Abstract
Purpose
To evaluate the potential causal associations between type 2 diabetes and fasting glucose and HbA1c levels and the risk of primary open-angle glaucoma (POAG) in European and East Asian populations.
Methods
We selected genetic variants (P < 5 × 10−8) for type 2 diabetes (898,130 Europeans; 433,540 East Asians), fasting glucose, and HbA1c (196,991 Europeans; 36,584 East Asians) from three meta-analyses of genome-wide association studies (GWAS). The GWAS for POAG provided summary statistics (192,702 Europeans; 46,523 East Asians). Mendelian randomization (MR) analysis was accomplished using the inverse variance–weighted method, weighted-median method, MR Egger method, and MR-Pleiotropy RESidual Sum and Outlier test.
Results
Genetically predicted type 2 diabetes was potentially positively associated with POAG in the European ancestry (body mass index [BMI]–unadjusted: odds ratio [OR] = 1.07, 95% confidence interval [CI], 1.01–1.14, P = 0.028; BMI-adjusted: OR = 1.07, 95% CI, 1.01–1.15, P = 0.035), but not in the East Asian ancestry (BMI-unadjusted: OR = 1.01, 95% CI, 0.95–1.06, P = 0.866; BMI-adjusted: OR = 1.00, 95% CI, 0.94–1.05, P = 0.882). There was no evidence to support a causal association of fasting glucose (European: OR = 1.19, P = 0.157; East Asian: OR = 0.94, P = 0.715) and HbA1c (European: OR = 1.27, P = 0.178; East Asian: OR = 0.85, P = 0.508) levels with POAG.
Conclusions
The causal effect of type 2 diabetes on the risk of POAG is different in European and East Asian populations. The point estimates of fasting glucose and Hb1Ac with POAG are large but not statistically significant, which prompts the question of statistical power.
Keywords: Mendelian randomization, single-nucleotide polymorphism, causal association, type 2 diabetes, primary open-angle glaucoma
Primary open-angle glaucoma (POAG) is a progressive optic neuropathy, characterized by an acquired loss of retinal ganglion cells and atrophy of the optic nerve, which is a leading cause of irreversible blindness in the world.1 A systematic review and meta-analysis suggested that the pooled global prevalence of POAG was 3.05% (95% confidence interval [CI], 1.69–5.27), and it estimated that 79.8 million people among the age group 40 to 80 years worldwide would experience POAG by 2040.2 As a progressive disease that results in progressive vision loss, it has a substantial negative impact on the quality of life and imposes huge socioeconomic effects and healthcare costs.3,4
Although previous studies have shown that many modifiable risk factors are associated with an increased risk of POAG,5–7 the potential causes and pathogenesis of this disease remain unclear. Nevertheless, defects in the retinal nerve fiber layer are frequently detected in subjects with diabetes,8 and individuals with high intraocular pressure (IOP) are more likely to have higher fasting blood glucose levels than healthy controls.9,10 This suggests that these metabolic dysregulations might be intrinsic to the pathogenesis of POAG.
Both the presence of type 2 diabetes and elevated fasting glucose and hemoglobin A1c (HbA1c) levels are associated with POAG in observational studies.11–13 However, whether these associations are causal remains unclear. Mendelian randomization (MR) is a valid inference method that uses genetic variants as instrumental variables (IVs) to determine whether an association between a risk factor and an outcome is consistent with a causal effect.14 It presents a valuable tool, especially when it is not feasible to conduct randomized controlled trials, for examining causality.15 Random distribution of genetic variants in natural populations avoids biased associations provided by traditional observational studies because of confounding or reverse causality. It is possible to perform an analysis of IVs to obtain an estimate of the magnitude of the causal effect of the exposure of interest on the outcome under investigation.16 Using multiple newly reported independent single-nucleotide polymorphisms (SNPs) of type 2 diabetes, fasting glucose, and HbA1c in recent genome-wide association studies (GWAS) as IVs,17–19 we can explore whether type 2 diabetes and fasting glucose and HbA1c levels casually affect the risk of POAG. To date, there have been no MR analyses addressing these questions.
Therefore, in this study, an MR approach was used to determine the causal effect of genetically predicted type 2 diabetes and fasting glucose and HbA1c levels on the risk of POAG in European and East Asian populations.
Methods
The data used for analysis were obtained from published studies. The patients were informed about the sample collection and had signed informed consent forms. The collection of data adhered to the Declaration of Helsinki and ethical approval had been obtained in all original studies.
