2) | 2.0 (−8.9 to 12.9) | 4.1 (−16.2 to 24.3) | −7.4 (−31.1 to 16.3) | −6.6 (−20.4 to 7.2) |
| Patient perception of lifetime cancer risk | p = .81 | p = .72 | p = .68 | p = .58 | p = .27 |
| Very likely vs neutral | 0.1 (−10.6 to 10.9) | −0.4 (−3.5 to 2.6) | 1.5 (−4.2 to 7.2) | −1.8 (−8.4 to 4.8) | 0.6 (−3.3 to 4.5) |
| Likely vs neutral | 0.5 (−6.4 to 7.4) | −0.7 (−2.6 to 1.3) | 0.4 (−3.3 to 4.0) | −2.1 (−6.3 to 2.2) | 1.9 (−0.6 to 4.4) |
| Unlikely vs neutral | −0.2 (−9.7 to 9.3) | −0.8 (−3.5 to 1.9) | 2.8 (−2.3 to 7.9) | −1.3 (−7.2 to 4.6) | −0.5 (−4.0 to 2.9) |
| Very unlikely vs neutral | −7.0 (−22.1 to 8.1) | 1.3 (−3.0 to 5.5) | 1.1 (−6.9 to 9.1) | 2.7 (−6.6 to 12.0) | 1.9 (−3.6 to 7.4) |
| Patient Perception of cancer curability with early stage | p = .93 | p = .85 | p = .96 | p = .71 | p = .71 |
| Very likely vs neutral | −1.8 (−11.1 to 7.6) | 0.3 (−2.4 to 2.9) | −0.1 (−5.1 to 4.9) | −0.3 (−6.1 to 5.5) | 1.9 (−1.5 to 5.3) |
| Likely vs neutral | −1.5 (−8.4 to 5.5) | 0.8 (−1.1 to 2.8) | −0.5 (−4.2 to 3.2) | 0.6 (−3.7 to 4.9) | 0.5 (−2.0 to 3.1) |
| Unlikely vs neutral | −1.4 (−13.4 to 10.6) | 0.1 (−3.2 to 3.5) | −1.8 (−8.2 to 4.6) | 2.3 (−5.1 to 9.8) | 0.8 (−3.6 to 5.2) |
| Very unlikely vs neutral | −4.6 (−18.5 to 9.4) | 0.3 (−3.6 to 4.3) | −0.0 (−7.5 to 7.4) | 4.0 (−4.6 to 12.6) | 0.3 (−4.8 to 5.4) |
| Patient perception of the chance of detecting cancer at an early stage with surveillance | p = .96 | p = .24 | p = .90 | p = .82 | p = .54 |
| All of the time vs most of the time | −1.8 (−11.0 to 7.4) | 1.4 (−1.1 to 4.0) | −0.3 (−5.2 to 4.6) | 1.1 (−4.6 to 6.8) | −0.4 (−3.8 to 2.9) |
| Some of the time vs most of the time | 0.7 (−12.0 to 13.4) | 1.7 (−1.9 to 5.2) | 1.7 (−5.0 to 8.5) | −2.3 (−10.1 to 5.6) | −1.8 (−6.4 to 2.8) |
| Rarely vs most of the time | −0.0 (−7.2 to 7.1) | 1.1 (−0.9 to 3.1) | 0.5 (−3.3 to 4.3) | −0.3 (−4.8 to 4.1) | −1.2 (−3.9 to 1.4) |
| Child-Pugh class | p = .60 | p = .95 | p = .27 | p = .94 | p = .97 |
| B vs A | −0.3 (−8.1 to 7.6) | 0.1 (−2.2 to 2.3) | 0.7 (−3.5 to 4.9) | −0.3 (−5.2 to 4.5) | −0.2 (−3.1 to 2.7) |
| C vs A | −4.5 (−16.1 to 7.1) | −0.4 (−3.6 to 2.9) | 3.8 (−2.3 to 10.0) | 0.8 (−6.4 to 8.0) | 0.2 (−4.0 to 4.5) |
| MELD score | p = .80 | p = .22 | p = .44 | p = .69 | p = .88 |
| MELD score: per 5 points | −0.3 (−2.8 to 2.3) | −0.3 (−1.1 to 0.4) | 0.4 (−1.0 to 1.8) | 0.2 (−1.3 to 1.8) | −0.1 (−1.0 to 0.9) |
NOTE. Attribute “importance” is the estimated average relative importance participants placed on that attribute when making product selection decisions. For each participant, attribute importance (%) is calculated as the range of their part-worth utilities for that attribute, divided by the sum of the ranges for all attributes multiplied by 100 (ie, ). Reported values in the first row above are the average importances across all 179 participants. CI, confidence interval; HCC, hepatocellular carcinoma; MELD, Model for End-Stage Liver Disease; UT Southwestern, University of Texas Southwestern Medical Center.
