Abstract
A universe of transcription factors (TFs), cofactors, as well as chromatin remodeling and modifying enzymes combine or compete on chromatin to control transcription. Measuring quantitatively how these proteins dynamically interact is required in order to formulate models with predictive ability to elucidate transcription control mechanisms. Single molecule tracking (SMT) provides a powerful tool towards this goal: it is a fluorescence microscopy approach that measures the location and mobility of individual TF molecules, as well as their rates of association with and dissociation from chromatin in the physiological context of the living cell. Here we review SMT principles, and discuss key TF properties uncovered by live-cell SMT, such as fast turnover (seconds), and formation of clusters that locally increase activity.
The transcription cycle
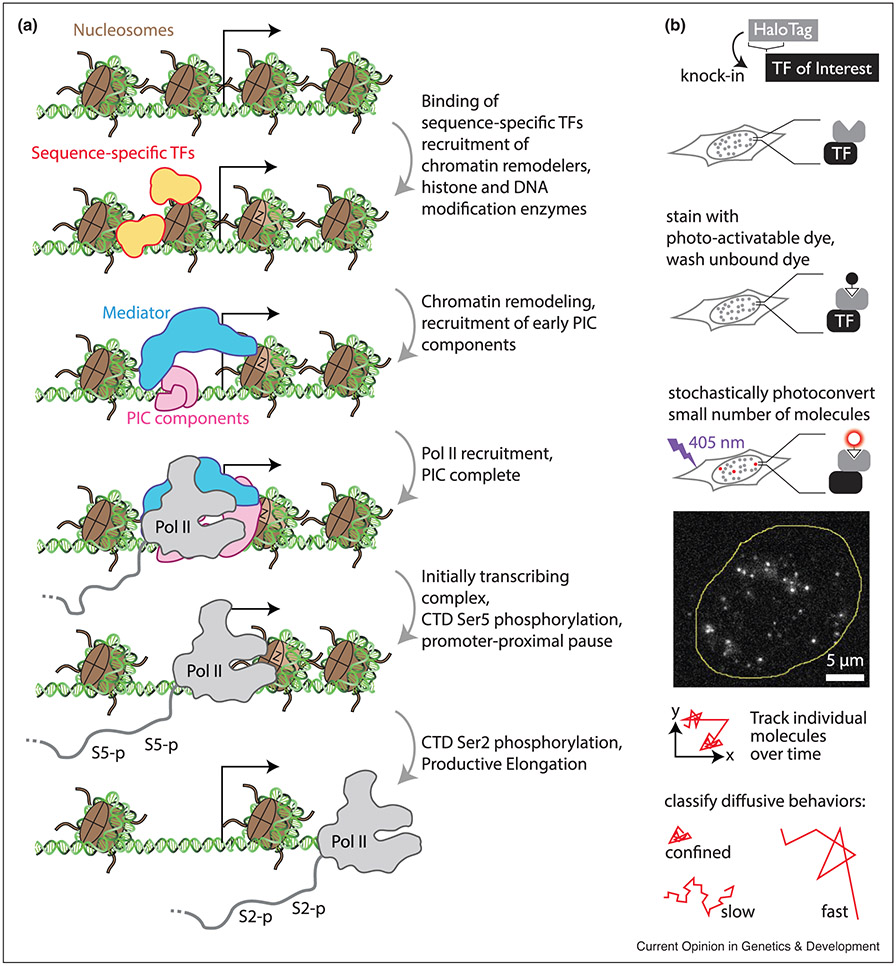
Transcription starts with the binding of sequence-specific TFs to the promoter of a gene or enhancer sequences regulating its expression (Figure 1) [1]. In eukaryotes, nucleosome organization can impede access to cognate DNA sequences, requiring cross-talk between ‘pioneer’ TFs and chromatin remodeling and histone modification enzymes to increase site accessibility (e.g. DNAse hypersensitive sites; DHS) [2]. TFs also recruit a host of co-activator proteins, as well as components of the pre-initiation complex (PIC). The PIC is an assembly of proteins that act as a recruitment platform for RNA Polymerase II (Pol II), responsible for transcription of protein-coding genes. Once the PIC has fully assembled onto the promoter, transcription initiation starts with the phosphorylation of the C-terminal Domain (CTD) of Pol II at the serine 5 position. The CTD is a long unstructured domain that consists of repeats of the consensus motif YSPTSPS and is required for transcription activity and viability [3••,4•]. Shortly after initiation, metazoan Pol II undergoes a short pause ~50–80 bp downstream of the promoter. Release from this promoter-proximal pause requires phosphorylation of the CTD at the serine 2 position by the kinase CDK9, part of the P-TEFb complex. After exit from the pause, Pol II transcribes the gene while processing events occur on nascent RNA (capping, splicing, polyadenylation), transiently displacing downstream nucleosomes until termination.
Figure 1.
(a) Stages of the transcription cycle. Transcription occurs in a series of steps, starting with the binding of sequence-specific Transcription Factors (TFs), that recruit co-activators, chromatin remodelers (for instance depositing histone variants such as H2A.Z, marked with Z) and eventually members of the Pre-Initiation Complex (PIC). Upon formation of the complete PIC containing Pol II, transcription starts, marked by Ser5 phosphorylation of the CTD of Pol II. Upon release from the promoter-proximal pause (marked by phosphorylation of the CTD at Ser2), productive elongation of RNA transcripts. (b) Single-Molecule Tracking workflow. A Transcription Factor (TF) of interest is fused to the self-labeling tag HaloTag. Cells containing the fusion protein are labeled with a HaloTag ligand conjugated to a photoactivatable fluorophore, and unbound ligands are washed out. Upon a low intensity pulse of blue light excitation, ~1–20 TF molecules turn fluorescent (example image shows individual molecules of H2B-HaloTag labeled with PA-JF646; cell nucleus outlined in yellow). The motion of those molecules is subsequently recorded until they leave the focal plane or photobleach; a new cycle is initiated by another pulse of blue light that turns on a fresh subset of molecules. Subsequent analysis of molecule trajectories (x,y positions over time) enables classifying TF molecules into different mobility populations.
Multiple control points punctuating the transcription cycle provide numerous opportunities for regulation, opening the door to rich dynamic behaviors. Indeed, transcripts encoded by an individual gene are not produced as a steady stream, but rather synthesized during bursts of activity interspersed by long periods of inactivity [5•]. A major challenge is to understand how transcription factors collectively generate complex bursting dynamics. As most players typically only dwell for seconds at the promoter [6•], one key question is how relatively short-lived interactions can lead to expression programs that are sustained over days.
What can live-cell SMT reveal about transcription, and what are its limitations?