Data Sources
Two meta-analyses of GWAS of European and East Asian ancestry provided the summary statistics for type 2 diabetes.17,18 Two studies performed meta-analyses with and without adjustment for body mass index (BMI). On the one hand, the meta-analysis involving European ancestry comprised 74,124 cases and 824,006 controls and consisted of studies from 32 GWAS, including the UK Biobank, Framingham Heart Study, and deCODE Genetics. On the other hand, the meta-analysis involving East Asian ancestry comprised 433,540 participants from 23 GWAS, including BioBank Japan, China Health and Nutrition Survey, and Korean Biobank Array. Physician diagnosis, self-report, medication usage, and electronic medical records were important methods for identifying patients with type 2 diabetes. The specific diagnostic criteria included the American Diabetes Association diagnostic criteria, International Classification of Diseases-9 (ICD-9), and World Health Organization guidelines. In general, patients with type 2 diabetes were identified by fasting glucose ≥7.0 mmol/L or 2-hr glucose ≥11.1 mmol/L in the oral glucose tolerance test or HbA1c ≥6.5%.
For fasting glucose and HbA1c, SNPs were obtained from a trans-ancestral GWAS meta-analysis.19 Approximately 196,991 European and 36,584 East Asian individuals without type 1 or type 2 diabetes were included in this GWAS. The duration of fasting was defined as ≥6 hours or ≥8 hours or overnight. The units of analysis for fasting glucose and HbA1c were millimoles per liter and percent, respectively. In the studies included in the meta-analysis, the mean values for fasting glucose and HbA1c ranged from 3.95 to 6.30 mmol/L and from 4.09% to 5.81%, respectively. Fasting glucose levels were measured by fasting serum, plasma, and whole blood. In contrast, non-fasting whole blood, plasma, and red blood cells were added to measure HbA1c.
Summary statistics for POAG were obtained from a recent meta-analysis of the GWAS.20 We extracted two ancestral groups from Europe (15,229 cases; 177,473 controls) and East Asia (6935 cases; 39,588 controls). We excluded data from the UK Biobank to ensure the independence of the sample in terms of European ancestry. However, there was a 7.7% sample overlap, which could not be excluded because of access restrictions, in the East Asian ancestry from the BioBank Japan and Singapore Chinese Eye Study. The identification of participants with POAG was based on the ICD-9 and ICD-10 diagnosis in most studies. POAG is a chronic, progressive optic neuropathy in which there is a characteristic acquired atrophy of the optic nerve and loss of retinal ganglion cells and their axons.21 Such atrophy and loss develop in the presence of open anterior chamber angles, characteristic visual field abnormalities, and raised IOP, with no other underlying disease.22 Participants underwent slit-lamp microscopy, fundoscopy, cup-disc ratio measurement, intraocular pressure measurement, and gonioscopy for assessment of the iridocorneal angle. The diagnostic criteria for POAG included visual field loss, open anterior chamber angle, IOP ≥22 mm Hg, cup-disc ratio ≥0.6 or 0.7, and inter-eye asymmetry >0.2. Those with secondary, angle-closure, or congenital glaucoma were not included.
Detailed information about the GWAS of type 2 diabetes, fasting glucose, HbA1c, and POAG is shown in Supplementary Tables S1 to S3. In each study, association testing was performed in a regression framework with adjustment for study-specific covariates, such as age, sex, and region.
Selection of Instrumental Variables
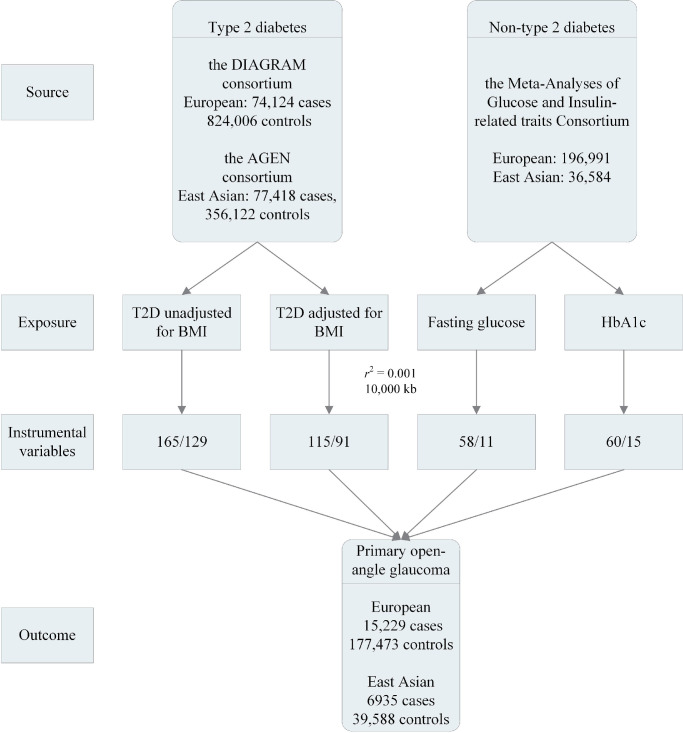
Figure 1 shows a flowchart of the selection of IVs. We applied stringent clumping criteria to select independent SNPs (LD cutoff r2 < 0.001, 10,000 kb) from sentinel SNPs (P < 5 × 10−8). Effect estimates of the associations between SNPs and type 2 diabetes were categorized as unadjusted for BMI and adjusted for BMI in European and East Asian ancestry. In total, 165 and 129 independent SNPs for type 2 diabetes unadjusted for BMI were selected as IVs for European and East Asian ancestry, respectively (Supplementary Tables S4 and S5). In contrast, the numbers of independent SNPs for type 2 diabetes adjusted for BMI were 115 and 91 in European and East Asian ancestry, respectively. Nevertheless, rs62469016 was not available in the summary statistics for POAG in East Asian ancestry; thus, rs10952994 (r2 = 0.99) was included as a proxy SNP.