Overall P value.
P < .01.
Attribute importance varied by several factors, including race-ethnicity, socioeconomic status, and educational attainment (Table 3, Supplementary Table 5). Compared with their counterparts, Hispanic patients, patients of high socioeconomic status, and patients with low educational attainment each placed less importance on sensitivity for early HCC detection and more importance on convenience. Hispanic patients also placed increased importance on potential financial harms than non-Hispanics. We did not find significant differences in patient preferences by other characteristics including location, age, sex, health insurance, perception of HCC risk, liver disease etiology, or liver disease severity.
Preference Share Simulator Model
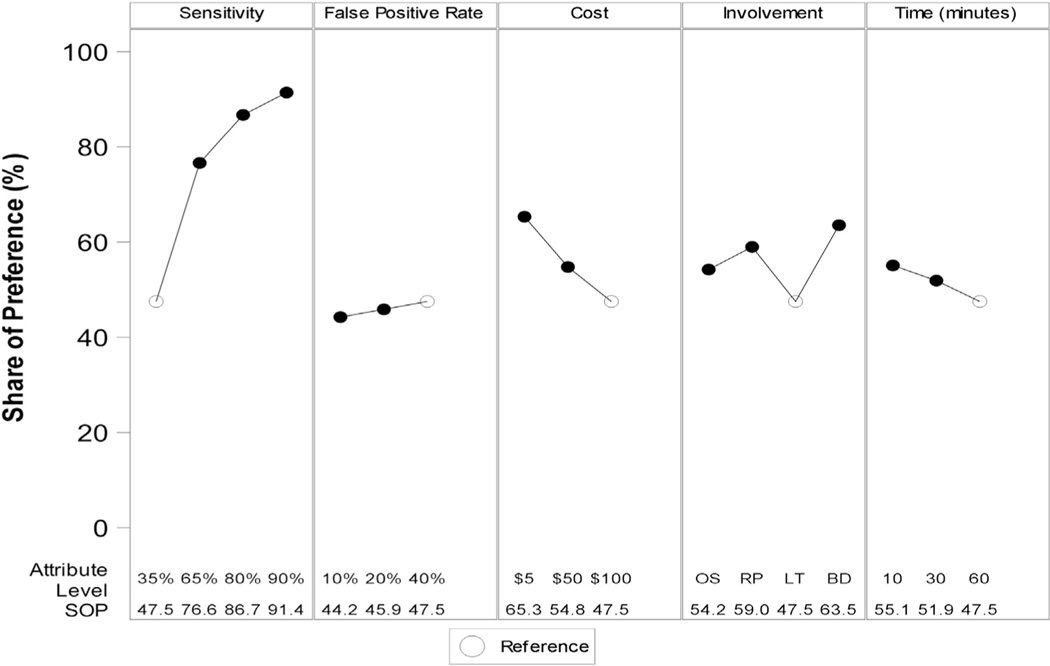
Figure 3 reports simulation analyses to characterize patient willingness to perform HCC surveillance with varying surveillance attributes. Simulations were compared with a reference modality with 35% sensitivity for early HCC detection, 40% false positive rate, $100 out-of-pocket expense, 60-minute duration, and requiring one to lay flat in an enclosed tube (ie, a surveillance test with the most undesirable characteristics across attributes). With these attributes, 47.5% of participants preferred to undergo HCC surveillance, with the remaining 52.5% preferring not to undergo HCC surveillance. Figure 3 shows how preference for undergoing surveillance changed when a single attribute was changed at a time. For example, if sensitivity for early HCC detection was increased from 35% to 65%, the proportion of patients preferring HCC surveillance would increase from 47.5% to 76.6%, with the remaining 24.4% continuing to prefer no surveillance.