Single-Molecule Tracking (SMT) in living cells provides precise measurements of the location and motions of nuclear factors, complementing genome localization-sequencing technologies that can be subject to side-effects of chemical cross-linking [7]. It is highly technical, requiring experience in optical physics, biophysics, computer science, molecular genetics and cell biology [8,9]. SMT directly observes the kinetic behaviors of fluorescently labeled protein factors at an x–y resolution approaching the 10 nm-diameter nucleosomes on chromatin fibers (~25 nm × 25 nm) but considerably lower z-resolution (>70 nm). At a minimum, a wide field epifluorescence microscope with additional high laser power and ultrasensitive camera components monitors the location over time of an individual protein (spot) undergoing free diffusion in the nucleoplasm, or paused due to association with chromatin or other nuclear structures. Movies recording a temporal sequence of spots (a trajectory) within a circumscribed nuclear area – under conditions where neighboring spots are absent or suffi-ciently distant to avoid confounding identities – are limited to a few minutes because of fluorophore photo-bleaching and chromatin motions out of the focal plane, which leads to disproportionate loss of long trajectories. Nonetheless, one can extrapolate binding events beyond the SMT observable window by calibrating photo-bleaching kinetics [10], or by normalizing the measured lifetime distribution to a chromosomal histone with low bulk turnover outside of S-phase [11, 12]. Repeated imaging of multiple cells over an hour or two records a statistically significant number of molecules, usually >1000, to inform the overall distribution of kinetic behaviors of the population.
From raw trajectories at fast frame rates (30 ms and under), one can extract the number and frequency of dynamic states [13•]. Because histones are known to be largely incorporated in chromatin, the slowest diffusive histone fraction provides a reference for the average diffusion coefficient D of bulk chromatin, although D values can vary depending on specific gene states or chromosomal locations (see below) [14]. Tightly associating chromatin and transcription factors may have similar diffusive properties as incorporated histones, but factor binding to other nuclear structures should also impede free diffusion. The use of DNA-binding or histone-binding mutants is necessary to assist functional assignment of diffusive populations [14]. In addition, transcription factors could exhibit a spectrum of diffusive behaviors between stably incorporated and free. Diffusion Coefficients alone do not fully capture the complexity of single-molecule behaviors, and complementary spatial metrics can be computed from trajectories in order to provide deeper insight into TF behavior. For instance, trajectories of chromatin-bound factors remain confined within a certain radius (in the 20–100 nm range) that tends to be shorter in nuclear lamin-associated domains compared to other chromatin regions [20,21,22•,25], and observation or recurring motion may allow calculation of a spring constant [16]. Displacement anisotropy (i.e. the tendency of a trajectory to feature U-turns) is a signature of how a factor explores the nucleus: either in a compact fashion that involves local oversampling, or more global exploration [15]. Anisotropy biases that appear at distinct time intervals, as observed for CTCF, suggest that the underlying nuclear space is heterogenous [16]. Since individual molecules might sample different diffusive states in their trajectory (e.g. binding, followed by dissociation and free diffusion), the transitions between states can be identified using Hidden Markov Models (e.g. vbspt [17]), or more recently using deep learning approaches [18,19].
Slower tracking (100 ms and above) motion blurs freely diffusing molecules in order to preferentially capture the chromatin-bound fraction and measure stable and transient dwell times of chromatin-bound molecules. Together, these allow one to calculate the frequency and duration of TF occupancy [18]. Because of the complexity of the data and the stochasticity of the pro-cesses at play, computational modeling and simulations are required to predict how the spatiotemporal dynamics of multiple transcription-related components can produce a defined RNA output [3••].
Visualization of the entire nuclear distribution of molecular behaviors by SMT can uncover novel subpopulations that would otherwise be masked by population averaging [9]. Furthermore, two-color, region-specific imaging (e.g. heterochromatin/euchromatin) and locus-specific marking and imaging with new target-lock [27••] or orbital tracking optical technologies [28••,29••] point the way to visualizing dynamics of gene-specific chromatin and interacting factors (see chapter by BC Chen). Continuous improvements in labeling chemistry enabling brighter fluorophores with higher affinity and specificity for protein tags extend SMT precision and raise the temporal detection limit [30•]. However, for abundant proteins such as histones or RNA polymerases, the necessity for sparse labeling to image individual molecules makes it difficult to simultaneously visualize molecular interactions between two different species. A constant concern for DNA damage imposed by short-wavelength laser light is partially alleviated by orange and red illumination [31], but SMT data interpretation is conditioned by other explicit and implicit biophysical assumptions that complicates biological conclusions. For example, intrinsic ‘blinking’ of a single fluorophore can produce apparent clusters from the same molecule, requiring a corrective computational approach [32•]. Especially worrisome is the real possibility that fusion of a ~30 kD protein tag (chemically labeled or genetically engineered fluorescent protein) and/or protein overexpression can adversely perturb function and produce misleading diffusive behaviors [33,34]. The advent of gene editing by CRISPR technology now enables rigorous functional validation of protein fusions expressed as the sole source under natural promoter/enhancer control [11,35•].
Dynamic chromatin binding and dissociation is compatible with high target occupancy
Recent SMT experiments have provided measurements on the residence times of chromatin-bound factors averaged across the entire nucleus. While most TF interactions with chromatin typically last <1 s (‘transient’), a small fraction display longer binding by an order of magnitude (‘stable’), often approximating an exponential decay with a half-life on the order of 2–20 s in yeast [28••,36•] and 10–20 s in metazoans [12,14,29••,37-39,40•]. Factors lacking a functional DNA-binding domain usually interact only transiently, suggesting that these represent non-specific interactions. Residence times are visualized using long exposure times in order to motion-blur chromatin-free molecules (100–500 ms per frame: slow tracking), while shorter exposures (10–50 ms per frame: fast-tracking) allow imaging of fast diffusing TFs through the nucleoplasm. These two regimes enable estimation of the search time tsearch: the time it takes upon dissociation for a molecule to find the next accessible genomic target based on the classic facilitated diffusion model [14,41], which posits that TFs find their targets by sequential searches in 1D (sliding along the double helix for a duration of t1D) and 3D (diffusing in nuclear space for a duration of t3D). After NTrials trials, the TF eventually finds a cognate site, where it resides longer (10–20 s). tsearch = (NTrials − 1) * (t3D + t1D) + t3D. In contrast With other modes of nuclear exploration (e.g. 3D diffusion or 1D scanning alone), facilitated diffusion successfully predicts the high association rates observed experimentally [41]. Search times range from ~7 s in yeast [36•] to ~6 min for a mammalian Sox2 molecule to find any of its ~7000 target sites in ES cells [14]. SMT of the lac repressor provided the first evidence supporting 1D sliding during facilitated diffusion in live bacteria [42], and a new in vitro study now reveals that LacI rotates as it slides on a microsecond time scale, hopping in and out of the DNA groove [43•].
The short-lived stable residence time of TFs on chromatin measured by SMT seems at first paradoxical [6•]: sequence-specific TFs display robust steady-state binding profiles in genomic assays such as ChIP-seq [14], and can drive developmental programs lasting days. Recent competition experiments for Ascl1, a TF in the nondividing Xenopus oocyte suggest a residence time of hours [44]. However, this does not necessarily conflict with SMT studies in cultured cells, because chromatin and transcription biology might differ between systems. Indeed, studies using endogenous knock-in of fluorescent tags validate that tags do not induce phenotypes, indicating that 10–20 s binding times are sufficient for TF function [11,35•]. Orthogonal techniques imaging for hour-long periods (e.g. FRAP, Fluorescence Recovery After Photobleaching) also show that TF binding is highly dynamic, convergent with SMT results [11,45]. These findings argue that the 10–20 s stable residence times do not represent an artifactual temporal boundary set by SMT. Moreover, locus-specific imaging of yeast Gal4 molecules measures binding events lasting on average 12–35 s [28••], peaking ~14 s before the onset of transcription, demonstrating that seconds-long interactions are sufficient to trigger transcription [28••]. Collectively, SMT results indicate that binding for just a few seconds might be sufficient for a TF to efficiently initiate PIC recruitment, whereas interactions <1 s are unproductive. The stable dwell times of <5 s for most PIC components in yeast provide a consistent timescale for this process [101]. TF residence times can be regulated by signaling [28••,29••,39], and longer residence times lead to higher transcription output [29••,37,38,102]. Interestingly, some TFs show a continuum of dwell times [12,29••,46], which could reflect binding to a variety of DNA sequences with graded affinities.
