SUMMARY
Few countries routinely collect comprehensive encephalitis data, yet understanding the epidemiology of this condition has value for clinical management, detecting novel and emerging pathogens, and guiding timely public health interventions. When this study was conducted there was no standardized diagnostic algorithm to aid identification of encephalitis or systematic surveillance for adult encephalitis. In July 2012 we tested three pragmatic surveillance options aimed at identifying possible adult encephalitis cases admitted to a major Australian hospital: hospital admissions searches, clinician notifications and laboratory test alerts (CSF herpes simplex virus requests). Eligible cases underwent structured laboratory investigation and a specialist panel arbitrated on the final diagnosis. One hundred and thirteen patients were initially recruited into the 10-month study; 20/113 (18%) met the study case definition, seven were diagnosed with infectious or immune-mediated encephalitis and the remainder were assigned alternative diagnoses. The laboratory alert identified 90% (102/113) of recruited cases including six of the seven cases of confirmed encephalitis suggesting that this may be a practical data source for case ascertainment. The application of a standardized diagnostic algorithm and specialist review by an expert clinical panel aided diagnosis of patients with encephalitis.
Key words: Encephalitis, emerging infectious disease, epidemiology, surveillance
INTRODUCTION
Encephalitis is inflammation of the brain parenchyma. It is a rare complication following infection with a wide range of infectious agents and/or autoimmune illness, often manifested by fever, reduced neurological function, altered consciousness, seizures, radiological and histopathological changes. Many of these signs and symptoms occur in other medical conditions. Encephalitis can result in lengthy hospitalization and has a case-fatality rate of up to 12% [1–3]. Survivors face possible long-term sequelae [4].
Confirming the cause of encephalitis is challenging and often not rigorously pursued [5]. Studies indicate that less than 50% of all encephalitis cases achieve a laboratory-confirmed diagnosis [2, 6, 7]. A large British study which applied a structured approach to pathology testing, doubled the proportion of cases with a confirmed diagnosis but still left 37% of patients without an aetiology [1]. Although treatment options are limited, it is important to achieve a laboratory-confirmed diagnosis to allow optimal clinical management and for patient prognosis. Additionally, understanding the nature of the agent may guide specific public health interventions, for instance mosquito-borne diseases can be prevented by environmental control measures, personal awareness and, for some diseases, vaccination. Recently, a number of emerging diseases that may present with an encephalitis syndrome have been recognized including: West Nile virus (in Australia the Kunjin clade has been reported), lyssaviruses including Australian bat lyssavirus (ABLV) and European bat lyssavirus, Henipah viruses (Hendra and Nipah virus), phlebovirus and the expanded geographical range of Japanese encephalitis [8]. Recognition of an emerging disease with encephalitic symptoms would be difficult without systematic workup of cases [5]. Achieving a confirmed diagnosis could aid in identifying new public health threats.
Obtaining robust encephalitis data is the basis for epidemiological study and surveillance. This is reliant on accurate case ascertainment from within a population inevitably containing numerous encephalitis mimickers. Here we report a pilot study undertaken in 2012–2013 in northern New South Wales, Australia to develop sentinel surveillance for adult encephalitis cases using a previously reported case definition [1] to explore:
potential data sources for identifying adult encephalitis patients within a hospital setting;
the feasibility of establishing encephalitis surveillance at a sentinel site;
the practicality of using a standardized case definition, testing algorithm and review panel to improve diagnosis of encephalitis cases.
METHODS
The study was conducted over 10 months between 1 July 2012 and 30 April 2013. Adult patients with suspected encephalitis attending the John Hunter Hospital, Newcastle, Australia were recruited into the study. This is the principal neurology referral facility for much of northern New South Wales which has a population of approximately 875 000 people.
The recruitment case definition was:
patient hospitalized at the John Hunter Hospital;
age ⩾18 years;
-
suspected encephalitis, consisting of acute febrile illness, with:
altered behaviour/consciousness, or
new onset seizures, or
new focal neurological signs.
Case ascertainment consisted of three non-independent approaches:
Infectious disease, neurology, intensive care and emergency department clinicians were advised of the project and encouraged to report potential encephalitis cases to the researchers using a standardized form.
