OBJECTIVES:
1) Characterize the prevalence of ventilator liberation protocol use in international PICUs, 2) identify the most commonly used protocol elements, and 3) estimate an international extubation failure rate and use of postextubation noninvasive respiratory support modes.
DESIGN:
International cross-sectional study.
SUBJECTS:
Nontrainee pediatric medical and cardiac critical care physicians.
SETTING:
Electronic survey.
INTERVENTION:
None.
MEASUREMENTS AND MAIN RESULTS:
Responses represented 380 unique PICUs from 47 different countries. Protocols for Spontaneous Breathing Trial (SBT) practice (50%) and endotracheal tube cuff management (55.8%) were the only protocols used by greater than or equal to 50% of PICUs. Among PICUs screening for SBT eligibility, physicians were most commonly screened (62.7%) with daily frequency (64.2%). Among those with an SBT practice protocol, SBTs were most commonly performed by respiratory therapists/physiotherapists (49.2%) and least commonly by nurses (4.9%). Postextubation respiratory support protocols were not prevalent (28.7%). International practice variation was significant for most practices surveyed. The estimated median international extubation failure was 5% (interquartile range, 2.3–10%). A majority of respondents self-reported use of planned high-flow nasal cannula in less than or equal to 50% (84.2%) and planned noninvasive ventilation in less than or equal to 20% of extubations (81.6%).
CONCLUSIONS:
Variability in international pediatric ventilation liberation practice is high, and prevalence of protocol implementation is generally low. There is a need to better understand elements that drive clinical outcomes and opportunity to work on standardizing pediatric ventilation liberation practices worldwide.
Keywords: clinical pathway, extubation, mechanical ventilation, pediatric intensive care unit, pediatrics, respiratory therapy
ICU liberation is an evidence-based bundle associated with improved outcomes in critically ill adults (1, 2). A key part of the Society of Critical Care Medicine ICU liberation bundle is the “B” component, which typically involves extubation readiness tests (ERTs). An ERT commonly includes a Spontaneous Breathing Trial (SBT) and other variables that clinicians use to determine extubation readiness. SBTs decrease duration of invasive mechanical ventilation (IMV), complications, and cost in mechanically ventilated adults (3).
Although there are limited controlled data surrounding SBTs and ERTs in critically ill children, these tools are important to promote timely ventilator liberation. Many PICUs have implemented protocols surrounding the ICU liberation bundle, including standardized methods to assess for extubation readiness, often including ERTs, with mixed methodology and success (4–14). There is significant variability in the approach and comprehensiveness of ventilator liberation protocols among PICUs. Some of this variability relates to provider preferences, patient population, and the lack of clear clinical practice guidelines (15–19). However, there are likely PICU-specific resources and characteristics, which may result in unwarranted variation in ventilator liberation practices (20).
We sought to characterize PICU-specific practices with regard to pediatric ventilation liberation, with a specific focus on the use of protocols for ventilator weaning and extubation readiness evaluation. The objectives were: 1) to characterize the prevalence of ventilator liberation protocols in international PICUs, 2) to identify the most commonly used protocol elements, and 3) to estimate an international extubation failure rate and use of postextubation noninvasive respiratory support modalities.
MATERIALS AND METHODS
Survey Development
We developed a pediatric ventilation liberation practice survey (J.M.L., S.A.-S., R.G.K.), which was reviewed by national and international pediatric critical care experts. The survey content included descriptors of the PICU including type, location, size, staffing, and resources. Unit-level and practitioner-level ventilation liberation practice patterns were surveyed. Fifteen core research questions served as the framework for survey content (Supplemental Table 1, http://links.lww.com/CCX/B5), which are related to different elements of pediatric ventilator liberation. ERT elements queried included protocols and practices relevant to: SBT eligibility screening, SBT performance/practice, endotracheal tube (ETT) cuff management throughout the IMV course, and steroid prescription to prevent postextubation upper airway obstruction (UAO). Postextubation support and outcome elements queried included: postextubation respiratory support with high-flow nasal cannula (HFNC) and noninvasive ventilation (NIV) as well as extubation failure rates. Respondents were asked to self-report the extubation failure rate for their ICU over the last 12 months using a continuous scale between 0% and 40%. Planned extubation to HFNC or NIV was specifically queried regarding personal practice and extrapolated to unit-based practice. Use of other protocols and practices relevant to the ICU liberation bundle, but not included in the “B” element, was also evaluated. Respondents were asked to consider patients requiring IMV for more than 24 hours and exclude children with cyanotic heart disease.
The survey was developed in English and then translated into Spanish and Portuguese by fluent pediatric critical care physicians (J.C., S.B.). Informal testing occurred with fluent pediatric critical care physicians unaffiliated with the project to ensure cross-cultural relevance and accuracy. Within the user interface, respondents were not forced to provide some answers as they progressed through the survey, making it possible to omit some questions. All surveys were completed between June and August 2021 via the Qualtrics (Provo, Utah) platform. The need for informed consent was waived following review by the institutional review board at the University of Alabama at Birmingham (300007311).