Figure 3.
Sensitivity analysis of share of preference based on simulations. BD, a single blood draw; LT, lay flat in a small enclosed tube that makes loud noises; OS, lay flat in an open space; RP, roll in different positions while a technician uses a probe; SOP, share of preference.
The proportion of patients preferring to undergo surveillance increased with improved sensitivity for early HCC detection (from 35% to 90%) from 47.5% to 91.4%; lower out-of-pocket costs (from $100 to $5) from 47.5% to 65.3%; decreased test duration (from 60 minutes to 10 minutes) from 47.5% to 55.1%; and test logistics (lying flat in an enclosed tube vs single blood draw) from 47.5% to 63.5%. The preference for surveillance did not significantly change with decreasing false positive rate (from 40% to 10%), with the proportion remaining between 44.2% and 47.5% of patients.
Multiproduct competitive market simulations, comparing HCC surveillance tests, were performed to determine which HCC surveillance method would dominate if patients knew the details of the available tests and values that directed surveillance selection (Table 4). In our base scenario with commonly available surveillance methods (AFP alone, US alone, US + AFP, computed tomography scan, and MRI), MRI had a preference share of 52.2%. The dominance of MRI vs other options appeared to be primarily driven by higher sensitivity for early HCC detection.
Table 4.
Ranked Simulation Results of Share of Preference
| Procedure | Early HCC Sensitivity (%) | Risk of Physical Harm (%) | Out-of-Pocket Costs, (2019 US$) | Test Logistics | Convenience/Test Duration (min) | Share of Preference (%) (95% CI) |
|---|---|---|---|---|---|---|
|
| ||||||
| Simulation 1 | ||||||
| MRI | 84 | 10 | 99.76 | LT | 37 | 52.2 (47.0–57.4) |
| US+AFP | 63 | 16 | 32.61 | RP | 35 | 15.0 (13.0–17.1) |
| AFP alone | 39 | 24 | 5.00 | BD | 10 | 12.8 (10.4–15.2) |
| CT scan | 63 | 12 | 54.71 | OS | 20 | 9.3 (8.1–10.5) |
| None | — | — | — | — | — | 5.4 (3.2–7.6) |
| US alone | 45 | 10 | 28.47 | RP | 25 | 5.3 (4.4–6.2) |
| Simulation 2 | ||||||
| MRI | 84 | 10 | 99.76 | LT | 37 | 40.0 (35.3–44.7) |
| Biomarker | 68 | 10 | 35.95 | BD | 10 | 26.9 (24.1–29.8) |
| US+AFP | 63 | 16 | 32.61 | RP | 35 | 10.6 (8.9–12.2) |
| AFP alone | 39 | 24 | 5.00 | BD | 10 | 7.8 (6.4–9.3) |
| CT scan | 63 | 12 | 54.71 | OS | 20 | 6.0 (5.2–6.7) |
| None | — | — | — | — | — | 4.8 (2.7–6.9) |
| US alone | 45 | 10 | 28.47 | RP | 25 | 3.9 (3.2–4.6) |
| Simulation 3 | ||||||
| aMRI | 84 | 10 | 99.76 | LT | 17 | 35.1 (32.1–38.0) |
| MRI | 84 | 10 | 99.76 | LT | 37 | 27.4 (25.0–29.8) |
| US+AFP | 63 | 16 | 32.61 | RP | 35 | 11.5 (9.5–13.5) |
| AFP alone | 39 | 24 | 5.00 | BD | 10 | 10.2 (7.9–12.4) |
| CT scan | 63 | 12 | 54.71 | OS | 20 | 6.9 (5.9–7.9) |
| None | — | — | — | — | — | 4.6 (2.6–6.6) |
| US alone | 45 | 10 | 28.47 | RP | 25 | 4.3 (3.5–5.2) |
| Simulation 4 | ||||||
| aMRI | 84 | 10 | 99.76 | LT | 17 | 29.0 (26.3–31.7) |
| MRI | 84 | 10 | 99.76 | LT | 37 | 23.3 (21.0–25.7) |
| Biomarker | 68 | 10 | 35.95 | BD | 10 | 20.9 (18.3–23.5) |
| US+AFP | 63 | 16 | 32.61 | RP | 35 | 8.8 (7.2–10.5) |
| AFP alone | 39 | 24 | 5.00 | BD | 10 | 5.5 (4.3–6.8) |
| CT scan | 63 | 12 | 54.71 | OS | 20 | 4.9 (4.2–5.6) |
| None | — | — | — | — | — | 4.1 (2.3–6.0) |
| US alone | 45 | 10 | 28.47 | RP | 25 | 3.4 (2.7–4.1) |
AFP, alpha-fetoprotein; aMRI, abbreviated magnetic resonance imaging; BD, a single blood draw; CI, confidence interval; CT, computed tomography; MRI, magnetic resonance imaging; LT, lay flat in a small enclosed tube that makes loud noises; OS, lay flat in an open space; RP, roll in different positions while a technician uses a probe; SOP, share of preference; US, ultrasound.
