Abstract
To assess the genetic diversity in Cryptosporidium parvum, we have sequenced the small subunit (SSU) rRNA gene of seven Cryptosporidium spp., various isolates of C. parvum from eight hosts, and a Cryptosporidium isolate from a desert monitor. Phylogenetic analysis of the SSU rRNA sequences confirmed the multispecies nature of the genus Cryptosporidium, with at least four distinct species (C. parvum, C. baileyi, C. muris, and C. serpentis). Other species previously defined by biologic characteristics, including C. wrairi, C. meleagridis, and C. felis, and the desert monitor isolate, clustered together or within C. parvum. Extensive genetic diversities were present among C. parvum isolates from humans, calves, pigs, dogs, mice, ferrets, marsupials, and a monkey. In general, specific genotypes were associated with specific host species. A PCR-restriction fragment length polymorphism technique previously developed by us could differentiate most Cryptosporidium spp. and C. parvum genotypes, but sequence analysis of the PCR product was needed to differentiate C. wrairi and C. meleagridis from some of the C. parvum genotypes. These results indicate a need for revision in the taxonomy and assessment of the zoonotic potential of some animal C. parvum isolates.
Cryptosporidiosis is a coccidian infection of humans, domestic animals, and other vertebrates. More than 20 Cryptosporidium species have been described in various animal hosts (30). The validity of most species, however, has not been established, because cross-transmission studies indicate that some isolates of Cryptosporidium are infective to several animal species (31). Only six to eight species (C. parvum, C. wrairi, C. felis, and C. muris in mammals, C. baileyi and C. meleagridis in birds, C. serpentis in reptiles, and C. nasorum in fish) are considered valid Cryptosporidium species by most researchers (15, 30). The validity of these six or eight species has been questioned recently by another group of researchers because a genetic analysis failed to support their classification as different species (41).
Because C. parvum is generally considered to be the parasite responsible for infection in most mammals, efforts have been made to examine the biologic and molecular diversity of this parasite. Although C. parvum isolates from humans, farm animals, companion animals, and rodents are morphologically and developmentally similar, differences in host specificity, prepatent and patent periods, and pathogenicity have been observed. For example, many isolates from humans are not infective for calves, mice, or guinea pigs. In contrast, bovine isolates are infective for humans, neonatal calves, and mice (32). Similarly, although isolates from wild, adult house mice can easily infect wild, uninfected adult mice (22), C. parvum bovine isolates are infective only for neonatal mice (35, 40). Differences in host specificity have been used previously as the basis for identifying C. wrairi and C. felis as unique species (11, 19). It remains to be determined how these biologic differences in other isolates relate to species differentiation.
Other than genetic differences between human and bovine isolates of C. parvum oocysts (5–7, 25, 26, 32, 38, 39), the inter- and intraspecies biological differences in Cryptosporidium have been infrequently substantiated by molecular studies. We recently sequenced the complete small subunit (SSU) rRNA genes of various Cryptosporidium isolates and used them in a phylogenetic analysis (46). Results of our study have shown that Cryptosporidium parasites are a multispecies complex containing at least four species, C. parvum, C. baileyi, C. muris, and C. serpentis. C. felis, C. nasorum and C. meleagridis were not studied. Differences were also observed between human and bovine isolates. Recently, based on sequences from the acetyl-coenzyme A synthetase gene, the internal transcribed spacer of rRNA, and a 298-bp region of the SSU rRNA genes, several new genotypes (pig, mouse, and koala) have been identified (27, 28).
In the present study, we have extended the phylogenetic analysis to include C. felis, C. meleagridis, and some other C. parvum (dog, pig, kangaroo, ferret, mouse, and monkey) isolates. We have included C. meleagridis in this study because a recent diagnostic report suggested that C. meleagridis may be closely related to C. parvum (9). The objectives of the present study were to test observations on the multispecies nature of Cryptosporidium parasites and to determine if C. parvum is much more diverse than previously believed. Findings from this study may contribute to a rationale for the revision of the taxonomy of the genus Cryptosporidium.
MATERIALS AND METHODS
Cryptosporidium isolates.
Isolates used in this study were from humans, cattle, dogs, cats, mice, pigs, turkeys, ferrets, a monkey, and a desert monitor. With the exception of C. meleagridis, which was originally isolated from a turkey and has been passaged in 1- to 2-week-old turkey pouts, all isolates were from naturally infected animals or humans and had no other identifiable parasites. Cryptosporidium species were determined by oocyst morphology, host origin, and traditional classification of Cryptosporidium parasites. Accordingly, the parasites with small-type oocysts from humans, calves, mice, ferrets, dogs, pigs, marsupials, and the monkey were all identified as C. parvum (15, 30, 33). Whenever possible, multiple isolates from the same host or closely related hosts were characterized to confirm the identity of parasites and the accuracy of data. To assess the relationship of these parasites to other Cryptosporidium parasites, sequences of C. wrairi, C. baileyi, C. muris, and C. serpentis previously obtained by us (46) were also used in the phylogenetic analysis. A complete list of isolates and sources is shown in Table 1.
TABLE 1.
