Abstract
Background:
Premature infants and their parents experience significant stress related to separation and lifesaving procedures. While evidence suggests that skin-to-skin contact (SSC) is a stress-reducing intervention for both neonates and parents, the mechanisms that underlie its efficacy are not well understood.
Objective:
Purpose of this systematic review is to summarize the current state of knowledge on changes in biomarkers (ie, oxytocin [OT], cortisol, hypoxanthine, xanthine, uric acid, and allantoin), associated with SSC in premature infants and parents, that may reflect physiologic responses to stress.
Methods:
A comprehensive literature search was conducted from 1990 to 2020. Studies were selected using prespecified inclusion and exclusion criteria.
Results:
Of the 175 studies identified, only 19 are included in this review. Ten studies evaluated only infants, 2 evaluated only parents, and 7 evaluated for changes in biomarkers in both infants and parents. Cortisol was the most common biomarker evaluated. While changes in infants' cortisol levels were highly variable, in 55% of the parent studies, parent cortisol levels decreased following SSC. In both parents and infants, OT levels decreased following SSC. Only 1 study found that allantoin levels were significantly lower in infants who received SSC.
Implications for Practice and Research:
While evidence suggests the numerous benefits of SSC, additional research is needed to identify the optimal biomarker to determine the mechanisms that underlie these effects. The use of novel biomarkers (eg, gene expression changes microbiome) may provide new insights into the mechanisms that underlie the efficacy of SSC.
Video Abstract available at: https://journals.lww.com/advancesinneonatalcare/Pages/videogallery.aspx?autoPlay=false&videoId=48
Keywords: allantoin, biochemical markers, cortisol, hypoxanthine, kangaroo mother care, neonatal intensive care unit (NICU), oxidative stress, oxytocin, skin-to-skin contact, xanthine
Premature infants enter the world facing the dual disadvantages of being deprived of the ability to fully develop within their mother's womb and of being physically separated from their mothers. Western maternal/neonatal care is to a large extent based on routine physical separation that is considered necessary to deliver the highly technological critical care that ensures premature infants' survival. However, parent–infant separation is associated with lifelong negative health consequences that impose a significant burden on both families and society.1–3 The cost of caring for premature infants in the United States totaled $25 billion in 2019.4
In developed countries, modern technology has increased the survival rates of premature infants and pushed the boundaries of viability so that infants as young as 23 to 24 weeks' gestation can survive. However, the provision of this care and the associated physical separation of infants from their mothers contributes to the inevitable risk of poor infant–mother interactions that have significant negative effects on both neonates and mothers.2 While the impact of early physical separation is bidirectional, research on infants' outcomes suggests that premature separation is associated with increases in neurodevelopmental and behavioral problems, maladjusted cognition, and learning disabilities that have a negative impact on emotional and psychological well-being of infants across their lifespan.5,6
The purpose of this systematic review is to summarize the current state of knowledge on changes in biomarkers (ie, oxytocin [OT], salivary or plasma cortisol, hypoxanthine [Hx], xanthine [Xa], uric acid [UA], and allantoin), associated with skin-to-skin contact (SSC) in premature infants and parents, that may reflect physiologic responses to stress.
BACKGROUND
Skin-to-skin contact and kangaroo mother care (KMC) are often incorrectly and synonymously used as being one the same. However, KMC is a wider term that includes breastfeeding and early discharge from the hospital as a step-down way to care for growing infants before discharge home. SSC is used as an intermittent intervention immediately after birth that is part of the conventional care in postbirth settings.7 SSC is a model of care that aims to improve the interactions between premature infants and their mothers to minimize the negative consequences of physical separation.8 This care model involves a parent/representative (eg, mother, father, and partner) holding an unclothed infant on his/her bare chest with just a diaper between them for extended periods. Evidence suggests that SSC reduces morbidity and mortality among premature infants.8 Some of the direct effects of SSC for the infants include improved temperature stability, stabilization of breathing, and improved oxygen saturation and heart rate.9 In addition, research findings suggest improvements in infants' brain development, better motor and mental development, as well as better behavioral responses including state regulation and motor regulation.10,11 For the mothers, SSC is associated with improvements in milk production and breastfeeding.12
Bergman and Bergman12 described the mother's body as the optimal environment that allows for the development of regulatory processes that ultimately provide physiologic stability for the infant. While these in utero benefits are well documented, the neurobiological mechanisms that underlie the benefits of SSC remain unclear. The current hypothesized mechanism suggests that direct SSC connects sensory nerve pathways between the mother and infant, which optimizes the premature infant's neurosensory pathway development. The observed improvements in infants' physiologic stability, associated with SSC, suggest that stress is being reduced in response to SSC.2
Research on SSC began in the 1970s with physician scientists Edgar Rey Sanabria and Hector Martinez-Gomez in the neonatal unit at San Juan de Dios Hospital in Bogotá, Colombia, South America.13 These researchers found it difficult to treat preterm low-birth-weight infants because of the lack of technology and resources. They developed the KMC program as a simple and low-cost method to care for these premature infants.13 In 1984, a method of increased contact emerged in Europe that was described as “extra contact” between the mother and infant.14 However, it is not clear whether this approach included SSC or simple forms of contact (eg, touching, holding, and physical closeness with the mother).
