Summary
Background
Early, rapid detection of SARS-CoV-2 is essential in healthcare settings in order to implement appropriate infection control precautions and rapidly assign patients to care pathways. Rapid testing methods, such as SARS-CoV-2 rapid antigen testing (RAT) may improve patient care, despite a lower sensitivity than real-time PCR (RT-PCR) testing.
Methods
Patients presenting to an Emergency Department (ED) in Melbourne, Australia, were risk-stratified for their likelihood of active COVID-19 infection, and a non-randomised cohort of patients were tested by both Abbott Panbio™ COVID-19 Ag test (RAT) and SARS-CoV-2 RT-PCR. Patients with a positive RAT in the ‘At or High Risk’ COVID-19 group were moved immediately to a COVID-19 ward rather than waiting for a RT-PCR result. Clinical and laboratory data were assessed to determine test performance characteristics; and length of stay in the ED was compared for the different patient cohorts.
Findings
Analysis of 1762 paired RAT/RT-PCR samples demonstrated an overall sensitivity of 75.5% (206/273; 95% CI: 69·9-80·4) for the Abbott Panbio™ COVID-12 Ag test, with specificity of 100% (1489/1489; 95% CI: 99·8-100). Sensitivity improved with increasing risk for COVID-19 infection, from 72·4% (95% CI: 52·8-87·3) in the ‘No Risk’ cohort to 100% (95% CI: 29·2-100) in the ‘High Risk’ group. Time in the ED for the ‘At/High Risk’ group decreased from 421 minutes (IQR: 281, 525) for those with a positive RAT result to 274 minutes (IQR:140, 425) for those with a negative RAT result, p = 0.02.
Interpretation
The positive predictive value of a positive RAT in this setting was high, allowing more rapid instigation of COVID-19 care pathways and an improvement in patient flow within the ED.
Funding
Royal Melbourne Hospital, Melbourne, Australia.
Keywords: SARS-CoV-2, Rapid antigen testing, Abbott panbio, Emergency department
Research in context.
Evidence before this study
Meta-analyses of studies assessing the performance of antigen tests have demonstrated sensitivities of 75·1-76·7% for SARS-CoV-2 rapid antigen tests (RAT), and specificities of 99-99·5%. A small number of studies have reported the implementation of RAT in Emergency Departments, with sensitivity ranging from 69·2–85%, and excellent specificity 97-100%. Although the reduced sensitivity of RAT compared to RT-PCR is potentially offset by significantly improved result turn-around times, few studies have reported on the impact of having timelier test results in the Emergence Department.
Added value of this study
This study is one of the largest to report the implementation of rapid antigen testing for symptomatic patients presenting to an Emergency Department, and highlights the potential impacts of rapid testing on length of stay. High numbers of patients tested (1,762) as well as a large number of COVID-19 PCR confirmed cases (273) allows robust understanding of test performance in this setting. Findings support a role for RAT to streamline COVID-19 positive patient flow in a hospital setting.
Implications of all the available evidence
Previously reported performance characteristics (∼ 75% sensitivity and >99% specificity) are maintained when tests are performed at point of care in the Emergency Department. With at least moderate COVID-19 case numbers, the excellent specificity and positive predictive value supports clinicians initiating patient COVID-19 specific management and infection control protocols based on a positive RAT result. Further study is needed as to how to best apply RAT to maximise clinical and health system outcomes.
Alt-text: Unlabelled box
Introduction
Rapid diagnosis of SARS-CoV-2 infection is essential for informing clinical care and public health action. As the COVID-19 pandemic has evolved, diagnostic testing has expanded beyond laboratory-based reverse transcriptase–polymerase chain reaction (RT-PCR), with rapid point-of-care antigen lateral flow devices (RATs) for SARS-COV-2 now widely available. The key advantage of RATs is the provision of rapid results, usually within 15 minutes, enabling timely decisions about individual treatment pathways, infection control measures and public health action.1,2 Several studies have assessed the utility of RATs in schools and community settings, largely to detect asymptomatic individuals with COVID-19 in order to interrupt viral transmission.3,4
The clinical utility and positive predictive value of antigen testing are greatest in high-prevalence settings.5 Guidelines from the Infectious Diseases Society of America (IDSA) recommend that, in symptomatic individuals with suspected COVID-19, an RT-PCR test is preferred over a rapid antigen test, particularly when the implications of a false negative result may have adverse consequences e.g., in healthcare settings.6 However, early, rapid detection of SARS-CoV-2 is essential in healthcare settings in order to implement appropriate infection control precautions and rapidly assign patients to appropriate care pathways. The need for urgent patient triage is greatest in the hospital emergency department, where patients with diverse clinical conditions are located, and where bottlenecks in patient flow can occur as patients await appropriate assessment and test results.