Isolates of Cryptosporidium parasites used in this studya
| Isolate | Species | Host | Location |
|---|---|---|---|
| HFL2 | C. parvum | Human | Florida |
| HFL5 | C. parvum | Human | Florida |
| CRPM1 | C. parvum | Rhesus monkey | Georgia |
| BOH6 | C. parvum | Calf | Ohio |
| CPF1 | C. parvum | Ferret | Georgia |
| CPD1 | C. parvum | Dog | Ohio |
| CPM1 | C. parvum | Mouse | Maryland |
| P1 | C. parvum | Pig | Australia |
| K2 | C. parvum | Red Kangaroo (Macropus rufus) | Australia |
| GP1 | C. wrairi | Guinea pig | Michigan |
| CMEL1 | C. meleagridis | Turkey | North Carolina |
| C8 | C. felis | Cat | Australia |
| CSP01 | C. serpentis | Corn snake (Elaphe g. guttata) | Kansas |
| IDVS-811 | C. muris | Cow | Idaho |
| CBA01 | C. baileyi | Chicken | Alabama |
| CSP06 | Cryptosporidium sp. | Desert monitor (Varanus griseus) | Missouri |
Full sequences were also obtained from 2 additional C. parvum bovine isolates, 1 human isolate, 2 C. muris isolates, and 3 C. serpentis isolates. Partial sequences covering the most polymorphic regions (about 820 bp) were also obtained from additional 48 C. parvum human genotype isolates, 20 bovine genotype isolates, 4 murine isolates, 3 dog isolates, 3 ferret isolates, 2 pig isolates, 2 koala isolates with the kangaroo sequence, 1 snake isolate with the desert monitor sequence, 5 C. felis isolates, 2 C. meleagridis isolates, and 2 C. baileyi isolates.
Oocyst isolation and DNA extraction.
Oocysts were purified from fecal samples by a combination of discontinuous density sucrose gradient centrifugation and isopycnic Percoll centrifugation or cesium chloride gradient centrifugation (1, 2). After treatment in 5.25% sodium hypochlorite solution (100% commercial bleach) at 4°C for 10 min, oocysts were washed five times by suspending in 10 ml of sterile water, centrifuging at 1,500 × g for 10 min, decanting supernatant, and resuspending in sterile water. DNA was extracted from purified oocysts by a previously published technique (21).
PCR and DNA sequencing.
The entire SSU rRNA gene was amplified from samples by conventional polymerase chain reaction by using the forwarding primer 5′-AACCTGGTTGATCCTGCCAGTAGTC-3′ and reverse primer 5′-TGATCCTTCTGCAGGTTCACCTACG-3′. Each PCR consisted of 35 cycles of denaturation at 94°C for 45 s, annealing at 60°C for 45 s, and extension at 72°C for 60 s, with an initial denaturation at 94°C for 5 min and a final extension at 72°C for 10 min. After PCR amplification, the PCR fragment was sequenced by using an ABI377 autosequencer. Sequence accuracy was confirmed by two-directional sequencing, by the sequence of a second PCR product, and in most cases (see footnote for Table 1), by sequencing of the most polymorphic regions (about 820 bp) of multiple isolates.
Sequence analyses.
Multiple alignment of the DNA sequences was done with the Wisconsin package version 9.0 from Genetics Computer Group, Madison, Wisconsin, with manual adjustment. Two types of phylogenetic analysis were used on the aligned sequences to assess relationships among isolates, the distance-based neighbor-joining analysis and parsimony analysis. For the former, neighbor-joining trees (34) were constructed with the program TreeconW (42), based on the evolutionary distances between different isolates calculated by Kimura 2-parameter analysis. For the latter, a CONSENSE parsimony tree was made by using the phylogenetic analysis software PHYLIP version 3.5 (17). For neighbor-joining tree construction, an initial analysis used sequences from Eimeria tenella (GenBank accession no. AF026388) as an outgroup to assess the relationship among different Cryptosporidium spp. A subsequent analysis used C. muris and C. serpentis as the outgroup to assess the relatedness of isolates within the C. parvum group. Tree reliability was assessed by the bootstrap method (16) with 1,000 pseudoreplicates. We considered a value of 95% to be statistically significant (14); however, values above 50% are reported, since bootstrap may be a conservative estimate for the reliability of a clade (18). The multiple sequence alignment was deposited in GenBank and is retrievable by using the accession number for any sequence from this study.
PCR-RFLP analysis.
We previously developed a PCR-restriction fragment length polymorphism (RFLP) technique for species and genotype-specific diagnosis of Cryptosporidium parasites (46). Because only three C. parvum genotypes (bovine, human, and C. wrairi) were used in the initial technical development, we evaluated the performance of this technique in differentiating various genotypes of C. parvum. A PCR product of about 1,325 bp was amplified first by primary PCR with primers 5′-TTCTAGAGCTAATACATGCG-3′ and 5′-CCCTAATCCTTCGAAACAGGA-3′. The PCR contained 10 μl of Perkin-Elmer (Norwalk, Conn.) 10× PCR buffer, 6 mM MgCl2, 200 μM (each) deoxynucleoside triphosphate, 100 nM (each) primer, 2.5 U of Taq polymerase, and 0.25 to 1 μl of DNA template in a total 100-μl reaction mixture. A total of 35 cycles were carried out, each consisting of 94°C for 45 s, 55°C for 45 s, and 72°C for 1 min, with an initial hot start at 94°C for 3 min and a final extension at 72°C for 7 min. A secondary PCR product of 826 to 864 bp (depending on isolates) was then amplified from 2 μl of the primary PCR with primers 5′-GGAAGGGTTGTATTTATTAGATAAAG-3′ and 5′-AAGGAGTAAGGAACAACCTCCA-3′. The PCR and cycling conditions were identical to primary PCR, except that 3 mM MgCl2 was used in the PCR.
