PURPOSE
A dose-adjusted etoposide, doxorubicin, and cyclophosphamide with vincristine and prednisone plus rituximab (DA-EPOCH-R) regimen has been shown to deliver excellent survival for adults with primary mediastinal large B-cell lymphoma (PMLBL) without the use of radiotherapy. No international prospective evaluation of this regimen has previously been reported in children and adolescents.
PATIENTS AND METHODS
We conducted an international single-arm phase II trial involving patients younger than age 18 years with PMLBL who were to receive six courses of DA-EPOCH-R. The primary end point was event-free survival (EFS). Overall survival and toxicity were also assessed. This trial was registered (ClinicalTrials.gov identifier: NCT01516567).
RESULTS
Analyses were based on 46 patients. The median age was 15.4 years (interquartile range: 14-16 years). The median follow-up was 59.0 months (interquartile range: 52.6-69.2 months). Fourteen events were observed (eight relapses or progressions (including three parenchymal CNS relapses), four residual lymphoma, and two second malignancies). The 4-year EFS was 69.6% (95% CI, 55.2 to 80.9), which did not differ from the rate observed historically (P = .59). Seven deaths occurred (six disease-related and one second malignancy). The overall survival was 84.8% (95% CI, 71.8 to 92.4). Twenty-two patients (48%) reached dose levels ≥ 4. Nonhematologic adverse events grade ≥ 3 or cardiac adverse events grade ≥ 2 occurred in 47 of 276 (17%) courses and 30 of 46 patients (65%).
CONCLUSION
DA-EPOCH-R did not improve the EFS compared with a historical control in this first prospective multisite international study of children and adolescents with PMLBL. Further studies are required to determine the optimum therapy for children and adolescents with this lymphoma.
INTRODUCTION
Primary mediastinal large B-cell lymphoma (PMLBL) is a distinct pathologic entity characterized by an anterior mediastinal mass thought to arise from a thymic medullary B cell, accounting for approximately 2% of pediatric mature B-cell lymphoma. Pathologically, the disease is indistinguishable from that seen in adult patients.1 Children with PMLBL are older than children with other Non-Hodgkin Lymphoma (NHL), and there is a female predominance.2 Historically, PMLBL has had a poorer outcome in pediatric patients compared with other mature B-NHL with a 5-year event-free survival (EFS) of 65%-75% using a chemotherapy-only approach in several international series.2-5
CONTEXT
Key Objective
Primary Mediastinal Large B-cell Lymphoma (PMLBL) in children and adolescents has a poorer outcome than other mature B-cell non-Hodgkin lymphomas when treated with the similar regimens. A dose-adjusted etoposide, doxorubicin, and cyclophosphamide with vincristine and prednisone plus rituximab (DA-EPOCH-R) regimen has been reported to give outstanding survival in adults with PMLBL. This prospective and multicenter study evaluated the efficacy of DA-EPOCH-R in children and adolescents.
Knowledge Generated
DA-EPOCH-R was not shown to be superior to historical chemotherapy regimens in children and adolescents with PMLBL. However, the emergence of CNS relapses and acute cardiac toxicity were observed—events unseen and only rarely seen with other pediatric regimens.
Relevance
DA-EPOCH-R has been adopted widely in many countries to treat PMLBL in children and adolescents. However, it should not be considered the optimum therapy for children and adolescents with this disease and further prospective trials of other regimens and new agents are required.
More recently, outstanding survival for adult patients with PMLBL has been reported without the need for routine irradiation through the use of the chemoimmunotherapy regimen, dose-adjusted etoposide, doxorubicin, and cyclophosphamide with vincristine and prednisone plus rituximab (DA-EPOCH-R). The single-institution, uncontrolled phase II report of 51 adult patients from the US National Cancer Institute (NCI) showed a 5-year EFS of 93% with an overall survival (OS) of 97%, and 16 adult patients treated at Stanford had an EFS and OS of 100%.6
Given the outstanding published reports of the efficacy of DA-EPOCH-R, a randomized trial was not considered feasible. We thus conducted an international, prospective single-arm phase II trial (Inter-B-NHL ritux 2010) to establish whether DA-EPOCH-R could improve EFS compared with historical control in children and adolescents with PMLBL.
PATIENTS AND METHODS
Trial Oversight and Design
This phase II trial was an academic international study of two cooperative groups—the European Intergroup for Childhood Non-Hodgkin Lymphoma and the Children's Oncology Group (COG)—involving nine countries, sponsored by Gustave Roussy (for countries of the European Intergroup for Childhood Non-Hodgkin Lymphoma) and COG (for Australia, Canada, and the United States). F. Hoffmann-La Roche-Genentech provided partial funding and rituximab at no cost but had no part in the trial design, conduct of the study, or in the preparation of the manuscript.
Parents or patients (if appropriate) signed the informed consent and assent forms before enrollment in the trial. In each country, the Protocol (online only) was approved by the relevant ethics and regulatory bodies. An independent data and safety monitoring committee monitored trial progress.
Patients
Eligible patients were age 6 months to 18 years with newly diagnosed PMLBL. Pathology slides were reviewed at national level in each country in Europe or centrally within COG, but this was not mandatory before enrollment. CSF cytology was mandatory at initial workup, and patients with CNS disease were excluded. Other main exclusion criteria were congenital immunodeficiency, prior organ transplantation, previous malignancy, known positive HIV serology, and prior exposure to rituximab. Because of potential rituximab-induced immunodeficiency, patients with severe active viral infection, especially hepatitis B virus (HBV) or HBV carrier history and/or positive HBV serology (except immunized status), were not eligible. There were no exclusion criteria on the basis of organ function with recommended dose guidelines for organ dysfunction provided in the Protocol.
Initial workup within 8 days before registration was performed for staging including clinical examination, chest and abdominal computed tomography (CT), or magnetic resonance imaging according to tumor site, bilateral bone marrow aspirates and biopsy, CSF cytology, and lactate dehydrogenase level. 18F-fluorodeoxyglucose positron emission tomography-CT (PET-CT) was recommended, but staging was not based on its result.
Treatment
All patients received six courses of DA-EPOCH-R with granulocyte colony-stimulating factor (G-CSF), and dose escalation of doxorubicin, etoposide, and cyclophosphamide followed previously reported schedule (Data Supplement, online only).6,7 A prephase of low-dose cyclophosphamide, vincristine, and prednisone was allowed 1 week before commencement of DA-EPOCH-R for patients requiring urgent treatment while awaiting histologic confirmation. 18F-fluorodeoxyglucose PET-CT was recommended at diagnosis, after two courses, and for complete remission assessment after the sixth course of DA-EPOCH-R. No treatment decisions were to be based on the PET-CT results. However, at the end of therapy, if PET-CT was positive, or a large residual tumor remained, then biopsy or removal of the residual mass was recommended.
End Points
The primary end point was EFS defined as the interval from registration in the trial to the presence of viable cells in any residual mass after the sixth DA-EPOCH-R course, relapse, progressive disease, second malignancy, or death from any cause. All events were validated by the Steering Committee.
Secondary End Points
Secondary end points were OS (defined as the time between random assignment and death from any cause or the last follow-up contact for patients who were alive), complete remission rate at the assessment time, acute and long-term toxicity, adverse events (AEs) graded according to NCI-CTC V4: nonhematologic AE grade ≥ 3 and cardiac AE grade 2-5, abnormal left ventricular ejection fraction or abnormal left ventricular shortening fraction, rituximab infusion reactions, and immune reconstitution assessed by immunoglobulin (G, A, and M) levels and lymphocyte counts at 1 year and every year during follow-up until normal level, postvaccination antibody levels, and need for immunoglobulin infusion.
