SUMMARY
The aims of this study were to identify Staphylococcus aureus nasal colonization prevalence, behavioural risk factors, and to determine staphylococcal protein A (spa) types in community-based injection drug users (IDUs). Nasal swabs were collected and methicillin susceptibility testing and spa/SCCmec typing were performed on S. aureus isolates. Generalized estimating equations were used to report adjusted odds ratios and 95% confidence intervals. Of the 440 participants, 24·1% were colonized and 5·7% had methicillin-resistant S. aureus (MRSA). Colonization was associated with age, employment/marital status, and the presence of scabs but not with sexually transmitted disease co-infection, HIV status, antibiotic use, hospitalization, or drug treatment programme participation. The USA300 MRSA clone spa types were most common, but 15/49 spa types were new to one of the international databases. Community-based IDUs appear to have different risk factors compared to IDUs from clinical studies. In addition, the number of newly identified spa types indicates a diverse, understudied population.
Key words: Drug users, methicillin-resistant Staphylococcus aureus, prevalence, staphylococcal epidemiology, Staphylococcus aureus
INTRODUCTION
Staphylococcus aureus is a common human pathogen frequently responsible for skin and soft-tissue infections, endocarditis and septicaemia [1]. Several anatomical sites are colonized by S. aureus, including the skin, nasopharynx, and perianal region. Although the nasal mucosa represents a preferred niche, colonization can be influenced by the host's immune system, underlying health conditions, and exposure to healthcare settings [2–4]. Nasal colonization of S. aureus is epidemiologically associated with an increased risk of invasive infections, and higher rates of invasive infections as well as outbreaks have been described in specific populations including injection drug users (IDUs) [3, 5–8]. Several studies have failed to confirm an association between previously identified risk factors and S. aureus colonization in IDUs [9–12]. Currently, it remains unknown what community-based risk factors (e.g. sexual behaviours and drug-use habits) are associated with higher colonization rates. Furthermore, data regarding S. aureus nasal colonization in IDUs are inconsistent. For example, it was previously demonstrated that the S. aureus nasal colonization prevalence estimates in IDUs ranged between 20% and 61·4% [7, 9, 10, 13–16]. By contrast, nasal colonization rates (persistent/intermittent carriers) in the general population range between 20% and 30% [8, 17]. Previous studies regarding methicillin-resistant Staphylococcus aureus (MRSA) colonization rates in IDUs ranged between 2% and 29·2% compared to MRSA colonization rates of 0·4–3·8% in the general population [9, 13, 15, 18–20].
Previous colonization studies conducted in healthcare settings (rather than in community-based environments) were subject to considerable biases in the context of obtaining samples representative of IDUs, including an underrepresentation of those who do not regularly obtain healthcare and identification of clinical risk factors that may not be applicable in the community setting. Appropriate surveillance of S. aureus nasal colonization prevalence, molecular characterization of the bacterial isolates, and the identification of community-associated risk factors for a population at high risk for invasive S. aureus outbreaks is necessary to inform healthcare professionals and in identifying potential behavioural prevention methods.
We conducted a cross-sectional study by observing a subsample of participants from the National HIV Behavioral Surveillance (NHBS) System (NHBS-IDU3) in Houston, Texas. The aims of this study were to: (1) determine methicillin-sensitive S. aureus (MSSA) and MRSA nasal colonization prevalence rates; (2) determine behavioural risk factors for S. aureus nasal colonization; and (3) determine the staphylococcal protein A (spa) types of isolates prevalent in respective community-based IDUs.
METHODS
Data collection
Subjects were recruited between September 2012 and December 2012 into this cross-sectional study following completion of data collection in the NHBS-IDU3 study. NHBS is an HIV/AIDS surveillance system managed by the Centers for Disease Control and Prevention (CDC) in coordination with local health departments/universities. The objectives of this system are to monitor HIV prevalence and risk behaviours in US cities, including Houston, Texas. The surveillance system focuses on three groups at high-risk for HIV including IDUs.
NHBS utilized respondent-driven sampling (RDS), which is a sampling methodology used to access hard-to-reach populations to identify community-based IDUs [21, 22]. Initially, a sample of initial participants (i.e. ‘seeds’) was identified and approached. Eligible ‘seed’ participants received five coupons (with identification numbers) for distribution. Referrals were invited to participate if enrolment/behavioural inclusion criteria were satisfied and received three coupons upon study completion. Criteria for enrolment included: (1) presenting a valid coupon; (2) not having participated in the NHBS-IDU3 cycle; (3) age ⩾18 years; (4) lived in the area; (5) had injected (non-prescription) drugs (past 12 months); and (6) completed the interview in English/Spanish. Additional criteria for ‘seed’ participants included: (1) recruited by NHBS staff and (2) were male/female (not transgender). Behavioural eligibility requirements included: (1) having physical evidence of recent injection (e.g. fresh track marks, etc.) or (2) knowledge of drug preparation, injection, and needles/syringes. Participants were asked to provide an oral specimen for a rapid HIV-1/2 antibody test (Oraquick Advance®, OraSure Technologies Inc., USA). Confirmatory tests were performed for presumptive-positive HIV participants. Participants were included in this colonization study if consent to a one-time nasal swab was obtained. Colonization outcomes, HIV status, and NHBS survey responses were linked via identification numbers.
