Abstract
Humans being unable to synthesize beta-carotene, the provitamin A, depend on external sources as its supplement. Health benefits and dietary requirements of beta-carotene are interrelated. This orange-red coloured pigment has been enormously examined for its capacity to alleviate several chronic diseases including various types of cancer, cystic fibrosis, as well as COVID-19. However, this class of phytoconstituents has witnessed a broad research gap due to several twin conclusions that have been reported. Natural sources for these compounds along with their extraction methods have been mentioned. The current communication aims at contributing to the global scientific literature on beta-carotene’s application in prevention and treatment of lifestyle diseases.
Graphical abstract

Keywords: Lifestyle diseases, Disease prevention, Disease treatment, Phytocompounds, Natural pigment, Antioxidant, Cancer, Cardiovascular diseases
Introduction
Carotenoids are the class of natural pigments belonging to the tetraterpenoids (C40 atoms) consisting of a long polyene chain with alternative conjugated double bonds with hefty scientific attention due to their substantial properties. Generally, carotenoids are classified as hydrocarbon carotenes, composed of only carbon and hydrogen (lycopene, alpha-carotene, and β-carotene etc.) and oxygenated carotenoids (xanthophylls), which contain an epoxy- (violaxanthin, neoxanthin, fucoxanthin), hydroxy- (lutein and zeaxanthin), keto- (canthaxanthin and astaxanthin), and methoxy- (spirilloxanthin) functional groups (Lakshminarayana et al. 2022). Out of around 600 structurally and functionally diverse natural types, three major provitamin As are the alpha, beta, and gamma isomers. The beta isomers get actively cleaved at the centre in an O2-dependent manner to produce retinal (Liang et al. 2013; PubChem 2022). Plants and algae are considered the best sources of these bright orange-red coloured pigments, such as β-carotene (1). These hydrocarbonated carotenes are fat-soluble and highly hydrophobic due to the conjugated double bonds and central symmetry (Rodriguez-Amaya 2016). These have been reported to be safe for consumption, as nutritional supplements as well as food additives (Akram et al. 2021).
β-Carotene (1, C40H56) is an oxygen-lacking isoprene-containing compounds, the characteristic colour imparted due to the presence of double bonds (PubChem 2022). Both the ends of the molecule have cyclic rings. During isolation, cis-isomers of carotenoids are the most common; however, they readily undergo cis ↔ trans isomerization in polar environments (Bartalucci et al. 2008). Due to the number of therapeutic and preventive effects that β-carotene offers, it has been recognized as a functional component of food.

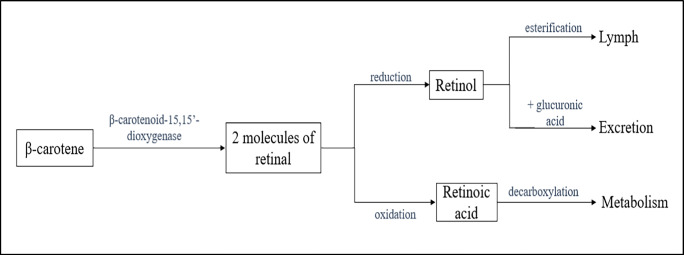
Upon oral administration of β-carotene, a hike of up to 60% in serum concentration is reported. It is the trans-isomer of β-carotene that is higher in serum than the cis-isomer. The concentration of β-carotene is different in parts of the body, being 2.2–122.7 μg/dl in serum, 0.21–6.3 μg/g in the liver, 0.05–1.5 μg/g in the kidney, and 0.05–0.86 μg/g in the lungs (Institute of Medicine 2000). However, the levels of β-carotene do not change in the kidneys, liver, or heart (DrugBank 2022). It is found that the carotenoid content of tissues with a greater number of low-density lipoprotein receptors (LDL-R) is greater, due to non-specific uptake (Maria et al. 2015). The intact β-carotene that is ingested has several metabolic fates to undergo, which are depicted in Fig. 1. However, research states that the lower the concentration of β-carotene, the more biologically effective is the pigment mixture of carotenoids (Lohan et al. 2018).
Fig. 1.
Fate of ingested β-carotene
As per James Olson, the activity of carotenes can be studied better upon the categorization of its properties as, “functions, actions, and associations”. The equivalence amongst these three would imply greater confidence in studies (Cooper 2004). Functions would be the indispensable role of β-carotene, to convert into vitamin A. Actions are demonstrations noted upon the administration of β-carotene in clinical subjects, minimized photosensitivity and so on. Associations could be simply understood as the correlation of β-carotene to any medical condition, like cancer or diabetes (Bendich and Olson 1989; Olson 1993; Hercberg 2005; Druesne-Pecollo et al. 2010; Marcelino et al. 2020)
This paper reviews the currently recognized potential of β-carotene in disease treatment and prevention. The carotenoid class of umbrella term nutraceuticals is underrated in the sense of its capabilities due to the literature gap and the research void. These points have been discussed in the paper in brief bringing its unnoticed therapeutic characteristics to light.
Search Strategies
Search keywords associated with β-carotene for various lifestyle disorders like cardiovascular diseases, cancer, COVID-19, and other diseases were used in the following order: β-carotene, sources of β-carotene, extraction technique, cancer, COVID-19, and cardiovascular diseases. The following list of biological databases was used to search the data for writing this article: PubMed, PubMed Central, Google Scholar, Springer, Science Direct, Wiley Online Library, LiebertPub, Nature. A time filter was used to retrieve articles from 2015 to 2021 in PubMed.
Discussion
Physicochemical Properties
β-Carotene, a type of secondary metabolite produced by plants and algae, is a dark red to brown-coloured solid substance. The brightness of the colour depends upon the level of double bond cis ↔ trans isomerization, as a result of exposure to high temperatures (Bogacz-Radomska and Harasym 2018). The melting point of β-carotene is around 178 °C, having variable solubility in non-polar organic solvents but water-insoluble. The substance is heat-labile and photosensitive, making the storage condition preferably in dark at 4 °C. The lipophilic properties of β-carotene make it localized in lipoproteins and cell membranes. Hence, the extracted β-carotene is also found dissolved in the lipid phase (Bogacz-Radomska and Harasym 2018).