For restriction fragment analysis, 20 μl of the secondary PCR product was digested in a total of 50 μl of reaction mixture, consisting of 20 U of SspI (New England BioLabs, Beverly, Mass.) for species diagnosis or VspI (GIBCO BRL, Grand Island, N.Y.) for genotyping of C. parvum and 5 μl of respective restriction buffer at 37°C for 1 h, under conditions recommended by the supplier. The digested products were fractionated on 2.0% agarose gel and visualized by ethidium bromide staining.
Nucleotide sequence accession number.
The nucleotide sequences of the SSU rRNA gene of Cryptosporidium parasites have been deposited in the GenBank database under accession no. AF093489 to AF093499, AF112569 to AF112576, AF115377, and AF115378.
RESULTS
In our previous study, we evaluated the species structure of the genus Cryptosporidium by using SSU rRNA gene sequences from C. parvum (from cattle and humans), C. wrairi, C. baileyi, C. muris, and C. serpentis and showed that C. parvum, C. baileyi, C. muris, and C. serpentis differed from each other at distances comparable to or greater than those among different species of apicomplexans (46). In the present study, we obtained complete SSU rRNA gene sequences from two additional Cryptosporidium species (C. felis and C. meleagridis), C. parvum isolates from mice, pigs, dogs, ferrets, a monkey, and a kangaroo, and an isolate from a desert monitor with small-type oocysts (4 to 5 μ). These sequences were used in more extensive phylogenetic analyses to evaluate the validity of Cryptosporidium speciation, relationships among various species, and genetic diversity within C. parvum.
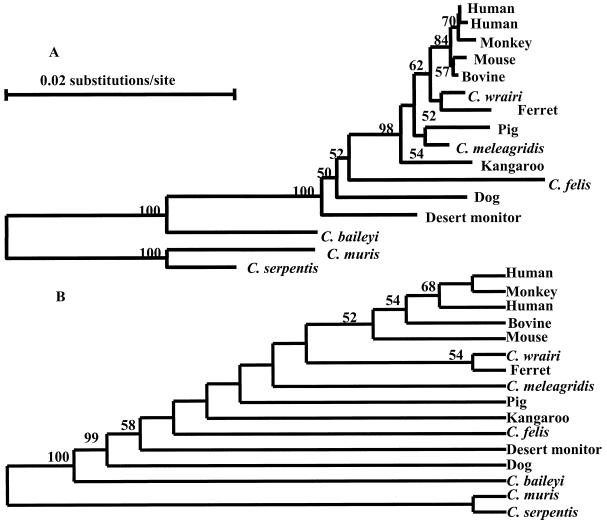
Both the neighbor-joining and the parsimony methods were used in the phylogenetic analyses of the SSU rRNA gene of the seven Cryptosporidium species. In an initial neighbor-joining analysis, E. tenella was used as an outgroup. This analysis confirmed the previous observation that Cryptosporidium species formed two groups, with full statistical reliability. One group contained C. muris and C. serpentis. The other group contained C. baileyi, C. felis, C. meleagridis, C. wrairi, all C. parvum isolates, and the isolate from a desert monitor (data not shown). A second analysis was made with C. muris and C. serpentis as an outgroup to assess the relationship among C. parvum, C. wrairi, C. meleagridis, and C. felis (Fig. 1A). In both phylogenetic analyses, C. baileyi diverged from C. parvum, C. meleagridis, and C. wrairi (100% bootstrap). C. wrairi and C. meleagridis, however, clustered within the different isolates of C. parvum. C. felis was separated from the majority of C. parvum isolates, as was true for the isolate from a desert monitor. Parsimony analysis revealed similar phylogenetic relationships among different Cryptosporidium isolates, with C. muris, C. serpentis, and C. baileyi well separated from the rest (Fig. 1B).
FIG. 1.
Phylogenetic relationship of Cryptosporidium parasites within the broad C. parvum group by neighbor-joining (A) and parsimony (B) analyses. Bootstrap values above 50% from 1,000 pseudoreplicates are also shown.
Extensive diversity was observed in the large C. parvum cluster (Fig. 1). Neighbor-joining analysis showed that the human and monkey isolates of C. parvum formed a monophylatic clade, which originated from the same sources with another monophylatic clade of bovine and murine isolates (Fig. 1A). The isolate from the ferret clustered together with C. wrairi, forming a sister group to the human and bovine C. parvum group. A kangaroo isolate was distant from the majority of members in the C. parvum group, as was the parasite from dogs. The pig isolate, on the other hand, grouped together with C. meleagridis. The Cryptosporidium isolate from a desert monitor also clustered together the C. parvum group. A similar observation regarding genetic diversity within the broad C. parvum group was made by parsimony analysis (Fig. 1B). The relationship among different isolates, however, was less well defined, and the bootstrap values were lower.
SSU rRNA sequences unique to particular genotypes were identified in the C. parvum group (C. parvum, C. wrairi, C. meleagridis, C. felis, and the desert monitor isolate). There were eight genotypes of C. parvum, which differed from each other primarily in four areas of the SSU rRNA gene (Table 2). The closely related Cryptosporidium species, C. wrairi, C. meleagridis, C. felis, and the isolate from a desert monitor, also had unique nucleotide sequences in these four areas. C. felis, the C. parvum dog genotype, and the desert monitor isolate also differed from C. parvum in other areas of the SSU rRNA gene (data not shown). The majority of the differences within the C. parvum group, however, were found in the first half of the SSU rRNA gene.
