Abstract
Single-cell recording, electrolytic lesion and electrical stimulation studies have indicated that the pontomedullary reticular formation (PMRF) plays a role in head movement (HM) control. However, recent studies utilizing excitotoxin lesions of the PMRF have reported no effect on HM. In the present study, we have examined the acute and chronic motor effects of injecting ibotenic acid (IBO) into the nucleus reticularis pontis oralis, nucleus reticularis pontis caudalis and rostral medullary nucleus gigantocellularis of the feline PMRF. IBO injections in all of these regions induced tonic flexion of the head toward the ipsilateral side. This effect lasted 4–16 h. It was followed by a second phase in which head flexion and whole body circling were directed toward the contralateral side. Although this forced contralateral head turning disappeared within two days, the tendency to turn contralaterally and the impaired ability to make rapid ipsilateral HMs were present throughout survival periods lasting more than 4 months. Unilateral IBO PMRF lesions reduced the amplitude of vestibular induced quick phase (anti-compensatory) HMs toward the ipsilateral side and resulted in abnormally large and persistent slow compensatory HMs toward the contralateral side. Following IBO injections, the threshold intensity for HMs evoked by electrical stimulation at the injection site was elevated, and the amplitude and velocity of evoked HMs reduced. Histological data indicated that the reticular area involved in HM control was relatively large and probably extended beyond the PMRF region examined here. However, lesions including the nucleus reticularis pontis caudalis (NRPC) produced more severe and persistent HM deficits than those including the nucleus reticularis gigantocellularis. These data together with available anatomical and electrophysiological evidence indicate that PMRF neurons play a critical role in the generation of fast horizontal HMs toward the ipsilateral side.
Keywords: Ibotenic acid, Pontomedullary reticular formation, Head movement, Vestibular nystagmus, Electrical stimulation
INTRODUCTION
Several lines of evidence implicate the pontomedullary reticular formation (PMRF) in the generation and control of head movements (HMs). It is well known that electrical stimulation of the PMRF induces ipsiversive head turning in various animals3,13,24,32,43,56 Bilateral electrolytic PMRF lesions were reported to produce severe deficits in spontaneous HMs14,28,29. Unilateral electrolytic lesions of the medial PMRF in rats abolished or severely impaired not only spontaneous HMs toward the ipsilateral side, but also the quick phase of vestibular and optokinetic head nystagmus toward the same side51,52. Previous studies in our laboratory on the behavioral correlates of discharge in PMRF neurons in unrestrained cats, have shown that the most common cell type in this area was maximally active in relation to directionally specific HMs47–49. PMRF neurons active in relation to HMs have also been described by others in rabbits11,12, cats16 and monkeys62.
A large number of anatomical and electrophysiological studies have described efferent and afferent connections of PMRF cells appropriate for HM control34. PMRF cells have extensive spinal projections and some make monosynaptic connections with neck motor neurons39. Reticulospinal neurons receive monosynaptic inputs from several HM-related areas including the motor cortex1,30,36, superior colliculus36 and vestibular nuclei35.
Despite this evidence, several studies have questioned the role of PMRF cells in HM control. Massive kainic acid lesions of the feline PMRF were reported to have no effect on head or other movements10,46. Smaller lesions in weanling rats were also reported not to alter motor control57. Electrical stimulation of PMRF regions may produce HM by activation of the descending projections of the tectospinal, rubrospinal, vestibulospinal or other descending pathways passing through or adjacent to the PMRF. Motor deficits after electrolytic lesions could be due to the interruption of these fibers of passage. It has been speculated that the apparent HM correlates of PMRF cells were secondary to an underlying relation to eye movements5. Furthermore, the kinds (voluntary, vestibularly induced, etc.) and parameters (direction, amplitude, velocity, etc.) of HMs controlled by PMRF neurons are unknown.
We approached these questions by investigating the acute and chronic effects, on spontaneous and evoked HMs, of unilateral or bilateral microinjections of the excitotoxin, ibotenic acid (IBO), into the nucleus reticularis pontis oralis (NRPO), nucleus reticularis pontis caudalis (NRPC) and rostral medullary nucleus gigantocellularis (NGC) of the PMRF. IBO, a naturally occurring glutamate analog, is known to cause initial hyperexcitation and subsequent destruction of neuronal cell bodies while sparing fibers of passage and nerve terminals7.
MATERIALS AND METHODS
Surgery
Two groups of adult cats (2.7–4.3 kg) were used. One group (Group A) underwent the electrode-cannula implantation surgery before any IBO injection, while the other group (Group B) received unilateral IBO injections in the first surgery. Two of the Group B cats had a subsequent implantation surgery performed. Under pentobarbital anesthesia (35 mg/kg, i.p.), cats were implanted chronically with (1) cannula-electrode assemblies into the PMRF, (2) EMG wires into neck muscles, and (3) screw electrodes for electrooculogram (EOG) and electroencephalogram (EEG) recording. The guide cannulas were made of 24 gauge stainless-steel tubing, 25 mm in length. The guide cannulas allowed the placement of lesions in unanesthetized animals, and minimized cerebellar damage at the time of reticular lesions. A stainless steel macroelectrode (150 μm diameter, tip sharpened) or an etched microelectrode (A-M systems, no. 5170, 5 MΩ at 1 kHz) was glued to the side of each guide cannula so that the electrode tip was located 10 mm below the guide cannula tip. Cannula-electrode assemblies were stereotaxically implanted into the PMRF (co-ordinates for electrode tip targets, P3 to 8, L1.5, H–5 to −7.5) either unilaterally or bilaterally. A 25 mm stylet made from 250 μm diameter tungsten rod was inserted into each guide cannula to maintain patency. For bipolar EMG recording, pairs of multi-stranded stainless-steel wires (Cooner Wire Co., CA518-30), deinsulated for 1–3 mm, were sewn into each of 3 neck muscles (splenius, biventer cervicis, complexus) bilaterally. The distance between the two deinsulated wires within a muscle was 2–5 mm. Stainless-steel screws were placed mediolaterally into the supraorbital bone for AC EOG recording. DC EOG recording was performed with bitemporal skin electrodes (silver/silver chloride, Beckman no. 217412). Screws were placed over the sensorimotor cortex to monitor EEG. The exposed portion of the cannulas was enclosed and protected by a hollow plastic cylinder (19 mm diameter), the base of which was fixed to the skull with acrylic. The top of the cylinder was threaded so that a matching plastic cap could be secured.
IBO injection
IBO (Sigma) was dissolved in 0.1 M phosphate buffer (pH = 7.4) to a concentration of 10 μg/μl. Except for the acute injection procedure in Group B cats, the unanesthetized animal was placed in a cloth bag and IBO was injected through the implanted cannulas. A mild anesthetic dose of ketamine (20 mg/kg, i.m.) was administered if adverse reactions such as continuous whole body rolling were observed. A 29 gauge injection cannula, connected to a 10 μl microsyringe with Teflon tubing, was inserted through the guide cannula. The injection cannula had a 2 mm long ‘stop’, made from a 24 gauge cannula soldered to its dorsal end to position its tip at the stereotaxically calculated target site. A 4 mm length of polyethylene tubing held the injection cannula to the guide cannula during the injection procedure. At each site, 1 μl IBO was slowly infused over 10 min, and the injection cannula was withdrawn 10 min after the end of the infusion. In Group B cats, the acute injection surgery was performed under halothane anesthesia. The cat was placed in a stereotaxic instrument and a small hole drilled into the skull over the cerebellum. The IBO injection procedure was the same as above, except that the injection cannula, held in a stereotaxic holder, was inserted without the use of guide cannulas. When there was more than one injection session per animal, two successive sessions were separated by at least a week. As a control procedure, a 1 μl vehicle solution was injected into one site in two unanesthetized cats and into two sites in one anesthetized cat. Because we have found cells with similar ipsilateral head movement relations in NRPO, NRPC, and NGC49, we placed lesions, and monitored the acute effects of the ibotenic acid injections, at each of these sites (P5, L1.5, H–6.0; P6, L1.5, H–6.0; P7, L1.5, H–6.5; P8, L1.5, H–6.5).
General observations
The animal was observed during and after IBO injection. After being released from the bag, the cat was monitored in a 3.7 × 4.2 m enclosed area. Foods favored by the cat were held with forceps and moved in the horizontal plane to elicit HM. Spontaneous behaviors were observed with the experimenter out of the observation area. Videotaping was employed in the first 48 h post-IBO, to document changes in the cat’s behavior. Supraorbital EOG, EEG, and neck electromyograph (EMG) were recorded on a Grass polygraph (Model 78D) for 1 h before injection, for the first 12 h after injection and for 2 h periods at 12, 24, 48 and 72 h after injection. Further recordings were made at weekly intervals until sacrifice.
Nystagmus test
The nystagmus test was performed before and at least two days after IBO injection. The cat was first put in a cloth bag which was secured to a large turntable (100 cm diameter) such that the cat’s head was located in the center. The table was rotated to an angular velocity of 250°/s in 3 s. After 10 rotations the table was braked to a complete stop in 1 s. Pilot data from normal cats indicated that, with these rotation parameters, both per- and post-rotatory nystagmus of the head and eyes could be induced reliably. Clockwise and counter-clockwise rotation trials were run alternately for a total of 20 trials per session with 60 s between trials. During the nystagmus test, the cat was videotaped through a camera placed above the turntable. Videotaped data were analyzed frame by frame (30 frames/s) to obtain head angular deviations (HADs) in the horizontal plane before, during and after rotation. Video analysis was found to be inadequate to examine the quick phase of head nystagmus because of its high frequency (~5 Hz) and low amplitude (<10°). We recorded nystagmus HMs by means of an accelerometer (Kistler Instruments, Model 815A5) attached to the cat’s head 6 cm anterior to the atlanto-axial joint. The cylindrical accelerometer (14 mm in diameter, 34 mm in length, 14 g in weight) was positioned so as to respond to horizontal acceleration of the head. The accelerometer had a sensitivity of 48 mV/g and a linear response between 4 Hz and 6 kHz.
Recording of head and eye movement
Spontaneous HMs and HMs evoked by PMRF stimulation were recorded with a system we developed using Hall generators (F.W. Bell, Inc., FH-540). The system is described in detail elsewhere33. The Hall generator produces an output voltage proportional to the sine of the angle between its sensitive plane and the magnetic field generated by a high power, cobalt-samarium magnet (Edmond Scientific, 14 × 15 × 58 mm, 100 g) attached to the cat’s headplug (6 cm anterior to the atlanto-axial joint). The cat was put in the cloth bag and then gently secured such that the magnet was centered between the two Hall generators attached to each side of a wooden frame (34 × 34 cm). The Hall device output was amplified, digitized and arcsine transformed by an LSI-11 (MINC) computer. The computer also generated the first derivative (i.e. horizontal angular velocity, HAV) of HAD. In the Group A cats, spontaneous HMs before and after IBO injection were compared.