Dietary Requirements
Even being categorized as a micronutrient, due to its requirement in minute amounts as compared to other requirements of the body, β-carotene is considered to be very essential. β-Carotene conversion to vitamin A has moved from 6:1 to the current ratio of 28:1. The reason is the single nucleotide polymorphisms in the β-carotene-15′,15-monooxygenase (BCMO) enzyme, responsible for converting provitamin A into retinol. This means that, in the affected individuals, 28 mg of β-carotene is converted into 1 mg of retinol. This exposes the population to a greater risk of developing vitamin A deficiency and associated disorders, as well as the inability to utilize provitamin A from the diet (Gröber and Holick 2022).
To date, no potential adverse effects of β-carotene consumption have been reported other than ‘carotenodermia’ which is skin discolouration due to elevated concentrations of carotenoids. β-Carotene did not appear to be mutagenic, carcinogenic, nor teratogenic in any of the assays carried out (Milani et al. 2017; Pham et al. 2014). In fact, no increase in serum retinol level was found even after long-term β-carotene supplementation in people with already adequate levels of vitamin A (Nierenberg et al. 1997). For prolonged serum and tissue accumulation of β-carotene, its administration must be with dietary fats (Kwatra and Modi 2020). However, people who smoke are better not subjected to high supplementations of β-carotene as this might put them at higher risk of lung cancer and an enhanced chance of mortality (Bohn et al. 2019). Most of the absorbed β-carotene is converted by the gut microbes into unknown complex compounds, and the remainder gets excreted. The general absorption, distribution, metabolism, and excretion route of β-carotene in humans is illustrated in Fig. 2.
Fig. 2.
ADME (absorption, distribution, metabolism and excretion) of β-carotene
Sources and Production
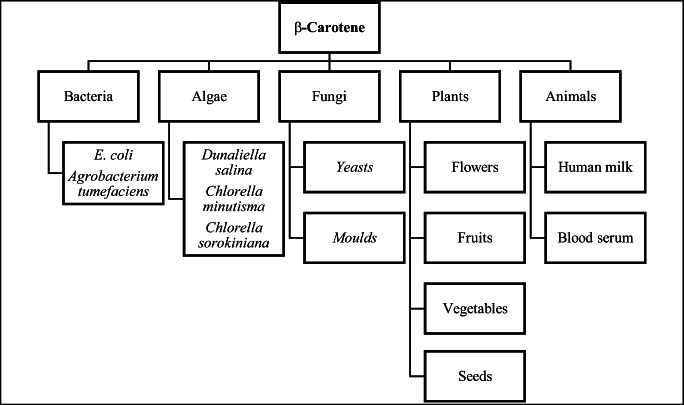
Mammals lack the de novo carotenoid synthesis capability and hence totally depend on external sources for their supplementation (Huang et al. 2018) (Fig. 3). These yellow-, red-, and orange-coloured pigments are naturally found in algae, higher plants (in fruits and flowers as esters), fungi, and animals as illustrated in Fig. 4 (Rodriguez-Amaya 2016). Even human milk has a carotenoid component, which is easily alterable, depending on the manipulations in the mother’s diet (Institute of Medicine 2000). This intake of β-carotenoids is reflected in serum levels which is also an indicator of the health parameters. A natural pigment, so promising, should have been the centre of the bio-pharmaceutical industry. However, it is not. Out of all the β-carotenoids in the market, only 3% of it comprise the bio-synthesized type and the rest comes under the synthetic β-carotene category. There is no strict difference between the function of the two, natural and synthetic. But the latter costs more. Switching to microalgae for β-carotene extraction is both scientifically and economically sensible. A lot of research has been done on the optimization of pigment in various species, along with special extraction techniques for cost-effective and enhanced yields (Marino et al. 2020).
Fig. 3.
Different natural sources of β-carotene
Fig. 4.
A representation of the necessary balance of antioxidant properties of β-carotene
There are several extraction methods of β-carotene, depending upon the source selected. A lot of research is involved in the optimization of pigment production as well as its extraction. From a broader perspective, the extraction methods can be divided into the organic solvent-based, enzymatic method as well as mechanical extraction. Some collaborative extraction techniques also exist which involve clubbing of the existing methods, as well as some innovation (Chen et al. 2021).
Extraction
Solvent-based extraction includes the use of aprotic organic solvents such as hexane, tetrahydrofuran, and acetone for the extraction of β-carotene from algae post- and pre-treatment. These extraction solvents are useful for the extraction of β-carotene from algae, fungi, and plants (Chen et al. 2021). Supercritical fluids reduce the extraction time of β-carotene as well as enhance the efficiency of extraction. Atmospheric liquid extraction with the help of maceration is used for extraction purposes without the use of sophisticated instruments and is simple to carry out. Soxhlet extraction is much simpler and most widely used for the extraction of carotenoids such as β-carotene. This method provides the highest recovery rates and does not require sophisticated instruments. This method uses large amounts of solvents along with the starter material for extraction purposes which makes the extraction procedure highly expensive (Saini and Keum 2018). Supercritical fluids are used for the extraction of β-carotene from plants and algae (Baysal et al. 2000). Various physical methods such as ultrasonic waves, microwave-assisted extraction, pressurized liquid extraction, bead milling, and high-pressure homogenization are also used for the extraction of β-carotene. These methods prevent thermal as well as chemical damage to pigment. This method is used for the extraction of β-carotene from algae, yeast, and plants (Dey and Rathod 2013; Yu and Rupasinghe 2013). There are several enzymatic approaches including pectinolytic and cellulolytic approaches which can be used in combination with other extraction methods for cell disruption and β-carotene extraction. These methods can be used for the extraction of β-carotene from carrots, bagasse, apples, and other juice-extracted leftover (Macedo et al. 2014). The selection of an appropriate method for β-carotene extraction depends upon the efficacy and the targeted purpose (Saini and Keum 2018).