A daily electronic search was conducted for hospital admissions with presenting symptoms compatible with encephalitis based on the International Statistical Classification of Diseases 10 (ICD-10) coding, as described by Huppatz et al. [2].
A daily alert list captured laboratory requests for herpes simplex virus (HSV) polymerase chain reaction (PCR) testing on cerebrospinal fluid (CSF) specimens.
Electronic medical records were available to review treatment, diagnostic procedures, results and discharge notes.
Following an extensive literature search, and in consultation with subject matter experts, a structured diagnostic management approach was developed, based on the study by Granerod et al. [1] and tailored to Australian conditions (Tables 1 and 2). The agents included were those likely to present as either meningo-encephalitis or encephalitis, but they may also occur in cases of meningitis.
Table 1.
First tier pathology tests
| Aetiology | Specimen | Test request | Comments |
|---|---|---|---|
| Bacteria (β-haemolytic streptococci, Haemophilus influenzae, Listeria monocytogenes, Neisseria meningitidis, Streptococcus pneumoniae) | CSF | Routine culture, cell count, Gram stain, protein, glucose, lactate | N. meningitidis PCR if clinically indicated |
| Cryptococcus | CSF | Antigen detection | |
| EBV, CMV, HHV6, HHV7 | CSF | PCR | If HSV1/2 negative |
| Enterovirus | CSF | Enterovirus PCR | |
| HIV | Blood | Antibody detection | |
| HSV1/2, VZV | CSF | PCR | |
| RRV, BFV, MVE, Kunjin, JEV | Paired sera | Antibody detection | |
| NMDAR and VGKC receptor antibody | CSF | Antibodies | |
| Stored specimens | Serum, CSF, throat, faeces | Store | Bank ~5 ml blood |
BFV, Barmah Forest virus; CMV, cytomegalovirus; CT, computerized tomography; EBV, Epstein–Barr virus; HHV, human herpes virus; HSV, herpes simplex virus; JEV, Japanese encephalitis virus; MRI, magnetic resonance imaging; MVE, Murray Valley encephalitis; NMDAR, Anti-N-methyl d-aspartate receptor; PCR, polymerase chain reaction; RRV, Ross River virus; VGKC, voltage-gated potassium channels; VZV, varicella zoster virus.
Table 2.
Second tier pathology tests
| Aetiology | Specimen | Test request | Comments |
|---|---|---|---|
| Secondary bacteria including: Borrelia burgdorferi, Bartonella henselae, Brucella species, Leptospira species, Mycobacterium tuberculosis, Tropheryma whipplei | CSF | PCR | |
| Chlamydia, Chlamydophila | CSF | PCR | |
| Mycoplasma pneumoniae | CSF | PCR | |
| Rickettsia, Coxiella | CSF | PCR | |
| Other bacteria | 16S rRNA gene PCR | ||
| Parastrongylus (Angiostrongylus) cantonensis | CSF | CSF and peripheral eosinophilia, microscopy of CSF, | Travel history |
| Blood | Serology | ||
| Toxoplasma | CSF | PCR | If radiology indicates |
| Adenovirus | CSF | PCR | |
| Influenza | CSF | PCR | |
| Lyssavirus (ABLV) | CSF, saliva | PCR, antibodies | |
| Mumps, measles, Hendra virus | CSF, blood, urine | PCR, serology | |
| Parvovirus B19 | CSF | PCR | |
| RSV | Throat swab (CSF if positive) | PCR | |
| Rubella | CSF | PCR | Paired sera |
| Other viruses | CSF | Viral culture, SCID mice (PC3 laboratory) | Sequencing and identification by BLAST |
| Brain/post mortem tissue | |||
| Viruses | Unfixed brain tissue | Viral culture, histochemistry/ immunohistochemistry | |
| ADEM | Brain tissue | Histology | MRI |
ABLV, Australian bat lyssavirus; ADEM, acute disseminated encephalomyelitis; BLAST, Basic Local Alignment Search Tool; EEG, electro-encephalography; RNA, ribonucleic acid; RSV, respiratory syncytial virus; SCID, severe combined immunodeficiency.