Horizontal DC EOG was recorded from a pair of skin electrodes attached to the outer canthi (see above). EOG calibration was obtained by moving the cat’s head in the horizontal plane at 0.5–2 Hz with an amplitude of 20–40° (measured by the Hall Effect device) and by recording the vestibulo-ocular reflex (VOR), which is known to have a near unity gain at this frequency and amplitude range in the alert cat9. Head acceleration, velocity and displacement, EOG and EMG were recorded simultaneously on a Grass polygraph (Model 78D) and on magnetic tape.
Electrical stimulation
Constant-current, rectangular cathodal pulses of 0.2 ms duration were delivered monopolarly through implanted PMRF electrodes. The anodal electrode was a skull screw. In an initial stimulation session, one-second trains of 400 Hz pulses were applied to the unrestrained cat to observe evoked movements and their threshold intensities. Evoked horizontal HMs were recorded by means of the Hall device. At each stimulation site, several intensities and durations (250 ms, 500 ms, 750 ms, 1 s) of 400 Hz stimulation were applied 10 times at each intensity–duration combination. The stimulation data were obtained before and at least two days after an IBO injection at the stimulation site. Stimulation data from intact sites contralateral to the IBO injection site were also examined.
Histology
After the experiment, the cats were deeply anesthetized with sodium pentobarbital and iron deposited at each stimulation electrode tip by passing 20 μA anodal DC current for 20 s. The animals were then perfused through the left ventricle with 0.9% saline and a 5% potassium ferrocyanide solution followed by 10% formalin. The brains were removed, sectioned coronally and stained with Cresyl violet or Carbolfuchsin red. Selected sections were also stained for fiber tracts with the Weil method.
RESULTS
Ibotenic acid lesions
Data were derived from 6 cats, 2 belonging to Group A (L-1, ST-5) and 4 to Group B (IB-2, IB-3, IB-5, IB-7). Cats (IB-2, IB-3) from Group B underwent the cannula-electrode implantation surgery as described above.
IBO (10 μg/μl) injected into the PMRF typically produced a spheroidal lesion extending 2–2.5 mm mediolaterally and rostrocaudally, and 2.5–3 mm dorsoventrally. As shown in Fig. 1, an IBO-induced lesion was characterized by a marked loss of neuronal cell bodies and a proliferation of glial cells. Although both of these characteristics were present in a sample with the shortest survival time (two weeks), the latter characteristic was more pronounced in samples with longer survival times. Injections of the phosphate buffer vehicle did not produce any lesions beyond the cannula tracks.
Fig. 1.
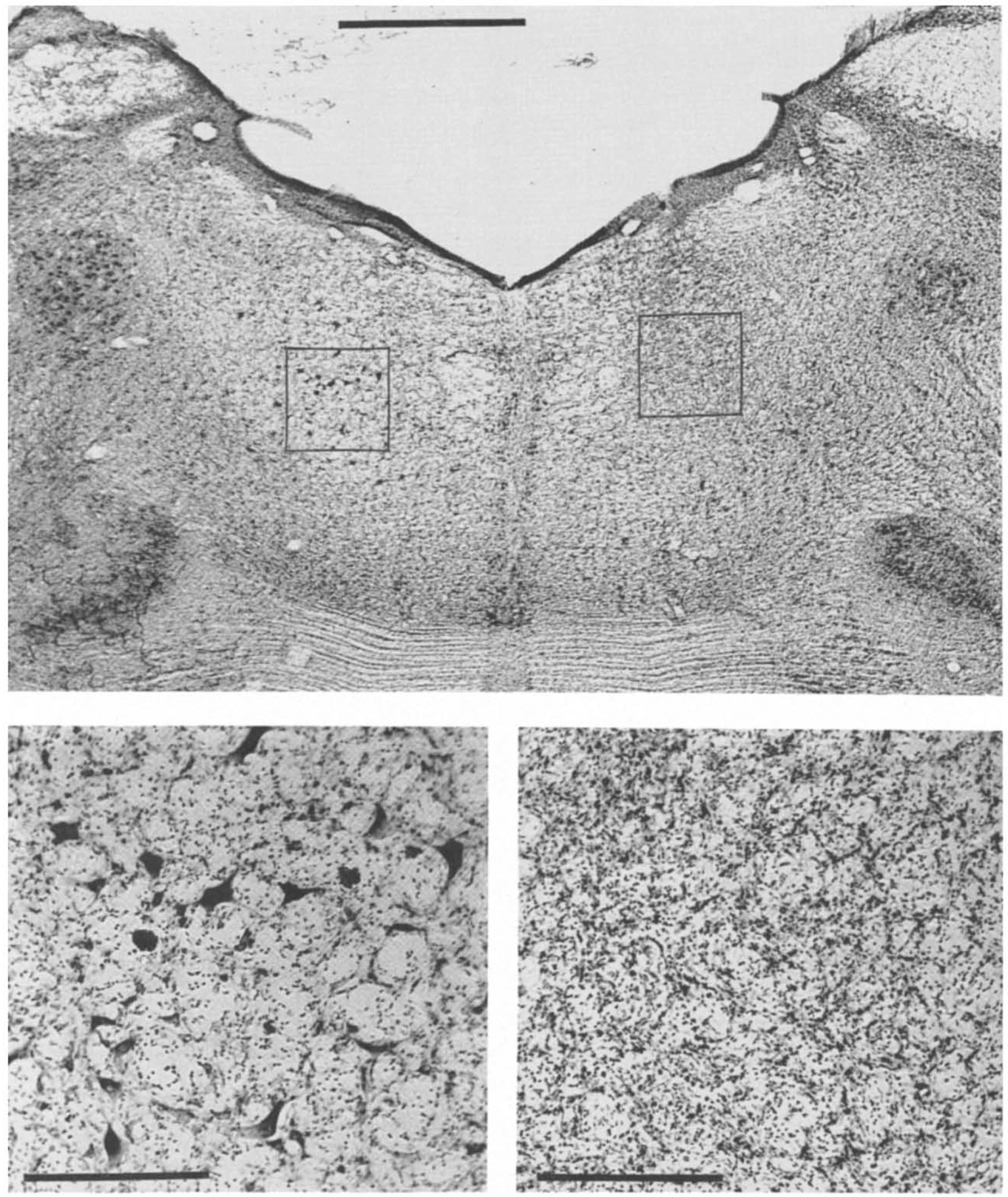
Photomicrographs of coronal sections (Cresyl violet stain) of pontine reticular formation showing IBO-lesioned (right on figure, left in brain) side and intact (left) side in cat IB-2. Bottom two sections are magnifications of lesioned and intact parts indicated on top section. Scale bar is 4 mm for top section and 0.2 mm for bottom sections.
The extent of IBO-induced lesions in five cats is schematically presented in Fig. 2. (The remaining cat, IB-5, had only vehicle injections.) The mediolateral and dorsoventral extents were relatively similar in separate lesions. As planned, the A–P levels of the lesions ranged from P2 to P8. Lesions were placed in the caudal NRPO and/or NRPC in the left sides of L-1, IB-2, IB-3, and IB-7, and both sides of ST-5. Lesions were centered in the rostral portions of the nucleus reticularis gigantocellularis (NRGC) in the right side of L-1 and IB-3. The abducens nucleus was damaged bilaterally in L-1 and unilaterally in IB-7. The nucleus praepositus hypoglossi was damaged unilaterally in IB-7, but was spared in the other cats. The medial and lateral vestibular nuclei and medial longitudinal bundle were not damaged. The dorsal tegmental nucleus was damaged bilaterally in ST-5. Therefore, the common areas with extensive damage included only NRPO, NRPC and rostral NGC.
Fig. 2.
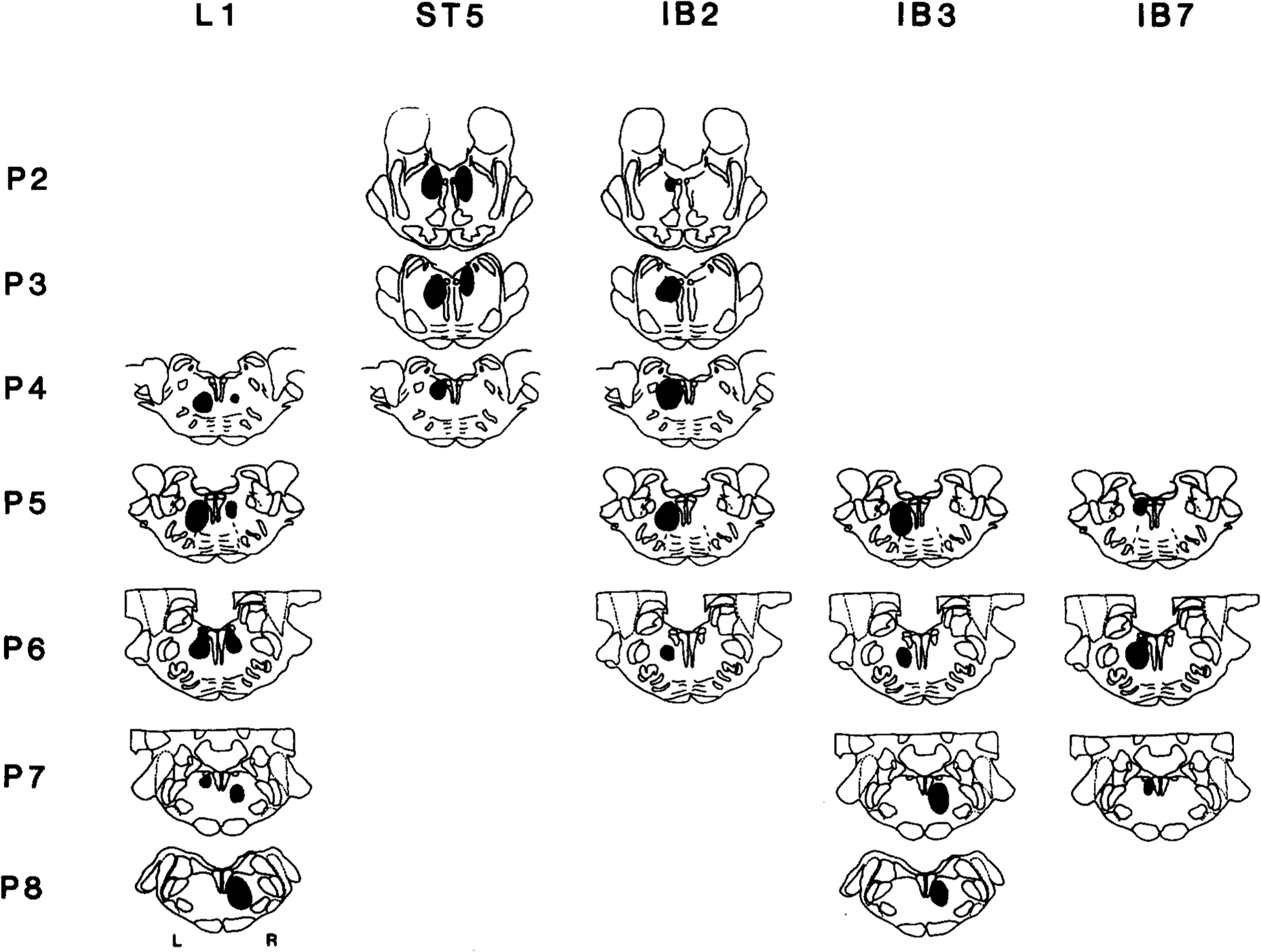
Schematic representations of coronal brain stem sections showing IBO-induced lesions in each cat. Black regions were free of neuronal somas.