Synthesis
Genetic engineering approaches such as the insertion of functional genes such as crtI, crtE, crtY, and crtYB enhance the production of β-carotene and block the expression of repressor genes like crgA which blocks the synthesis of β-carotene. These approaches have been applied to red yeast and fungus so far (Zhang et al. 2016; Wu et al. 2020). There are various environmental conditions such as organism-based culture condition, optimization of light intensity, salt stress, pH, and carbon sources that can be optimized for enhanced production of β-carotene. These optimizations have been carried out on microalgae, plants, and fungi for enhanced production of β-carotene (Chen et al. 2021). One of the key methods for enhanced production of β-carotene is the construction of polyene chains using Wittig’s reaction, such as two 15 carbons containing phosphonium salt molecule along with one 10 carbons containing dialdehyde molecule, and using Grignard’s reaction involving 2 molecules of methanol and diketone (Farfán et al. 2019).
Quantification
The spectrophotometric analysis method is an inexpensive, sensitive, simple, and rapid procedure for the estimation of β-carotene. This method employs the use of smaller amounts of toxic chemical solvent for the analysis of β-carotene. Spectroscopic methods have widely been employed in commercial settings; however, this method faces certain disadvantages such as being applicable for certain food components only (Biswas et al. 2011). Recently, many liquid chromatography (LC) techniques have also been developed for the analysis of β-carotene. Amongst all the LC techniques, high-performance liquid chromatography (HPLC) is the most suitable method for analysis (separation and quantification) of β-carotene. HPLC has a requirement for high sample purification steps for the analysis of β-carotene present in the sample (Olives-Barba et al. 2006). Column chromatography is also one of the most important methods for the separation and detection of various carotenoids including β-carotene. In the case of column chromatography, silicic acid and alumina are used as stationary phases in order to separate different carotenoids from the extracts (Starek et al. 2015; Ganea et al. 2016).
Disease Prevention and Treatment
β-Carotene not only treats or prevents the likeliness of disease development but also improves its prognosis. It has been found to play roles like antioxidant and anti-inflammation, upon administration in varying amounts:
-
i)
In a study, subjects with a generous amount of β-carotene intake, about 12–25 mg/day, reported a decrease in DNA strand breakage, along with a reduction in Cu-induced LDL oxidation. There was also subsequent supplementation of vitamins C and E (Pool-Zobel and Bub 1997; Mosca et al. 1997). An amount of 10 mg/day was reported in increased CuZn-superoxide dismutase activity (Hininger et al. 1997). In subjects with increased oxidative stress, smokers, and sufferers of cystic fibrosis, administered β-carotene resulted in depleted lipid peroxidation as compared to control. The biological markers responded inconsistently when the amounts of β-carotene were higher than 25 mg/day (Institute of Medicine 2000).
-
ii)
In vitro studies have demonstrated a stimulation of cellular communication through gap junctions (Sies and Stahl 1997). A study concluded the positive effect of β-carotene leading to increased intercellular communications in rats (Novo et al. 2013).
-
iii)
The degree of conversion of the consumed provitamin A carotenes to retinol is the essential measure to which the deficiency of vitamin A, common in children, can be treatable. This is because vitamin A deficiency is indirectly related to poor immunity of the body, night blindness, and so on. In older men, β-carotene increased the activity of natural killer cells (Hughes et al. 1997).
-
iv)
There is a contrary association between the pigment levels in serum and systemic inflammation markers like neutrophil to lymphocyte ratio and resistance to insulin as well as dysfunctional beta cells (Huang et al. 2018).
-
v)
The carotenoid-derived metabolites have been found to participate in gene alterations by interactions with nuclear and retinoic acid receptors (Piga et al. 2014).
Various studies have been carried out to identify the actions of β-carotene intake; CARET study was carried out for the prevention of lung cancer using β-carotene along with retinol. The study reported a twin conclusion that daily consumption of 30 mg of β-carotene along with retinol supplementation showed no harm as well as no benefit in preventing lung cancer (NLM NCT00712647 2022). Another study named as ATBC (α-tocopherol, β-carotene cancer prevention) has been carried out to access the incidence rate of cancer and mortality in male smokers using β-carotene and α-tocopherol. The study reported that these compounds can be used as a “chemo-preventive” for lung cancer (NLM NCT00342992 2022; ATBC cancer prevention study group 1994).
Depending upon the extent of oxidative stress in the environment to which the pigment is exposed, the actions can be beneficial or damaging. The accumulation of very reactive products released upon carotenoid breakdown contributes to pro-oxidation. This involves the production of very reactive organic compounds like aldehydes. Such products are termed carotene breakdown products (CBPs) (Siems et al. 2009). A representation of the necessary balance of antioxidant properties of β-carotene is depicted in Fig. 4. Hence, it can be summarized that the pigment offers several therapeutic effects in the context of acting as a provitamin A: antioxidants neutralizing ROS, regulating connexin expression, thus improving communications through gap junctions, activating macrophages, and triggering an immune response (Chen et al. 2021). Various therapeutic effects of β-carotene are recapitulated in Fig. 5. A combination of magnesium along with vitamins having antioxidant properties has been used to treat noise-induced hearing loss (NIHL) in animals, which is assumed to scavenge oxidative radicals produced due to trauma (le Prell et al. 2011; Eroglu and Harrison 2013; Álvarez et al. 2014; Maurya et al. 2021).
Fig. 5.
Therapeutic effects of β-carotene
Bioavailability and Absorption
The lipophilic β-carotene like all other lipids and lipid-associated compounds is absorbed in the mammalian small intestine for further transport to the peripheral tissues. Despite the presence of β-carotene-cleaving enzymes in the small intestine, about half of the uncleaved β-carotene enters the circulatory system. The readily available provitamin A absorbed by human intestinal epithelia can be estimated by the concentration of intact plasma-β-carotene (Shete and Quadro 2013). The other factors affecting the bioavailability of β-carotene are genetic factors like pigment-cleaving gene polymorphisms and mutations, the type and lipid content of the food and its matrix, its digestibility and interactions, and subjective variations referring to individuals’ endogenous digestive enzymes (Reboul 2013).
