Abstract
Ethnopharmacological relevance
The coronavirus disease (COVID-19) has relentlessly spread all over the world even after the advent of vaccines. It demands management, treatment, and prevention as well with utmost safety and effectiveness. It is well researched that herbal medicines or natural products have shown promising outcomes to strengthen immunity with antiviral potential against SARS-COV-2.
Aim of the review: Our objective is to provide a comprehensive insight into the preventive and therapeutic effects of herbal medicines and products (Ayurvedic) for pre-and post-COVID manifestations.
Material and method
The database used in the text is collected and compiled from Scopus, PubMed, Nature, Elsevier, Web of Science, bioRxiv, medRxiv, American Chemical Society, and clinicaltrials.gov up to January 2022. Articles from non-academic sources such as websites and news were also retrieved. Exploration of the studies was executed to recognize supplementary publications of research studies and systematic reviews. The keywords, such as “SARS-COV-2, coronavirus, COVID-19, herbal drugs, immunity, herbal immunomodulators, infection, herbal antiviral drugs, and WHO recommendation” were thoroughly searched. Chemical structures were drawn using the software Chemdraw Professional 15.0.0.160 (PerkinElmer Informatics, Inc.).
Result
A plethora of literature supports that the use of herbal regimens not only strengthen immunity but can also treat SARS-COV-2 infection with minimal side effects. This review summarizes the mechanistic insights into herbal therapy engaging interferons and antibodies to boost the response against SARS-COV-2 infection, several clinical trials, and in silico studies (computational approaches) on selected natural products including, Ashwagandha, Guduchi, Yashtimadhu, Tulsi, etc. as preventive and therapeutic measures against COVID. We have also emphasized the exploitation of herbal medicine-based pharmaceutical products along with perspectives for unseen upcoming alike diseases.
Conclusion
According to the current state of art and cutting-edge research on herbal medicines have showed a significant promise as modern COVID tools. Since vaccination cannot be purported as a long-term cure for viral infections, herbal/natural medicines can only be considered a viable alternative to current remedies, as conceived from our collected data to unroot recurring viral infections.
Keywords: Coronavirus, Herbal medicine, Immunomodulation, Pharmaceutical product, SARS-COV-2
Graphical abstract
Abbreviations
- Antibody-secreting cells
ASCs
- Angiotensin convertase enzyme 2
ACE-2
- Coronavirus
CoV
- Coronavirus disease of 2019
COVID-19
- Cyclooxygenase-2
COX-2
- Dendritic Cells
DC
- Follicular helper T
Tfh
- Food and Drug Administration
FDA
- Herpes simplex virus
HSV
- Interferon
IF
- Interleukin
IL
- Interleukin-1 receptor-associated kinase
IRAK
- Macrophage inflammatory protein-1-alpha
MIP-1- α
- Mitogen-activated protein kinases
MAPKs
- Monocyte chemoattractant protein
MCP
- Natural killer cells
NKc
- NF-κB-inducing kinase
NIK
- Non-structural proteins
Nsp
- Once in a day
O.D.
- Oral nutrition supplement
ONS
- Phospholipase A2
PLA2
- Receptor binding domain
RBD
- Replication/transcription complex
RTC
- RNA-dependent RNA polymerase
RDRP
- Severe acute respiratory syndrome coronavirus 2
SARS-COV-2
- Tablespoon
tbsp
- Teaspoon
tsp
- T-helper
th
- Three times a day
T.I.D.
- Tissue necrosis factor
TNF
- Tool like receptors
TLRs
- Transmembrane protease, serine
TMPRSS
- Twice a day
B.I.D.
- Vascular endothelial growth Factor
VEGF
- World health organization
WHO
1. Introduction
The current pandemic of CoV disease is exceptional not just because of its occurrence by a novel coronavirus, but also because of the diverse and unforeseen innate immune responses (Balkrishna, 2020; Jain et al., 2021). The amplitude of the pandemic necessitates an urgent harnessing of all knowledge systems available globally to conquer the disease efficiently. However, WHO has proposed guidelines to prevent oneself from effecting by social distancing to avoid direct contact of virus, and patient isolation at an early stage, circulating the evidences accurately to the public, and curtailing the social and financial influence on them. Since the start of the COVID-19 outbreak, traditional herbal therapies have been vigorously used among the people as the home remedies (Mousavi et al., 2021). Ayurveda has shown substantial potential towards prevention and treatment of COVID-19 (Singh et al., 2022; Xu et al., 2020). The involvement of Ayurveda to manage the COVID-19 challenge is not limited but is potentially well known to support health services. SARS-COV-2 infection generally affects people with a weak immune system. So, it is a primary goal to enhance immunity using the Ayurvedic system for the treatment of COVID-19 patients. Ayurveda intrusions become even more pertinent by the fact that there is a broad explanation of the causation and management of the COVID-19 pandemic in Ayurveda. The identification and the use of phytochemicals obtained from medicinal herbs could help to lower the infection rate and successfully have become a new approach for the treatment of viral SARS-COV-2 infection (Anand et al., 2021; Islam et al., 2021). Some risk factors, for instance, age, diabetes, cardiac diseases, reduced oxygen level, and psychosis, are the chief hurdles seen in the treatment of SARS-COV-2.
The recommended ayurvedic regimens for the prophylaxis of COVID-19 include the use of Guduchi (giloy) (Tinospora cordifolia (Willd.) Miers.), chyawanprash, golden milk, kadha, nutritional supplement, and the use of warm water, etc. A recent study proposed that the application of Ashwagandha (Withania somnifera (L.) Dunal), Tulsi (Ocimum sanctum L.), and Guduchi constituents are very effective against COVID-19, owing to their potential to target the ACE-2 receptor. The ACE-2 protein attached to a human immunoglobulin G Fc domain (ACE-2-Fc) of SARS-COV-2 patients may assist traditional neutralizing antibodies, which could allow for the treatment of the infection. ACE-2-Fc might play an imperative role in the treatment of COVID-19 if the function of ACE-2-Fc is inhibited (Adhikari et al., 2021; Gajewski et al., 2021; Vellingiri et al., 2020). For the period of viral infection, host factors provoke an immune response, contrary to the viruses. T cells, specifically CD4+ and CD8+, are crucial for an antiviral role to battle the pathogens and raise the risk of evolving autoimmunity/inflammation. The CD4+ T cells produce viral-specific antibodies by stimulating T cell-dependent B cells. Nevertheless, CD8+ T cells are cytotoxic and kill virus-infected cells. It has been observed that the patients infested with SARS-COV-2 showed CD8+T cells in the pulmonary interstitium which are 80% of the total inflammatory cells and they play a vital role in eradicating the CoV from infected cells and recovering immune damage. (Cecere et al., 2012; Maloir et al., 2018).
Having a healthy immune system is just that abundance of vibrancy, vitality, and health. The developed vaccines and identified medicines are not effective on a long-term basis to prevent COVID-19 from spreading among the population. Moreover, the mutating nature of a virus limits the effectiveness of the developed vaccine against them. During the various waves of this pandemic, most of the countries were suffered severely while the spread was not in abundance in India due to the use of herbal immunity boosters which is protecting our body by magnifying the immunity.
2. Spread of SARS-COV-2
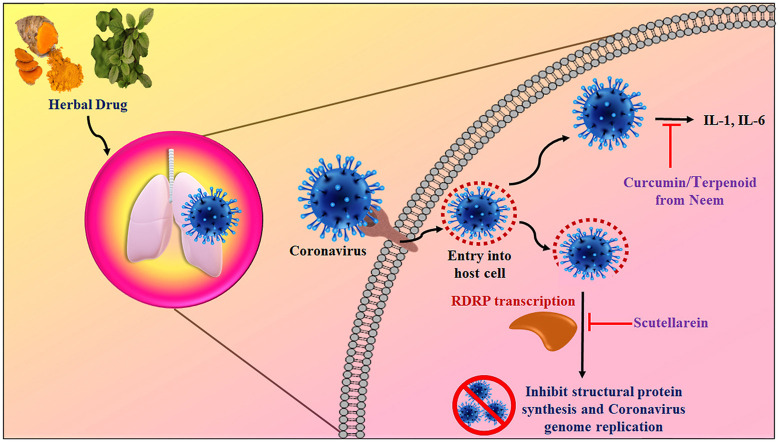
The Ayurveda system is well known for its management to treat various ailments. The medicines which are obtained from a natural source are widely utilized for their efficacy as antiviral, antibacterial, neurostimulation, and for its immune-boosting property, which have potential to fight against foreign pathogens. Viruses are an intracellular parasite that lives in the host cell for their survival. The drug/bioactive interfere with the cellular mechanisms of the virus to hinder its further growth. Therefore, antiviral drugs are vital for the survival of host cells without any adverse effects on the host cell. RDRP is an enzyme that catalyzes RNA replication, and these are essential proteins encoded in the genomes of all RNA-rich viruses without having a DNA stage. RDRP is responsible for the multiplication, growth, and survival of the virus. Hence, RDRP lately arose as a capable target to inhibit viral replication and its high conservation among viral strains (Balkrishna, 2020).
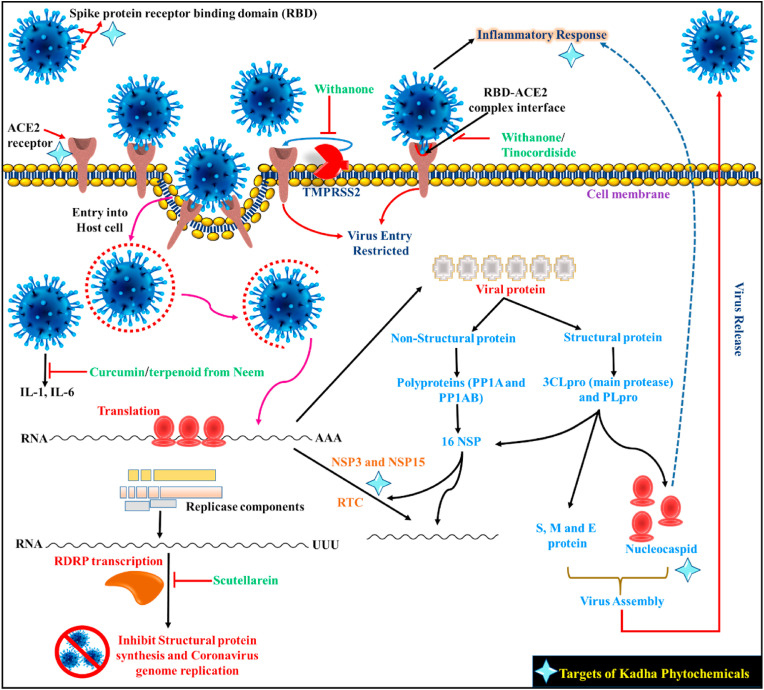
The entrance of SARS-COV-2 into the hosting cell depends on the interaction with the ACE-2 receptor (Kumar et al., 2020; Seyran et al., 2021). The S protein of the spike attaches with ACE-2; as a result, the virus gets cleaved and is enabled for its annexation into the membrane of the host cell, which is facilitated by the TMPRSS-2 (Hasan et al., 2021; Hoffmann et al., 2020). The clinical assessment of the affected patient with SARS-COV-2 has shown a high level of cytokines and chemokines within the human plasma, which is the responsible factor for multi-organ failure and higher mortality of patients (Huang, F. et al., 2020). In the mild to moderate SARS-COV-2 patients, immunological alterations such as an increase in the number of activated CD4+ helper T cells and CD8+ killer T cells, Tfh) cells, ASCs, and antibodies particularly IgG and IgM were observed (Khanna et al., 2021; Thevarajan et al., 2020). COX-2, PLA2, NIK, and IRAK play a crucial role in SARS-COV-2-induced inflammatory response and can be used to screen anti-inflammatory molecules. The SARS-COV-2 spike protein has two domains S1 and S2. S1 is behind the insertion of the virus into the host cell while S2 mediates the fusion of the host and the viral membrane to facilitate the viral RNA genome within the host. Thus, these play a pivotal role in drug design. Many phytochemicals inhibit the entry of the viral genome to the host. The spikes proteins are also active in modulating the immunity of host cells toward CoVs (Dubey and Dubey, 2022) and S is a significant antigen on the virus surface (Yuan et al., 2017). Another Nsp3 protein responsible for CoV is RTC formation. It releases Nsp1, Nsp2, and interacts with other Nsps and RNA to form RTC (Hardenbrook and Zhang, 2022; Wu et al., 2022).
3. Chemical constituents and properties of selected phytochemicals
Since the coronavirus outbreak, computer docking models have been used to screen beneficial constituents from phytochemicals against coronavirus in virto and in vivo research. Natural polyphenols such as Tulsi, Ashwagandha, Amla, Piperine, Guduchi, and others have demonstrated to be effective against COVID 19 activity in the studies listed above (Españo et al., 2021).
Tulsi contains phenolic compounds like apigenin, circimaritin, rosameric acid, isothymusin, cirsilineol, and a substantial amount of eugenol. The volatile oil content of Tulsi is about 0.7%, it comprises about eugenol (71%) and methyl eugenol (20%); carvacrol and sesquiterpene hydrocarbon, caryophyllene, and ursolic acid. Orientin and vicenin are flavonoids present in Tulsi that display different pharmacological activities. Eugenol (phenylpropanoid) is a derivative of guaiacol with an allyl chain substituted para to the hydroxy group (Orlo et al., 2021). The animal and human experimentation data reveal the antimicrobial (including antibacterial, antiviral, antimalarial), anti-diarrheal, anti-oxidant, anti-inflammatory, hepato-protective, cardio-protective, reno-protective, analgesic, antipyretic, immune-modulatory properties. Ursolic acid is a pentacyclic triterpenoid with three terpene units which is constructed by an isoprene unit (2-methylbutadiene).It shows antiviral activity by protease inhibition due to the presence of hydroxyl and carboxylic acid present within the pentacyclic ring (Mishra et al., 2021; Xiao et al., 2018). These compound found in Tulsi helps to increase hemoglobin concentration, SRBC agglutinin titers, upregulates IL-2, IFN-γ, and TNF-α, downregulates IL-1β and decreases the cyclo-oxygenase (CoX)-2 and lipoxygenase (LOX)-5 enzymes activity, NF-κB classical pathway and produces the SRBC antigen-specific antibodies. These activities help to enhance the immune response and boost the defense mechanism of the body (Gautam et al., 2020). Tulsi is positively recommended as an immunomodulator and antiviral agent to treat the CoV infection (Balkrishna et al., 2021a; Devpura et al., 2021; Mohapatra et al., 2021; Shree et al., 2022). Curcumin a chief phytoconstituent of turmeric constitutes around 77% of the available curcuminoids in spice preparations, 17% of demethoxycurcumin and 3% of bisdemethoxycurcumin (Prajapati et al., 2021; R Cundell and Wilkinson, 2014). Computational chemistry suggested that curcumin has optimal planer molecular arrangement, and its several derivatives are responsible for immunomodulation. The immunomodulatory activities of turmerones (α and aromatic) isolated from curcumin are also scrutinized with the help of human peripheral blood mononuclear cells (Andrin'iranto et al., 2021).
Ashwagandha comprises withanolides having steroidal lactone and ergostane lactone skeleton (Choudhary et al., 2013; Saggam et al., 2021). It has a C29 steroidal nucleus and a C9 side chain with a six-membered lactone ring (Choudhary et al., 2013; Dalvi et al., 2015; Kulkarni and Dhir, 2008). The chief constituents of ashwagandha are withanosides, sitoindosides, alkaloids, saponins, amino acids, and phenolic compounds which shows therapeutic effects in a different type of complexities such as viral infections, communicable diseases cancer, organ failure, diabetes, cardiovascular diseases, etc. to the human body (Elsakka et al., 1990; Mishra et al., 2000). Its activity is due to withaferin-A having aenone ring responsible for its biological activity.
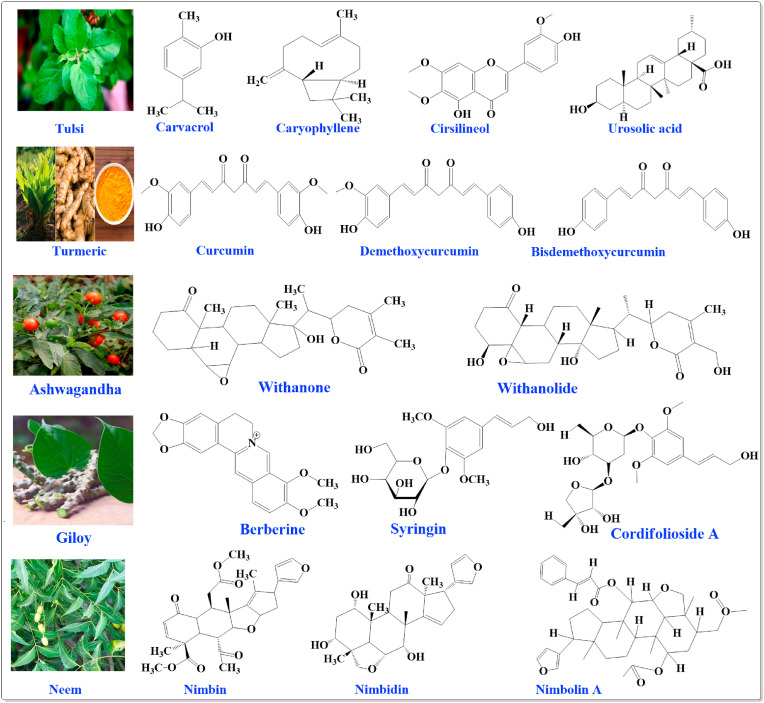
Piperine is a chief phytoconstituent of Pepper or kalimirch (Piper nigrum L.) comprised of an aromatic ring with a methylenedioxy bridge, a conjugated dienone system, and a piperidine ring composed of an amide bond which provides biological activities (Pal Singh and Choudhary, 2015). Black pepper is used as medicine to treat digestive and respiratory tract-related diseases caused by a viral infection such as acute respiratory infection, asthma, chronic indigestion, and fever. Pepper is well known to boost antioxidant property, bioavailability enhancer, enzymatic activity, lipid peroxidation, and immunomodulatory effect (Kumar et al., 2021). Garlic (Allium sativum L.) is one of the well-known immunity boosters that contains 33 sulfur compounds, 17 amino acids, and nutrients such as calcium, copper, germanium, iron, magnesium, potassium, selenium, zinc, and vitamins A, B1, and C (Afifah et al., 2021). Out of these germanium, selenium, and zinc are the major immunostimulants (Ali et al., 2007; Bižanov et al., 2018). It directly affects cell-mediated immunity, including macrophages and T cells (Abdullah et al., 1988). The availability of different garlic chemical constituents are found to be present on their different part (1) fresh bulbs contain S-allyl-L-cysteine sulfoxide (alliin); (2) powder and dried garlic contains alliin and disulfide; (3) ground garlic (macerates) are enriched with sulfide family compounds, dithiines, and ajoene compounds; (4) in steam distillation oil sulfides are main; (5) ethanolic solution of chopped, slathered and aged garlic contain S-allyl-L-cysteine and S-allyl mercaptocysteine. Another herbal medicine is amla (Phyllanthus emblica L.) which contains tannins, alkaloids, and phenols. The amla fruit constitutes alkaloids (phyllantine, phyllembein, phyllantidine); hydrolyzable tannins (emblicanin A and B, punigluconin, pedunculagin, chebulinic acid, chebulagic acid, corilagin, geraniin, and ellagotannin); phenolic compounds (gallic acid, methyl gallate, ellagic acid, trigallayl glucose), amino acids, carbohydrates, organic acids, flavonoids, and vitamins. The antioxidant activity is due to the presence of ascorbic acid-containing hydrolyzable tannins. Emblicanin A, emblicanin B, punigluconin, and pedunculagin are some chief constituents that accounted for antioxidant activity. Licorice (Glycyrrhiza glabra L.) is one of the well-known immunomodulator containing flavonoids, glycosides, cinnamic acid, and coumarin derivatives are among the phenolic chemicals. Liquiritin, isoliquiritin, glucosides, triterpenoid saponins such as glycyrrhizin, a potassium and calcium salt of glycyrrhizinic acid, and others are the primary active components. Compounds that have been extracted from neem (Azadirachta indica A. Juss.) are nimbin, nimbinin, and nimbidin. The compounds that are isolated from the aqueous extract of bark are gallic acid, catechin, epicatichin, margolonone, polysaccharides (G1A, G1B, G2A, and G3A), and peptidoglucan. The essential compounds found in the neem leaves are nimbanene, nimbandiol, nimbin, nimbiol, 6-desacetlynimbinene, ascorbic acid, nimbolide, amino acid, n-hexacosanol, 7-desacetly-7-benzoylgedunin, 7-desacetly-7-benzoylazadiradione, and 17-hydroxyazadiradione (Latif et al., 2020). The phytochemicals of neem have already It directly affects been known for antiviral and antibacterial activity. Pomegranates (Punica granatum L.) are high in polyphenols including ellagitannins, which include mainly α and β isomers of PC, anthocyanins, ellagic acid, gallic acid, glycosylated derivatives (Sharma et al., 2021; Tito et al., 2021). The chemical structures of chief immunomodulatory phytoconstituents are depicted in Fig. 1 .