Statistical Analysis
Historically, a long-term (≥ 4 years) EFS of 67% was established (on the basis of analysis of a merged data set of 114 pediatric patients with PMLBL, treated with chemotherapy used for other pediatric B-NHL, in published series from European and American studies8), with most events occurring in the first 2 years (a 1-year rate of 75% and a 2-year rate of 69%). As no disease relapses occurred after 4 years, EFS at this point represents a cure fraction. The efficacy of DA-EPOCH-R therapy was assessed by comparing the EFS for the sample of children and adolescents included in the trial with a fixed outcome, reflecting the historical survival. The null hypothesis was that the EFS for these patients is EFS(t) = 0.67 + 0.33{exp(–1.5t)} versus the alternative with EFS(t) = [0.67 + 0.33{exp(–1.5t)}]R, where R is < 1.0. A one-sample log-rank test9 was used to compare the EFS experience with the fixed null outcome. Testing was performed at the 0.10 level of statistical significance (one-sided). A sample size of 40 patients would provide 90% power to detect a true long-term EFS of 84.6% and 80% power to detect a true long-term EFS of 82.4%. Further details are given in the Data Supplement.
RESULTS
Patients
Between April 2012 and April 2016, 48 patients were registered in the study: There was a suspension of enrollment between March 19 and June 16, 2015 because of pending approval of an amendment to increase the total number of patients from 40 to 47 because of a protocol error that resulted in the first seven patients receiving only 50% of the intended dose of prednisolone (60 mg/m2/d in two divided doses on days 1-5 instead of 120 mg/m2/d in two divided doses on days 1-5; Data Supplement). Two patients were deemed ineligible and excluded: one with a diagnosis of Burkitt leukemia who did not receive any trial therapy as the error was noted quickly and another patient whose diagnosis was Hodgkin Lymphoma on national pathologic review. The patient was treated with DA-EPOCH-R and relapsed at 2.8 years from diagnosis. As patients who received full dose of prednisolone did not have better outcome than the patients who received half dose (Data Supplement), the analyses were based on the 46 eligible patients registered with a diagnosis of PMLBL.
Patient characteristics (Table 1) revealed a predominance (57%) of female patients. The median age was 15.4 years (interquartile range [IQR]: 14-16 years). Thirty-one (67%) patients had large (> 10 cm) mediastinal masses. Initial staging confirmed Ann Arbor stage II disease in 31 patients, stage III in one patient, and stage IV in 12; data were missing for two patients.
TABLE 1.
Baseline Characteristics
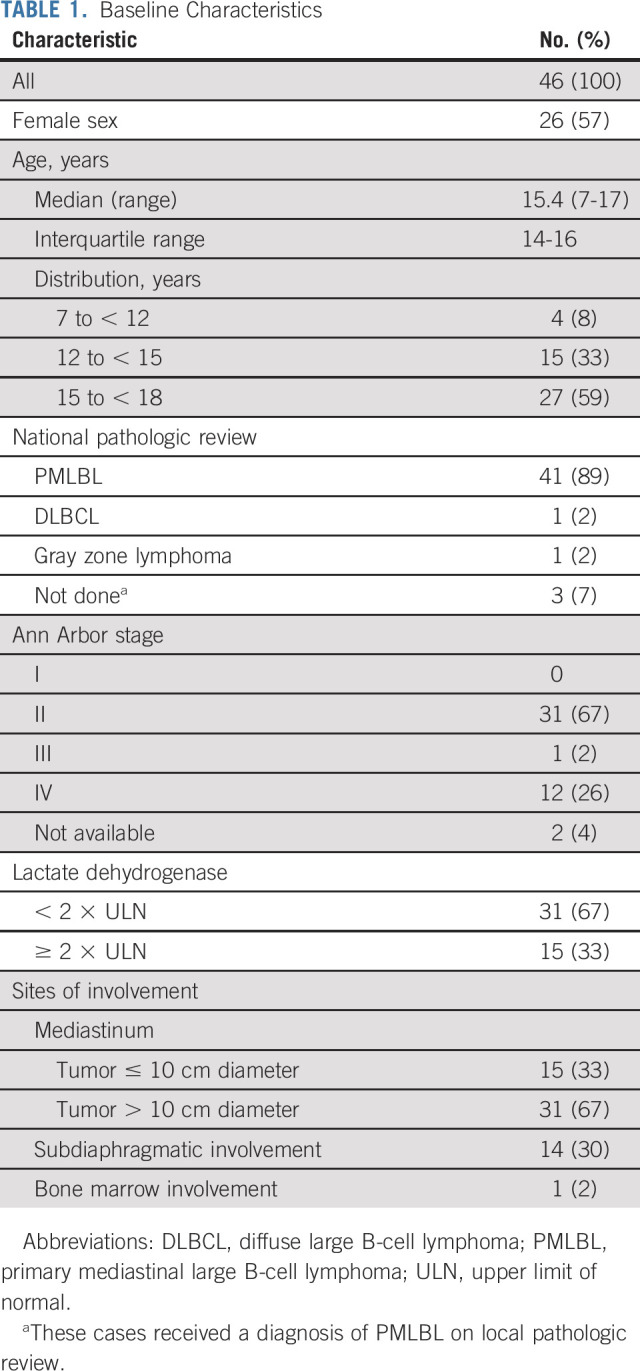
National pathologic review was performed for 43 patients, and PMLBL was confirmed for 41 patients. Diffuse large B-cell lymphoma, centroblastic variant, was diagnosed in one patient (no event), and Gray zone NHL was diagnosed in one patient (event at 7 months and died at 21.5 months). The remaining three patients had a local pathologic diagnosis of PMLBL.
Treatment
All patients received six courses of DA-EPOCH-R. In addition, 19 (41%) received a cyclophosphamide, vincristine, and prednisone course before the first DA-EPOCH-R course. Twenty-two of 46 patients (48%) reached at least dose level 4. Data were available to assess the adherence to the dose escalation rules for DA-EPOCH-R in 42 of 46 (91%) patients. Twelve of 42 (29%) patients should have received a dose escalation in at least one course of DA-EPOCH-R, and 4 of 42 (10%) patients should have had a dose reduction in at least one course of DA-EPOCH-R. Thirty-three (72%) and 11 (24%) patients received ≥ 300 mg/m2 and ≥ 350 mg/m2 of total cumulative dose of doxorubicin, respectively.
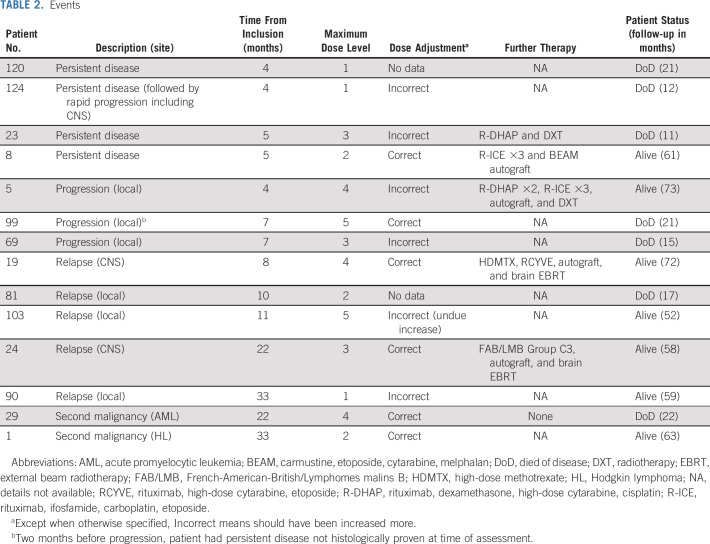
Efficacy
The median follow-up was 59.0 months (IQR 52.6-69.2 months). There were a total of 14 events (Table 2). There were four inadequate response with viable cells in the residual mass, eight progressions or relapses, and two second malignancies (Hodgkin Lymphoma and acute promyelocytic leukemia). Among the progression or relapses, three involved the CNS parenchyma (two isolated including one with blasts in CSF and one combined with thoracic progression; all three patients had correct DA-EPOCH-R dose escalation). The EFS at 4 years was 69.6% (95% CI, 55.2 to 80.9; Fig 1A). The comparison of the observed EFS with historical EFS = [0.67 + 0.33{exp(–1.5t)}] was not significant (P value = .59). DA-EPOCH-R did not, therefore, significantly improve EFS as compared with the historical rate. The 4-year EFS of the 12 patients who should have received a dose escalation in at least one course of DA-EPOCH-R was 58.3% (95% CI, 32.0 to 80.7), and it was 76.7% (95% CI, 59.1 to 88.2) for the 30 other patients (log-rank test P value = .15). There were seven deaths, six because of progression or relapse and one because of second malignancy (acute promyelocytic leukemia). The OS was 84.8% (95% CI, 71.8% to 92.4%) at 4 years (Fig 1B; Sensitivity analyses are given in the Data Supplement).