In simulations adding abbreviated MRI (aMRI), aMRI had the highest preference share: aMRI 35.1% vs 27.4% for full MRI. Preference share was primarily driven by maintaining highest sensitivity for early HCC detection, while decreasing exam duration. After introducing potential novel biomarker panels, market share was split between these 3 options (preference share: aMRI 28%, MRI 23%, biomarker-based surveillance 21%). Blood-based biomarker surveillance approached similar preference shares as MRI-based surveillance despite lower sensitivity for early HCC detection due to higher convenience and a short time commitment.
Discussion
Patients with cirrhosis undergoing HCC surveillance strongly prioritize early tumor detection over surveillance-related risks and convenience. Among surveillance-related risks and logistics, patients place greater importance on test logistics and potential financial harms over time commitment and physical harms. These relationships are maintained regardless of patient demographics and background. These data are particularly important in light of US’s suboptimal sensitivity for early HCC detection, and highlight patients’ desire for novel imaging- and blood-based surveillance strategies.
HCC surveillance from the patient’s perspective is more nuanced than potential benefits (eg, early cancer detection) or a single type of surveillance harm. This point is important because current literature has exclusively focused on potential benefits and risk of physical harms.8,10,26,27 While surveillance benefits were highly valued, the risk of a physical harms was the least important HCC surveillance attribute among patients in our study. There have been limited data on financial or psychological harms of HCC surveillance, despite literature suggesting that these can be common in other cancer surveillance programs as well as lead to lower utilization of HCC surveillance in clinical practice.28,29 Overall, a comprehensive assessment of benefits, harms, and patient barriers are important to consider when attempting to align patient values with choice of a surveillance plan.30
Surveillance strategies provide the best value when there is a clear balance between benefits and harms vs patient-centered values. In all of our simulations, patients had lower preference for US alone or US with AFP compared with abbreviated MRI, full MRI, and novel biomarker-based surveillance. Abbreviated MRI was the most preferred method due to its high sensitivity for early HCC detection and high convenience. There are several emerging biomarker panels that are awaiting large-scale validation (eg, GALAD score, methylated DNA marker panels), and our study affirms that patients demonstrate a preference for the convenience of such testing, despite potentially lower early HCC sensitivity than MRI-based modalities. Prior studies indicate direct patient involvement in decisions regarding HCC surveillance improves satisfaction and adherence.31 These findings and simulation tools can be used as a way for providers to engage with patients when making decisions to build a personalized surveillance plan and improve surveillance utilization in clinical practice.
In our study, we found patient variation in surveillance attribute weights. For example, patients of Hispanic ethnicity placed less importance on early HCC sensitivity and greater importance on financial harms and test convenience. These data are important given Hispanics’ experience a disproportionately high burden of HCC, in terms of not only incidence and mortality, but also decreased surveillance utilization.1,32–35 These data reflect how socioeconomic barriers and perceived surveillance inconveniences affect healthcare decision making and this population. These data help understand how perceptions and inconveniences related to surveillance can contribute to racial-ethnic disparities in early detection and overall survival.