Fig. 1.
Chemical structures of chief immunomodulatory phytoconstituents.
4. Antiviral and immunomodulatory mechanisms of recommended bioactive compounds
The Nsp15 is a protein that acts as an endoribonuclease and preferentially cleaves 3’ of uridylates through a ribonuclease A (RNase A) like mechanism and also facilitates viral replication and transcription (Zrieq et al., 2021). A study predicted that many of the phytochemicals of the Kadha have a significant binding affinity with Nsp3 and Nsp15. Green tea extract also increases the lymphoblast to induce the production of IL-1α, IL-1β, monocytes, and lymphocytes. The EGCG and EGC may prevent inflammation; impede proliferation, proinflammation, and activation of NF-кB. These conditions reduce the levels of IL-8 and enhance immunity (Di Lorenzo et al., 2013). Thus, the phytochemicals (berberinepigenin, curcumin, piperine, quercetin, tinosporide, urosolic acid, withonolide, withaferin, and withanone, etc.) may disrupt the formation of RTC and stop the viral genome replication (Maurya and Sharma, 2020; Zrieq et al., 2021). It is well known that NF-κB is the master regulator for several genes such as COX-2, VEGF, pro-inflammatory cytokines (IL-1, IL-2, IL-6, and TNFα), chemokines (e.g., IL-8, MIP-1α, and MCP-1), adhesion molecules, immunoreceptors, growth factors, and other agents associate in proliferation and invasion. NF-κB activation mediates through two of the redox signaling pathways. One pathway includes NIK/IKK, whereas the second pathway involves MAPKs, and both cause the induction of transcriptional activation of NF-κB. In the non-canonical NF-κB signaling pathway, NIK is a pivotal mediator. The main activity of NF-κB is that its transcription factor acts directly on oxidative stress. Therefore, oxidative stress activates nuclear factor-inducing kinase (NIK)/IκB kinase (IKK) and MAPKs. NIK/IKK and MAPK pathways activate NF-κB and migrate to the nucleus and bind to κ elements on DNA at enhancers and promoter regions. Many herbs suppress inflammatory diseases by controlling the pro-inflammatory pathways by producing pro-inflammatory mediators (Maurya and Sharma, 2020). There is also evidence that phytochemicals found in the Kadha have a significant binding affinity with NIK, which can stop NF-κB mediated downstream events (Fig. 2 ). Recently, Huang et al. (2020b) showed that the patients infected with SARS-COV-2 had high levels of IL1, IFNγ, IP10, and MCP1, which can mediate cytokine storm-related multi-organ damage. At the same time, SARS-COV-2 infection also initiates the secretion of Th2 cytokines (eg, IL4 and IL10) which suppress inflammation (Huang et al., 2020). The increased secretion of inflammatory mediators was also associated with the moderation of helper T cell responses in COVID-19 patients. In this series, the entire tulsi plant is useful for the immunomodulatory effect. The myricetin and scutellarein found in tulsi potentially inhibited SARS-COV helicase by inhibition of ATPase activity (Islam et al., 2021). Scutellarein is a natural flavonoid obtained from tulsi. The scutellarein docks well into the cavity of the RDRP enzyme (catalyzes RNA replication of SARS-COV) with a docking score of −8.3 kcal/mol. It may perturb Motif B, which is used by the coronavirus for its polymerization (Balkrishna, 2020). Thus, the hang-up of RDRP offers a striking means to control pathogenicity and the spread of COVID-19. Tulsi possibly minimizes the IgG1 level and increases the level of IgG2a to set up a defending immune response. Few phyto-compounds present in tulsi may hit the catalytic cleft of the RDRP (Fig. 2).
Fig. 2.
Viral replication and their inhibition by phytoconstituents.
Many studies have been conducted showing the significant effect of tulsi in enhancing the immune response. The immune-boosting ability of tulsi assessed in cattle with subclinical mastitis shows improved IL-2 gene expression and IL-2 production in male Wistar rats (Goel et al., 2010; Mukherjee et al., 2005). The possible mechanism for improving immunity is a modulation of the GABA pathway (Goothy et al., 2020). Tulsi enhances immune response by increasing T-helper and NK cells; phagocytic activity and index with the rise in lymphocyte count, neutrophil count, and antibody titer (Gautam et al., 2022). It has been observed that when 3 ml/kg of tulsi oil was administered by the intraperitoneal route, the rat showed a high augmentation in the anti-sheep red blood cell antibody, and decreased histamine release at the peritoneal mast cell confirmed the humoral immune response (Godhwani et al., 1988). Further, similar dosing of tulsi has shown a potent antiviral effect by avoiding the entry of the virus into the cell. In other research, Paidi et al. (2021) screened the various component of the tulsi leaf. By the Alpha Screen assay, it was observed that the eugenol significantly inhibited the interaction between spike S1 and ACE2. In silico and thermal shift assays reported that the eugenol is associated with spike S1, but it did not have any impact on ACE2. Consequently, eugenol strongly suppressed the entry of pseudo typed SARS-COV-2 and decreased SARS-COV-2 spike S1-induced activation of NF-κB and in the human A549 lung cells, the expression of IL-6, IL-1β, and TNFα was also found to be reduced, confirming its potential for selective targeting of spike S1 of SARS-COV-2 (Paidi et al., 2021).
The ashwagandha significantly increases the cell-mediated immune response in normal mice. The root extract of ashwagandha raises the level of IF-γ, IL-2, and granulocyte-macrophage colony-stimulating factors in mice, the raised level indicates that it is capable of augmenting immunity and myelo-protective effects. Ashwagandha potentially increases the nitric oxide synthetase activity of the macrophages, which in response enhances the microbial assassination potential of these immune cells (Iuvone et al., 2003; Tiwari et al., 2014, 2018). Withanone is a natural phytochemical of ashwagandha that acts on viral RBD and the host's ACE-2 receptor complex. It was disclosed in docking to the binding edge of the AEC-2-RBD complex which moved slightly towards the interface center on simulation (Varshney et al., 2020). Withanone noticeably reduced the electrostatic constituent of binding free energies of the ACE-2-RBD complex (Fig. 2). Such types of disruptions may potentially block COVID-19 entrance or deteriorate and prevent subsequent infectivity (Varshney et al., 2020). Withaferin-A bound with the DNA polymerase of the HSV and inhibited it to evident the antiviral mechanism of withaferin A (Grover et al., 2011). In the context of COVID-19, withanone showed interaction with the highly conserved protease of CoVs. The molecular simulation study for the N3 inhibitor showed the potential to inhibit the functional activity of SARS-COV-2 protease (Balkrishna, 2020). Moreover, the activity of withanone and withaferin-A was investigated against TMPRSS2. The TMPRSS2 helps to invade the virus and target the host cell. In this, the molecular modeling revealed that both compounds significantly bind and steadily intermingle at the catalytic site of TMPRSS2. At the TMPRSS2 catalytic site, withanone interacted potentially compared to withaferin-A and induce changes at the allosteric site (Kumar et al., 2020). Two constituents from Ashwagandha with glycowithanoloid i.e. sitoindoside IX and sitoindoside X assessed for immunomodulatory activity (Khare and Naharwar, 2020). By this evidence, we can say that ashwagandha is a major natural compound for improving immunity and antiviral efficacy. Past evidence of the use of Withania with other herbal medicines may play a pivotal role to boost immunity and bring enormous changes to the asymptomatic people of the community.
The aqueous extract of black pepper causes an increment in splenocyte proliferation in a dose-dependent synergistic manner. The rise in Th1 cytokine release by splenocytes and suppression of Th2 cytokine release by splenocytes strongly endorses black pepper for the immune-modulatory effect (Majdalawieh and Carr, 2010). Pepper showed antiviral activity against two viruses “vesicular stomatitis virus and human parainfluenza virus” on HeLa cell lines (Priya and Kumari, 2017). Piperine is a major alkaloid of the cinnamamides group that is produced from an ethanolic extract of black pepper. Piperine has a considerable anti-inflammatory effect and can thus be repurposed for the inhibition of COVID-19-induced hyper inflammation. Piperine stimulates phagocytic activity, which enhances innate immunity. It has been shown to inhibit LPS-induced IRF-1 and IRF-7 mRNA expression, STAT-1 activity, type 1IFN mRNA, and phosphorylation of IRF-3 (Mrityunjaya et al., 2020). Black pepper enhances antioxidant activity, and the immunomodulatory effect enhances the bioavailability, raises lipid peroxidation, and inhibits adipogenesis (Kumar et al., 2021). Curcumin inhibits the transcription factors such as NF-κB and AP-1. It plays a central role in the inhibition of T-cell proliferation, cytokine production, and inflammation (Liu and Ying, 2020; Rattis et al., 2021). Curcumin (Curcuma aeruginosa Roxb.) also affects post-transcriptional and post-translational modifications (Zahedipour et al., 2020). These mechanisms cover either a direct interference of viral replication machinery or suppression of cellular signaling pathways necessary for viral replication, such as PI3K/Akt and NF-κB (Mathew and Hsu, 2018). In a study, Li et al. (2020) evaluated the potential of curcumin. Curcumin repressed the proliferation of TGEV and the expression of viral protein expression in a dose-dependent manner. The cell viability gradually decreased with the rise in the concentration of curcumin. The adsorption assay revealed that curcumin reduced the viral titer of 3.55 log TCID50 ml−1 in a concentration of 40 μM revealing its significant inhibitory effect on the TGEV adsorption (Li et al., 2020).
Studies also confirm the amla's property of boosting immunity where it inhibits chromium induced free radical and restored the antioxidant property by resolving DNA fragmentation and apoptosis. It also showed the immunosuppressive effect of chromium on lymphocyte proliferation and even restored the IL-2 and IFNγ production (Ram et al., 2002). Mechanisms of action of some herbal immunomodulators are summarized in Table 1 .
Table 1.
Mechanisms of action of selected herbal immunomodulators.
| Drug | Reported mechanisms | References |
|---|---|---|
| Garlic extract |
|
Ota et al. (2012) |
| Allicin |
|
Bruck et al. (2005) |
| Glycyrrhizin |
|
(Xie, Y.-C. et al., 2009) |
| Cinnamon (Cinnamomum verum J. Presl) |
|
Kim et al. (2018) |
| Long pepper (Piper longum L.) |
|
Bui et al. (2017) |
| n- Gingerol and 6-gengerol |
|
(Ahui et al., 2008; Tripathi et al., 2007) |
| Guduchi |
|
Nair et al. (2008) |
5. Application potential of recommended treatment to fight SARS-COV-2
5.1. Liquorice (Mulethi)
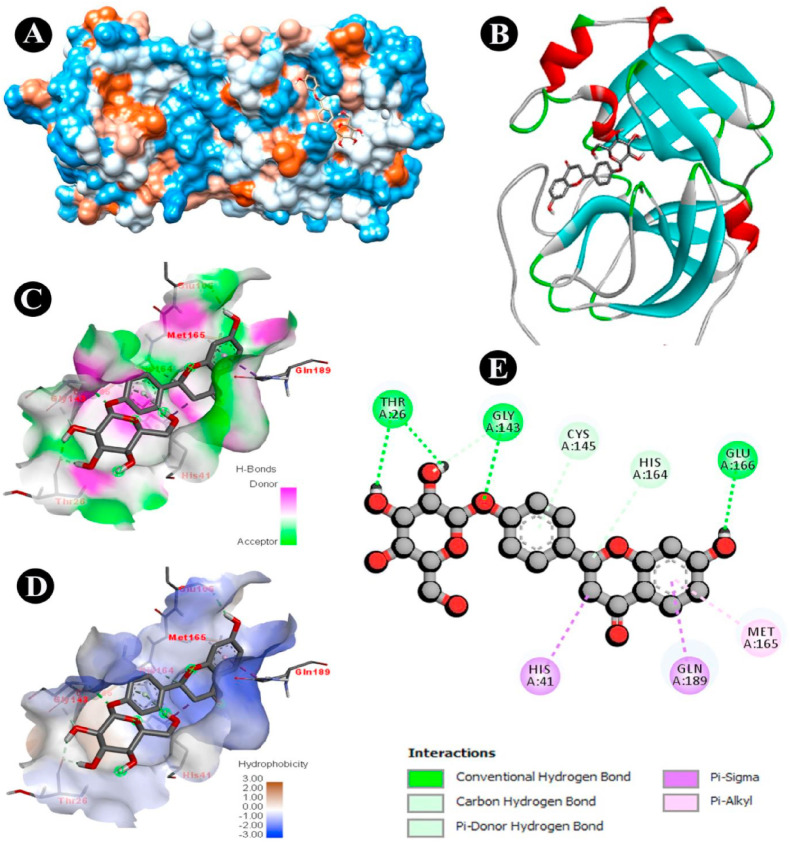
Liquorice (Glycyrrhiza glabra Linn) is also known as “Yashtimadhu” and “Mulethi” in India (Sharma and Agrawal, 2013). Liquorice root powder is an effective expectorant that is used in Ayurvedic medicine for the treatment of upper respiratory tract and throat infections since prehistoric times. The active compound, glycyrrhizinic acid has shown antiviral activity (van de Sand et al., 2021). It has also been used to inhibit the replication of SARS-COV. This active compound can immobilize the virus due to its potent immunomodulatory activity. The polysaccharide fraction of liquorice stimulates the macrophages. It also elevates and assists immune stimulation (Wagner and Jurcic, 2002). N-acetylmuramoyl is a glycyrrhizin analog that has shown the potential for in vitro immune stimulation (Dissanayake et al., 2020). In a study, Cinatl et al. (2003) compared the antiviral efficacy of 6-azouridine, glycyrrhizic acid, mycophenolic acid, pyrazofurin, and ribavirin, on SARS-COV. The experimental results showed that glycyrrhizic acid has a better antiviral activity as compared to the other four drugs in viral adsorption and penetration (Cinatl et al., 2003). It was found that glycyrrhizin possesses the ability to stimulate endogenous defense mechanisms (Arora et al., 2011). Another study suggested that neutrophils' alcoholic extract of Glycyrrhiza glabra enhances phagocytic activity (Al-Snafi, 2018). A different study has indicated that SARS-COV-2, as well as SARS-COV, have the same receptor ACE-2. Glycyrrhizic acid can potentially bind to this receptor and may produce a therapeutic effect on SARS-COV-2 (Zheng et al., 2021). Molecular docking of glycyrrhizic acid revealed its antiviral potency on SARS-COV-2 with potential interaction with ACE-2. The binding energy of glycyrrhizic acid and ACE-2 used in molecular docking was −7.0 kcal/mol (Huang, F. et al., 2020). The intake of Glycyrrhiza glabra root powder (0.1, 0.2, 0.3, and 0.4 mg) with warm water, may produce a lucrative effect on COVID-19 infection. Factor Xa (FXa) inhibitors effectively block the coagulation cascade and have evolved as a crucial part of the prevention and treatment of thromboembolic diseases and in therapeutic protocols involved in many clinical trials in COVID-19. Ibrahim et al. (2021) performed in-silico studies. When examined in-vitro, licorice flavonoids were found to have significant inhibitory action on coagulation factor Xa. The compounds of liquorice, i.e., liquiritin, naringenin 5-O-glucoside, 3,3′,4,4′-tetrahydroxy-2-methoxychalcone, and 7-hydroxy-4′-methoxyisoflavone identified as hopeful anticoagulant agents for the design of FXa inhibitors. These drugs have high oral bioavailability and can be regarded as possible candidates for building orally active direct FXa inhibitors, according to in silico ADMET predictions. Most of the substances examined had superior oral absorption predictions and fewer metabolic interactions compared to that of synthetic drug “Endoxaben”. Glabridin and 7-hydroxy-4′-methoxyisoflavone showed significant oral absorption (100%) (Ibrahim et al., 2021). Vanzara et al. (2021) evaluated liquiritin and compared with dexamethasone, remdesivir, hydroxychloroquine, and azithromycin. The In-Silico evaluation was carried out by Autodock 4.2.6. The liquiritin showed −6.62 kcal/mol binding energy. Liquiritin showed binding formation with six active residues viz. THR26, GLY143, CYS145, HIS 164, GLU166 and GLN189 (Fig. 3 E). out of these four, three, four and two residues were similar to that of the dexamethasone, remdesivir, hydroxychloroquine and azithromycin, respectively. Liquiritin, a naturally active chemical, demonstrated the presence of a typical hydrogen bond with THR26, GLY143 (Fig. 3C). Liquritin also possesses hydrophobic (Fig. 3D) and electrostatic interaction. Liquiritin and the synthetic medication dexamethasone have similar drug abilities, according to an ADMET analysis (Vanzara et al., 2021).
Fig. 3.