Diagnostic testing for clinical purposes was initiated by the attending medical officer according to accepted hospital practice. In addition, when the patient was identified to researchers through the daily surveillance system, supplementary pathology testing was encouraged according to Table 1. Neurology research nurses liaised with medical staff to obtain case information and encourage pathology testing, according to the recommended lists (Tables 1 and 2), and assisted in the exclusion of patients with a condition mimicking encephalitis. The patient or an accompanying person, where the patient was incapacitated, was asked to provide case history details including a travel history, information on animal exposures, outdoor activities and symptoms.
Once possible encephalitis cases were identified through the recruitment case definition, a more rigorous study case definition was applied by a review panel consisting of neurology, infectious disease, epidemiology, pathology and public health specialists, and the attending medical officer. The review panel met monthly and members were provided with an extract of the patient's past medical history, discharge summary, laboratory results, radiological findings, Glasgow Outcome Score and other relevant details to enable them to determine study eligibility and determine a final diagnosis. Additionally, when clinically indicated, the panel offered advice on further testing.
The study case definition was:
- Clinical features: altered level of consciousness persisting for >24 h and including lethargy, irritability or a change in personality and behaviour. With ⩾2 of the following:
- fever or history of fever (⩾38 °C);
- seizures and/or focal neurological findings (with evidence of brain parenchyma involvement);
- CSF pleocytosis (>4 white blood cells/μl when the white cell:red cell ratio was ⩾1:500);
- electroencephalogram findings compatible with encephalitis;
- abnormal results of neuroimaging in keeping with encephalitis.
Exclusions, as determined by the review panel, included any of the following: patient not admitted to hospital; no CSF collected; history of prior neurological impairment, epilepsy, or surgery; syncope; or evidence of a transient ischaemic attack or stroke.
When a diagnosis was achieved to the satisfaction of the attending clinician and review panel, further testing was discontinued.
Using the review panel final diagnosis as the ‘gold standard’, the sensitivity, specificity and positive predictive value (PPV) were calculated for the three surveillance methodologies – clinician reporting, ICD-10 admission coding, and laboratory HSV alert list. A case definition or surveillance method is sensitive if it identifies all cases of a disease or condition, and is specific if it excludes individuals without the disease. The PPV is the proportion of reported cases that actually have the health-related event under surveillance [9, 10]. An earlier retrospective clinical audit, at the same hospital as the pilot study, examining primary discharge ICD-10 codes for encephalitis, documented that ‘more than 85% of cases had no specific aetiology confirmed’ [5]; hence the use of discharge coding as the comparator ‘gold standard’ in preference to review panel diagnosis was considered inappropriate.
Pathology screen
Standard investigations at the recruitment hospital for encephalitis presentations include CSF collection for cell count, Gram stain, direct examination, culture, biochemical analysis (glucose, protein and lactate) and testing for HSV. In our study, if pathology tests requested at admission failed to identify a causal agent, first tier pathology tests (Table 1) were offered. Agents that predominantly cause meningitis but occasionally result in encephalitis were included in the first tier testing regimen to cover possible alternative diagnoses. When a causal agent was not identified through these tests the consultant infectious diseases physician or review panel made additional testing recommendations based on the second tier of pathology tests (Table 2) and known risk factors. Additionally, specimens were stored for future testing, including a sample of ‘acute-phase’ serum to enable demonstration of seroconversion if indicated.
Ethics approval
This research was approved by the Hunter New England Health Research Ethics Committee, reference number 12/05/16/5·01. Individual patient consent was not required as the study was classified as ‘best clinical practice’ and all suspected encephalitis patients, who met the recruitment case definition, were included.
RESULTS
An average of 13 patient extracts were received daily from the ICD-10 admission search and 1·3 from the laboratory alert list. Searches included patients who were outside of the study frame, such as paediatric cases. Each patient was then checked in the health service's ‘Clinical Applications Portal’ for admission diagnosis, presenting features and laboratory results. Determining whether a patient would meet the recruitment case definition varied considerably in time depending on the presenting features and completeness of information. This process took an average of 7 min per patient. As there were ten times the number of patients identified by the admission search than by the laboratory alert the process was tenfold more time-consuming.