Survey Distribution
The survey was distributed to an international group of pediatric critical care attending physicians. Trainees and other licensed independent providers were instructed not to complete the survey. E-mail solicitation consisted of an initial e-mail followed by one additional e-mail 2 weeks or 1 month afterward. Solicitations were distributed by the Australia and New Zealand Intensive Care Society, Brazilian Research Network in Pediatric Intensive Care, European Society of Pediatric and Neonatal Intensive Care, Groupe Francophone de Réanimation et d’Urgences Pédiatricques, Latin American Pediatric Collaborative, Pediatric Acute and Critical Care Medicine Asian Network, Pediatric Acute Lung Injury and Sepsis Investigators, Sociedad Latino Americana de Cuidados Intensivos Pediatricos, and the World Federation of Pediatric Intensive and Critical Care Societies. The survey was also promoted by one of the authors on social media using a link to the survey (Twitter, San Francisco, CA).
Unique PICU Identification
First, responses without answers to nondemographic questions were excluded. Next, high-risk duplicate responses were identified. This was determined by identifying identical responses to all of the following: hospital name, hospital city, hospital country, length of clinical practice, percent clinical practice time, PICU type, and division chief/medical director status. For high-risk duplicate responses, the response with the fewest answers was excluded. Where both respondents answered the same number of questions, the second response was excluded. Next, multiple responses from the same PICU were identified. PICUs were considered the same if the country, city, hospital name, and PICU type were all identical. If any of these identifiers were missing, the response was excluded from this analysis. Where multiple responses existed from the same PICU, first preference was given to division chief/medical director responses. Where none existed, the response(s) with the fewest questions answered was(were) excluded.
Outcomes and Data Definitions
The primary outcome was the prevalence of ventilation liberation protocols and practices. Secondary outcomes included regional variation in self-reported use of those protocols, extubation failure rates, and rates of planned HFNC/NIV use postextubation. For the sake of the survey, a protocol was defined as a mutually agreed upon and documented clinical pathway or approach that standardizes work for the majority of patients in the PICU. Extubation failure was defined as replacement of an ETT for any reason other than a planned procedure within 48 hours of a planned extubation attempt. For the purposes of the secondary outcome analyses, HFNC and NIV responses were considered to similarly reflect PICU-level practice. A large academic PICU was defined as PICUs with greater than 1,000 annual admissions plus nonattending general and/or pediatric critical care trainees.
Statistical Analysis