Docking analysis of SARS-CoV-2 Mpro binding with Liquiritin (A) hydrophobicity surface 3D representation (B) 3D representation of Liquiritin-SARS-CoV-2 Mpro interaction (C) Interactions of Liquiritin through H-bond in a pocket site of SARS-CoV-2 Mpro (D) Interactions of Liquiritin through hydrophobic bond in a pocket site of SARS-CoV-2 Mpro (E) 2D representation of Liquiritin in the active site of SARS-CoV-2 Mpro (CC BY NC ND 4.0 License) (Vanzara et al., 2021).
5.2. Tulsi
Tulsi is also known as “The Queen of Herbs” and contains Flavones and flavonoids in their extract. Tulsi contains a flavone called scutellarein (Chaudhary et al., 2020). Tulsi is useful for various therapeutic benefits like antiviral, antifungal, hepatoprotective, anti-diarrheal, antioxidants, analgesics, antipyretics, anti-inflammatory, anti-asthmatic, antiallergic, and produce immuno-modulatory effects (Palai and Giri, 2021). Tulsi drop is a well-regarded substance that has been shown to improve immunity (Jamshidi and Cohen, 2017). It assists in different infections such as fever, cold, sore throat, and cough and helps in building new blood cells. The efficacy of tulsi drop in the prophylactic treatment of SARS-COV-2 can be enhanced by combining it with other immunity-boosting extracts such as Guduchi, turmeric, ginger (Zingiber officinale Roscoe), etc. For this purpose, a proper guideline should be followed for testing the efficacy in different concentration of combined formulation. Three to four washed tulsi leaves should be used with tea or any sort of kwath, according to the AYUSH ministry (Palai and Giri, 2021). The dose of each herb is mentioned in API (The Ayurvedic Pharmacopoeia of India) as 2–3 g powder of Tulsi leaves, 1–3 g powder of cinnamon bark, 250 mg–1 g powder form of black pepper, and 1–2 g powder of ginger.
5.3. Ashwagandha
The immunomodulatory effect of the root extract of Ashwagandha was evaluated in the three myelosuppression models in mice such as azathioprine, cyclophosphamide, or prednisolone. The study revealed that the Ashwagandha increased the red blood cells, white blood cells, platelet count, and body weight in mice as compared to untreated control mice. The estimated dose found in rats 200 mg/kg/day. The histopathology of the liver, stomach, kidney, spleen, and lungs was observed normal after the treatment at this dose (Yan et al., 2018). Another study also investigates the production of IL-1 and TNF-α after the administration of Ashwagandha to mice treated for carcinogenic ochratoxin A (Mishra et al., 2000). Ashwagandha showed immunomodulatory effects at doses of 150 and 300 mg/kg in IgE-mediated anaphylaxis as a reduction of ovalbumin-induced paw edema in comparison to the drug disodium cromoglycate (Agarwal et al., 1999). Ashwagandha produces toxicological effects in our body in overdose condition. A recent study has reported a strategy that blocks or weakens the COVID-19 viral infections. Ashwagandha most potential phytochemical for controlling COVID-19 infection by disrupting the spike protein's electrostatic interaction and lowering the binding free energy between the viral RBD and its AEC-2 receptor. This was proved by a disease where phytocompound withanone was screened by molecular docking and showed a distinct effect on RBD and ACE-2 receptor complex. It was observed from the study that withanone significantly reduces the electrostatic component of binding free energies between viral RBD and its AEC-2 receptor. These disruptions of the electrostatic interaction results in interference of viral protein entry and other manifestations via the ACE-2 receptor (Varshney et al., 2020). Two salt bridges were also occupied at the interface. When withanone incorporated, it subverted salt bridges and reduced their possessions. This study focused on the most applicable option for controlling CoV entry into host cells. Thus, we can say, these studies provide an alternative and attractive means for the successful treatment of COVID-19 infection (Varshney et al., 2020). Ashwagandha is available in various forms like powder, capsules, and extract (liquid). The standardized extract dose of ashwagandha is 100–200 mg twice per day. We can also take the dried powder of ashwagandha (1–2 g) daily one to three-time for therapeutic effect in COVID-19 (Shah and Krishnamurthy, 2013).
5.4. Garlic
Garlic is also called Lahsan (Hindi) belonging to the family Alliaceae (Yasmin et al., 2020). They show beneficial effects on various diseases like viral, fungal, parasitic, and anti-inflammatory properties. A key advantage of garlic for COVID-19 patients is that it protects against various inflammatory diseases like allergic-airway inflammation, inflammatory bowel disease, and many more (Moutia et al., 2018). Garlic preparation may be liquid (aqueous, oil, or solvent) or solid (dried power and fresh cataplasm). Garlic is efficient in maintaining the homeostasis of the immune system and prevents immune dysfunction. The garlic boosts the function of the immune system by provoking the cells, such as lymphocytes, macrophages, NKc, DC, and eosinophils. It works by modulation of immunoglobulin production, cytokinin secretion, macrophage activation, and phagocytosis (Arreola et al., 2015). Several studies reported the immunomodulatory and antiviral effects of garlic and its components (Prajapati et al., 2021; Vathsala and Murthy, 2020). Some researchers used the molecular docking method and investigated that alliin may be a functional inhibitor of SARS-COV-2 as compared to remdesivir, chloroquine, and ritonavir (Cheng and Li, 2020). If garlic and its active constituents are used in proportionate quantities, then it may produce beneficial effects against COVID-19. Suitable dosing and dosage form can be achieved by employing drug delivery advances in nanotechnology.
5.5. Neem
Neem is a tree belonging to the family Meliaceae. Neem possesses immunomodulatory as well as anti-inflammatory, antioxidant, antiviral, antibacterial, and anti-tumor activity. Each part of the neem plant is useful in developing strong immunity (Alzohairy, 2016). One such study suggested that neem oil potentially induced the production of IFγ by Y-lymphocytes. It activates the macrophages to enhance phagocytic ability. Neem oil is efficient in antigen uptake and cellular activation (Upadhyay et al., 1992). The research study investigated the immunomodulatory and growth-promoting effects of neem leaves. 50 mL/L of fresh neem leaves infusion on broiler chicks showed that neem infusion successfully improved antibody titer, growth performance, and gross return water (Durrani et al., 2008). In another study, broilers, and dry leaves powder of neem showed humoral as well as cell-mediated immune responses. It was observed that neem (2 g/kg) significantly enhances the antibody titers against the new castle disease virus (NCDV) antigen (Alzohairy, 2016; Sadekar et al., 1998). Application of neem oil on the body or intake of 1 gm neem powder daily with lukewarm water enhances the immunity power and produces a beneficial effect on fighting SARS-COV.
5.6. Guduchi
Guduchi is a shrub belonging to the family Menispermaceae, and shows immunomodulatory effects against several microbial infections. Commonly it is known as Amrita, Guduchi, Gurach, Tinospora. This plant mainly constitutes choline, berberine, tinosporic acid, tinosporin, tinocordifolioside, tinosporon, tinosporaside, and tembeterine (Sarangi and Soni, 2013). These active phytochemicals helps Guduchi to fight against various diseases such as jaundice, gout, urinary disorder, asthma, inflammation, urinary infections, and pyrexia. It is an antiallergic that provides substantial relaxation from nasal pruritus, nasal discharge, and sneezing (Badar et al., 2005). A study revealed that G1-4A is a type of polysaccharides having a molecular weight of 2.2 × 106 Da obtained from Guduchi, which modulates the levels of pro-inflammatory cytokines such as TNF-α, IL-β, IL-6, IL-12, IFNγ. The result indicates that G1-4A is a potent therapeutic agent that inhibits Mycobacterium tuberculosis through the immunomodulatory effect both in-vivo and in-vitro (Gupta et al., 2016). Guduchi is also used against several viral diseases such as herpes HIV, etc. it is suggested to be a potent agent in monitoring COVID-19 due to its immunomodulatory and antiviral nature.
6. Pharmaceutical product developments for immunity-boosting against COVID-19
Researchers from all over the world are seeking to find a cure for COVID-19. Health experts have recommended promoting the body's immune system with ayurvedic herbs which may help to minimize viral load and speed up the recovery from the disease. For this, there are various marketed preparations available in different forms to promote immunity.
6.1. Nanoparticles
Nanoparticles (NPs) have emerged as a novel vehicle for the targeted delivery of drugs, vaccines, bioimaging, and enhanced bioavailability. Because of its unique features of water solubility, biocompatibility, cost-effective manufacturing, and low toxicity, NPs are the preferred carrier (Dheyab et al., 2021; Mohammadzadeh et al., 2022; Prajapati et al., 2019). Due to their structural similarities, synthetic NPs can precisely imitate the virus and interface extensively with its proteins. As a result, NP-based techniques for combating this virus possess a good prospect. Earlier, NPs had been proven to be successful against a variety of viruses, particularly those belonging to the Coronaviridae family (Medhi et al., 2020; Mosselhy et al., 2021). Recently, NPs are emerging as a carrier for drug delivery in the treatment of COVID-19. Zhao et al. (2021) synthesized glycyrrhizic acid NPs (GANPs) to inhibit the proliferation of the murine coronavirus MHV-A59 and reduce proinflammatory cytokine production caused by MHV-A59 or the N protein of SARS-COV-2. GANPs revealed their targeting efficiency in the severe inflammation area such as the lung and had antiviral and anti-inflammatory impacts, reducing organ failure and providing a substantial survival benefit to diseased mice (Zhao et al., 2021).
6.2. Tablets
Recently, the Coronil tablet has received certification from AYUSH Ministry as per the WHO certification scheme. The company (Patanjali Ayurved Limited) claimed that it is the first evidence-based medicine to fight Covid-19. Coronil tablet is the combination of several herbal ingredients including Ashwagandha, Konch Shuddha (Mucuna pruriens (L.) DC.), Satavar (Asparagus racemosus Willd.), Safed Musli (Chlorophytum arundinaceum Baker), Javitri (Myristica dactyloides Gaertn.), Jaiphal (Myristica fragrans Houtt.), Shuddhkuchla (Strychnos nux-vomica L.), Akarkara (Anacyclus pyrethrum (L.) Lag.), Jundbedaster (Castorium), Swarn Bhasma (Incinerated oxide of gold), Praval Pishti (Coral), Vang Bhasma (Thermprocessedstanum), Shilajit Shuddh Asphaltum, Pan (Piper betle L.) Ras. Two tablets per day are recommended for the age group between 15 and 80 years and 1 tablet between 6 and 14 years old with warm water, half an hour after a meal (Tiwari and Talreja, 2020). Guduchi ghan vati is an ayurvedic preparation of Guduchi extract, known for its prophylaxis and treatment of COVID-19. In a clinical trial (NCT04480398), Guduchi Ghan Vati was administered orally to SARS-COV-2 patients [2 tablets (500 mg each) B.I.D]. after meal for 28 days. The clinical efficacy was evaluated on 91 patients which revealed positive results in controlling the COVID infection (Radhakrishnan, 2020). In a randomized clinical trial, 1 g of Guduchi Ghan Vati (1 g) and tulsi Ghan Vati (0.5 g) Ashwagandha (0.5 g), and swasari sas (2 g) were administered orally to the patients in the treatment group twice per day (B.I.D.) for 7 days. Medicines were given in the form of tablets (each of 500 mg), while the swasari ras in powder form. Patients in the treatment group also received Anu taila. Then the viral load was detected by the RT-qPCR test. It was observed that 71.3% patients of the treatment group and 50.0% of patients in the placebo group were recovered on day 3 while 100% of patients in the treatment group were recovered on day 7. Only 60% of patients in the placebo group were recovered. The recovery proportion was more in the treatment group. In the treatment group on day 7, average serum levels of hs-CRP, IL-6, and TNF-α were respectively, 12.4, 2.5, and 20 times lesser compared to the placebo group. Ayurvedic therapy can accelerate virological clearance, and recovery and reduced the risk of viral proliferation (Devpura et al., 2021). Based on clinical improvement, the profit of an Ayurvedic regime in SARS-COV-2 patients was studied by Wanjarkhedkar et al. (2020). The Ayurvedic formulation Dasamool kaduthrayam Kashaya and Guluchyadi Kwatham tablet were administered as an add-on to Standard of Care (SoC) in patients with mild to moderate symptoms, in this perspective, open-label, comparative study. While the control group received SoC only. Patients getting ayurvedic tablets i.e., Dasamoolkaduthrayam Kashaya and Guluchyadi Kwatham together with SoC exhibited a quick recovery from dyspnoea with reduced ageusia. Patients of this group could be discharged earlier than the control group patient. The above-mentioned ayurvedic tablet with SoC hastened the recovery of COVID-19 hospitalized patients (Wanjarkhedkar et al., 2020). The possibility of ayurvedic intervention in COVID-19 patients was assessed by Kulkarni et al. (2021). Ashwagandha, Guduchi, and Tulsi were administered orally in tablet form in the dose range of 250 mg to 5 g, 500 mg to 1g, and 500 mg-1grespectively. The average recovery time was 4.5 (SD 1.8) days in the group treated with ayurvedic tablets while the control group took 13.5 (SD 6.4) days for recovery. Full recovery on day 3 was seen in 10 patients, on the 5th day 22 patients, and 26 patients on the 7th day. The results of the Ayurveda intervention significantly reduced the symptoms to prevent COVID-19 complications (Kulkarni et al., 2021).
Some more tablet formulations are available in the market which can help to fight COVID-19 like symptoms such as respiratory tract infection, asthma, severe cold and cough, viral infections, and communicable diseases. Kerala Ayurveda's “Resigest Tablets” are intended for the smooth function of the respiratory tract and help in suppressing viral infection. It is an improved form of the classical ‘Padthyashadangam Kwath’ (Abraham et al., 2020). There is another tablet “Imugest” from the same ayurvedic company having ingredients ashwagandha, Guduchi, Brahmi (Bacopa monnieri (L.) Wettst.) Mandukaparni (Centella asiatica (L.) Urb.), Draksha (Vitis vinifera L.) Haridra, Jhandu (Tagetes erecta L.). Imugest tablet is indicated for immune-deficient conditions with recognized immunomodulatory activities. For adults and geriatric, 1–2 tablets B.I.D. are recommended with lukewarm water and/or as directed by the physician. Besides the immunomodulatory effect, it is also a good antioxidant that maintains tridosha balance and prevents recurrent infections. The AYUSH ministry has recommended that a few of the naturally derived immunity-boosting measures be supplied in 3-g powder form and administered with hot water (150 ml). However, it is not clear whether the recommended dose is similar for all age groups (pediatrics, adults, and geriatrics); if so, this dose can be toxic to pediatrics and do not document any guidelines for the patients who are affected by any type of acute and chronic diseases symptoms.
6.3. Capsules
Recently, capsules of Neem were studied by Nesari et al. (2021) for the prophylactic treatment of SARS-COV-2 infection. Participants received 50 mg of neem-leaf extract or placebo orally in capsules, B.I.D. for 28 days. Participants who administered neem capsules had a lower chance of COVID-19 infection, indicating that it might be used as a preventive therapy for SARS-COV-2 infection (Nesari et al., 2021). FLUCOMUNE™ (90) is the brand name of the capsule which contains Emblica Officinalis. It is a natural source of bioflavonoids and ascorbinogen which is having strong antioxidant activity with immune modulator properties, as well as being an adaptogen. Vasaka (Justicia adhatoda L.), tulsi, guduchi, and liquorice are involved in this capsule for respiratory health support, immune-supportive properties, antispasmodic support, and healthy bronchial function, respectively. Trikatu acts are used as a digestive agent which increases the bioavailability of nutrients in the digestive tract. The dose is one capsule daily for all age groups (no specification regarding dose for the different age groups) with not many herbs recommended by the AYUSH ministry for COVID-19 like ginger, turmeric, ashwagandha, etc. The second product is “Inlife Ashwagandha Capsules 60's” which contains Withanolides >7% as the active constituent. It has 500 mg of ashwagandha extract in each capsule. Further, the dose prescribed on the pack is two capsules in a day. “I-IMMUNE” is a capsule brand that has the blend natural constituents of triphala (78 mg), haldi (32 mg), trikatu (45 mg), mulethee (55 mg), papaya leaves (62 mg) (Carica papaya L.), shigru (36 mg) (Moringa oleifera Lam.), manjishtha (65 mg) (Rubia cordifolia L.), ashwagandha (50 mg), wheatgrass (17 mg) (Triticum aestivum L.), and guduchisatva (60 mg). The prescribed dose of this product is 1–2 capsules three times a day for 2–3 months continuously for boosting immunity, and the dose is not mentioned according to the age group.
Another capsule developed with the name “Astha 15” capsule, a polyherbal combination can be used as prophylactic against CoV by boosting immunity, it shows bronchodilator properties and acts as a lung detoxifier (Rao et al., 2021). It can suppress infections and regulate allergic reactions. AYUSH 15 mainly acts on the nasal mucosa of the respiratory tract surrounding the walls of the lungs which acts in airways. It has an anti-inflammatory effect that reduces inflammation and congestion inside the lungs. The capsule of tulsi (200 mg; B.I.D.) was given to 41 patients and the data was compared with the standard drug salbutamol. Tulsi showed significant bronchodilator activity by diminishing the levels of IgG1 and IgG2a pointing toward a humoral immune response (Vinaya, 2017). Natural immunomodulatory Curcumet capsules have been developed by Lactonova Nutripharm (P) Ltd, Hyderabad. The active constituents present are flavonoid curcumin (diferuloylmethane) and volatile oils, including atlantone, tumerone, and zingiberone for COVID-19 treatment (Shukla, G. et al., 2021; Shukla, Rao et al., 2021).
6.4. Syrups
To overcome CoV infection researchers at the Central Institute of Medicinal and Aromatic Plants (CIMAP), Lucknow, produced two herbal products (CIM-Paushak and herbal Cough Syrup) which modulate immunity by decreasing dry cough. The formulation comprises twelve powerful Ayurvedic ingredients such as Puranva (Boerhavia diffusa L.), Ashwagandha, Mulethi, Harad (Terminalia chebula Retz.), Baheda (Terminalia bellirica (Gaertn.) Roxb., and Sataver (Asparagus racemosus Willd.) known for their nutritive values with Emblica Officinalis and sugar as the base. These are developed based on the latest guidelines of the AYUSH ministry, and it has been prepared based on the ‘Tridosha’ principle of Ayurveda.
6.5. Nasal oil
Conventional Ayurvedic nasal oil hydrates, soothes the nasal cavity, relieves stress, heals supraclavicular problems, and improves health. Based on the clinical symptoms seen in COVID-19 patients, the treatments must also aim to reduce inflammation of the upper respiratory tract (especially the nasal mucosa). 2-2 drops of Pratimarsha Nasya of Tila taila Anu taila, and Sarshap taila can be administered daily in each nostril. Pratimarsha Nasya (Anu taila/sesame oil) is crucial in reducing and treating Nasobronchial illnesses, as well as strengthening respiratory immunity (Shah et al., 2020). Anti-inflammatory phytocompounds exist in the herbal constituents of Anu taila (the nasal drop), which keep pro-inflammatory cytokines in control (Devpura et al., 2021). Nyasa is an Ayurvedic practice in which oil is instilled through the nostrils to boost immunity. It is a therapy for the nose, sinuses, throat, and head. The oil is in the form of Desi ghee and Anu taila. One can also use natural Ayurvedic oils with herbs like Brahmi, and Basil (in small quantities). SINUREX NASAL DROP” includes the property to relieve sinusitis and boosts immunity due to the presence of Shrigru (Moringa oleifera Lam.), Guda (Jaggery i.e., Saccharum officinarum L.), Shunthi (Zingiber officinale Roscoe), Tailaparna taila (Oil of Eucalyptus globulus Labill.), Tila Taila (Oil of Sesamum indicum L.), Trivrit (Operculina turpethum (L.) Silva Manso). The suggested dose is 4–8 drops in each nostril B.I.D. Nasal oil application probably forms a biofilm that helps by creating a blockade to prevent the entry of viruses or other pathogens (Tillu et al., 2020).