Across these early studies,14,15 while only full-term infants were included and the assessments were done in the first1 to 2 hours post birth, the behaviors of mothers toward their infants improved. In 1979, the term SSC appeared in the literature.15 In 1983, an evaluation of the Colombia program, which was done in conjunction with the United Nations International Children's Emergency Fund (UNICEF), was published.13 This report received significant attention from British neonatal researchers who traveled to Bogotá to analyze the data on the efficacy of the KMC program. In a Lancet publication,13 these researchers reported that KMC was effective in treating low-birth-weight infants in Colombia.13 Two nurse scientists, Susan Ludington-Hoe and Gene Cranston Anderson, were instrumental in introducing SSC into the United States.16 While several systematic reviews and meta-analyses have documented the benefits of SSC for preterm infants,17–19 data suggest that infants in the United States receive less than 1 hour of SSC per day.20,21 In addition, no systematic reviews have evaluated the effects of SSC on biomarkers of stress in infants and parents who used this intervention. Evidence of physiologic effects on stress responses may increase the use of SSC.
What This Study Adds
A focused review of the scientific evidence on various biomarkers that were used to evaluate the effects of skin-to-skin contact (SSC) on physiologic responses to stress in both neonates and their parents.
Identification of the need to develop a longitudinal randomized clinical trial with rigorous methodologies and a comprehensive set of biomarkers to be able to identify the mechanisms that underlie the benefits of SSC in infants and mothers.
METHODS
In collaboration with a medical librarian (M.L.F.), 5 electronic databases (PubMed, EMBASE, American Psychological Association [PsycINFO], Web of Science [WoS], and the Cumulative Index to Nursing and Allied Health Literature [CINAHL]) were searched. Systematic search strategies were designed using a combination of MeSH/Emtree terms and various key words to identify peer-reviewed studies related to the effects of SSC on biomarkers of stress in preterm. Articles written in English were included if published between 1990 and 2020. All of the studies were reviewed by D.F. and C.M. Any discrepancies were resolved through discussion. Details of sample search strategies are included as Appendix (available at: http://links.lww.com/ANC/A95). In addition, Web of Science was searched to review citing references, cited references, and related articles of included studies for additional studies.
Studies were included if they met all of the following criteria: (1) evaluated a biomarker of short-term physiologic stress in conjunction with the administration of SSC; (2) evaluated these biomarkers in a preterm infant and/or a mother or father; and (3) evaluated 1 or more of the following biomarkers: cortisol, OT, Hx, Xa, UA, and allantoin.
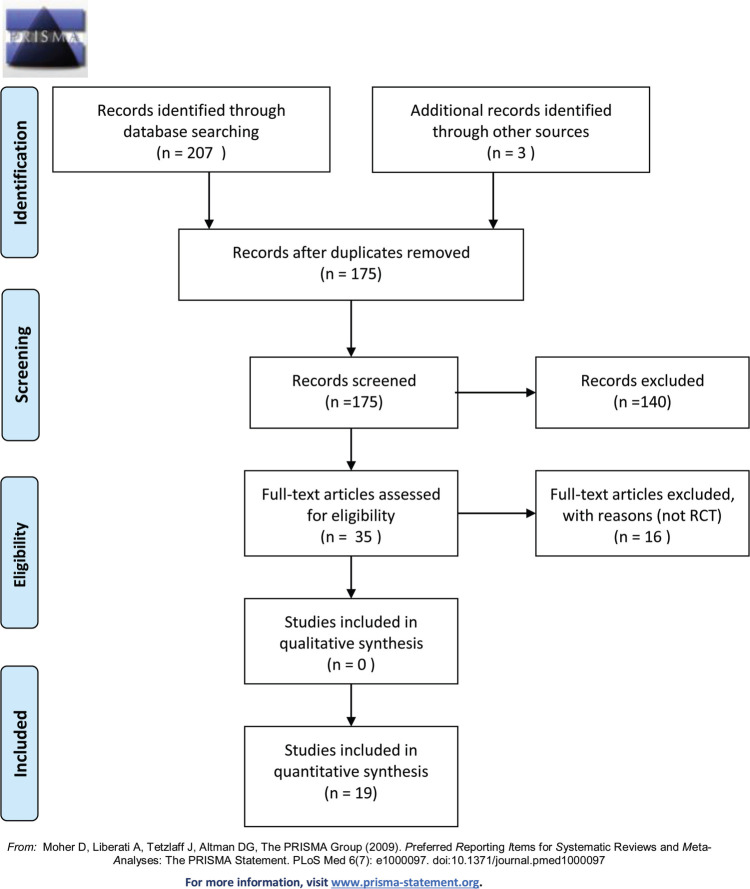
The search strategy yielded 35 studies identified in PubMed, 65 in EMBASE, 29 in PsycINFO, 50 in WoS, and 28 in CINAHL (Figure 1). After duplicates were removed, the abstracts from 175 studies were evaluated. Of these 175 studies, full articles for 35 studies were reviewed. After eliminating studies that did not meet our prespecified inclusion criteria, 19 studies are included in this systematic review. Of these 19 studies, 10 (52.6%) evaluated only infants,21–30 2 (10.5%) evaluated only parents,31,32 and 7 (36.9%) evaluated both infants and parents.33–39
FIGURE 1.