TABLE 2.
Events
FIG 1.
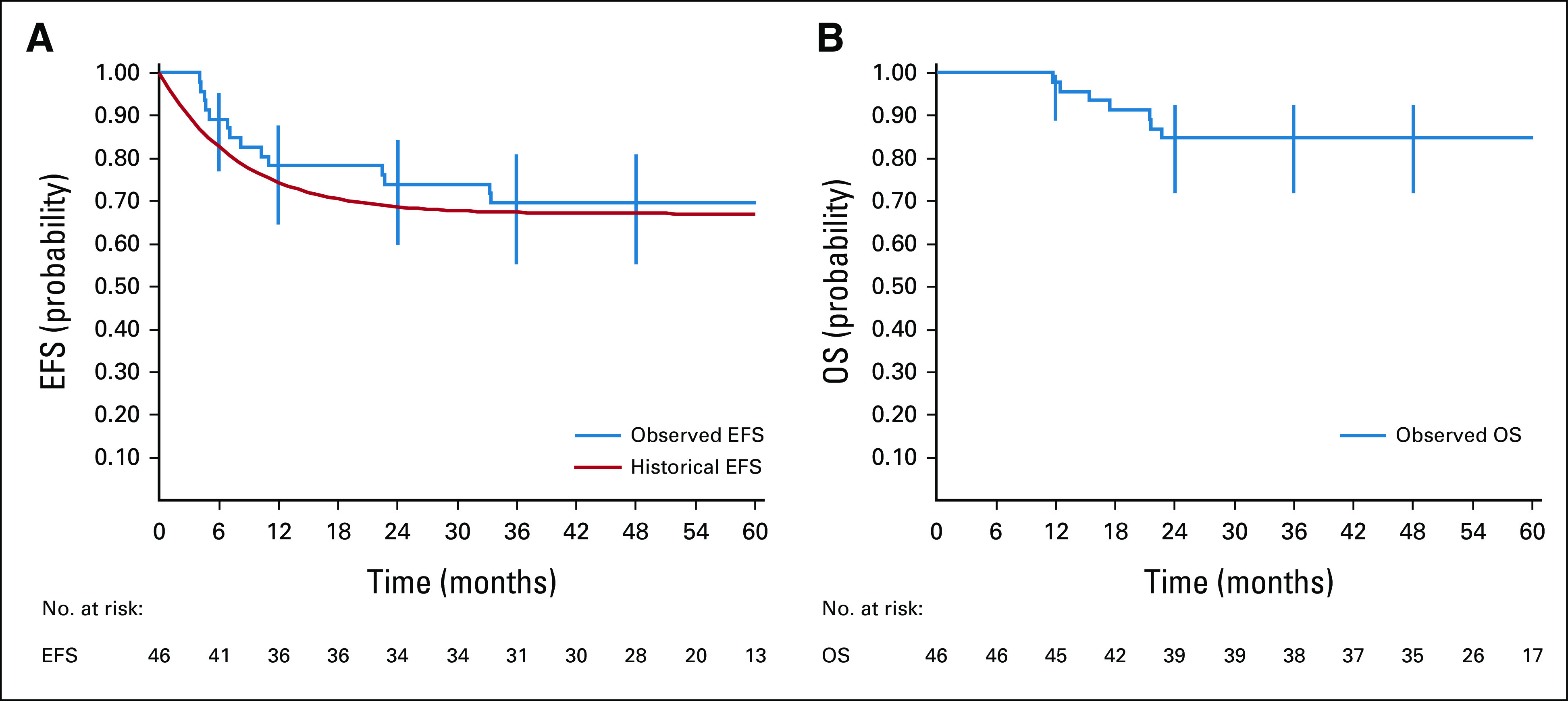
Kaplan-Meier estimates of (A) EFS and (B) OS in PMLBL. Vertical lines represent the Rothman 95% CIs; point estimates of 12-, 24-, 36-, and 48-month EFS and OS with 95% CIs are shown. EFS, event-free survival; OS, overall survival; PMLBL, primary mediastinal large B-cell lymphoma.
Response to Treatment and PET Imaging
After the completion of the sixth course of DA-EPOCH-R, 33 of 46 (72%) patients had a residual mass. In total, 19 of 33 had biopsies or excisions or partial excisions: six had viable tumor cells (median residual mass size: 52 mm, range, 11-86) and for 13 patients, the histology revealed complete necrosis (median residual mass size: 56 mm, range, 15-104). Thirty-nine patients (85%) were considered to have achieved complete remission. Of the remaining patients, four (9%) had persistent disease histologically proven, two had disease progression histologically proven, and one had persistent disease not histologically proven (residual mass of 100 mm followed by early progression).
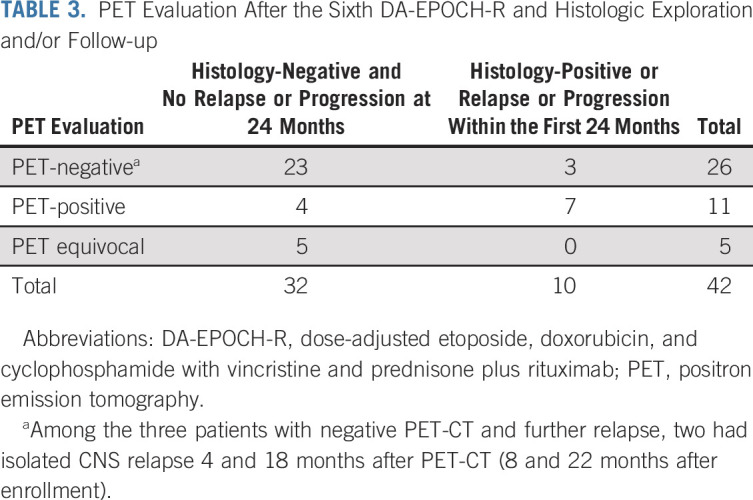
Forty-two patients (91%) had PET-CT after the end of therapy (Table 3). The negative predictive value of PET-CT was found to be 23/26 = 88.5% (95% CI, 69.9 to 97.6), whereas the positive predictive value was 7/11 = 63.6% (95% CI, 30.8 to 89.1). Of note, among the three patients with negative end-of-therapy PET-CT and subsequent relapse, two had isolated CNS relapse 4 and 18 months after PET-CT (8 and 22 months after enrollment).
TABLE 3.