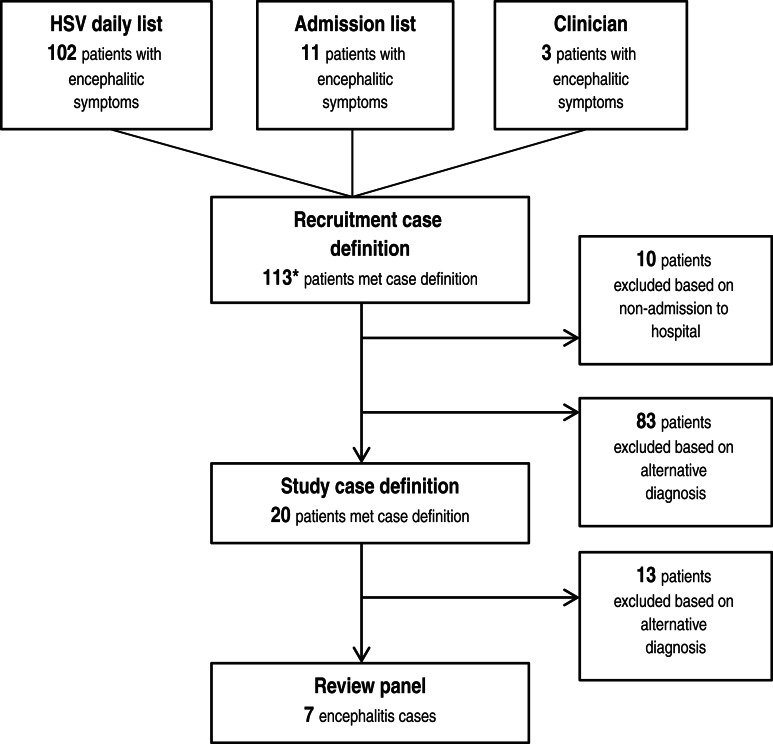
Screening was conducted on 113 patients who met the recruitment definition (Fig. 1). Of these, 102/113 (90%) were identified from the daily laboratory HSV alert list, 11/113 (10%) were found by the ICD-10 daily admission search (two were found by both methods) and 3/113 (3%) were reported directly by the clinician, although one of these was also on the laboratory list. Of these patients, 83/113 (73%) had an alternative diagnosis identified in the medical notes and in consultation with the attending clinician. Admissions with presentations initially suggestive of encephalitis included: viral meningitis (18/83), post-ictal state (10/83), toxic/metabolic conditions(11/83), subarachnoid haemorrhage (3/83), bacterial sepsis (3/83), other non-infectious neurological diseases (25/83), multi-system failure (9/83), and other conditions of head and neck (4/83). Care was taken to ensure these conditions were not a manifestation of encephalitis symptoms. Overall 10/113 (9%) were excluded as they did not meet the case definition and were not admitted to hospital [five cases of headache for investigation and one case each of viral meningitis (not proven), anti-phospholipid syndrome, head injury, shortness of breath/chest infection, dizziness]. Twenty (18%) of 113 met the study definition as potential encephalitis cases and were carefully reviewed. This resulted in seven encephalitis cases (7/113, i.e. 6% of those recruited) that met the study definition as determined by the review panel (Table 3).
Fig. 1.
Recruitment of encephalitis cases into study. *A small number of patients were recruited through multiple sources (see text).
Table 3.
Encephalitis cases identified through different surveillance methods and case definitions, including sensitivity, specificity and positive predictive value (PPV)
| Method | Recruitment case definition | Study case definition | Review panel | Sensitivity % (95% CI) | Specificity % (95% CI) | PPV |
|---|---|---|---|---|---|---|
| HSV daily list | 102 (90%) | 19 (95%) | 6 (86%) | 86% (60–112) | 9% (4–15) | 6% |
| ICD-10 daily admission list | 11 (10%) | 1 (5%) | 1 (14%) | 14% (12–40) | 91% (85–96) | 9% |
| Clinician reporting | 3 (3%) | 1 (5%) | 1 (14%) | 14% (12–40) | 98% (96–101) | 33% |
| Total* | 113 | 20 | 7 | − | − | − |
CI, Confidence interval.