PRISMA 2009 flow diagram.
For the purposes of this review, the findings from the infant (see Supplementary Table 1, available at: http://links.lww.com/ANC/A93)21–30,33–39 and parent (see Supplementary Table 2, available at: http://links.lww.com/ANC/A94)31–39 studies are presented separately. Standardized criteria were developed to review the 2 groups of studies. Across both groups of studies, information was obtained on author, year, purpose, study design, sample size, study procedures (ie, SSC procedure, amount of time for SSC), how and the frequency with which biomarker specimens were collected, major findings, strengths, and limitations. For the infant studies, specific information was obtained on gestational age, gender, ethnicity, birth weight, Apgar score, Score for Neonatal Acute Physiology with Perinatal Extension II (SNAPPE II), and birth mode. For the parent studies, specific information was obtained on age, ethnicity, employment status, education level, and marital/partner status.
RESULTS OF THE INFANT STUDIES
Description of the Studies
As shown in Supplementary Table 1 (http://links.lww.com/ANC/A93), 11 (64.7%) of the 17 studies that evaluated biomarkers in preterm infants were prospective randomized controlled trials (RCTs) of SSC.22–27,30,34–36,38 Across the 17 studies, 5 studies were conducted in the United States,21,22,30,36,38 2 each in Sweden33,34 and the Netherlands,28,29 and 1 each in Canada,35 the United Kingdom,25 Germany,24 Serbia,37 Egypt,27 Iran,26 Israel,23 and Brazil.39 Sample sizes ranged from 1733 to 14623 patients and 5 studies28,29,33,35,38 had fewer than 30 patients.
Across 14 studies, the grand mean for the infant's gestational age was 30.5 weeks. Two of the studies33,37 reported median gestational age and 1 study34 reported postmenstrual age of the infants. Across the 15 studies that reported weight, the grand mean birth weight was 1488.9 g. Across the 6 studies that reported 5-minute Apgar scores, the grand mean score was 8.4. Across the 14 studies that reported gender, the grand mean percentage of females was 44%. Of the 8 studies that reported type of delivery, the grand mean rate of cesarean delivery was 69.0%. All 17 studies were conducted in the intensive care nursery.
Characteristics of SSC
Across the 17 studies, the duration of SSC was extremely variable (ie, 2025-12027,30,37 minutes per session). The total number of sessions varied from 126,29 to 56.36 The initiation of SSC varied across the studies (ie, immediately after delivery,34 140-1421 days after birth, or when the infant was considered stable37). In terms of the comparator groups in the RCTs, a variety of approaches were used including having some opportunity to provide SSC,22 mothers being present for visitation and breastfeeding only,27 and mothers performing other care functions.26 The various timing of sample collection noted across the studies made it difficult to make specific or uniformed generalizations.
In terms of biomarkers, cortisol was the most common one evaluated.23–27,30,33–37,39 Four studies examined OT,21,28,29,38 and 1 study evaluated both cortisol and OT.38 One study examined biochemical markers of adenosine triphosphate (ATP) degradation (i.e., Hx, Xa, UA) and allantoin as a marker of oxidative stress.22 In 8 studies,22,26,27,29,34,35,38,39 specimens were collected before and after an SSC session. In 12 studies,22,23,26–30,34–36,38,39 changes over time in the various biomarkers were evaluated. Cortisol and OT measures were obtained from salivary specimens using a cotton swab,25,29,33,35,38 eye sponge,30,37 filter paper,36 or direct aspiration from the floor of the mouth23,26,27 and in 1 study from plasma.39 The biochemical markers were assayed from urine obtained from cotton balls placed in the infant's diaper.22
Biomarker Outcomes in Infants
In terms of changes in infants' cortisol levels in response to SSC, the results were highly variable. In 2 of the studies that utilized a pre-/posttest design,33,39 no differences in cortisol levels were found. In 6 of the RCTs,24–27,30,35 no differences in cortisol levels were found between the intervention and control groups. In 1 study,38 cortisol levels decreased following a session of SSC. The various study procedures (ie, SSC procedure, amount of time for SSC), the method, and the frequency with which biomarker specimens were collected could have contributed to the high variability in the results across the studies.