PET Evaluation After the Sixth DA-EPOCH-R and Histologic Exploration and/or Follow-up
Safety
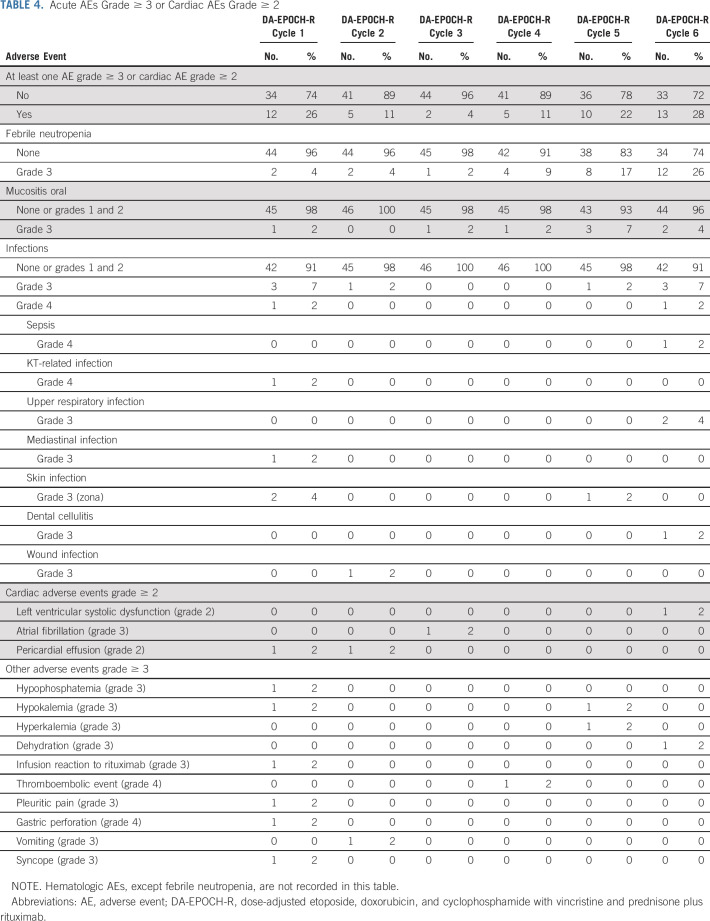
Acute AEs were assessed in all patients and all 276 courses except neutropenia and thrombopenia that were assessed in 42 patients (91%) and 252 courses. No toxic deaths were reported. Grade 4 neutropenia occurred in 106 of 251 courses (42%), and grade 4 thrombocytopenia occurred in eight of 252 courses (3%). Nonhematologic toxicities of grade ≥ 3 or cardiac toxicity grade ≥ 2 occurred in 47 of 276 courses (17%) among 30 of 46 patients (65%; Table 4). The most frequent AE was febrile neutropenia with 29 episodes among 276 courses (10.5%) in 21 patients (46%). Ten infections (3.6%) of grade ≥ 3 were observed in eight patients (17%).
TABLE 4.
Acute AEs Grade ≥ 3 or Cardiac AEs Grade ≥ 2
Cardiac Toxicity
There were four adverse cardiac events (grade ≥ 2) in four (8.7%) patients reported during treatment. Two patients had had a pericardial effusion (grade 2) likely disease-related. A further patient developed atrial fibrillation (grade 3) after the third course of chemotherapy, and the final patient had left ventricular systolic dysfunction (grade 2) after the fifth cycle of therapy. Twenty-six of 36 patients with continuous first complete remission (72%) have had echocardiographic evaluation at 1 year following the last chemotherapy; of these, one (3.8%) patient met cardiac toxicity criteria with a Shortening Fraction of 26%. Long-term cardiac outcome requires further follow-up. One last patient had severe idiopathic pleuroparenchymal fibroelastosis that occurred 3 years after enrollment and 2 years after relapse and required lung transplantation but had normal cardiac function.
Immunity Status
At the end of treatment, 19 of 30 (63%) evaluable patients had low immunoglobulin G (IgG; less than the lower limit of the normal range), whereas at 1 year after inclusion in the study, 11 of 22 (50%) evaluable patients had low IgG. Six patients received immunoglobulin infusions, all of them for low IgG levels without infection, and, for four patients, immunoglobulin infusions started after treatment failure (relapse or progression or persistent disease). The occurrence of late infections after longer follow-up has not been evaluated.
DISCUSSION
In this prospective, multisite international phase II study of DA-EPOCH-R in pediatric and adolescent patients with PMLBL, the 4-year EFS was 69.6% (95% CI, 55.2 to 80.9) and the long-term OS was 84.8% (95% CI, 71.8 to 92.4). No improvement in EFS over historical controls with pediatric chemotherapy-only regimens was observed with the use of the regimen DA-EPOCH-R. There have been few prospective trials of this regimen in the pediatric or adult populations, and comparison with monocentric or retrospective trials is difficult.
The characteristics of the patients included in this study differed from those included in the NCI phase II study6 only with respect to age (median 15 v 30 years); in other respects, the patients were similar: sex distribution (57% v 59% female), large mediastinal masses (> 10 cm, 67% v 65%), and stage IV disease (27% v 29%). Similarly, with regard to treatment, more than 50% of patients in the NCI study achieved dose escalation ≥ 4% and 48% in the current study. Residual masses were observed in 36 of 51 (71%) in the NCI study and 33 of 46 (72%) in the current study. In the NCI study, viable tumor on biopsy of residual mass was not counted as an event for EFS analysis and neither was second malignancy. A reanalysis of our data using the NCI event criteria gives only a small increase of the 4-year EFS to 73.9% (95% CI, 59.7 to 84.4; Data Supplement). We examined the adherence to dose escalation rules and found that 29% of patients should have received a higher dose in at least one course of DA-EPOCH-R, but these data are not reported in other studies so it is not possible to assess whether this might have contributed to the outcome that we have observed. Among the 10 relapses that occurred locally (or were due to persistent disease), failure to escalate as per the Protocol was observed in five (one was due to gastric perforation and therefore clinically justified; Table 2). In the NCI study, five of 51(10%) patients who had evidence of continuous response between cycles 4 and 6 received eight cycles of DA-EPOCH-R. A major consideration for children is that eight cycles deliver a maximal possible cumulative anthracycline dose of approximately 600 mg/m2, which is unacceptable because of high risk of long-term cardiac damage.10
The only prospective randomized trial of DA-EPOCH-R including patients with PMLBL was the Phase III Intergroup Trial Alliance/CALGB 50303 trial of DA-EPOCH-R versus R-CHOP for diffuse large B-cell lymphoma, 35 patients with PMLBL were included, and there was no difference in outcome between those treated in either arm.11 Two retrospective studies of children and adolescents with PMLBL treated with DA-EPOCH-R have been reported.12,13 The small number of patients (15) in the initial Berlin-Frankfurt-Munster report on DA-EPOCH-R makes further comment difficult; they observed a 2-year EFS of 92.8%. The maximum cumulative dose of doxorubicin was capped at 360 mg/m2, and additional intrathecal therapy was given.11 Of note was the inclusion of one isolated CNS relapse (6.7%) in the report consistent with the rate that we observed in the current study (6.5%). In the previous FAB/LMB experience with PMLBL,3 using intrathecal therapy and other CNS-directed therapy such as high-dose methotrexate and aracytine, there were no CNS relapses at first relapse. Moreover, in the French LMB2001 prospective trial, among the 42 patients with PMLBL, none had CNS relapse, and among the 22 of 42 patients treated with rituximab in addition to LMB-based chemotherapy, only one relapsed (mediastinum).14
In the other retrospective series of children and adults with PMLBL treated with DA-EPOCH-R,13 there were 38 children who experienced a 3-year EFS of 81%. The characteristics of the children included were somewhat different from what we observed (more large mediastinal masses, greater proportion with elevated lactate dehydrogenase, and fewer stage IV in our trial). When combined with the adults in the study, there was no prognostic significance found for dose level achieved and a positive PET-CT at the end of therapy was associated with a poor prognosis. The exploratory findings of our study cast doubt over their conclusion that the total cumulative dose of doxorubicin can be capped at 360 mg/m2 as adopted by the Berlin-Frankfurt-Munster group, with no detriment to efficacy. Moreover, and as reported by others,15 the positive predictive value of PET-CT at the end of therapy was low (64%) in our prospective trial. PET-CT was not used for clinical decision making in this study, and central review was not undertaken. It was not, therefore, possible to refine the level of PET-CT positivity for those without a complete metabolic response after six cycles of DA-EPOCH-R using Deauville score.
The data from our trial confirm that DA-EPOCH-R has a favorable acute toxicity profile compared with the combination of rituximab with chemotherapy for other pediatric B-NHL.15 However, long-term evaluation of cardiac toxicity is required since 72% and 24% of patients received ≥ 300 mg/m2 and ≥ 350 mg/m2 of total cumulative dose of doxorubicin, respectively. The impact of rituximab on immunity is consistent with that seen in the trial for non-PMLBL patients.16
Although DA-EPOCH-R did not improve EFS in our study in comparison with historical controls treated with (LMB and other) chemotherapy only, it has been clearly demonstrated in adult patients with PMLBL that the addition of rituximab improves outcome.17 It is possible that the failure to demonstrate an increase in survival with rituximab over historical control in the current study is related to the efficacy of the chemotherapy part of the regimen in children and adolescents; however, our study was not designed to assess the efficacy of adding rituximab to chemotherapy or the addition of rituximab to DA-EPOCH in children. Why we did not reproduce the outstanding NCI results is not clear although multisite evaluation of a regimen does not always replicate data generated by single institutions.