TABLE 2.
Differences among isolates in the C. parvum group (C. parvum, C. wrairi, C. felis, and C. meleagridis) in four areas of the SSU rRNA gene
| Source or genotype | Location of mutations in the SSU rRNA genea
|
|||
|---|---|---|---|---|
| 182–189 | 273–286 | 639–656 | 689–699 | |
| Bovine | AAACTCGA | ATTAA------A | AAATATTTTGATGAATATT-----TATATAAT | ACTA------------TATATTTTAGT |
| Human | AAACTCGA | AATTA------A | AAATATTTTGATGAATATT-----TATATAAT | ACTA---------TTTTTTTTTTTAGT |
| Monkey | AAACTCGA | AATTA------A | ATATATTTTGATGAATATT-----TATATAAT | ACTA-----------TTTTTTTTTAGT |
| Ferret | AGGCCTGA | ATAAA------T | AAATATTTTGATTAATATT-----TATATAAT | ACTA---------AATTTTTGTTTGGT |
| Guinea pigb | AGGCCCGA | ATAAA------T | TAATATTTTGA-AAATATT-----TATATAAT | ACTA-----------TATATTTTTAGT |
| Turkeyc | AAACCTGA | AATTT------A | TAATA-TTTGATTAATATT-----TATATAAT | ACTA------------AATTTATTAGT |
| Mouse | AAACTCGA | ATTAA------A | AAATATTTTAATTAATATT-----TATATAAT | ACTA--------TAATTATTTTTTAGT |
| Pig | AAACCTAA | ATTTTTA----A | TAATATTTT--T-AATATT-----TATATAAT | ACTA---------TAATTTTTATTAGT |
| Kangaroo | GA-CCTGA | ATAAATA----A | TTATACTTTTTAAGGTGTT-----TATATAAT | ACTA-----------TATTTTTTTAGT |
| Lizard | AGGCCTGA | AATTAT-----T | TAATATTACG----GTATT-----TATATAAT | ACT-----------TTATTTTTAGAGT |
| Dog | AAACCTGA | ATTTT------A | TAATATTT---AACATATT-----TATATAAT | ACTA---------------TTTATAGT |
| Catd | GA-CCCTA | AATAATTTATTT | TAATATTTTTTTTTTAAATATTAATATGTAAG | TTTAAGACTGAATTTTTAGTTTTGATA |
| C. baileyi | AGACCCGA | ATTT-------A | CAATACCACG----GTATT-----TATATAAC | ACT--------------TATTTAAAGT |
Nucleotide positions in the aligned sequences of all Cryptosporidium species. Actual positions in individual sequences vary because of the introduction of gaps (dashes) in the aligned sequences (1,757 bp).
C. wrairi.
C. meleagridis.
C. felis.
Based on the SSU rRNA gene sequences from C. parvum, C. baileyi, C. muris, and C. serpentis, we previously developed a nested PCR-RFLP technique for species and genotype differentiation (46). Species diagnosis was made by digesting the secondary PCR product (826 to 864 bp) with SspI, and differentiation of C. parvum genotypes by digestion with VspI. In the present study, we evaluated the ability of this technique to differentiate extended members of the C. parvum group. Digestion of the secondary PCR products from the C. parvum group parasites with SspI resulted in four predicted restriction patterns. The C. parvum human, monkey, bovine, mouse, ferret, and kangaroo isolates, C. wrairi, and C. meleagridis showed an identical restriction pattern, with three visible bands of 109 to 112, 254, and 441 to 461 bp in size (Table 3). The C. parvum dog isolate and the desert monitor isolate also had a three-band pattern, but with a smaller (417- to 418-bp) upper band. In contrast, the C. parvum pig isolate and C. felis each had only two visible bands (365 and 453 bp and 390 and 426 bp, respectively) that were different in size from the two-band patterns of C. baileyi (254 and 572 bp), C. muris (385 and 448 bp), and C. serpentis (370 and 414 bp). Electrophoresis of the digested secondary products largely confirmed the predicted restriction patterns, except for that of the C. parvum kangaroo isolate, which consistently showed partial digestion of the upper band (Fig. 2). It was possible to differentiate by SspI digestion the C. parvum bovine genotype A gene from the B gene, which yielded a larger lower band (119 versus 108 bp) than the A gene (Fig. 2).
TABLE 3.
RFLP (in base pairs) in the SSU rRNA gene of various Cryptosporidium spp. and genotypes
| Species | Source | PCR fragment no. | SspI digestiona | VspI digestiona |
|---|---|---|---|---|
| C. muris | Cattle, camel, hyrax | 833 | 385, 448 | 102, 731 |
| C. serpentis | Snake | 831 | 14, 33, 370, 414 | 102, 729 |
| C. baileyi | Chicken | 826 | 254, 572 | 102, 104, 620 |
| C. felis | Cat | 864 | 15, 33, 390, 426 | 102, 104, 182, 476 |
| C. meleagridis | Turkey | 833 | 11, 11, 108, 254, 449 | 102, 104, 171, 456 |
| C. wrairi | Guinea pig | 834 | 11, 11, 109, 254, 449 | 102, 104, 628 |
| Cryptosporidium sp. | Desert monitor | 834 | 19, 33, 109, 255, 418 | 102, 104, 628 |
| C. parvum | Human | 837 | 11, 12, 111, 254, 449 | 70, 102, 104, 561 |
| C. parvum | Monkey | 835 | 11, 109, 254, 461 | 70, 102, 104, 559 |
| C. parvum | Bovine, A gene | 834 | 11, 12, 108, 254, 449 | 102, 104, 628 |
| C. parvum | Bovine, B gene | 831 | 9, 119, 254, 449 | 102, 104, 625 |
| C. parvum | Mouse | 838 | 11, 12, 112, 254, 449 | 102, 104, 175, 457 |
| C. parvum | Dog | 829 | 20, 33, 105, 254, 417 | 94, 102, 633 |
| C. parvum | Ferret | 837 | 11, 12, 111, 254, 449 | 102, 104, 174, 457 |
| C. parvum | Pig | 838 | 9, 11, 365, 453 | 102, 104, 632 |
| C. parvum | Kangaroo, koala | 837 | 33, 109, 254, 441 | 102, 104, 631 |
Numbers in bold are the sizes of bands visible on the electrophoresis gel.