Four studies21,28,29,38 reported on changes in OT levels in response to SSC. In 3 of these studies21,29,38 significant increases in OT were reported. In the other study,28 OT levels decreased during SSC. In the only study that evaluated for biomarkers of ATP degredation,22 mean allantoin levels were significantly lower in the infants who received SSC.
RESULTS OF THE PARENT STUDIES
Description of the Studies
As shown in Supplemental Table 2 (available at: http://links.lww.com/ANC/A94), 5 of the 9 studies that evaluated biomarkers in parents were prospective RCTs of SSC.32,35,36,38,39 Across these 9 studies, 3 were conducted in the United States,32,36,38 2 in Canada,31,35 2 in Sweden,33,34 1 in Brazil,39 and 1 in Serbia.37 Sample sizes ranged from 1733 to 7936 participants. Across the 9 studies, the grand means for maternal and paternal ages were 30.1 and 33.4 years, respectively. In the 2 studies that reported employment status,31,38 94.5% of the fathers and 69.0% of the mothers were employed. In the 6 studies that reported on the educational level of the parents,31–34,38,39 47.0% of the mothers and 60.0% of the fathers had 4 years or more of college. For the 4 studies that reported marital status,32,34,38,39 83% of mothers and 87% of fathers were married or partnered.
Characteristics of SSC
Across the 9 studies, the duration and number of SSC sessions were extremely heterogeneous. The duration of each session ranged from 3032,39 to 120 minutes.37 The number of sessions ranged from 131 to 56.36 In terms of biomarkers, 7 studies31,33–37,39 evaluated cortisol and 2 studies32,38 evaluated both cortisol and OT. The salivary specimens were collected using an eye sponge,37 oral swabs,31,35,39 filter paper,36 cotton tip applicators,33,34 and passive drool38 methods.
Biomarker Outcomes in Parents
In terms of changes in parents' cortisol levels in response to SSC, the results were inconsistent. While in 4 studies34,35,38,39 no significant differences were found, in the other 5 studies,31–33,36,37 cortisol levels decreased following SSC. In the one study that found decreases in cortisol in both mothers and fathers during SSC,32 while maternal levels continued to drop in the 30 minutes following SSC, paternal levels of cortisol rose during the same period.
In the 2 studies that evaluated for changes in OT,32,38 measures were done in both mothers and fathers. In both studies and for both mothers and fathers, OT levels increased during SSC. However, in 1 study,32 while mothers' OT levels decreased, fathers' levels remained stable in the 30 minutes following SSC. In the other study,38 the pattern of change was the same for both parents, namely OT levels increased during SSC and then decreased post-SSC.
DISCUSSION
This review is the first to summarize the findings from studies that evaluated for changes in biomarkers of physiologic stress (ie, cortisol, OT, Hx, Xa, UA, and allantoin) in response to SSC in both preterm infants and their parents. Of our prespecified biomarkers, the most common one evaluated was cortisol (ie, 76.5% of infant and 100% of parent studies). Cortisol is considered a major biomarker of stress for both infants41 and adults.32 In the fetus, the hypothalamic-pituitary-adrenal (HPA) axis, which regulates the release of cortisol, is functional by the second trimester.42 In healthy, full-term infants, beginning at 1 month of age, cortisol is secreted in a circadian rhythm pulsatile fashion, peaking in the morning and showing a nadir in the evening.42 In preterm infants, the data suggest that the circadian rhythm for cortisol is established by 1 month corrected age.42 Of note, the establishment of the circadian rhythm for cortisol is more related to gestational age than postnatal age, illness, antenatal steroid administration, environmental stressors, and varying levels of neurodevelopmental care.17 However, high and prolonged levels of cortisol during acute and chronic stress contribute to dysregulation of allostatic processes that mediate unhealthy and inadequate adaptations to stress.43
Across 90% of the studies, the findings regarding changes in infants' cortisol levels in response to SSC were highly variable (see Supplemental Table 1, available at: http://links.lww.com/ANC/A93). In contrast, in 55% of the parent studies, cortisol levels decreased following SSC. Across both sets of studies, the variability in the findings may be related to: the relatively small sample sizes; the timing of the cortisol measures in relationship to diurnal variability; the wide range in infants' gestational ages; and a lack of consistency in the SSC procedures (ie, duration, frequency, position, and timing of the initiation of the intervention in relationship to the infant's birth). While these findings suggest that cortisol may be an appropriate biomarker to evaluate the biological mechanisms that underlie SSC in adults who have a more established circadian rhythm, its use as a biomarker in infants warrants additional consideration. Researchers who propose to use this biomarker may want to establish rigorous inclusion and exclusion criteria for the infants, particularly in terms of gestational age and standardization of specimen collection that accounts for circadian rhythm.