6.6. Swasari ras
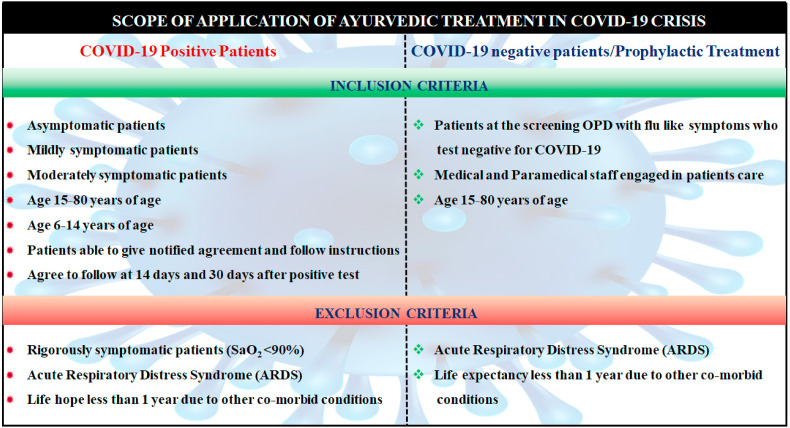
Swsari ras is used for the treatment of severe cough, cold, Bronchopneumonia, and other respiratory infections. It helps to remove congestion in the chest and lungs and activates the oxygen supply to the lungs. Predominantly in allergic conditions of asthma, cough, cold, and sinusitis, it escalates the immune response by improving the respiratory system (Balkrishna et al., 2020b). Swasari ras is also active against bacteria and viruses. It is recommended as a prophylactic treatment for persons who are directly in contact with COVID-19 patients during their treatment or are in the contentment zone (Balkrishna et al., 2021b). In a study performed by Balkrishna et al. (2020b), Divya-Swasari-Vati was tested on A549 cell xenotransplant in the swim bladder of zebrafish which was injected with recombinant spike protein of SARS-COV-2. In a dose-dependent manner, Swasari-Vati repressed the pro-inflammatory cytokines, IL-6 and TNF-α, and reduces the inflammatory damage triggered by the viral spike protein while increasing the survival of the experimental fish (Balkrishna et al., 2020a). The proposed prophylactic and symptomatic treatments recommended by the AYUSH health ministry are depicted in Table 3. Ayurvedic treatment in COVID-19 positive and negative people with the inclusion and exclusion criteria is shown in Fig. 4 .
Table 3.
Symptomatic and prophylactic recommendation by AYUSH for SARS-COV-2.
| Traditional medicine practice | Medicinal Plant | Purpose | Form of extract | Preparation | Recommended usage | Effective against | Reference |
|---|---|---|---|---|---|---|---|
| Ayurveda | Guduchi | Prophylactic | Aqueous | Samshamani Vati 500 gm with warm water | 15 days B.I.D. |
|
(Kalikar et al., 2008; More and Pai, 2011; Sharma et al., 2014) |
| Ashwagandha | Prophylactic | Tablet of extract | With Lukewarm | 500 mg B.I.D. |
|
(Mandlik and Namdeo, 2020; Tillu et al., 2020) | |
| Swasari ras (Polyherbal tablet) | Prophylactic | Tablet | With lukewarm water | 500mg/tablet B.I.D. (two tablets in the morning and two in the evening |
|
Balkrishna et al. (2020a) | |
| AYUSH-64 | Symptomatic | Tablet | 2 tablets B.I.D. |
|
|||
| Agastya Haritaki | Symptomatic | Powder | 5 gm in warm water | B.I.D. |
|
Hegde (2020) | |
| Jujube red date (Ziziphus abyssinica Hochst. ex A.Rich.) | Prophylactic | Tablet | with lukewarm water | 500 mg tablet B.I.D. |
|
(Gao et al., 2013; Zou et al., 2018) | |
| Anu Thaila (Sesame oil) | Nasal drop | 2 drops in each nostril |
|
Dalvi et al. (2015) | |||
| Unani | Quince (Cydonia oblonga Mill.) | Prophylactic | Aqueous | Behidana – 3 gm Unnab – 5 Nos Sapistan – 9 Nos Boil these 3 in 250 ml water, boil it until it remains half, and filter. |
14 days B.I.D. |
|
(Ad-Dahhan, 2010; Afzal et al., 2007; AlBayaty and AlTahan, 2008; Hamauzu et al., 2005) |
| Jujube red date | |||||||
| Lasura (Cordia myxa L.) | |||||||
| Khashkhash (Poppy seeds) (Papaver somniferum L.) | Prophylactic | Aqueous | Add a tablespoon of honey and a tablespoon of KhasKhas to eight tablespoons of coconut milk and drink it before retiring to bed | 12 gm |
|
Raut and Ghotankar (2020) | |
| Homeopathic | Arsenicum album | Prophylactic | Tablet | 3 days O.D. in empty stomach (repeat for one month till the infection persist) |
|
Khurana (2020) | |
| Siddha | Adathodai Manapagu (Justicia adhatoda L.) | Prophylactic | Syrup | 10 ml syrup | B.I.D. |
|
Kudineer (2013) |
| Vishasura Kudineer | Prophylactic | Tablet | Decoction 60 ml | B.I.D. |
|
Shailaja et al. (2017) | |
| KabaSura Kudineer | Prophylactic | Tablet | Decoction 60 ml | B.I.D. |
|
Saravanan et al. (2018) |
Fig. 4.
Scope of application of Ayurvedic treatments in COVID-19 crisis.
6.7. Chyawanprash
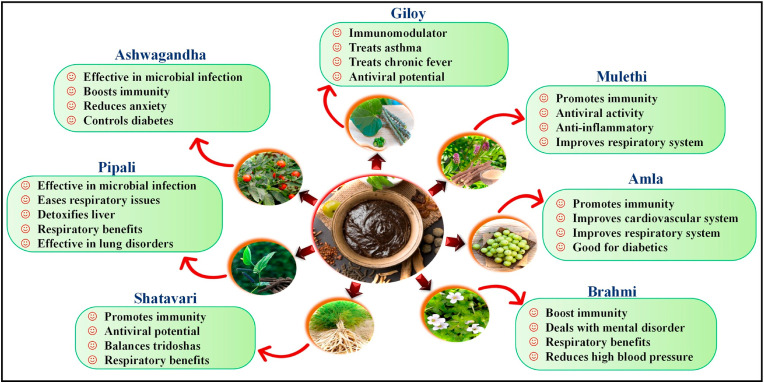
Chyawanprash is also known as Chyavanprasha, chyawanaprasam, etc. It is made from two lexes, “Chyawan” and Prasha”. The word Chyawan means sage, and symbolizes ‘degenerative change’ and Prasha indicates ftalla drug or foodstuff that presents consumption. Chyawanprash is a combination of more than 40 herbs and is made by different Ayurveda companies such as Dabur, Patanjali, etc. (Fig. 5 ). The primary ingredient of Chyawanprash is amla fruit pulp, which is useful in respiratory tract infections and tuberculosis. Amla possesses immunostimulatory and inflammatory properties (Muthuraman et al., 2011), and other ingredients such as Ashwagandha and Guduchi have also been known due to their immunostimulatory properties (Aranha et al., 2012; Yamada et al., 2011). It is used as a metabolic tonic used to foster immunity and prevent various ailments (Khan et al., 2016; Sharma et al., 2019). It possesses properties that are essential to boost our health such as, it clears respiratory passages, enhances energy, good for cholesterol balance, protects the body against infection, normalizes blood pressure, improving complexion, purifies the blood, and eliminates toxins. A regular intake of Chyawanprash strengthens the trachea-bronchial tree, boosts the secretions of TNF-α and MIP-1 α, stimulation in IL-1β levels, and increases phagocytic activity, and henceforth develops immunity. Clinical studies also confirmed that Chyawanprash modulates IgE and immunity markers C3 and C4 levels, and improved pulmonary functions (Gupta et al., 2021).
Fig. 5.
The immunity-boosting potential of chief ingredients of Chyawanprash.
The common dosage of Chyawanprash is 12–28 g can be taken with milk (about 100–250 ml) in the morning on an empty stomach to improve immunity. Those people suffering from respiratory diseases are advised to take lukewarm water. A study reported the immunomodulatory effect of Chyawanprash, the in vitro assay showed the secretion of cytokinin such as TNF-α -1β, IL-1β, and MIP-1- α from murine bone marrow-derived DC. The effects of Chyawanprash on phagocytosis in murine macrophages (RAW264.7) and NKc activity were also reported. Chyawanprash at a non-cytotoxic concentration (20–500 μg/ml), increased the cytokinin secretion from DC. Another research study states that chloroform extract of Chyawanprash (Dabur India Ltd., Uttar Pradesh, India) and hydrolyzed Chyawanprash show antimicrobial activity as compared to the standard drug ampicillin (20 mg/ml) (Khan et al., 2016). Several research works have been carried out, but there is no evident information available on its toxicity. A randomized controlled study (open-label) was conducted by Gupta et al. (2021) on 193 health care professionals (ages 25–60) who had a high risk of contracting COVID-19. Group I received standard palliative care, whereas group II received standard preventive protocol with Chyawanprash (12 gm, B.I.D.) for 30 days. The major assessment tool was indeed the number of confirmed COVID-19 cases in both groups. At the end of one month, no one in either group was COVID-19 positive out of 193 people who finished the experiment. 4 and 2 COVID-19 positive individuals were found in Groups I and 2 of Group II, respectively post-intervention follow-ups. During the trial, no major adverse events or adverse medication reactions have been recorded. Before and after the trial, there was no clinically drastic change observed in the safety measures. In Group II, there was a dramatic increase in serum IgG, but no other inflammatory or immunological indicators showed a statistically significant change. All of the individuals in the intervention group accepted it well, but a longer-term clinical trial with bigger sample size is needed to show its apoptogenic impact and effectiveness as an add-on to standard treatments in avoiding the recurrence of COVID-19 (Gupta et al., 2021). In a randomized community-based study, Godatwar et al. (2021) evaluated the prophylactic activity of Chyawanprash in which 771 people were screened for the research. Participants in the DCP group aged 13–70 years old, and children aged 5–12 years, were instructed to take 12 gm and 6 gm of Chyawanprash, respectively. The control group was instructed to drink one cup of milk twice a day. In the DCP group, one subject, and in the Control group 8 subjects were found to be COVID positive. The DCP group's quality of life (QoL) score was 66.79 14.75 at the start of the trial and increased to 70.83 13.57on completion, whereas the control group's QoL score was 66.52 13.60 at the start of the trial and drastically dropped to 61.48 12.33 by the end of the study. QoL score using Chyawanprash was 2.25 times higher than the control group suggesting the prophylaxis potential of Chyawanprash for COVID-19 prevention. The favorable results might be owing to the synergetic effect of the powerful herbs, which are known to enhance immunity among healthy people (Godatwar et al., 2021).
6.8. Extract of herbs
6.8.1. Green tea/decoction
Green tea (Camellia sinensis (L.) Kuntze) contains plenty of catechins and polyphenols. Polyphenols such as epicatechin-3-gallate, epicatechin, epigallocatechin-3-gallate (EGCG), and epigallocatechin (EGC) enhance the cell-mediated and humoral immunity. Oral Intake of EGCG and EGC influences the proliferation of lymphocyte T and cytokine production (Tallei et al., 2021). EGCG can re-establish the natural immunological homeostasis in various autoimmune diseases (Menegazzi et al., 2020). The innate immune response is an important element in disease progression. Evidence suggests that tea polyphenols and micronutrients strengthen defensive mechanisms, such as infection resistance by altering immunological modulation; this might have a significant impact on COVID-19 cytokine storm management (Cena and Chieppa, 2020). Catechins show numerous pharmacological properties such as antimicrobial, antiviral, and antioxidants (Kumar and Sharma, 2010). The uptake of viruses on red blood cells was mostly hindered by these compounds of decoction (Li et al., 2005). Results indicate that green tea extract showed high immunomodulatory effects against Candida albicans infection (Rahayu et al., 2018). The efficacy of EGCG (Green tea polyphenol) was evaluated by Jang et al. (2021). The findings indicated that EGCG protects host cells. Treatment with EGCG increased the viability of infected cells while decreasing plaque formation. Furthermore, EGCG administration reduced the expression of viral protein. All these results confirm that green tea may be used as a preventive measure against COVID-19 (Jang et al., 2021). In another study, Zhang et al. (2021) reported that EGCG suppresses the ACE2 and TMPRSS2 via activating Nrf2. Through inhibition of SARS-COV-2 main protease, EGCG may inhibit viral reproduction. EGCG owing to its wide antioxidant action may protect against SARS-COV-2-induced mitochondrial ROS and neutrophil extracellular trap-induced ROS burst. EGCG can impede the SARS-COV-2 life cycle by reducing ER-resident GRP78 activity and expression. SARS-COV-2-induced cytokine storm, sepsis, thrombosis, and lung fibrosis may all be reduced by EGCG (Zhang et al., 2021). In a proof of principle study, Bettuzzi et al. (2021) reported the efficacy of green tea catechin. During their stay in the hospital, ten swab-positive individuals with SARS-COV-2 COVID-19 symptoms were treated effectively at home for 15 days with two sessions of inhalation and three capsules each day having total 840 mg total catechins: 595 mg total EGCG. At a median of 9 days, with a range of 7–15 days, all patients had healed and had no complications. At a median of 9 days, seven flipped to a negative SARS-COV-2 nasopharyngeal swab test, with a range of 6–13 days. One of the three patients who remained swab-positive showed a significant reduction in infection, dropping to a “very low” SARS-COV-2 nucleic acid load after five days. Because they were clear of symptoms at the end of treatment, all the patients were released from quarantine. The inflammatory markers −1 antitrypsin, C-reactive protein, and eosinophils had all reduced considerably. In seven out of ten patients, IL-6 and erythrocyte sedimentation rates were reduced. These findings confirm the potential role of green tea against COVID 19 (Bettuzzi et al., 2021). To make green tea, combine one or half a gram of dried green tea leaf with 200 ml boiling water and steep for 15 min. Herbal tea, according to Table 2 , can assist to improve immunity.
Table 2.
Different types of herbal tea with immune-boosting potential.
| Type | Effect | Procedure |
|---|---|---|
| Haldi tea | It boosts immunity due to its antiviral, antibacterial, and antioxidant properties. | In 50 ml of boiling water, add some Haldi powder, ginger slices, and pepper. Then boil for 15 min. Filter the water and add some lemon juice and honey. |
| Ginger tea | Ginger tea can help in the treatment of respiratory issues and nasal congestion. | In boiling water add some slices of ginger. Strain the tea and discard the pieces of ginger. Then add some honey, cinnamon, haldi, or lemon juice. |
| Tulsi tea | It can prevent respiratory illnesses and reduce phlegm when you have a cough. | Add 2-inch ginger, 12 holy basil leaves, and 100 mg cardamom powder in 2 cups boiling water. After boiling for 10 min, strain the water. Add some honey and ½ lemon. You can also get goodness using tulsi leaves. |
| Lemon tea | Lemon is a rich source of vitamin C that boosts immunity. It relieves chest infections and phlegm when suffering from a cold and cough. | In boiling water, add turmeric and add lime juice, and a dash of honey. Take this tea in the morning. |
| Peppermint tea | Peppermint is free from caffeine and boasts antibacterial and antiviral properties. So, it helps to fight clogged sinuses due to allergies or due to frequent coughs and colds. | Take 2 cups of boiling water and add some mint leaves and stay for boiling 10 min till the water turns pale yellow. Filter the tea and add some honey and lime. It may be taken in the morning as well as at night. |
| Hibiscus tea | It improves the immune system and is easy to make at home. | Firstly, Separate the flower from the calyx. Then wash the petals and steep the petals in hot water and some honey and lime juice. |
6.8.2. Ayurvedic Kadha
India's health ministry, AYUSH, has announced several immunity-boosting measures, Kadha is one of them. Kadha has been used for a long time and has the potential to assist the body in recovery from cold and flu infection with enhanced immunity. Distinct mixtures of herbs are being used to make different variations of regular Kadha (Maurya and Sharma, 2020) and some of these are recommended by the Ministry of AYUSH as ingredients in ‘AYUSH Kwath’ ‘AYUSH Joshanda’ or ‘AYUSH Kudineer’ (Charan et al., 2021). The AYUSH Kwath concludes with dozens of health benefits such as antiviral, antioxidant, anti-platelet, reno-protective properties, anti-atherosclerotic anti-inflammatory, hepato-protective, and enhances immunity to regulate viral infections such as COVID-19. The ingredients are powdered and tea bags or sachets of 3 gm of powder in each or 500 mg tablet of the aqueous extract is recommended with 150 ml boiled water O.D. or B.I.D. It is generally taken with Draksh (Resins)/Gud (Jaggery) and/or with lemon juice. The AYUSH Kwath has demonstrated its importance in boosting immunity and preventing the spread of SARS-CoV-2 during the non-severe stage of COVID-19 (Gautam et al., 2022). The dose of each herb is mentioned in the Ayurvedic Pharmacopoeia of India) in the dose range of 2–3 g powder of Tulsi leaves, 1–3 g powder of cinnamon bark (Cinnamomum cassia (L.) J.Presl), and 1–2 g powder of ginger, 250 mg-1 g powder of form of black pepper (Ayurvedic Pharmacopoeia of India, 2016). Shukla et al. (2021) conducted an In Silico analysis to assess the targets of ayurvedic decoction. The findings revealed that two of the 26 active compounds in Ayurvedic decoctions are significant binders for spike protein and the related Mpro, which plays a vital role in viral replication and transcription, making it a potential antiviral therapeutic target. One molecule was discovered to be the most active among the 26 components (Rao et al., 2021, Shukla et al., 2021) .