Until early 2022 in Australia, most diagnostic testing has been conducted using RT-PCR, with Australia having one of the highest testing rates in the world.7 However, as community transmission of the Delta and Omicron variants has become endemic in parts of Australia during 2021 and 2022, alternative testing modalities such as antigen testing have been increasingly utilised. Previous work by our group assessed the feasibility of using RATss to rapidly detect SARS-CoV-2 in hospital emergency departments in Melbourne, Australia.8 This work provided valuable information on the logistical and operational challenges associated with implementing rapid antigen tests in our setting. However, at the time of our study in late 2020, there was limited community transmission of SARS-CoV-2, precluding detailed assessment of the clinical performance of antigen testing for rapid patient diagnosis.8 Here, we describe the performance and utility of rapid antigen testing in the emergency department at a time of high community transmission of the SARS-CoV-2 Delta variant in Melbourne. Specifically, the aims of the study were to: (i) determine the clinical sensitivity and specificity of the Abbott PanBio™ COVID-19 Ag test and (ii) describe the clinical pathway of patients who received rapid antigen testing in the emergency department.
Methods
Study setting and patient population
The Royal Melbourne Hospital (RMH) is a tertiary academic public hospital situated in Melbourne, Victoria, Australia. During the early phase of ‘COVID Peak’ (a Victorian Department of Health (DH) designated risk-rating based on COVID-19 daily case numbers, beginning 23/08/2021 and current at the time of this study on 20/12/2021)9, the number of daily COVID-19 cases in Victoria increased from 48 on 23th August to 1,231 on the 20th December. In addition to local community RMH ED presentations, RMH was initially one of three designated ‘COVID-19 Streaming Hospital’ for Victoria, a designation that was applied in a stepwise fashion to health services as the outbreak progressed. Under this model all confirmed COVID-19 cases in Victoria requiring hospital assessment or management were referred to one of the COVID-19 Streaming Hospitals.
Throughout this period of ‘COVID-19 Peak’, all of the RMH Emergency Department (ED) was considered a ‘suspected COVID-19’ ward. All emergency admissions to the hospital were required to have a SARS-CoV-2 RT-PCR test to determine: (i) their COVID-19 status, and (ii) their destination within the hospital (e.g., to a designated COVID or non-COVID ward). For patients who were not admitted to hospital, SARS-CoV-2 RT-PCR was undertaken according to clinical need as determined by the treating clinician.
To assess the utility of rapid antigen testing for improving patient triage and flow through the emergency department during ‘COVID Peak’, we undertook a six-week prospective implementation study between 13th September to 26th October 2021, inclusive. All individuals undertaking COVID-19 PCR were eligible to be offered rapid antigen testing, and therefore eligible for inclusion. Limitations in access to rapid antigen test kits, and availability of staff trained in their administration, meant not all those with COVID-19 PCR received additional RAT. Participants were excluded if they did not, or were not able to, consent to an additional nasal swab for rapid antigen testing.
SARS-CoV-2 risk stratification
All patients underwent a standardised clinical and epidemiological screening process on initial presentation to the emergency department (Supp. Figure SF1). Based on this screening process, patients were classified into five risk categories as: (i) Known COVID-19 (positive PCR in last 14 days); (ii) High Risk (epidemiology and compatible clinical syndrome); (iii) At Risk (epidemiology without a compatible clinical syndrome); (iv) Low Risk (compatible clinical syndrome without epidemiology, or un-assessable patient), (v) No Risk Identified (neither epidemiology, nor compatible clinical syndrome) (Supp. Figure SF1; Supplementary Table 1). Known COVID-19 cases were admitted to dedicated COVID-19 wards. High risk and At Risk cases were managed on suspected COVID-19 (sCOVID) wards once a negative PCR was obtained. Low Risk patients were generally kept in ED until a PCR result was available to re-stratify them as No Risk or Known COVID-19. No Risk Identified cases were managed on routine inpatient wards with no added infection control precautions. Prior to this study therefore, all risk categories other than “No Risk” were required to wait in ED until a SARS-CoV-2 RT-PCR result was returned.
SARS-CoV-2 rapid antigen testing
The Abbott PanBio™ COVID-19 Ag Rapid Test (RAT) Device was selected for use in this study based on previously reported performance characteristics, and familiarity with the device following a pilot program within the RMH ED in 2020.8,10, 11, 12 Mid-turbinate nasal swabs were collected by specifically trained ED staff from patients, according to the manufacturer's instructions for use. In the initial stages of the project, rapid antigen testing was limited to daytime ED shifts when adequate training and supervision levels were available.
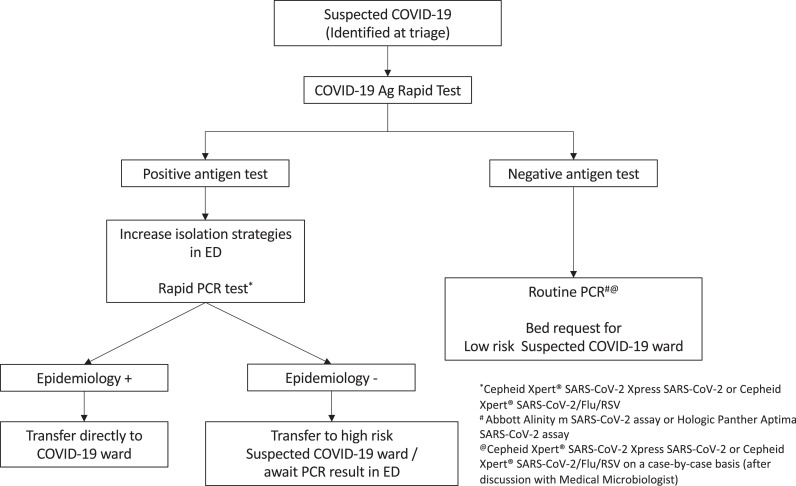
RAT results were checked by two staff members and recorded in the patient electronic medical record. Results were considered invalid if the control line was not present; positive if the test line was visible within 15-20 minutes, and negative if a test line failed to appear by 20 minutes. If more than one test was undertaken on a patient within the same ED presentation episode, the majority result was recorded as the final result. Episodes with one positive and one negative result recorded were considered indeterminate. Clinical decisions related to patient triage, flow and infection control measures were made based on the antigen test result (Figure 1). The initially assigned COVID-19 risk category was not de-escalated based on antigen test result.