Questions without answers or with “don’t know/not sure” selected were excluded from denominators where applicable. Descriptive analyses included medians with interquartile ranges (IQRs) and frequency distributions. Comparisons employed the chi-square, Fisher exact, Mann-Whitney U, or Student t tests, as appropriate. As the focus study was descriptive analysis and hypothesis generation, we did not control for multiple comparisons in these univariate analyses. All hypothesis tests were two-tailed, and a p value of less than 0.05 was used to indicate statistical significance. All analyses used SPSS (Version 25, IBM, Armonk, NY).
RESULTS
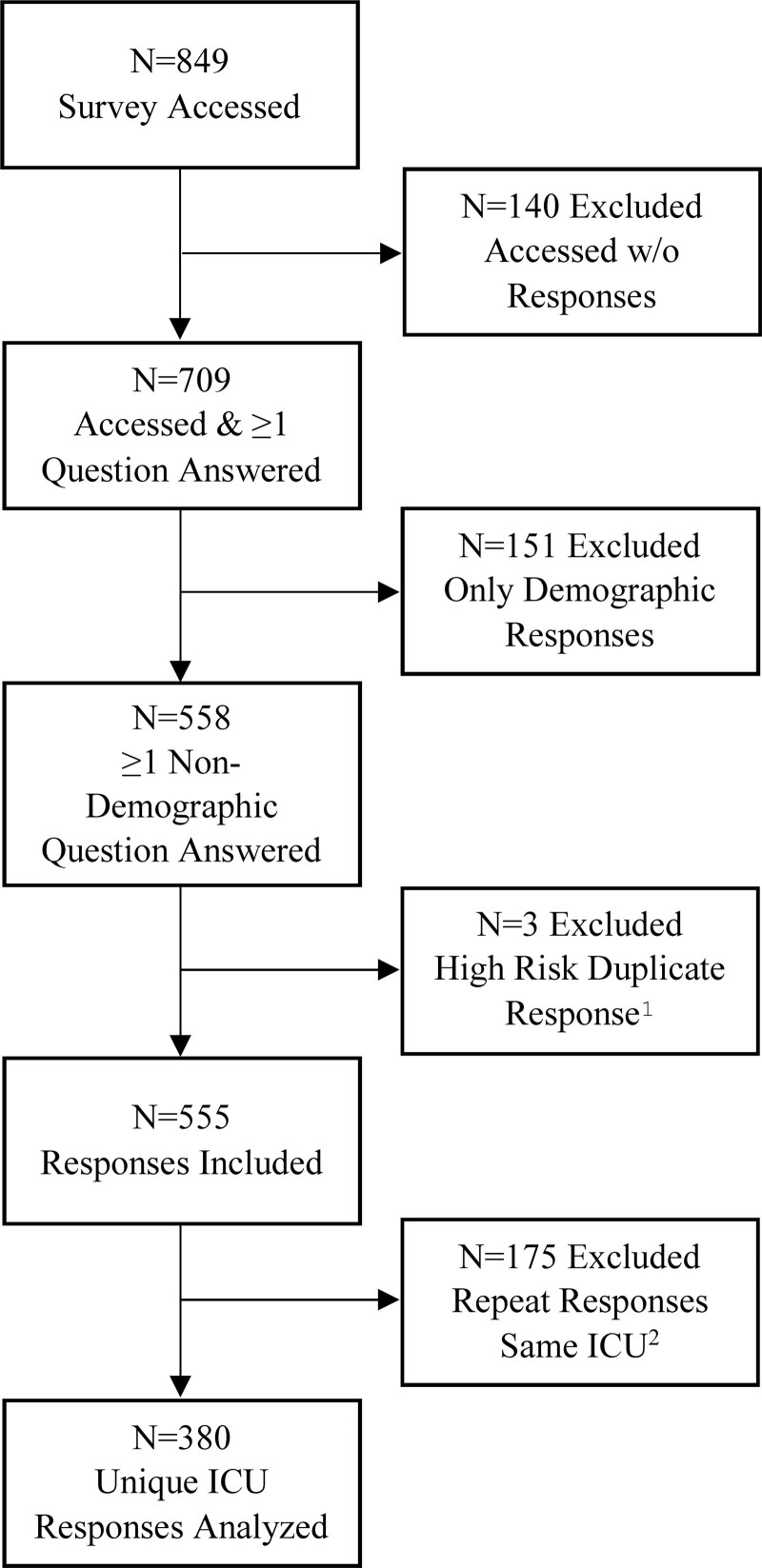
After exclusion of 151 responses for only demographic answers and three for high risk of a duplicate response, there were 555 total responses representing 380 unique PICUs from 47 different countries (Fig. 1). Descriptive statistics are shown in Table 1. There were 67 (19.1%) large academic PICUs represented (n = 30 excluded for missing classification data). At the respondent level, 40.3% were either the medical director or division chief. Most (60.3%) reported being in clinical practice for greater than 10 years and spending at least 50% time in direct patient care (82.6%).
Figure 1.
CONSORT diagram, describing survey responses and exclusions leading to final cohort of unique ICU responses. 1High-risk duplicate response status was determined by comparing duplicate responses to all of the following: hospital name, hospital city, hospital country, length of clinical practice, percent clinical time, PICU type, and division chief/medical director status. For high-risk duplicate responses, the response with the fewest questions answered was excluded. Where both responses had the same number of questions answered, the second response was excluded. 2Where multiple responses existed from the same ICU, first preference was given to division chief/medical director responses. Where none existed, the response with the most questions answered was included. CONSORT = Consolidated Standards of Reporting Trials.
TABLE 1.
Descriptive Characteristics of Unique PICUs (n = 380)
| Location of ICU (%) | Value |
|---|---|
| South America/Central America/Mexico | 174 (45.8) |
| United States/Canada | 79 (20.8) |
| Europe | 67 (17.6) |
| Asia | 34 (9) |
| Australia/New Zealand | 7 (1.8) |
| Middle East/Africa/Caribbean | 19 (5) |
| ICU type (%) | |
| General Medical/Surgical ICU | 174 (45.8) |
| Mixed Medical/Surgical/Cardiac ICU | 155 (40.8) |
| Medical ICU only | 32 (8.4) |
| Cardiac ICU only | 16 (4.2) |
| Other | 3 (0.8) |
| Median maximum patient capacity (IQR) | 13 (9–20) |
| Median attending physician-to-patient ratio (IQR) | 1:8 (1:5–1:10) |
| Prescriber team composition (%) | |
| Nonattending physician general pediatric trainees | 277 (72.9) |
| Nonattending physician pediatric critical care trainees | 209 (55) |
| Nonphysician licensed independent providers | 191 (50.3) |
| None of the above | 34 (8.9) |
| ICU-dedicated respiratory therapist/physiotherapist | 266 (59.5) |
| Average annual ICU admissions (%) | |
| Less than 500 | 166 (43.7) |
| 500–1,000 | 109 (28.7) |
| 1,001–2,000 | 62 (16.3) |
| More than 2,000 | 13 (3.4) |
| Unsure/do not know | 30 (7.9) |
| Median admissions requiring invasive ventilation, % (IQR) | 35 (20–52) |
| Noninvasive respiratory support capabilities (%) | |
| High-flow nasal cannula | 325 (85.5) |
| Noninvasive positive pressure | 362 (95.3) |
| None of the above | 5 (1.3) |
IQR = interquartile range.