The lower acute toxicity of DA-EPOCH-R with similar EFS to historical pediatric regimens that did not include rituximab would commend it as a new standard; however, the observation of isolated and combined CNS relapse with its use sounds a note of caution as these are very rarely reported in historical pediatric series (but being recognized in protocols more commonly used in adults with a rate of 3%-4%10). Further prospective trials are required to define optimal treatment for pediatric and adolescent PMLBL, and it is likely that alternative regimens such as chemotherapy used for other pediatric B-NHL or novel agents (eg, NF-kB pathway inhibitors or anti-PD1 therapies) will be required to enable children and adolescents to realize the survival outcomes that have been observed in some adults treated with DA-EPOCH-R.
ACKNOWLEDGMENT
The authors are indebted to the children and families that participated in this research. The authors thank the members of the national and international review panels (Andrew Wotherspoon, Peggy Dartigues, Thierry Molina, Jacques Bosq, Sherrie Perkins, Keith McCarthy, Antonio Ferrández, Olga Balagué, Jason C. So, Emanuele S. G. d'Amore, Florence Loong); to the national data managers (Rita Banusz, Elisa Carraro, Andrew Raxworthy Cooper, Verwer Femke, Olivia Hung, An Michiels, Marta Peiró, Jacqueline Vreijling, Olivia Wajsen, Tim Yu); to Giséle Goma and Anthony Mangin for the data management of the EICNHL patients; to Jim Anderson, Allen Buxton, Dave Hall, Lauren Saguilig, Alejandra Miranda, Taneesa Hlaing, and Giselle Galit for the data management of the COG patients; to Anne Tulard, Delphine Vuillier, and Jonathan Rubino from Gustave Roussy clinical research team; and to Salim Laghouati for the pharmacovigilance. The authors are also grateful to the Roche team. A full list of investigators and institutions is given in Appendix 1.
APPENDIX 1. Full List of Investigators and Institutions
List of Independent Data Monitoring Committee Members
Richard Sposto, Department of Preventive Medicine, Keck School of Medicine, University of Southern California, Los Angeles, CA
François Pein, Department of Clinical Research and Innovation, Institut Cancérologique de l'Ouest, Saint Herblain, France
Ross Pinkerton, Hummingbird House Children's Hospice, Brisbane, Australia
Marcus Robert, Kings College Hospital, London, UK
List of Principal Investigators
Listed in alphabetical order by name
For EICNHL (Belgium, France, Italy, Spain, the Netherlands, United Kingdom).
Aladjidi, Nathalie, Chu Pellegrin, Bordeaux, France
Almazan, Franscisco, Hospital Universitari Germans Trias I Pujol, Badalona, Spain
Andión Catalan, Maitane, Hospital Universitario Niño Jesús, Madrid, Spain
Astigarraga Aguirre, Itziar, Hospital Universitario Cruces, Baracaldo, Spain
Beishuizen, Auke, Erasmusmc, Rotterdam, the Netherlands
Bertolini, Patrizia, Az. Osp. Di Parma, Parma, Italy
Bonneau, Jacinthe, Chru De Rennes, Rennes, France
Brennan, Bernadette, Royal Manchester Children's Hospital, Manchester, United Kingdom
Brichard, Benedicte, Cliniques Universitaires Saint-Luc (Ucl), Brussel Ucl, Belgium
Bruin, Marrie, Umcu, Utrecht, the Netherlands
Buffardi, Salvatore, Ospedale “Pausilipon,” Napoli, Italy
Bulian, Pietro, IRCCS Centro Di Riferimento Oncologico—Aviano, Pordenone, Italy
Burke, Amos, Cambridge University Hospitals, Cambridge, United Kingdom
Burnelli, Roberta, Università Di Ferrara, Ferrara, Italy
Carausu, Liana, CHRU Brest, Brest, France
Carbone Baneres, Ana, Hospital Miguel Servet, Zaragoza, Spain
Casale, Fiorina, Ii Ateneo Di Napoli, Napoli, Italy
Celis, Veronica, Hospital Sant Joan De Déu, Barcelona, Spain
Cesar, Simone, Policlinico “G.B. Rossi,” Verona, Italy
Chalmers, Elizabeth, Royal Hospital For Sick Children (Yorkhill), Glasgow, United Kingdom
Clerico, Anna, Università “La Sapienza,” Roma, Italy
Connor, Philip, Cardiff and Vale University Health Board, Cardiff, United Kingdom
Consarino, Caterina, Az. Osp. “Pugliese-Ciaccio,” Catanzaro, Italy
Cosmi, Carlo, Università Di Sassari, Sassari, Italy
Couillault, Gérard, Chu Le Bocage, Dijon, France
Couselo Sanchez, Jose Miguel, Hospital Santiago, Santiago De Compostela, Spain
Dalle, Jean-Hugues, Hôpital Robert Debre, Paris, France
Dandapani, Madhumita, Nottingham University Hospitals, Nottingham, United Kingdom
D'angelo, Paolo, Ospedale Civico, Pad. 17/C, Palermo, Italy
Daw, Stephen, University College London Hospitals, London, United Kingdom
De Bont, Eveline, Umgc, Gronigen, the Netherlands
De Santis, Raffaela, Ospedale “Casa Sollievo Della Sofferenza,” San Giovanni Rotondo, Italy
Devalck, Christine, ULB, Brussel, Belgium
Devoldere, Catherine, CHU D'amiens—Hôpital Nord, Amiens, France
Dupuy-Poiree, Marilyne, CHU De Nice, Nice, France
Edgar, Angela, NHS Lothian—Royal Hospital For Sick Children, Edinburgh, United Kingdom
Elliott, Martin, Leeds Teaching Hospitals, Leeds, United Kingdom
Escobosa Sanchez, Olga, Hospital Carlos Haya, Málaga, Spain
Fagioli, Franca, Ospedale Infantile Regina Margherita, Torino, Italy
Favre, Claudio, Ospedale S. Chiara, Pisa, Italy
F-Delgado, Rafael, Hospital Clínico, Valencia, Spain
Fernandez Navarro, José, Hospital La Fe, Valencia, Spain
Foa, Robin, Università “La Sapienza,” Roma Umberto, Italy
Galera Minano, Ana Mª, Hospital Virgen De Arrixaca, El Palmar (Murcia), Spain