Figure 1.
Workflow for the testing and management of COVID-19 Ag Rapid Tests results in the Emergency Department. Suspected COVID-19 included patients with any symptoms (may or may not be consistent with COVID-19) or an epidemiological risk factor for COVID-19 exposure.
Real-time SARS-CoV-2 PCR testing
ED staff collected combined nasal and oropharyngeal swabs were tested in an onsite laboratory at RMH by one or more commercial SARS-CoV-2 RT-PCR assays (Cepheid Xpert® SARS-CoV-2 Xpress SARS-CoV-2; Cepheid Xpert® SARS-CoV-2/Flu/RSV; Abbott Alinity m SARS-CoV-2 assay or Hologic Panther Aptima SARS-CoV-2 assay).13, 14, 15, 16 All assays met appropriate performance characteristics for RT-PCR testing in either FDA or comparator studies.17, 18, 19 Samples were confirmed as SARS-CoV-2 PCR positive if they were positive on two or more different SARS-CoV-2 gene targets, as per local guidelines.20 In the event of discordant test results, a third gene target was tested, with the result determined by the result of the third assay.
Data analysis and statistics
All patient demographic, clinical and epidemiological data as well as antigen test result were extracted from the electronic medical record. RT-PCR test result information was extracted from the laboratory information system and matched to ED presentation episode by patient identifier and date of test. Sensitivity, specificity and positive and negative predictive values (PPV and NPV) were calculated by comparing the results of the Abbott PanBio COVID-19 Ag test with RT-PCR. Where appropriate, results were reported with 95% confidence intervals by the ‘exact’ method of Clopper and Pearson. The Mann-Whitney U test was used to test the null hypothesis for the non-normally distributed data for samples with positive versus negative RAT results (with respect to PCR cycle threshold (Ct) values, days from symptom onset and ED length of stay). Logistic regression was performed in GraphPad Prism to determine at what Ct value the probability of a positive RAT result was 50%. Statistical analyses and data visualisation were performed using either R Studio (version 1.4) or GraphPad Prism (version 9.0).
Ethical declaration
This study was approved as a quality assurance activity with Human Research Ethic Committee approval from the Royal Melbourne Hospital (QA2020085).
Role of the funding source
This work was funded by the Royal Melbourne Hospital, Melbourne, Australia. The funder was not involved in data collection, analysis or manuscript preparation. KB, EW and DAW are also supported by NHMRC grants.
Results
Utility of antigen testing in patient triage
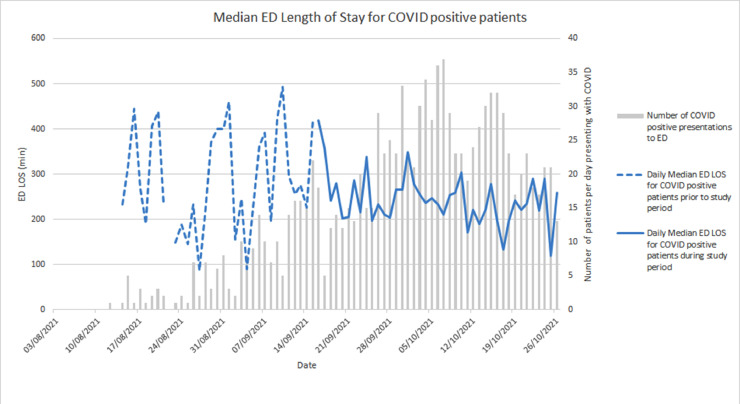
During the study period, 8,802 patients presented a total of 10,618 times to the ED. Daily COVID-19 positive case numbers increased over 6 weeks preceding the study and in the early study period, and are presented with the associated ED median length of stay in Figure 2. SARS-CoV-2 RT-PCR testing was undertaken on 4,636/10,618 (43·7%) occasions, and both RT-PCR and RAT on 1,773/4,636 (38·2%) occasions. Median turn-around time for all samples from collection to a validated PCR result was 2.7hrs (IQR 1.7, 4.7). Eleven paired RT-PCR and RAT were excluded from subsequent analyses (six had invalid RAT results, and five RAT results were indeterminate), providing two final datasets of (i) 1,762 paired RT-PCR and antigen tests from 1,703 individuals (Supp. Figure SF2) and (ii) 2,863 RT-PCR tests without antigen tests from 2,579 individuals. Females accounted for 48% of each cohort and the age distribution was relatively well matched, (Supp. Table 1). More patients presenting as Triage Category 1 or 2; who described COVID-19 contact; or presented with High Risk symptoms underwent RAT. Length of stay in the ED was not meaningfully different between the two groups.