General descriptions of acute and chronic effects
Acute effects were observed following each of 6 IBO injections in 3 unanesthetized cats (L-1, at R-P6 (right-posterior 6), R-P8 and L-P8; ST-5 at L-P3 and R-P3; IB3 at R-P7). Despite the A–P range of the injection site, these injections produced fundamentally similar acute effects. In all cases, the most conspicuous effect of IBO injection into the PMRF was tonic flexion of the head to the ipsilateral side. This effect appeared during or immediately after IBO injection and lasted 4–16 h. Fig. 3A presents a typical head posture following IBO injection to the right PMRF. During maximal head flexion, which occurred within one hour after IBO injection, head angular deviation (HAD) in the horizontal plane was 120–150° toward the ipsilateral side. Head flexion was often accompanied by turning and circling in the same direction. In ST-5, following injection into L-P3 or R-P3, initial horizontal head twisting gradually developed into a head posture with a strong roll component in the vertical plane. In the case where IBO was injected to the right side (ST-5 at R-P3), the cat’s head was tilted such that its chin was pointed to its left shoulder after the initial ipsilateral flexion. The contralateral forelimb and sometimes hindlimb were extended, as if to prevent rolling toward the ipsilateral side. However, when such head tilting became severe, the cat rolled on the floor in the ipsilateral direction. This happened to Cat ST-5 at about 30 min after IBO injections to L-P3 and R-P3. On both occasions ketamine (20 mg/kg, i.m.) was injected and it stopped such whole body rolling. However, even under ketamine, the typical head/body posture was maintained, though to a much weaker degree. Cats rarely vocalized or showed any other indications of distress after injection. These acute effects were also observed in milder form in the initially anesthetized cat when recovering from anesthesia (IB-2 at L-P3 and L-P5; IB-3 at L-P5; IB-7 at L-P6) (see Fig. 3B, left).
Fig. 3.
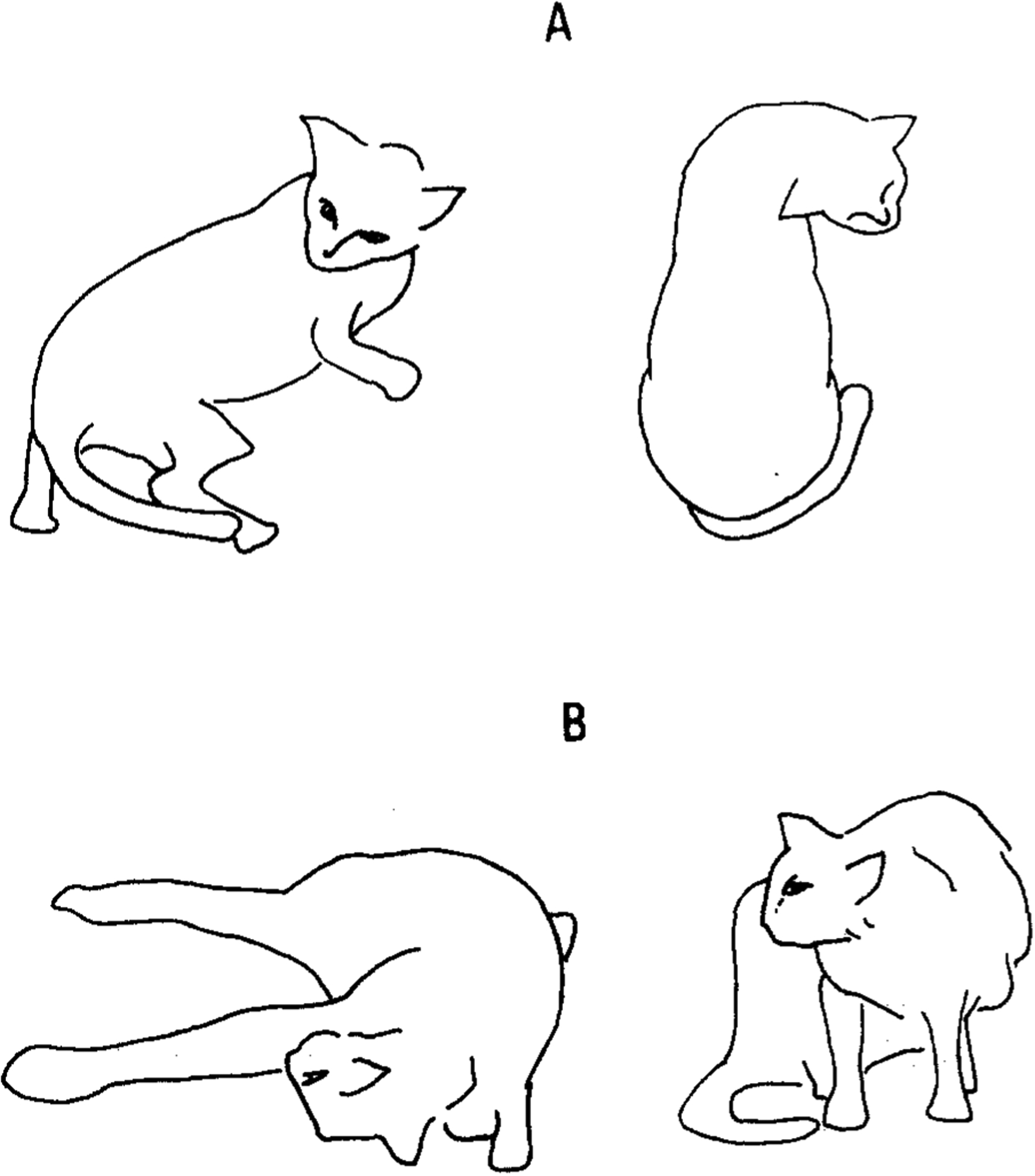
Drawings made from videotapes showing acute effects of IBO injections into pontomedullary reticular formation on head and body posture in unanesthetized cat (IB-3), and in halothane-anesthetized cat (IB-2). A: 30 min after IBO injection into R-P7 (right posterior 7, cat IB 3). Lateral view (A, left) and top view (A, right) show relatively pure horizontal head flexion to ipsilateral side. B, left: 15 min after IBO injection into L-P3 and L-P5 (cat IB2), and 5 min after removal from stereotaxic instrument and cessation of halothane administration (Acute phase I). Note that (1) chin is directed toward right shoulder even while contractions occur mainly in left neck muscles, and (2) contralateral fore- and hindlimbs are extended. B, right: 15 h after IBO injections (Acute phase II). Head is mildly twisted to contralateral (right) side.
The above syndrome (tonic ipsilateral head flexion, Acute Phase I) disappeared within 4–16 h after IBO injection and was followed by a second acute syndrome in which head flexion and whole body circling were directed toward the contralateral side (Acute Phase II). This effect was observed in all the cats given IBO with or without anesthesia. During the maximal effect, which occurred within 6 hours after the cessation of Acute Phase I, the cat’s head was tonically flexed to the contralateral side with HAD of 60–120° (see Fig. 3B, right). This head posture was a mirror image of the Acute Phase I head posture, though milder than the latter. The Acute Phase II syndrome gradually weakened and disappeared within 48 h after IBO injection.
These observations are consistent with EMG data. Fig. 4 shows polygraphic recordings of EEG, EOG and EMGs from the 3 neck muscles before and various times after injection (Acute Phase I), when the cat’s head was flexed to left with elevated EMGs in the left neck muscles. At 42 h after injection, when the Acute Phase II syndrome disappeared, neck EMGs were bilaterally balanced at rest.
Fig. 4.