Lipoproteins and cholesterols facilitate the transport and distribution of the non-polar β-carotenes in the organism. They can be found in the hydrophobic cores of organic compounds like various sub-types of lipoproteins, and cholesteryl-esters (Shete and Quadro 2013). This β-carotene flowing from blood can be taken up by tissues for either storage or metabolism. The most efficient reservoir of β-carotene in the human body is the liver, followed by muscles, kidneys, and skin, as well as glands like adrenal and mammary glands. It has also been found in the placenta and the yolk sac. Hence, it is comparatively very widely distributed in the body as compared to the other classes of carotenoids (Renzi et al. 2012). The cleavage, transport, and distribution of β-carotene in humans are similar to that in ruminants, and hence, they are considered a good study model (Bohn et al. 2019).
Being water-insoluble, the bioavailability of β-carotene through the gastrointestinal tract is very low. Due to its vulnerability to physiochemical degradation during processing, storage, and post-consumption, its protected delivery becomes necessary. Nanotechnology offers better solubility, storage, target delivery, encapsulated protection stability, and dispersion properties to the β-carotene. Some such nano-engineered forms of β-carotene are nanostructure- or solid-lipid-carriers, microemulsions, nano-spheres, and capsules, inter alia. The polymer-based and lipid-based delivery systems are the most adopted delivery systems for β-carotene. These nano-engineered pigments have to be targeted to the gastrointestinal tract fluid for them to be absorbed by the enterocytes for subsequent assimilation. For this purpose, micelles and niosomes have been widely exploited. However, the interaction of these nano-pigments with the gastrointestinal tract cells and the environment within must be wisely researched before their incorporation into functional foods.
Cancer
The confirmation of the anti-cancer properties of fruits and vegetables rich in carotenes comes from epidemiological studies. According to the observations, the pigment-rich food preceded the direct intake of supplements in preference. This protection against cancer that carotene provides is due to metabolisms like singlet-oxygen quenchers, immunity enhancement, intracellular communications through gap junctions, and inhibition of cell division (Cooper 2004).
Communication that is regulated amongst cells means coordination in metabolism and growth. The failed signalling would eventually lead to uncontrolled cell growth and ultimately cancer. Hence, the cell-signalling regulation is another mode of action, through which β-carotene offers anti-cancer properties (Institute of Medicine (US) Panel on Dietary Antioxidants and Related Compounds, 2000). In fact, β-carotene supplementation can be useful in the treatment of site-specific cancers categorized as that of high incidence. For example, gastric cancer, which has complex aetiology has been under research against this natural pigment by many scientists. Varied research methods and subject areas have been used for epidemiological and cohort studies on patients suffering from gastric cancer. Several molecular mechanisms of the pigment are established for the probable result in the treatment of such patients in the past years (Chen et al. 2021). It also exhibits apoptotic as well as anti-proliferative effects on tumour cells (Kacar et al. 2022).
Cardiovascular Diseases
Diet rich in β-carotene has been found to be preventive for heart-related problems (Yang et al. 2022). The pigment is fat-soluble and is transported primarily in low-density lipoproteins (LDL). This association protects LDL from getting oxidized which would otherwise upon oxidation cause atherogenesis. A decrease in the thickness of “intima-media” resembles decreased risk of atherosclerosis, which was a result of increased total carotenoid concentration in serum (Mikkilä et al. 2009; Bhat et al. 2019). There is a lowered risk of CVD and related mortality cases due to the antioxidant effect of β-carotene which modifies the LDL oxidation and peroxidation mechanism (Carroll et al. 2000).
The β-carotene in blood plasma effectively increases the bioavailability of nitrous oxide (NO) along with levels of cyclic guanosine monophosphate (cGMP). This leads to a decline in endothelial cells’ NF-κB-dependent molecules responsible for adhesion. The pigment also can enhance drug activity and function in the downregulation of genes that are involved in metabolizing cholesterol. Hence, there is a reduced chance of developing atherogenesis or other cardiac-related issues (Aizawa et al. 2003; Shaish et al. 2006).
COVID-19
Medical professionals confirm that the deficiency of vitamin A is associated with infections of the respiratory tract (Calder 2020). On the other hand, deficiency of vitamin D, due to less exposure to the sun, has been the reason for viral epidemics in winters. Muscogiuri et al. (2020) has described the interrelation of quarantine periods practised during COVID-19 with vitamin deficiency in patients. Doctors recommended strict diets to patients infected with corona virus, inclusive of β-carotene supplementing vegetables and fruits along with those supplementing vitamin C and other micronutrients. On the other hand, β-carotene due to its antioxidant activity stimulates responses from lymphocytes, interleukin production, and the activity of natural killer cells and, thus, stimulating the overall immune system (Muscogiuri et al. 2020; Gröber and Holick 2022).
Other Diseases
Patients suffering from diseases like cystic fibrosis (CF) and non-CF-associated bronchiectasis have lower levels of plasma-β-carotene as well as vitamin E. They have been found to be disposable to damage by oxidation, which can be prevented by β-carotene supplementation (Çobanogcarlu et al. 2002). The different roles played by β-carotene contribute to different therapeutic effects in the management of several diseases including cancer, cardiovascular disorders, COVID-19, cystic fibrosis, and bronchiectasis. In the case of cancer, β-carotene acts as an antioxidant and anti-inflammatory, enhances immunity, and increases intracellular signalling. In addition to these effects, β-carotene also exhibits anti-apoptotic and anti-progressive effects on tumour (Middha et al. 2019). β-Carotene exhibits antioxidative properties and enhances drug activity in the case of cardiovascular diseases (Huang et al. 2018). In the case of cystic fibrosis, β-carotene helps to reduce the levels of TNF-α (tumour necrosis factor) and helps to increase the levels of vitamin E; also, it acts as an antioxidant (Çobanogcarlu et al. 2002). In bronchiectasis, β-carotene has been reported to reduce the levels of malondialdehydes and also serves as an antioxidant (Çobanogcarlu et al. 2002; Bartoli et al. 2011). In COVID-19 disease, β-carotene has been reported to stimulate cellular immune responses as well as act as an antioxidant (Muscogiuri et al. 2020).