The NHBS-IDU3 study was conducted in the 5th ward of Houston, Texas. The project protocol was approved by the Committee for the Protection of Human Subjects at the University of Texas Health Science Center at Houston.
Microbiological processing and methicillin susceptibility testing
One nasal swab per participant was obtained and each swab was plated twice to ensure the isolation of any S. aureus present on the swab as described below. Specimens were first plated on mannitol salt agar followed by culturing the CultureSwab® LQ Stuart tips (BD, USA) in tryptic soy broth at 37 °C for 48 h. The tips were then plated on mannitol salt agar and incubated for 24 h. Presumptive S. aureus-positive colonies were identified based on morphology and mannitol fermentation. Single colonies were then streaked on both blood and tryptic soy agar plates and incubated for 24 h. A S. aureus isolate was confirmed if both the catalase and coagulase tests were positive. A total of 106 participants were positive for S. aureus. Of these, 31 participants yielded single S. aureus isolates and 75 participants yielded two isolates, thus 181 isolates were submitted for methicillin resistance testing and molecular characterization.
Susceptibility to methicillin was determined by plating 100 μl of S. aureus re-suspended in phosphate-buffered saline (PBS, pH 7·0) at an optical density of 0·44–0·54 at 600 nm on Mueller–Hinton agar plates. Following the addition of an oxacillin E-test strip (AB Biodisk, Sweden), plates were incubated 37 °C for 24 h. The minimal inhibitory concentration (MIC) was identified as the lowest concentration of bacterial inhibition. MICs ⩾4 μg/ml were identified as MRSA.
DNA extraction
Frozen stocks of all S. aureus isolates (n = 181) were used to inoculate 5 ml of tryptic soy broth and cultured at 37 °C overnight. Genomic DNA was isolated from overnight cultures using the DNeasy blood and tissue culture kits (Qiagen, Germany) using manufacturer's instructions modified to include a digestion step with Lysostaphin (10 mg/ml; Sigma-Aldrich, USA).
spa- and SCCmec typing
The X-repeat region of the protein A gene (spa) was amplified by PCR with primers spa1095u 5′-AGACGATCCTTCGGTGA and spa1517d 5′-CAGCAGTAGTGCCGTTTG as described previously, and sequenced by SeqWright Inc. (USA) with the same primers [23]. Sequence traces were edited using SeqMan Pro (DNASTAR, USA). Both the eGenomics (www.egenomics.com) and Ridom (www.spaserver.ridom.de) spa sequence databases were queried to assign spa types, and newly identified types were submitted for database inclusion. All isolates were screened for the presence of the mecA gene, and the staphylococcal chromosomal cassette mec (SCCmec) genetic element was characterized for all mecA-positive isolates by scoring the mecA gene complex (mecA class A, B, C) and ccrABC gene complexes (ccrAB1–ccrAB4, ccrC) as described previously [24].
Social network diagram
Data regarding identification numbers, network size of IDUs, and coupon numbers were exported from RDSCM to NETDraw v. 2.123 (Borgatti SP, 2002, NetDraw Software for Network Visualization, Analytic Technologies, USA).
Statistical analysis
The method of generalized estimating equations (GEE) was used to control for the lack of independence between participants [25]. Independence between participants could not be inferred due to the use of RDS. In order to estimate the variance between participants, the recruiters for IDUs were specified and the network size (number of known IDUs per participant) was used as the control variable. Bivariate GEE analyses (controlling for network size of IDUs) were conducted to determine whether marginal associations (P < 0·25) exist between colonization status and risk factor(s). To determine a multivariable model, the quasi-likelihood information criterion (QIC) with the exchangeable correlation structure was used for model selection [26]. The best-fitting model was identified with the lowest QIC value and significant covariates (P < 0·05). To control for additional confounding, gender, employment, HIV status, and antiretroviral use were included in the final multivariate model. Adjusted odds ratios, 95% confidence intervals, and P values are reported.
RESULTS
Prevalence rates of S. aureus nasal carriage
The NHBS-IDU3 enrolled 596 eligible participants and a total of 443 (74·3%) participants consented to a one-time nasal swab. Three participants did not complete the NHBS core questionnaire and were excluded. All participants included in this study (N = 440) consented to a rapid, oral HIV test. A total of 106 participants (24·1%) were positive for S. aureus. The majority of S. aureus-positive participants carried MSSA (76·4%) and 25 participants (23·6% of carriers, or 5·7% of all participants) carried MRSA (data not shown). Of the 440 participants, 7·1% were HIV positive and only one HIV-positive participant was colonized with MRSA. The majority of non-colonized individuals were HIV negative (92·5%).