A small number of patients were identified through multiple sources (see text), hence totals may not summate.
The laboratory HSV alert list picked up all but one of the cases meeting the study case definition as determined by the review panel (a case of Neisseria meningitidis infection who presented with encephalitic symptoms was missed as exclusion of HSV was not deemed necessary by the neurologist). Table 4 demonstrates that the HSV list had high sensitivity [86%, 95% confidence interval (CI) 60–112] but low specificity (9%, 95% CI 4–15). The ICD-10 daily admission list had low sensitivity (14%, 95% CI 12–40) and high specificity (91%, 95% CI 85–96). Reporting by clinicians had low sensitivity (14%, 95% CI 12–40) and the highest specificity (98%, 95% CI 96–101) of all surveillance methods. All methods had a low PPV, the highest was with clinician reporting at 33%.
Table 4.
Diagnoses of encephalitis and non-encephalitis cases meeting study case definition (n = 20), as determined by review panel
| Diagnosis | Count |
|---|---|
| Diagnoses of encephalitis cases meeting study case definition criteria | |
| Neisseria meningitidis | 1 |
| Herpes simplex virus | 3 |
| Varicella zoster virus | 1 |
| Anti-N-methyl d-aspartate receptor antibody | 2 |
| Total | 7 |
| Diagnoses of non-encephalitis cases meeting study case definition criteria | |
| Post-ictal generalised seizure | 1 |
| Primary brain carcinoma | 1 |
| Dementia and unrelated febrile illness | 1 |
| Herpes zoster ophthalmicus | 1 |
| Cerebral malaria | 1 |
| Ulcerative colitis with deranged blood sugar levels | 1 |
| Ascending cholangitis | 1 |
| Secondary brain carcinoma | 1 |
| Acute psychosis in known mental health patient | 2 |
| Cerebrovascular incident | 1 |
| Sub-arachnoid haemorrhage | 1 |
| Varicella zoster meningitis | 1 |
| Total | 13 |
Thirteen cases who met the study case definition were subsequently excluded by the review panel with alternative diagnoses (Table 3). Two cases detected through the recruitment case definition were found to have an acute infectious aetiology (VZV and Plasmodium falciparum) but did not meet the case definition, more closely resembling meningitis. Conversely, a patient with N. meningitidis infection was accepted as an encephalitis case because of significant neurological changes consistent with parenchymal involvement.
DISCUSSION
Three surveillance approaches were employed to prospectively identify patients with encephalitis and 20 possible cases were identified over the 10-month study period. Taking into account the small numbers of possible and confirmed encephalitis cases in this pilot study, while no single system had a high sensitivity, specificity and PPV, the laboratory HSV list had a high sensitivity and proved to be a simple tool through which to collect data on potential encephalitis cases. The application of the recruitment case definition to the daily ICD-10 admission search was labour intensive, and only one of the confirmed encephalitis cases was identified by this method. While clinician reporting had a low sensitivity (14%) it is possible that the clinicians were aware that potential encephalitis cases had already been identified by other reported surveillance methods, and so did not notify. We did not attempt to identify why more encephalitis cases were not reported by clinicians directly as we were investigating sustainable data sources. When the study definition was rigorously applied by the review panel, only seven cases of encephalitis were identified: five with an infectious aetiology and two immune-mediated. Achieving a 100% diagnostic rate is unusual but may be explained by the small sample size, inclusion of a raised CSF white cell count in the case definition, encouraging thorough laboratory testing and involvement of a specialist review panel. As mentioned, there are limited options for treating encephalitis; however, specific medications are available for the majority of cases identified in our study adding weight to the value of pursuing a confirmed laboratory diagnosis.