Perspectives and Future Directions
Some of the physiological properties of β-carotene limit its effectiveness. Its poor bioavailability in crystalline form was reported, and is insoluble in water. Hence, its better delivery is obtained in oil/water emulsion, which also imparts the pigment and its stability (Liu et al. 2014). Its sensitivity to heat and light and liability to oxidation are other challenges that need to be addressed. The trend of involving low-on-fat products in food has been adversely affecting the absorption of β-carotene in the body. This might also be the reason for trial results not agreeing with the epidemiological observations. The span of the study and the dosage of β-carotene are all crucial in determining the pigment’s disease-preventing potential.
The effect of carotenes on diseases can be studied better by considering the epigenetic factors. The diet pattern is another factor. The complex evidence of research has to be translated into an effective lifestyle and dietary recommendation, to lower the risk of disease development. The existing pattern of public health will then witness a paradigm shift, post-recognition of carotenes not only in disease treatment but also in prevention.
It is important to identify the indicators of carotene adequacy in the system and evaluate them. Such biological markers could be strong evidence of β-carotene intake levels required to prevent diseases. The biomarkers, indicative of disease progression, should be ligand-specific and indicative of β-carotene intake with responsiveness. However, these biomarkers must be validated, engaging a broad range of analogues and subjects.
The biological activity of β-carotene is enhanced in an aqueous solution if it is present in the form of nanoparticles (Rocha et al. 2018). This is where the role of nanotechnology might come. β-Carotene can be applied as nutraceuticals, cosmeceuticals, and pharmaceuticals in the form of emulsions, with improved sensitivity, solubility, and bioavailability (Yuan et al. 2008). There are a lot of prospects for enhanced production of β-carotenoid engaging the technological advancements of genetic engineering and nanotechnology. Regulation of gene expression or transformation of genes involved in β-carotene biosynthesis in some ways is to be listed. Proteins and water-soluble nano-β-carotene particles can offer bioactivity beyond their limitations.
Conclusions
The human body has a very vigilant feedback mechanism that maintains a balance between the serum retinol and β-carotene concentration. When the body gets deficient in vitamin A, the pre-consumed available β-carotene is converted into retinol and the metabolic conversion stops as the levels reach optimum. Until now, no side effects of β-carotene consumption have been reported apart from the harmless skin pigmentation due to overdose. However, some studies suggest post-menopause women are at a higher risk of fractures if having an excess of supplemental vitamin A. This could be simply concluded as making a wise preference of choosing diet-based β-carotene over-supplementation. During the literature survey, it was found that not much work has been carried out on β-carotene in the past two decades. Most of the available research data date back to the mid-90s, which resembles the scientific gap that the carotenoid family has been subjected to.
Acknowledgements
The authors would like to acknowledge the Department of Biotechnology, Delhi Technological University, for their constant support.
Author Contribution
Conceptualization of the article, editing, and supervision were done by NB. Literature search, data compilation, and writing and editing of the article were done by RA and LM. All authors have read and approved the final submission.
Footnotes
Raksha Anand and Lalit Mohan contributed equally to this work.
References
- Aizawa T, Wei H, Miano JM, Abe J, Berk BC, Yan C. Role of phosphodiesterase 3 in NO/cGMP-mediated anti-inflammatory effects in vascular smooth muscle cells. Circ Res. 2003;93:406–413. doi: 10.1161/01.RES.0000091074.33584.F0. [DOI] [PubMed] [Google Scholar]
- Akram S, Mushtaq M, Waheed A. β-Carotene: beyond provitamin A. In: Mushtaq M, Anwar F, editors. A centum of valuable plant bioactives. London: Academic Pres; 2021. pp. 1–3. [Google Scholar]
- Álvarez R, Vaz B, Gronemeyer H, de Lera ÁR. Functions, therapeutic applications, and synthesis of retinoids and carotenoids. Chem Rev. 2014;114:1–125. doi: 10.1021/cr400126u. [DOI] [PubMed] [Google Scholar]
- ATBC cancer prevention study group The alpha-tocopherol, beta-carotene lung cancer prevention study: design, methods, participant characteristics, and compliance. Ann Epidemiol. 1994;4:1–10. doi: 10.1016/1047-2797(94)90036-1. [DOI] [PubMed] [Google Scholar]
- Bartalucci G, Delroy C, Fisher S, Helliwell M, Liaaen-Jensen S. 13- cis-β,β-carotene and 15- cis-β,β-carotene. Acta Crystallogr Structure. 2008;64:128–131. doi: 10.1107/S0108270108001583. [DOI] [PubMed] [Google Scholar]