Our work had several limitations. First, no preexisting conjoint instrument existed as a base for our survey instrument; however, we vetted survey content by nonauthor experts with expertise in survey design, hepatologists with expertise in HCC surveillance, and 5 patients with cirrhosis from each center for readability and survey content. Risk of bias in the design of the survey was minimized through use of professional conjoint software to create a near-orthogonal design with level balance and minimal attribute level overlap and administering the survey by a trained patient interviewer at each site. Second, survey studies are inherently limited by response and nonresponse biases; however, our study had an excellent response rate of approximately 90%. Third, our study was conducted in 4 health systems with a diverse patient population but may not be generalized to other types of patient populations followed in community practices of other types of integrated systems such as the Veterans Affairs health system. Future studies in broader patient populations are needed to validate our study findings. Fourth, our subgroup analyses were exploratory and likely underpowered to detect meaningful differences. Fifth, the part-worth utilities, attribute importances, and share of preference are based on number of attributes and attribute levels asked in the survey. Although our results could differ if different attributes or levels were included, we selected attribute levels based on the full range of HCC surveillance techniques at the time of study design. Although we believe we have accounted for all attributes that could affect the choices, future studies may choose wider attribute level ranges for variables such as costs, or include additional attributes. Finally, hypothetical scenarios and implications of factors such as physical harms may not have been fully understood by all patients. These weaknesses are outweighed by study strengths, including its unique and clinically important question, high response rate, and diverse patient population.
In conclusion, patient with cirrhosis undergoing HCC surveillance prioritize early HCC detection over surveillance-related risks and convenience. Although these relationships were generally maintained across patient subgroups, patients of Hispanic ethnicity placed greater importance on financial harms and convenience than their counterparts when determining preferred surveillance strategies. These data, and the corresponding simulator, are valuable for understanding patient preferences for a personalized HCC surveillance plan.
Supplementary Material
What You Need to Know.
Background
Hepatocellular carcinoma surveillance is recommended for improvement in the early detection of hepatocellular carcinoma (HCC). The preference of patients when considering surveillance modalities has not been studied.
Findings
Our prospective multicenter study shows that patients with cirrhosis eligible for HCC surveillance prefer surveillance sensitivity for early detection over other test attributes. Preferred strategies include magnetic resonance imaging and biomarker-based approaches.
Implications for patient care
With several available HCC surveillance methods, including ultrasound, magnetic resonance imaging, computed tomography, and several emerging imaging and blood based biomarkers, understanding what aspects of tests patients value may influence choice of surveillance methodology.
Funding
Amit G. Singal’s research is supported in part by National Institutes of Health U01 CA230694, R01 212008, and R01 MD12565. Neehar D. Parikh’s research is supported in part by U01 CA230669. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
Conflicts of Interest
These authors disclose the following: Amit G. Singal has served on advisory boards and as a consultant for Wako Diagnostics, Roche, Exact Sciences, Glycotest, and Bayer. Matthew Davenport has received unrelated royalties from Wolters Kluwer. Jonathan Troost owns stocks in Procter & Gamble and General Electric. Elliot Tapper has served as a consultant for Novartis, Axcella, Kaleido, Mallinckrodt, and Allergan; and on advisory boards for Mallinckrodt, Novo Nordisk, Takeda, and Rebiotix. Anjana Pillai has served on the Speakers Bureau for Simply Speaking Hepatitis and on the medical advisory board for Exelixis, Eisai, and Genentech. Neehar D. Parikh has served as a consultant for Bristol Myers Squibb, Exact Sciences, Eli Lilly, Freenome has served on advisory boards of Genentech, Eisai, Bayer, Exelixis, Wako/Fujifilm and has received research funding from Bayer, Target Pharmasolutions, Exact Sciences, and Glycotest.
Abbreviations used in this paper:
- AFP
alpha fetoprotein
- aMRI
abbreviated magnetic resonance imaging
- CI
confidence interval
- HCC
hepatocellular carcinoma
- MRI
magnetic resonance imaging
- US
ultrasound
Footnotes
Supplementary Material
Note: To access the supplementary material accompanying this article, visit the online version of Clinical Gastroenterology and Hepatology at www.cghjournal.org, and at https://doi.org/10.1016/j.cgh.2021.02.024.
