OBJECTIVES:
The hemodynamic profile of multisystem inflammatory syndrome in children (MIS-C)–related shock remains poorly defined and, therefore, challenging to support with pharmacotherapy in the ICU. We aimed to evaluate the hemodynamic profile and vasoactive medication management used in MIS-C patients presenting to the ICU in shock and provide data from high-fidelity continuous cardiac output monitoring.
DESIGN
Single-center retrospective case-cohort study.
SETTING:
Pediatric and cardiac ICU in a quaternary-care hospital.
PATIENTS:
All patients who met U.S. Centers for Disease Control and Prevention criteria for MIS-C and who were admitted to the ICU between March 2020 and May 2021 required vasoactive support and were placed on continuous cardiac index (CCI) monitoring. Patients requiring extracorporeal life support were excluded.
INTERVENTIONS:
None.
MEASUREMENTS AND MAIN RESULTS:
Among 52 children with MIS-C presenting in shock and requiring vasoactive support, 14 patients (27%) were placed on CCI monitoring. These 14 patients had hyperdynamic cardiac index (CI) and low indexed systemic vascular resistance (SVRi) in the first 24 hours with normalization of CI and improved SVRi within the subsequent 24 hours.
CONCLUSIONS:
Further studies are needed to evaluate the difference between the use of vasoconstrictor versus vasodilators in pediatric patients with MIS-C because a phenotype with high CI and low SVRi may be important.
Keywords: cardiac output, COVID-19, multisystem inflammatory syndrome in children, shock, vasoplegia
Multisystem inflammatory syndrome in children (MIS-C) following COVID-19 presents with a systemic hyperinflammatory response that, in severe form, leads to circulatory impairment and, ultimately, cardiogenic shock (1, 2). An estimated 50% of children with MIS-C admitted to a PICU require vasoactive support (1, 2). Although MIS-C is, in the majority, relatively short-lived and a reversible phenomenon, the hemodynamic profile associated with MIS-C–related shock is poorly defined. We hypothesize that with the use of continuous cardiac output monitoring, we will be able to describe the hemodynamic characteristics of this population, which may aid in better utilization of vasoactives.
MATERIALS AND METHODS
This study was approved by the Baylor College of Medicine Internal Review Board Protocol number H-49387 with a waiver of informed consent.
In this retrospective case-cohort study, we selected pediatric patients presenting to the PICUs at Texas Children’s Hospital, Texas (from March 2020 to May 2021), who met the U.S. Centers for Disease Control and Prevention criteria for MIS-C (3), required vasoactive support, and underwent hemodynamic monitoring with the use of continuous cardiac output monitorization. Invasive hemodynamic monitoring included invasive arterial blood pressure, central venous pressure, and arteriovenous oxygen saturation gradient measurements during the first 24 hours of PICU admission, compared with the subsequent 24 hours. Continuous cardiac index (CCI) data were obtained using high-fidelity arterial pulse contour monitors (Pulse Contour Cardiac Output [PiCCO], Getinge, Wayle, NJ, or FloTrac Edwards Lifescience, Irvine, CA), and stroke volume index and indexed systemic vascular resistance (SVRi) were analyzed during the same two time periods. Mean arterial pressure (MAP) was adjusted using median MAP reference values based on age and sex in critically ill children (4). Echocardiographic assessments were obtained and characterized by a pediatric cardiologist (in accordance with our institutional management protocol), and vasoactive-inotropic scores (VIS) (5) were calculated for all children. Patients requiring extracorporeal mechanical circulatory support during this time were excluded since they had different hemodynamics and vasoactive medication requirements. The data were analyzed using descriptive statistics (percentages or median [interquartile range]) or Mood median test for statistical significant (p < 0.05), as appropriate. Statistical analyses were performed using JMP (Version 16, SAS, Cary, NC).
RESULTS
We studied 52 patients with MIS-C requiring vasoactive support. Demographics, presenting symptoms, and clinical characteristics are described in Supplemental Table 1 (http://links.lww.com/PCC/C50). Admission echocardiography revealed moderate or severe left ventricular (LV) systolic dysfunction in 11 (21%) and moderate or severe right ventricular systolic dysfunction in six (12%).