- Bartoli ML, Novelli F, Costa F, Malagrinò L, Melosini L, Bacci E, Cianchetti S, Dente FL, di Franco A, Vagaggini B, Paggiaro PL. Malondialdehyde in exhaled breath condensate as a marker of oxidative stress in different pulmonary diseases. Mediat Inflamm. 2011;2011:891752. doi: 10.1155/2011/891752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baysal T, Ersus S, Starmans DAJ. Supercritical CO2 extraction of β-carotene and lycopene from tomato paste waste. J Agric Food Chem. 2000;48:5507–5511. doi: 10.1021/jf000311t. [DOI] [PubMed] [Google Scholar]
- Bendich A, Olson JA. Biological actions of carotenoids. FASEB J. 1989;3:1927–1932. doi: 10.1096/fasebj.3.8.2656356. [DOI] [PubMed] [Google Scholar]
- Bhat S, Mocciaro G, Ray S. The association of dietary patterns and carotid intima-media thickness: a synthesis of current evidence. Nutr Metab Cardiovasc Dis. 2019;29:1273–1287. doi: 10.1016/j.numecd.2019.08.014. [DOI] [PubMed] [Google Scholar]
- Biswas AK, Sahoo J, Chatli MK. A simple UV-Vis spectrophotometric method for determination of β-carotene content in raw carrot, sweet potato and supplemented chicken meat nuggets. LWT - Food Sci Technol. 2011;44:1809–1813. doi: 10.1016/j.lwt.2011.03.017. [DOI] [Google Scholar]
- Bogacz-Radomska L, Harasym J. β-Carotene-properties and production methods. Food Qual Saf. 2018;2:69–74. doi: 10.1093/fqsafe/fyy004. [DOI] [Google Scholar]
- Bohn T, Desmarchelier C, El SN, Keijer J, van Schothorst E, Rühl R, Borel P. β-Carotene in the human body: metabolic bioactivation pathways – from digestion to tissue distribution and excretion. Proc Nutr Soc. 2019;78:68–87. doi: 10.1017/S0029665118002641. [DOI] [PubMed] [Google Scholar]
- Calder PC. Nutrition, immunity and COVID-19. BMJ Nutr Prev & Health. 2020;3:74–92. doi: 10.1136/bmjnph-2020-000085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carroll Y, Corridan B, Morrissey P. Lipoprotein carotenoid profiles and the susceptibility of low-density lipoprotein to oxidative modification in healthy elderly volunteers. Eur J Clin Nutr. 2000;54:500–507. doi: 10.1038/sj.ejcn.1601046. [DOI] [PubMed] [Google Scholar]
- Chen Q-H, Wu B-K, Pan D, Sang L-X, Chang B. Beta-carotene and its protective effect on gastric cancer. World J Clin Cases. 2021;9:6591–6607. doi: 10.12998/wjcc.v9.i23.6591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Çobanogcarlu N, Özçelik U, Göçmen A, Kiper N, Doǧru D. Antioxidant effect of β-carotene in cystic fibrosis and bronchiectasis: clinical and laboratory parameters of a pilot study. Acta Paediatr. 2002;91:793–798. doi: 10.1080/08035250213212. [DOI] [PubMed] [Google Scholar]
- Cooper DA. Functions and actions of retinoids and carotenoids: building on the vision of James Allen Olson carotenoids in health and disease: recent Scientific Evaluations, Research Recommendations and the Consumer. J Nutr. 2004;134:220S–293S. doi: 10.1093/jn/134.1.220S. [DOI] [PubMed] [Google Scholar]
- Dey S, Rathod VK. Ultrasound assisted extraction of β-carotene from Spirulina platensis. Ultrason Sonochem. 2013;20:271–276. doi: 10.1016/j.ultsonch.2012.05.010. [DOI] [PubMed] [Google Scholar]
- Druesne-Pecollo N, Latino-Martel P, Norat T, Barrandon E, Bertrais S, Galan P, Hercberg S. Beta-carotene supplementation and cancer risk: a systematic review and metaanalysis of randomized controlled trials. Int J Cancer. 2010;127:172–184. doi: 10.1002/ijc.25008. [DOI] [PubMed] [Google Scholar]
- DrugBank (2022) Compound summary for DBAN DB06755, beta-carotene. Retrieved March 14, 2022 from https://go.drugbank.com/drugs/DB06755.
- Eroglu A, Harrison EH. Carotenoid metabolism in mammals, including man: formation, occurrence, and function of apocarotenoids. J Lipid Res. 2013;54:1719–1730. doi: 10.1194/jlr.R039537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Farfán P, Gómez S, Restrepo A. Dissection of the mechanism of the Wittig reaction. J Org Chem. 2019;84:14644–14658. doi: 10.1021/acs.joc.9b02224. [DOI] [PubMed] [Google Scholar]
- Ganea M, Moisa C, Cozma A, Bota S. Determination of carotenoids by thin layer chromatography. Analele Univ din Oradea, Fasc Protecția Mediu. 2016;26:247–252. [Google Scholar]
- Gröber U, Holick MF. The coronavirus disease (COVID-19) – a supportive approach with selected micronutrients. Int J Vitam Nutr Res. 2022;92:13–34. doi: 10.1024/0300-9831/a000693. [DOI] [PubMed] [Google Scholar]
- Hercberg S. The history of beta-carotene and cancers: from observational to intervention studies. What lessons can be drawn for future research on polyphenols? Am J Clin Nutr. 2005;81:218S–222S. doi: 10.1093/ajcn/81.1.218s. [DOI] [PubMed] [Google Scholar]
- Hininger I, Chopra M, Thurnham DI, Laporte F, Richard M-J, Favier A, Roussel A-M. Effect of increased fruit and vegetable intake on the susceptibility of lipoprotein to oxidation in smokers. Eur J Clin Nutr. 1997;51:601–606. doi: 10.1038/sj.ejcn.1600451. [DOI] [PubMed] [Google Scholar]
- Huang J, Weinstein SJ, Yu K, Männistö S, Albanes D. Serum beta carotene and overall and cause-specific mortality. Circ Res. 2018;123:1339–1349. doi: 10.1161/CIRCRESAHA.118.313409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hughes DA, Wright AJA, Finglas PM, Peerless ACJ, Bailey AL, Astley SB, Pinder AC, Norwich SS. The effect of 13-carotene supplementation on the immune function of blood monocytes from healthy male nonsmokers. J Lab Clin Med. 1997;129:309–317. doi: 10.1016/s0022-2143(97)90179-7. [DOI] [PubMed] [Google Scholar]