Galimberti, Daniela, Università Degli Studi Di Siena, Siena, Italy
Gallego Melcon, Soledad, Hospital Vall D'hebron, Barcelona, Spain
Garaventa, Alberto, Istituto “ G. Gaslini,” Genova, Italy
Garcia Miguel, Purificación, Hospital La Paz, Madrid, Spain
Garnier, Nathalie, Ihop, Lyon, France
Garrido Colino, Carmen, Hospital Gregorio Marañon, Madrid, Spain
Giraldi, Eugenia, U.O. Pediatrica—OO.RR Bergamo, Bergamo, Italy
Gomez, Pedro, Hospital Reina Sofia, Córdoba, Spain
Gonzalez Muniz, Soledad, Hospital Central De Asturias, Oviedo, Spain
Gray, Juliet, Southampton University Hospitals, Hampshire, United Kingdom
Hall, Georgina, Oxford Radcliff Hospitals—Children's Hospital, Oxford, United Kingdom
Haouy, Stéphanie, CHU Arnaud De Villeneuve, Montpellier, France
Hayden, James, Alder Hey Children's, Liverpool, United Kingdom
Hernandez, Isabel, Hospital Son Espases, Palma, Spain
Hobin, David, Birmingham Children’s Hospital, Birmingham, United Kingdom
Hoyoux, Claire, CHR De La Citadelle, Liege, Belgium
Jenkins, Anna, Sheffield Children's, Sheffield, United Kingdom
Johnston, Robert, The Royal Belfast Hospital For Sick Children, Belfast, United Kingdom
Jourdain, Anne, CHU De Tours, Tours, France
Kanold-Lastawiecka, Justyna, Chu Estaing, Clermont Ferrand, France
Kaspers, Gert-Jan, VUMC, Amsterdam, the Netherlands
King, Derek, Royal Aberdeen Children's Hospital, Aberdeen, United Kingdom
Kiss, Csongor, Dote Dept. Of Pediatrics; Hemato-Oncology Ward, Debrecen, Hungary
Lambilliotte, Anne, CHRU De Lille—Hôpital Jeanne De Flandre, Lille, France
Laureys, Geneviève, University Hospital Gent, Gent, Belgium
Lendinez Molinos, Francisco, Hospital Torrecardenas, Almeria, Spain
Lillo, Miguel, Complejo Hospitalario De Albacete, Albacete, Spain
Lo Nigro, Luca, Clinica Pediatrica, Catania, Italy
Locatelli, Franco, Ospedale “Bambino Gesù,” Roma, Italy
Loeffen, Jan, Umc St Radboud, Nijmegen, the Netherlands
Lopez Almaraz, Ricardo, Hospital Universitario De Canarias (Huc), La Laguna (Tenerife), Spain
Lopez Duarte, Monica, Hospital Marqués De Valdecilla, Santander, Spain
Lowis, Stephen, University Hospitals Bristol, Bristol, United Kingdom
Melo Valls, Montserrat, Hospital Parc Taulí., Sabadell, Spain
Menguy, Sandrine, CHU Saint-Etienne, Saint Etienne, France
Michon, Jean, Institut Curie, Paris, France
Millot, Frédéric, Chu De Poitiers, Poitiers, France
Minard-Colin, Véronique, Gustave Roussy, Villejuif, France
Minckes, Odile, CHU Côte De Nacre, Caen, France
Molina Garicano, Javier, Hospital Virgen Del Camino, Pamplona, Spain
Munzer, Martine, Hôpital Américain, Reims, France
Mura, Rosamaria, Ospedale Regionale Microcitemie, Cagliari, Italy
Ortega Acosta, Mª José, Hospital Virgen De Las Nieves, Granada, Spain
Paillard, Catherine, Hôpital De Haute Pierre, Strasbourg, France
Paolucci, Paolo, Azienda Policlinico Di Modena, Modena, Italy
Pelaez Pleguezuelos, Irene, Complejo Hospitalario De Jaén Avda, Jaén, Spain
Pellier, Isabelle, CHU D’angers, Angers, France
Pericoli, Roberta, Azienda Usl Rimini, Rimini, Italy
Perruccio, Katia, Ospedale “R. Silvestrini,” Perugia, Italy
Pession, Andrea, Ospedale Sant’orsola Malpighi, Bologna, Italy
Petit, Arnaud, G.H. Armand Trousseau, Paris, France
Philippet, Pierre, Chc Espérance, Liege, Belgium
Pierani, Paolo, Ospedale Dei Bambini “G. Salesi,” Ancona, Italy
Piguet, Christophe, CHU De Limoges, Limoges, France
Pillon, Marta, Azienda Ospedaliera-Università Di Padova, Padova, Italy
Plantaz, Dominique, CHU De Grenoble, Grenoble, France
Plat, Geneviève, Hôpital Des Enfants Toulouse, Toulouse, France
Plouvier, Emmanuel, Hôpital Saint- Jacques—CHR, Besancon, France
Porta, Fulvio, Clinica Pediatrica Ospedale Civile, Brescia, Italy
Quiroga Cantero, Eduardo, Hospital Virgen Del Rocio, Sevilla, Spain
Rao, Anupama, Great Ormond Street Hospital for Children, London, United Kingdom
Riccardi, Riccardo, Università Cattolica Di Roma, Roma, Italy
Riesco Riesco, Susana, Complejo Asistencial Universitario De Salamanca, Salamanca, Spain
Rizzari, Carmelo, Clinica Pediatrica Ospedale S. Gerardo, Monza, Italy
Santoro, Nicola, U.O. Pediatrica I Policlinico, Bari, Italy
Schmitt, Claudine, CHU De Nancy Brabois Hôpital D’enfants, Nancy, France
Spreafico, Filippo, Ist Nazionale Studio E Cura Tumori, Milano, Italy
Taj, Mary, The Royal Marsden, Surrey, United Kingdom
Tallón, María, CHUVI, Vigo, Spain
Tamaro, Paolo, Università Degli Studi Di Trieste, Trieste, Italy
Thomas, Caroline, Chu De Nantes—Hôpital Mère Enfant, Nantes, France
Tondo, Annalisa, Azienda “A.Meyer,” Firenze, Italy
Torrent Espanol, Montse, Hospital De Sant Pau, Barcelona, Spain
Uriz_Monaut, José, Hospital Donostia, San Sebastián, Spain
Uyttebroeck, Anne, University Hospitals Leuven, Leuven, Belgium
Van Der Werff Ten Bosch, Jutte, University Hospital Brussels, Brussel Uz, Belgium
Vannier, Jean-Pierre, CHU—Hôpitaux De Rouen, Rouen, France
Verschuur, Arnauld, Hopital La Timone Enfants, Marseille, France
Villa Alcazar, Marta, Hospital Monteprincipe, Madrid, Spain
Visser, Johannes, University Hospitals Of Leicester, Leicester, United Kingdom
Vivanco Martinez, José Luis, Hospital Universitario 12 De Octubre, Madrid, Spain
Vormoor, Josef, The Newcastle Upon Tyne Hospitals, Newcastle, United Kingdom
Zecca, Marco, Irccs, Policlinico San Matteo, Pavia, Italy
Zsiros, Jozsef, PMC, Utrecht, the Netherlands
For COG (Australia, Canada, and United States).