7. Clinical trials
A large number of clinical trials have been registered worldwide to evaluate the efficacy of herbal medicines and yoga for SARS-COV-2 infection. In a clinical study (NCT04323228), anti-inflammatory, and antioxidants are used in the treatment of COVID-19. The study was based on the modulation of cytokinin production, which is a novel concept through immune-nutrition. Specific nutrients such as n3-fatty acids, antioxidants, and vitamins modulate the host immune response and ameliorate the cytokinin storm associated with this disease. They divide the 30 participants into two groups i.e., the first intervention group and the second is the placebo group. The composition of ONS includes protein 14.8 g, fat 22.2 g, 25 g carbohydrate 355 Kcal, eicosapentaenoic acid 1.1g, docosahexaenoic acid 450 mg, gamma-linolenic acid 950 mg, 2840 IU vitamin A, β-carotene 1.2 mg, Vitamin C 205 mg, vitamin E 75 IU, Selenium 18 μg, and zinc 5.7 mg. This study revealed that the ONS group might help in the reduction of COVID-19 as compared to the other group based on biochemical parameters such as cytokine storm parameters (IL-6, TNF-α, and monocyte chemoattractant protein 1), total leukocyte count, and C-reactive protein, etc. Some recent clinical trials related to herbal/natural preparations are displayed in Table 4 .
Table 4.
Clinical trials of herbal medicines registered in (A) clinical trials registry in India (CTRI) and clinicaltrials.gov.
| Clinical trials registered in CTRI | ||||||||
|---|---|---|---|---|---|---|---|---|
| Clinical trial Id | Agent | Phase | Estimated starting date | Period | Patients | Age group | Dose | |
| CTRI/2020/05/025156 | AYUSH-64 | 3/4 | May 18, 2020 | 1 year | 60 | 18–60 year | 2 Tablets (500 mg each) T.I.D. after meal for 1 month | |
| CTRI/2020/05/025338 | AYUSH-64 | 2/3 | June 02, 2020 | 3 month 5 days | 40 | 18–60 year | 2 capsules (500 mg each) T.I.D. Orally for 14 days | |
| CTRI/2020/05/025341 | Kiratiktadi Kwath and Ashwagandha Churna with Yoga | N/A | June 02, 2020 | 3 months | 30 | <20 or >60 years | Gugguludhoopan B.I.D. in COVID-19 wards. AYUSH Kadha O.D. in the early morning. Kirtikatdi kwath: 30 ml B.I.D. Ashwagandha churna 5 gm O.D. with lukewarm water. Yoga sessions twice a day. |
|
| CTRI/2020/05/025171 | Samshamani Vati, Anutaila, rock salt, and turmeric | 2 | May 16, 2020 | 3 months | 5000 | 19–60 years | SamshamaniVati 250 mg B.I.D. after food with water. Application of Anutaila 2 drops in each nostril O.D. after bath. Gargle with warm water mixed with rock salt and turmeric. Yoga and Pranayama- for 8 weeks. |
|
| CTRI/2020/05/025425 | Chyawapanprash | 3/4 | June 02, 2020 | 2 month 15 days | 50 | 20–60 years | 12 gm Avaleha B.I.D. orally Onan empty stomach at least 1 h before breakfast and 2 h after dinner at night. Anupana: Warm water. Duration of therapy: 30 days. |
|
| CTRI/2020/05/025429 | Ashwagandha | 2/3 | June 10, 2020 | 3 months | 5000 | 18–68 years | Ashwagandha capsule with standard prophylactic care. | |
| CTRI/2020/05/025370 | Guduchi Ghan Vati | N/A | June 04, 2020 | 1 month 15 days | 40 | 18–60 years | 500 mg B.I.D. orally for 30 days. | |
| CTRI/2020/05/025093 | Yashtimadhu tablet | 2/3 | May 18, 2020 | 1 month 15 days | 1200 | 18–68 years | Yashtimadhu tablet −250 mg 2 tablets B.I.D. for 1 month. | |
| CTRI/2020/05/025166 | Ashwagandha tablet | 2/3 | May 19, 2020 | 6 months | 1200 | 18–68 years | Ashwagandha tablet (250 mg) two tablets twice daily for one month | |
| CTRI/2020/05/025088 | Guduchi tablet | ½ | May 20, 2020 | 6 months | 1200 | 18–68 years | Guduchi 500 mg B.I.D. for one month. | |
| CTRI/2020/05/025205 | Arsenicum album 30C | 2/3 | May 24, 2020 | 2 months | 33000 | 1–90 years | Arsenicum album 30C will be given to the high danger COVID-19 patients and those located in the containment zone, 4 pills of medicine will be given orally B.I.D. for 7 days. | |
| CTRI/2020/04/024882 | Kashaya (Decoction) | 3 | April 28, 2020 | 1 year | 60 | >18 years | 90–100 ml of Kashaya of Guduchi stem added with 2 gm of powdered dried long pepper B.I.D. | |
| CTRI/2020/05/025332 | Ashwagandha tablet | 2 | May 28, 2020 | 3 months | 400 | 20–69 years | 250 mg, 2 tablets B.I.D. for 12 weeks. | |
| Clinical trials registered in clinicaltrials.gov | ||||||||
| Trial Id | Status | Period | Study type | Participant | Age group | Description | ||
| NCT04716647 | Completed | Interventional | 28 | 20–70 years | In this trial, the therapeutic efficacy of ashwagandha and Guduchi was evaluated | |||
| NCT04480398 | Completed | Jun 2020–July 2020 | Interventional | 91 | 18–75 Year | Guduchi Ghan Vati was tested in asymptomatic COVID 19 patients. | ||
| NCT04920773 | Completed | May 2020–June 2020 | Interventional | 216 | 18–60 years | Guduchi Ghan Vati was tested as prophylaxis for COVID-19. | ||
| NCT04395976 | Not yet recruiting | Jun 2020–Oct 2020 | Interventional | 120 | 18 years and older (Adult, Older Adult) | The study is aimed to prevent people from COVID-19 and those who must met confirmed, COVID-19 patients. The treatment is based on the combination therapy by herbs, nutritional advice, food items, lifestyle, yoga along with standard approvals and it relies on symptoms and personality. | ||
| NCT04351542 | Completed | Mar 2020–Apr 2020 | Interventional | 32 | 18–60 years | The study is based on the Ayurveda-based assessment; self-managed approaches were recommended. | ||
| NCT04345549 | Completed | Feb 2020–Mar 2020 | Interventional | 18 | 18–60 years | In this, participants are advised to self-isolate for seven days and maintain self-care according to directions. The participants were advised to Constitution-based Ayurveda treatment using herbs, lifestyle, and yoga. Participants were suggested to use Ginger/lemon/turmeric/honey recommended as per individual. | ||
| NCT04387643 | Completed | Mar 2020–Apr 2020 | Observational | 52 | 18–60 years | Ayurvedic Kadha was suggested in this study. | ||
8. Computational approaches for identification of target
Computer-based tactics are being in use for the identification of the target and binding energies by molecular docking. Gandhi et al. (2020) performed in silico molecular docking study of Nagaradi Kashaya which is the mixture of Sunthi, Pushkarmool (Inula racemosa Hook.f.), Guduchi, Kantakari (Solanum virginianum L.) for combating COVID-19 using Pyrx Software with Autodock. The binding energy and inhibition of 6-gingesulphonic acid from ginger were found to be greater than HCQ and quinine. Also, the target prediction of selected phytoconstituents was performed. These were found to be effective against SARS-COV-2 which improved humoral immunity and showed antiviral activity (Gandhi et al., 2020). Molecular docking analysis was performed to analyze the efficacy of phytochemicals. A total of 57 phytochemicals were examined virtually against four structural protein targets of SARS-COV-2 viz. 3CLpro, ACE-2, spike glycoprotein and RdRp. Advantageously, two bioactive from each of the three plants i.e., apigenin-o-7-glucuronide and ellagic acid from Eucalyptus globulus; vasicolinone and anisotine from Malabar nut, eudesmol, and viridiflorene from Chinese chaste tree (Vitex negundo L.) were observed based on interaction energies, conventional hydrogen bonding numbers, and other non-covalent interactions. In this study, apigenin-o-7-glucuronide was found to be a promising inhibitor of both protease and polymerase because it interacts with their active sites and showed notably high binding affinity. Phytochemicals from selected traditional herbs showed their potential for steam inhalation therapy for COVID-19 (Gowrishankar et al., 2021). Khaerunnisa et al. (2020) assessed the activity of some phytochemical compounds, one of which was curcumin. The activity was analyzed by the molecular docking method. Curcumin exposed comparatively low binding energies and inhibition constants. They proposed that curcumin could inhibit COVID-19 Mpro and act as a therapeutic agent (Khaerunnisa et al., 2020). By the computational approach, Azim et al. (2020) performed screening and drug ability analysis of herbal compounds to evaluate their efficacy in COVID-19. Twenty-seven plant metabolites were screened against main protease proteins (MPP), spike receptor binding, domain, Nsp9 RNA binding protein, HR2 domain, and spike ectodomain. Among them, asiatic acid, avicularin, guajaverin, and withaferin exposed optimum binding affinity. The key binding sites and drug surface hotspots were unraveled for each viral protein. To examine the drug profile, these four metabolites were subsequently evaluated for ADME analysis, and none of the compounds rendered unsolicited fallouts that could reduce drug resemblance properties (Fig. 6 A-D.). The targets of these drugs were also evaluated in well explained in Fig. 6 (E-H) (Azim et al., 2020).
Fig. 6.
ADME analysis of top four metabolites; (A): Asiatic acid, (B): Guajaverin, (C): Avicularin, and (D): Withaferin; Prediction of drug targets for (E) asiatic acid, (F) guajaverin, (G) avicularin, and (H) withaferin [Adapted from (Azim et al., 2020) under CC BY-NC-ND 4.0].
Most drug targets were found to be on enzymes, oxidoreductases, phosphatases, kinase proteins, and lyases. In another study efficacy of catechin and curcumin (both are polyphenols) was evaluated by Jena et al. (2021) using computational. Both the catechin and curcumin potentially bind with ACE2 and viral S-protein with the binding energy of −10.5 kcal/mol and −8.9 kcal/mol, respectively. Compared to the curcumin, catechin showed a greater resemblance with the binding energy of −7.9 kcal/mol and - 7.8 kcal/mol respectively for catechin and curcumin. This in silico method suggested their potential for the prevention of COVID-19 (Jena et al., 2021).
Khanal et al. (2020) used a different database for the prediction of a protein-based target of withanolides. Their protein-based targets were determined employing DigepPred and the protein-protein interaction was analyzed using STRING. Withanoloid Q was predicted to control the maximum number of proteins, exhibited optimistic human intestinal absorption, and had the uppermost drug-likeness score. Likewise, mutual network interaction is indented withanolide Q to target the maximum number of proteins; RAC1 was chiefly modulated and regulated Fluid shear stress and atherosclerosis as a majorly regulated pathway. Among the numerous withanolides from ashwagandha, withanolide-D, -G, -M, and -Q were projected as a chief success based on drug-likeness score, modulated proteins, and docking score to boost the immune system and inhibit the COVID infection (Khanal et al., 2020). Tripathi et al. (2020) evaluated the potential of 40 constituents of Ashwagandha. The four constituents, namely Withanoside II Withanoside IV Withanoside V, and Sitoindoside IX showed maximum docking energy −11.30 kcal/mol, −11.02 kcal/mol, −8.96 kcal/mol, and −8.37 kcal/mol respectively. Additionally, Withanoside V showed better binding potential and hydrogen-bonding interactions with the protein active site and so indicating stability in the active site (Tripathi et al., 2020). Adem et al. (2020) performed in silico study of natural polyphenols to inhibit COVID-19 Mpro. In this, COVID-19 Mpro was docked with 80 flavonoid compounds. hesperidin, rutin, diosmin, apiin, diacetylcurcumin, (E)-1-(2-Hydroxy-4-methoxyphenyl)-3-[3-[(E)-3-(2-hydroxy-4- methoxyphenyl)-3-oxoprop-1-enyl] phenyl] prop-2-en-1-one, and beta, beta'-(4-Methoxy-1,3- phenylene) bis (2′-hydroxy-4′,6′-dimethoxyacrylophenone have been found as more effective on COVID-19 than nelfinavir. The effective flavonoids found in this study can be ordered as follows affinity hesperidin > rutin > diosmin > apiin > diacetylcurcumin (Adem et al., 2020). Lung et al. (2020) in the computational study evaluated the potential of theaflavin. The theaflavin showed a high docking score with good binding with the RBD in SARS-CoV-2. The driving forces of these interactions were hydrophobic interactions with hydrogen bonds at sites such as ARG454, ASN460, ASN501, GLN493, PHE456, CYS480, and VAL503 of SARS-CoV-2 RBD, near the ACE2-S protein. For the utmost promising interaction of theaflavin with the RBD in SARS-COV-2, the binding energy was found to be −8.53 kcal/mol (Lung et al., 2020). In a molecular docking study, Shree et al. (2020) worked to identify the SARS-COV-2 Mpro (Main protease) inhibitors. A molecular docking study showed six probable inhibitors against SARS-COV-2 Mpro (Main protease). In their study, one from Guduchi, two from ashwagandha (Withanoside V and Somniferin), and three from of Tulsi (Vicenin, Isorientin 4′-O-glucoside 2″-O-p-hydroxybenzoate, and Ursolic acid) were found to inhibit SARS-COV-2 Mpro. The best-docked phytochemicals from this study were found to be safe and had drug-like effects, according to ADMET profile assessment (Shree et al., 2020).
9. Pitfalls and pharmaceutical perspectives
Medicine is prepared by natural constituents does not assure that it is effective and safe. The Government of India's Ministry of AYUSH has recommended prophylactic and symptomatic treatment for the management of health in the COVID-19 epidemic. The advisory created is very helpful in fighting SARS-COV-2, but it has some shortcomings which need further improvement for the safe and effective use of ancient Indian medicinal science. FDA has not approved herbal medicine. It means that the herbal medicines have not passed the stringent criteria of safety and efficacy and are not even recommendations for emergency use. In the recommended advisory, the dose criteria for any age group have not been suggested. As any medicine could have potentially toxic effects and maybe lead to severe toxicity to the liver or kidney. Still, no severe side effects have been reported. Most of the home remedies such as turmeric, methi (Trigonella foenum-graecum L.), and vitamin D3 are used for vitamin C, Zinc, Ginger, ‘Kadhas’, many herbals, homeopathic e.g., Arsenicum alba and Ayurvedic e.g., ashwagandha, to boost up the immunity have been taken which may cause side effects. Licorice supplementation may lead to an elevation in blood pressure due to its effect on the renin-angiotensin-aldosterone system. The overdose of ashwagandha may stimulate the thyroid and causes thyrotoxicosis in some humans and mice (Das et al., 2014). Some common side effects such as constipation, cough, headache, and abdominal pain may be seen in patients taking herbal immune boosters.
The toxic effects of these sources used in traditional cultures have ordinarily not been notified, and this is in the later stage mentioned in terms of safety. Amongst the dozens of approaches, Arsenicum album-30 (homeopathic treatment) is recommended for prophylactic therapy for three days for the prevention form SARS-COV-2 infection. Generally, it is the drug prescribed for the prevention of influenza or similar infections. No evidence confirms its efficacy in the prevention of SARS-COV-2. Similarly, the use of Khas Khas (12 gm) is recommended, but the way to intake and dose according to age group or vulnerable population comprising patients having some existing disease or disorder or dysfunction is still not warranted. It is well appreciated that it helps promote immunity and also relieves cough and sore throat. However, the recommendation does not verify that the use of Khas Khas may fight SARS-COV-2. In the case of the kadha or other herbs there must be specific regarding the number of ingredients, and whether it needs boiling or not, if yes, then it's boiling time and quantity to be taken otherwise, it may lead to serious health concerns. Such concerns should be diagnosed in the advisory, and they must include the possible side effects and drug interactions. There are many such recommendations with insufficient guidelines without any scientific methodologies. The advisory must include proper dosing criteria with a suitable dosage form and regimen. Sometimes self-medication may pose a risk to the patients. These may cause drug-related interaction issues with the other ongoing treatment and potentiate the effect of each other or can cause toxicities. There must not be a communication gap between doctors and patients on the topic of alternative medicine, well this gap is due to the patients being embarrassed or the misunderstanding the potency of these compounds both the patient and physicians need to understand that these drugs have the potential to cause problems while you are taking these for prophylactic or symptomatic treatment. Work should be done in the direction to prepare; besides, these are economical and biocompatible. There is an urgent need to look into the pharmacokinetics of these complex mixtures of bioactive. India is a leading player in ayurvedic medicines at the world level, and premature decisions may lead to untoward aftermaths on the long-term use of these natural plants in raw form. Their active constituents are properly either admixed or transformed into a suitable formulation so that dosing and dosage regimen may be decided. With the advent of nanotechnology, nanocarriers have earmarked tremendous progress in improving the safety and efficacy of potent drugs. These advances can be utilized by formulators working at laboratory, research, and development sections at industries and scientists in collaboration with clinical practitioners to bring upon a fruitful sight of nano-ayurvedic medicines.
For a global fight against this pandemic, approval from national and international authorities is required. Without following proper guidelines for the development of universally accepted means to pacify the needs of patients and approving authorities in terms of bioavailability and bioequivalence studies, these ayurvedic magic bullets will remain unseen and of no use for the betterment of mankind. The remedies from herbal derivatives need an influential and profound evaluation of their pharmacological profile and safety; it is possible by the use of techniques such as pharmacogenomics, metabolic and microarray tactics. The beneficial roles of phytochemicals recommended in advisory can be explored by in silico methods, and molecular docking, to identify the chief compound against viral or host proteins. rational drug design against COVID-19 may shorten the time and reduce the cost based on the genomic information and pathological features of the virus (Wu et al., 2020). In the preclinical phase, it is well-known that pharmacology offers a prevailing perspective for drug discovery, but still, there is a need for research and development endeavors to outrun a drug to the market from the clinical stage, and it is only possible with the proper experiments and their documentation.
Natural products target multiple sites, and about 35% of natural drugs are known for being active against more than one target (Xie, L. et al., 2009). Such type of activity may affect the overall treatment of SARS-COV-2 infection and may cause toxicities. For example, curcumin, a natural polyphenol product isolated from, at least, has ten molecular targets associated with antiviral, anti-cancer, antiarthritic, antioxidant, anti-inflammatory, and immunomodulatory properties (Pan et al., 2013). Similarly, Guduchi, tulsi, and garlic show multiple efficacies. In a case study of a 43-year-old patient, who is a banker in New York Ayurvedic Vaidya in Chennai, India. The symptoms associated were cough, loss of smell and taste, body and abdominal pain. Fever (Jwara) is well known in Ayurveda and COVID-19 is one such. The therapeutic intervention involved three implementations, viz., medicines, diet, and regimen. The patient had been self-quarantined from the first day of fever. The treatment given to the patient for the management of COVID-19 and their mechanism are as follows: (i) Sudarsana Churna: diminish all the three doshas; treat different kinds of fevers such as Sannipata jwara, and Agantujajwara, etc., (ii) Dhanvantara Gutika: cures Svasa, Kasa; Vaataanulomana (aiding the normal flow of vayu), Talisadi Churna: cures Jwara, svasa, kasa, aruchi (loss of taste); Deepanam (stimulates digestion). The symptoms were decreased on day 7, except for the loss of smell and taste. The last sign to normalize was his sense of smell, which reversed on Day 16. The solution to fever was spotted on Day 7 (Girija and Sivan, 2020). The WHO has insisted countries assign financial support for traditional practitioners to evolve traditional medical systems. It is required to access both traditional and Western systems of medicine to accomplish elementary health care needs (Parasuraman, 2018). The Ministry of AYUSH, India has taken ways to resist the unscrupulous systems, and the Ayurvedic Pharmacopoeia of India has standardized the protocol for the use of herbal materials (Sahoo and Manchikanti, 2013).