Figure 1.
The flowchart of the selection of instrumental variables. T2D, type 2 diabetes.
In individuals without diabetes, independent SNPs for fasting glucose and HbA1c were selected as IVs (European: fasting glucose 58, HbA1c 60; East Asian: fasting glucose 11, HbA1c 15) (Supplementary Tables S6 and S7). The rs57884925 in fasting glucose was replaced by rs4237150 (r2 = 0.91) because it was not found in the summary statistics for POAG in East Asian ancestry. The rs28671200 (rs4453594, r2 = 0.99) and rs7190771 (rs17640009, r2 = 1) were replaced by proxy SNPs in the association between HbA1c and POAG in European ancestry.
Statistical Analyses
The inverse-variance-weighted (IVW) method was used as the main analysis to evaluate the causal associations between type 2 diabetes, fasting glucose, and HbA1c and the risk of POAG. The IVW estimate of the causal effect combines the ratio estimates of each variant in a meta-analysis model, and the standard error of the ratio estimates is approximated using the delta method.23 The results of the IVW method are robust if all the IVs are valid, or if any horizontal pleiotropy is balanced.24 The Cochran Q statistic and I-squared were used to assess the heterogeneity of the IVW estimations (I2). To evaluate the robustness of our findings, we performed the weighted-median approach, MR-Egger method, and MR-Pleiotropy RESidual Sum and Outlier (PRESSO) test for sensitivity analyses.
If valid IVs account for at least 50% of the weight variance, the weighted-median method provides a consistent estimate.25 The MR-Egger regression method was used to identify potential directional pleiotropy.26 If the intercept from the MR-Egger analysis is not equal to zero (P for intercept < 0.05), there is potential directional pleiotropy. Conversely, an intercept equal to zero means that the IVW method can provide a consistent estimate of the causal effect. The MR-PRESSO test can identify outliers that are potentially horizontally pleiotropic and give the estimate of causal association after excluding outliers.27 Causal estimates were converted to odds ratio (OR), representing the average change in POAG per 2.72-fold increase in the prevalence of type 2 diabetes and per 1 SD increase in fasting glucose and HbA1c.28 In this MR study, the statistical power to identify OR of 1.10 per corresponding change in exposure was computed using an online tool with a type 1 error rate of 0.05 (https://shiny.cnsgenomics.com/mRnd/).29 P < 0.0125 (0.05/4, accounting for multiple tests for four exposures and one outcome) was considered statistically significant. P < 0.05 but above the Bonferroni-corrected significance threshold suggested a potential association.
All analyses were conducted in R version 4.0.1. MR analyses were performed using “MendelianRandomization,” “TwoSampleMR,” and “MRPRESSO” R packages.
Results
The Causal Associations Between Type 2 Diabetes and POAG
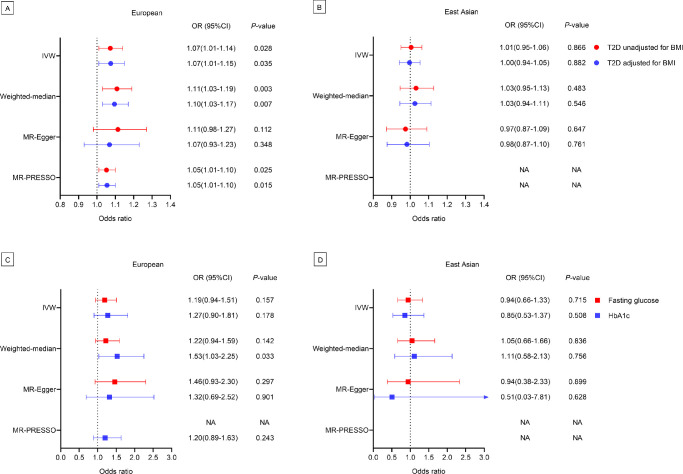
Genetically predicted type 2 diabetes was potentially positively associated with POAG using the IVW method for European ancestry (BMI-unadjusted: OR = 1.07, 95% CI, 1.01–1.14, P = 0.028; BMI-adjusted: OR = 1.07, 95% CI, 1.01–1.15, P = 0.035) (Fig. 2A). The weighted-median method provided statistically significant estimates (BMI–unadjusted: OR = 1.11, 95% CI, 1.03–1.19, P = 0.003; BMI–adjusted: OR = 1.10, 95% CI, 1.03–1.17, P = 0.007). After excluding three outlier SNPs detected by the MR–PRESSO test, the estimates of the causal effect of type 2 diabetes on the risk of POAG were potentially significant (BMI–unadjusted: OR = 1.05, 95% CI, 1.01–1.10, P = 0.025; BMI–adjusted: OR = 1.05, 95% CI, 1.01–1.10, P = 0.015).