International Unit-Based Practices for All Unique Units
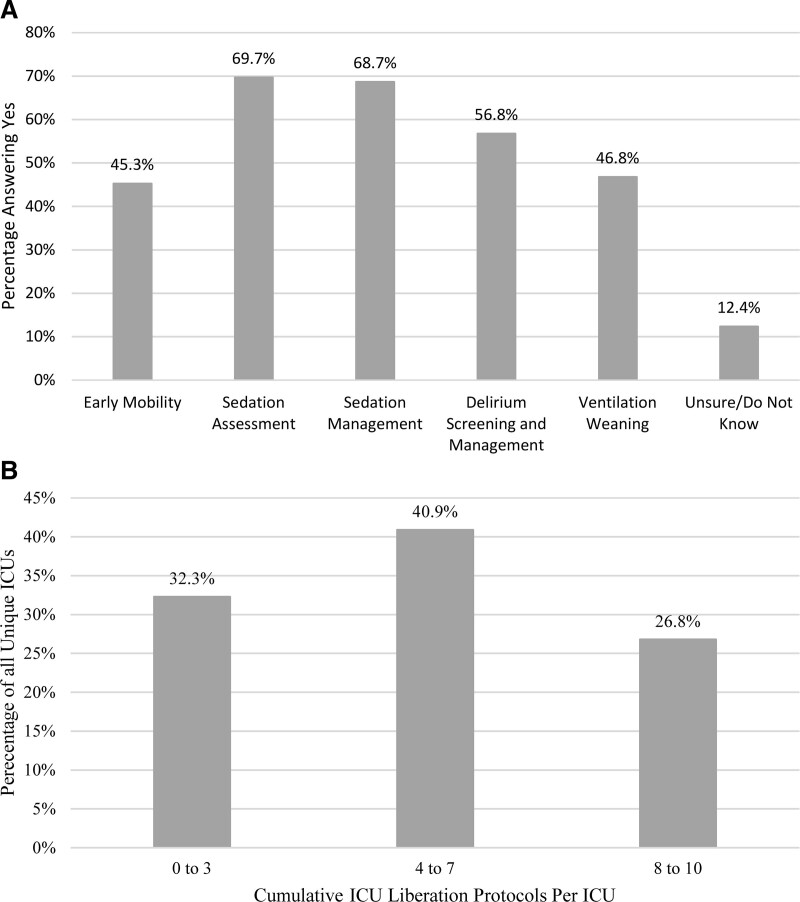
Self-reported ventilation liberation protocols and practices are shown in Supplemental Figure 1 (http://links.lww.com/CCX/B5). ERT protocols were endorsed as follows: SBT eligibility screening (46.6%), standardized performance of SBTs (50%), ETT cuff management (55.8%), and protocols for steroid use to prevent postextubation UAO (41.6%). Among PICUs using a SBT eligibility screen, most relied on physicians or other licensed independent providers to perform the screen (62.7%) with daily frequency (64.2%) being most prevalent. Among PICUs using an SBT practice protocol, SBTs were most commonly performed by respiratory therapists (RT)/physiotherapists (49.2%) and least commonly by nurses (4.9%). Postextubation respiratory support protocols were present in 28.7% of ICUs. Figure 2 shows use of other ICU liberation bundle protocols for children requiring IMV. Sedation assessment (69.7%), sedation management (n = 261, 68.7%), and delirium screening/management (56.8%) were the most common. In addition to SBT practice protocols, these were the only ICU liberation protocols meeting greater than or equal to 50% prevalence. Early mobility protocols were the least prevalent (45.3%). The greatest proportion of PICUs reported simultaneous employment of four to seven ICU liberation-related protocols (40.9%).
Figure 2.
Self-reported use of other (non-ERT) ICU liberation protocols. A, Other ICU liberation protocols for children requiring invasive mechanical ventilation. B, Cumulative concurrent ICU liberation protocols used in each ICU (Spontaneous Breathing Trial/extubation readiness test protocols excluded).