Fourteen of 52 patients (27%) underwent CCI monitoring. In the patients who received CCI monitoring, median age was 13 years (10–17 yr) and weight was 67.6 kg (44.8–96.5 kg). Common electrocardiographic (ECG) abnormalities at admission included abnormal T wave morphology in 10 of 14 (71%) and ST-segment abnormalities in four of 14 (29%). Atrioventricular block was not observed on initial ECG. Initial echocardiogram was performed within 3 hours (2–9 hr) from hospital admission and demonstrated moderate or severe LV systolic dysfunction in six of 14 patients (43%). Of the 14 patients, 12 underwent coronary artery evaluation with one patient identified with coronary dilation greater than 2.5 z score (Supplemental Table 2, http://links.lww.com/PCC/C51).
Patients received fluid resuscitation within 1 hour (<1 to 13 hr) of hospital admission with a median of 14 mL/kg (8–26 mL/kg) of crystalloids within the first 6 hours. Vasoactive therapy was started within a median of 6 hours (1–10 hr) from hospital admission, and all were prior to CCI monitoring. Two or more vasoactive agents were required in 11 of 14 patients (79%) (epinephrine 100%, norepinephrine 57%, milrinone 21%, and vasopressin 21%) with a median duration of vasoactive support of 61 hours (38–98 hr). MIS-C therapy was started 8 hours (4–13 hr) from admission with all but two of 14 receiving therapy before CCI monitoring (Supplemental Fig. 1, http://links.lww.com/PCC/C52 [legend, http://links.lww.com/PCC/C53]; and Supplemental Table 3, http://links.lww.com/PCC/C54).
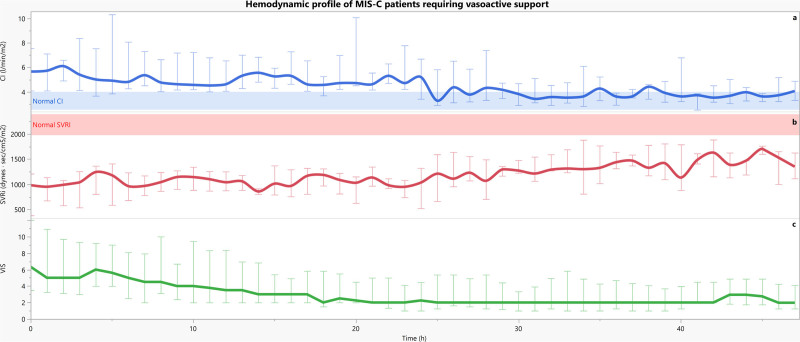
CCI monitoring was started at a median of 11 hours (4–17 hr) from PICU admission and initiated based on provider discretion. Repeat echocardiogram within 24 hours of CCI monitoring demonstrated improvement in LV systolic function (Supplemental Table 2, http://links.lww.com/PCC/C51). During CCI monitoring, patients remained normotensive based on age reference for MAP (Table 1). Their cardiac index (CI) at initiation (Fig. 1) of monitoring was elevated at 5.0 L/min/m2 (4.2–6.6 L/min/m2) in the setting of low SVRi, 1,039 dynes s/cm5/m2 (833–1,239 dynes s/cm5/m2). CI subsequently normalized to a median of 3.7 L/min/m2 (3.2–4.8 L/min/m2) with an improved, but still low, SVRi of 1,330 dynes s/cm5/m2 (1,113–1,560 dynes s/cm5/m2) during the subsequent 24 hours (both p < 0.001). Patients had significantly higher median VIS (4 [2–7] vs 2 [1–4]; p < 0.001) and maximum VIS (6 [4–12] vs 2 [2–5]) at the initiation of CCI monitoring when compared with the subsequent 24 hours (p = 0.003).
TABLE 1.