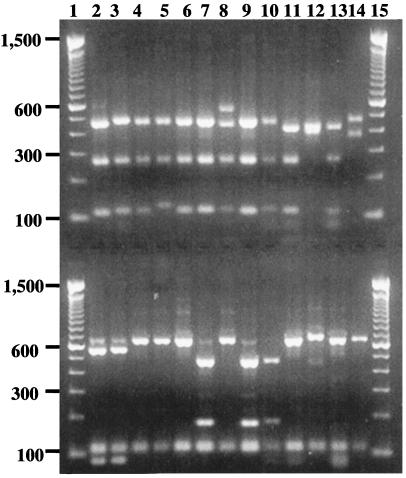
FIG. 2.
Genotyping of the C. parvum group parasites by a nested PCR-RFLP procedure based on the SSU rRNA gene sequences. Lanes 1 and 15, molecular size markers; lane 2, C. parvum human genotype; lane 3, C. parvum monkey genotype; lane 4, C. parvum bovine genotype, A gene; lane 5, C. parvum bovine genotype, B gene; lane 6, C. wrairi; lane 7, C. parvum ferret genotype; lane 8, C. parvum kangaroo genotype; lane 9, C. meleagridis; lane 10, C. parvum mouse genotype; lane 11, Cryptosporidium sp. from a desert monitor; lane 12, C. felis; lane 13, C. parvum dog genotype; lane 14, C. parvum pig genotype. The upper lanes show SspI digestion products, and the lower lanes show VspI digestion products. Only partial digestion could be obtained with the kangaroo isolate by SspI and the C. felis isolate by VspI.
Digestion of the secondary PCR products from the C. parvum group parasites with VspI yielded three additional patterns as follows: (i) C. parvum human and monkey isolates; (ii) C. parvum bovine, dog, pig, and kangaroo isolates, C. wrairi, C. felis, and the desert monitor isolate; and (iii) C. parvum mouse and ferret isolates and C. meleagridis. Restriction digestion with SspI and VspI could differentiate the C. parvum human and monkey genotypes from all other genotypes, but DNA sequencing was needed to differentiate the C. parvum bovine genotype from the kangaroo genotype and C. wrairi (Table 2). Electrophoresis of digested products confirmed the predicted restriction patterns, with the exception of C. felis isolates, which consistently showed partial digestion by VspI (Fig. 2).
DISCUSSION
Results of this study confirm the heterogeneous nature of Cryptosporidium parasites. Based on SSU rRNA sequences, the genus Cryptosporidium contains at least four species: C. muris, C. serpentis, C. baileyi, and C. parvum. Several Cryptosporidium species considered to be valid by biologic characteristics, including C. meleagridis, C. felis, and C. wrairi, cluster together or within different C. parvum isolates. Their separate species status may need to be reexamined. Although C. felis is within the broad C. parvum clade in phylogenetic analyses, it is genetically different from the majority of C. parvum genotypes to such an extent that it may indeed represent a valid species. The Cryptosporidium parasites in dogs and pigs are traditionally classified as C. parvum (15, 30, 33). They are, however, genetically distant from the majority of C. parvum isolates in this study, and they may be cryptic species, especially if C. wrairi and C. meleagridis retain species status. Despite strong bootstrap support for some of the groupings, the relationships within the groups containing C. parvum and closely related isolates may be better resolved by the use of other genes such as the rRNA internal transcribed spacers. Biologic studies in addition to other genetic characterizations of various isolates are apparently needed before the taxonomic status of members in the broad C. parvum group can be clarified.
The isolate from a desert monitor is also more related to C. parvum than to any other species. Previously, C. parvum-like parasites have been seen in reptiles, but these parasites were found to be genetically identical to the C. parvum murine genotype, presumably as a result of ingesting a C. parvum-infected prey (29). The desert monitor had been in captivity for at least 6 years (acquired as an adult in 1992), and feeder mice that were used as the major diet were found to be infected (2 of 10) with the murine genotype of C. parvum. In addition, the desert monitor was shedding a large number of oocysts. Because the oocysts from the desert monitor were genetically different from the C. parvum murine genotype normally seen in mice, it is unlikely that the oocysts were from ingested prey. Recently, a new Cryptosporidium species, C. saurophilum, has been described from lizards, Schneider’s skink (Eumeces schneideri), and desert monitors. The new species differs from C. serpentis by having smaller oocysts, developing in the intestine, and an inability to infect snakes (23). It is unclear whether the oocysts from the desert monitor in the present study also belong to the same new Cryptosporidium species. Genetic characterization of C. saurophilum is in progress by the original researchers and will help address this issue.