In terms of the findings related to OT, the relationships between stress and OT are extremely complex. OT is produced by the hypothalamus and released from the posterior pituitary gland or other parts of the brain and spinal cord, where it binds to receptors to influence both physiology and behavior.43 Oxytocin is released during both eustress and distress. Its release elicits differential effects on the HPA axis and the sympathetic adrenal medullary system based on the type, severity, and duration of the stressor.43
SSC is hypothesized to activate this oxytocinergic system in both infants and parents with associated decreases in stress and anxiety.44 For example, if the release of OT during SSC decreased social anxiety in the dyad, socially based behaviors and emotions during parental–infant engagement would be facilitated.44 In addition, it is known that during periods of decreased stress (eg, during SSC), the hypothalamus stimulates the posterior pituitary gland to release OT, which acts on the nucleus ambiguus.45 This action can decrease muscle tone in the infant's head and face, which facilitates eye contact, observation of facial expressions, and the ability of the infant to differentiate human voices. These changes facilitate engagement cues with parents. In addition, the enhancement of the muscle tone of the pharynx, soft palate, and larynx leads to better sucking, swallowing, and rhythmic breathing during feeding. Enhanced muscle tone in the gastrointestinal tract results in increased tone of the esophageal sphincter and increased motility. This effect results in decreases in reflux and constipation, as well as increased tolerance to enteral feeding.46 Finally, OT stimulates the release of gastrointestinal enzymes that improves digestion and overall growth of the infant.47
In the 3 studies that evaluated for changes in OT in response to SSC,28,32,38 in both parents and infants OT levels increased following SSC. Unlike cortisol, OT levels increase during the sleep, and then rapidly decline upon awakening to basal levels. Of note, OT is known to modulate cortisol levels during stressful conditions.48 While only 3 studies have evaluated this biomarker,28,32,38 the relatively consistent findings suggest that SSC may influence levels of OT in both infants and parents. While the data suggest that OT may be a useful biomarker to evaluate the effects of SSC, 150 μL of saliva is required to conduct the assay. In addition, OT needs to be placed on ice and frozen in a −80°C freezer within an hour after specimen collection.
Only 1 study evaluated for changes in urinary markers of ATP degradation (ie, Hx, Xa, and UA) and oxidative stress (ie, allantoin) in response to SSC.22 In this pilot study, mean allantoin levels over 2 days were significantly lower in the SSC group than in the control group. These results provide preliminary evidence that SSC reduces neonatal oxidative stress processes and that allantoin may be a noninvasive marker for future studies. In terms of ATP degradation, the stress and associated morbidities experienced by preterm infants may be partially explained by an oxygen supply that cannot meet the body's demands and the switch from aerobic to anaerobic metabolism. As a result of this metabolic switch, ATP is broken down to generate energy.49 The degradation of ATP results in elevated levels of adenosine monophosphate and free adenosine that are catabolized into inosine monophosphate and inosine, respectively. Then, inosine and inosine monophosphate are converted into Hx, Xa, and UA. Uric acid acts as an antioxidant by scavenging reactive oxygen species, which converts the UA compound mainly to allantoin, a marker of oxidative stress in humans.50 Interventions to reduce anaerobic metabolism and oxidative stress, such as SSC, may partially blunt the occurrence and severity of preterm morbidities. These biochemical markers may be a more objective measure of stress and can be combined with the study of cortisol and OT to determine the mechanisms that underlie the benefits of SSC.
LIMITATIONS
This systematic review has a number of limitations that warrant consideration. First, only studies published in English were included in the review. One of the primary purposes of this review was to examine the current state of the knowledge on changes in biomarkers associated with SSC in preterm infants and their parents that may reflect physiologic responses to stress. These findings from the limited number of studies showed inconsistent results. The inconsistent findings may be related to the relatively small samples sizes; the considerable variability in the duration of each SSC session, as well as the total number of sessions; and the lack of a control group in some studies. Given this heterogeneity, a meta-analysis was not done.
IMPLICATIONS FOR RESEARCH
Currently, the optimal biomarker to evaluate the effects of SSC on short- or long-term physiologic responses to stress is unknown. Randomized controlled trials that control for infant's gestational age, the timing of the initiation of SSC in relationship to the infant's birth, type and duration of SSC, timing of biomarker collection in relationship to circadian variability in the various biomarkers, and standardization of control group procedures are needed to determine the physiologic mechanisms that underlie the benefits of SSC. Research is needed that investigate the effects of SSC in the neonatal intensive care unit (NICU) environment on the infant's long-term structural brain development, stress reactivity, and additional neurodevelopmental outcomes. Longitudinal analyses of biobehavioral outcomes, as well as biomarkers of stress in the dyad, are vital to determine the effects of SSC on parenting quality, maternal/paternal health, infant neurodevelopment, and dyadic attachment over time. Sophisticated analytic methods and multisite studies that allow for larger sample sizes will be needed to parse out the effects associated with SSC and infant health, maternal/paternal caregiving, and the physical environment in the NICU. Consideration needs to be given to an evaluation of additional biomarkers including genetic and epigenetic markers.