NHBS-IDU3 recruitment diagram and S. aureus nasal colonization
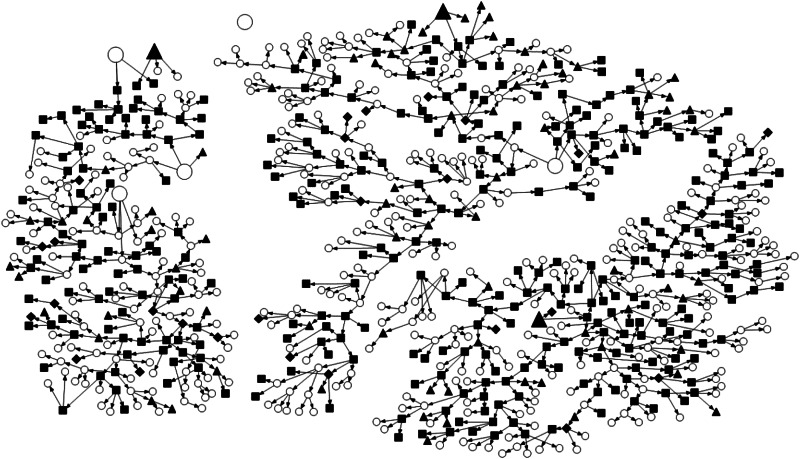
Figure 1 represents a visualization of NHBS-IDU3 recruitment and S. aureus nasal screening protocol. A highly linked IDU population with no apparent pattern regarding MRSA or MSSA colonization status was observed. All ‘seed’ participants (n = 3) in this study were nasally colonized with MSSA. The majority of linked nodes were non-colonized participants and 30·19% of colonized participants were terminal nodes.
Fig. 1.
NHBS-IDU3 recruitment chains and Staphylococcus aureus nasal colonization status. Not included in colonization study (open circle); not colonized (filled square); methicillin-sensitive Staphylococcus aureus (filled triangle); methicillin-resistant Staphylococcus aureus (filled diamond); seed participants (larger shapes).
Demographic and behavioural risk factors of S. aureus nasal carriage
The mean age of colonized participants was significantly lower than that of non-colonized participants (Table 1). The IDU population consisted mostly of males (78·9%), that identified as never married (42·5%), and 58·6% indicated their race as Black/African American. Most participants had some high school education (73·4%) and a large percentage had been unemployed (72·3%) or homeless (63·2%) at least once in their lives. A higher percentage of colonization was observed in females (25·7%), participants that were unemployed (77·4%), and those that never married (47·2%). Colonization status did not significantly differ with regards to sexually transmitted disease/hepatitis co-infection, HIV status, antibiotic use, hospitalization, or participation in a drug treatment programme (Table 1). Significant differences were observed in participants with scabs present at any body site (P = 0·023); however, the presence of abscesses did not significantly differ by colonization status.
Table 1.
Association of Staphylococcus aureus nasal colonization status and demographic and clinical risk factors in NHBS-IDU3 participants (N = 440)
| Colonized (n = 106), No. (%) | Not colonized (n = 334), No. (%) | |
|---|---|---|
| Age (mean, s.d.) | 43·73 ± 12·57* | 47·58 ± 11·13* |
| Sex Male Female |
78 (74·3) 27 (25·7) |
269 (81·0) 63 (19) |
| Race Hispanic/Latino White Black Other |
18 (17) 26 (24·5) 59 (55·7) 3 (2·8) |
54 (16·2) 64 (19·2) 199 (59·6) 17 (5·1) |
| Education Grades 1–11 Grade 12 or GED Some college or more |
36 (34) 44 (41·5) 26 (24·5) |
103 (30·8) 141 (42·2) 90 (27) |
| Employment status Employed Unemployed Not currently in the workforce |
24 (22·6)* 82 (77·4)* 0 (0)* |
80 (24)* 236 (70·7)* 18 (5·4)* |
| Marital status Married/common-law Separated Divorced Widowed Never married |
16 (15·1) 11 (10·4) 27 (25·5) 2 (1·9) 50 (47·2) |
38 (11·4) 43 (12·9) 95 (28·4) 21 (6·3) 137 (41·0) |
| History of homelessness | 63 (59·4) | 215 (64·4) |
| History of incarceration† | 37 (34·9) | 128 (38·3) |
| Any STD‡ | 8 (7·6) | 34 (10·2) |
| Hepatitis | 37 (34·9) | 109 (32·7) |
| HIV positive | 6 (5·7) | 25 (7·5) |
| Currently using antibiotics | 5 (4·7) | 27 (8·1) |
| Antiretroviral use | 2 (1·9) | 12 (3·6) |
| Hospitalized§ | 20 (18·9) | 59 (17·7) |
| Drug treatment programme† | 25 (23·6) | 57 (17·1) |
| Scabs present | 20 (18·9)* | 35 (10·5)* |
| Abscesses present | 12 (11·3) | 42 (12·6) |
GED, General education diploma; STD, sexually transmitted disease.
Some percentages may not total 100 due to rounding/missing data.
Past 12 months.
Not including HIV.
Past 6 months.
P < 0·05.
Colonization status did not differ significantly in the investigated sex- and drug-related behaviours. A high percentage of participants stated that at their last sexual encounter, unprotected vaginal sex (63·9%), and sex for money/drug exchange (40·9%) was practised. Regarding injecting practices, participants reported using heroin (87·5%) most frequently, and the majority of participants injected at least once a day (46·6%). Colonization rates were higher for participants who injected heroin (92·5% colonized vs. 85·9% not colonized); those that did not inject heroin resulted in lower rates (25·5% colonized vs. 32·9% not colonized).