Figure 2.
Number of presentations to the Emergency Department with RT-PCR confirmed SARS-CoV-2 and daily median length of stay for all patients.
Of 1,762 presentations, 273 recorded a positive PCR result (15·5%), of which 3 samples required confirmation by 3 targets. Length of stay was lower for PCR positive cases if they had concurrent RAT testing, however, did not reach statistical significance (Table 2). Patients with a positive RAT result, and thus eligible for immediate transfer to a COVID-19 ward, were in the ED for shorter periods compared to those with negative RAT result, which reached statistical significance for the High Risk or At Risk group (Median length of stay (LOS) in ED 274 minutes (Interquartile range (IQR): 140, 425) vs 421 minutes (IQR: 281,525), p = 0·02)).
Table 2.
Length of stay in the Emergency Department for PCR positive patients according to Panbio Rapid Antigen Test and Result Status.
| Length of stay IN THE emergency department for PCR POSITIVE CASES, Minutes median (IQR) |
||||
|---|---|---|---|---|
| Rapid Antigen Test Status | High or At Risk Group[n] | p value | Low or No Risk Group[n] | p value |
| RAT not done | 352 (218, 445) [9] |
0·66 | 366 (285, 539) [51] |
0·23 |
| RAT done | 287 (189, 458) [74] |
345 (215, 505) [124] |
||
| RAT Positive | 274 (140, 425) [61] |
0·02 | 335 (191, 484) [96] |
0·16 |
| RAT Negative | 421 (281, 525) [13] |
359 (282, 538) [28] |
||
Clinical performance of the Abbott PanBio™ COVID-19 Ag rapid test device
When compared to RT-PCR, sensitivity for the RAT was 75.5% (95%CI: 69·9-80·4%), with decreasing sensitivity associated with lower assigned risk categories (Table 1). Cases with confirmed COVID-19 prior to presentation to ED had the lowest RAT sensitivity at 67.3% (95% CI: 52·9-79·7). Within the limitations of comparing cycle threshold (Ct) values across test platforms, the sensitivity for RAT decreased with rising cycle threshold (Ct) values, from 100% (95% CI: 78.2-100) for samples with Ct values < 15; to 22·2% (95% CI: 6·4-47·6%) for Ct values between 30-34·9 (Supp. Table S2). Sensitivity of the RAT was 80% (95% CI: 72.0-87.1) for samples collected within 4 days of symptom onset, compared to 70% (95% CI: 59.2-80.0) for samples collected more than 7 days from symptom onset, (p =0·13 for <4 days vs 4-7 days vs > 7 days) (Supp. Table S3). The sensitivity of the RAT for 9 asymptomatic individuals was 33% (95% CI: 7.5-70). No false positive RAT results were encountered, therefore we observed 100% specificity and positive predictive value (PPV) across all risk categories. The predictive value of a negative RAT (NPV) was 95.7% (95% CI: 94.6-96.7).
Table 1.
Performance characteristics of the Abbott PanBio COVID-19 Ag Rapid Test Device in different patient cohorts presenting to the Emergency Department.
| Risk group | Sensitivity (%) (95%CI)[TP/TP+FN] | Specificity (%) (95%CI)[TN/TN+FP] | PPV (%)(95%CI)[TP/TP+FP] | NPV (%)(95%CI)[TN/TN+FN] |
|---|---|---|---|---|
|
All Cases (n = 1762) |
75.5 (69·9-80·4) [206/273] |
100 (99·8-100) [1489/1489] |
100 (98·6-100) [206/206] |
95·7 (94·6-96·7) [1489/1556] |
|
All Cases with unknown COVID-19 status (n = 1697) |
77·4 (71·3-82·7) [171/271] |
100 (99·8-100) [1476/1476] |
100 (98.3-100) [171/171] |
96·7 (95·7-97·6) [1476/1526] |
|
Confirmed cases (n = 65) Positive SARS-CoV-2 PCR result |
67·3 (52·9-79·7) [35/52] |
100 (79.4-100) [13/13] |
100 (91.8-100) [35/35] |
43·3 (25·5-62·6) [13/30] |
|
High Risk (n = 20) Symptoms and relevant epidemiology |
100 (29·2-100) [3/3] |
100 (83.8-100) [17/17] |
100 (36.8-100) [3/3] |
100 (80·5-100) [17/17] |
|
At Risk (n = 173) Relevant epidemiology with no symptoms |
81·7 (70·7-89·9) [58/71] |
100 (97.1-100) [102/102] |
100 (95.0-100) [58/58] |
88·7 (81·5-93·8) [102/115] |
|
Low Risk (n = 1055) Symptoms with no relevant epidemiology* |
75·4 (66·7-82·9) [89/118] |
100 (99·7-100) [937/937] |
100 (96.7-100) [89/89] |
97 (95·7-98) [937/966] |
|
No Risk (n = 449) No compatible symptoms or relevant epidemiology |
72·4 (52·8-87·3) [21/29] |
100 (99·3-100) [420/420] |
100 (86.7-100) [21/21] |
98·1 (96·4-99·2) [420/428] |
*Includes those with unknown risk (unconscious or unable to ascertain symptoms or epidemiological risk factors); TP = True Positive, TN = True Negative; FP = False Positive; FN = False Negative with reference to SARS-CoV-2 Real-Time PCR as the gold standard.