Summary of Recommendations for Practice and Research.
| What we know: |
|
| What needs to be studied: |
|
| What we can do today: |
|
IMPLICATIONS FOR PRACTICE
While this systematic review did not identify the optimal biomarker to evaluate the effects of SSC, one needs to note that the increased stress experienced by both parents and infants is a risk factor for poor infant outcomes. Clinicians need to employ effective strategies to implement SSC as a stress reducing intervention. Considering the potential benefits of this relatively simple intervention, nursing staff should explain the benefits of SSC to parents and support them to be close to their infant. Clinicians should continue to provide guidance, education, and support to parents regarding SSC with the ultimate goal of reducing stress and promoting well-being in both preterm infants and parents.
CONCLUSIONS
In this systematic review, we evaluated 26 studies from 11 countries. Across these studies, the findings on the associations between SSC and biomarkers of stress were inconsistent and highly variable. In terms of the infants' cortisol levels in response to SSC, no statistically significant differences in cortisol levels were found on 2 pre-/posttest design studies33,39 and in 6 RCTs.24–27,30,35 In terms of parents' cortisol levels in response to SSC, in 4 studies34,35,38,39 no significant differences were found, and in 5 31–33,36,37 studies cortisol levels decreased following SSC. In yet another study,32 maternal levels of cortisol dropped 30 minutes following SSC, while paternal cortisol levels rose during the same period.
The reported changes in OT levels among parents and infants were more consistent in 2 studies32,38 that found statistically significant increases followed by significant decreases in OT levels in response to SSC. In a single pilot study,22 allantoin showed promise as a potential biomarker of ATP degradation. The findings from this systematic review support the use of biomarkers in future RCTs to determine the underlying mechanisms for SSC. The addition of other biomarkers (eg, changes in gene expression, metabolomics, and microbiome) may provide new and novel insights into the mechanisms that underlie this important therapeutic intervention for both infants and parents.
Supplementary Material
Footnotes
Supplemental digital content is available for this article. Direct URL citations appear in the printed text and are provided in the HTML and PDF versions of this article on the journal's Web site (www.advancesinneonatalcare.org).
Supported by NINR (T32NR016920).
The authors declare no conflicts of interest.
Contributor Information
Dorothy Forde, Email: dforde78@gmail.com.
Min Lin Fang, Email: Min-Lin.Fang@ucsf.edu.
Christine Miaskowski, Email: christine.miaskowski@ucsf.edu.
References
- 1.Craig BM, Hartman JD, Owens MA, Brown DS. Prevalence and losses in quality-adjusted life years of child health conditions: a burden of disease analysis. Matern Child Health J. 2016;20(4):862–869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bergman NJ. Birth practices: maternal-neonate separation as a source of toxic stress. Birth Defects Res. 2019;111(15):1087–1109. [DOI] [PubMed] [Google Scholar]
- 3.Bystrova K, Ivanova V, Edhborg M, et al. Early contact versus separation: effects on mother-infant interaction one year later. Birth. 2009;36(2):97–109. [DOI] [PubMed] [Google Scholar]
- 4.March of Dimes. Report Card. https://www.marchofdimes.org/mission/reportcard.aspx.
- 5.Sutton PS, Darmstadt GL. Preterm birth and neurodevelopment: a review of outcomes and recommendations for early identification and cost-effective interventions. J Trop Pediatr. 2013;59(4):258–265. [DOI] [PubMed] [Google Scholar]
- 6.Cirulli F, Berry A, Alleva E. Early disruption of the mother-infant relationship: effects on brain plasticity and implications for psychopathology. Neurosci Biobehav Rev. 2003;27(1-2):73–82. [DOI] [PubMed] [Google Scholar]
- 7.Linner A, Westrup B, Lode-Kolz K, et al. Immediate Parent-infant skin-to-skin study (IPISTOSS): study protocol of a randomised controlled trial on very preterm infants cared for in skin-to-skin contact immediately after birth and potential physiological, epigenetic, psychological and neurodevelopmental consequences. BMJ Open. 2020;10(e038938):1–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Conde-Agudelo A, Diaz-Rossello JL. Kangaroo mother care to reduce morbidity and mortality in low birthweight infants. Cochrane Database Syst Rev. 2016;2016(8):CD002771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Campbell-Yeo ML, Disher TC, Benoit BL, Johnston CC. Understanding kangaroo care and its benefits to preterm infants. Pediatric Health Med Ther. 2015;6:15–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Feldman R, Eidelman AI. Skin-to-skin contact (kangaroo care) accelerates autonomic and neurobehavioural maturation in preterm infants. Devl Med Child Neurol. 2003;45(4):274–281. [DOI] [PubMed] [Google Scholar]