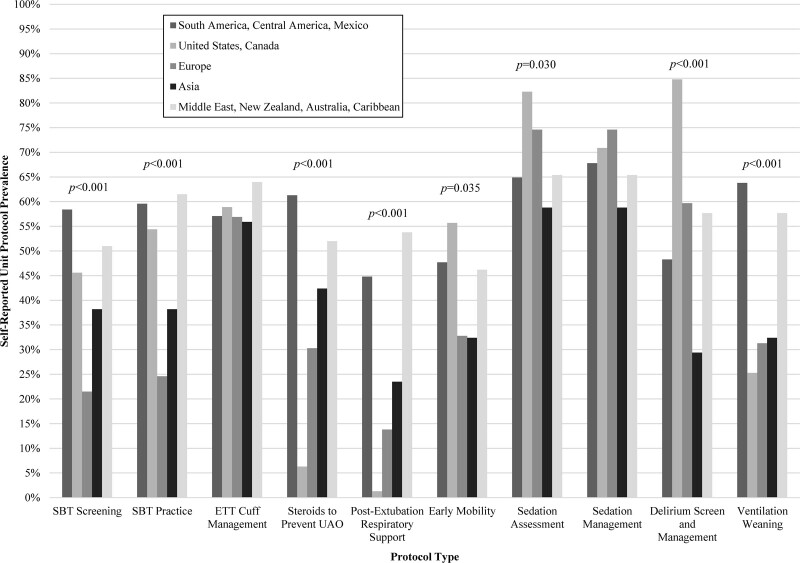
International Unit-Based Practices Stratified by Region
Protocols and practices were compared according to PICU region with significant variation (Fig. 3; Table 2). Most responses were from South/Central America and Mexico (45.8%) followed by the United States and Canada (20.8%). SBT eligibility-screening protocols were most common in the South/Central American and Mexico region (58.4%) and least common in Europe (21.5%) (p < 0.001). Physicians or other licensed independent providers most commonly performed the screening in all regions except the United States and Canada where RT/physiotherapists predominated (p < 0.001). Daily screening frequency was most common with the exception of Europe where more frequent screening was endorsed (p = 0.045). Similar to screening, the United States and Canada were most likely to have RT/physiotherapists perform the SBT independently (p < 0.001). The United States and Canada less commonly used protocols for postextubation respiratory support (1.3%; p < 0.001) and steroid use for UAO-prevention (6.3%; p < 0.001). Asian PICUs reported fewer cumulative ICU liberation-related protocols (p < 0.001).
Figure 3.
ICU and ventilation liberation-relevant protocol prevalence stratified by region (if regional differences were statistically significant, the p value is reported). ETT = endotracheal tube, SBT = Spontaneous Breathing Trial, UAO = upper airway obstruction.
TABLE 2.
Unit-Based Ventilation Liberation Practices Stratified by Region
| Variable | South America, Central America, and Mexico | United States and Canada | Europe | Asia | Middle East, New Zealand, Australia, Africa, and Caribbean | p |
|---|---|---|---|---|---|---|
| Total unique ICUs (%) | 174 (45.8) | 79 (20.8) | 67 (17.6) | 34 (9) | 26 (6.8) | N/A |
| SBT screening responsibility (%)a | ||||||
| Physician or other LIP | 74 (73.3) | 7 (19.4) | 8 (57.1) | 11 (84.6) | 11 (84.6) | < 0.001 |
| Respiratory therapist or physiotherapist | 23 (22.8) | 28 (77.8) | 0 (0) | 2 (15.4) | 2 (15.4) | |
| Other | 4 (4) | 1 (2.8) | 6 (42.9) | 0 (0) | 0 (0) | |
| SBT screening frequency (%)b | ||||||
| Daily | 67 (66.3) | 21 (58.3) | 5 (35.7) | 11 (91.7) | 9 (69.2) | 0.045 |
| Greater than once daily | 34 (33.7) | 15 (41.7) | 9 (64.3) | 1 (8.3) | 4 (30.8) | |
| SBT practice responsibility (%)c,d | ||||||
| Physician or other LIP | 48 (49) | 4 (9.5) | 8 (50) | 10 (76.9) | 13 (81.3) | < 0.001 |
| Respiratory therapist or physiotherapist | 50 (51) | 37 (88.1) | 8 (50) | 2 (15.4) | 2 (12.5) | |
| Other | 0 (0) | 1 (2.4) | 0 | 1 (7.7) | 1 (6.3) | |
| Cumulative ICU liberation protocols per ICU (%)e | ||||||
| 0–3 | 48 (28.7) | 17 (23.3) | 27 (42.2) | 18 (54.5) | 7 (28) | < 0.001 |
| 3–7 | 55 (32.9) | 48 (65.8) | 30 (46.9) | 7 (21.2) | 8 (32) | |
| 8–10 | 63 (38.3) | 8 (11) | 7 (10.9) | 8 (24.2) | 10 (40) | |
| 48-hr extubation failure rate (%)f | ||||||
| < 3% | 32 (25.8) | 11 (23.4) | 13 (28.9) | 6 (27.3) | 2 (11.1) | 0.002 |
| 3–8% | 38 (30.6) | 30 (63.8) | 23 (51.1) | 9 (40.9) | 9 (50) | |
| > 8% | 54 (43.5) | 6 (12.8) | 9 (20) | 7 (31.8) | 7 (38.9) | |
| Planned high flow nasal cannula use postextubation (%)g | ||||||
| ≤ 25% | 50 (45.5) | 36 (49.3) | 28 (48.3) | 13 (50) | 14 (82.4) | 0.248 |
| 26–50% | 44 (40) | 22 (30.1) | 21 (36.2) | 9 (34.6) | 2 (11.8) | |
| ≥ 51% | 16 (14.5) | 15 (20.5) | 9 (15.5) | 4 (15.4) | 1 (5.9) | |
| Planned noninvasive ventilation use postextubation (%)h | ||||||
| ≤ 10% | 62 (44.6) | 37 (50.7) | 29 (48.3) | 11 (39.3) | 7 (43.8) | 0.785 |
| 11–20% | 53 (38.1) | 20 (27.4) | 21 (35) | 13 (46.4) | 5 (31.3) | |
| ≥ 21% | 24 (17.3) | 16 (21.9) | 10 (16.7) | 4 (14.3) | 4 (25) | |
LIP = licensed independent provider, SBT = Spontaneous Breathing Trial.