Results of this study confirm the heterogeneity of C. parvum. In addition to the previously described human, bovine, pig, mouse, and kangaroo genotypes, three additional genotypes (dog, ferret, and monkey) have been characterized. To date, with the exception of the bovine genotypes (27), each of these genotypes occurs only in their respective hosts, suggesting that host specificity may exist among these genotypes. Limited cross-transmission studies with the human and murine genotypes confirm the existence of host specificity (22, 32). Differences in host specificity were used as the basis for the separation of C. wrairi and C. felis from C. parvum. If the same standard is used in species designation, many of the other isolates from the larger C. parvum group may be considered distinct species. Extensive biologic characterization is needed to address this issue.
The significance of this genetic diversity in the C. parvum group is not clear. Companion animals and rodents have been frequently suggested as a source of infection for humans and farm animals (4, 8, 10, 20, 22, 24, 43, 44). It has been recently suggested that all Cryptosporidium isolates, including those from lower vertebrates, should be considered as hazardous to humans (41). In view of the genetic heterogeneity and associated host specificity, this point of view needs to be reassessed, especially the infectivity of these parasites to immunocompetent humans. Thus far, only the human and bovine genotypes of C. parvum have been found in humans (3, 5–7, 28, 32, 36, 37, 39, 45, 47). A C. baileyi-like parasite was reported in a patient with AIDS (13), but the identity of this parasite has been subsequently questioned by the original investigators (12). Even though studies conducted to date have only identified the human and bovine genotypes of C. parvum in humans, further studies with larger sample sizes are needed to test if nonparvum Cryptosporidium spp. or other genotypes of C. parvum are infectious in humans, especially immunocompromised individuals. The use of PCR and sequencing tools as shown in this study would make these studies possible.
ACKNOWLEDGMENTS
This work was supported in part by an interagency agreement (no. DW75937730-01-0) between the Centers for Disease Control and Prevention (CDC) and U.S. Environmental Protection Agency and by funding from CDC’s Opportunistic Infectious Diseases program.
We thank Bruce Anderson at the University of Idaho, Richard Montali at the National Zoological Park (Washington, D.C.), and Mark Kombert and Randall Junge at the St. Louis Zoo (St. Louis, Mo.) for providing specimens.
REFERENCES
- 1.Arrowood M J, Donaldson K. Improved purification methods for calf-derived Cryptosporidium parvum oocysts using discontinuous sucrose and cesium chloride gradients. J Eukaryot Microbiol. 1996;43:89S. doi: 10.1111/j.1550-7408.1996.tb05015.x. [DOI] [PubMed] [Google Scholar]
- 2.Arrowood M J, Sterling C R. Isolation of Cryptosporidium oocysts and sporozoites using discontinuous sucrose and isopycnic Percoll gradients. J Parasitol. 1987;73:314–319. [PubMed] [Google Scholar]
- 3.Awad-El-Kariem F M, Robinson H A, Petry F, McDonald V, Evans D, Casemore D. Differentiation between human and animal isolates of Cryptosporidium parvum using molecular and biological markers. Parasitol Res. 1998;84:297–301. doi: 10.1007/s004360050399. [DOI] [PubMed] [Google Scholar]
- 4.Bajer A, Bednarska M, Sinski E. Wildlife rodents from different habitats as a reservoir for Cryptosporidium parvum. Acta Parasitol. 1997;42:192–194. [Google Scholar]
- 5.Bonnin A, Fourmaux M N, Dubremetz J F, Nelson R G, Gobet P, Harly G, Buisson M, Puygauthier-Toubas D, Gabriel-Pospisil G, Naciri M, Camerlynck P. Genotyping human and bovine isolates of Cryptosporidium parvum by polymerase chain reaction-restriction fragment length polymorphism analysis of a repetitive DNA sequence. FEMS Microbiol Lett. 1996;137:207–211. doi: 10.1111/j.1574-6968.1996.tb08107.x. [DOI] [PubMed] [Google Scholar]
- 6.Carraway M, Tzipori S, Widmer G. Identification of genetic heterogeneity in the Cryptosporidium parvum ribosomal repeat. Appl Environ Microbiol. 1996;62:712–716. doi: 10.1128/aem.62.2.712-716.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Carraway M, Tzipori S, Widmer G. A new restriction fragment length polymorphism from Cryptosporidium parvum identifies genetically heterogeneous parasite populations and genotypic changes following transmission from bovine to human hosts. Infect Immun. 1997;65:3958–3960. doi: 10.1128/iai.65.9.3958-3960.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Chalmers R M, Sturdee A P, Bull S A, Miller A, Wright S E. The prevalence of Cryptosporidium parvum and C. muris in Mus domesticus, Apodemus sylvaticus and Clethrionomys glareolus in an agricultural system. Parasitol Res. 1997;83:478–482. doi: 10.1007/s004360050283. [DOI] [PubMed] [Google Scholar]