The use of a two-step case definition followed the method employed by Granerod et al. [1]. This improves the specificity of the surveillance system by screening out patients with cerebral dysfunction that is not encephalitis, e.g. intoxication, electrolyte imbalance, trauma, stroke, psychosis, liver or renal disease, hypoxia, malignancies, and non-encephalitic sepsis. It is possible that a small proportion of these presentations masked an underlying encephalitis case which may possibly only be recognized through astute clinical judgment. A prospective surveillance system must consider the management of encephalopathy cases in its design. We excluded patients who were not admitted or did not warrant a lumbar puncture as this suggested minor symptoms outside of the case definition of an acute illness with altered behaviour/consciousness. On rare occasions a lumbar puncture is clinically contraindicated and these patients would be missed by a laboratory-based surveillance system. CSF examination is one of the most helpful diagnostic tools and is highly recommended for all cases of suspected encephalitis. If lumbar puncture is not possible at presentation it may still provide valuable diagnostic information if CSF is collected later in the disease process, when intracranial pressure has reduced, although sensitivity may be compromised by empirical treatment.
Retrospective data collection using discharge coding has been used to inform the epidemiology of encephalitis [2, 11]; however, the delay between admission and coding makes this impractical for recognition of disease clusters and timely public health interventions. It also fails to address the weakness of a low rate of confirmed diagnosis.
Without the benefit of an exhaustive clinical, radiological and laboratory workup the ability to achieve a diagnosis for accurate surveillance is limited and encephalitis is grossly under-reported [12]. Identifying suspected cases, applying a case definition and confirming the diagnosis are pivotal in obtaining reliable epidemiological information [12]. It is evident that developing an accurate definition for encephalitis that can be consistently applied is not straightforward. This could help explain the high proportion of ‘encephalitis’ cases for which no aetiology is identified.
Limitations of this study included small case numbers restricting the usefulness of extrapolating the findings to a broader setting. The surveillance methodologies were not independent and we could not exclude the possibility that clinicians did not report because they believed that cases had already been recruited by other methods. The use of a review panel, in preference to discharge coding, for final diagnosis as the gold standard may have affected study results.
Research nurses liaised with researchers and clinicians to ensure the correct specimens were collected and pathology tests completed. Without this assistance, it is unlikely that encephalitis surveillance at sentinel sites would be feasible as doctors may not always pursue a definitive diagnosis. As the study progressed, and clinicians became more aware of our interest in encephalitis cases, we found that infectious disease physicians were more likely to be consulted and were directly involved in achieving a diagnosis in two cases.
The cost of performing a comprehensive diagnostic test panel on suspect cases needs to be balanced against the potential reduction in morbidity and mortality achieved through targeted treatment and the opportunity of rapidly instituting effective public health interventions where indicated [5]. Since implementation of this study, an international encephalitis consortium has published diagnostic algorithms [12] that include experiences gained from this study and a complementary Australian paediatric study (P. Britton & C. A. Jones, personal communication). Australian guidelines have been accepted for publication and should assist doctors achieve a diagnosis which will aid data accuracy. We encourage the development of guidelines tailored to defined geographical areas to ensure locally endemic disease agents are given appropriate consideration.
Recognition of highly pathogenic emergent infectious diseases such as infection with ABLV and Japanese encephalitis virus in Australia; and West Nile virus infection in Europe and North America warrant appropriate surveillance. In Australia, ABLV has caused three known deaths but testing is rarely considered by clinicians unless there is an explicit history of bat exposure. As a result, it is likely that ABLV and other causes of encephalitis are under-diagnosed [8], a situation that could be improved if clinicians adhere to a systematic testing approach as described here [13]. It is recognized that achieving a laboratory-confirmed diagnosis is often challenging and a range of variables interplay such as the timing of specimen collection, specimen site and test method. In addition, the identification of a microorganism per se is not necessarily proof of causality [14].
Autoimmune encephalitis is now well recognized and the finding of two cases in our relatively short study time-frame is not surprising. Other autoantibodies besides those listed in the tables should be considered if clinically indicated [15].
Our experiences in conducting encephalitis surveillance identified the following challenges that would impact on case ascertainment, particularly if encephalitis notifications relied on clinicians unfamiliar with the surveillance complexities of this condition:
Difficulty separating patients with encephalitis from patients with meningitis or encephalopathic presentations.
Consistent application of an internationally accepted case definition and case workup.
Access to a group of specialist clinicians willing to review potential cases.