Hemodynamic Profiles of Multisystem Inflammatory Syndrome in Children Patients Admitted to the ICU Within 48 hr and Requiring Vasoactive Support and Invasive Monitoring
| Hemodynamic Profile of 14 Children From the Onset of Continuous Cardiac Index Monitoring | |||
|---|---|---|---|
| Variable | 0–24 hr | 25–48 hr | p |
| Age-adjusted mean arterial pressure (% from median) | 1 (–8 to 11) | 1 (–6 to 11) | 0.934 |
| Central venous pressure (mm Hg) | 8 (6–11) | 7 (4–11) | 0.140 |
| Stroke volume index (mL/mm2/beat) (normal: 37–47) | 48 (40–66) | 43 (33–53) | 0.036 |
| Cardiac index (L/min/m2) (normal: 2.5–4.0) | 5.0 (4.2–6.6) | 3.7 (3.2–4.8) | < 0001a |
| Systemic vascular resistance index (dynes s/cm5/m2) (normal: 1,970–2,390) | 1,039 (833–1,239) | 1,330 (1,113–1,560) | < 0.001a |
| Median vasoactive doseb | |||
| Epinephrine | 0.01 (0.2–0.05) | 0.02 (0.01–0.02) | < 0.001a |
| Norepinephrine | 0.02 (0.02–0.05) | 0.04 (0.01–0.06) | 0.691 |
| Vasopressin | 0.01 (0.1–0.02) | 0.01 (0.01–0.02) | 0.952 |
| Milrinone | 0 | 0.25 (0.25–0.28) | — |
| Dobutamine | 3 (3–3) | 1 (1–1) | 0.003a |
| Median VIS | 4 (2–7) | 2 (1–4) | < 0.001a |
| Max VIS | 6 (4–12) | 2 (2–5) | 0.003a |
| Arteriovenous oxygen saturation gradient difference (%) | 11 (8–20) | 17 (15–19) | 0.002a |
| Left ventricular functionc | n = 12 | n = 8 | |
| Normal | 6 (50) | 6 (75) | 0.378 |
| Mild | 5 (42) | 1 (13) | |
| Moderate | 1 (8) | 1 (13) | |
| Severe | 0 | 0 | |
VIS = vasoactive-inotropic score.
p < 0.003.
Vasoactive medication units: epinephrine (µg/kg/min), vasopressinU/kg/min), norepinephrine (µg/kg/min), milrinone (µg/kg/min), dopamine (µg/kg/min), and dobutamine (µg/kg/min).
Left ventricular ejection fraction < 30% severely depressed, 30–40% moderately depressed, 40–48% mildly depressed, and > 48% normal.
Hemodynamics were collected as a continuous variable and described as a median (interquartile range) or n (%).
Figure 1.
Hemodynamic progression of the 14 patients with continuous cardiac index (CCI) monitoring beginning at the start of CCI measurement. First row = There is initial hyperdynamic cardiac output and a low systemic vascular resistance index (SVRi) state (second row) within the first 24 hr with normalization of CI and improved, but still low, SVRi in the next 24 hr (p < 0.001 for both). Third row = Similarly, this cohort required high vasoactive support initially with a decrease over time. CI = cardiac index, MIS-C = multisystem inflammatory syndrome in children, VIS = vasoactive-inotropic score.
Patients were further subdivided based on their initial LV systolic function assessment (Supplemental Table 4, http://links.lww.com/PCC/C55). CI remained elevated in both groups but was more hyperdynamic in the normal/mild LV systolic function group in the setting of lower SVRi when compared with the moderate/severe LV systolic dysfunction patients. All patients survived to hospital discharge.
DISCUSSION
Involvement of the cardiovascular system occurs in 80–100% of patients with MIS-C (6–8). Our study describes the hemodynamic profile and vasoactive requirements of critically ill children with MIS-C and shock. The laboratory profiles and markers of illness severity, including echocardiographic findings, of our cohort are similar to previously published reports (9, 10). The hemodynamic profile of our cohort highlights a subset of critically ill MIS-C patients who present with vasoplegia and hyperdynamic cardiac output during the first 24 hours of illness. These observations are followed by normalization of hemodynamic parameters, albeit with the use of critical care interventions and immunomodulation.
This work also highlights the use of echocardiographic assessment of ejection fraction as an indicator of contractility, which may not translate into changes in stroke volume. Ejection fraction remains dependent on ventricular loading conditions and would require knowledge of end-diastolic volume to properly assess cardiac output (11, 12). This finding is evident in the patients presenting with LV systolic dysfunction in whom cardiac output remained elevated in the setting of low systemic vascular resistance (13).
Our study has some obvious limitations: it was retrospective, and we did not have hemodynamic data before the initiation of invasive monitoring. Comparison of hemodynamic data collected by both the PiCCO system and FloTrac may be challenging as cardiac output is calculated in different manners. PiCCO requires calibration with transpulmonary thermodilution to account for difference in arterial compliance for the individual patient. This may potentially reduce accuracy in the setting of large variations in arterial compliance between calibrations (14). FloTrac devices carry a proprietary algorithm that evaluates pulse-contour properties to determine compliance requiring no external calibration but may overestimate cardiac output in extreme hemodynamic states (15).