- 9.Champliaud D, Gobet P, Naciri M, Vagner O, Lopez J, Buisson J C, Varga I, Harly G, Mancassola R, Bonnin A. Failure to differentiate Cryptosporidium parvum from C. meleagridis based on PCR amplification of eight DNA sequences. Appl Environ Microbiol. 1998;64:1454–1458. doi: 10.1128/aem.64.4.1454-1458.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Chen Y G, Yao F B, Li H S, Shi W S, Dai M X, Lu M. Cryptosporidium infection and diarrhea in rural and urban areas of Jiangsu, People’s Republic of China. J Clin Microbiol. 1992;30:492–494. doi: 10.1128/jcm.30.2.492-494.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Chrisp C E, Suckow M A, Fayer R, Arrowood M J, Healey M C, Sterling C R. Comparison of the host ranges and antigenicity of Cryptosporidium parvum and Cryptosporidium wrairi from guinea pigs. J Protozool. 1992;39:406–409. doi: 10.1111/j.1550-7408.1992.tb01471.x. [DOI] [PubMed] [Google Scholar]
- 12.Ditrich O, Kopacek P, Kucerova Z. Antigenic characterization of human isolates of cryptosporidia. Folia Parasitol. 1993;40:301–305. [PubMed] [Google Scholar]
- 13.Ditrich O, Palkovic L, Sterba J, Prokopic J, Loudova J, Giboda M. The first finding of Cryptosporidium baileyi in man. Parasitol Res. 1991;77:44–47. doi: 10.1007/BF00934383. [DOI] [PubMed] [Google Scholar]
- 14.Efron B, Halloran E, Holmes S. Bootstrap confidence levels for phylogenetic trees. Proc Natl Acad Sci USA. 1996;93:13429–13434. doi: 10.1073/pnas.93.23.13429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Fayer R, Speer C, Dubey J. The general biology of Cryptosporidium. In: Fayer R, editor. Cryptosporidium and cryptosporidiosis. Boca Raton, Fla: CRC Press; 1997. pp. 1–41. [Google Scholar]
- 16.Felsenstein J. Confidence limits on phylogenies: an approach using bootstrap. Evolution. 1985;39:783–791. doi: 10.1111/j.1558-5646.1985.tb00420.x. [DOI] [PubMed] [Google Scholar]
- 17.Felsenstein J. PHYLIP: phylogeny inference package (version 3.2) Cladistics. 1989;5:164–166. [Google Scholar]
- 18.Hillis D M, Bull J J. An empirical test of bootstrapping as a method for assessing confidence in phylogenetic analysis. Syst Biol. 1993;8:189–191. [Google Scholar]
- 19.Iseki M. Cryptosporidium felis sp. n. (Protozoa: Eimeriorina) from the domestic cat. Jpn J Parasitol. 1979;28:285–307. [Google Scholar]
- 20.Iseki M, Maekawa T, Moriya K, Uni S, Takada S. Infectivity of Cryptosporidium muris (strain RN 66) in various laboratory animals. Parasitol Res. 1989;75:218–222. doi: 10.1007/BF00931279. [DOI] [PubMed] [Google Scholar]
- 21.Kim K, Gooze L, Petersen C, Gut J, Nelson R G. Isolation, sequence and molecular karyotype analysis of the actin gene of Cryptosporidium parvum. Mol Biochem Parasitol. 1992;50:105–113. doi: 10.1016/0166-6851(92)90248-i. [DOI] [PubMed] [Google Scholar]
- 22.Klesius P H, Haynes T B, Malo L K. Infectivity of Cryptosporidium sp. isolated from wild mice for calves and mice. J Am Vet Med Assoc. 1986;189:192–193. [PubMed] [Google Scholar]
- 23.Koudela B, Modry D. New species of Cryptosporidium (Apicomplexa, Cryptosporidiidae) from lizards. Folia Parasitol. 1998;45:93–100. [Google Scholar]
- 24.Laakkonen J, Soveri T, Henttonen H. Prevalence of Cryptosporidium sp. in peak density Microtus agrestis, Microtus oeconomus and Clethrionomys glareolus populations. J Wildl Dis. 1994;30:110–111. doi: 10.7589/0090-3558-30.1.110. [DOI] [PubMed] [Google Scholar]
- 25.Morgan U M, Constantine C C, Forbes D A, Thompson R C. Differentiation between human and animal isolates of Cryptosporidium parvum using rDNA sequencing and direct PCR analysis. J Parasitol. 1997;83:825–830. [PubMed] [Google Scholar]
- 26.Morgan U M, Constantine C C, O’Donoghue P, Meloni B P, O’Brien P A, Thompson R C A. Molecular characterization of Cryptosporidium isolates from humans and other animals using random amplified polymorphic DNA analysis. Am J Trop Med Hyg. 1995;52:559–564. doi: 10.4269/ajtmh.1995.52.559. [DOI] [PubMed] [Google Scholar]
- 27.Morgan, U. M., P. Deplazes, D. A. Forbes, F. Spano, H. Hertzberg, K. D. Sargent, A. Elliot, and R. C. A. Thompson. Sequence and PCR-RFLP analysis of the internal transcribed spacers of the rDNA repeat unit in isolates of Cryptosporidium from different hosts. Parasitology, in press. [DOI] [PubMed]
- 28.Morgan U M, Sargent K D, Deplazes P, Forbes D A, Spano F, Hertzberg H, Elliot A, Thompson R C A. Molecular characterization of Cryptosporidium from various hosts. Parasitology. 1998;117:31–37. doi: 10.1017/s0031182098002765. [DOI] [PubMed] [Google Scholar]
- 29.Morgan, U. M., L. Xiao, R. Fayer, T. K. Graczyk, A. A. Lal, P. Deplazes, and R. C. A. Thompson. Phylogenetic analysis of 18S rDNA sequence data and RAPD analysis of Cryptosporidium isolates from captive reptiles. J. Parasitol. in press. [PubMed]