Figure 2.
The forest plot showing the causal association between type 2 diabetes and POAG in European (A) and East Asian ancestry (B); the causal association of fasting glucose and HbA1c with POAG in European (C) and East Asian ancestry (D). T2D, type 2 diabetes. NA, not available.
There was no evidence to support a causal association between genetically predicted type 2 diabetes and POAG according to the IVW method for East Asian ancestry (BMI-unadjusted: OR = 1.01, 95% CI, 0.95–1.06, P = 0.866; BMI–adjusted: OR = 1.00, 95% CI, 0.94–1.05, P = 0.882) (Fig. 2B). Similar results were obtained using the weighted-median method (BMI-unadjusted: OR = 1.03, 95% CI, 0.95–1.13, P = 0.483; BMI-adjusted: OR = 1.03, 95% CI, 0.94–1.11, P = 0.546) and MR-Egger method (BMI-unadjusted: OR = 0.97, 95% CI, 0.87–1.09, P = 0.647; BMI-unadjusted: OR = 0.98, 95% CI = 0.87–1.10, P = 0.761). No outlier SNPs were detected using the MR-PRESSO test.
The Causal Associations Between Fasting Glucose and HbA1c Levels and POAG
The results indicated that genetically predicted fasting glucose level was not associated with POAG using the IVW method (OR = 1.19, 95% CI, 0.94–1.51, P = 0.157), weighted-median method (OR = 1.22, 95% CI, 0.94–1.59, P = 0.142), and MR-Egger method (OR = 1.46, 95% CI, 0.93–2.30, P = 0.297) for European ancestry. The potential association between genetically predicted HbA1c levels and POAG was only found in the weighted-median method (OR = 1.53, 95% CI = 1.03–2.25, P = 0.033) (Fig. 2C).
For East Asian ancestry, there were no statistically significant associations of genetically predicted fasting glucose (all P > 0.715) and HbA1c (all P > 0.508) levels with POAG in all methods (Fig. 2D).
Heterogeneity, Directional Pleiotropy, and Statistical Power
Heterogeneity, directional pleiotropy, and statistical power are presented in the Table. The I2 ranged from 49.8% to 72.6% in European ancestry and 0.0% to 18.9% in East Asian ancestry. The heterogeneity was significant in European ancestry (P < 0.001), but not in East Asian ancestry (P > 0.066). Meanwhile, the potentially pleiotropic effect was equal to zero, as shown by the MR-Egger regression analysis (all P for intercept > 0.531). The statistical power to detect an OR of 1.10 ranged from 0.24 to 0.93.
Table.
Heterogeneity and Directional Pleiotropy
| Ancestry | Exposure | N SNPs | Q Statistic | I 2 | P Value | P for Intercept | Statistical Power |
|---|---|---|---|---|---|---|---|
| European | |||||||
| T2D unadjusted for BMI | 165 | 507.2 | 67.7% | <0.001 | 0.531 | 0.93 | |
| T2D adjusted for BMI | 115 | 416.3 | 72.6% | <0.001 | 0.932 | 0.88 | |
| Fasting glucose | 58 | 113.5 | 49.8% | <0.001 | 0.297 | 0.80 | |
| HbA1c | 60 | 416.3 | 51.9% | <0.001 | 0.901 | 0.59 | |
| East Asian | |||||||
| T2D unadjusted for BMI | 129 | 144.2 | 11.2% | 0.156 | 0.532 | 0.85 | |
| T2D adjusted for BMI | 91 | 111.0 | 18.9% | 0.066 | 0.790 | 0.69 | |
| Fasting glucose | 11 | 5.7 | 0.0% | 0.838 | 0.987 | 0.24 | |
| HbA1c | 15 | 12.3 | 0.0% | 0.584 | 0.708 | 0.35 |
T2D, type 2 diabetes.