At any given accessible, DNase hypersensitive (DHS) promoter, the TF occupancy, defined as % occupancy in a particular time period, and not necessarily the residence time, is likely the key parameter regulating transcription levels. For a simple binding reaction TF + DHS → TF*DHS, the equilibrium dissociation constant Kd = [TF] [DHS]/[TF*DHS] indicates that the concentration of the TF-promoter complex [TF*DHS], is proportional to [TF] and [DHS] and inversely proportional to Kd. The important contribution of SMT is to enable calculation of Kd in living cells from measurable rate constants (koff /kon = Kd), where koff is the inverse of the residence time and kon is inversely proportional to tsearch, and Nsites the number of cognate DHSs in the genome. [TF] can be quantified roughly [47•] and varies depending on expression level, nuclear import, nuclear volume, and inhomogeneity or local clustering near a promoter [37,48•], while Nsites can be estimated from genomic technologies and is dependent on ‘pioneer’ TFs, and chromatin remodeling and modification enzymes regulating transitions between DHSs and canonical nucleosomes. Thus, TF occupancy depends on any combination of changes in factor concentration, number and accessibility of cognate sites, residence and search times, and additional influences such as TF association with a ligand partner or protein partner, or competitive displacement by a nuclear enzyme. Accordingly, even relatively short residence times (10–20 s) are compatible with target motifs being occupied a large percentage of the time, provided that there are compensatory mechanisms.
Protein clustering depends on IDRs in live cells: is phase transition the underlying mechanism?
The growing evidence that TFs cluster spatially as foci in the nucleus via multi-valent, low-affinity interactions between intrinsically disordered regions (IDRs), also called low complexity domains (LCDs), a ubiquitous feature of TFs, suggests a mechanism to increase local concentration and thus drive up occupancy at key regulatory sites [27••,49••,50•,51•,52-58]. Clustering near DNA or in the nucleoplasm may foster local niches or ‘hubs’ with unique diffusive properties [23,59••]. Promoter/Enhancer clustering increases the effective size of a target site, potentially attracting other TFs harboring IDRs, although the rules governing the specificity or promiscuity of IDR–IDR interactions are not well understood [3••,35•,49••,52,60•]. Clustering would likely provide an extra assist to the search process beyond 1D scanning and 3D diffusion [6•]
Short-lived clustering (seconds to minutes) [35•,50•,61] parallels the propensity of IDRs to phase separate in vitro into ‘condensates’ or droplets [49••,50•,51•,52,62•,63•,64]. However, in vitro phase separation assays often require TF concentrations orders of magnitude higher than physiological levels, and thus as a group, should be interpreted with caution, since alternative mechanisms can also increase local TF concentration at physiological levels in the cell, for example, locally enhanced DNA binding [59••,65•,66•,67].
The CTD of Pol II offers an important paradigm of how clustering impacts transcription [53]. CTD propensity to cluster scales with its length [62•]. CTDs shorter or longer than their WT lengths display dramatic phenotypes, while truncated CTDs fused to an unrelated IDR are functional, suggesting that the transcription machinery operates in a narrow range of clustering potential [3••,4•]. CTD phosphorylation regulates Pol II affinity for itself and other IDR-containing partners such as Mediator [53,62•,64,68•]. These observations suggest that transient and stable Pol II clusters [27••,50•] are recruited via CTD interactions, and that subsequent CTD phosphorylation frees elongating Pol II molecules [69]. The observation that the number of mRNAs produced per burst scales with CTD length and Pol II cluster size further support this model [3••,61]. Of interest, nuclear actin, whose function has long been speculated, is implicated in Pol II clustering [70], while nuclear myosin movement on actin filaments appears to facilitate long-range chromosome rearrangements during transcription [20,71].
For other chromatin regulators, conserved IDR residues of the Cbx2 subunit of the Polycomb Repressive Complex PRC1 play a key role in forming foci in living cells and condensates in vitro [72]. SMT studies reveal dynamics of PRC1 and PRC2 subunits [40•,73], showing stable dwell times of ~10 s, comparable to mammalian TFs [40•], An unexpected low level of PRC1 site occupancy argues against a physical mode of repression [74•]. Similarly, the groucho/TLE family of transcription repressors exhibits puncta in living Ciona with properties of phase-separated condensates [75], and the silencing factor Sir3 promotes long-range contacts between distant loci [76]. Alanine or glutamine repeat expansions in IDRs of human sequence-specific transcription factors and the TATA-Binding Protein TBP change the capacity to form phase-separated condensates in vitro, and alter the composition of heterotypic puncta in live cells [77•]. The HP1 heterochromatin protein oligomerizes via interactions between IDRs and forms phase-separated droplets in vitro [78,79], but another study reports a weak propensity for droplet formation in cells and proposes an alternative polymer collapse model [65•,80].
Local and global chromatin movements on short and long timescales
Even when chromatin-bound, individual transcription factor molecules exhibit a wide range of mobility and confinement behaviors [24•], possibly reflecting local dynamics of chromatin sites. Chromatin mobility can be assessed locally, by fluorescent labeling of a genomic locus [81,82••], or globally, by tracking fluorescent histones [24•,83•,84•]. Transcription activity at a locus generally correlates with increased mobility at short timescales (<5 s) and is dependent on Pol II initiation and elongation activity [82••]. However, an ectopically integrated locus responding to Estrogen Receptor activation shows constrained mobility at longer timescales (>5 s) independent of Pol II elongation [81].
Global tracking of chromatin motion provides a complementary view. An approach termed Dense Flow reConstruction and Correlation (DFCC) shows that at short time scales, small chromatin domains move independently from one another, while at longer time scales (>10 s) micron-scale domains (up to the size of an entire chromosome) appear to move coordinately [85]. Nucleosomes within lamin-associated and heterochromatic regions move slower and are more confined [24•]. Counterintuitively, shutting down transcription globally induces an increase in histone mobility [83•,86•]. These apparently conflicting observations may be reconciled by a model where active transcription – and associated chromatin remodeling [26,67] – stiffens the periphery of chromosomal domains decorated with RNA [87-89], which constrains the motion of internal heterochromatin domains, forcing large regions (possibly entire chromosomes) to move in sync. Blocking transcription releases that motion constraint. A caveat common to many chromatin tracking experiments is that ectopically expressed fluorescent H2B might display behavior reflecting incorporation into chromatin domains that do not fully represent endogenous H2B synthesized in S-phase. Indeed, DNA fluorescently labeled by intercalating dyes displays distinct nuclear mobility from a fluorescent H2B fusion expressed in mammalian cells [85]. Ideally, a fluorescent H2B fusion should be the sole source of the histone, expressed under natural promoter control as shown for yeast [84•] but this is technically challenging for expression in metazoans, due to multiple H2B gene copies.