References
- 1.Altekruse SF, Henley SJ, Cucinelli JE, et al. Changing hepatocellular carcinoma incidence and liver cancer mortality rates in the United States. Am J Gastroenterol 2014;109:542–553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Ryerson AB, Eheman CR, Altekruse SF, et al. Annual Report to the Nation on the Status of Cancer, 1975–2012, featuring the increasing incidence of liver cancer. Cancer 2016; 122:1312–1337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Chen VL, Singal AG, Tapper EB, et al. Hepatocellular carcinoma surveillance, early detection and survival in a privately insured US cohort. Liver Int 2020;40:947–955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Singal AG, Mittal S, Yerokun OA, et al. Hepatocellular carcinoma screening associated with early tumor detection and improved survival among patients with cirrhosis in the US. Am J Med 2017;130:1099–1106.e1. [DOI] [PubMed] [Google Scholar]
- 5.Zhang BH, Yang BH, Tang ZY. Randomized controlled trial of screening for hepatocellular carcinoma. J Cancer Res Clin Oncol 2004;130:417–422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.European Association for the Study of the Liver. EASL Clinical Practice Guidelines: management of hepatocellular carcinoma. J Hepatol 2018;69:182–236. [DOI] [PubMed] [Google Scholar]
- 7.Heimbach JK, Kulik LM, Finn RS, et al. AASLD guidelines for the treatment of hepatocellular carcinoma. Hepatology 2018; 67:358–380. [DOI] [PubMed] [Google Scholar]
- 8.Singal AG, Patibandla S, Obi J, et al. Benefits and harms of hepatocellular carcinoma surveillance in a prospective cohort of patients with cirrhosis. Clin Gastroenterol Hepatol 2020. Sep 10 [E-pub ahead of print]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Konerman MA, Verma A, Zhao B, et al. Frequency and outcomes of abnormal imaging in patients with cirrhosis enrolled in a hepatocellular carcinoma surveillance program. Liver Transpl 2019;25:369–379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Atiq O, Tiro J, Yopp AC, et al. An assessment of benefits and harms of hepatocellular carcinoma surveillance in patients with cirrhosis. Hepatology 2017;65:1196–1205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Parikh ND, Mehta AS, Singal AG, et al. Biomarkers for the early detection of hepatocellular carcinoma. Cancer Epidemiol Biomarkers Prev 2020;29:2495–2503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Vietti Violi N, Lewis S, Liao J, et al. Gadoxetate-enhanced abbreviated MRI is highly accurate for hepatocellular carcinoma screening. Eur Radiol 2020;30:6003–6013. [DOI] [PubMed] [Google Scholar]
- 13.Marrero JA, Kulik LM, Sirlin CB, et al. Diagnosis, staging, and management of hepatocellular carcinoma: 2018 Practice Guidance by the American Association for the Study of Liver Diseases. Hepatology 2018;68:723–750. [DOI] [PubMed] [Google Scholar]
- 14.Software S. Discover-CBC: how and why it differs from SSI Web’s CBC Software, 2014. Available at: http://www.sawtoothsoftware.com/download/SaaS_CBC_White_Paper.pdf. Accessed July 1, 2020. [Google Scholar]
- 15.Best J, Bechmann LP, Sowa JP, et al. GALAD score detects early hepatocellular carcinoma in an international cohort of patients with nonalcoholic steatohepatitis. Clin Gastroenterol Hepatol 2020;18:728–735.e4. [DOI] [PubMed] [Google Scholar]
- 16.Pocha C, Dieperink E, McMaken KA, et al. Surveillance for hepatocellular cancer with ultrasonography vs computed tomography – a randomised study. Aliment Pharmacol Ther 2013; 38:303–312. [DOI] [PubMed] [Google Scholar]
- 17.Kim SY, An J, Lim YS, et al. MRI with liver-specific contrast for surveillance of patients with cirrhosis at high risk of hepatocellular carcinoma. JAMA Oncol 2017;3:456–463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Tzartzeva K, Obi J, Rich NE, et al. Surveillance imaging and alpha fetoprotein for early detection of hepatocellular carcinoma in patients with cirrhosis: a meta-analysis. Gastroenterology 2018;154:1706–1718.e1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Cubanski J, Neuman T, Damico A, et al. Medicare beneficiaries’ out-of-pocket health care spending as a share of income now and projections for the future. Henry J Kaiser Family Foundation, 2018. Available at: https://www.kff.org/medicare/report/medicare-beneficiaries-out-of-pocket-health-care-spending-as-a-share-of-income-now-and-projections-for-the-future/. Accessed July 16, 2019. [Google Scholar]