Note: Symptoms included fever or chills in absence of an alternative diagnosis, acute respiratory infection symptoms (cough, sore throat, shortness of breath, rhinorrhoea, loss of smell or loss of taste), and atypical symptoms in the elderly (functional decline, delirium, exacerbation of underlying conditions, nausea, vomiting, diarrhoea, myalgia and headache) and were based on the Victorian Department of Health testing criteria;31 Relevant epidemiology was considered close contact with a confirmed case, patients instructed to quarantine or isolate, having lived in or visited an exposure site or release from a quarantine facility in the previous 7 days. The relevance of work in specific industries including border control and quarantine services varied throughout the period in line with state health department advice and hospital advisory group decisions.
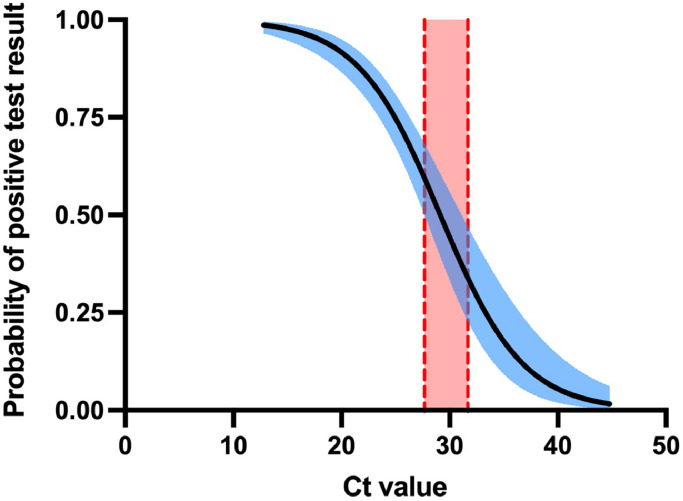
Of 273 positive PCR tests, 67 (24.5%) were RAT negative and thus considered false negative RAT results. The mean cycle threshold (Ct) value for false negative RAT results was significantly higher than for positive RAT results, 29·8 versus 20·8 respectively (95% CI: 7·45-10·4, p < 0·0001); however 25% (17/67) of false negative RAT were seen at relatively low Ct values of less than 25, 52% (35/67) were seen at Ct values less than 30. Of the 273 samples with a positive PCR test, 267 had an N gene Ct value available from testing on the Cepheid Xpert® SARS-CoV-2 Xpress. Binomial regression determined that the 50% likelihood for a RAT returning a positive result was reached at a N gene Ct value of 27.7-31.0 (Figure 3). There was no difference in the median day of testing relative to symptom onset for PCR confirmed cases testing RAT positive or negative (median 4·3 vs 5·3 days post symptom onset for RAT, p = 0·15) (Supp. Table S3). Nine PCR positive patients did not have any symptoms described in the clinical notes, of which 3 were also RAT positive.
Figure 3.
Binomial regression for 267 positive Rapid Antigen Results according to N gene cycle threshold values (Cepheid Xpert® Xpress) for positive SARS-CoV-2 PCR samples. Shaded area represents 95% confidence interval. Vertical shaded area represents 95% confidence interval for the Ct value corresponding to 50% probability of a positive rapid antigen test result (Ct 27.66-30.99).
Discussion
Here, we assessed the performance of the PanBio COVID-19 Ag Rapid Test in individuals presenting to a busy tertiary hospital emergency department during a period with rising COVID-19 case numbers. Although the overall sensitivity of 75·5% was less than the minimum suggested threshold of 80% for SARS-CoV-2 diagnostic assays, as recommended by the World Health Organisation, testing in high-risk clinical and epidemiological groups did reach this threshold, with clinical sensitivities of 100% (95% CI, 29·2-100) and 81·7% (95% CI, 70·7-89·9) in the high-risk and at-risk groups, respectively. These findings further highlight the utility of targeted, rather than indiscriminate, use of rapid antigen tests.11 However, even in high risk groups, the sensitivity of rapid antigen testing is insufficient as a definitive ‘rule out’ test, with approximately 50% of false negative results in our study from RT-PCR positive patients having Ct values of <30 (35 of 67, 52%) or (ii) time from symptom onset less than 7 days (33 of 67, 49.3%). Thus, in a sensitive setting, with a vulnerable population for which the consequences of COVID-19 infection may be high (such as a hospital, aged care facility, immunocompromised population), RT-PCR testing is likely to remain important to exclude COVID-19 infection. Sensitivity was lowest for previously confirmed COVID-19 cases at 67·3% (95% CI:52·9-79·7), presumably relating to this cohort presenting due to complications later in the course of their illness when viral loads in the nasopharynx are lower. In our study, specificity of the RAT was 100%, with no false positive results recorded. This observation suggests antigen testing could be employed as a ‘rule in’ test, which is likely to be helpful in situations where high case numbers require rapid triage. Prior to this study there was concern within Australia about the proportion of false positive RAT results in a population with very low prevalence, with initial estimates of up to 7 to 9 out of 10 positive tests potentially returning as false positive results.21, 22, 23 However, in our experience the false positive proportion was much lower than expected, (zero cases). This discrepancy may relate to the difficulty of calculating accurate prevalence based estimates such as PPV or NPV in a dynamic outbreak environment, or in applying national or state based case data to the local hospital catchment level. Our findings highlight the importance of trialling diagnostic interventions within the local target population rather than extrapolating or generalising from external data.