Discussion
This is the first study to use MR analysis to estimate the causal associations of type 2 diabetes and fasting glucose and HbA1c levels with the risk of POAG. This MR study showed that a genetic predisposition to type 2 diabetes was associated with an increased risk of POAG in the European population without pleiotropy regardless of whether type 2 diabetes is unadjusted or adjusted for BMI. However, no statistically significant association was observed between genetically predicted type 2 diabetes and the risk of POAG in the East Asian population. We did not find any association between genetically predicted fasting glucose and HbA1c levels and the risk of PAOG.
Several observational studies have reported an association between type 2 diabetes and POAG. For example, the Los Angeles Latino Eye Study demonstrated that the risk of POAG was 40% higher in participants with type 2 diabetes than in those without type 2 diabetes.30 Similarly, women with type 2 diabetes were independently associated with an 82% increased risk of incident primary POAG in the Nurses’ Health Study.31 Moreover, a meta-analysis performed in 2014 found that the pooled risk ratio of the association between diabetes mellitus and POAG, based on the risk estimates of the six cohort studies, was 1.40 (95% CI, 1.25–1.57).32 Therefore our study supported the causal association that patients with type 2 diabetes were more likely to have incident POAG among Europeans. It should be noted that type 2 diabetes is a binary risk factor in this MR study. The estimate of type 2 diabetes represents the average causal effect on the subgroup of individuals only under a plausible monotonicity assumption, which is that an increase in the number of risk alleles does not lower the likelihood of type 2 diabetes for any individual.33 Although the underlying pathways between type 2 diabetes and POAG remain unclear, several mechanisms have been proposed. One possible explanation is that type 2 diabetes may be related to elevated IOP,34 which is the only widely recognized modifiable risk factor for POAG.6 In this regard, a genome-wide meta-analysis indicated that most of the risk loci associated with POAG have also been associated with IOP or vertical cup-to-disc ratio.35 The pooled mean values estimated that participants with diabetes have higher 0.18 mm Hg of IOP than those without diabetes.36 The increasing glucose levels may increase IOP by increasing fibronectin production in the bovine trabecular meshwork.37 In addition, type 2 diabetes increases corneal stiffness and central corneal thickness, which increases IOP measurement values artificially.38 Another possible explanation for popularity is vascular mechanisms. Diabetes mellitus may lead to microvascular structural and functional damage. Hence, dysfunction in these vessels induces poor vascular autoregulation of the retina and optic nerve to protect against IOP and blood pressure fluctuations.39,40 In addition to these vascular changes, diabetes can impair physiological glial and neuronal function in the retina, which may increase the susceptibility of retinal ganglion cells to glaucomatous damage.41 Nonetheless, the potential mechanisms underlying this association need to be evaluated more thoroughly.
However, in contrast to several observational studies,13,42 this MR study found that there was no significant causal association between type 2 diabetes and POAG among the East Asian population. On the one hand, the variety of SNPs associated with type 2 diabetes between East Asian and European ancestries caused different effect sizes. On the other hand, the differences in clinical characteristics across ethnic groups were an important interpretation method for the subtle discrepancy in the association between type 2 diabetes and POAG. For example, there is a preponderance of normal-tension glaucoma over high-tension glaucoma in Asians compared with the White population.43 Furthermore, the statistical power may be an important explanation for such variance, since there was a relatively small sample size (6935 POAG cases) and limited valid IVs of type 2 diabetes in the East Asia population compared to European ancestry. An accurate estimate of the causal association between type 2 diabetes and the occurrence of POAG is difficult in observational studies. Except for POAG, type 2 diabetes results in many ocular diseases; thus patients with type 2 diabetes are more likely to receive more ophthalmological examinations.36 This may lead to an overestimation of the association between type 2 diabetes and POAG. In addition, it is possible that diabetes complications could lead to retinal diseases and visual field defects, resulting in overdiagnosis of POAG. It is also possible that the effect sizes of such observational studies were influenced by various confounding factors. Therefore confounding, reverse causation, and various biases in these studies may generate unreliable indicators of the causal effects of type 2 diabetes on the risk of POAG. Analogous to randomized controlled trials, the MR analysis assumes that the alleles of interest are randomly and equally distributed in the population of interest and can infer causality to some extent. Given the largely asymptomatic nature of early glaucoma and the long latent phase of the disease, the results of this study provide valuable information for the screening and early detection of POAG among patients with type 2 diabetes. It is important for the management of type 2 diabetes and prevention of POAG.