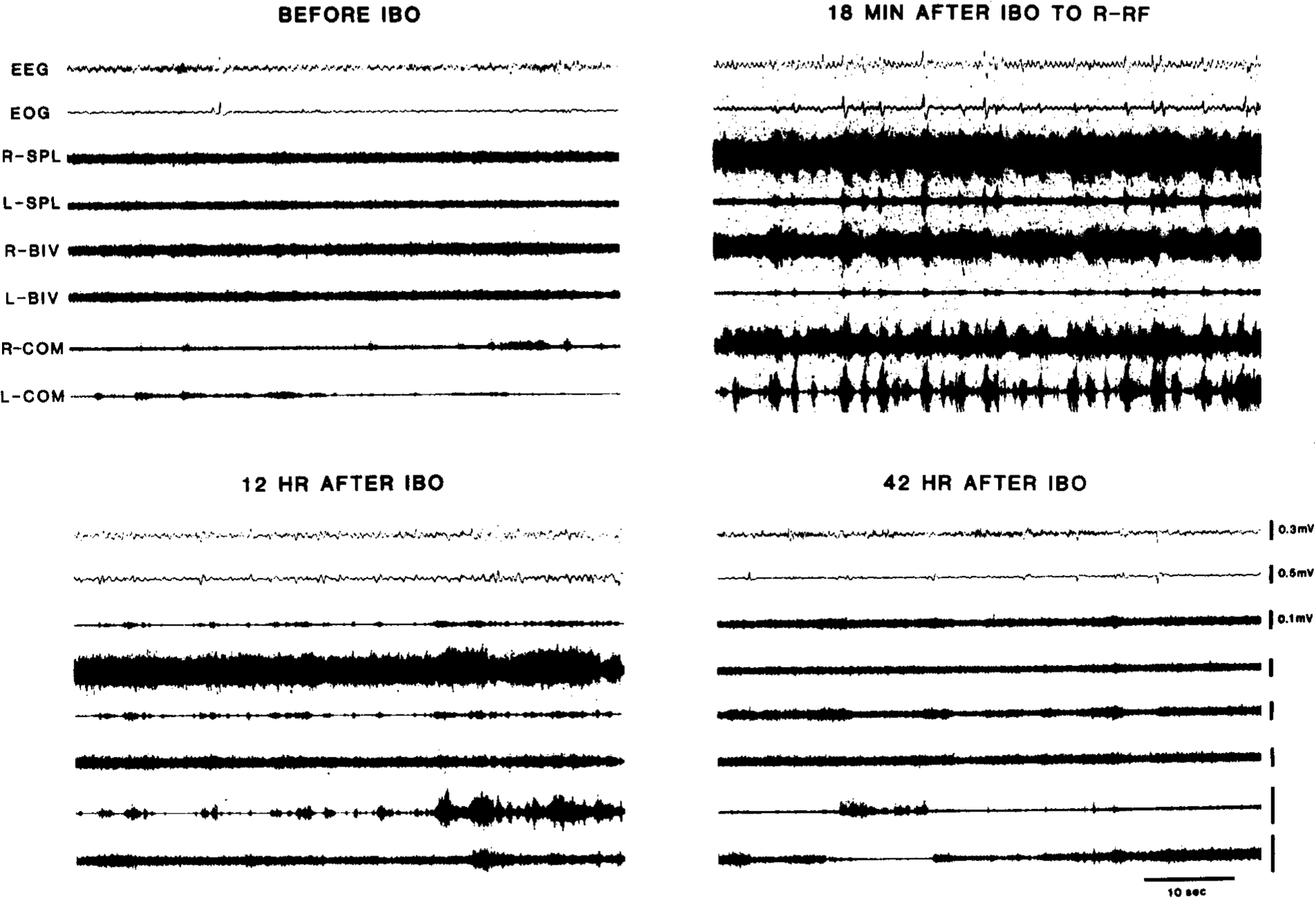
Polygraphic records of EEG, EOG, and EMGs from 3 neck muscles before and various times after unilateral IBO injection into R-P3 in unanesthetized cat (ST-5). Note that after IBO injection, excitation of ipsilateral neck muscles (top right, Acute phase I) is followed by that of contralateral muscles (bottom left, Acute phase II) and then by normal EMGs (bottom right, Chronic phase). R, right; L, left; SPL, splenius; Biv, biventer cervicis; Com, complexus. Voltage calibration for R-SPL also applies to other EMG traces.
Although a relatively normal head posture returned within 48 h after unilateral IBO injection, the tendency to turn contralaterally and the impaired ability to make rapid HMs to the ipsilateral side were observed in all cases with various degrees of severity and persistence (Chronic Phase). In the most severe case (IB-2) with large lesions located mainly in NRPC, the deficits did not diminish during the survival period lasting more than 4 months. This cat could not turn its head voluntarily beyond the midline to the left side when food was presented to that side. In such a situation the animal often made a complete circle to the right to obtain the food. In cat IB-7 with a smaller and more caudal lesion, HM deficits were milder and less persistent. The food presentation tests given to this cat during the first post-injection week revealed a mild deficit, in that the animal could move its head to the lesioned side even though more slowly and awkwardly than to the intact side. However, this deficit was no longer obvious in subsequent weeks. The chronic effects of unilateral lesions were also observed in the other cats (L-1 at R-P6 and R-P8, ST-5 at L-P3, IB-3 at L-P5) before they received lesions to the other side. In these cases, the rostral lesions produced more severe and persistent deficits than the caudal lesions. In ST-5 and IB-3 the second lesion was made to the contralateral side (right) when the effects of the first lesion to the left side were still evident (46 and 81 days after the first lesion, respectively). The acute effects of the second IBO injection were similar to those described above, though the Acute Phase II syndrome was weaker than after unilateral IBO injection. Following the acute effects, both cats showed bilateral head movement deficits. Namely, they were deficient in head turning not only to the first lesioned side (left) but also to the second lesioned side (right). However, probably due to the asymmetry in size (ST-5) or A-P level (IB-3) of the bilateral lesions, the chronic HM deficits were larger to the left than to right in both cases. The acute and chronic effects of IBO injection into the PMRF are summarized in Table I.
TABLE I.
A summary of the effects of unilateral IBO injections into the pontomedullary reticular formation (PMRF) on head posture and movement
| Phase | Time after IBO | Syndrome | Hypothesized cause |
|---|---|---|---|
| Acute I | 0–16 h | Forced head twisting to ipsilateral side | Hyperexcitation of affected PMRF cells controlling ipsilateral neck motor units |
| Acute II | 4–48 h | Mildly forced head twisting to contralateral side | Imbalance of neck muscle activity due to inactivation of ipsilateral cells |
| Chronic | 2 days permanent | Loss of rapid ipsilateral head turning, tendency to turn contralaterally, and relatively normal head posture at rest | Degeneration of affected PMRF cells controlling ipsilateral neck motor units. Compensatory changes in remaining systems preventing abnormal postures |
| Recovery | 1 week | Seen only after small lesions. Recovery of posture and head movement abilities | Compensatory changes in remaining PMRF neurons and postsynaptic cells. |
Effects on vestibular head nystagmus
In the vestibular test before IBO injection, no asymmetry in head movement or posture was observed between clockwise and counterclockwise rotation. The tonic compensatory head posture (HAD) was 50–90° away from the rotatory direction during rotation, and toward the rotatory direction after rotation. The postrotatory compensatory HAD reached its maximum within 2 s and gradually declined to baseline (zero) then shifted to the other direction within 20 s. Table II summarizes data from 4 cats on the duration of post-rotatory compensatory head posture before and after IBO lesions of the PMRF. Following unilateral lesions in the left PMRF, this duration was significantly longer (HAD to left) in all the cats examined. Further lesions to the contralateral side (right) in two cats eliminated this asymmetry.
TABLE II. Duration of postrotatory compensatory head posture before and after ibotenic acid lesions of the pontomedullary reticular formation.
Each cat was rotated on a turntable as described in Materials and Methods. The duration of post-rotatory compensatory head posture defined as a horizontal angular deviation (HAD) of the head toward the direction of rotation was measured. CW and CCW refer to clockwise rotation and counter-clockwise rotation, respectively. The post-rotatory HAD is to right after CCW and to left after CW. Numberszz indicate mean durations (n = 10) in seconds and those in parentheses, ranges.
| Cat | Before lesion | After 1st unilateral lesion (left) | After 2nd lesion to contralateral side | ||||
|---|---|---|---|---|---|---|---|
| CW | CCW | CW | CCW | CW | CCW | ||
| ST-5 | 15.9 | 16.3 | 29.3 | 9.0 | 13.7 | 15.7 | |
| (13–23) | (9–23) | (14–51) | * | (5–13) | (7–19) | (8–20) | |
| IB-2 | – | – | 30.3 | * | 10.0 | – | – |
| (10–75) | (4–16) | ||||||
| IB-3 | 5.8 | 5.8 | 80.3 | * | 16.5 | 26.5 | 27.8 |
| (2–11) | (4–8) | (10–120) | * | (3–29) | (7–63) | (18–57) | |
| IB-7 | – | – | 18.5 | * | 4.7 | – | – |
| (11–65) | (2–12) | ||||||
Significantly different from each other at P < 0.01 by the Mann–Whitney U-test.
Unilateral lesions increased not only the duration of compensatory HAD to the intact side, but also its amplitude during and after rotation. This is illustrated in Fig. 5 where HADs before, during, and after clockwise/counterclockwise rotation in two cats (ST-5, IB-2) are plotted. In both cases the HAD during rotation was 100–140° to the intact side and 40–80° to the lesioned side. After rotation the HAD was also much larger (and persisted longer) to the intact side than to the lesioned side.
Fig. 5.
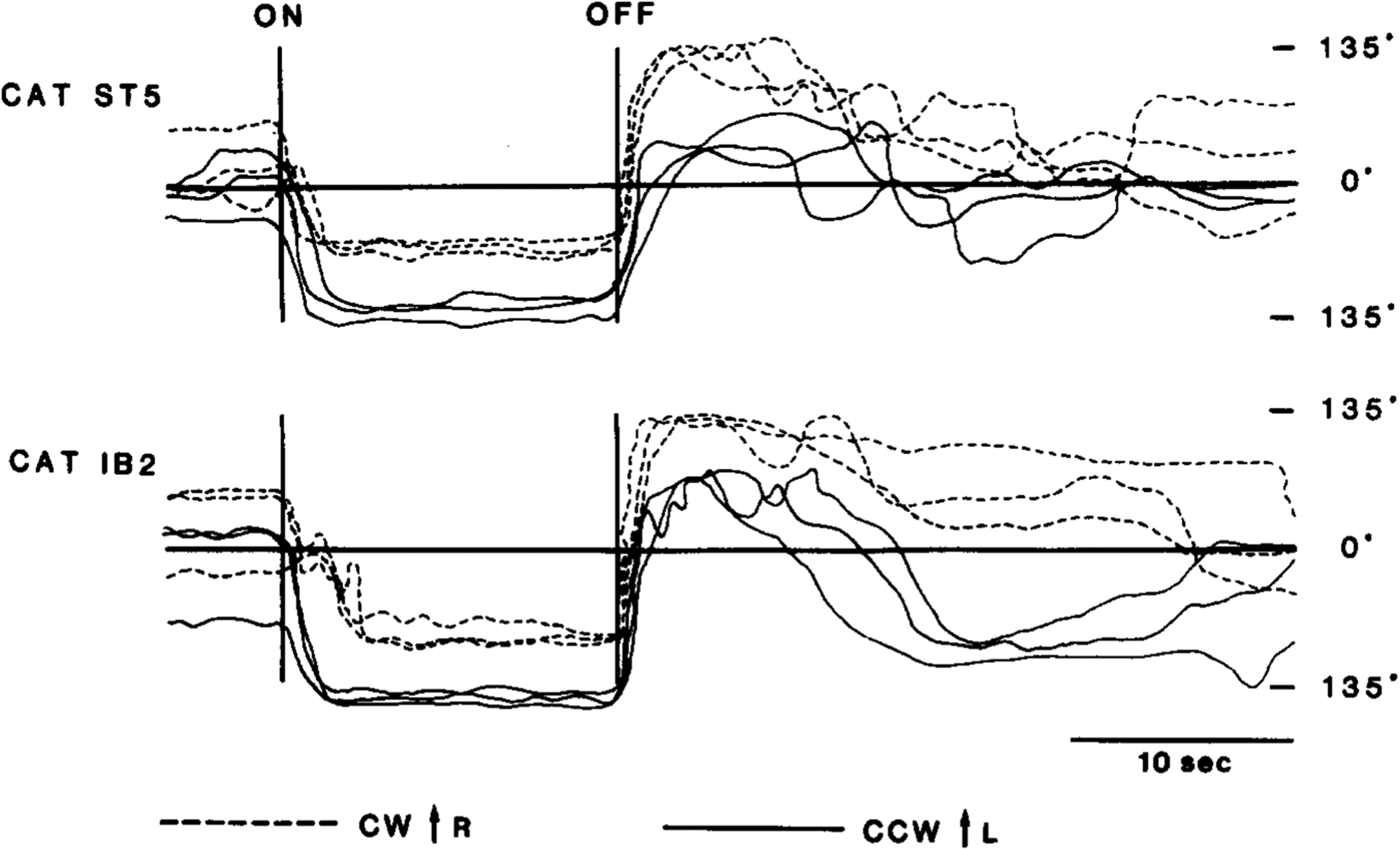
Horizontal head angular deviations (HADs) during and after clockwise and counterclockwise rotations in two cats with IBO-induced lesions of left pontomedullary reticular formation. HADs are much larger toward contralateral side than toward ipsilateral side during counterclockwise rotation and after clockwise rotation. For comparison HADs to right are plotted upward for clockwise rotation but downward for counterclockwise rotation. ‘On’ and ‘Off’ indicate onset and offset of rotation, respectively. CW, clockwise rotation; CCW, counterclockwise rotation.