Future directions: temporal order of events and convergence with in vitro SMT biochemistry
Despite recent progress in SMT at select loci [27••,28••,29••,61], sparse labeling constraints make it impractical so far to measure interactions between different protein species at the single-molecule level. Future progress in tracking two or more factors simultaneously will help address longstanding questions of how accessible promoters and enhancers integrate inputs from multiple TFs [90], the order of in vivo assembly of the transcription machinery, and how promoter-enhancer dynamics [91•,92•,93•], TF binding [28••,35•,94], and chromatin modification or remodeling [26,67,84•,95•,96,97•] are dynamically coupled with transcription activation. In vitro approaches using DNA or reconstituted nucleosome templates offer a tantalizing glimpse of how multi-color SMT and well-controlled experimental perturbations can deepen understanding of fundamental transcription mechanisms lying beyond the reach of current live-cell imaging [98•,99,100]. Furthermore, recapitulating the crowded nuclear environment and native chromatin templates for SMT in vitro will provide powerful means to uncover key reaction intermediates transiently populating the transcription pathway. This is an inescapable challenge for the future.
Acknowledgements
T.L. is supported by N.I.H. grant R01GM127538. C.W. is supported by NIH Grant R01 GM132290 and a Bloomberg distinguished professorship. Authors thank members of the Lionnet and Wu labs for comments on the manuscript.
Footnotes
Conflict of interest statement
C.W. declares no conflicts of interest. T.L. is co-inventor on a patent whose value may be affected by this publication (US-2019107534-A1).
References and recommended reading
Papers of particular interest, published within the period of review, have been highlighted as:
• of special interest
•• of outstanding interest
- 1.Cramer P: Organization and regulation of gene transcription. Nature 2019, 573:45–54. [DOI] [PubMed] [Google Scholar]
- 2.Thurman RE, Rynes E, Humbert R, Vierstra J, Maurano MT, Haugen E, Sheffield NC, Stergachis AB, Wang H, Vernot B et al. : The accessible chromatin landscape of the human genome. Nature 2012, 489:75–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.••. Quintero-Cadena P, Lenstra TL, Sternberg PW: RNA Pol II length and disorder enable cooperative scaling of transcriptional bursting. Mol Cell 2020, 79:207–220.e8 This study quantifies the impact of Pol II CTD-driven clustering on transcription bursting, demonstrating that the clustering function of the CTD can be partially rescued by unrelated IDRs.
- 4.•. Lu F, Portz B, Gilmour DS: The C-terminal domain of RNA Polymerase II is a multivalent targeting sequence that supports Drosophila development with only consensus heptads. Mol Cell 2019, 73:1232–1242.e4 This study shows that a truncated pol II CTD harboring only canonical repeats supports Drosophila viability, while canonical replacement of divergent motifs in the native CTD is defective, suggesting a novel function for motif divergence.
- 5.•. Rodriguez J, Larson DR: Transcription in living cells: molecular mechanisms of bursting. Annu Rev Biochem 2020, 89:189–212 This review covers the evidence linking bursting dynamics with different biochemical steps of the transcription cycle proposed to explain this phenomenon.
- 6.•. Suter DM: Transcription factors and DNA play hide and seek. Trends Cell Biol 2020, 30:491–500 The author presents a comprehensive and current review of the mechanisms by which transcription factors search the nucleus to reach their specific binding sites.
- 7.Festuccia N, Owens N, Papadopoulou T, Gonzalez I, Tachtsidi A, Vandoermel-Pournin S, Gallego E, Gutierrez N, Dubois A, Cohen-Tannoudji M et al. : Transcription factor activity and nucleosome organization in mitosis. Genome Res 2019, 29:250–260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Elf J, Barkefors I: Single-molecule kinetics in living cells. Annu Rev Biochem 2019, 88:635–659. [DOI] [PubMed] [Google Scholar]
- 9.Liu Z, Tjian R: Visualizing transcription factor dynamics in living cells. J Cell Biol 2018, 217:1181–1191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Gebhardt JC, Suter DM, Roy R, Zhao ZW, Chapman AR, Basu S, Maniatis T, Xie XS: Single-molecule imaging of transcription factor binding to DNA in live mammalian cells. Nat Methods 2013. 10:421–426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hansen AS, Pustova I, Cattoglio C, Tjian R, Darzacq X: CTCF and cohesin regulate chromatin loop stability with distinct dynamics. eLife 2017, 6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Garcia DA, Fettweis G, Presman DM, Paakinaho V, Jarzynski C, Upadhyaya A, Hager Gl: A new model for single-molecule tracking analysis of transcription factor dynamics. bioRxiv 2020. 10.1101/637355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.•. Hansen AS, Woringer M, Grimm JB, Lavis LD, Tjian R, Darzacq X: Robust model-based analysis of single-particle tracking experiments with Spot-On. eLife 2018, 7 This work describes SPOT-ON, a user-friendly, web-based tool where users can input single-molecule trajectories and fit their datasets to mixed-population models of diffusion.
- 14.Chen J, Zhang Z, Li L, Chen BC, Revyakin A, Hajj B, Legant W, Dahan M, Lionnet T, Betzig E et al. : Single-molecule dynamics of enhanceosome assembly in embryonic stem cells. Cell 2014, 156:1274–1285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Izeddin I, Recamier V, Bosanac L, Cisse II, Boudarene L, Dugast-Darzacq C, Proux F, Benichou O, Voituriez R, Bensaude O et al. : Single-molecule tracking in live cells reveals distinct target-search strategies of transcription factors in the nucleus. eLife 2014. 3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hansen AS, Amitai A, Cattoglio C, Tjian R, Darzacq X: Guided nuclear exploration increases CTCF target search efficiency. Nat Chem Biol 2020, 16:257–266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Persson F, Lindén M, Unoson C, Elf J: Extracting intracellular diffusive states and transition rates from single-molecule tracking data. Nat Methods 2013, 10:265–269. [DOI] [PubMed] [Google Scholar]
- 18.Granik N, Weiss LE, Nehme E, Levin M, Chein M, Perlson E, Roichman Y, Shechtman Y: Single-particle diffusion characterization by deep learning. Biophys J 2019, 117:185–192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Möckl L, Roy AR, Moerner WE: Deep learning in single-molecule microscopy: fundamentals, caveats, and recent developments [Invited]. Biomed Opt Express 2020, 11:1633–1661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Nora EP, Caccianini L, Fudenberg G, So K, Kameswaran V, Nagle A, Uebersohn A, Hajj B, Le Saux A, Coulon A, Mirny LA, Pollard KS, Dahan M, Bruneau BG: Molecular basis of CTCF binding polarity in genome folding. Nat Commun 2020, 11:5612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Oomen ME, Hansen AS, Liu Y, Darzacq X, Dekker J: CTCF sites display cell cycle–dependent dynamics in factor binding and nucleosome positioning. Genome Res 2019, 29:236–249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.•. Shukron O, Seeber A, Amitai A, Holcman D: Advances using single-particle trajectories to reconstruct chromatin organization and dynamics. Trends Genet 2019, 35:685–705 This review covers a large number of SMT analyses applied to chromatin dynamics, and provides a comprehensive description of each approach’s principle, strengths and caveats.