In contrast to cross-sectional studies,11,12 which found that higher levels of fasting glucose, fasting insulin, HbA1c, and homeostatic model assessment of insulin resistance (HOMA-IR) were associated with a higher prevalence of open-angle glaucoma in subjects with good glycemic control, we found no evidence of a causal relationship between fasting glucose and HbA1c levels and the risk of POAG. Dysglycemia alone does not explain the increased risk of POAG in this study. There are distinct physiological mechanisms that regulate fasting glucose and HbA1c.44,45 It is likely that the benefits of type 2 diabetes therapies, such as metformin, on POAG occur via mechanisms, such as fibrosis and mitochondrial bioenergetic dysfunction other than improved glycemic control.46,47 Importantly, the genetic variants associated with fasting glucose and HbA1c we used in our analyses only account for a small proportion of the variance in these parameters. Besides, no significant association was found between genetically predicted glucose metabolism and POAG, perhaps due to the selection of fasting glucose and HbA1c related SNPs from non-diabetic populations. Thus we may have been underpowered to detect a smaller effect, as has been observed in traditional observational data. The significant associations of fasting glucose and HbA1c per 1-SD changes with POAG may require larger statistical power.
Large sample sizes were used to examine the causal association between type 2 diabetes and fasting glucose and HbA1c levels and POAG among European and East Asian populations. Thus, this was one of the strengths of this MR analysis. Despite this, sample sizes from multiple studies might have contributed to the increased heterogeneity in European ancestry. We were cautious in selecting IVs to decrease potential pleiotropy. Genetic correlations between BMI and POAG were unclear, but the evidence from a prospective cohort study showed that each unit increase in BMI was associated with a 6% reduced risk of type 2 diabetes (P = 0.01).48 Meanwhile, several SNPs were significantly associated with both type 2 diabetes and BMI. Therefore we selected two categories of SNPs, BMI-unadjusted and BMI-adjusted, as IVs to compare the effect of BMI on POAG. The potentially pleiotropic effect was equal to zero in this MR analysis according to MR-Egger regression analysis.
This study has some limitations. First, we did not analyze the genetic correlation between type 2 diabetes and fasting glucose HbA1c levels and POAG. Second, there was some sample overlap in the East Asian population. The sample overlap might have inflated type 1 error rates,49 but we did not observe a statistically significant association in the East Asian population. Third, genetic diversity complicates causal inference across races, and our conclusions do not apply to African and South Asian populations. Fourth, this was a two-sample MR study design, and we adjusted for BMI only in the selection of IVs for type 2 diabetes. However, we did not find a significant difference between the adjusted and unadjusted BMI models, and multivariate MR was a more feasible approach. Fifth, we used the data from the meta-analysis of GWAS, which helped to increase the sample size but might also contain heterogeneity and biases, such as recall bias from self-report data and overdiagnosis bias from electronic medical records. Sixth, phenotypic associations of type 2 diabetes, fasting glucose, and Hb1Ac with POAG were not assessed in this study due to constraints in data access.
In conclusion, type 2 diabetes is causally associated with the risk of POAG in European instead of East Asian populations. The point estimates of fasting glucose and Hb1Ac with POAG are large but not statistically significant, which prompts the question of statistical power.
Supplementary Material
Acknowledgments
The authors thank the DIAGRAM, MAGIC, and AGEN consortium for providing summary statistics data for the analyses.
Disclosure: Z. Hu, None; F. Zhou, None; A.C. Kaminga, None; H. Xu, None
References
- 1. Distelhorst JS, Hughes GM.. Open-angle glaucoma. Am Fam Physician. 2003; 67: 1937–1944. [PubMed] [Google Scholar]
- 2. Tham YC, Li X, Wong TY, Quigley HA, Aung T, Cheng CY.. Global prevalence of glaucoma and projections of glaucoma burden through 2040: a systematic review and meta-analysis. Ophthalmology. 2014; 121: 2081–2090. [DOI] [PubMed] [Google Scholar]
- 3. Quaranta L, Riva I, Gerardi C, Oddone F, Floriani I, Konstas AG.. Quality of life in glaucoma: a review of the literature. Adv Ther. 2016; 33: 959–981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Sheybani A, Scott R, Samuelson TW, et al.. Open-angle glaucoma: burden of illness, current therapies, and the management of nocturnal IOP variation. Ophthalmol Ther. 2020; 9: 1–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Marcus MW, de Vries MM, Junoy Montolio FG, Jansonius NM. Myopia as a risk factor for open-angle glaucoma: a systematic review and meta-analysis. Ophthalmology. 2011; 118: 1989–1994.e2. [DOI] [PubMed] [Google Scholar]