In the vestibular nystagmus test before IBO injection, clockwise and counterclockwise rotations produced no asymmetry in anticompensatory, quick-phase HMs during or after the rotations. These HMs, recorded with the accelerometer attached to the head, were nystagmic in that they were coordinated with nystagmic eye movements. As shown in Fig. 6, unilateral lesions of the left PMRF greatly reduced the magnitude of anticompensatory HMs toward the lesioned side during counterclockwise rotation and after clockwise rotation. On the other hand, relatively normal anticompensatory HMs were observed to the intact side during clockwise rotation and after counterclockwise rotation. As was the case with the lesion effects on spontaneous HMs, rostral lesions (ST-5, IB-2) appeared to produce more severe and persistent deficits in quick-phase movements of the eyes toward the ipsilateral side, to various degrees depending on the location and extent of the lesions. Lesions involving the periabducens area (IB-2, IB-3, IB-7) were more effective than other lesions (ST-5 (see Fig. 7)).
Fig. 6.
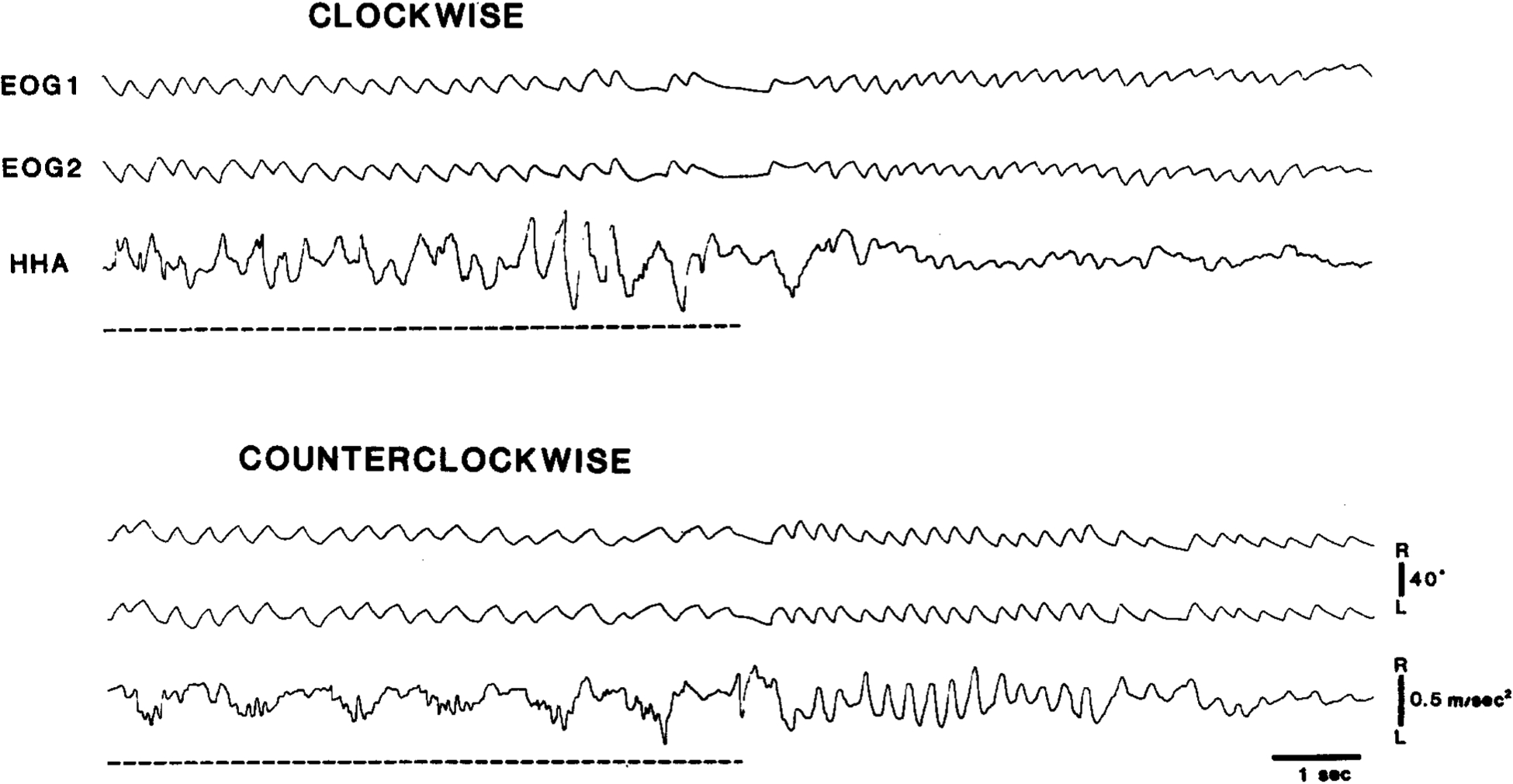
Differential effects of unilateral lesion (L-P3) of pontine reticular formation on eye and head nystagmus (cat ST-5). Quick-phase head movements to ipsilateral side (left – up on record) during counterclockwise rotation and after clockwise rotation are greatly reduced, while quick-phase eye movements are less dramatically affected by lesion. Interrupted lines indicate periods of rotation. EOG 1, bitemporal DC EOG; EOG 2, supraorbital AC EOG with time constant of 0.3 s; HHA, horizontal head angular acceleration.
Fig. 7.
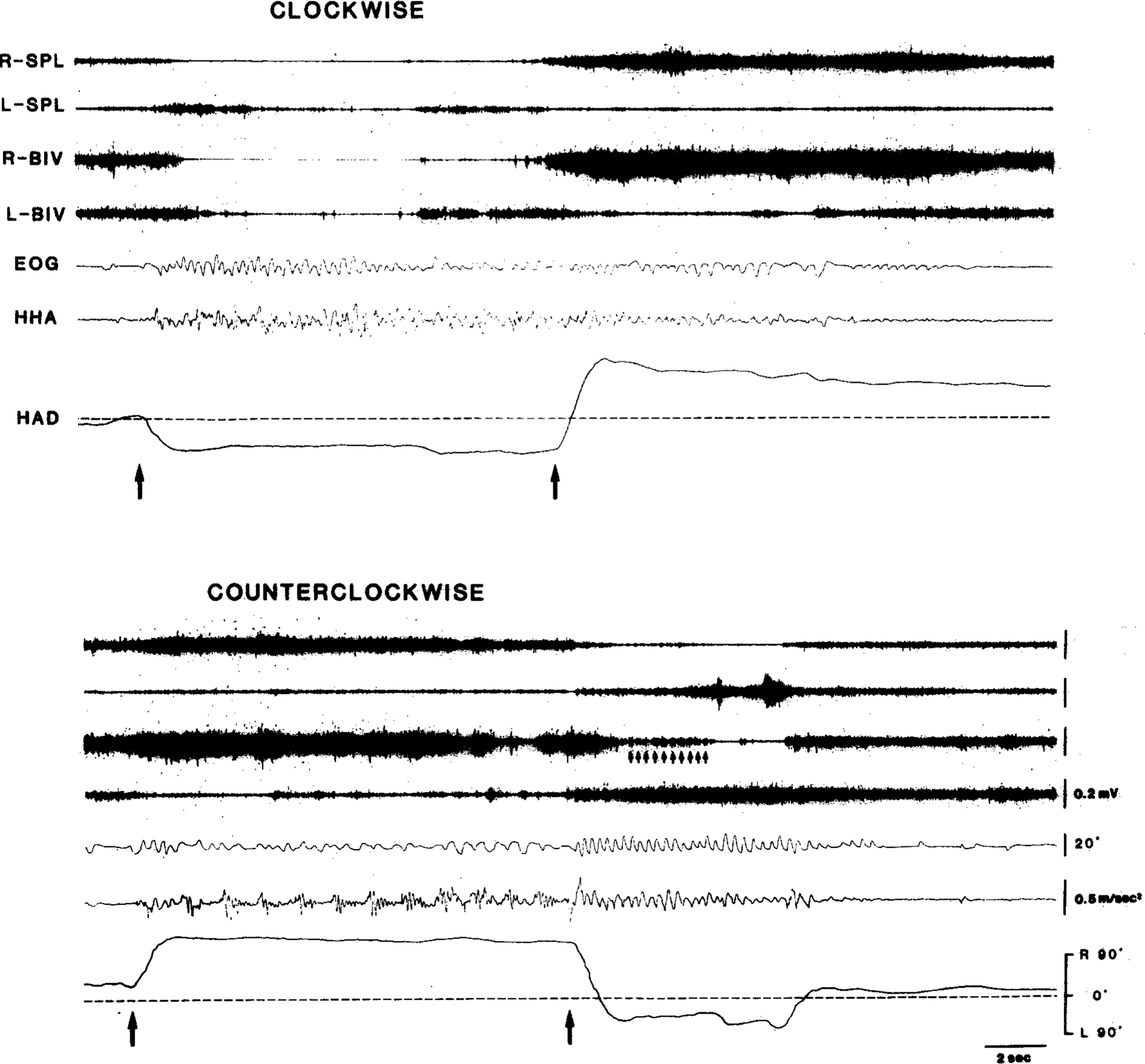
Polygraphic records of horizontal per/postrotatory eye (AC EOG) and head movement after unilateral lesions of the left pontine reticular formation (cat IB-2). Cat on turntable was rotated during period indicated by the two arrows. Note that quick-phase anticompensatory movements of eyes and head toward ipsilateral side (left) during counterclockwise rotation are much weaker than those toward contralateral side during clockwise rotation and after counterclockwise rotation. Also, note that HAD representing compensatory head posture is abnormally large toward contralateral side during counterclockwise rotation and after clockwise rotation. Small arrows in bottom panel indicate EMG bursts corresponding to postrotatory nystagmic, quick-phase eye-movements toward intact side. Upward deflections in both EOG and HHA traced indicate movements to right. HHA, horizontal head angular acceleration; HAD, horizontal head angular deviation.