an = 4 unsure/do not know excluded.
bn = 1 no answer excluded.
cn = 5 unsure/do not know excluded.
dn = 5 no answer excluded.
en = 18 unsure/do not know excluded.
fn = 124 no answer excluded.
gn = 55 no HFNC capability and n = 41 no answer excluded.
hn = 18 no NIV capability and n = 46 no answer excluded.
The “n” for each variable is based on the total responses to each survey question. A response may have been missing or not apply to a given variable based on responses to previous questions. As such, total responses may not be equal to the total unique ICUs represented for each region.
Extubation Failure and Postextubation Support Practices
The median international self-reported extubation failure rate was 5% (IQR, 2.3–10; min-max, 0–39; n = 256 PICUs reporting). The greatest percentage of extubation failure rates greater than 8% were reported in the South/Central America and Mexico region. In contrast, the highest percentage of PICUs reporting extubation failure rates 3–8% was in the United States and Canada region (p = 0.002). Excluding those without HFNC or NIV capability in the PICU, a majority used planned HFNC in less than or equal to 50% (84.2%) and planned NIV in less than or equal to 20% of extubations (81.6%). There were no statistically significant differences in international practices for either planned HFNC or NIV use postextubation.
DISCUSSION
This study is the first in-depth description of international pediatric ventilation liberation practices. Self-reported international pediatric ventilation liberation practice is highly variable with only about 50% of PICUs implementing ventilator liberation protocols. In addition, the sedation assessment and delirium elements of the ICU liberation bundle were more commonly present than the ERT and early mobility elements. Although these findings are consistent with recent international practice data, our study adds a more in-depth understanding of international practice relevant to pediatric ventilation liberation and the “B” bundle element (21). These results reinforce an opportunity to standardize pediatric ventilation practices in an effort to eliminate noise and discriminate strategies that drive outcomes.
Ventilation liberation begins with suspicion or diagnostic triggering that a patient may be nearing the time of weaning and/or extubation (22). Less than half of PICUs surveyed use of a screening protocol for SBT eligibility with most being prescriber-driven and only performed daily. This structure is prone to bias and competing demands of the critical care environment. Additionally, it is possible to miss readiness by as much as 24 hours. The optimal screening frequency is not known and must balance competing demands of delayed identification and excessive workload. Only half of all responding ICUs used an SBT practice protocol and international variation was significant. SBT eligibility screening and SBT practice represent two important leverage points for improving pediatric IMV outcomes. Protocols driven by bedside providers may identify eligible patients earlier and result in improved clinical outcomes (5, 6, 8). Other PICU liberation initiatives that heavily relied on nonphysician members of the multidisciplinary have also shown safety and efficacy (10, 23, 24). In any improvement strategy, harnessing the skills of the multidisciplinary is likely to be critical.
Cuffed ETTs are most commonly used in the pediatric population (25). Excessively high cuff pressures are associated with complications such as subglottic UAO, and measured pressures are often outside the recommended range of 20–30 cm H2O (26–29). Cuff pressure monitoring protocols may decrease postextubation subglottic UAO (30). Only half of responding units reported presence of a cuff management protocol. Prophylactic corticosteroid prescription to prevent postextubation subglottic UAO is controversial, but its use in children was supported by a recent meta-analysis and may be particularly important for higher risk patients (31). A majority of PICUs do not have a protocol for corticosteroid prescription. Identifying high-risk populations for subglottic UAO that might benefit from preextubation administration of corticosteroids as well as the most appropriate dosing regimens is an important element to consider in these protocols.
There is a dose-dependent response between bundle element compliance and ICU outcomes, including mortality, in adults (32). Although unproven in children, it is reasonable to hypothesize that a similar relationship may be present. However, both ICU and ventilation liberation protocols are not widely adopted. Therefore, such a study cannot be undertaken with acceptable external validity. The reasons for low adoption are speculative, but likely include diverse practice patterns, limited randomized controlled evidence, differential unit resources, and regional cultural differences. To determine if protocol use directly impacts ventilation liberation outcomes, more widespread standardization of practices, outcome definitions, and data collection is needed.