- 20.Berhane S, Toyoda H, Tada T, et al. Role of the GALAD and BALAD-2 Serologic Models in Diagnosis of Hepatocellular Carcinoma and Prediction of Survival in Patients. Clin Gastroenterol Hepatol 2016;14:875–886.e6. [DOI] [PubMed] [Google Scholar]
- 21.Besa C, Lewis S, Pandharipande PV, et al. Hepatocellular carcinoma detection: diagnostic performance of a simulated abbreviated MRI protocol combining diffusion-weighted and T1-weighted imaging at the delayed phase post gadoxetic acid. Abdom Radiol (NY) 2017;42:179–190. [DOI] [PubMed] [Google Scholar]
- 22.Tillman B, Gorman J, Hru J, et al. Diagnostic per-lesion performance of a simulated gadoxetate disodium-enhanced abbreviated MRI protocol for hepatocellular carcinoma screening. Clin Radiol 2018;73:485–493. [DOI] [PubMed] [Google Scholar]
- 23.Hauber AB, González JM, Groothuis-Oudshoorn CG, et al. Statistical methods for the analysis of discrete choice experiments: a report of the ISPOR Conjoint Analysis Good Research Practices Task Force. Value Health 2016;19:300–315. [DOI] [PubMed] [Google Scholar]
- 24.Kuhfeld W. Marketing Research Methods in SAS. Techinical report, SAS Institute Inc, 2010. Available at: http://support.sas.com/resources/papers/tnote/tnote_marketresearch.html. Accessed November 3, 2019. [Google Scholar]
- 25.Lahiri K, Gao J. Bayesian analysis of nested logit model by Markov chain Monte Carlo. J Econom 2002;111:103–133. [Google Scholar]
- 26.Kanwal F, Singal AG. Surveillance for hepatocellular carcinoma: current best practice and future direction. Gastroenterology 2019;157:54–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Singal AG, Pillai A, Tiro J. Early detection, curative treatment, and survival rates for hepatocellular carcinoma surveillance in patients with cirrhosis: a meta-analysis. PLoS Med 2014;11: e1001624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Singal AG, Lok AS, Feng Z, et al. Conceptual model for the Hepatocellular Carcinoma Screening Continuum: current status and research agenda. Clin Gastroenterol Hepatol 2020. Sep 19 [E-pub ahead of print]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Wolf E, Rich NE, Marrero JA, et al. Utilization of hepatocellular carcinoma surveillance in patients with cirrhosis: a systematic review and meta-analysis. Hepatology 2021;73:713–725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Heleno B, Thomsen MF, Rodrigues DS, et al. Quantification of harms in cancer screening trials: literature review. BMJ 2013; 347:f5334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Singal AG, Volk ML, Rakoski MO, et al. Patient involvement in healthcare is associated with higher rates of surveillance for hepatocellular carcinoma. J Clin Gastroenterol 2011; 45:727–732. [DOI] [PubMed] [Google Scholar]
- 32.Ha J, Chaudhri A, Avirineni A, et al. Burden of hepatocellular carcinoma among hispanics in South Texas: a systematic review. Biomark Res 2017;5:15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Ha J, Yan M, Aguilar M, et al. Race/ethnicity-specific disparities in cancer incidence, burden of disease, and overall survival among patients with hepatocellular carcinoma in the United States. Cancer 2016;122:2512–2523. [DOI] [PubMed] [Google Scholar]
- 34.Mathur AK, Osborne NH, Lynch RJ, et al. Racial/ethnic disparities in access to care and survival for patients with early-stage hepatocellular carcinoma. Arch Surg 2010;145:1158–1163. [DOI] [PubMed] [Google Scholar]
- 35.Rich NE, Hester C, Odewole M, et al. Racial and ethnic differences in presentation and outcomes of hepatocellular carcinoma. Clin Gastroenterol Hepatol 2019;17:551–559.e1. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.