Our findings for the performance of the Abbott PanBio COVID-19 Ag Rapid Test in symptomatic individuals are in keeping with two meta-analyses, which reported sensitivity of 75·1-76·7% with similar incremental improvements for sensitivity results according to Ct value, and specificities of 99-99·5%.10,11 A number of other studies have examined the utility of RAT in emergency departments, although this study has screened one of the largest population of patients with high numbers of confirmed COVID-19 cases, and demonstrated the utility of the test in conjunction with a standardised clinical and epidemiological risk stratification.24, 25, 26, 27, 28 Sensitivities in symptomatic patients presenting to the ED were similar across studies at 69·2–85%, and all showed excellent specificity 97-100%. Two studies did not demonstrate false negative results for paired PCR samples with high viral loads or low Ct values < 25,25,28 however two others showed similar results to this study where a number of low Ct and/or early infections were missed.26,27
Given the initial uncertainty about the performance characteristics in our setting, RAT was incorporated as a rapid triage tool designed to maximise patient flow. Our study showed RAT testing to be effective for this purpose, with a 147 minute reduction in length of stay in the Emergency Department for patients of the High Risk or At Risk group who were RAT positive. There were no significant changes to our PCR testing algorithm, and no therapeutic decisions were instituted on receipt of a positive RAT. This makes it challenging to demonstrate wider clinical or system-based benefits of RAT use in our cohort. Caruna et. al. incorporated RAT in a similar workflow to ours, and were able to save 271 rapid RT-PCR tests for 572 patients, a beneficial outcome in periods of high demand and reagent shortages.25 In addition, we were able to minimise or avert the need for contact trace events for 21 patients who were not considered at risk of COVID-19 and would have otherwise been managed without appropriate personal protective equipment and isolation strategies.
A limitation of this study was the lack of randomised design, and therefore bias in comparator groups which limited our capacity to robustly evaluate clinical and hospital symptom-based outcomes. While a reduced ED LOS was seen for RAT positive patients stratified to ‘High’ or ‘At Risk’, we were not able to demonstrate a reduced LOS in general for patients within the ED who received RAT (positive or negative) in addition to a PCR test, however our groups were not randomised, and there were significant baseline differences between the two cohorts. This is reflective of the challenges of undertaking clinical research in an outbreak setting, with an intense workload and time constraints, as well as operational and service delivery requirements which take precedence over optimised trial design. There may be some less tangible benefits that RAT may add by providing earlier diagnostic clarity to clinical decision-making, in the future this could be captured by seeking formal qualitative feedback from ED staff.
The clinical utility of diagnostic assays is dependent upon intrinsic test performance characteristics (sensitivity, specificity) and prevalence of the condition of interest, in addition to implementation aspects such as turn-around time, ease of sample collection and test use. Here we demonstrate practical utility of rapid antigen testing, largely due to high specificity in a period of moderate-high prevalence, coupled with rapid test results and ease of use. While a systematic cost-benefit analysis is necessary to determine the optimal approach to implementation, a potential workflow, in periods of high demand on the PCR testing system, may be to undertake RAT for symptomatic presentations to ED with subsequent PCR testing only in those who test negative. Ideally a targeted group of RAT positive individuals would also undertake further sampling to allow for genomic sequencing (WGS) of their SARS-CoV-2 RNA, to facilitate epidemiological surveillance for variants of concern or resistance mutations. Alternate approaches to improving sensitivity such as collection of a combined nose and throat swab, similar to our approach for PCR testing, have been suggested but have not demonstrated utility, and are not currently recommended by most manufacturers.29,30 The interplay of frequently and rapidly changing SARS-CoV-2 prevalence, the emergence of new SARS-CoV-2 variants, and the large number of available test kits on the market, can make it challenging to determine the optimal implementation strategy for rapid antigen testing.
Conclusion
When deployed for high-risk patient presentations to an Emergency Department during moderate to high COVID-19 prevalence, the Abbott PanBio™ demonstrated good sensitivity and excellent specificity. Sensitivity of rapid antigen testing improved with increasing pre-test probability of COVID-19, as determined by standardised clinical and epidemiological risk stratification. The high positive predictive value for RAT suggests that clinical and therapeutic decisions could be made earlier based on a positive RAT result without the need for confirmatory RT-PCR, thereby preserving RT-PCR testing for situations of high clinical and public health need, such as peaks of infections due to the ongoing emergence of SARS-CoV-2 variants.