- 11.Ferber SG, Makhoull IR. Neurobehavioural assessment of skin-to-skin effects on reaction to pain in preterm infants: a randomized, controlled within-subject trial. Acta Paediatr. 2008;97(2):171–176. [DOI] [PubMed] [Google Scholar]
- 12.Bergman J, Bergman N. Whose choice? Advocating birthing practices according to baby's biological needs. J Perinat Educ. 2013;22(1):8–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Whitelaw A, Sleath K. Myth of the marsupial mother: home care of very low birth weight babies in Bogota, Colombia. Lancet. 1985;1(8439):1206–1208. [DOI] [PubMed] [Google Scholar]
- 14.de Château P, Wiberg B. Long-term effect on mother–infant behaviour of extra contact during the first hour post partum III: follow-up at one year. Scand J Soc Med. 1984;12(2):91–103. [DOI] [PubMed] [Google Scholar]
- 15.Thomson ME, Hartsock TG, Larson C. The importance of immediate postnatal contact: its effect on breastfeeding. Can Fam Physician. 1979;25:1374–1378. [PMC free article] [PubMed] [Google Scholar]
- 16.Ludington-Hoe S. Kangaroo Care: The Best You Can Do to Help Your Preterm Infant. 1st ed. New York, NY: Bantam; 1993. [Google Scholar]
- 17.Casavant SG, Cong X, Fitch RH, Moore J, Rosenkrantz T, Starkweather A. Allostatic load and biomarkers of stress in the preterm infant: an integrative review. Biol Res Nurs. 2019;21(2):210–223. [DOI] [PubMed] [Google Scholar]
- 18.Pados BF, Hess F. Systematic review of the effects of skin-to-skin care on short-term physiologic stress outcomes in preterm infants in the neonatal intensive care unit. Adv Neonatal Care. 2019;20(1):48–58. [DOI] [PubMed] [Google Scholar]
- 19.Cleveland L, Hill CM, Pulse WS, DiCioccio HC, Field T, White-Traut R. Systematic review of skin-to-skin care for full-term, healthy newborns. J Obstet Gynecol Neonatal Nurs. 2017;46(6):857–869. [DOI] [PubMed] [Google Scholar]
- 20.Reynolds LC, Duncan MM, Smith GC, et al. Parental presence and holding in the neonatal intensive care unit and associations with early neurobehavior. J Perinatol. 2013;33(8):636–641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Weber A, Harrison TM, Sinnott L, Shoben A, Steward D. Associations between nurse-guided variables and plasma oxytocin trajectories in premature infants during initial hospitalization. Adv Neonatal Care. 2018;18(1):E12–E23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Forde D, Deming DD, Tan JC, et al. Oxidative stress biomarker decreased in preterm neonates treated with kangaroo mother care. Biol Res Nurs. 2020;22(2):188–196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Feldman R, Rosenthal Z, Eidelman AI. Maternal-preterm skin-to-skin contact enhances child physiologic organization and cognitive control across the first 10 years of life. Biol Psychiatry. 2014;75(1):56–64. [DOI] [PubMed] [Google Scholar]
- 24.Mehler K, Hucklenbruch-Rother E, Trautmann-Villalba P, Becker I, Roth B, Kribs A. Delivery room skin-to-skin contact for preterm infants—a randomized clinical trial. Acta Paediatr. 2020;109(3):518–526. doi:10.1111/apa.14975. [DOI] [PubMed] [Google Scholar]
- 25.Miles R, Cowan F, Glover V, Stevenson J, Modi N. A controlled trial of skin-to-skin contact in extremely preterm infants. Early Hum Dev. 2006;82(7):447–455. [DOI] [PubMed] [Google Scholar]
- 26.Mirnia K, Arshadi Bostanabad M, Asadollahi M, Hamid Razzaghi M. Paternal skin-to-skin care and its effect on cortisol levels of the infants. Iran J Pediatr. 2016;26(5):e5743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.El-Farrash RA, Shinkar DM, Ragab DA, et al. Longer duration of kangaroo care improves neurobehavioral performance and feeding in preterm infants: a randomized controlled trial. Pediatr Res. 2020;87(4):683–688. doi:10.1038/s41390-41019-40558-41396. [DOI] [PubMed] [Google Scholar]
- 28.Kommers D, Broeren M, Oei G, Feijs L, Andriessen P, Bambang Oetomo S. Oxytocin levels in the saliva of preterm infant twins during kangaroo care. Biol Psychol. 2018;137:18–23. [DOI] [PubMed] [Google Scholar]