CONCLUSIONS
Understanding the specific shock phenotype associated with MIS-C allows clinicians to devise a tailored approach to vasoactive medication management of this cardiovascular pathophysiology. The use of CCI in this population provides a more complete physiologic profile than echocardiogram alone and allows for continuous monitoring and evaluation of responsiveness to therapies.
Supplementary Material
Footnotes
Supplemental digital content is available for this article. Direct URL citations appear in the printed text and are provided in the HTML and PDF versions of this article on the journal’s website (http://journals.lww.com/pccmjournal).
Dr. Vogel received support for article research from the NIH (R61HD105593). She disclosed the off-label product use of anakinra for rare hyperinflammatory condition. The remaining authors have disclosed that they do not have any potential conflicts of interest.
REFERENCES
- 1.Valverde I, Singh Y, Sanchez-de-Toledo J, et al. ; AEPC COVID-19 Rapid Response Team*: Acute cardiovascular manifestations in 286 children with multisystem inflammatory syndrome associated with COVID-19 infection in Europe. Circulation 2021; 143:21–32 [DOI] [PubMed] [Google Scholar]
- 2.Feldstein LR, Rose EB, Horwitz SM, et al. ; Overcoming COVID-19 Investigators; CDC COVID-19 Response Team: Multisystem inflammatory syndrome in U.S. children and adolescents. N Engl J Med 2020; 383:334–346 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Centers for Disease Control and Prevention: CDC Health Alert Network. Emergency Preparedness and Response: Multisystem Inflammatory Syndrome in Children (MIS-C) Associated With Coronavirus Disease 2019 (COVID-19). Health Advisory. 2020. Available at: https://emergency.cdc.gov/han/2020/han00432.asp. Accessed September 2, 2021
- 4.Eytan D, Goodwin AJ, Greer R, et al. : Heart rate and blood pressure centile curves and distributions by age of hospitalized critically ill children. Front Pediatr 2017; 5:52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Gaies MG, Gurney JG, Yen AH, et al. Vasoactive–inotropic score as a predictor of morbidity and mortality in infants after cardiopulmonary bypass*. Pediatr Crit Care Med 2010; 11:234–238 [DOI] [PubMed] [Google Scholar]
- 6.Godfred-Cato S, Bryant B, Leung J, et al. ; California MIS-C Response Team: COVID-19-associated multisystem inflammatory syndrome in children - United States, March-July 2020. MMWR Morb Mortal Wkly Rep 2020; 69:1074–1080 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Cantarutti N, Battista V, Adorisio R, et al. : Cardiac manifestations in children with SARS-COV-2 infection: 1-year pediatric multicenter experience. Children (Basel) 2021; 8:717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Alsaied T, Tremoulet AH, Burns JC, et al. : Review of cardiac involvement in multisystem inflammatory syndrome in children. Circulation 2021; 143:78–88 [DOI] [PubMed] [Google Scholar]
- 9.Matsubara D, Kauffman HL, Wang Y, et al. : Echocardiographic findings in pediatric multisystem inflammatory syndrome associated with COVID-19 in the United States. J Am Coll Cardiol 2020; 76:1947–1961 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Radia T, Williams N, Agrawal P, et al. : Multi-system inflammatory syndrome in children & adolescents (MIS-C): A systematic review of clinical features and presentation. Paediatr Respir Rev 2021; 38:51–57 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Konstam MA, Abboud FM: Ejection fraction: Misunderstood and overrated (changing the paradigm in categorizing heart failure). Circulation 2017; 135:717–719 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Rusinaru D, Bohbot Y, Ringle A, et al. : Impact of low stroke volume on mortality in patients with severe aortic stenosis and preserved left ventricular ejection fraction. Eur Heart J 2018; 39:1992–1999 [DOI] [PubMed] [Google Scholar]
- 13.Vieillard-Baron A: Septic cardiomyopathy. Ann Intensive Care 2011; 1:6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Monnet X, Anguel N, Naudin B, et al. : Arterial pressure-based cardiac output in septic patients: Different accuracy of pulse contour and uncalibrated pressure waveform devices. Crit Care 2010; 14:R109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hadian M, Kim HK, Severyn DA, et al. : Cross-comparison of cardiac output trending accuracy of LiDCO, PiCCO, FloTrac and pulmonary artery catheters. Crit Care 2010; 14:R212. [DOI] [PMC free article] [PubMed] [Google Scholar]