- 23.Cisse II, Izeddin I, Causse SZ, Boudarene L, Senecal A, Muresan L, Dugast-Darzacq C, Hajj B, Dahan M, Darzacq X: Real-time dynamics of RNA Polymerase II clustering in live human cells. Science 2013, 341:664–667. [DOI] [PubMed] [Google Scholar]
- 24.•. Lerner J, Gomez-Garcia PA, McCarthy RL, Liu Z, Lakadamyali M, Zaret KS: Two-parameter mobility assessments discriminate diverse regulatory factor behaviors in chromatin. Mol Cell 2020, 79:677–688.e6 This study presents a two-parameter SMT approach to report the movements of incorporated histone H2B, and use the varied chromatin motion landscape to compare the behaviors of heterochromatin proteins and pioneer transcription factors.
- 25.Wieser S, Schütz GJ: Tracking single molecules in the live cell plasma membrane-Do’s and Don’t’s. Methods 2008, 46:131–140. [DOI] [PubMed] [Google Scholar]
- 26.Basu S, Shukron O, Ponjavic A, Parruto P, Boucher W: Live-cell 3D single-molecule tracking reveals how NuRD modulates enhancer dynamics. bioRxiv 2020. 10.1101/2020.04.03.003178.2020.04.03.003178. [DOI] [Google Scholar]
- 27.••. Li J, Dong A, Saydaminova K, Chang H, Wang G, Ochiai H, Yamamoto T, Pertsinidis A: Single-molecule nanoscopy elucidates RNA Polymerase II transcription at single genes in live cells. Cell 2019, 178:491–506.e28 This study uses a target-locking microscope to specifically measure recruitment and dwell times of factors at the locus of an actively transcribing gene, demonstrating rapid turnover and clustering of pluripotency TFs.
- 28.••. Donovan BT, Huynh A, Ball DA, Patel HP, Poirier MG, Larson DR, Ferguson ML, Lenstra TL: Live-cell imaging reveals the interplay between transcription factors, nucleosomes, and bursting. EMBO J 2019, 38 This study elegantly uses orbital tracking microscopy to directly correlate single-molecule events of Gal4 binding and transcription bursts of its target GAL10, demonstrating that rapid rounds of binding (seconds) are sufficient to induce productive transcription.
- 29.••. Stavreva DA, Garcia DA, Fettweis G, Gudla PR, Zaki GF, Soni V, McGowan A, Williams G, Huynh A, Palangat M et al. : Transcriptional bursting and co-bursting regulation by steroid hormone release pattern and transcription factor mobility. Mol Cell 2019, 75:1161–1177.e11 This study correlates the mobility and DNA binding of the Glucocorticoid Receptor to the bursting dynamics of a target gene, correlating TF residence time to burst duration, and overall fraction of TF bound to burst frequency.
- 30.•. Jradi FM, Lavis LD: Chemistry of photosensitive fluorophores for single-molecule localization microscopy. ACS Chem Biol 2019, 14:1077–1090 A chemical review of the history of fluorescent probes for SMT with an updated list of probes with demonstrated utility.
- 31.Wäldchen S, Lehmann J, Klein T, van de Linde S, Sauer M: Light-induced cell damage in live-cell super-resolution microscopy. Sci Rep 2015, 5:15348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.•. Bohrer CH, Yang X, Weng X, Tenner B, Thakur S: A pairwise Distance Distribution Correction (DDC) algorithm to eliminate blinking-caused artifacts in super-resolution microscopy. bioRxiv 2020. 10.1101/768051.768051 This study presents a new ‘Distance Distribution Correction’ approach to manage fluorophore blinking in single-molecule localization microscopy.
- 33.Wisniewski J, Hajj B, Chen J, Mizuguchi G, Xiao H, Wei D, Dahan M, Wu C: Imaging the fate of histone Cse4 reveals de novo replacement in S phase and subsequent stable residence at centromeres. eLife 2014, 3:e02203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Landgraf D, Okumus B, Chien P, Baker TA, Paulsson J: Segregation of molecules at cell division reveals native protein localization. Nat Methods 2012, 9:480–482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.•. Mir M, Stadler MR, Ortiz SA, Hannon CE, Harrison MM, Darzacq X, Eisen MB: Dynamic multifactor hubs interact transiently with sites of active transcription in Drosophila embryos. eLife 2018, 7 The authors use lattice light-sheet microscopy for SMT of transcription factors Bicoid and Zelda in the Drosophila embryo, showing high chromatin unbinding rates and heterogenous subnuclear distribution in short-lived ‘hubs.’
- 36.•. Mehta GD, Ball DA, Eriksson PR, Chereji RV, Clark DJ, McNally JG, Karpova TS: Single-molecule analysis reveals linked cycles of RSC chromatin remodeling and Ace1p transcription factor binding in yeast. Mol Cell 2018, 72:875–887.e9 This study shows that yeast Ace1 transcription factor binds dynamically to its specific CUP1 array target, facilitated by the RSC chromatin remodeler, which speeds up the target search process and increases promoter occupancy.
- 37.Callegari A, Sieben C, Benke A, Suter DM, Fierz B, Mazza D, Manley S: Single-molecule dynamics and genome-wide transcriptomics reveal that NF-kB (p65)-DNA binding times can be decoupled from transcriptional activation. PLoS Genet 2019, 15:e1007891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Clauß K, Popp AP, Schulze L, Hettich J, Reisser M, Escoter Torres L, Uhlenhaut NH, Gebhardt JCM: DNA residence time is a regulatory factor of transcription repression. Nucleic Acids Res 2017, 45:11121–11130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Hipp L, Beer J, Kuchler O, Reisser M, Sinske D, Michaelis J, Gebhardt JCM, Knöll B: Single-molecule imaging of the transcription factor SRF reveals prolonged chromatin-binding kinetics upon cell stimulation. Proc Natl Acad Sci U S A 2019, 116:880–889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.•. Tatavosian R, Duc HN, Huynh TN, Fang D, Schmitt B, Shi X, Deng Y, Phiel C, Yao T, Zhang Z et al. : Live-cell single-molecule dynamics of PcG proteins imposed by the DIPG H3.3K27M mutation. Nat Commun 2018, 9:2080. This study characterizes highly dynamic binding and dissociation of PRC2 and Cbx7-PRC1, the effector of H3.3K27me3 in mouse ES cells, and shows how the DIPG mutation H3.3K27M blocks PRC2 activity in vitro and reprograms genomic occupancy of H3K27me3 and PRC2 in cells.