EMG data as shown in Fig. 7, suggest that the reduction in quick-phase HMs to the lesioned side is causally related to the increased amplitude and duration of compensatory HMs to the intact side. Namely, in the cat with unilateral lesions of the left PMRF, EMGs in the right neck muscles were tonically elevated after clockwise rotation. On the other hand, after counterclockwise rotation, normal nystagmic contractions of the right neck muscles rapidly centered the head which had been flexed to the lesioned (left) side. The fact that the cat could move the head to the lesioned side in this situation demonstrated that the vestibularly induced slow-phase HMs were relatively unaffected by the PMRF lesions.
Effects on electrically-evoked head movements
Ten PMRF sites in three cats (ST-5, IB-2, IB-3) were electrically stimulated with 250–1000 ms trains of 400 Hz pulses. Stimulation of 8 sites evoked ipsiversive HMs at intensities less than 100 μA. Threshold intensities were 10–20 μA. The amplitude and velocity of evoked HMs were measured by means of the Hall device before and after IBO injections into the vicinity of 3 stimulation sites. In all cases, evoked HMs were reduced in amplitude and velocity following IBO-induced lesions of local neuronal somata. Fig. 8 depicts representative polygraphic data showing HAD, HAV, EOG and bilateral EMGs from 3 neck muscles before and 8 days after the IBO injection into the stimulation site. Fig. 9 presents the amplitude of HMs as a function of stimulus intensities, before and after IBO lesion of the stimulation site shown in Fig. 8. It is clear that the stimulation effect was greatly reduced following the neurotoxin injection. On the other hand, HMs evoked by control stimulation at the contralateral (intact) site were not altered appreciably by the unilateral lesion.
Fig. 8.
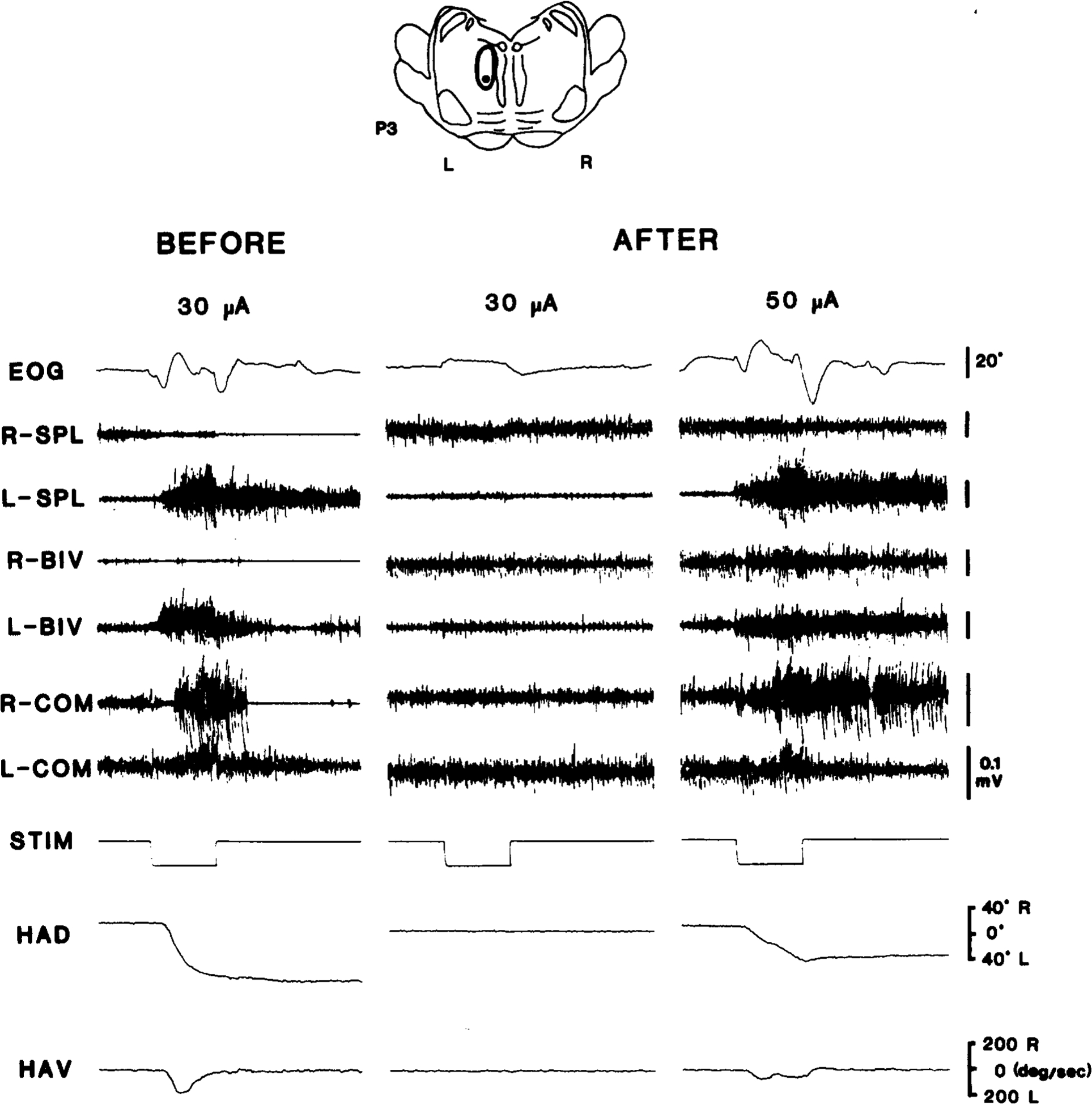
Effects on electrically evoked head movements of IBO-induced lesion (L-P3) of stimulation site (cat ST-5). Stimulation site (dot) and lesioned area (encircled) are indicated on coronal section of pontine reticular formation (top). Bottom two panels are polygraphic records of electrically evoked head movements showing EOG, neck EMGs, HAD and HAV before and eight days after IBO lesion of stimulation site. Stimulation (STIM) were 1 s trains of 400 pulses (30 μA). HAD, horizontal head angular deviation; HAV, horizontal head angular velocity.
Fig. 9.
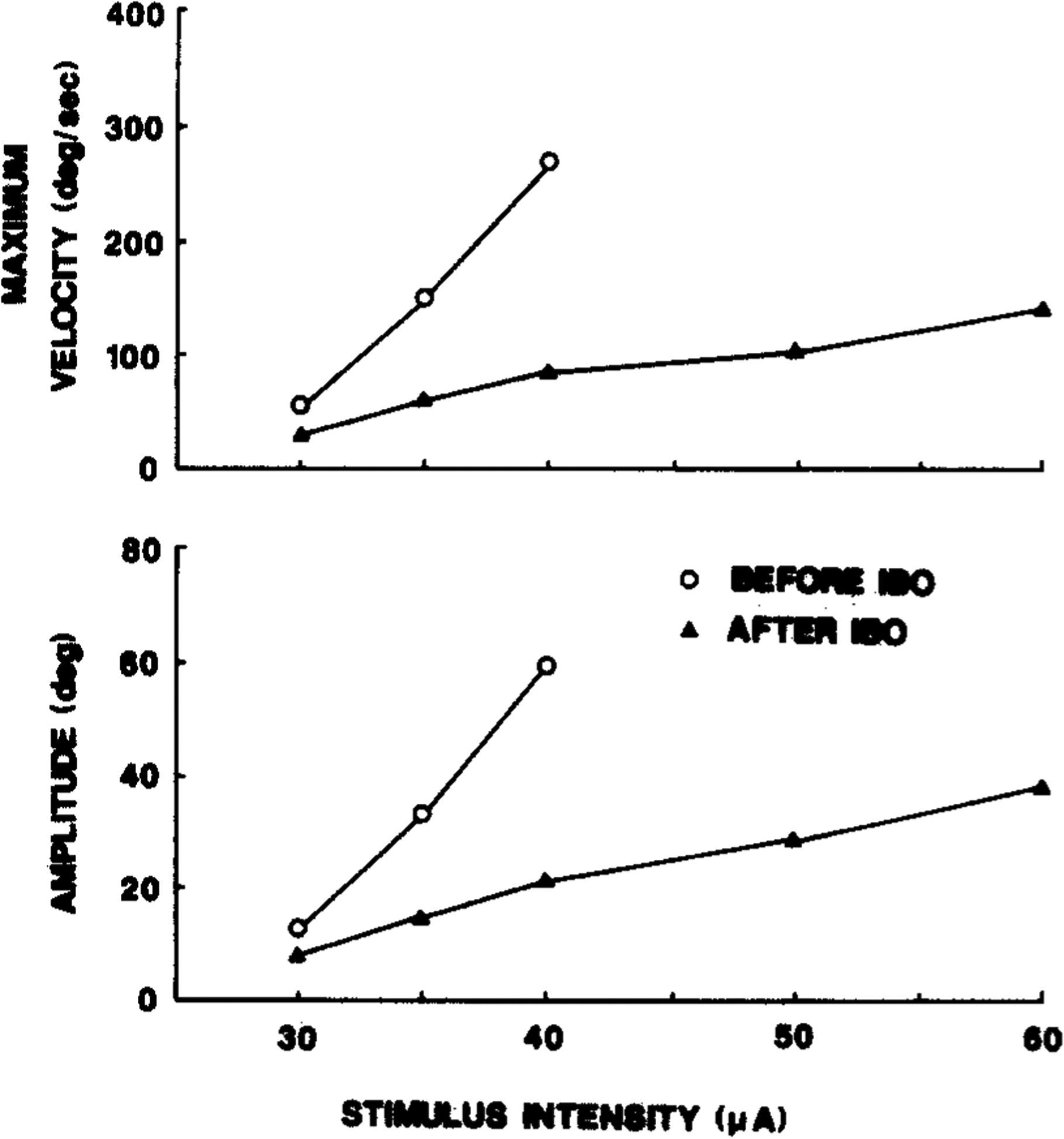
Amplitude and velocity of horizontal head movements evoked by electrical stimulation at different intensities of left pontine reticular formation site before and eight days after IBO-induced lesion of area containing stimulation site (see Fig. 8).