GEE logistic analyses yielded 11 covariates that were marginally associated with colonization status when controlling for the size of the recruitment network and included: age, marital status, employment status, and participants that presented with scabs, gender, HIV-positive status, antiretroviral use, participation in a drug treatment programme, participation in vaginal sex, and heroin (injection and non-injection) drug use (Table 2). Of particular interest, those with scabs at any body site were more likely to be colonized with S. aureus; however, the same was not observed for individuals presenting with abscesses [adjusted odds ratio (aOR) 0·96, 95% confidence interval (CI) 0·85–1·09].
Table 2.
Exchangeable generalized estimating equations logistic regression analyses of risk factors for Staphylococcus aureus nasal colonization status controlling for size of recruitment network in NHBS-IDU3 participants (N = 437)
| aOR (95% CI) | |
|---|---|
| Age (mean, s.d.) | 0·99 (0·99–1·00)* |
| Gender Male Female |
Ref. 1·07 (0·98–1·18)† |
| Marital status Married/common-law Separated Divorced Widowed Never married |
1·04 (0·99–1·00) 0·94 (0·83–1·07) 0·96 (0·87–1·05) 0·83 (0·70–1·00)* Ref. |
| Employment status Employed Unemployed Not currently in the workforce |
0·96 (0·88–1·06) Ref. 0·78 (0·64–0·95)* |
| Any sexually transmitted disease‡ | 0·95 (0·83–1·08) |
| HIV positive | 0·90 (0·77–1·05)† |
| Currently using antibiotics | 0·92 (0·79–1·08) |
| Antiretroviral use | 0·84 (0·66–1·06)† |
| Drug treatment programme§ | 1·08 (0·97–1·19)† |
| Scabs present | 1·13 (1·00–1·27)* |
| Abscesses present | 0·96 (0·85–1·09) |
| Vaginal sex at last sex | 0·91 (0·81–1·02)† |
| Injection drug use§ Speedball Heroin¶ Cocaine|| Crystal meth Oxycontin |
1·02 (0·94–1·10) 1·10 (0·98–1·24)† 0·98 (0·89–1·09) 0·99 (0·89–1·10) 1·01 (0·90–1·13) |
| Non-injection drug use§ Marijuana Heroin¶ Cocaine|| Crystal meth |
0·98 (0·90–1·06) 0·93 (0·86–1·12)† 0·98 (0·90–1·06) 1·06 (0·96–1·18) |
aOR, Adjusted odds ratio; CI, confidence interval; GED, general education diploma.
Values represent the adjusted odds ratio, 95% CI (in parentheses), and P values. Three transgender individuals were excluded.
Not including HIV.
Past 12 months.
By itself.
Powdered, crack.
P < 0·05
P < 0·25.
Age (aOR 0·99, 95% CI 0·99, 1·00), heroin injection (aOR 1·13, 95% CI 1·01–1·27), and heroin non-injection (aOR 0·90, 95% CI 0·83–0·98) drug use was significantly associated with colonization when controlling for gender, employment status, HIV status, and antiretroviral use (data not shown).
Molecular characterization of S. aureus isolates
Of the 181 isolates, nine were non-typable in spa sequence. Of the remaining 172 isolates, a total of 49 unique spa types were observed; surprisingly, four of these types were new to the Ridom database and 15 were new to the eGenomics database. The most common spa types were t008 (25·6%, found in 26 participants), t189 (8·7%, 10 participants), t688 (5·2%, six participants), and t050 (4·7%, five participants).
Of 32 MRSA isolates with a typable SCCmec element, all were SCCmec type IV, and 30 of these 32 isolates were t008 and related spa types (Table 3). This spa-SCCmec combination is characteristic of the USA300 MRSA clone, which has been documented in nearly 36 countries and is associated with outbreaks in specific populations [27]. Known for its virulence, USA300 is currently the most prevalent community-associated MRSA in the United States and is a major source of healthcare-associated infection [27, 28]. The other two MRSA isolates with SCCmec type IV were spa t9197, which differs by a single base pair from the spa t002 typically seen in the USA100 and USA800 MRSA clones of clonal complex 5 [29–31].
Table 3.