Athale, Uma, McMaster Children's Hospital at Hamilton Health Sciences, Hamilton, Canada
Balagtas, Jay Michael, Lucile Packard Children's Hospital Stanford University, Palo Alto, United States (US)
Balis, Frank, Children's Hospital of Philadelphia, Philadelphia, US
Barbaric, Draga, Sydney Children's Hospital, Randwick, Australia
Barnette, Phillip, Primary Children's Hospital, Salt Lake City, US
Barredo, Julio, University of Miami Miller School of Medicine-Sylvester Cancer Center, Miami, US
Bartels, Ute, Hospital for Sick Children, Toronto, Canada
Batra, Sandeep, Riley Hospital for Children, Indianapolis, US
Bautista-Otanez, Felipe, Lehigh Valley Hospital-Cedar Crest, Bethlehem, US
Becton, David, Arkansas Children's Hospital, Little Rock, US
Bell, Jessica, Novant Health Presbyterian Medical Center, Charlotte, US
Bhakta, Manoo, T C Thompson Children's Hospital, Chattanooga, US
Boklan, Jessica, Phoenix Children's Hospital, Phoenix, US
Borinstein, Scott, Vanderbilt University/Ingram Cancer Center, Nashville, US
Bradfield, Scott, Nemours Children's Clinic-Jacksonville, Jacksonville, US
Brown, Evangeline, Nemours Children’s Clinic—Pensacola, Pensacola, US
Bryant, Nichole, BI-LO Charities Children's Cancer Center, Greenville, US
Campbell, Laura, Kaiser Permanente-Oakland, Oakland, US
Casillas, Jacqueline, Miller Children's and Women's Hospital Long Beach, Long Beach, US
Caywood, Emi, Alfred I du Pont Hospital for Children, Wilmington, US
Chamdin, Aghiad, Michigan State University Clinical Center, East Lansing, US
Clark, Jennifer, Rocky Mountain Hospital for Children-Presbyterian Saint Luke's Medical Center, Denver, US
Cooper, Robert, Kaiser Permanente Downey Medical Center, Downey, US
De Santes, Kenneth, University of Wisconsin Hospital and Clinics, Madison, US
Dome, Jeffrey, Children's National Medical Center, Washington, US
Fixler, Jason, Sinai Hospital of Baltimore, Baltimore, US
Friedmann, Alison, Massachusetts General Hospital Cancer Center, Boston, US
Gidvani-Diaz, Vinod, Methodist Children's Hospital of South Texas, San Antonio, US
Golden, Carla, Children's Hospital and Research Center at Oakland, Oakland, US
Goldman, Stanton, Medical City Dallas Hospital, Dallas, US
Greene Welch, Jennifer, Rhode Island Hospital, Providence, US
Gregory, John, Morristown Medical Center, Morristown, US
Halligan, Gregory, Saint Christopher's Hospital for Children, Philadelphia, US
Hansford, Jordan, Royal Children's Hospital, Parkville, Australia
Hartman, Lisa, El Paso Children's Hospital, El Paso, US
Hawkins, Douglas, Seattle Children's Hospital, Seattle, US
Hayashi, Robert, Washington University School of Medicine, Saint Louis, US
Irving, Helen, Queensland Children's Hospital, South Brisbane, Australia
Isakoff, Michael, Connecticut Children's Medical Center, Hartford, US
Jasty, Rama, Mercy Children’s Hospital, Toledo, US
Kheradpour, Albert, Loma Linda University Medical Center, Loma Linda, US
Kim, Julie, Dartmouth-Hitchcock Medical Center/Norris Cotton Cancer Center, Lebanon, US
Kram, David, Wake Forest University Health Sciences, Winston-Salem, US
Kraveka, Jacqueline, Medical University of South Carolina, Charleston, US
Kuerbitz, Steven, Children's Hospital Medical Center of Akron, Akron, US
Kutny, Matthew, Children's Hospital of Alabama, Birmingham, US
Kuttesch, John, University of New Mexico Cancer Center, Albuquerque, US
Kyono, Wade, Kapiolani Medical Center for Women and Children, Honolulu, US
Law, Jason, Floating Hospital for Children at Tufts Medical Center, Boston, US
Leavey, Patrick, UT Southwestern/Simmons Cancer Center-Dallas, Dallas, US
Lee, Alice, NYP/Columbia University Medical Center/Herbert Irving Comprehensive Cancer Center, New York, US
Libes, Jaime, Saint Jude Midwest Affiliate, Peoria, US
Long, Catherine, Saint Vincent Hospital Cancer Center Green Bay, Green Bay, US
Madhusoodhan, Pillai Pallavi, Mount Sinai Hospital, New York, US
Majlessipour, Fataneh (Fae), Cedars-Sinai Medical Center, Los Angeles, US
Mallory, Samantha, Blank Children's Hospital, Des Moines, US
Maloney, Kelly, Children's Hospital Colorado, Aurora, US
Manalang, Michelle, Marshfield Medical Center-Marshfield, Marshfield, US
Martin, Alissa, Wayne State University/Karmanos Cancer Institute, Detroit, US
Massey, Gita, Virginia Commonwealth University/Massey Cancer Center, Richmond, US
McFall, Rebecca, Advocate Children's Hospital-Oak Lawn, Oak Lawn, US
McNall-Knapp, Rene, University of Oklahoma Health Sciences Center, Oklahoma City, US
Michon, Bruno, CHU de Quebec-Centre Hospitalier de l'Universite Laval (CHUL), Quebec, Canada
Mitchell, David, The Montreal Children's Hospital of the MUHC, Montreal, Canada
Mody, Rajen, C S Mott Children's Hospital, Ann Arbor, US
Monteleone, Philip, State University of New York Upstate Medical University, Syracuse, US
Nagasubramanian, Ramamoorthy, Nemours Children's Hospital, Orlando, US
Padhye, Bhavna, The Children's Hospital at Westmead, Westmead, Australia
Perentesis, John, Cincinnati Children's Hospital Medical Center, Cincinnati, US
Phillips, Marianne, Perth Children's Hospital, Perth, Australia
Rabin, Karen, Baylor College of Medicine/Dan L Duncan Comprehensive Cancer Center, Houston, US
Radulescu, Vlad, University of Kentucky/Markey Cancer Center, Lexington, US
Raj, Ashok, Norton Children's Hospital, Louisville, US
Ramdas, Jagadeesh, Geisinger Medical Center, Danville, US
Rangaswami, Arun, UCSF Medical Center-Mission Bay, San Francisco, US
Razzouk, Bassem, Saint Vincent Hospital and Health Care Center, Indianapolis, US
Roberts, William, Rady Children's Hospital—San Diego, San Diego, US
Samson, Yvan, Centre Hospitalier Universitaire Sainte-Justine, Montreal, Canada
Sato, Mariko, University of Iowa/Holden Comprehensive Cancer Center, Iowa City, US
Schorin, Marshall, Inova Fairfax Hospital, Falls Church, US
Scothorn, Douglas, Mission Hospital Inc-Memorial Campus, Asheville, US
Shaw, Peter, Johns Hopkins All Children's Hospital, Saint Petersburg, US
Shusterman, Suzanne, Dana-Farber/Harvard Cancer Center, Boston, US
Silva, Mariana, Kingston Health Sciences Centre, Kingston, Canada
Smith, Amy, Arnold Palmer Hospital for Children, Orlando, US
Stearns, Duncan, Rainbow Babies and Children's Hospital, Cleveland, US
Stork, Linda, Oregon Health and Science University, Portland, US
Suh, Eugene, Loyola University Medical Center, Maywood, US
Twist, Clare, Roswell Park Cancer Institute, Buffalo, US
Wagner, Kayelyn, Sanford United StatesD Medical Center—Sioux Falls, Sioux Falls, US
Walterhouse, David, Ann and Robert H Lurie Children's Hospital of Chicago, Chicago, US
Weintraub, Lauren, Albany Medical Center, Albany, US
G. A. Amos Burke
Consulting or Advisory Role: Roche, Takeda, Oxford Immune Algorithmics, Novartis
Veronique Minard-Colin
Research Funding: F. Hoffmann-La Roche-Genentech
Consulting or Advisory Role: Novartis, Roche, BMS, Pfizer
Anne Aupérin
Consulting or Advisory Role: MSD
Research Funding: F. Hoffmann-La Roche-Genentech
Catherine M. Bollard
Leadership: Cabaletta Bio
Consulting or Advisory Role: Mana Therapeutics, Catamaran Bio
Keith Wheatley
Research Funding: Bio-Cancer Treatment International, EUSA Pharma, Bayer
Donald A. Barkauskas
Employment: Genentech (I)
Stock and Other Ownership Interests: Genentech (I)
Patents, Royalties, Other Intellectual Property: US patent on the basis of PhD research in glioblastoma (I)
Peter C. Adamson
Employment: Sanofi
Stock and Other Ownership Interests: Gilead Sciences, McKesson, Molina Healthcare, Thermo Fisher Scientific, UnitedHealthcare, AbbVie, Medtronic, Sanofi
Gilles Vassal
Consulting or Advisory Role: Bayer, Roche/Genentech, AstraZeneca, Bristol Myers Squibb, Lilly, Ipsen, Novartis
Travel, Accommodations, Expenses: Bristol Myers Squibb, Roche
No other potential conflicts of interest were reported.
SUPPORT
Supported by the Clinical Research Hospital Program of the French Ministry of Health, Enfants Cancers Santé (ECS), NCTN Operations Center Grant U10CA180886, NCTN Statistics & Data Center Grant U10CA180899, Cancer Research UK, and National Institute for Health Research Clinical Research Network (UK).
CLINICAL TRIAL INFORMATION
NCT01516567 (Registry name, Inter B-NHL Ritux 2010 phase II).
G.A.A.B. and V.M.-C. share first authorship. C.P. and T.G.G. share last authorship.