DISCUSSION
In contrast to previous reports indicating no effect of cytotoxin lesions of the medial reticular formation on motor capabilities10,46, we find dramatic and consistent defects in ipsilateral HM after ibotenic acid lesions of the reticular formation. The previous studies employed lesions at least as large as those reported here, therefore lesion size cannot account for the reported lack of motor deficits. An important difference in technique is the use of single stage bilateral lesions in these prior studies. Extrapolating from our lesion results, one would expect that these one stage bilateral lesions would cause a bilateral deficit in rapid HM initiation, as occurred with our 2 stage bilateral lesions. However, this deficit would not be obvious without systematic testing, and could easily be missed in a standard neurological examination. Unilateral lesions allow the detection of more subtle deficits, since the normal contralateral side makes the asymmetry of motor capabilities obvious. Systematic baseline measurements of motor capabilities were also not taken in these prior studies, which were primarily concerned with the effects of these lesions on sleep control.
We see deficits in rapid ipsilateral HMs occurring in a variety of situations. The amplitude and velocity of spontaneous and vestibularly triggered (quick phase) ipsilateral HMs are both deficient after cytotoxin lesions. Despite these defects, HM velocity and amplitude to the contralateral side in animals with unilateral lesions was within the range of baseline values. Similar deficits have recently been reported in visually triggered orienting after unilateral kainic acid lesions of the NRPC and NGC25. In this study, it was reported that lesions produced an inability to make visually triggered head movements in some trials and reduced speed and amplitude in visually triggered head movements on the remaining trials. We found that head posture after lesions was normal, in contrast to the abnormalities in head posture reported after electrolytic lesions of the PMRF in monkeys60. Fiber damage or species differences may explain these results.
The abnormally large and persistent compensatory head twisting to the contralateral side during per- and post-rotatory nystagmus following unilateral lesion may be due to the loss of quick-phase HMs to the ipsilateral side. A similar phenomenon was seen after electrolytic lesions of the NRPC51. Our lesions had a relatively selective effect on quick ipsilateral HMs, as was demonstrated by the loss of rapid ipsilateral anticompensatory and spontaneous HMs, while slow compensatory and slow spontaneous HMs to the ipsilateral side were maintained (see Figs. 5 and 7).
The acute effects of IBO injection are hypothesized to result from the selective excitation of cell bodies within the medial PMRF. These acute effects, in their initial stage, were in a direction opposite to the chronic effects; i.e. injection always elicited an ipsilateral deviation of the head. This posture was caused by a tonic activation of the ipsilateral musculature. Thus, activation of somas in either NRPO, NRPC or NRGC is sufficient to trigger ipsilateral head movement.
The initial ipsilateral HM was followed by a period of contralateral HM with a relative excitation of the appropriate muscles. Reduction in neuronal discharge and morphological changes in cells at the injection site have been reported to occur two or more hours after excitotoxin injection26,42. Therefore this change in posture would correspond approximately to the time period in which cells at the injection site would become inactive. Accordingly, the contralateral HM may be seen as resulting from the unopposed activity of the contralateral neck musculature, after the inactivation of cells mediating ipsilateral HM. One must also consider the possibility that the death of the ipsilateral cells resulted in a loss of inhibition on the contralateral reticulospinal neurons. While decussating reticulo-reticulo inhibitory connections have been identified31, most such connections are excitatory. By 48 h post-lesion, normal head and body posture was seen in all the animals, indicating that the medial reticular formation, while required for the initiation of rapid ipsilateral movements, is not required for the maintenance of the static, head centered posture.
Unilateral lesions of the NRPC region produced more persistent behavioral deficits than more caudal lesions. This is somewhat surprising, since the caudal regions have a much higher proportion of reticulospinal cells6,38,54. However it has long been known that the medullary-spinal projections are bilaterally organized40,58,59. Therefore, unilateral lesions of NRGC might be expected to produce a less lateralized deficit in head movement than similar lesions of NRPC. Since direct NRPC spinal connections are less dense than those of RGC, one may hypothesize that descending NRPC-NGC projections17,31,44,45 mediate some HM effects.
The acute and chronic effects of IBO are consistent with studies of medial reticular unit activity in the behaving animal. Studies in our laboratory, utilizing a systematic analysis of behavioral correlates of discharge, have identified a number of cell types in the PMRF with specific movement correlates. Cell types related to facial, tongue, limb and other movements were reported49,50, in addition to the intensively studied reticular eye movement cells2,15,41. However, the most common cell type was maximally active in relation to rapid ipsilateral HM49. This relation was maintained whether the movement was emitted spontaneously or was elicited by vestibular stimulation. These cells were concentrated in the same NRPC region whose destruction we now report causes the most consistent deficit in HM. However, they were also found in lesser numbers throughout the medial portions of NRPO and NGC49, consistent with the present finding that activation of these regions with ibotenic acid produces ipsilateral HM. In more recent studies55 (Suzuki et al., in preparation), we have found that many of these reticulospinal cells related to head movement receive monosynaptic input from superior colliculus, motor cortex, and vestibular nuclei. Previous studies have also described medial PMRF neurons which discharge in relation to HMs in rabbits11 or in relation to neck EMG activities in head-restrained cats8,19,20,22,23,61. Thus, the present data are in agreement with previous physiological and anatomical data indicating a central role for these neurons in the mediation of axial movement27–29,34,37.
While further testing might well reveal subtle changes in limb movement after PMRF lesions, we could see no obvious deficit in such movements. This is also consistent with previous behavioral analyses which found that only 7% of feline medial PMRF cells discharged maximally in association with limb movement, while 38% discharged maximally in association with head or neck movement49. This is in marked contrast to feline rubrospinal cells, a majority of which are maximally active in relation to limb movement18.
It is well known that electrical stimulation of the NRPO, NRPC and NGC evokes ipsiversive head turning. We found that IBO-induced lesions containing the stimulation site reduced the amplitude and velocity of evoked HMs. This effect was not non-specific, in that the HMs evoked by stimulation of the control (intact) side were unaffected by the lesion. Thus neuronal somas within the medial PMRF and their associated axons contribute to the evocation of head movement by electrical stimulation.
Lesions in the NRPC also impair rapid ipsilateral eye movements (saccades and quick-phase eye movements), so this region may be involved in coordinated eye–HMs4. As we find with HM, the eye movement deficit is restricted to rapid movements, with slow movements elicited by vestibular stimulation normal and symmetrical after unilateral pontine lesions21. A number of anatomically localized pontine cell subpopulations, pausing or firing in bursts before eye movement have been identified2,15,41,53. One may speculate that the reticular cells can be similarly divided into specific subpopulations mediating various aspects of HM control. In the present study, differential effects on eye and head nystagmus (Fig. 6) demonstrate that neurons controlling the two types of nystagmic movements are not completely co-localized.
ACKNOWLEDGEMENTS
Supported by the Medical Research Service of the Veterans Administration, USPHS Grants NS14610 and MH43811.
REFERENCES
- 1.Alstermark B, Pinter MJ and Sasaki S, Pyramidal effects in dorsal neck motoneurons of the cat, J. Physiol. (Lond.), 363 (1985) 287–302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bender MB, Brain control of horizontal and vertical eye movements: a survey of the structural and functional correlates, Brain, 103 (1980) 23–69. [DOI] [PubMed] [Google Scholar]
- 3.Bender MB and Shanzer S, Oculomotor pathways defined by electric stimulation and lesions in the brainstem of the monkey. In Bender MB (Ed.), The Oculomotor System, Hoeber, New York, 1964, pp. 81–140. [Google Scholar]
- 4.Berthoz A and Grantyn A, Neuronal mechanisms underlying eye-head coordination. In Freund HJ, Buttner U, Cohen B and Noth J (Eds.), Progress in Brain Research, Amsterdam, Elsevier, 1986, pp. 325–343. [DOI] [PubMed] [Google Scholar]
- 5.Cohen B, Pontine reticular formation neurons and motor activity, Science, 199 (1978) 207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Coulter JD, Bowker RM, Wise SP, Murray EA, Castiglioni AJ and Westlund KN, Reflex control of posture and movements, Prog. Brain Res, 50 (1979) 265–279. [DOI] [PubMed] [Google Scholar]
- 7.Coyle JT and Schwarcz R, The use of amino acids as selective neurotoxins. In Björklund A and Hökfelt T (Eds.), Handbook of Chemical Neuroanatomy, Methods in Chemical Neuroanatomy, Elsevier, Amsterdam, 1983, pp. 508–527. [Google Scholar]
- 8.Curthoys IS, Nakao S and Markham CH, Cat medial pontine reticular neurons related to vestibular nystagmus: firing pattern, location and projection, Brain Research, 222 (1981) 75–94. [DOI] [PubMed] [Google Scholar]
- 9.Donaghy M, The cat’s vestibulo-ocular reflex, J. Physiol. (Lond.), 300 (1980) 337–351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Drucker-Colin R and Bernal-Pedraza JGB, Kainic acid lesions of gigantocellular tegmental field (FTG) neurons does not abolish REM sleep, Brain Research, 272 (1983) 387–391. [DOI] [PubMed] [Google Scholar]