There were significant regional differences in self-reported extubation failure rates. It is difficult to fully interpret these differences without including the unit-level case mix data, such as illness severity and capacity issues. The self-reported extubation failure rates are prone to recall bias but provide a starting point for benchmarking in quality improvement collaboration. However, it is important to note that a single extubation failure rate is unlikely to be “correct” or externally valid for all PICUs. As an example, a PICU that predominantly cares for low illness severity should not have the same failure rate benchmark as one with higher illness severity. Furthermore, there may be an optimal balance between the competing outcomes of IMV duration and extubation failure rates that yields the best outcomes. This is a key evidence gap and an area for future study. Instead of an extubation failure rate benchmark, such a ratio may be more useful in driving improvement in outcomes. Continuous monitoring of an international extubation failure rate balanced with IMV duration and NIV/HFNC use post extubation is critical.
A key strength of this study is the sample size and regional representation. Additionally, the study included a wide spectrum of PICUs. The findings presented here should serve as a foundation for implementing and improving practices in consideration of the upcoming pediatric ventilation liberation guidelines. In addition to those mentioned previously, this study has several limitations. First, the survey design introduces reporting bias. A mitigation strategy was the a priori preference for responses from medical directors and/or division chiefs who may have ready access to objective unit data. Second, despite efforts to accurately identify unique PICUs and eliminate duplicate responses, complete accuracy cannot be assured. Third, these data represent self-reported estimates for practice, and often there is a difference between what practitioners think they do and what occurs (recall bias). However, this survey sets the stage for larger observational studies using actual patient data to understand the relationship between practice patterns and outcomes. Finally, there are many unmeasured confounders particularly related to illness severity and case mix.
CONCLUSIONS
Variability in international pediatric ventilation liberation practice is high. Standardization was low with less than 50% of ICUs utilizing protocols for ventilator liberation. Standardizing pediatric ventilation liberation practices may lead to a better understanding of which elements drive outcomes.
Supplementary Material
Footnotes
Supplemental digital content is available for this article. Direct URL citations appear in the printed text and are provided in the HTML and PDF versions of this article on the journal’s website (http://journals.lww.com/ccejournal).
The authors have disclosed that they do not have any potential conflicts of interest.
This work was performed at the University of Alabama at Birmingham.
REFERENCES
- 1.Ely EW: The ABCDEF bundle: Science and philosophy of how ICU liberation serves patients and families. Crit Care Med 2017; 45:321–330 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Pun BT, Balas MC, Barnes-Daly MA, et al. : Caring for critically ill patients with the ABCDEF bundle: Results of the ICU liberation collaborative in over 15,000 adults. Crit Care Med 2019; 47:3–14 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ely EW, Baker AM, Dunagan DP, et al. : Effect on the duration of mechanical ventilation of identifying patients capable of breathing spontaneously. N Engl J Med 1996; 335:1864–1869 [DOI] [PubMed] [Google Scholar]
- 4.Farias JA, Retta A, Alía I, et al. : A comparison of two methods to perform a breathing trial before extubation in pediatric intensive care patients. Intensive Care Med 2001; 27:1649–1654 [DOI] [PubMed] [Google Scholar]
- 5.Foronda FK, Troster EJ, Farias JA, et al. : The impact of daily evaluation and spontaneous breathing test on the duration of pediatric mechanical ventilation: A randomized controlled trial. Crit Care Med 2011; 39:2526–2533 [DOI] [PubMed] [Google Scholar]
- 6.Abu-Sultaneh S, Hole AJ, Tori AJ, et al. : An interprofessional quality improvement initiative to standardize pediatric extubation readiness assessment. Pediatr Crit Care Med 2017; 18:e463–e471 [DOI] [PubMed] [Google Scholar]
- 7.Loberger JM, Jones RM, Prabhakaran P: A respiratory therapist-driven pathway improves timeliness of extubation readiness assessment in a single PICU. Pediatr Crit Care Med 2020; 21:e513–e521 [DOI] [PubMed] [Google Scholar]
- 8.Randolph AG, Wypij D, Venkataraman ST, et al. ; Pediatric Acute Lung Injury and Sepsis Investigators (PALISI) Network: Effect of mechanical ventilator weaning protocols on respiratory outcomes in infants and children: A randomized controlled trial. JAMA 2002; 288:2561–2568 [DOI] [PubMed] [Google Scholar]
- 9.Farias JA, Alía I, Esteban A, et al. : Weaning from mechanical ventilation in pediatric intensive care patients. Intensive Care Med 1998; 24:1070–1075 [DOI] [PubMed] [Google Scholar]