- Institute of Medicine (US) Panel on Dietary Antioxidants and Related Compounds . Dietary reference intakes for vitamin C, vitamin E, selenium, and carotenoids. Washington (DC): NAP (US); 2000. [PubMed] [Google Scholar]
- Kacar S, Sariisik E, Sahinturk V. Beta-carotene exerted anti-proliferative and apoptotic effect on malignant mesothelioma cells. Naunyn-Schmiedeb Arch Pharmacol. 2022;395:407–415. doi: 10.1007/s00210-022-02214-6. [DOI] [PubMed] [Google Scholar]
- Kwatra B, Modi R. Therapeutic potentials and applications of folic acid and beta carotene. Int J Sci Res Sci Technol. 2020;7:271–282. doi: 10.32628/IJSRST1207481. [DOI] [Google Scholar]
- Lakshminarayana R, Vijay K, Ambedkar R, Ranga Rao A, Ravishankar GA. Biological activities and health benefits of seaweed carotenoids with special reference to fucoxanthin. In: Ranga Rao A, Ravishankar GA, editors. Sustainable global resources of seaweeds Volume 2. Cham: Springer; 2022. pp. 539–558. [Google Scholar]
- le Prell C, Lindblad A, Ulfendahl M, Green G, Miller J, Johnson A, Skjönsberg Å, Guire K, Campbell KCM. Increased vitamin plasma levels in Swedish military personnel treated with nutrients prior to automatic weapon training. Noise Health. 2011;13:432–443. doi: 10.4103/1463-1741.90317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liang R, Huang Q, Ma J, Shoemaker CF, Zhong F. Effect of relative humidity on the store stability of spray-dried beta-carotene nanoemulsions. Food Hydrocoll. 2013;33:225–233. doi: 10.1016/j.foodhyd.2013.03.015. [DOI] [Google Scholar]
- Liu Y, Hou Z, Yang J, Gao Y. Effects of antioxidants on the stability of β-carotene in O/W emulsions stabilized by Gum Arabic. J Food Sci Technol. 2014;52:3300–3311. doi: 10.1007/s13197-014-1380-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lohan SB, Vitt K, Scholz P, Keck CM, Meinke MC. ROS production and glutathione response in keratinocytes after application of β-carotene and VIS/NIR irradiation. Chem Biol Interact. 2018;280:1–7. doi: 10.1016/j.cbi.2017.12.002. [DOI] [PubMed] [Google Scholar]
- Macedo M, Robrigues RDP, Pinto GAS, de Brito ES. Influence of pectinolyttic and cellulotyc enzyme complexes on cashew bagasse maceration in order to obtain carotenoids. J Food Sci Technol. 2014;52:3689–3693. doi: 10.1007/s13197-014-1411-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marcelino G, Machate DJ, de Freitas KC, Hiane PA, Maldonade IR, Pott A, Asato MA, Candido CJ, Guimarães RCA. β-Carotene: preventive role for type 2 diabetes mellitus and obesity: a review. Molecules. 2020;25:5803. doi: 10.3390/molecules25245803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maria AG, Graziano R, Nicolantonio D. Carotenoids: potential allies of cardiovascular health? Food Nutr Res. 2015;59:26762. doi: 10.3402/fnr.v59.26762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marino T, Casella P, Sangiorgio P, Verardi A, Ferraro A, Hristoforou E, Molino A, Musmarra D. Natural beta-carotene: a microalgae derivate for nutraceutical applications. Chem Eng Trans. 2020;79:103–108. doi: 10.3303/CET2079018. [DOI] [Google Scholar]
- Maurya VK, Shakya A, Aggarwal M, Gothandam KM, Bohn T, Pareek S. Fate of β-carotene within loaded delivery systems in food: state of knowledge. Antioxidants. 2021;10:426. doi: 10.3390/antiox10030426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Middha P, Weinstein SJ, Männistö S, Albanes D, Mondul AM. β-Carotene supplementation and lung cancer incidence in the alpha-tocopherol, beta-carotene cancer prevention study: the role of tar and nicotine. Nicotine Tob Res. 2019;21:1045–1050. doi: 10.1093/ntr/nty115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mikkilä V, Räsänen L, Laaksonen MML, Juonala M, Viikari J, Pietinen P, Raitakari OT. Long-term dietary patterns and carotid artery intima media thickness: the cardiovascular risk in Young Finns Study. Br J Nutr. 2009;102:1507–1512. doi: 10.1017/S000711450999064X. [DOI] [PubMed] [Google Scholar]
- Milani A, Basirnejad M, Shahbazi S, Bolhassani A. Carotenoids: biochemistry, pharmacology and treatment. Br J Pharmacol. 2017;174:1290–1324. doi: 10.1111/bph.13625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mosca L, Rubenfire M, Mandel C, Rock C, Tarshis T, Tsai A, Pearson T. Antioxidant nutrient supplementation reduces the susceptibility of low density lipoprotein to oxidation in patients with coronary artery disease. J Am Coll Cardiol. 1997;30:392–399. doi: 10.1016/S0735-1097(97)00188-5. [DOI] [PubMed] [Google Scholar]
- Muscogiuri G, Barrea L, Savastano S, Colao A. Nutritional recommendations for CoVID-19 quarantine. Eur J Clin Nutr. 2020;74:850–851. doi: 10.1038/s41430-020-0635-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nierenberg DW, Dain BJ, Mott LA, Baron JA, Greenberg ER. Effects of oral supplementation with 3-carotene on serum concentrations of retinol, tocopherol, and five carotenoids. Am J Clin Nutr. 1997;66:315–319. doi: 10.1093/ajcn/66.2.315. [DOI] [PubMed] [Google Scholar]
- NLM NCT00342992 Clinicaltrials.gov (2022) Carotene and retinol efficacy trial. [online] Available at: https://clinicaltrials.gov/ct2/show/study/NCT00342992 [Accessed 14 Mar 2022]
- NLM NCT00712647 Clinicaltrials.gov (2022) Carotene and retinol efficacy trial. [online] Available at: https://clinicaltrials.gov/ct2/show/study/NCT00712647 [Accessed 14 Mar 2022].