- 6. Quigley HA. Open-angle glaucoma. N Engl J Med. 1993; 328: 1097–1106. [DOI] [PubMed] [Google Scholar]
- 7. Gordon MO, Beiser JA, Brandt JD, et al.. The Ocular Hypertension Treatment Study: baseline factors that predict the onset of primary open-angle glaucoma. Arch Ophthalmol. 2002; 120: 714–720; discussion 829-30. [DOI] [PubMed] [Google Scholar]
- 8. Choi JA, Ko SH, Park YR, Jee DH, Ko SH, Park CK.. Retinal nerve fiber layer loss is associated with urinary albumin excretion in patients with type 2 diabetes. Ophthalmology. 2015; 122(5): 976–981. [DOI] [PubMed] [Google Scholar]
- 9. Cui Y, Yang X, Zhang G, et al.. Intraocular pressure in general and diabetic populations from Southern China: the Dongguan Eye Study. Invest Ophthalmol Vis Sci. 2019; 60: 761–769. [DOI] [PubMed] [Google Scholar]
- 10. Chua J, Chee ML, Chin CWL, et al.. Inter-relationship between ageing, body mass index, diabetes, systemic blood pressure and intraocular pressure in Asians: 6-year longitudinal study. Br J Ophthalmol. 2019; 103: 196–202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Choi JA, Park YM, Han K, Lee J, Yun JS, Ko SH.. Fasting plasma glucose level and the risk of open angle glaucoma: Nationwide population-based cohort study in Korea. PLoS One. 2020; 15(9): e0239529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Zhao D, Cho J, Kim MH, Friedman D, Guallar E.. Diabetes, glucose metabolism, and glaucoma: the 2005-2008 National Health and Nutrition Examination Survey. PLoS One. 2014; 9(11): e112460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Jung Y, Han K, Park HL, Park CK.. Type 2 diabetes mellitus and risk of open-angle glaucoma development in Koreans: an 11-year nationwide propensity-score-matched study. Diabetes Metab. 2018; 44: 328–332. [DOI] [PubMed] [Google Scholar]
- 14. Emdin CA, Khera AV, Kathiresan S. Mendelian randomization. JAMA. 2017; 318: 1925–1926. [DOI] [PubMed] [Google Scholar]
- 15. Sekula P, Del Greco MF, Pattaro C, Köttgen A. Mendelian randomization as an approach to assess causality using observational data. J Am Soc Nephrol. 2016; 27: 3253–3265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Davey Smith G, Hemani G. Mendelian randomization: genetic anchors for causal inference in epidemiological studies. Hum Mol Genet. 2014; 23(R1): R89–R98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Mahajan A, Taliun D, Thurner M, et al.. Fine-mapping type 2 diabetes loci to single-variant resolution using high-density imputation and islet-specific epigenome maps. Nat Genet. 2018; 50: 1505–1513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Spracklen CN, Horikoshi M, Kim YJ, et al.. Identification of type 2 diabetes loci in 433,540 East Asian individuals. Nature. 2020; 582(7811): 240–245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Chen J, Spracklen CN, Marenne G, et al.. The trans-ancestral genomic architecture of glycemic traits. Nat Genet. 2021; 53(6): 840–860. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Gharahkhani P, Jorgenson E, Hysi P, et al.. Genome-wide meta-analysis identifies 127 open-angle glaucoma loci with consistent effect across ancestries. Nat Comm. 2021; 12(1): 1258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Prum BE, Rosenberg LF, Gedde SJ, et al.. Primary open-angle glaucoma preferred practice pattern® guidelines. Ophthalmology. 2016; 123(1): P41–P111. [DOI] [PubMed] [Google Scholar]
- 22. Kwon YH, Fingert JH, Kuehn MH, Alward WLM.. Primary open-angle glaucoma. N Engl J Med. 2009; 360: 1113–1124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Burgess S, Butterworth A, Thompson SG.. Mendelian randomization analysis with multiple genetic variants using summarized data. Genet Epidemiol. 2013; 37: 658–665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Hartwig FP, Davey Smith G, Bowden J. Robust inference in summary data Mendelian randomization via the zero modal pleiotropy assumption. Int J Epidemiol. 2017; 46: 1985–1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Bowden J, Davey Smith G, Haycock PC, Burgess S. Consistent estimation in mendelian randomization with some invalid instruments using a weighted median estimator. Genet Epidemiol. 2016; 40: 304–314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Burgess S, Thompson SG.. Interpreting findings from Mendelian randomization using the MR-Egger method. Eur J Epidemiol. 2017; 32: 377–389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Verbanck M, Chen C-Y, Neale B, Do R. Detection of widespread horizontal pleiotropy in causal relationships inferred from Mendelian randomization between complex traits and diseases. Nat Genet. 