AUTHOR CONTRIBUTIONS
Conception and design: G. A. Amos Burke, Veronique Minard-Colin, Anne Aupérin, Marta Pillon, Keith Wheatley, Peter C. Adamson, Gilles Vassal, Catherine Patte, Thomas G. Gross
Administrative support: Catherine M. Bollard, Gilles Vassal
Provision of study materials or patients: G. A. Amos Burke, Veronique Minard-Colin, Sarah Alexander, Marta Pillon, Rafael Delgado, József Zsíros, Anne Uyttebroeck, Bernarda Kazanowska, Alan K. Chiang, Catherine M. Bollard, Monika Csoka, Keith Wheatley, Catherine Patte, Thomas G. Gross
Collection and assembly of data: G. A. Amos Burke, Veronique Minard-Colin, Marta Pillon, Rafael Delgado, József Zsíros, Anne Uyttebroeck, Peggy Dartigues, Rodney R. Miles, Bernarda Kazanowska, Alan K. Chiang, Stéphanie Haouy, Monika Csoka, Donald A. Barkauskas, Thomas G. Gross
Data analysis and interpretation: G. A. Amos Burke, Veronique Minard-Colin, Sarah Alexander, Anne Aupérin, Keith Wheatley, Donald A. Barkauskas, Peter C. Adamson, Thomas G. Gross, Catherine Patte
Manuscript writing: All authors
Final approval of manuscript: All authors
Accountable for all aspects of the work: All authors
AUTHORS' DISCLOSURES OF POTENTIAL CONFLICTS OF INTEREST
Dose-Adjusted Etoposide, Doxorubicin, and Cyclophosphamide With Vincristine and Prednisone Plus Rituximab Therapy in Children and Adolescents With Primary Mediastinal B-Cell Lymphoma: A Multicenter Phase II Trial
The following represents disclosure information provided by authors of this manuscript. All relationships are considered compensated unless otherwise noted. Relationships are self-held unless noted. I = Immediate Family Member, Inst = My Institution. Relationships may not relate to the subject matter of this manuscript. For more information about ASCO's conflict of interest policy, please refer to www.asco.org/rwc or ascopubs.org/jco/authors/author-center.
Open Payments is a public database containing information reported by companies about payments made to US-licensed physicians (Open Payments).
G. A. Amos Burke
Consulting or Advisory Role: Roche, Takeda, Oxford Immune Algorithmics, Novartis
Veronique Minard-Colin
Research Funding: F. Hoffmann-La Roche-Genentech
Consulting or Advisory Role: Novartis, Roche, BMS, Pfizer
Anne Aupérin
Consulting or Advisory Role: MSD
Research Funding: F. Hoffmann-La Roche-Genentech
Catherine M. Bollard
Leadership: Cabaletta Bio
Consulting or Advisory Role: Mana Therapeutics, Catamaran Bio
Keith Wheatley
Research Funding: Bio-Cancer Treatment International, EUSA Pharma, Bayer
Donald A. Barkauskas
Employment: Genentech (I)
Stock and Other Ownership Interests: Genentech (I)
Patents, Royalties, Other Intellectual Property: US patent on the basis of PhD research in glioblastoma (I)
Peter C. Adamson
Employment: Sanofi
Stock and Other Ownership Interests: Gilead Sciences, McKesson, Molina Healthcare, Thermo Fisher Scientific, UnitedHealthcare, AbbVie, Medtronic, Sanofi
Gilles Vassal
Consulting or Advisory Role: Bayer, Roche/Genentech, AstraZeneca, Bristol Myers Squibb, Lilly, Ipsen, Novartis
Travel, Accommodations, Expenses: Bristol Myers Squibb, Roche
No other potential conflicts of interest were reported.
REFERENCES
- 1.Oschlies I Burkhardt B, Salaverria I, et al. : Clinical, pathological and genetic features of primary mediastinal large B-cell lymphomas and mediastinal gray zone lymphomas in children. Haematologica 96:262-268, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Burkhardt B, Zimmermann M, Oschlies I, et al. : The impact of age and gender on biology, clinical features and treatment outcome of non-Hodgkin lymphoma in childhood and adolescence. Br J Haematol 131:39-49, 2005 [DOI] [PubMed] [Google Scholar]
- 3.Gerrard M, Waxman IM, Sposto R, et al. : Outcome and pathologic classification of children and adolescents with mediastinal large B-cell lymphoma treated with FAB/LMB96 mature B-NHL therapy. Blood 121:278-285, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Seidemann K, Tiemann M, Lauterbach I, et al. : Primary mediastinal large B-cell lymphoma with sclerosis in pediatric and adolescent patients: Treatment and results from three therapeutic studies of the Berlin-Frankfurt-Münster Group. J Clin Oncol 21:1782-1789, 2003 [DOI] [PubMed] [Google Scholar]
- 5.Pillon M, Di Tullio MT, Garaventa A, et al. : Long-term results of the first Italian Association of Pediatric Hematology and Oncology protocol for the treatment of pediatric B-cell non-Hodgkin lymphoma (AIEOP LNH92). Cancer 101:385-394, 2004 [DOI] [PubMed] [Google Scholar]
- 6.Dunleavy K, Pittaluga S, Maeda LS, et al. : Dose-adjusted EPOCH-rituximab therapy in primary mediastinal B-cell lymphoma. N Engl J Med 368:1408-1416, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Wilson WH, Grossbard ML, Pittaluga S, et al. : Dose-adjusted EPOCH chemotherapy for untreated large B-cell lymphomas: A pharmacodynamic approach with high efficacy. Blood 99:2685-2693, 2002 [DOI] [PubMed] [Google Scholar]
- 8.Patte C, Reiter A, Rosolen A, et al. : Primary mediastinal large B-cell lymphoma (PMLBL) in chidren/adolescents. Data of European and American Groups. Ann Oncol 16:61-62, 2005 [Google Scholar]
- 9.Finkelstein DM, Muzikansky A, Schoenfeld DA. Comparing survival of a sample to that of a standard population. J Natl Cancer Inst 95:1434-1439, 22003 [DOI] [PubMed] [Google Scholar]
- 10.van Dalen EC, van der Pal HJ, Kremer LC. Different dosage schedules for reducing cardiotoxicity in people with cancer receiving anthracycline chemotherapy. Cochrane Database Syst Rev 3:CD005008, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Bartlett NL, Wilson WH, Jung SH, et al. : Dose-adjusted EPOCH-R compared with R-CHOP as Frontline therapy for diffuse large B-cell lymphoma: Clinical outcomes of thePhase III Intergroup Trial Alliance/CALGB 50303. J Clin Oncol 37:1790-1799, 2019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Woessmann W, Lisfeld J, Burkhardt B, et al. : Therapy in primary mediastinal B-cell lymphoma. N Engl J Med 369:282-284, 2013 [DOI] [PubMed] [Google Scholar]
- 13.Giulino-Roth L, O'Donohue T, Chen Z, et al. : Outcomes of adults and children with primary mediastinal B-cell lymphoma treated with dose-adjusted EPOCH-R. Br J Haematol 179:739-747, 2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Dourthe ME, Phulpin A, Auperin A, et al. : Rituximab in addition to LMB-modified chemotherapy regimen in pediatric patients with primary mediastinal large B-cell lymphoma: Results of the French LMB2001 prospective study. Pediatr Blood Cancer 66:S38-S, 2019 [Google Scholar]
- 15.Melani C, Advani R, Roschewski M, et al. : End-of-treatment and serial PET imaging in primary mediastinal B-cell lymphoma following dose-adjusted EPOCH-R: A paradigm shift in clinical decision making. Haematologica 103:1337-1344, 2018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Minard-Colin V, Auperin A, Pillon M, et al. : Rituximab for high-risk, mature B-cell non-Hodgkin's lymphoma in children. N Engl J Med 382:2207-2219, 2020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Rieger M, Österborg A, Pettengell R, et al. : Primary mediastinal B-cell lymphoma treated with CHOP-like chemotherapy with or without rituximab: Results of the Mabthera International Trial Group study. Ann Oncol 22:664-670, 2011 [DOI] [PubMed] [Google Scholar]