- 11.Duensing F and Schaefer KP, Die Aktivität einzelner Neurone der Formatio reticularis des nicht gefesselten Kaninchens bei Kopfwendungen und vestibalaren Reizen, Arch. Psychiatr. Nervenkr, 201 (1960) 97–122. [DOI] [PubMed] [Google Scholar]
- 12.Duensing F and Schaffer KP, Die Neuronenaktivität in der Formatio reticularis des Rhombencephalons beim Vestibularen Nystagmus, Archiv. Psychiatr. Nervenkr, 196 (1957) 265–290. [DOI] [PubMed] [Google Scholar]
- 13.Faulkner RF and Hyde JE, Coordinated eye and body movements evoked by brain stem stimulation in decerebrate cats, J. Neurophysiol, 21 (1958) 171–182. [DOI] [PubMed] [Google Scholar]
- 14.Friedman L and Jones BE, Computer graphics analysis of sleep-wakefulness state changes after pontine lesions, Brain Res. Bull, 13 (1984) 53–68. [DOI] [PubMed] [Google Scholar]
- 15.Fuchs AF, Kaneko CRS and Scudder CA, Brainstem control of saccadic eye movements, Annu. Rev. Neurosci, 8 (1984) 307–337. [DOI] [PubMed] [Google Scholar]
- 16.Fukushima Y, Igusa Y and Yoshida K, Characteristics of responses of medial brain stem neurons to horizontal head angular acceleration and electrical stimulation of the labyrinth in the cat, Brain Reseach, 120 (1977) 564–570. [DOI] [PubMed] [Google Scholar]
- 17.Gallager DW and Pert A, Afferents to brain stem nuclei (Brain stem raphe, nucleus reticularis pontis caudalis and nucleus giganto-cellularis) in the rat as demonstrated by microiontophoretically applied horseradish peroxidase, Brain Res, 144 (1978) 257–275. [DOI] [PubMed] [Google Scholar]
- 18.Ghez C and Kubota K, Activity of red nucleus neurons associated with a skilled forelimb movement in the cat, Brain Res, 131 (1977) 383–388. [DOI] [PubMed] [Google Scholar]
- 19.Grantyn A and Berthoz A, Reticulo-spinal neurons participating in the control of synergic eye and head movements during orienting in the cat. I. Behavioral properties, Exp. Brain Res, 66 (1987) 339–354. [DOI] [PubMed] [Google Scholar]
- 20.Grantyn A, Ong-Meang Jacques V and Berthoz A, Reticulo-spinal neurons participating in the control of synergic eye and head movements during orienting in the cat. II. Morphological properties as revealed by intraaxonal injections of horseradish peroxidase, J. Exp. Brain Res, 66 (1987) 355–377. [DOI] [PubMed] [Google Scholar]
- 21.Henn V, Lang W, Hepp K and Reisine H, Experimental gaze palsies in monkeys and their relation to human pathology, Brain, 107 (1984) 619–636. [DOI] [PubMed] [Google Scholar]
- 22.Hikosaka O and Kawakami T, Inhibitory reticular neurons related to the quick phase of vestibular nystagmus - their location and projection, Exp. Brain Res, 27 (1977) 377–396. [DOI] [PubMed] [Google Scholar]
- 23.Igusa Y, Sasaki S and Shimazu H, Excitatory premotor burst neurons in the cat pontine reticular formation related to the quick phase of vestibular nystagmus, Brain Res, 182 (1980) 451–456. [DOI] [PubMed] [Google Scholar]
- 24.Ingram WR, Ranson SW, Hannett FI, Zeiss FR and Terwilliger EH, Results of stimulation of the tegmentum with the Horsley-Clarke stereotaxic apparatus, Arch. Neurol. Psychiat, 28 (1932) 513–541. [Google Scholar]
- 25.Isa T and Sasaki S, Effects of lesion of paramedian pontomedullary reticular formation by kainic acid injection on the visually triggered horizontal orienting movements in the cat, Neurosci. Lett, 87 (1988) 233–239. [DOI] [PubMed] [Google Scholar]
- 26.Kohler C and Schwarcz R, Comparison of ibotenate and kainate neurotoxicity in rat brain: a histological study, Neuroscience, 8 (1983) 819–835. [DOI] [PubMed] [Google Scholar]
- 27.Kuypers HGJM, The descending pathways to the spinal cord, their anatomy and function, Prog. Brain Res, 11 (1964) 178–202. [DOI] [PubMed] [Google Scholar]
- 28.Kuypers HGJM, Anatomy of descending pathways. In Brooks VB (Ed.), Handbook of Physiology, Section I, The Nervous System, Vol. II, Motor Control, American Physiological Society, Bethesda, MD, 1981, pp. 597–666. [Google Scholar]
- 29.Lawrence DG and Kuypers HGJM, The functional organization of the motor system in the monkey. II. The effects of lesions of the descending brain stem pathways, Brain, 261 (1968) 15–36. [DOI] [PubMed] [Google Scholar]
- 30.Magni F and Willis WD, Cortical control of brain stem reticular neurones, Arch. Ital. Biol, 102 (1964) 418–433. [PubMed] [Google Scholar]
- 31.McCarley RW, Ito K and Rodrigo-Angulo ML, Physiological studies of brainstem reticular connectivity. II. Responses of mPRF neurons to stimulation of mesencephalic and contralateral pontine reticular formation, Brain Research, 409 (1987) 111–127. [DOI] [PubMed] [Google Scholar]
- 32.Miliaressis E and Phillippe L, The pontine substrate of circling behavior, Brain Research, 293 (1984) 143–152. [DOI] [PubMed] [Google Scholar]
- 33.Nienhuis R and Siegel JM, Analysis of head movement and position using Hall effect devices, Physiol. Behav, in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Peterson BW, Reticulospinal projections to spinal motor nuclei, Annu. Rev. Physiol, 41 (1979) 127–140. [DOI] [PubMed] [Google Scholar]
- 35.Peterson BW and Abzug C, Properties of projections from vestibular nuclei to medial reticular formation in the cat, J. Neurophysiol, 38 (1975) 1421–1435. [DOI] [PubMed] [Google Scholar]
- 36.Peterson BW, Anderson ME and Filion M, Responses of ponto-medullary reticular neurons to cortical, tectal and cutaneous stimuli, Exp. Brain Res, 21 (1974) 19–44. [DOI] [PubMed] [Google Scholar]
- 37.Peterson BW, Anderson ME and Fillion M, The reticulospinal system and its role in the control of movement. In Barnes CD (Ed.), Brainstem Control of Spinal Cord Function, Academic Press, New York, 1984, pp. 28–86. [Google Scholar]
- 38.Peterson BW, Maunz RA, Pitts NG and Mackel RG, Patterns of projection and branching of reticulospinal neurons, Exp. Brain Res, 23 (1975) 333–351. [DOI] [PubMed] [Google Scholar]
- 39.Peterson BW, Pitts NG, Fukushima K and Mackel R, Reticulo-spinal excitation and inhibition of neck motoneurons, Exp. Brain Res, 32 (1978) 417–489. [DOI] [PubMed] [Google Scholar]
- 40.Petras JM, Cortical, tectal and tegmental fiber connections in the spinal cord of the cat, Brain Research, 6 (1967) 275–324. [DOI] [PubMed] [Google Scholar]
- 41.Raphan T and Cohen B, Brainstem mechanisms for rapid and slow eye movements, Annu. Rev. Physiol, 40 (1978) 527–552. [DOI] [PubMed] [Google Scholar]
- 42.Rieke GK and Bowers DE, Acute effects of the neurotoxin kainic acid on neurons of the pigeon basal ganglia, Acta Neuropathol, 56 (1982) 123–135. [DOI] [PubMed] [Google Scholar]
- 43.Robinson TE, Electrical stimulation of the brain stem in freely moving rats: I. Effects on behavior, J. Physiol. Behav, 21 (1977) 223–231. [DOI] [PubMed] [Google Scholar]
- 44.Rose JD, Projections to the caudolateral medulla from the pons, midbrain and diencephalon in the cat, Exp. Neurol, 72 (1981) 413–428. [DOI] [PubMed] [Google Scholar]
- 45.Sakai K, Sastre JP, Salvert D, Touret M, Tohyama M and Jouvet M, Tegmentoreticular projections with special reference to the muscular atonia during paradoxical sleep in the cat: an HRP study, Brain Research, 176 (1979) 233–254. [DOI] [PubMed] [Google Scholar]
- 46.Sastre JP, Sakai K and Jouvet M, Are the giganto-cellular tegmental field neurons responsible for paradoxical sleep?, Brain Research, 229 (1981) 147–161. [DOI] [PubMed] [Google Scholar]
- 47.Siegel JM and McGinty DJ, Brainstem neurons without spontaneous unit discharge, Science, 193 (1976) 240–242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Siegel JM and McGinty DJ, Pontine reticular formation neurons: relationship of discharge to motor activity, Science, 196 (1977) 678–680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Siegel JM and Tomaszewski KS, Behavioral organization of reticular formation: Studies in the unrestrained cat. I. Cells related to axial, limb, eye, and other movements, J. Neurophysiol, 50 (1983) 696–716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Siegel JM, Tomaszewski KS and Wheeler RL, Behavioral organization of reticular formation: Studies in the unrestrained cat: II. Cells related to facial movements, J. Neurophysiol, 50 (1983) 717–723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Sirkin DW, Schallert T and Teitelbaum P, Involvement of the pontine reticular formation in head movements and labyrinthine righting in the rat, Exp. Neurol, 69 (1980) 435–457. [DOI] [PubMed] [Google Scholar]
- 52.Sirkin DW, Zedek Y and Teitelbaum P, Effects of pontine reticular formation lesions on optokinetic head nystagmus in rats, Exp. Brain Res, 58 (1985) 503–509. [DOI] [PubMed] [Google Scholar]
- 53.Strassman A, Highstein SM and McCrea RA, Anatomy and physiology of saccadic burst neurons in the alert squirrel monkey. II. Inhibitory burst neurons, J. Comp. Neurol, 249 (1986) 358–380. [DOI] [PubMed] [Google Scholar]
- 54.Suzuki K, An HRP study in the cat of brainstem projections to the spinal cord, with particular reference to sacral afferents, Arch. Ital. Biol, 123 (1985) 155–170. [PubMed] [Google Scholar]
- 55.Suzuki SS and Siegel JM, Pontomedullary reticular formation neurons related to head movement in behaving cats: converging inputs from motor cortex, deep superior colliculus, midbrain tegmentum, vestibular nuclei, and spinal cord, Soc. Neurosci. Abstr, 12 (1986) 1545. [Google Scholar]
- 56.Tehovnik EJ and Yoemans JS, Two converging brainstem pathways mediating circling behavior, Brain Research, 385 (2) (1986) 329–342. [DOI] [PubMed] [Google Scholar]
- 57.Thompson R, Gibbs RB, Ristic GA, Cotman CW and Yu J, Learning deficits in rats with early neurotoxic lesions to the globus pallidus, substantia nigra, median raphe or pontine reticular formation, Physiol. Behav, 37 (1986) 141–151. [DOI] [PubMed] [Google Scholar]
- 58.Tohyama M, Sakai K, Salvert D, Touret M and Jouvet M, Spinal projections from the lower brain stem in the cat as demonstrated by the horseradish peroxidase technique. I. Origins of the reticulospinal tracts and their funicular trajectories, Brain Research, 173 (1979) 383–403. [DOI] [PubMed] [Google Scholar]
- 59.Torvik A and Brodai A, The origin of reticulo-spinal fibers in the cat, Anat. Rec, 128 (1957) 113–137. [DOI] [PubMed] [Google Scholar]
- 60.Uemura T and Cohen B, Effects of vestibular nuclei lesions on vestibulo-ocular reflexes and posture in monkeys, Acta Oto-laryngol., 315 (1973) 1–71. [DOI] [PubMed] [Google Scholar]
- 61.Vidal PP, Corvisier J and Berthoz A, Eye and neck motor signals in periabducens reticular neurons of the alert cat, Exp. Brain Res, 53 (1983) 16–28. [DOI] [PubMed] [Google Scholar]
- 62.Whittington DA, Lestienne F and Bizzi E, Behaviour of preoculomotor burst neurons during eye-head coordination, Exp. Brain Res, 55 (1984) 215–222. [DOI] [PubMed] [Google Scholar]