- 30.O’Donoghue P J. Cryptosporidium and cryptosporidiosis in man and animals. Int J Parasitol. 1995;25:139–195. doi: 10.1016/0020-7519(94)e0059-v. [DOI] [PubMed] [Google Scholar]
- 31.O’Donoghue P J, Tham V L, de Saram W G, Paull K L, McDermott S. Cryptosporidium infections in birds and mammals and attempted cross-transmission studies. Vet Parasitol. 1987;26:1–11. doi: 10.1016/0304-4017(87)90071-9. [DOI] [PubMed] [Google Scholar]
- 32.Peng M M, Xiao L, Freeman A R, Arrowood M J, Escalante A A, Weltman A C, Ong C S, MacKenzie W R, Lal A A, Beard C B. Genetic polymorphism among Cryptosporidium parvum isolates: evidence of two distinct human transmission cycles. Emerg Infect Dis. 1997;3:567–573. doi: 10.3201/eid0304.970423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Riggs M W. Cryptosporidiosis in cats, dogs, ferrets, raccoons, opossums, rabbits, and non-human primates. In: Dubey J P, Speer C A, Fayer R, editors. Cryptosporidiosis of man and animals. Boston, Mass: CRC Press; 1990. pp. 113–123. [Google Scholar]
- 34.Saitou N, Nei M. The neighbor-joining method: a new method for reconstructing phylogenetic trees. Mol Biol Evol. 1987;4:406–425. doi: 10.1093/oxfordjournals.molbev.a040454. [DOI] [PubMed] [Google Scholar]
- 35.Sherwood D, Angus K W, Snodgrass D R, Tzipori S. Experimental cryptosporidiosis in laboratory mice. Infect Immun. 1982;38:471–475. doi: 10.1128/iai.38.2.471-475.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Spano F, Putignani L, Crisanti A, Sallicandro P, Morgan U M, Leblancq S M, Tchack L, Tzipori S, Widmer G. Multilocus genotypic analysis of Cryptosporidium parvum isolates from different hosts and geographical origins. J Clin Microbiol. 1998;36:3255–3259. doi: 10.1128/jcm.36.11.3255-3259.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Spano F, Putignani L, Guida S, Crisanti A. Cryptosporidium parvum—PCR-RFLP analysis of the TRAP-C1 (thrombospondin-related adhesive protein of Cryptosporidium-1) gene discriminates between two alleles differentially associated with parasite isolates of animal and human origin. Exp Parasitol. 1998;90:195–198. doi: 10.1006/expr.1998.4324. [DOI] [PubMed] [Google Scholar]
- 38.Spano F, Putignani L, McLauchlin J, Casemore D P, Crisanti A. PCR-RFLP analysis of the Cryptosporidium oocyst wall protein (COWP) gene discriminates between C. wrairi and C. parvum, and between C. parvum isolates of human and animal origin. FEMS Microbiol Lett. 1997;150:209–217. doi: 10.1016/s0378-1097(97)00115-8. [DOI] [PubMed] [Google Scholar]
- 39.Sulaiman I M, Xiao L, Yang C, Escalante L, Moore A, Beard C B, Arrowood M J, Lal A A. Differentiating human from animal isolates of Cryptosporidium parvum. Emerg Infect Dis. 1998;4:681–685. doi: 10.3201/eid0404.980424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Tarazona R, Blewett D A, Carmona M D. Cryptosporidium parvum infection in experimentally infected mice: infection dynamics and effect of immunosuppression. Folia Parasitol. 1998;45:101–107. [PubMed] [Google Scholar]
- 41.Tzipori S, Griffiths J K. Natural history and biology of Cryptosporidium parvum. Adv Parasitol. 1998;40:5–36. doi: 10.1016/s0065-308x(08)60116-5. [DOI] [PubMed] [Google Scholar]
- 42.Van de Peer Y, De Wachter R. TREECON for Windows: a software package for the construction and drawing of evolutionary trees for the Microsoft Windows environment. Comput Appl Biosci. 1994;10:569–570. doi: 10.1093/bioinformatics/10.5.569. [DOI] [PubMed] [Google Scholar]
- 43.Webster J P. Wild brown rats (Rattus norvegicus) as a zoonotic risk on farms in England and Wales. Communicable Disease Report CDR Rev. 1996;6:R46–R49. [PubMed] [Google Scholar]
- 44.Webster J P, Macdonald D W. Cryptosporidiosis reservoir in wild brown rats (Rattus norvegicus) in the U.K. Epidemiol Infect. 1995;115:207–209. doi: 10.1017/s0950268800058271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Widmer G, Tzipori S, Fichtenbaum C J, Griffiths J K. Genotypic and phenotypic characterization of Cryptosporidium parvum isolates from people with AIDS. J Infect Dis. 1998;178:834–840. doi: 10.1086/515373. [DOI] [PubMed] [Google Scholar]
- 46.Xiao L, Escalante L, Yang C, Sulaiman I, Escalante A A, Montali R J, Fayer R, Lal A A. Phylogenetic analysis of Cryptosporidium parasites based on the small subunit ribosomal RNA gene locus. Appl Environ Microbiol. 1999;65:1578–1583. doi: 10.1128/aem.65.4.1578-1583.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Xiao L, Sulaiman I, Fayer R, Lal A A. Species and strain-specific typing of Cryptosporidium parasites in clinical and environmental samples. Mem Inst Oswaldo Cruz. 1998;93:687–691. doi: 10.1590/s0074-02761998000500022. [DOI] [PubMed] [Google Scholar]