2018; 50: 693–698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Burgess S, Labrecque JA.. Mendelian randomization with a binary exposure variable: interpretation and presentation of causal estimates. Eur J Epidemiol. 2018; 33: 947–952. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Brion MJ, Shakhbazov K, Visscher PM.. Calculating statistical power in Mendelian randomization studies. Int J Epidemiol. 2013; 42: 1497–1501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Chopra V, Varma R, Francis BA, Wu J, Torres M, Azen SP.. Type 2 diabetes mellitus and the risk of open-angle glaucoma the Los Angeles Latino Eye Study. Ophthalmology. 2008; 115: 227–232.e1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Pasquale LR, Kang JH, Manson JE, Willett WC, Rosner BA, Hankinson SE.. Prospective study of type 2 diabetes mellitus and risk of primary open-angle glaucoma in women. Ophthalmology. 2006; 113: 1081–1086. [DOI] [PubMed] [Google Scholar]
- 32. Zhou M, Wang W, Huang W, Zhang X.. Diabetes mellitus as a risk factor for open-angle glaucoma: a systematic review and meta-analysis. PLoS One. 2014; 9(8): e102972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Skrivankova VW, Richmond RC, Woolf BAR, et al.. Strengthening the reporting of observational studies in epidemiology using mendelian randomisation (STROBE-MR): explanation and elaboration. BMJ (Clin Res Ed). 2021; 375: n2233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Oh SW, Lee S, Park C, Kim DJ.. Elevated intraocular pressure is associated with insulin resistance and metabolic syndrome. Diabetes Metab Res Rev. 2005; 21: 434–440. [DOI] [PubMed] [Google Scholar]
- 35. Gharahkhani P, Jorgenson E, Hysi P, et al.. Genome-wide meta-analysis identifies 127 open-angle glaucoma loci with consistent effect across ancestries. Nat Commun. 2021; 12: 1258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Zhao D, Cho J, Kim MH, Friedman DS, Guallar E.. Diabetes, fasting glucose, and the risk of glaucoma: a meta-analysis. Ophthalmology. 2015; 122: 72–78. [DOI] [PubMed] [Google Scholar]
- 37. Sato T, Roy S.. Effect of high glucose on fibronectin expression and cell proliferation in trabecular meshwork cells. Invest Ophthalmol Vis Sci. 2002; 43: 170–175. [PubMed] [Google Scholar]
- 38. Su DH, Wong TY, Wong WL, et al.. Diabetes, hyperglycemia, and central corneal thickness: the Singapore Malay Eye Study. Ophthalmology. 2008; 115: 964–968.e1. [DOI] [PubMed] [Google Scholar]
- 39. Feke GT, Bex PJ, Taylor CP, et al.. Effect of brimonidine on retinal vascular autoregulation and short-term visual function in normal tension glaucoma. Am J Ophthalmol. 2014; 158: 105–112.e1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Flammer J, Mozaffarieh M.. Autoregulation, a balancing act between supply and demand. Can J Ophthalmol. 2008; 43: 317–321. [DOI] [PubMed] [Google Scholar]
- 41. Nakamura M, Kanamori A, Negi A.. Diabetes mellitus as a risk factor for glaucomatous optic neuropathy. Ophthalmologica. 2005; 219: 1–10. [DOI] [PubMed] [Google Scholar]
- 42. Ishikawa M, Sawada Y, Sato N, Yoshitomi T.. Risk factors for primary open-angle glaucoma in Japanese subjects attending community health screenings. Clin Ophthalmol. 2011; 5: 1531–1537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Cho HK, Kee C.. Population-based glaucoma prevalence studies in Asians. Surv Ophthalmol. 2014; 59: 434–447. [DOI] [PubMed] [Google Scholar]
- 44. Saxena R, Hivert MF, Langenberg C, et al.. Genetic variation in GIPR influences the glucose and insulin responses to an oral glucose challenge. Nat Genet. 2010; 42: 142–148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Soranzo N. Genetic determinants of variability in glycated hemoglobin (HbA(1c)) in humans: review of recent progress and prospects for use in diabetes care. Curr Diabetes Rep. 2011; 11: 562–569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Hurley DJ, Irnaten M, O'Brien C. Metformin and glaucoma-review of anti-fibrotic processes and bioenergetics. Cells. 2021; 10: 2131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Shen L, Walter S, Melles RB, Glymour MM, Jorgenson E.. Diabetes pathology and risk of primary open-angle glaucoma: evaluating causal mechanisms by using genetic information. Am J Epidemiol. 2016; 183: 147–155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Pasquale LR, Willett WC, Rosner BA, Kang JH.. Anthropometric measures and their relation to incident primary open-angle glaucoma. Ophthalmology. 2010; 117: 1521–1529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Burgess S, Davies NM, Thompson SG.. Bias due to participant overlap in two-sample Mendelian randomization. Genet Epidemiol. 2016; 40: 597–608. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.