10. Conclusion and future perspectives
CoV infection is rising day by day at a very rapid pace. But there is no medicine or vaccine available up till now for their treatment. Various Allopathic, as well as natural therapy such as Ayurveda, Siddha, Unani, Homeopathic as well as Yogasan, are being used in the treatment of CoV infection. Some antiviral medicines or immunomodulators are proposed in high doses which may cause severe health illnesses. Herbal medicinal products supremely have different effects and are supportive in a diversity of ailments, for valuable health effects. Globally, the use of herbal medicine as immunostimulants is traditionally not limited to India, but it has been in use in almost all civilizations of Asia, America, Africa, etc. The department of AYUSH has issued a guideline because of the problem of the SARS-COV-2 pandemic. The advisory suggested the use of various Ayurvedic, Homeopathic, and Unani medicines for the prophylactic and symptomatic treatment of SARS-COV-2 infection. Various herbal medicinal plant and their product such as Ashwagandha, Mulethi, Anuthaila, Tulsi, herbal tea, Golden milk, etc. are in use to cure this Global disease due to their couple of profits including immunomodulatory and antiviral effects. There are ample opportunities to focus on research for the treatment of COVID-19 using herbal medicine. During viral infections, imbalances in minerals and micronutrients are linked to poor clinical outcomes. The nutrients such as zinc, vitamin C, Vitamin D, Vitamin A, B vitamins, omega-3 polyunsaturated fatty acids, and iron, should be considered in the evaluation of micronutrients in COVID-19 patients. Thus, providing daily vitamin and trace element intakes is imperative for malnourished individuals. (Ağagündüz et al., 2021; El Zakhem et al., 2021). There is also a wide prospect of research, and it is the right time to execute translational and operational research on Ayurvedic medicines on COVID- 19 as preventive and curative measures on a large scale. The use of nanotechnology can cure many problems associated with the treatment of this disease due to their targeting potential, and subsequently, it will reduce the dose and dosing frequency which directly reduces the toxicity concerns. Researchers should be encouraged to evaluate their potential in terms of efficacy and toxicity. Medical researchers need to investigate precisely how specific herbal preparations affect the body, unlike prescription medications, alternative medicines are not subject to strict guidelines the FDA has for prescription drugs. The manufacturer of herbal preparation is neither required to prove efficacy nor to track complications caused by the use of such preparations. Recently various clinical studies of herbal medicines and the medicines recommended in the AYUSH advisory are being conducted to ascertain their potency in SARS-COV-2 infection.
CRediT authorship contribution statement
Shiv Kumar Prajapati: Writing – original draft, Formal analysis. Akanksha Malaiya: Data Collection, Writing. Gaurav Mishra: Formal analysis. Dolly Jain: Data Collection, Writing. Payal Kesharwani: Review & Editing. Nishi Mody: Review & Editing. Amirhossein Ahmadi: Formal analysis. Rishi Paliwal: Formal analysis. Ankit Jain: Conceptualization, Supervision, Formal analysis.
Declaration of Competing interest
All authors declare no financial/commercial conflicts of interest.
Acknowledgment
Dr. Ankit Jain is grateful for the financial support as the CV Raman postdoctoral fellowship under the Institution of Eminence at Indian Institute of Science, Bangalore (Karnataka), India.
References
- Abdullah T.H., Kandil O., Elkadi A., Carter J. Garlic revisited: therapeutic for the major diseases of our times? J. Natl. Med. Assoc. 1988;80(4):439. [PMC free article] [PubMed] [Google Scholar]
- Abraham A., Samuel S., Mathew L. Phytochemical analysis of Pathyashadangam kwath and its standardization by HPLC and HPTLC. J. Ayurveda Integr. Med. 2020;11(2):153–158. doi: 10.1016/j.jaim.2017.10.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ad-Dahhan H.A. Detection of Immunomodulatory activity of alcoholic extract ofCordia myxa (L.) fruit. journal of al-qadisiyah for pure science (quarterly) 2010;15(4):14–21. [Google Scholar]
- Adem S., Eyupoglu V., Sarfraz I., Rasul A., Ali M. Identification of potent COVID-19 main protease (Mpro) inhibitors from natural polyphenols: an in silico strategy unveils a hope against CORONA. Preprints. 2020:2020030333. doi: 10.1016/j.compbiomed.2022.105452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Adhikari B., Marasini B.P., Rayamajhee B., Bhattarai B.R., Lamichhane G., Khadayat K., Adhikari A., Khanal S., Parajuli N. Potential roles of medicinal plants for the treatment of viral diseases focusing on COVID-19: a review. Phytother Res. 2021;35(3):1298–1312. doi: 10.1002/ptr.6893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Afifah D., Arief M., Al-Arif M. IOP Publishing; 2021. The Effect of Garlic (Allium Sativum) and Turmeric (Curcuma Longa) Extract Addition in Commercial Feed on Feeding Rate, Feed Efficiency and Feed Conversion Ratio of Gouramy Fish (Osphronemus Gouramy), IOP Conference Series: Earth and Environmental Science; p. 12073. [Google Scholar]
- Afzal M., Obuekwe C., Khan A., Barakat H. Antioxidant activity of Cordia myxa L. and its hepatoprotective potential. Electron. J. Environ. Agric. Food Chem. 2007;6(8):2236–2242. [Google Scholar]
- Ağagündüz D., Çelik M.N., Dazıroğlu M.E.Ç., Capasso R. Emergent drug and nutrition interactions in COVID-19: a comprehensive narrative review. Nutrients. 2021;13(5):1550. doi: 10.3390/nu13051550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Agarwal R., Diwanay S., Patki P., Patwardhan B. Studies on immunomodulatory activity of Withania somnifera (Ashwagandha) extracts in experimental immune inflammation. J. Ethnopharmacol. 1999;67(1):27–35. doi: 10.1016/s0378-8741(99)00065-3. [DOI] [PubMed] [Google Scholar]
- Ahui M.L.B., Champy P., Ramadan A., Van L.P., Araujo L., Andre K.B., Diem S., Damotte D., Kati-Coulibaly S., Offoumou M.A. Ginger prevents Th2-mediated immune responses in a mouse model of airway inflammation. Int. Immunopharm. 2008;8(12):1626–1632. doi: 10.1016/j.intimp.2008.07.009. [DOI] [PubMed] [Google Scholar]
- Al-Snafi A.E. Glycyrrhiza glabra: a phytochemical and pharmacological review. IOSRPHR. 2018;8(6):1–17. doi: 10.1002/ptr.6178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- AlBayaty M.A., AlTahan F.J.A.F.J. Mechanism of the tracheal smooth muscle relaxant activity of the Cordia myxa plant extract in sheep. Iraqi J. Veterinary Med. 2008;32(2):214–226. [Google Scholar]
- Ali M.M., Noaman E., Kamal S., Soliman S., Ismail D.A. Uloga kompleksa germanija s L-cysteinom i α-tokoferolom kao stimulatora antioksidativnog obrambenog sustava štakora izloženih gama-zračenju. Acta Pharm. 2007;57(1):1–12. [Google Scholar]
- Alzohairy M.A. Therapeutics role of Azadirachta indica (Neem) and their active constituents in diseases prevention and treatment. Evid Based Complement Alternat Med. 2016;2016:7382506. doi: 10.1155/2016/7382506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anand A.V., Balamuralikrishnan B., Kaviya M., Bharathi K., Parithathvi A., Arun M., Senthilkumar N., Velayuthaprabhu S., Saradhadevi M., Al-Dhabi N.A. Medicinal plants, phytochemicals, and herbs to combat viral pathogens including SARS-CoV-2. Molecules. 2021;26(6):1775. doi: 10.3390/molecules26061775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Andrin'iranto R.A., Narindra R., Jules R., Rokiman L., Octavie R.E. EDXRF and GC Characterization of Curcuma longa L.(Zingiberaceae) Rhizome from Madagascar. Discovery Phytomedicine. 2021;8(1):15–23. [Google Scholar]
- Aranha I., Clement F., Venkatesh Y.P. Immunostimulatory properties of the major protein from the stem of the Ayurvedic medicinal herb, guduchi (Tinospora cordifolia) J. Ethnopharmacol. 2012;139(2):366–372. doi: 10.1016/j.jep.2011.11.013. [DOI] [PubMed] [Google Scholar]
- Arora R., Chawla R., Marwah R., Arora P., Sharma R., Kaushik V., Goel R., Kaur A., Silambarasan M., Tripathi R. Potential of complementary and alternative medicine in preventive management of novel H1N1 flu (Swine flu) pandemic: thwarting potential disasters in the bud. Evid Based Complement Alternat Med. 2011;2011 doi: 10.1155/2011/586506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arreola R., Quintero-Fabián S., López-Roa R.I., Flores-Gutiérrez E.O., Reyes-Grajeda J.P., Carrera-Quintanar L., Ortuño-Sahagún D. Immunomodulation and anti-inflammatory effects of garlic compounds. J. Immunol. Res. 2015;2015:401630. doi: 10.1155/2015/401630. 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Azim K.F., Ahmed S.R., Banik A., Khan M.M.R., Deb A., Somana S.R. Screening and druggability analysis of some plant metabolites against SARS-CoV-2: an integrative computational approach. Inform. Med. Unlocked. 2020;20:100367. doi: 10.1016/j.imu.2020.100367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Badar V., Thawani V., Wakode P., Shrivastava M., Gharpure K., Hingorani L., Khiyani R. Efficacy of Tinospora cordifolia in allergic rhinitis. J. Ethnopharmacol. 2005;96(3):445–449. doi: 10.1016/j.jep.2004.09.034. [DOI] [PubMed] [Google Scholar]
- Balkrishna A. Vol. 13. Patanjali Research Institute; 2020. (Indian Traditional Ayurvedic Treatment Regime for Novel Coronavirus, COVID-19). [Google Scholar]
- Balkrishna A., Solleti S.K., Singh H., Tomer M., Sharma N., Varshney A. Calcio-herbal formulation, Divya-Swasari-Ras, alleviates chronic inflammation and suppresses airway remodelling in mouse model of allergic asthma by modulating pro-inflammatory cytokine response. Biomed. Pharmacother. 2020;126:110063. doi: 10.1016/j.biopha.2020.110063. [DOI] [PubMed] [Google Scholar]
- Balkrishna A., Solleti S.K., Singh H., Verma S., Sharma N., Nain P., Varshney A. Herbal decoction Divya-Swasari-Kwath attenuates airway inflammation and remodeling through Nrf-2 mediated antioxidant lung defence in mouse model of allergic asthma. Phytomedicine. 2020;78:153295. doi: 10.1016/j.phymed.2020.153295. [DOI] [PubMed] [Google Scholar]
- Balkrishna A., Bhatt A.B., Singh P., Haldar S., Varshney A. Comparative retrospective open-label study of ayurvedic medicines and their combination with allopathic drugs on asymptomatic and mildly-symptomatic COVID-19 patients. J. Herb. Med. 2021;29:100472. doi: 10.1016/j.hermed.2021.100472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Balkrishna A., Raj P., Singh P., Varshney A. Influence of patient-reported treatment Satisfaction on Psychological health and quality of life among patients receiving divya-swasari-Coronil-kit against COVID-19: findings from a cross-Sectional “SATISFACTION COVID” survey. Patient Prefer. Adherence. 2021;15:899. doi: 10.2147/PPA.S302957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bettuzzi S., Gabba L., Cataldo S. Efficacy of a polyphenolic, standardized green tea extract for the treatment of COVID-19 syndrome: a proof-of-principle study. COVID. 2021;1(1):2–12. [Google Scholar]
- Bižanov G., Normantienė T., Jonauskienė I., Vyšniauskis G. Evaluation of herb extracts and germanium oxide as immunological adjuvants in quails (Coturnix coturnix japonica) Rev. MVZ Córdoba. 2018;23(3):6838–6849. [Google Scholar]
- Bruck R., Aeed H., Brazovsky E., Noor T., Hershkoviz R. Allicin, the active component of garlic, prevents immune‐mediated, concanavalin A‐induced hepatic injury in mice. Liver Int. 2005;25(3):613–621. doi: 10.1111/j.1478-3231.2005.01050.x. [DOI] [PubMed] [Google Scholar]
- Bui T.T., Piao C.H., Song C.H., Shin H.S., Shon D.-H., Chai O.H. Piper nigrum extract ameliorated allergic inflammation through inhibiting Th2/Th17 responses and mast cells activation. Cell. Immunol. 2017;322:64–73. doi: 10.1016/j.cellimm.2017.10.005. [DOI] [PubMed] [Google Scholar]
- Cecere T.E., Todd S.M., LeRoith T. Regulatory T cells in arterivirus and coronavirus infections: do they protect against disease or enhance it? Viruses. 2012;4(5):833–846. doi: 10.3390/v4050833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cena H., Chieppa M. Coronavirus disease (COVID-19–SARS-CoV-2) and nutrition: is infection in Italy suggesting a connection? Front. Immunol. 2020;11:944. doi: 10.3389/fimmu.2020.00944. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Charan J., Bhardwaj P., Dutta S., Kaur R., Bist S.K., Detha M.D., Kanchan T., Yadav D., Mitra P., Sharma P. Use of complementary and alternative medicine (CAM) and home remedies by COVID-19 patients: a telephonic survey. Indian J. Clin. Biochem. 2021;36(1):108–111. doi: 10.1007/s12291-020-00931-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chaudhary A., Sharma S., Mittal A., Gupta S., Dua A. Phytochemical and antioxidant profiling of Ocimum sanctum. J. Food Sci. Technol. 2020;10:3852–3863. doi: 10.1007/s13197-020-04417-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheng B., Li T. Discovery of alliin as a putative inhibitor of the main protease of SARS-CoV-2 by molecular docking. Biotechniques. 2020;69(2):108–112. doi: 10.2144/btn-2020-0038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Choudhary M., Yousuf S., Rahman A. In: Withanolides: Chemistry and Antitumor Activity, Natural Products Ramawat KG. Merillon J.M., editor. Springer; Berlin, Heidelberg: Springer-Verlag: 2013. pp. 3465–3495. [Google Scholar]
- Cinatl J., Morgenstern B., Bauer G., Chandra P., Rabenau H., Doerr H. Glycyrrhizin, an active component of liquorice roots, and replication of SARS-associated coronavirus. Lancet. 2003;361(9374):2045–2046. doi: 10.1016/S0140-6736(03)13615-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dalvi P., Kulkarni M., Jadhav S. Literary review of Anu taila Nasya. Unique J. Ayurvedic Herbal Med. 2015;3(2):42–45. [Google Scholar]
- Das S., Bordoloi R., Newar N. A review on immune modulatory effect of some traditional medicinal herbs. J. Pharm. Chem. Biol. Sci. 2014;2(1):33–42. [Google Scholar]
- Devpura G., Tomar B.S., Nathiya D., Sharma A., Bhandari D., Haldar S., Balkrishna A., Varshney A. Randomized placebo-controlled pilot clinical trial on the efficacy of ayurvedic treatment regime on COVID-19 positive patients. Phytomedicine. 2021;84:153494. doi: 10.1016/j.phymed.2021.153494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dheyab M.A., Khaniabadi P.M., Aziz A.A., Jameel M.S., Mehrdel B., Oglat A.A., Khaleel H.A. Focused role of nanoparticles against COVID-19: diagnosis and treatment. Photodiagnosis Photodyn. Ther. 2021:102287. doi: 10.1016/j.pdpdt.2021.102287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Di Lorenzo C., Dell'Agli M., Sangiovanni E., Dos Santos A., Uberti F., Moro E., Bosisio E., Restani P. Correlation between catechin content and NF-κB inhibition by infusions of green and black tea. Plant Foods Hum. Nutr. 2013;68(2):149–154. doi: 10.1007/s11130-013-0354-0. [DOI] [PubMed] [Google Scholar]
- Dissanayake K., Wmtdn W., Perera W. Root/Stem extracts of Glycyrrhiza glabra; as a medicinal plant against disease forming microorganisms. Int. J. Sci. Basic Appl. Res. 2020;51(1):1–11. [Google Scholar]
- Dubey R., Dubey K. Springer; 2022. SARS-CoV-2: Potential Drug Targets and its Virtual Screening, Modeling, Control and Drug Development for COVID-19 Outbreak Prevention; pp. 203–244. [Google Scholar]
- Durrani F., Sultan A., Jan M., Chand N., Durrani Z. Immunomodulatory and growth promoting effects of Neem (Azadirachta indica) leaves infusion in broiler chicks. Sarhad J. Agric. 2008;24(4):661–664. [Google Scholar]
- El Zakhem A., Chalhoub M.A., Bassil M. The role of herbal and nutritional treatments in the fight against COVID-19 and other respiratory tract infections. Int. J. Environ. Res. Publ. Health. 2021;18(22):12001. doi: 10.3390/ijerph182212001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elsakka M., Grigorescu E., Stănescu U., Dorneanu V. New data referring to chemistry of Withania somnifera species. Rev. Med.-Chir. Soc. Med. Nat. Iasi. 1990;94(2):385–387. [PubMed] [Google Scholar]
- Españo E., Kim J., Lee K., Kim J.K. Phytochemicals for the treatment of COVID-19. J. Microbiol. 2021 Nov;59(11):959–977. doi: 10.1007/s12275-021-1467-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gajewski A., Kośmider A., Nowacka A., Puk O., Wiciński M. Potential of herbal products in prevention and treatment of COVID-19. Literature review. Biomed. Pharmacother. 2021;143:112150. doi: 10.1016/j.biopha.2021.112150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gao Q.-H., Wu C.-S., Wang M. The jujube (Ziziphus jujuba Mill.) fruit: a review of current knowledge of fruit composition and health benefits. J. Agric. Food Chem. 2013;61(14):3351–3363. doi: 10.1021/jf4007032. [DOI] [PubMed] [Google Scholar]