Contributors
K.B., B.S, E.G., N.W, M.P and D.W were responsible for the conceptualisation, methodology, project administration and supervision of the project. B.S., E.G., and K.L. were involved in undertaking the investigation, while K.B., B.S., K.L., E.W. and M.P were involved in data curation. K.B, M.P and D.W undertook formal analysis of the data. K.B., B.S., M.P and D.W. were responsible for visualisation and for writing the original draft. All authors were involved in review and editing of the manuscript, and had access to the original data.
Declaration of interests
The authors certify that they have no affiliations with or involvement in any organization or entity with any financial interest or non-financial interest in the subject matter or materials discussed in this manuscript.
Acknowledgments
Data sharing statement
The data that support the findings of this study are available from the corresponding author, KB, upon reasonable request.
Acknowledgements
The authors would like to thank all the Emergency Department and Microbiology staff at RMH for their contribution to this study.
Funding and disclosures
This study was funded by the Royal Melbourne Hospital, Melbourne, Australia. DAW is supported by an Investigator Grants from the National Health and Medical Research Council (NHMRC) of Australia (APP1174555). KB and EW are supported by NHMRC Postgraduate Scholarships (GNT1191321 and GNT2005380).
Footnotes
Supplementary material associated with this article can be found in the online version at doi:10.1016/j.lanwpc.2022.100486.
Appendix. Supplementary materials
References
- 1.Larremore DB, Wilder B, Lester E, et al. Test sensitivity is secondary to frequency and turnaround time for COVID-19 screening. Sci Adv. 2021;7(1):eabd5393. doi: 10.1126/sciadv.abd5393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Mina MJ, Peto TE, García-Fiñana M, Semple MG, Buchan IE. Clarifying the evidence on SARS-CoV-2 antigen rapid tests in public health responses to COVID-19. Lancet. 2021;397(10283):1425–1427. doi: 10.1016/S0140-6736(21)00425-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.García-Fiñana M, Hughes DM, Cheyne CP, et al. Performance of the Innova SARS-CoV-2 antigen rapid lateral flow test in the Liverpool asymptomatic testing pilot: population based cohort study. BMJ. 2021:n1637. doi: 10.1136/bmj.n1637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Young BC, Eyre DW, Kendrick S, et al. Daily testing for contacts of individuals with SARS-CoV-2 infection and attendance and SARS-CoV-2 transmission in English secondary schools and colleges: an open-label, cluster-randomised trial. Lancet. 2021;398(10307):1217–1229. doi: 10.1016/S0140-6736(21)01908-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.World Health Organization. Antigen-detection in the diagnosis of SARS-CoV-2 infection [Internet]. Available from:https://www.who.int/publications/i/item/antigen-detection-in-the-diagnosis-of-sars-cov-2infection-using-rapid-immunoassays
- 6.Hanson KE, Caliendo AM, Arias CA, et al. The infectious diseases society of America guidelines on the diagnosis of COVID-19: molecular diagnostic testing. Clinic Infect Dis. 2021:ciab048. [Google Scholar]
- 7.University of Oxford. Our World in Data, Statistics and Research Coronavirus (COVID-19) Testing [Internet]. Available from: https://ourworldindata.org/coronavirus-testing
- 8.Muhi S, Tayler N, Hoang T, et al. Multi-site assessment of rapid, point-of-care antigen testing for the diagnosis of SARS-CoV-2 infection in a low-prevalence setting: a validation and implementation study. Lancet Regional Health - Western Pacific. 2021;9 [Google Scholar]
- 9.Victorian Department of Health. Victorian health service guidance and response to COVID-19 risks [Internet]. Available from:https://www.health.vic.gov.au/covid-19/victorian-health-service-guidance-and-response-to-covid-19-risks
- 10.Brümmer LE, Katzenschlager S, Gaeddert M, et al. Accuracy of novel antigen rapid diagnostics for SARS-CoV-2: a living systematic review and meta-analysis. Suthar AB, editor. PLoS Med. 2021;18(8) [Google Scholar]
- 11.Dinnes J, Deeks JJ, Berhane S, et al. Rapid, point-of-care antigen and molecular-based tests for diagnosis of SARS-CoV-2 infection. Cochrane Infectious Diseases Group, editor. Cochrane Database System Rev [Internet]. 2021 Mar 24 [cited 2021 Dec 14];2021(4). Available from: http://doi.wiley.com/10.1002/14651858.CD013705.pub2
- 12.Peto T, Affron D, Afrough B, et al. COVID-19: Rapid antigen detection for SARS-CoV-2 by lateral flow assay: A national systematic evaluation of sensitivity and specificity for mass-testing. EClinicalMedicine. 2021;36 [Google Scholar]