Encephalitis causes significant morbidity and mortality but substantial gaps remain in our understanding of its aetiology and epidemiology. This pilot study suggests that effective case ascertainment can feasibly be achieved through monitoring CSF HSV test requests and that a systematic approach to achieving a diagnosis is possible through a tiered testing regimen. The use of a review panel to arbitrate on case eligibility is resource-intensive but strengthens data quality. Data collection through sentinel sites may be possible but is unlikely to be sustainable without additional resourcing and is not without challenges.
ACKNOWLEDGEMENTS
We are grateful for the assistance of research officers Gemma Kitsos, Lara Kaauwai, Erin Kerr, Kristy Morris and Angela Royan. Thanks to members of the Microbiology Laboratory of Pathology North–Hunter New England, Newcastle, NSW, especially Dr Stephen Graves and Shaunus Brousek for assistance with laboratory assays. We thank Dr Adeniya Borire, neurologists, clinicians and patients for their cooperation.
Funding for this project was supported by a Hunter Medical Research Institute grant donated by Ann and Eric Bone. B. J. Paterson was funded as a Research Fellow through the Hunter Medical Research Institute, University of Newcastle.
DECLARATION OF INTEREST
None.
REFERENCES
- 1.Granerod J, et al. Causes of encephalitis and differences in their clinical presentations in England: a multicentre, population-based prospective study. Lancet Infectious Diseases 2010; 10: 835–844. [DOI] [PubMed] [Google Scholar]
- 2.Huppatz C, et al. Etiology of encephalitis in Australia, 1990–2007. Emerging Infectious Diseases 2009; 15: 1359–1365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Khetsuriani N, Holman RC, Anderson LJ. Burden of encephalitis-associated hospitalizations in the United States, 1988–1997. Clinical Infectious Diseases 2002; 35: 175–182. [DOI] [PubMed] [Google Scholar]
- 4.Chaudhuri A, Kennedy PG. Diagnosis and treatment of viral encephalitis. Postgraduate Medical Journal 2002; 78: 575–583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Huppatz C, et al. Should there be a standardised approach to the diagnostic workup of suspected adult encephalitis? A case series from Australia. BMC Infectious Diseases 2010; 10: 353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Granerod J, et al. Challenge of the unknown. A systematic review of acute encephalitis in non-outbreak situations. Neurology 2010; 75: 924–932. [DOI] [PubMed] [Google Scholar]
- 7.Tack DM, et al. Trends in encephalitis-associated deaths in the United States, 1999–2008. Neuroepidemiology 2014; 43: 1–8. [DOI] [PubMed] [Google Scholar]
- 8.Paterson BJ, et al. A review of the epidemiology and surveillance of viral zoonotic encephalitis and the impact on human health in Australia. NSW Public Health Bulletin 2011; 22: 99–104. [DOI] [PubMed] [Google Scholar]
- 9.Buehler JW, et al. Framework for evaluating public health surveillance systems for early detection of outbreaks: recommendations from the CDC Working Group. Morbidity and Mortality Weekly Report. Recommendations and Reports 2004; 53: 1–11. [PubMed] [Google Scholar]
- 10.German RR, et al. Updated guidelines for evaluating public health surveillance systems: recommendations from the Guidelines Working Group. Morbidity and Mortality Weekly Report. Recommendations and Reports 2001; 50: 1–35. [PubMed] [Google Scholar]
- 11.Bernard S, Mailles A, Stahl JP. Epidemiology of infectious encephalitis, differences between a prospective study and hospital discharge data. Epidemiology & Infection 2013; 141: 2256–2268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Venkatesan A, et al. Case definitions, diagnostic algorithms, and priorities in encephalitis: consensus statement of the International Encephalitis Consortium. Clinical Infectious Diseases 2013; 57: 1114–1128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Skull SA, et al. A retrospective search for lyssavirus in humans in the Northern Territory. Australian and New Zealand Journal of Public Health 1999; 23: 305–308. [DOI] [PubMed] [Google Scholar]
- 14.Granerod J, et al. Causality in acute encephalitis: defining aetiologies. Epidemiology & Infection 2010; 138: 783–800. [DOI] [PubMed] [Google Scholar]
- 15.Singh T, Fugate J, Rabinstein A. The spectrum of acute encephalitis: causes, management, and predictors of outcomes. Neurology 2015; 84: 359–366. [DOI] [PubMed] [Google Scholar]