- 10.Blackwood B, Tume LN, Morris KP, et al. ; SANDWICH Collaborators: Effect of a sedation and ventilator liberation protocol vs usual care on duration of invasive mechanical ventilation in pediatric intensive care units: A randomized clinical trial. JAMA 2021; 326:401–410 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Krawiec C, Carl D, Stetter C, et al. : Challenges with implementation of a respiratory therapist-driven protocol of spontaneous breathing trials in the pediatric ICU. Respir Care 2017; 62:1233–1240 [DOI] [PubMed] [Google Scholar]
- 12.Rivera R, Tibballs J: Complications of endotracheal intubation and mechanical ventilation in infants and children. Crit Care Med 1992; 20:193–199 [DOI] [PubMed] [Google Scholar]
- 13.Glau CL, Conlon TW, Himebauch AS, et al. : Progressive diaphragm atrophy in pediatric acute respiratory failure. Pediatr Crit Care Med 2018; 19:406–411 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Johnson RW, Ng KWP, Dietz AR, et al. : Muscle atrophy in mechanically-ventilated critically ill children. PLoS One 2018; 13:e0207720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Newth CJ, Hotz JC, Khemani RG: Ventilator liberation in the pediatric ICU. Respir Care 2020; 65:1601–1610 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Newth CJ, Venkataraman S, Willson DF, et al. ; Eunice Shriver Kennedy National Institute of Child Health and Human Development Collaborative Pediatric Critical Care Research Network: Weaning and extubation readiness in pediatric patients. Pediatr Crit Care Med 2009; 10:1–11 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Mhanna MJ, Anderson IM, Iyer NP, et al. : The use of extubation readiness parameters: A survey of pediatric critical care physicians. Respir Care 2014; 59:334–339 [DOI] [PubMed] [Google Scholar]
- 18.Little LA, Koenig JC, Jr, Newth CJ: Factors affecting accidental extubations in neonatal and pediatric intensive care patients. Crit Care Med 1990; 18:163–165 [DOI] [PubMed] [Google Scholar]
- 19.Kurachek SC, Newth CJ, Quasney MW, et al. : Extubation failure in pediatric intensive care: A multiple-center study of risk factors and outcomes. Crit Care Med 2003; 31:2657–2664 [DOI] [PubMed] [Google Scholar]
- 20.Krasinkiewicz JM, Friedman ML, Slaven JE, et al. : Extubation readiness practices and barriers to extubation in pediatric subjects. Respir Care 2021; 66:582–590 [DOI] [PubMed] [Google Scholar]
- 21.Ista E, Redivo J, Kananur P, et al. ABCDEF bundle practices for critically ill children: An international survey of 161 PICUs in 18 countries. Crit Care Med 2022; 50:114–125 [DOI] [PubMed] [Google Scholar]
- 22.Tobin MJ, Jubran A: Weaning from mechanical ventilation. In: Principles and Practice of Mechanical Ventilation. Third Edition. Tobin MJ. (Ed), Chicago, IL, McGraw Hill, 2013, pp 1307–1351 [Google Scholar]
- 23.Shildt N, Traube C, Dealmeida M, et al. : “Difficult to Sedate”: Successful implementation of a benzodiazepine-sparing analgosedation-protocol in mechanically ventilated children. Children (Basel) 2021; 8:348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Di Nardo M, Boldrini F, Broccati F, et al. : The liberAction project: Implementation of a pediatric liberation bundle to screen delirium, reduce benzodiazepine sedation, and provide early mobilization in a human resource-limited pediatric intensive care unit. Front Pediatr 2021; 9:788997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Chambers NA, Ramgolam A, Sommerfield D, et al. : Cuffed vs. uncuffed tracheal tubes in children: A randomised controlled trial comparing leak, tidal volume and complications. Anaesthesia 2018; 73:160–168 [DOI] [PubMed] [Google Scholar]
- 26.Tobias JD: Pediatric airway anatomy may not be what we thought: Implications for clinical practice and the use of cuffed endotracheal tubes. Paediatr Anaesth 2015; 25:9–19 [DOI] [PubMed] [Google Scholar]
- 27.Zuckerburg AL, Nichols DG: Airway management in pediatric critical care. In: Textbook of Pediatric Intensive Care. Third Edition. Rogers MC, Nichols DG. (Eds), Philadelphia, PA, Wolters Kluwer, 1996, pp 51–76 [Google Scholar]
- 28.Tobias JD, Schwartz L, Rice J, et al. : Cuffed endotracheal tubes in infants and children: Should we routinely measure the cuff pressure? Int J Pediatr Otorhinolaryngol 2012; 76:61–63 [DOI] [PubMed] [Google Scholar]
- 29.Felten ML, Schmautz E, Delaporte-Cerceau S, et al. : Endotracheal tube cuff pressure is unpredictable in children. Anesth Analg 2003; 97:1612–1616 [DOI] [PubMed] [Google Scholar]
- 30.Schneider J, Mulale U, Yamout S, et al. : Impact of monitoring endotracheal tube cuff leak pressure on postextubation stridor in children. J Crit Care 2016; 36:173–177 [DOI] [PubMed] [Google Scholar]
- 31.Kimura S, Ahn JB, Takahashi M, et al. : Effectiveness of corticosteroids for post-extubation stridor and extubation failure in pediatric patients: A systematic review and meta-analysis. Ann Intensive Care 2020; 10:155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Hartmann SM, DiBlasi RM: Implementing spontaneous breathing trials - but a piece of the puzzle. Respir Care 2017; 62:1368–1371 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.