- Gautam S., Gautam A., Chhetri S., Bhattarai U. Immunity against COVID-19: potential role of ayush Kwath. J. Ayurveda Integr. Med. 2020 Aug 17:100350. doi: 10.1016/j.jaim.2020.08.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gautam S., Gautam A., Chhetri S., Bhattarai U. Immunity against COVID-19: potential role of ayush Kwath. J. Ayurveda Integr. Med. 2022;13(1):100350. doi: 10.1016/j.jaim.2020.08.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Girija P., Sivan N. Ayurvedic treatment of COVID-19/SARS-CoV-2: a case report. J. Ayurveda Integr. Med. 2020;13(1):100329. doi: 10.1016/j.jaim.2020.06.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Godatwar P.K., Deshpande S., JoshiDeshmukh P.S., Deshpande V.S., Ghungralekar R., Tamoli S., Gupta A., Vedula S., Rugvedi P., Rai R.K. Clinical evaluation of Chyawanprash as a preventive measure during the COVID-19 pandemic: an open-label, multicentric, randomized, comparative, prospective, and interventional community-based clinical study on healthy individuals. J. Indian Syst. Med. Res. 2021;9(2):104. [Google Scholar]
- Godhwani S., Godhwani J., Was D. Ocimum sanctum—a preliminary study evaluating its immunoregulatory profile in albino rats. J. Ethnopharmacol. 1988;24(2–3):193–198. doi: 10.1016/0378-8741(88)90151-1. [DOI] [PubMed] [Google Scholar]
- Goel A., Singh D.K., Kumar S., Bhatia A.K. Immunomodulating property of Ocimum sanctum by regulating the IL-2 production and its mRNA expression using rat's splenocytes. Asian Pac. J. Trop. Med. 2010;3(1):8–12. [Google Scholar]
- Goothy S.S.K., Goothy S., Choudhary A., Potey G., Chakraborty H., Kumar A.H., Mahadik V. Ayurveda's holistic lifestyle approach for the management of Coronavirus disease (COVID-19): possible role of tulsi. Int. J. Res. Pharm. Sci. 2020:16–18. [Google Scholar]
- Gowrishankar S., Muthumanickam S., Kamaladevi A., Karthika C., Jothi R., Boomi P., Maniazhagu D., Pandian S.K. Promising phytochemicals of traditional Indian herbal steam inhalation therapy to combat COVID-19–An in silico study. Food Chem. Toxicol. 2021;148:111966. doi: 10.1016/j.fct.2020.111966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grover A., Agrawal V., Shandilya A., Bisaria V.S., Sundar D. Non-nucleosidic inhibition of Herpes simplex virus DNA polymerase: mechanistic insights into the anti-herpetic mode of action of herbal drug withaferin A. BMC Bioinformatics. BioMed Central. 2011:S22. doi: 10.1186/1471-2105-12-S13-S22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gupta P.K., Chakraborty P., Kumar S., Singh P.K., Rajan M., Sainis K.B., Kulkarni S. G1-4A, a polysaccharide from Tinospora cordifolia inhibits the survival of Mycobacterium tuberculosis by modulating host immune responses in TLR4 dependent manner. PLoS One. 2016;11(5) doi: 10.1371/journal.pone.0154725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gupta A., Madan A., Yadav B., Singhal R., Mundada P.S., Pandey Y.K., Agarwal R., Rana R., Tripathi A., Sharma B.S. medRxiv; 2021. Chyawanprash for the Prevention of COVID-19 Infection Among Healthcare Workers: A Randomized Controlled Trial. [Google Scholar]
- Hamauzu Y., Yasui H., Inno T., Kume C., Omanyuda M. Phenolic profile, antioxidant property, and anti-influenza viral activity of Chinese quince (Pseudocydonia sinensis Schneid.), quince (Cydonia oblonga Mill.), and apple (Malus domestica Mill.) fruits. J. Agric. Food Chem. 2005;53(4):928–934. doi: 10.1021/jf0494635. [DOI] [PubMed] [Google Scholar]
- Hardenbrook N.J., Zhang P. A structural view of the SARS-CoV-2 virus and its assembly. Curr. Opin. Virol. 2022;52:123–134. doi: 10.1016/j.coviro.2021.11.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hasan A., Paray B.A., Hussain A., Qadir F.A., Attar F., Aziz F.M., Sharifi M., Derakhshankhah H., Rasti B., Mehrabi M. A review on the cleavage priming of the spike protein on coronavirus by angiotensin-converting enzyme-2 and furin. J. Biomol. Struct. Dyn. 2021;39(8):3025–3033. doi: 10.1080/07391102.2020.1754293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hegde P.L. Agasthya Harithaki as Naimithika Rasayana in Tamaka svasa: a review. Int. J. Health Sci. Res. 2020;10(1):101–103. [Google Scholar]
- Hoffmann M., Kleine-Weber H., Schroeder S., Krüger N., Herrler T., Erichsen S., Schiergens T.S., Herrler G., Wu N.-H., Nitsche A. SARS-CoV-2 cell entry depends on ACE2 and TMPRSS2 and is blocked by a clinically proven protease inhibitor. Cell. 2020;181(2):271–280. doi: 10.1016/j.cell.2020.02.052. e278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang C., Wang Y., Li X., Ren L., Zhao J., Hu Y., Zhang L., Fan G., Xu J., Gu X. Clinical features of patients infected with 2019 novel coronavirus in Wuhan, China. Lancet. 2020;395(10223):497–506. doi: 10.1016/S0140-6736(20)30183-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang F., Li Y., Leung E.L.-H., Liu X., Liu K., Wang Q., Lan Y., Li X., Yu H., Cu L. Pharmacol Res; 2020. A Review of Therapeutic Agents and Chinese Herbal Medicines against SARS-COV-2 (COVID-19) p. 104929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ibrahim R.S., Mahrous R.S., EL-Khair R.M.A., Ross S.A., Omar A.A., Fathy H.M. Biologically guided isolation and ADMET profile of new factor Xa inhibitors from Glycyrrhiza glabra roots using in vitro and in silico approaches. RSC Adv. 2021;11(17):9995–10001. doi: 10.1039/d1ra00359c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Islam S.S., Midya S., Sinha S., Saadi S.M.A.I. Natural medicinal plant products as an immune-boosters: a possible role to lessen the impact of Covid-19. Case Studies Chem. Environ. Eng. 2021;4:100105. doi: 10.1016/j.cscee.2021.100105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iuvone T., Esposito G., Capasso F., Izzo A.A. Induction of nitric oxide synthase expression by Withania somnifera in macrophages. Life Sci. 2003;72(14):1617–1625. doi: 10.1016/s0024-3205(02)02472-4. [DOI] [PubMed] [Google Scholar]
- Jain A., Prajapati S.K., Tripathi M., Raichur A.M., Kanwar J.R. Exploring the room for repurposed hydroxychloroquine to impede COVID-19: toxicities and multipronged combination approaches with pharmaceutical insights. Expet Rev. Clin. Pharmacol. 2021;4(6):715–734. doi: 10.1080/17512433.2021.1909473. [DOI] [PubMed] [Google Scholar]
- Jamshidi N., Cohen M.M. The clinical efficacy and safety of Tulsi in humans: a systematic review of the literature. Evid Based Complement Alternat Med. 2017 doi: 10.1155/2017/9217567. 2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jang M., Park R., Park Y.-I., Cha Y.-E., Yamamoto A., Lee J.I., Park J. EGCG, a green tea polyphenol, inhibits human coronavirus replication in vitro. Biochem. Biophys. Res. Commun. 2021;547:23–28. doi: 10.1016/j.bbrc.2021.02.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jena A.B., Kanungo N., Nayak V., Chainy G.B.N., Dandapat J. Catechin and curcumin interact with S protein of SARS-CoV2 and ACE2 of human cell membrane: insights from computational studies. Sci. Rep. 2021;11(1):2043. doi: 10.1038/s41598-021-81462-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kalikar M., Thawani V., Varadpande U., Sontakke S., Singh R., Khiyani R. Immunomodulatory effect of Tinospora cordifolia extract in human immuno-deficiency virus positive patients. Indian J. Pharmacol. 2008;40(3):107. doi: 10.4103/0253-7613.42302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khaerunnisa S., Kurniawan H., Awaluddin R., Suhartati S., Soetjipto S. Potential inhibitor of COVID-19 main protease (Mpro) from several medicinal plant compounds by molecular docking study. Preprints. 2020;2020:2020030226. [Google Scholar]
- Khan M.A., Kumar S., Gupta A., Ahmad S. Antimicrobial activity of sugar-based semisolid polyherbal ayurvedic formulation: Chyawanprash. Drug Dev. Ther. 2016;7(1):31–33. [Google Scholar]
- Khanal P., Duyu T., Patil B., Dey Y.N., Pasha I., Wanjari M., Gurav S.S., Maity A. Network pharmacology of AYUSH recommended immune-boosting medicinal plants against COVID-19. J. Ayurveda Integr. Med. 2020 doi: 10.1016/j.jaim.2020.11.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khanna K., Kohli S.K., Kaur R., Bhardwaj A., Bhardwaj V., Ohri P., Sharma A., Ahmad A., Bhardwaj R., Ahmad P. Herbal immune-boosters: substantial warriors of pandemic Covid-19 battle. Phytomedicine. 2021;85:153361. doi: 10.1016/j.phymed.2020.153361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khare C., Naharwar A.V. Ashwagandha (Withania somnifera (L.) Dunal): a scientific review with respect to Ayurvedic perspectives. Ann phytomedicine. 2020;9(2):134–141. [Google Scholar]
- Khurana A. Homoeopathy in epidemics: Bridging the gap. Indian J. Res. Homoeopathy. 2020;14(2):77. [Google Scholar]
- Kim M.E., Na J.Y., Lee J.S. Anti-inflammatory effects of trans-cinnamaldehyde on lipopolysaccharide-stimulated macrophage activation via MAPKs pathway regulation. Immunopharmacol. Immunotoxicol. 2018;40(3):219–224. doi: 10.1080/08923973.2018.1424902. [DOI] [PubMed] [Google Scholar]
- Kudineer N. 2013. A Combinatino of Nilavembu Kudineer and Adathodai Manapagu in the Management of Dengue Fever. [Google Scholar]
- Kulkarni S., Dhir A. Withania somnifera: an Indian ginseng. Prog. Neuro-Psychopharmacol. Biol. Psychiatry. 2008;32(5):1093–1105. doi: 10.1016/j.pnpbp.2007.09.011. [DOI] [PubMed] [Google Scholar]
- Kulkarni V., Sharma N., Modi D., Kumar A., Joshi J., Krishnamurthy N. 2021. A Community-Based Participatory Research to Assess the Feasibility of Ayurveda Intervention in Patients with Mild-To-Moderate COVID-19. medRxiv. [Google Scholar]
- Kumar V., Sharma A. Neutrophils: Cinderella of innate immune system. Int. Immunopharm. 2010;10(11):1325–1334. doi: 10.1016/j.intimp.2010.08.012. [DOI] [PubMed] [Google Scholar]
- Kumar V., Dhanjal J.K., Kaul S.C., Wadhwa R., Sundar D. Withanone and caffeic acid phenethyl ester are predicted to interact with main protease (Mpro) of SARS-CoV-2 and inhibit its activity. J. Biomol. Struct. Dyn. 2020:1–17. doi: 10.1080/07391102.2020.1772108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kumar G., Kumar D., Singh N.P. Therapeutic approach against 2019-nCoV by inhibition of ACE-2 receptor. Drug Res. 2021;71(4):213–218. doi: 10.1055/a-1275-0228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Latif M.J., Hassan S.M., Mughal S.S., Aslam A., Munir M., Shabbir N., Mushtaq M., Perveiz S. Therapeutic potential of Azadirachta indica (neem) and their active phytoconstituents against diseases prevention. J. Chem Cheml Sci. 2020;10(3):98–110. [Google Scholar]
- Li Y., But P.P., Ooi V.E. Antiviral activity and mode of action of caffeoylquinic acids from Schefflera heptaphylla (L.) Frodin. Antivir. Res. 2005;68(1):1–9. doi: 10.1016/j.antiviral.2005.06.004. [DOI] [PubMed] [Google Scholar]
- Li Y., Wang J., Liu Y., Luo X., Lei W., Xie L. Antiviral and virucidal effects of curcumin on transmissible gastroenteritis virus in vitro. J. Gen. Virol. 2020;101(10):1079–1084. doi: 10.1099/jgv.0.001466. [DOI] [PubMed] [Google Scholar]
- Liu Z., Ying Y. The inhibitory effect of curcumin on virus-induced cytokine storm and its potential use in the associated severe Pneumonia. Front. Cell Dev. Biol. 2020;8 doi: 10.3389/fcell.2020.00479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lung J., Lin Y.-S., Yang Y.-H., Chou Y.-L., Chang G.-H., Tsai M.-S., Hsu C.-M., Yeh R.-A., Shu L.-H., Cheng Y.-C., Liu H.T., Wu C.-Y. The potential SARS-CoV-2 entry inhibitor. bioRxiv. 2020 2020.2003.2026.009803. [Google Scholar]
- Majdalawieh A.F., Carr R.I. In vitro investigation of the potential immunomodulatory and anti-cancer activities of black pepper (Piper nigrum) and cardamom (Elettaria cardamomum) J. Med. Food. 2010;13(2):371–381. doi: 10.1089/jmf.2009.1131. [DOI] [PubMed] [Google Scholar]
- Maloir Q., Ghysen K., Louis R., Guiot J. Acute respiratory distress revealing antisynthetase syndrome. Rev. Med. Liege. 2018;73(7–8):370–375. [PubMed] [Google Scholar]
- Mandlik D.S., Namdeo A.G. Pharmacological evaluation of Ashwagandha highlighting its healthcare claims, safety, and toxicity aspects. J. Diet. 2020;Suppl:1–44. doi: 10.1080/19390211.2020.1741484. [DOI] [PubMed] [Google Scholar]
- Mathew D., Hsu W.-L. Antiviral potential of curcumin. J. Funct.Foods. 2018;40:692–699. [Google Scholar]
- Maurya D.K., Sharma D. Evaluation of traditional ayurvedic preparation for prevention and management of the novel Coronavirus (SARS-CoV-2) using molecular docking approach. Chem. Rxiv. 2020 doi: 10.1080/07391102.2020.1852119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Medhi R., Srinoi P., Ngo N., Tran H.-V., Lee T.R. Nanoparticle-based strategies to combat COVID-19. ACS Appl. Nano Mater. 2020;3(9):8557–8580. doi: 10.1021/acsanm.0c01978. [DOI] [PubMed] [Google Scholar]
- Menegazzi M., Campagnari R., Bertoldi M., Crupi R., Di Paola R., Cuzzocrea S. Protective effect of epigallocatechin-3-gallate (EGCG) in diseases with uncontrolled immune activation: could such a scenario be helpful to counteract COVID-19? Int. J. Mol. Sci. 2020;21(14):5171. doi: 10.3390/ijms21145171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mishra L.-C., Singh B.B., Dagenais S. Scientific basis for the therapeutic use of Withania somnifera (ashwagandha): a review. Alternative Med. Rev. 2000;5(4):334–346. [PubMed] [Google Scholar]
- Mishra P., Sohrab S., Mishra S.K. A review on the phytochemical and pharmacological properties of Hyptis suaveolens (L.) Poit. Future J. Pharm. Sci. 2021;7(1):1–11. doi: 10.1186/s43094-021-00219-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mohammadzadeh V., Barani M., Amiri M.S., Yazdi M.E.T., Hassanisaadi M., Rahdar A., Varma R.S. Applications of plant-based nanoparticles in nanomedicine: a review. Sustain Chem. Pharm. 2022;25:100606. [Google Scholar]
- Mohapatra P.K., Chopdar K.S., Dash G.C., Mohanty A.K., Raval M.K. In silico screening and covalent binding of phytochemicals of Ocimum sanctum against SARS-CoV-2 (COVID 19) main protease. J. Biomol. Struct. Dyn. 2021:1–10. doi: 10.1080/07391102.2021.2007170. [DOI] [PubMed] [Google Scholar]
- More P., Pai K. Immunomodulatory effects of Tinospora cordifolia (Guduchi) on macrophage activation. Biol. Med. 2011;3(2):134–140. [Google Scholar]
- Mosselhy D.A., Virtanen J., Kant R., He W., Elbahri M., Sironen T. COVID-19 pandemic: what about the safety of anti-coronavirus nanoparticles? Nanomaterials. 2021;11(3):796. doi: 10.3390/nano11030796. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mousavi S.S., Karami A., Haghighi T.M., Tumilaar S.G., Idroes R., Mahmud S., Celik I., Ağagündüz D., Tallei T.E., Emran T.B. In silico evaluation of Iranian medicinal plant phytoconstituents as inhibitors against main protease and the receptor-binding domain of SARS-CoV-2. Molecules. 2021;26(18):5724. doi: 10.3390/molecules26185724. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moutia M., Habti N., Badou A. In vitro and in vivo immunomodulator activities of Allium sativum L. Evid Based Complement Alternat Med. 2018 doi: 10.1155/2018/4984659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mrityunjaya M., Pavithra V., Neelam R., Janhavi P., Halami P., Ravindra P. Immune-boosting, antioxidant and anti-inflammatory food supplements targeting pathogenesis of COVID-19. Front. Immunol. 2020;11 doi: 10.3389/fimmu.2020.570122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mukherjee R., Dash P., Ram G. Immunotherapeutic potential of Ocimum sanctum (L) in bovine subclinical mastitis. Res. Vet. Sci. 2005;79(1):37–43. doi: 10.1016/j.rvsc.2004.11.001. [DOI] [PubMed] [Google Scholar]
- Muthuraman A., Sood S., Singla S.K. The antiinflammatory potential of phenolic compounds from Emblica officinalis L. in rat. Inflammopharmacology. 2011;19(6):327–334. doi: 10.1007/s10787-010-0041-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nair P.R., Melnick S.J., Ramachandran C. Materials and methods for immune system stimulation. Google Patents. 2008 [Google Scholar]
- Nesari T.M., Bhardwaj A., ShriKrishna R., Ruknuddin G., Ghildiyal S., Das A., Pandey A.K., Chaudhary N., Soman G., Barde M. Neem (Azadirachta indica A. Juss) capsules for prophylaxis of COVID-19 infection: a pilot, double-blind, randomized controlled trial. Alternative Ther. Health Med. 2021;27(S1):196–203. [PubMed] [Google Scholar]
- Orlo E., Russo C., Nugnes R., Lavorgna M., Isidori M. Natural methoxyphenol compounds: antimicrobial activity against foodborne pathogens and food Spoilage bacteria, and role in antioxidant Processes. Foods. 2021;10(8):1807. doi: 10.3390/foods10081807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ota N., Takano F., Muroga S., Kawabata T., Ishigaki Y., Yahagi N., Ohta T. Garlic extract and its selected organosulphur constituents promote ileal immune responses ex vivo. J. Funct.Foods. 2012;4(1):243–252. [Google Scholar]
- Paidi R.K., Jana M., Raha S., McKay M., Sheinin M., Mishra R.K., Pahan K. Eugenol, a component of holy Basil (tulsi) and common spice Clove, inhibits the interaction between SARS-CoV-2 spike S1 and ACE2 to induce therapeutic responses. J. Neuroimmune Pharmacol. 2021;16(4):743–755. doi: 10.1007/s11481-021-10028-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pal Singh I., Choudhary A. Piperine and derivatives: trends in structure-activity relationships. Curr. Top. Med. Chem. 2015;15(17):1722–1734. doi: 10.2174/1568026615666150427123213. [DOI] [PubMed] [Google Scholar]