- 29.Kommers DR, Broeren M, Andriessen P, Oei SG, Feijs L, Bambang Oetomo S. Pilot study demonstrates that salivary oxytocin can be measured unobtrusively in preterm infants. Acta Paediatr. 2017;106(1):34–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Mitchell AJ, Yates CC, Williams DK, Chang JY, Hall RW. Does daily kangaroo care provide sustained pain and stress relief in preterm infants? J Neonatal Perinatal Med. 2013;6(1):45–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Varela N, Tessier R, Tarabulsy G, Pierce T. Cortisol and blood pressure levels decreased in fathers during the first hour of skin-to-skin contact with their premature babies. Acta Paediatr. 2018;107(4):628–632. [DOI] [PubMed] [Google Scholar]
- 32.Cong X, Ludington-Hoe SM, Hussain N, et al. Parental oxytocin responses during skin-to-skin contact in pre-term infants. Early Hum Dev. 2015;91(7):401–406. [DOI] [PubMed] [Google Scholar]
- 33.Mörelius E, Theodorsson E, Nelson N. Salivary cortisol and mood and pain profiles during skin-to-skin care for an unselected group of mothers and infants in neonatal intensive care. Pediatrics. 2005;116(5):1105–1113. [DOI] [PubMed] [Google Scholar]
- 34.Mörelius E, Örtenstrand A, Theodorsson E, Frostell A. A randomised trial of continuous skin-to-skin contact after preterm birth and the effects on salivary cortisol, parental stress, depression, and breastfeeding. Early Hum Dev. 2015;91(1):63–70. [DOI] [PubMed] [Google Scholar]
- 35.Srinath BK, Shah J, Kumar P, Shah PS. Kangaroo care by fathers and mothers: comparison of physiological and stress responses in preterm infants. J Perinatol. 2016;36(5):401–404. [DOI] [PubMed] [Google Scholar]
- 36.Neu M, Hazel NA, Robinson J, Schmiege SJ, Laudenslager M. Effect of holding on co-regulation in preterm infants: a randomized controlled trial. Early Hum Dev. 2014;90(3):141–147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Janevski MR, Maglajić Đukić S. Salivary cortisol as a biomarker of stress in mothers and their low birth weight infants and sample collecting challenges. J Med Biochem. 2016;35(2):118–122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Vittner D, McGrath J, Robinson J, et al. Increase in oxytocin from skin-to-skin contact enhances development of parent-infant relationship. Biol Res Nur. 2018;20(1):54–62. [DOI] [PubMed] [Google Scholar]
- 39.Castral TC, Warnock F, Dos Santos CB, et al. Maternal mood and concordant maternal and infant salivary cortisol during heel lance while in kangaroo care. Eur J Pain. 2015;19(3):429–438. [DOI] [PubMed] [Google Scholar]
- 40.Shorey S, He H-G, Morelius E. Skin-to-skin contact by fathers and the impact on infant and paternal outcomes: an integrative review. Midwifery. 2016;40:207–217. [DOI] [PubMed] [Google Scholar]
- 41.Jansen J, Beijers R, Riksen-Walraven M, de Weerth C. Cortisol reactivity in young infants. Psychoneuroendocrinology. 2010;35(3):329–338. [DOI] [PubMed] [Google Scholar]
- 42.Ivars K, Nelson N, Theodorsson A, Theodorsson E, Stromgren JO, Morelius E. Development of salivary cortisol circadian rhythm in preterm infants. PLoS One. 2017;12(8):1–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Pados BF. Physiology of stress and use of skin-to-skin care as a stress-reducing intervention in the NICU. Nurs Womens Health. 2019;23(1):59–70. [DOI] [PubMed] [Google Scholar]
- 44.Gimpl G, Fahrenholz F. The oxytocin receptor system: structure, function, and regulation. Physiol Rev. 2001;81(2):629–683. [DOI] [PubMed] [Google Scholar]
- 45.McCance KL, Huether SE.Pathophysiology—E-Book: The Biologic Basis for Disease in Adults and Children. St Louis, MO: Elsevier Health Sciences; 2018. [Google Scholar]
- 46.Porges SW, ed. The Polyvagal Theory: Neurophysiological Foundations of Emotions, Attachment, Communication, and Self-regulation. 1st ed. New York, NY; W.W. Norton; 2011. [Google Scholar]
- 47.Farre R, Tack J. Food and symptom generation in functional gastrointestinal disorders: physiological aspects. Am J Gastroenterol. 2013;108:698–706. [DOI] [PubMed] [Google Scholar]
- 48.Van Dam JM, Garrett AJ, Schneider LA, et al. Variability of the cortisol awakening response and morning salivary oxytocin in late adolescence. J Neuroendocrinol. 2018;30(11):e12645. [DOI] [PubMed] [Google Scholar]
- 49.Plank MS, Boskovic DS, Tagge E, et al. An animal model for measuring the effect of common NICU procedures on ATP metabolism. Biol Res Nurs. 2011;13(3):283–288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Kand'ár R, Záková P, Muzáková V. Monitoring of antioxidant properties of uric acid in humans for a consideration measuring of levels of allantoin in plasma by liquid chromatography. Clin Chim Acta. 2006;365(1/2):249–256. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.