Summary of spa types in NHBS-IDU3 participants (n = 172 isolates)
| No. of isolates | Ridom spa type |
eGenomics spa type |
eGenomics spa repeat profile | Resistance profile (no.) |
|---|---|---|---|---|
| 2 | t002 | 2 | T1-J1-M1-B1-M1-D1-M1-G1-M1-K1 | MSSA (2) |
| 44 | t008 | 1 | Y1-H1-G1-F1-M1-B1-Q1-B1-L1-O1 | MSSA (12), MRSA (32) |
| 2 | t012 | 33 | W1-G1-K1-A1-K1-A1-O1-M1-Q1-Q1 | MSSA (2) |
| 1 | t015 | 73 | X1-K1-A1-K1-B1-E1-M1-B1-K1-B1 | MSSA (1) |
| 2 | t040 | 62 | A2-A1-K1-E1-M1-B1-K1-B1 | MSSA (2) |
| 8 | t050 | 212 | X1-K1-A1-K1-B1-B1-M1-B1-K1-B1 | MSSA (8) |
| 2 | t065 | 42 | A2-A1-K1-B1-E1-M1-B1-K1-B1 | MSSA (2) |
| 2 | t084 | 21 | U1-J1-G1-B1-B1-G1-G1-J1-A1-G1-J1 | MSSA (2) |
| 4 | t091 | 111 | U1-J1-F1-M1-B1-G1-J1-A1-G1-J1 | MSSA (4) |
| 2 | t094 | 51 | U1-J1-G1-B1-B1-G1-G1-J1 | MSSA (2) |
| 2 | t121 | 245 | Y1-H1-F1-M1-B1-Q1-B1-L1-O1 | MRSA (2) |
| 5 | t148 | 193 | U1-J1-G1-F1-G1-M1-D1-M1-G1-G1-M1 | MSSA (5) |
| 4 | t160 | 141 | U1-J1-F1-Q1-P1-L1-M1 | MSSA (4) |
| 2 | t164 | 414 | U1-G2-M1-F1-B1-B1-L1-B1 | MSSA (2) |
| 2 | t185 | 108 | Z1-D1-M1-J1-D1-M1 | MSSA (2) |
| 15 | t189 | 122 | U1-J1-G1-F1-M1-B1 | MSSA (15) |
| 2 | t209 | 37 | U1-K1-G1-J1-B1 | MSSA (2) |
| 3 | t216 | 17 | Z1-D1-M1-D1-M1-N1-K1-B1 | MSSA (3) |
| 2 | t228 | 151 | I2-G1-B1-B1-G1-G1-J1-A1-G1-J1 | MSSA (2) |
| 3 | t340 | 1526* | X1-E1-M1-B1-K1-B1 | MSSA (3) |
| 5 | t359 | 92 | U1-J1-G1-F1-M1-B1-B1-P1-B1 | MSSA (5) |
| 1 | t362 | 187 | A2-B1 | MSSA (1) |
| 1 | t528 | 493 | Z1 | MSSA (1) |
| 2 | t548 | 23 | T1-J1-M1-B1-M1-D1-M1-G1-K1 | MSSA (2) |
| 1 | t571 | 109 | X1-K1-A1-O1-A1-O1-B1-O1 | MSSA (1) |
| 2 | t622 | 364 | Y1-H1-G1-F1-M1-B1-L1-O1 | MSSA (2) |
| 9 | t688 | 176 | T1-J1-M1-B1-M1-K1 | MSSA (7), MRSA (2) |
| 5 | t701 | 154 | Y1-C2-F1-M1-B1-Q1-B1-L1-O1-O1 | MSSA (5) |
| 1 | t779 | 419 | X1 | MSSA (1) |
| 1 | t967 | 810 | Y1-H1-G1-F1-M1-B1-Q1-B1-Q1-B1-L1-O1 | MRSA (1) |
| 1 | t1476 | 456 | Y1-C2-M1-B1-Q1-B1-L1-O1 | MSSA (1) |
| 2 | t2016 | 1513* | U1-J1-G1-F1-G1-U2-D1-M1-G1-G1-M1 | MSSA (2) |
| 2 | t3087 | 1536* | Z1-F1-G1-U2-D1-M1-G1 | MSSA (2) |
| 1 | t3092 | 879 | U1-J1-G1-F1-G1-M1-D1-M1-G1-M1 | MSSA (1) |
| 3 | t3169 | 152 | J1-J1-F1-G1-M1-D1-M1-G1-G1-M1 | MSSA (3) |
| 4 | t3277 | 1519* | U1-M1-F1-B1-B1-L1-B1 | MSSA (4) |
| 3 | t3914 | 1507* | U1-G2-M1-G1-F1-B1-B1-L1-B1 | MSSA (3) |
| 2 | t4214 | 1100 | I2-Z2-Z2-G1-M1-J1-H2-M1 | MSSA (2) |
| 1 | t5444 | 1544* | I2-G1-P1-L1-M1 | MSSA (1) |
| 2 | t6862 | 1531* | Y1-H1-G1-F1-M1-B1-Q1-B1-M1-B1-Q1-B1-L1-O1 | MSSA (2) |
| 1 | t8317 | 1511* | U1-J1-F1-G1-M1-D1-M1-G1-M1 | MSSA (1) |
| 2 | t9197 | 1503* | T1-J1-J5-B1-M1-D1-M1-G1-M1-K1 | MRSA (2) |
| 2 | t9987 | 1504* | T1-J1-M1-B1-M1-M1-M1-K1 | MSSA (2) |
| 2 | t 10 754 | 969 | I2-G1-G1-J1-A1-G1-J1 | MSSA (2) |
| 1 | t 12 699 | 1518* | U1-M1-D1-M1-G1-G1-M1 | MSSA (1) |
| 1 | t 13 026* | 1520* | W1-G1-G1-G1-G1-K1-A1-K1-A1-O1-M1-Q1-Q1 | MSSA (1) |
| 1 | t 13 027* | 1510* | U1-J1-F1-G1-M1-D1-M1-G1-G1 | MRSA (1) |
| 2 | t 13 028* | 1501* | A2-A1-K1-A1-K1-E1-E1-M1-B1-B1 | MSSA (2) |
| 2 | t 13 039* | 1502* | T1-J1-G1-F1-M1-E1-B1-B1-P1-B1 | MSSA (2) |
MSSA, Methicillin-sensitive S. aureus; MRSA, methicillin-resistant S. aureus.