- Novo R, Azevedo PS, Minicucci MF, Zornoff LAM, Paiva SAR. Effect of beta-carotene on oxidative stress and expression of cardiac connexin 43. Arq Bras Cardiol. 2013;101:233–239. doi: 10.5935/abc.20130160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olives-Barba AI, Cámara-Hurtado M, Sánchez-Mata MC, Fernández-Ruiz V, López-Sáenz de Tejada M. Application of a UV-vis detection-HPLC method for a rapid determination of lycopene and β-carotene in vegetables. Food Chem. 2006;95:328–336. doi: 10.1016/j.foodchem.2005.02.028. [DOI] [Google Scholar]
- Olson JA. Molecular actions of carotenoids. Ann N Y Acad Sci. 1993;691:156–166. doi: 10.1111/j.1749-6632.1993.tb26167.x. [DOI] [PubMed] [Google Scholar]
- Pham MA, Byun HG, Kim KD, Lee SM. Effects of dietary carotenoid source and level on growth, skin pigmentation, antioxidant activity and chemical composition of juvenile olive flounder Paralichthys olivaceus. Aquaculture. 2014;431:65–72. doi: 10.1016/j.aquaculture.2014.04.019. [DOI] [Google Scholar]
- Piga R, van Dartel D, Bunschoten A, van der Stelt I, Keijer J. Role of Frizzled6 in the molecular mechanism of beta-carotene action in the lung. Toxicology. 2014;320:67–73. doi: 10.1016/j.tox.2014.03.002. [DOI] [PubMed] [Google Scholar]
- Pool-Zobel BL, Bub A. Consumption of vegetables reduces genetic damage in humans: first results of a human intervention trial with carotenoid-rich foods. Carcinogenesis. 1997;18:1847–1850. doi: 10.1093/carcin/18.9.1847. [DOI] [PubMed] [Google Scholar]
- PubChem 2022- National Center for Biotechnology Information (2022) PubChem Compound Summary for CID 5280489, beta-Carotene. Retrieved March 1, 2022 from https://pubchem.ncbi.nlm.nih.gov/compound/beta-Carotene
- Reboul E. Absorption of vitamin A and carotenoids by the enterocyte: focus on transport proteins. Nutrients. 2013;5:3563–3581. doi: 10.3390/nu5093563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Renzi LM, Hammond BR, Dengler M, Roberts R. The relation between serum lipids and lutein and zeaxanthin in the serum and retina: results from cross-sectional, case-control and case study designs. Lipids Health Dis. 2012;11:33. doi: 10.1186/1476-511X-11-33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rocha F, Yumi Sugahara L, Leimann FV, de Oliveira SM, da Silva BE, Calhelha RC, Barreiro MF, Ferreira ICFR, Porto Ineu R, Gonçalves OH. Nanodispersions of beta-carotene: effects on antioxidant enzymes and cytotoxic properties. Food Funct. 2018;9:3698–3706. doi: 10.1039/C8FO00804C. [DOI] [PubMed] [Google Scholar]
- Rodriguez-Amaya DB. Structures and analysis of carotenoid molecules. Subcell Biochem. 2016;79:71–108. doi: 10.1007/978-3-319-39126-7_3. [DOI] [PubMed] [Google Scholar]
- Saini RK, Keum Y-S. Carotenoid extraction methods: a review of recent developments. Food Chem. 2018;240:90–103. doi: 10.1016/j.foodchem.2017.07.099. [DOI] [PubMed] [Google Scholar]
- Shaish A, Harari A, Hananshvili L, Cohen H, Bitzur R, Luvish T, Ulman E, Golan M, Ben-Amotz A, Gavish D, Rotstein Z, Harats D. 9-cis β-Carotene-rich powder of the alga Dunaliella bardawil increases plasma HDL-cholesterol in fibrate-treated patients. Atherosclerosis. 2006;189:215–221. doi: 10.1016/j.atherosclerosis.2005.12.004. [DOI] [PubMed] [Google Scholar]
- Shete V, Quadro L. Mammalian metabolism of β-carotene: gaps in knowledge. Nutrients. 2013;5:4849–4868. doi: 10.3390/nu5124849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Siems W, Salerno C, Crifò C, Sommerburg O, Wiswedel I. β-Carotene degradation products – formation, toxicity and prevention of toxicity. Forum Nutr. 2009;61:75–86. doi: 10.1159/000212740. [DOI] [PubMed] [Google Scholar]
- Sies H, Stahl W. Carotenoids and intercellular communication via gap junctions. Int J Vitam Nutr Res. 1997;67:364–367. [PubMed] [Google Scholar]
- Starek M, Guja A, Dąbrowska M, Krzek J. Assay of β-carotene in dietary supplements and fruit juices by TLC-densitometry. Food Anal Methods. 2015;8:1347–1355. doi: 10.1007/s12161-014-0019-0. [DOI] [Google Scholar]
- Wu Y, Yan P, Li Y, Liu X, Wang Z, Chen T, Zhao X. Enhancing β-carotene production in Escherichia coli by perturbing central carbon metabolism and improving the NADPH supply. Front Bioeng Biotechnol. 2020;8:585. doi: 10.3389/fbioe.2020.00585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang J, Zhang Y, Na X, Zhao A. β-Carotene supplementation and risk of cardiovascular disease: a systematic review and meta-analysis of randomized controlled trials. Nutrients. 2022;14:1284. doi: 10.3390/nu14061284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu LJ, Rupasinghe HV. Improvement of cloud stability, yield and β-carotene content of carrot juice by process modification. Food Sci Technol Int. 2013;19:399–406. doi: 10.1177/1082013212455342. [DOI] [PubMed] [Google Scholar]
- Yuan Y, Gao Y, Mao L, Zhao J. Optimisation of conditions for the preparation of β-carotene nanoemulsions using response surface methodology. Food Chem. 2008;107:1300–1306. doi: 10.1016/j.foodchem.2007.09.015. [DOI] [Google Scholar]
- Zhang Y, Navarro E, Cánovas-Márquez JT, Almagro L, Chen H, Chen YQ, Zhang H, Torres-Martínez S, Chen W, Garre V. A new regulatory mechanism controlling carotenogenesis in the fungus Mucor circinelloides as a target to generate β-carotene over-producing strains by genetic engineering. Microb Cell Factories. 2016;15:99. doi: 10.1186/s12934-016-0493-8. [DOI] [PMC free article] [PubMed] [Google Scholar]