- 41.von Hippel PH, Berg OG: Facilitated target location in biological systems. J Biol Chem 1989, 264:675–678. [PubMed] [Google Scholar]
- 42.Hammar P, Leroy P, Mahmutovic A, Marklund EG, Berg OG, Elf J: The lac repressor displays facilitated diffusion in living cells. Science 2012, 336:1595–1598. [DOI] [PubMed] [Google Scholar]
- 43.•. Marklund E, van Oosten B, Mao G, Amselem E, Kipper K, Sabantsev A, Emmerich A, Globisch D, Zheng X, Lehmann LC et al. : DNA surface exploration and operator bypassing during target search. Nature 2020, 583:858–861 This recent in vitro study uses a cutting-edge combination of single-molecule confocal laser tracking and fluorescence correlation spectroscopy to show that LacI explores DNA in 1D by coupling sliding and rotation, effectively advancing one turn per ~40 bp traveled through hoppings in the subms timescale regime.
- 44.Gurdon JB, Javed K, Vodnala M, Garrett N: Long-term association of a transcription factor with its chromatin binding site can stabilize gene expression and cell fate commitment. Proc Natl Acad Sci U S A 2020, 117:15075–15084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Paakinaho V, Presman DM, Ball DA, Johnson TA, Schiltz RL, Levitt P, Mazza D, Morisaki T, Karpova TS, Hager GL: Single-molecule analysis of steroid receptor and cofactor action in living cells. Nat Commun 2017, 8:15896. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Normanno D, Boudarene L, Dugast-Darzacq C, Chen J, Richter C, Proux F, Benichou O, Voituriez R, Darzacq X, Dahan M: Probing the target search of DNA-binding proteins in mammalian cells using TetR as model searcher. Nat Commun 2015, 6:7357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.•. Cattoglio C, Pustova I, Walther N, Ho JJ, Hantsche-Grininger M, Inouye CJ, Hossain MJ, Dailey GM, Ellenberg J, Darzacq X et al. : Determining cellular CTCF and cohesin abundances to constrain 3D genome models. eLife 2019, 8 The authors develop mammalian cell lines with accurately measured protein levels for CTCF and cohesin; the method can be conveniently applied to determine the number of molecules of any cellular Halo-tagged protein.
- 48.•. Reisser M, Palmer A, Popp AP, Jahn C, Weidinger G, Gebhardt JCM: Single-molecule imaging correlates decreasing nuclear volume with increasing TF-chromatin associations during zebrafish development. Nat Commun 2018, 9:5218. SMT analysis using light-sheet microscopy of the developing zebrafish embryo shows that increasing chromatin binding correlates with decreasing nuclear volume.
- 49.••. Chong S, Dugast-Darzacq C, Liu Z, Dong P, Dailey GM, Cattoglio C, Heckert A, Banala S, Lavis L, Darzacq X et al. : Imaging dynamic and selective low-complexity domain interactions that control gene transcription. Science 2018, 361 This study uses induced recruitment of IDRs and TFs to confirm that hubs of TFs are dynamic, and establish that IDRs exhibit specificity in their affinity for one another.
- 50.•. Cho W-K, Spille J-H, Hecht M, Lee C, Li C, Grube V, Cisse II: Mediator and RNA Polymerase II clusters associate in transcription-dependent condensates. Science 2018, 361:412–415 The authors use SMT and light-sheet imaging to show that Mediator coactivator and Pol II form small transient and large stable clusters in live ES cells with properties of phase-separated condensates.
- 51.•. Boija A, Klein IA, Sabari BR, Dall’Agnese A, Coffey EL, Zamudio AV, Li Ch, Shrinivas K, Manteiga JC, Hannett NM et al. : Transcription factors activate genes through the phase-separation capacity of their activation domains. Cell 2018, 175:1842–1855.e16 This study established that clustering of TFs with Mediator at sites of active transcription parallels their potential to phase-separate in vitro.
- 52.Zamudio AV, Dall’Agnese A, Henninger JE, Manteiga JC, Afeyan LK, Hannett NM, Coffey EL, Li CH, Oksuz O, Sabari BR et al. : Mediator condensates localize signaling factors to key cell identity genes. Mol Cell 2019, 76:753–766.e6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Kwon I, Kato M, Xiang S, Wu L, Theodoropoulos P, Mirzaei H, Han T, Xie S, Corden JL, McKnight SL: Phosphorylation-regulated binding of RNA Polymerase II to fibrous polymers of low-complexity domains. Cell 2013, 155:1049–1060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Mir M, Reimer A, Haines JE, Li X-Y, Stadler M, Garcia H, Eisen MB, Darzacq X: Dense Bicoid hubs accentuate binding along the morphogen gradient. Genes Dev 2017, 31:1784–1794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Tsai A, Muthusamy AK, Alves MR, Lavis LD, Singer RH, Stern DL, Crocker J: Nuclear microenvironments modulate transcription from low-affinity enhancers. eLife 2017, 6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Andrews JO, Conway W, Cho W-K, Narayanan A, Spille J-H, Jayanth N, Inoue T, Mullen S, Thaler J, Cissé II: qSR: a quantitative super-resolution analysis tool reveals the cell-cycle dependent organization of RNA Polymerase I in live human cells. Sci Rep 2018, 8:7424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Tsai A, Alves MR, Crocker J: Multi-enhancer transcriptional hubs confer phenotypic robustness. eLife 2019, 8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Hur W, Kemp JP, Tarzia M, Deneke VE, Marzluff WF, Duronio RJ, Talia Di: S: CDK-regulated phase separation seeded by histone genes ensures precise growth and function of histone locus bodies. Dev Cell 2020, 54:379–394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.••. McSwiggen DT, Hansen AS, Teves SS, Marie-Nelly H, Hao Y, Heckert AB, Umemoto KK, Dugast-Darzacq C, Tjian R, Darzacq X: Evidence for DNA-mediated nuclear compartmentalization distinct from phase separation. eLife 2019, 8 This study demonstrates that clusters of Pol II in cells form via mechanisms distinct from phase separation in Herpes Simplex Virus replication compartments, where locally enhanced DNA binding increases Pol II concentration.
- 60.•. Brodsky S, Jana T, Mittelman K, Chapal M, Kumar DK, Carmi M, Barkai N: Intrinsically disordered regions direct transcription factor in vivo binding specificity. Mol Cell 2020, 79:459–471 This work demonstrates that IDRs play a substantial role in selecting which promoters TFs occupy through low-affinity interactions distributed across the entire IDR.
- 61.Cho W-K, Jayanth N, English BP, Inoue T, Owen Andrews J, Conway W, Grimm jB, Spille J-H, Lavis lD, Lionnet T et al. : RnA Polymerase II cluster dynamics predict mRNA output in living cells. eLife Sci 2016, 5:e13617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.•. Boehning M, Dugast-Darzacq C, Rankovic M, Hansen AS, Yu T, Marie-Nelly H, McSwiggen DT, Kokic G, Dailey GM, Cramer P et al. : RNA Polymerase II clustering through carboxy-terminal domain phase separation. Nat Struct Mol Biol 2018, 25:833–840 This study shows that human and yeast Pol II CTD undergoes phase separation in droplets in vitro and has a CTD length-dependent role in forming Pol II clusters in live cells.