Newly identified Ridom or eGenomics spa type.
The molecular data also allow an estimate of the number of participants that were co-colonized with more than one strain. Of the 172 isolates with typable spa sequences, 70 pairs of isolates from the same participants can be compared. Of these pairs, spa types matched in 62 pairs and mismatched in eight pairs. None of the subjects with mismatched spa types was co-colonized with MSSA and MRSA. Thus, the probability of co-colonization, defined from mismatched pairs, is 8/70 (11·4%). However, this estimate may be underestimating the true number of participants that were co-colonized since multiple bacterial colonies from each participant were not investigated.
DISCUSSION
The IDU population identified in this study characteristically consisted of middle-aged individuals of low socioeconomic status and included participants that engaged in high-risk sexual practices. Prevalent and incident HIV-positive individuals were without physical evidence of immune suppression. This study identified a significantly higher MRSA colonization rate (5·7%) compared to rates described for the general population [18–20]. Commonly identified clinical risk factors such as HIV status or antibiotic use were not associated with S. aureus colonization. However, age and heroin (injection/non-injection) drug use was significantly associated with colonization. In addition, spa typing showed a surprising diversity of new types in this population, with 15/49 types never before observed in one or the other international spa databases. On the other hand, nearly all of the MRSA were USA300 and related variants, with the exception of two isolates that were similar to MRSA clones of clonal complex 5. Previous epidemiological work has shown an association between USA300 and injection drug use [32]. The discovery of new types of S. aureus in the United States suggests the need for continued monitoring. These findings identified a highly linked population in which transmission/propagation of strains associated with outbreaks is likely. Outbreaks in IDUs have been described and concerns regarding MRSA transmission into healthcare settings remains problematical [6, 33–35].
The association of heroin (injection/non-injection) use and S. aureus nasal colonization was anticipated since several studies have observed an association between injection drug use and colonization [7, 15, 35]. However, specific drug use and the route of administration had not been previously examined with regard to colonization/infection. Gwizdala et al. found that heroin drug use (injected or snorted) was associated with an increased colonization risk (aOR 12·2, 95% CI 1·5–98·8) [9]. However, Basseti et al. observed no association between heroin use (injected or smoked) and carriage status [36]. The terms ‘drug use’ or ‘illicit drug use’ used in previous colonization studies did not differentiate between administration methods and were overly inclusive, which may contribute to the variability in studies. The elevated odds of colonization may be attributed to repetitive skin breakage, shared drug paraphernalia, and overall poor hygiene. It is unclear why non-injection heroin use was protective. The possibility that heroin or heroin drug mixtures with an antibacterial or binding interference property should be considered as a previous study determined that street heroin possessed antibacterial effects against S. aureus [37]. These risk factors appear contradictory; however, it is plausible that the act of injecting heroin itself may not cause haematogenous seeding or the initial infection. Rather, infection/colonization of the injection site following the initial puncture may account for increased colonization. Additionally, the use of antibiotics or recent exposure to healthcare settings may be confounding as described in previous studies [3, 17]. These original findings need to be replicated and further explored.
In the current study, colonized participants were younger than non-colonized participants (P = 0·002). Several studies have identified older age and the presence of wounds/scabs as significant risk factors for colonization and infection in various populations [38, 39]; however, in IDUs age had not previously been identified as a significant risk factor [15, 36]. In this study, younger participants were more likely to participate in high-risk sexual activities and reported more frequent injection drug use (IDUs aged <35 years).
The present investigation has a number of strengths including the recruitment of community-based IDUs and the use of a validated core questionnaire (previously used in the NHBS-IDU1 and NHBS-IDU2 studies) assessing sex- and drug-related behaviours. The sampling methodology used here allowed for appropriate behavioural risk factor surveillance for this hard-to-access population [21]. Some limitations of the present investigation should also be noted. Due to the cross-sectional nature of this study, identified risk factors and colonization status were assessed at one time point, therefore temporal relationships cannot be determined. Furthermore, prevalence estimates may be underestimated because colonization status was assessed with one nasal swab and secondary colonization sites were not evaluated in this study. Roughly 20% of intermittent carriers may be missed when only a single sample is obtained [12]. Moreover, testing the anterior nares as the only site of colonization may underestimate the prevalence of extranasal colonization. An additional limitation was the potential for recall bias or inaccurate recollections. To minimize bias, questions ascertaining data ‘at last sex’ or a participant's most recent encounter were analysed. Last, we were statistically limited with regards to risk factor associations and outcomes could be a result of sampling error. Although our study has limitations, to our knowledge, this study is the only community-based nasal colonization study assessing S. aureus protein A (spa) types and specific sex- and drug-related behaviours in IDUs in the United States.