- Palai S.S., Giri A. Tulsi-A potential immune boosting functional food ingredient to combat COVID-19. Immunity Boosting Functional Foods to Combat COVID- 2021;19:27–36. [Google Scholar]
- Pan S.-Y., Zhou S.-F., Gao S.-H., Yu Z.-L., Zhang S.-F., Tang M.-K., Sun J.-N., Ma D.-L., Han Y.-F., Fong W.-F. New perspectives on how to discover drugs from herbal medicines: CAM's outstanding contribution to modern therapeutics. Evid Based Complement Alternat Med. 2013 doi: 10.1155/2013/627375. 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parasuraman S. Herbal drug discovery: challenges and perspectives. Curr. Pharmacogenomics Personalized Med. (CPPM) 2018;16(1):63–68. [Google Scholar]
- Prajapati S.K., Jain A., Jain A., Jain S. Biodegradable polymers and constructs: a novel approach in drug delivery. Eur. Polym. J. 2019;120:109191. [Google Scholar]
- Prajapati S.K., Mishra G., Malaiya A., Jain A., Mody N., Raichur A.M. Springer; 2021. Antimicrobial Application Potential of Phytoconstituents from Turmeric and Garlic, Bioactive Natural Products for Pharmaceutical Applications; pp. 409–435. [Google Scholar]
- Priya N., Kumari P.S. Antiviral activities and cytotoxicity assay of seed extracts of Piper longum and Piper nigrum on human cell lines. Int. J. Pharmaceut. Sci. Rev. Res. 2017;44(1):197–202. [Google Scholar]
- R Cundell D., Wilkinson F. Curcumin: powerful immunomodulator from turmeric. Curr. Immunol. Rev. 2014;10(2):122–132. [Google Scholar]
- Radhakrishnan S. ClinicalTrialsgov; 2020. Efficacy and Safety of Guduchi Ghan Vati for Covid-19 Asymptomatic Patients. [Google Scholar]
- Rahayu R.P., Prasetyo R.A., Purwanto D.A., Kresnoadi U., Iskandar R.P., Rubianto M. The immunomodulatory effect of green tea (Camellia sinensis) leaves extract on immunocompromised Wistar rats infected by Candida albicans. Vet. World. 2018;11(6):765. doi: 10.14202/vetworld.2018.765-770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ram M.S., Neetu D., Yogesh B., Anju B., Dipti P., Pauline T., Sharma S., Sarada S., Ilavazhagan G., Kumar D. Cyto-protective and immunomodulating properties of Amla (Emblica officinalis) on lymphocytes: an in-vitro study. J. Ethnopharmacol. 2002;81(1):5–10. doi: 10.1016/s0378-8741(01)00421-4. [DOI] [PubMed] [Google Scholar]
- Rao M.V.V., Juneja A., Maulik M., Adhikari T., Sharma S., Gupta J., Panchal Y., Yadav N. Emerging trends from COVID-19 research registered in the clinical trials registry-India. Indian J. Med. Res. 2021;153(1–2):26. doi: 10.4103/ijmr.IJMR_2556_20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rattis B.A.C., Ramos S.G., Celes M.R.N. Curcumin as a potential treatment for COVID-19. Front. Pharmacol. 2021;12 doi: 10.3389/fphar.2021.675287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raut R.A., Ghotankar A.M. Review of khuskhus (Khaskhas)(Seeds of Papaver Somniferum Linn.) with special reference of ayurveda medicine. J. Ayurveda Integrated Med. Sci. 2020;4(6):228–231. (ISSN 2456-3110) [Google Scholar]
- Sadekar R., Kolte A., Barmase B., Desai V. Immunopotentiating effects of Azadirachta indica (Neem) dry leaves powder in broilers, naturally infected with IBD virus. Indian J. Exp. Biol. 1998;36(11):1151–1153. [PubMed] [Google Scholar]
- Saggam A., Limgaokar K., Borse S., Chavan-Gautam P., Dixit S., Tillu G., Patwardhan B. Withania somnifera (L.) dunal: opportunity for clinical repurposing in COVID-19 management. Front. Pharmacol. 2021;12:835. doi: 10.3389/fphar.2021.623795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sahoo N., Manchikanti P. Herbal drug regulation and commercialization: an Indian industry perspective. J. Alternative Compl. Med. 2013;19(12):957–963. doi: 10.1089/acm.2012.0275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sarangi M.K., Soni S. A review on giloy: the magic herb. Inventi Rapid: Planta Activa. 2013;2013(2):1–5. [Google Scholar]
- Saravanan J., Devasia N., Gopalasatheeskumar K., Sanish Devan V., Thanga Kokila K., Sanjay M. Anti-inflammatory, antipyretic and antibacterial study of kabasura kudineer choornam. Int. J. Curr. Adv. Res. 2018;7(2):9992–9997. [Google Scholar]
- Seyran M., Takayama K., Uversky V.N., Lundstrom K., Palù G., Sherchan S.P., Attrish D., Rezaei N., Aljabali A.A., Ghosh S. The structural basis of accelerated host cell entry by SARS‐CoV‐2. FEBS J. 2021;288(17):5010–5020. doi: 10.1111/febs.15651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shah A., Krishnamurthy R. Swine flu and its herbal remedies. Int. J. Eng. Sci. 2013;2(2):68–78. [Google Scholar]
- Shah N., Deepshikha a., Garg G.P. Scope of ayurveda in preventing COVID-19. Int. J. Adv. Res. 2020;8:466–472. [Google Scholar]
- Shailaja R., Sugunthan S., Pitchiah Kumar M. A review on polyherbal formulation- Vishasura Kudineer Chooranam - a classical anti-viral drug used in Siddha system of medicine. European J. Pharmaceut. Med. Res. 2017;4(9):184–192. [Google Scholar]
- Sharma V., Agrawal R. Glycyrrhiza glabra-a plant for the future. Mintage J. Pharm. Med. Sci. 2013;2(3):15–20. [Google Scholar]
- Sharma R., Amin H., Prajapati P., Ruknuddin G. Therapeutic vistas of guduchi (Tinospora cordifolia): a medico-historical memoir. J. Res. Educ. Indian Med. 2014;20:113–128. [Google Scholar]
- Sharma R., Martins N., Kuca K., Chaudhary A., Kabra A., Rao M.M., Prajapati P.K. Chyawanprash: a traditional Indian bioactive health supplement. Biomolecules. 2019;9(5):161. doi: 10.3390/biom9050161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sharma K., Kesharwani P., Prajapati S.K., Jain A., Jain D., Mody N., Sharma S. An insight into Anticancer bioactives from Punica granatum (Pomegranate) Anti Cancer Agents Med. Chem. 2021;34315399 doi: 10.2174/1871520621666210726143553. [DOI] [PubMed] [Google Scholar]
- Shree P., Mishra P., Selvaraj C., Singh S.K., Chaube R., Garg N., Tripathi Y.B. Targeting COVID-19 (SARS-CoV-2) main protease through active phytochemicals of ayurvedic medicinal plants–Withania somnifera (Ashwagandha), Tinospora cordifolia (Giloy) and Ocimum sanctum (Tulsi)–a molecular docking study. J. Biomol. Struct. Dyn. 2020:1–14. doi: 10.1080/07391102.2020.1810778. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shree P., Mishra P., Selvaraj C., Singh S.K., Chaube R., Garg N., Tripathi Y.B. Targeting COVID-19 (SARS-CoV-2) main protease through active phytochemicals of ayurvedic medicinal plants–Withania somnifera (Ashwagandha), Tinospora cordifolia (Giloy) and Ocimum sanctum (Tulsi)–a molecular docking study. J. Biomol. Struct. Dyn. 2022;40(1):190–203. doi: 10.1080/07391102.2020.1810778. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shukla G., Yadav M., Kandala A., Kanade U.L., Kumar A. Pugos nutrition to boost immunity in covid-19 infection. Irish Interdiscip J. Sci. Res. 2021;5(2):55–60. [Google Scholar]
- Shukla R., Singh S., Singh A., Misra K. Two pronged approach for prevention and therapy of COVID-19 (Sars-CoV-2) by a multi-targeted herbal drug, a component of ayurvedic decoction. Eur. J. Integr. Med. 2021;43:101268. doi: 10.1016/j.eujim.2020.101268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singh S.K., Rajoria K., Sharma S. Principles of Rajayakshma management for COVID-19. J. Ayurveda Integr. Med. 2022;13(1):100349. doi: 10.1016/j.jaim.2020.08.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tallei T.E., Niode N.J., Idroes R., Zidan B., Mitra S., Celik I., Nainu F., Ağagündüz D., Emran T.B., Capasso R. A comprehensive review of the potential use of green tea polyphenols in the management of COVID-19. Evid Based Complement Alternat Med. 2021 doi: 10.1155/2021/7170736. 2021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thevarajan I., Nguyen T.H., Koutsakos M., Druce J., Caly L., van de Sandt C.E., Jia X., Nicholson S., Catton M., Cowie B. Breadth of concomitant immune responses prior to patient recovery: a case report of non-severe COVID-19. Nat. Med. 2020;26(4):453–455. doi: 10.1038/s41591-020-0819-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tillu G., Chaturvedi S., Chopra A., Patwardhan B. Public health approach of ayurveda and yoga for COVID-19 prophylaxis. J. Alternative Compl. Med. 2020;26(5):360–364. doi: 10.1089/acm.2020.0129. [DOI] [PubMed] [Google Scholar]
- Tito A., Colantuono A., Pirone L., Pedone E., Intartaglia D., Giamundo G., Conte I., Vitaglione P., Apone F. Pomegranate Peel extract as an inhibitor of SARS-CoV-2 spike binding to human ACE2 receptor (in vitro): a promising source of novel antiviral drugs. Front. Chem. 2021;9(231) doi: 10.3389/fchem.2021.638187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tiwari S., Talreja S. Importance of Cinnamomum Tamala in the treatment of various diseases. Phcog. J. 2020;12(6s) [Google Scholar]
- Tiwari R., Chakraborty S., Saminathan M., Dhama K., Singh S.V. Ashwagandha (Withania somnifera): role in safeguarding health, immunomodulatory effects, combating infections and therapeutic applications: a review. J. Biol. Sci. 2014;14(2):77–94. [Google Scholar]
- Tiwari R., Latheef S.K., Ahmed I., Iqbal H., Bule M.H., Dhama K., Samad H.A., Karthik K., Alagawany M., El-Hack M.E. Herbal immunomodulators-a remedial panacea for designing and developing effective drugs and medicines: current scenario and future prospects. Curr. Drug Metabol. 2018;19(3):264–301. doi: 10.2174/1389200219666180129125436. [DOI] [PubMed] [Google Scholar]
- Tripathi S., Maier K.G., Bruch D., Kittur D.S. Effect of 6-gingerol on pro-inflammatory cytokine production and costimulatory molecule expression in murine peritoneal macrophages. J. Surg. Res. 2007;138(2):209–213. doi: 10.1016/j.jss.2006.07.051. [DOI] [PubMed] [Google Scholar]
- Tripathi M.K., Singh P., Sharma S., Singh T.P., Ethayathulla A., Kaur P. Identification of bioactive molecule from Withania somnifera (Ashwagandha) as SARS-CoV-2 main protease inhibitor. J. Biomol. Struct. Dyn. 2020;39(15):5668. doi: 10.1080/07391102.2020.1790425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Upadhyay S.N., Dhawan S., Garg S., Talwar G. Immunomodulatory effects of neem (Azadirachta indica) oil. Int. J. Immunopharm. 1992;14(7):1187–1193. doi: 10.1016/0192-0561(92)90054-o. [DOI] [PubMed] [Google Scholar]
- van de Sand L., Bormann M., Alt M., Schipper L., Heilingloh C.S., Steinmann E., Todt D., Dittmer U., Elsner C., Witzke O. Glycyrrhizin effectively inhibits SARS-CoV-2 replication by inhibiting the viral main protease. Viruses. 2021;13(4):609. doi: 10.3390/v13040609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vanzara A., Patel R., Patel A., Patel N., Yadav K., Nagar P. Biological & Med Chem Preprint; 2021. Liquiritin from Glycirrhyza Glabra L (Fabaceae)-A Natural Derived Drug, as a Potential Inhibitor for SARS-CoV-2. [Google Scholar]
- Varshney A., Balkrishna A., Singh J. Withanone from Withania somnifera may inhibit novel coronavirus (COVID-19) entry by disrupting interactions between viral S-protein receptor binding domain and host ACE2 receptor. Res. Square. 2020:1–21. [Google Scholar]
- Vathsala P., Murthy P.K. Immunomodulatory and antiparasitic effects of garlic–arteether combination via nitric oxide pathway in Plasmodium berghei-infected mice. J. Parasit. Dis. 2020;44(1):49–61. doi: 10.1007/s12639-019-01160-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vellingiri B., Jayaramayya K., Iyer M., Narayanasamy A., Govindasamy V., Giridharan B., Ganesan S., Venugopal A., Venkatesan D., Ganesan H. Sci Total Environ; 2020. COVID-19: A Promising Cure for the Global Panic; p. 138277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vinaya M. Bronchodilator activity of Ocimum sanctum Linn.(tulsi) in mild and moderate asthmatic patients in comparison with salbutamol: a single-blind cross-over study. Int. J. Basic Clin. Pharmacol. 2017;6(3):511. [Google Scholar]
- Wagner H., Jurcic K. Immunological studies of Revitonil®, a phytopharmaceutical containing Echinacea purpurea and Glycyrrhiza glabra root extract. Phytomedicine. 2002;9(5):390–397. doi: 10.1078/09447110260571616. [DOI] [PubMed] [Google Scholar]
- Wanjarkhedkar P., Sarade G., Purandare B., Kelkar D. A prospective clinical study of an Ayurveda regimen in COVID 19 patients. J. Ayurveda Integr. Med. 2020 doi: 10.1016/j.jaim.2020.10.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu F., Zhao S., Yu B., Chen Y.-M., Wang W., Song Z.-G., Hu Y., Tao Z.-W., Tian J.-H., Pei Y.-Y., Yuan M.-L., Zhang Y.-L., Dai F.-H., Liu Y., Wang Q.-M., Zheng J.-J., Xu L., Holmes E.C., Zhang Y.-Z. A new coronavirus associated with human respiratory disease in China. Nature. 2020;579(7798):265–269. doi: 10.1038/s41586-020-2008-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu C.-r., Yin W.-c., Jiang Y., Xu H.E. Structure genomics of SARS-CoV-2 and its Omicron variant: drug design templates for COVID-19. Acta Pharmacol. Sin. 2022:1–13. doi: 10.1038/s41401-021-00851-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xiao S., Tian Z., Wang Y., Si L., Zhang L., Zhou D. Recent progress in the antiviral activity and mechanism study of pentacyclic triterpenoids and their derivatives. Med. Res. Rev. 2018;38(3):951–976. doi: 10.1002/med.21484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xie L., Li J., Xie L., Bourne P.E. Drug discovery using chemical systems biology: identification of the protein-ligand binding network to explain the side effects of CETP inhibitors. PLoS Comput. Biol. 2009;5(5) doi: 10.1371/journal.pcbi.1000387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xie Y.-C., Dong X.-W., Wu X.-M., Yan X.-F., Xie Q.-M. Inhibitory effects of flavonoids extracted from licorice on lipopolysaccharide-induced acute pulmonary inflammation in mice. Int. Immunopharm. 2009;9(2):194–200. doi: 10.1016/j.intimp.2008.11.004. [DOI] [PubMed] [Google Scholar]
- Xu B., Gutierrez B., Mekaru S., Sewalk K., Goodwin L., Loskill A., Cohn E.L., Hswen Y., Hill S.C., Cobo M.M. Epidemiological data from the COVID-19 outbreak, real-time case information. Sci. Data. 2020;7(1):1–6. doi: 10.1038/s41597-020-0448-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamada K., Hung P., Park T.K., Park P.J., Lim B.O. A comparison of the immunostimulatory effects of the medicinal herbs Echinacea, Ashwagandha and Brahmi. J. Ethnopharmacol. 2011;137(1):231–235. doi: 10.1016/j.jep.2011.05.017. [DOI] [PubMed] [Google Scholar]
- Yan L., Song F., Li H., Li Y., Li J., He Q.-Y., Zhang D., Wang F., Zhang M., Zhao H. Submicron emulsion of cinnamaldehyde ameliorates bleomycin-induced idiopathic pulmonary fibrosis via inhibition of inflammation, oxidative stress and epithelial-mesenchymal transition. Biomed. Pharmacother. 2018;102:765–771. doi: 10.1016/j.biopha.2018.03.145. [DOI] [PubMed] [Google Scholar]
- Yasmin A., Chia S., Looi Q., Omar A., Noordin M., Ideris A. Elsevier; 2020. Herbal Extracts as Antiviral Agents, Feed Additives; pp. 115–132. [Google Scholar]
- Yuan Y., Cao D., Zhang Y., Ma J., Qi J., Wang Q., Lu G., Wu Y., Yan J., Shi Y. Cryo-EM structures of MERS-CoV and SARS-CoV spike glycoproteins reveal the dynamic receptor binding domains. Nat. Commun. 2017;8:15092. doi: 10.1038/ncomms15092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zahedipour F., Hosseini S.A., Sathyapalan T., Majeed M., Jamialahmadi T., Al‐Rasadi K., Banach M., Sahebkar A. Potential effects of curcumin in the treatment of COVID‐19 infection. Phytother Res. 2020;34(11):2911–2920. doi: 10.1002/ptr.6738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Z., Zhang X., Bi K., He Y., Yan W., Yang C.S., Zhang J. Potential protective mechanisms of green tea polyphenol EGCG against COVID-19. Trends Food Sci. Technol. 2021 doi: 10.1016/j.tifs.2021.05.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao Z., Xiao Y., Xu L., Liu Y., Jiang G., Wang W., Li B., Zhu T., Tan Q., Tang L. Glycyrrhizic acid nanoparticles as antiviral and anti-inflammatory agents for COVID-19 treatment. ACS Appl. Mater. Interfaces. 2021;13(18):20995–21006. doi: 10.1021/acsami.1c02755. [DOI] [PubMed] [Google Scholar]
- Zheng W., Huang X., Lai Y., Liu X., Jiang Y., Zhan S. Glycyrrhizic acid for COVID-19: findings of targeting pivotal inflammatory pathways triggered by SARS-CoV-2. Front. Pharmacol. 2021;12 doi: 10.3389/fphar.2021.631206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zou M., Chen Y., Sun-Waterhouse D., Zhang Y., Li F. Immunomodulatory acidic polysaccharides from Zizyphus jujuba cv. Huizao: insights into their chemical characteristics and modes of action. Food Chem. 2018;258:35–42. doi: 10.1016/j.foodchem.2018.03.052. [DOI] [PubMed] [Google Scholar]
- Zrieq R., Ahmad I., Snoussi M., Noumi E., Iriti M., Algahtani F.D., Patel H., Saeed M., Tasleem M., Sulaiman S. Tomatidine and Patchouli Alcohol as inhibitors of SARS-CoV-2 enzymes (3CLpro, PLpro and NSP15) by molecular docking and molecular dynamics simulations. Int. J. Mol. Sci. 2021;22(19):10693. doi: 10.3390/ijms221910693. [DOI] [PMC free article] [PubMed] [Google Scholar]