- 63.•. Sabari BR, Dall’Agnese A, Boija A, Klein IA, Coffey EL, Shrinivas K, Abraham Bj, Hannett nM, Zamudio AV, Manteiga JC et al. : Coactivator condensation at super-enhancers links phase separation and gene control. Science 2018, 361 The authors demonstrate that Mediator and BRD4 coactivators form discrete puncta at superhancers in mouse ES cells, displaying properties of phase-separated condensates.
- 64. Guo YE, Manteiga JC, Henninger JE, Sabari BR, Dall’Agnese A, Hannett NM, Spille J-H, Afeyan LK, Zamudio AV, Shrinivas K et al. : Pol II phosphorylation regulates a switch between transcriptional and splicing condensates. Nature 2019, 572:543–548 The authors demonstrate that a histidine-rich IDR domain in the cyclin T1 subunit of P-TEFb is capable of phase separation and enhances P-TEFb binding to the Pol II CTD, promoting hyperphosphorylation and transcription elongation.
- 65.•. Erdel F, Rademacher A, Vlijm R, Tünnermann J, Frank L, Weinmann R, Schweigert E, Yserentant K, Hummert J, Bauer C et al. : Mouse heterochromatin adopts digital compaction states without showing hallmarks of HP1-driven liquid-liquid phase separation. Mol Cell 2020, 78:236–249.e7 This study compares the capacity of HP1 to form droplets in vitro, in the nucleoplasm and when tethered to chromatin. The authors find that HP1 does not form stable droplets in living cells.
- 66.•. McSwiggen DT, Mir M, Darzacq X, Tjian R: Evaluating phase separation in live cells: diagnosis, caveats, and functional consequences. Genes Dev 2019, 33:1619-1634 This review critically evaluates phase-separation studies in living cells, outlining caveats of common assays and suggesting rigorous criteria.
- 67.Kenworthy CA, Liou S-H, Chandris P, Wong V, Dziuba P, Lavis LD, Liu W-L, Singer RH, Coleman RA: PBAF regulates compartmentalization of actively transcribing chromatin hubs. bioRxiv 2019. 10.1101/111674.111674. [DOI] [Google Scholar]
- 68.•. Lu H, Yu D, Hansen AS, Ganguly S, Liu R, Heckert A, Darzacq X, Zhou Q: Phase-separation mechanism for C-terminal hyperphosphorylation of RNA Polymerase II. Nature 2018, 558:318–323 The authors demonstrate that a histidine-rich IDR domain in the cyclin T1 subunit of P-TEFb is capable of phase separation and enhances P-TEFb binding to the Pol II CTD, promoting hyperphosphorylation and transcription elongation.
- 69.Tantale K, Mueller F, Kozulic-Pirher A, Lesne A, Victor J-M, Robert M-C, Capozi S, Chouaib R, Bäcker V, Mateos-Langerak J et al. : A single-molecule view of transcription reveals convoys of RNA polymerases and multi-scale bursting. Nat Commun 2016, 7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Wei M, Fan X, Ding M, Li R, Shao S, Hou Y, Meng S, Tang F, Li C, Sun Y: Nuclear actin regulates inducible transcription by enhancing RNA Polymerase II clustering. Sci Adv 2020, 6: eaay6515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Große-Berkenbusch A, Hettich J, Kuhn T, Fili N, Cook AW, Hari-Gupta Y, Palmer A, Streit L, Ellis PJI, Toseland CP et al. : Myosin VI moves on nuclear actin filaments and supports long-range chromatin rearrangements. bioRxiv 2020. 10.1101/2020.04.03.023614. [DOI] [Google Scholar]
- 72.Tatavosian R, Kent S, Brown K, Yao T, Duc HN, Huynh TN, Zhen CY, Ma B, Wang H, Ren X: Nuclear condensates of the Polycomb protein chromobox 2 (CBX2) assemble through phase separation. J Biol Chem 2019, 294:1451–1463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Youmans DT, Schmidt JC, Cech TR: Live-cell imaging reveals the dynamics of PRC2 and recruitment to chromatin by SUZ12-associated subunits. Genes Dev 2018, 32:794–805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.•. Huseyin MK, Klose RJ: Live-cell single particle tracking of PRC1 reveals a highly dynamic system with low target site occupancy. bioRxiv 2020. 10.1101/2020.04.25.061358 The authors use live-cell SMT to show that PRC1 in mouse ES cells is highly dynamic, with surprisingly low occupancy at targets. Results suggest that histone modification, not physical occupancy by repressor, is central to function.
- 75.Treen N, Shimobayashi SF, Eeftens J, Brangwynne CP: Regulation of gene expression by repression condensates during development. bioRxiv 2020. 10.1101/2020.03.03.975680. [DOI] [Google Scholar]
- 76.Ruault M, Scolari VF, Lazar-Stefanita L, Hocher A, Loïodice I, Noûs C, Koszul R, Taddei A: The silencing factor Sir3 is a molecular bridge that sticks together distant loci. bioRxiv 2020. 10.1101/2020.06.29.178368. 2020.06.29.178368. [DOI] [Google Scholar]
- 77.•. Basu S, Mackowiak SD, Niskanen H, Knezevic D, Asimi V, Grosswendt S, Geertsema H, Ali S, Jerković I, Ewers H et al. : Unblending of transcriptional condensates in human repeat expansion disease. Cell 2020, 181:1062–1079.e30 This study reports that alanine repeat expansions in the HOXD13 TF alter its phase separation capacity and its capacity to co-condense with transcriptional co-activators.
- 78.Larson AG, Elnatan D, Keenen MM, Trnka MJ, Johnston JB, Burlingame AL, Agard DA, Redding S, Narlikar GJ: Liquid droplet formation by HP1α suggests a role for phase separation in heterochromatin. Nature 2017, 547:236–240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Strom AR, Emelyanov AV, Mir M, Fyodorov DV, Darzacq X, Karpen GH: Phase separation drives heterochromatin domain formation. Nature 2017, 547:241–245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Gitler AD, Shorter J, Ha T, Myong S: Just took a DNA test, turns out 100% not that phase. Mol Cell 2020, 78:193–194. [DOI] [PubMed] [Google Scholar]
- 81.Germier T, Kocanova S, Walther N, Bancaud A, Shaban HA, Sellou H, Politi AZ, Ellenberg J, Gallardo F, Bystricky K: Real-time imaging of a single gene reveals transcription-initiated local confinement. Biophys J 2017, 113:1383–1394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.••. Gu B, Swigut T, Spencley A, Bauer MR, Chung M, Meyer T, Wysocka J: Transcription-coupled changes in nuclear mobility of mammalian cis-regulatory elements. Science 2018, 359:1050–1055 The authors developed a chimeric array of gRNA oligonucleotides (CARGO) technique coupled with dCas9 imaging in live ES cells to demonstrate that enhancers and promoters increase mobility with transcriptional activation.
- 83.•. Nagashima R, Hibino K, Ashwin SS, Babokhov M, Fujishiro S, Imai R, Nozaki T, Tamura S, Tani T, Kimura H et al. : Single nucleosome imaging reveals loose genome chromatin networks via active RNA Polymerase II. J Cell Biol 2019, 218:1511–1530 The authors use SMT to demonstrate that active RNAPII globally constrains chromatin movements, and that Pol II inhibition or depletion released constraints and increased chromatin dynamics.