A number of factors contributing to S. aureus colonization and subsequent infections in IDUs exist. The complexity of this relationship is complicated by poorly characterized sex- and drug-related behaviours. Continual epidermal trauma, lack of hygiene, limited preventative healthcare, and overall poor health are all risk factors associated with frequent, acute hospitalizations [5]. The combination of high MRSA colonization rates and circulating epidemic MRSA strains observed in our study, coupled with repeated contact with healthcare settings are especially problematical for infection control practitioners, healthcare personnel, and patients. Introduction of epidemic strains of MRSA may have detrimental health and financial consequences to other vulnerable and immunocompromised patient populations. Preventative interventions, prophylactic care for S. aureus decolonization, and aggressive treatment for infections should be examined in IDUs. Potential prevention strategies to be studied in this population include implementing clean needle-exchange programmes and education regarding behavioural risk factors. Other biomedical prevention strategies currently being implemented such as post-exposure oral prophylaxis (PEP) and pre-exposure oral prophylaxis (PrEP) have been identified as reduceing HIV incidence and may be beneficial in preventing co-morbid conditions such as S. aureus invasive infections. In order to prevent outbreaks in IDUs, further research within this hard-to-access community must be conducted.
ACKNOWLEDGEMENTS
This work was supported by the Department of Health and Human Services Health Resources and Services Administration (HRSA) Public Health Traineeship (PHS no. HP 01 182) and discretionary funds from the University of Texas Health Science Center – School of Public Health. We thank the Houston Department of Health and Human Services and the CDC for their collaboration on this project. Additionally, we thank Dr Hafeez Rehman and Dr Marcia Wolverton for their collaboration on this work.
DECLARATION OF INTEREST
None.
Supplementary material
For supplementary material accompanying this paper visit https://doi.org/10.1017/S0950268814003227.
click here to view supplementary material
REFERENCES
- 1.Gordon RJ, Lowy FD. Pathogenesis of methicillin-resistant Staphylococcus aureus infection. Clinical Infectious Diseases 2008; 46 (Suppl. 5): S350–359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Nouwen J, et al. Human factor in Staphylococcus aureus nasal carriage. Infection and Immunity 2004; 72: 6685–6688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Wertheim HF, et al. The role of nasal carriage in Staphylococcus aureus infections. Lancet Infectious Diseases 2005; 5: 751–762. [DOI] [PubMed] [Google Scholar]
- 4.Williams RE. Healthy carriage of Staphylococcus aureus: its prevalence and importance. Bacteriological Reviews 1963; 27: 56–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bassetti S, Battegay M. Staphylococcus aureus infections in injection drug users: risk factors and prevention strategies. Infection 2004; 32: 163–169. [DOI] [PubMed] [Google Scholar]
- 6.Fleisch F, et al. Epidemic spread of a single clone of methicillin-resistant Staphylococcus aureus among injection drug users in Zurich, Switzerland. Clinical Infectious Diseases 2001; 32: 581–586. [DOI] [PubMed] [Google Scholar]
- 7.Tuazon CU, Sheagren JN. Increased rate of carriage of Staphylococcus aureus among narcotic addicts. Journal of Infectious Diseases 1974; 129: 725–727. [DOI] [PubMed] [Google Scholar]
- 8.von Eiff CB, et al. Nasal carriage as a source of Staphylococcus aureus bacteremia. New England Journal of Medicine 2001; 344: 11–16. [DOI] [PubMed] [Google Scholar]
- 9.Gwizdala RA, et al. Staphylococcus aureus colonization and infection among drug users: identification of hidden networks. American Journal of Public Health 2011; 101: 1268–1276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Holbrook KA, et al. Staphylococcus aureus nasal colonization in HIV-seropositive and HIV-seronegative drug users. Journal of Acquired Immune Deficiency Syndromes and Human Retrovirology 1997; 16: 301–306. [DOI] [PubMed] [Google Scholar]
- 11.Padoveze MC, et al. Staphylococcus aureus nasal colonization in HIV outpatients: persistent or transient? American Journal of Infection Control 2008; 36: 187–191. [DOI] [PubMed] [Google Scholar]
- 12.Shet A, et al. Colonization and subsequent skin and soft tissue infection due to methicillin-resistant Staphylococcus aureus in a cohort of otherwise healthy adults infected with HIV type 1. Journal of Infectious Diseases 2009; 200: 88–93. [DOI] [PubMed] [Google Scholar]
- 13.Al-Rawahi GN, et al. Methicillin-resistant Staphylococcus aureus nasal carriage among injection drug users: six years later. Journal of Clinical Microbiology 2008; 46: 477–7479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Berman DS, et al. Staphylococcus aureus colonization in intravenous drug abusers, dialysis patients, and diabetics. Journal of Infectious Diseases 1987; 155: 829–831. [DOI] [PubMed] [Google Scholar]
- 15.Miller M, et al. Staphylococcus aureus colonization in a community sample of HIV-infected and HIV-uninfected drug users. European Journal of Clinical Microbiology & Infectious Diseases . 2003; 22: 463–469. [DOI] [PubMed] [Google Scholar]