- 13.Cepheid G. Xpert(R) Xpress SARS-CoV-2 instructions for use [Internet]. [cited 2021 Dec 20]. Available from:https://www.cepheid.com/Package%20Insert%20Files/Xpert%20Xpress%20SARS-CoV-2%20Assay%20ENGLISH%20Package%20Insert%20302-3787%20Rev.%20B.pdf
- 14.Cepheid G. Xpert (R) Xpress SARS-CoV-2/Flu/RSV instructions for use [Internet]. [cited 2021 Dec 20 ]. Available from:https://www.cepheid.com/Package%20Insert%20Files/Xpert%20Xpress%20SARS-CoV-2%20Flu%20RSV%20HC%20English%20Package%20Insert%20302-5707%20Rev.%20A.pdf
- 15.Abbott. Alinity m SARS-CoV-2 assay - instructions for use [Internet]. [cited 2021 Dec 20 ]. Available from: https://www.fda.gov/media/137979/download
- 16.Hologic. SARS-CoV-2 Assay (Panther Fusion (R) System) instructions for use [Internet]. Available from: https://www.hologic.com/sites/default/files/2020-03/AW-21159-001_002_01.pdf
- 17.U.S. Food & Drug Administration. SARS-CoV-2 Reference Panel Comparative Data [Internet]. Available from:https://www.fda.gov/medical-devices/coronavirus-covid-19-and-medical-devices/sars-cov-2-reference-panel-comparative-data#table2
- 18.Perchetti GA, Pepper G, Shrestha L, et al. Performance characteristics of the Abbott Alinity m SARS-CoV-2 assay. J Clinic Virol. 2021;140 [Google Scholar]
- 19.Mostafa HH, Carroll KC, Hicken R, et al. Multicenter evaluation of the cepheid xpert xpress SARS-CoV-2/Flu/RSV test. McAdam AJ, editor. J Clin Microbiol. 2021;59(3) e02955-20. [Google Scholar]
- 20.Public Health Laboratory Network. PHLN guidance on laboratory testing for SARS-CoV-2 (the virus that causes COVID-19). 2021;2.1:21.
- 21.Royal College of Pathologists Australasia . 2021. Media Release: Emerging Real-World Evidence Highlights Risk of Missing COVID-19 Cases with Rapid Antigen Tests [Internet]https://www.rcpa.edu.au/News-and-Media-Releases/Media-Releases/Docs/Emerging-real-world-evidence-highlights-risk-of-mi [cited 2021 Dec 20]. Available from: [Google Scholar]
- 22.Royal College of Pathologists of Australasia . 2021. RCPA Position Statement COVID-19 Antigen and Point of Care Testing [Internet]https://www.rcpa.edu.au/Library/College-Policies/Position-Statements/COVID-19-Antigen-and-Point-of-Care-Testing.aspx Available from: [Google Scholar]
- 23.Public Health Laboratory Network . Joint Statement on SARS-CoV-2 Rapid Antigen Tests [Internet] 2021. Communicable diseases network Australia.https://www.rcpa.edu.au/Library/COVID-19-Updates/COVID-19-Useful-Resources/Docs/PHLN-Communicable-Diseases-Network-Australia-Joint Available from: [Google Scholar]
- 24.Turcato G, Zaboli A, Pfeifer N, et al. Rapid antigen test to identify COVID-19 infected patients with and without symptoms admitted to the Emergency Department. Amer J Emerg Med. 2022;51:92–97. doi: 10.1016/j.ajem.2021.10.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Caruana G, Croxatto A, Kampouri E, et al. Implementing SARS-CoV-2 rapid antigen testing in the emergency ward of a Swiss University hospital: the INCREASE study. Microorganisms. 2021;9(4):798. doi: 10.3390/microorganisms9040798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Cento V, Renica S, Matarazzo E, et al. Frontline screening for SARS-CoV-2 infection at Emergency Department Admission by third generation rapid antigen test: can we spare RT-qPCR? Viruses. 2021;13(5):818. doi: 10.3390/v13050818. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Leli C, Di Matteo L, Gotta F, et al. Performance of a SARS-CoV-2 antigen rapid immunoassay in patients admitted to the emergency department. Int J Infect Dis. 2021;110:135–140. doi: 10.1016/j.ijid.2021.07.043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Möckel M, Corman VM, Stegemann MS, et al. SARS-CoV-2 antigen rapid immunoassay for diagnosis of COVID-19 in the emergency department. Biomarkers. 2021;26(3):213–220. doi: 10.1080/1354750X.2021.1876769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Patriquin G, Davidson RJ, Hatchette TF, et al. Generation of false-positive SARS-CoV-2 antigen results with testing conditions outside manufacturer recommendations: a scientific approach to pandemic misinformation. 9(2):15.
- 30.U.S. FDA . 2022. Please Don't Go Sticking that #COVID19 Testing Swab Down Your Throat. Use Swabs as Instructed: Via the Nose. [Internet]https://twitter.com/US_FDA/status/1479835504199745537?ref_src=twsrc%5Etfw%7Ctwcamp%5Etweetembed%7Ctwterm%5E1479835504199745537%7Ctwgr%5E%7Ctwcon%5Es1_&ref_url=https%3A%2F%2Fwww.webmd.com%2Flung%2Fnews%2F20220111%2Fnose-throat-covid-test TwitterAvailable from: [Google Scholar]
- 31.Victorian Department of Health. Assessment and Testing Criteria for COVID-19 [Internet]. Available from: https://www.health.vic.gov.au/covid-19/assessment-and-testing-criteria-for-covid-19
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.