- 84.•. Ranjan A, Nguyen VQ, Liu S, Wisniewski J, Kim JM, Tang X, Mizuguchi G, Elalaoui E, Nickels TJ, Jou V et al. : Live-cell single particle imaging reveals the role of RNA Polymerase II in histone H2A.Z eviction. eLife 2020, 9 This study combines SMT with conditional depletion experiments to examine mechanisms of histone variant H2A.Z eviction from yeast promoter chromatin, and highlights the role of Pol II escape mediating this process. The authors functionally validate HaloTag-histone fusions by sole source expression under natural promoter control.
- 85.Shaban HA, Barth R, Bystricky K: Formation of correlated chromatin domains at nanoscale dynamic resolution during transcription. Nucleic Acids Res 2018, 46:e77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.•. Shaban HA, Barth R, Recoules L, Bystricky K: Hi-D: nanoscale mapping of nuclear dynamics in single living cells. Genome Biol 2020, 21:95. This paper introduces a new approach called high-resolution diffusion mapping (Hi-D) that combines a dense optical flow reconstruction and a Bayesian inference approach to classify local diffusion. Authors find that DNA compaction does not necessarily correlate with its dynamics.
- 87.Hilbert L, Sato Y, Kimura H, Jülicher F, Honigmann A, Zaburdaev V, Vastenhouw NL: Transcription organizes euchromatin similar to an active microemulsion. bioRxiv 2018. 10.1101/234112. [DOI] [Google Scholar]
- 88.Leidescher S, Nuebler J, Feodorova Y, Hildebrand E: Spatial organization of transcribed eukaryotic genes. bioRxiv 2020. 10.1101/2020.05.20.106591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Miron E, Oldenkamp R, Brown JM, Pinto DMS, Shan Xu C, Faria aR, Shaban HA, Rhodes JDp, Innocent C, de Ornellas S et al. : Chromatin arranges in chains of mesoscale domains with nanoscale functional topography independent of cohesin. Sci Adv 2020. EABA8811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Biddle JW, Nguyen M, Gunawardena J: Negative reciprocity, not ordered assembly, underlies the interaction of Sox2 and Oct4 on DNA. eLife 2019, 8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.•. Alexander JM, Guan J, Li B, Maliskova L, Song M, Shen Y, Huang B, Lomvardas S, Weiner OD: Live-cell imaging reveals enhancer-dependent Sox2 transcription in the absence of enhancer proximity. eLife 2019, 8 The authors image the spatial organization and transcriptional output of Sox2 and its essential enhancer, finding no evidence of enhanced spatial proximity correlated with transcription.
- 92.•. Benabdallah NS, Williamson I, Illingworth RS, Kane L, Boyle S, Sengupta D, Grimes GR, Therizols P, Bickmore WA: Decreased enhancer-promoter proximity accompanying enhancer activation. Mol Cell 2019, 76:473–484.e7 The authors combine 3D-FISH and chromosome conformation capture to observe increased separation between the Shh gene and distant Shh brain enhancers upon activation, evidence which is incompatible with looping models of enhancer function.
- 93.•. Rodriguez J, Ren G, Day CR, Zhao K, Chow CC, Larson DR: Intrinsic dynamics of a human gene reveal the basis of expression heterogeneity. Cell 2019, 176:213–226.e18 This study uses live-cell RNA imaging coupled with Hi-C to dissect the endogenous regulation of the estrogen-responsive TFF1 gene. Despite high induction, TFF1 shows short active periods and variable long inactive periods, accounting for expression heterogeneity between cells.
- 94.Hamilton WB, Mosesson Y, Monteiro RS, Emdal KB, Knudsen TE, Francavilla C, Barkai N, Olsen JV, Brickman JM: Dynamic lineage priming is driven via direct enhancer regulation by ERK. Nature 2019, 575:355–360. [DOI] [PubMed] [Google Scholar]
- 95.•. Sato Y, Hilbert L, Oda H, Wan Y, Heddleston JM, Chew T-L, Zaburdaev V, Keller P, Lionnet T, Vastenhouw N et al. : Histone H3K27 acetylation precedes active transcription during zebrafish zygotic genome activation as revealed by live-cell analysis. Development 2019, 146 The authors apply a Fab-based live endogenous modification labeling technique to monitor the changes in histone modification levels during zygotic genome activation (ZGA) in living zebrafish embryos, and show developmental changes for H3 Lys27 acetylation (H3K27ac) before transcription activation.
- 96.Stasevich TJ, Hayashi-Takanaka Y, Sato Y, Maehara K, Ohkawa Y, Sakata-Sogawa K, Tokunaga M, Nagase T, Nozaki N, McNally JG et al. : Regulation of RNA Polymerase II activation by histone acetylation in single living cells. Nature 2014, 516:272–275. [DOI] [PubMed] [Google Scholar]
- 97.•. Forero LS, Raymond W, Handa T, Saxton M, Morisaki T: Live-cell imaging reveals the spatiotemporal organization of endogenous RNA Polymerase II phosphorylation at a single gene. bioRxiv 2020. 10.1101/2020.04.03.024414 The authors reveal the timing, kinetics, and spatial organization of Pol II phosphorylation along a single-copy HIV-1 reporter gene. Using specific antibodies to endogenous states of phosphorylated Pol II, they report heterogenous distribution of Pol II along the gene length and clusters of Pol II Ser5-P spatiotemporally separated from nascent RNA.
- 98.•. Mivelaz M, Cao A-M, Kubik S, Zencir S, Hovius R, Boichenko I, Stachowicz AM, Kurat CF, Shore D, Fierz B: Chromatin fiber invasion and nucleosome displacement by the Rap1 transcription factor. Mol Cell 2020, 77:488–500.e9 This study uses a reconstituted yeast mononucleosome to characterize pioneer factor Rap1 single-molecule binding at cognate sites. The authors show that nucleosome organization shortens the residence time for Rap1 binding to nucleosomal DNA, interferes with higher order nucleosome stacking, and collaborates with the RSC remodeler for nucleosome displacement.
- 99.Revyakin A, Zhang Z, Coleman RA, Li Y, Inouye C, Lucas JK, Park SR, Chu S, Tjian R: Transcription initiation by human RNA Polymerase II visualized at single-molecule resolution. Genes Dev 2012, 26:1691–1702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Bryan LC, Weilandt DR, Bachmann AL, Kilic S, Lechner CC, Odermatt PD, Fantner GE, Georgeon S, Hantschel O, Hatzimanikatis V et al. : Single-molecule kinetic analysis of HP1-chromatin binding reveals a dynamic network of histone modification and DNA interactions. Nucleic Acids Res 2017, 45:10504–10517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Nguyen VQ, Ranjan A, Liu S, Tang X, Ling YH, Wisniewski J, Mizuguchi G, Li Ky, Jou V, Zheng Q, Lavis LD, Lionnet T, Wu C: Spatio-temporal coordination or transcription preinitiation complex assembly in live cells. bioRxiv 2020. 10.1101/2020.12.30.424853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Popp AP, Hettich J, Gebhardt JCM: Transcription factor residence time dominates over concentration in transcription activation. bioRxiv 2020. 10.1101/2020.11.26.400069. [DOI] [Google Scholar]