- 16.Miller M, et al. Incidence and persistence of Staphylococcus aureus nasal colonization in a community sample of HIV-infected and -uninfected drug users. Clinical Infectious Diseases 2007; 45: 343–346. [DOI] [PubMed] [Google Scholar]
- 17.Kluytmans J, van Belkum A, Verbrugh H. Nasal carriage of Staphylococcus aureus: epidemiology, underlying mechanisms, and associated risks. Clinical Microbiology Reviews 1997; 10: 505–520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Gorwitz RJ, et al. Changes in the prevalence of nasal colonization with Staphylococcus aureus in the United States, 2001–2004. Journal of Infectious Diseases 2008; 197: 1226–1234. [DOI] [PubMed] [Google Scholar]
- 19.Kuehnert MJ, et al. Prevalence of Staphylococcus aureus nasal colonization in the United States, 2001–2002. Journal of Infectious Diseases 2006; 193: 172–179. [DOI] [PubMed] [Google Scholar]
- 20.Williamson K, et al. The prevalence of methicillin-resistant Staphylococcus aureus colonization in emergency department fast track patients. World Journal of Emergency Medicine 2013; 4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Heckathorn D. Respondent-driven sampling: a new approach to the study of hidden populations. Social Problems 1997; 44: 174–199. [Google Scholar]
- 22.Heckathorn D. Extensions of respondent-driven sampling: analyzing continuous variables and controlling for differential recruitment. Sociological Methodology 2007; 37: 151–207. [Google Scholar]
- 23.Robinson DA, Enright MC. Evolutionary models of the emergence of methicillin-resistant Staphylococcus aureus. Antimicrobial Agents and Chemotherapy 2003; 47: 3926–3934. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Zhang L, et al. Multilocus sequence typing and further genetic characterization of the enigmatic pathogen, Staphylococcus hominis. PLoS ONE 2013; 8: e66496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Zeger SL, Liang KY. Longitudinal data analysis for discrete and continuous outcomes. Biometrics 1986; 42: 121–130. [PubMed] [Google Scholar]
- 26.Pan W. Akaike's information criterion in generalized estimating equations. Biometrics 2001; 57: 120–125. [DOI] [PubMed] [Google Scholar]
- 27.Nimmo GR. USA300 abroad: global spread of a virulent strain of community-associated methicillin-resistant Staphylococcus aureus. Clinical Microbiology and Infection 2012; 18: 725–734. [DOI] [PubMed] [Google Scholar]
- 28.Diekema DJ, et al. Continued emergence of USA300 methicillin-resistant Staphylococcus aureus in the United States: results from a nationwide surveillance study. Infection Control and Hospital Epidemiology 2014; 35: 285–292. [DOI] [PubMed] [Google Scholar]
- 29.Sola C, et al. Spread of epidemic MRSA-ST5-IV clone encoding PVL as a major cause of community onset staphylococcal infections in Argentinean children. PLoS ONE 2012; 7: e30487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Williamson DA, et al. Clinical and molecular epidemiology of methicillin-resistant Staphylococcus aureus in New Zealand: rapid emergence of sequence type 5 (ST5)-SCCmec-IV as the dominant community-associated MRSA clone. PLoS ONE 2013; 8: e62020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Caboclo RM, et al. Methicillin-resistant Staphylococcus aureus in Rio de Janeiro hospitals: dissemination of the USA400/ST1 and USA800/ST5 SCCmec type IV and USA100/ST5 SCCmec type II lineages in a public institution and polyclonal presence in a private one. American Journal of Infection Control 2013; 41: e21–26. [DOI] [PubMed] [Google Scholar]
- 32.Seybold U, et al. Emergence of community-associated methicillin-resistant Staphylococcus aureus USA300 genotype as a major cause of health care-associated blood stream infections. Clinical Infectious Diseases 2006; 42: 647–656. [DOI] [PubMed] [Google Scholar]
- 33.Gilbert M, et al. Outbreak in Alberta of community-acquired (USA300) methicillin-resistant Staphylococcus aureus in people with a history of drug use, homelessness or incarceration. Canadian Medical Association Journal 2006; 175: 149–154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Saravolatz LD, et al. Methicillin-resistant Staphylococcus aureus. Epidemiologic observations during a community-acquired outbreak. Annals of Internal Medicine 1982; 96: 11–16. [DOI] [PubMed] [Google Scholar]
- 35.Saravolatz LD, Pohlod DJ, Arking LM. Community-acquired methicillin-resistant Staphylococcus aureus infections: a new source for nosocomial outbreaks. Annals of Internal Medicine 1982; 97: 325–329. [DOI] [PubMed] [Google Scholar]
- 36.Bassetti S, et al. Carriage of Staphylococcus aureus among injection drug users: lower prevalence in an injection heroin maintenance program than in an oral methadone program. Infection Control and Hospital Epidemiology 2004; 25: 133–137. [DOI] [PubMed] [Google Scholar]
- 37.Tuazon CU, Miller H, Shamsuddin D. Antimicrobial activity of street heroin. Journal of Infectious Diseases 1980; 142: 944. [DOI] [PubMed] [Google Scholar]
- 38.Davis MF, et al. Household risk factors for colonization with multidrug-resistant Staphylococcus aureus isolates. PLoS ONE 2013; 8: e54733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Lu SY, et al. Methicillin-resistant Staphylococcus aureus nasal colonization among adult patients visiting emergency department in a medical center in Taiwan. PLoS ONE 2011; 6: e18620. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
For supplementary material accompanying this paper visit https://doi.org/10.1017/S0950268814003227.
click here to view supplementary material