SUMMARY
Group B streptococcus (GBS) is an increasing cause of disease in adults. We present long-term trends in incidence of overall infections and identify characteristics of patients with GBS cellulitis, bone and joint infections. Active, population-based surveillance was conducted from 1995–2012 in three California counties and the data were analysed retrospectively. All cases had isolation of GBS from a normally sterile site. Cases of cellulitis were classified based on clinical diagnosis. GBS bone or joint infection was defined as isolation of GBS from a bone or joint or a diagnosis of osteomyelitis or septic arthritis. Medical charts were reviewed for demographic and clinical information. There were 3917 cases of GBS; the incidence of disease increased from 5·8 to 8·3 cases/100 000 persons (P < 0·001) from 1995 to 2012. In adults aged ⩾40 years, the overall incidence of GBS increased from 8·5 to 14·2 cases/100 000 (P < 0·001) persons during the study period. The incidence of cellulitis increased from 1·6 to 3·8 cases/100 000 (P < 0·001), bone infection increased from 0·7 to 2·6 cases/100 000 (P < 0·001), and the incidence of joint infection remained approximately constant at an average rate of 1·0 case/100 000. The highest incidence rates were observed in men, persons aged ⩾80 years, non-Hispanic blacks and Hispanics. Diabetes was the most common underlying condition (51·2% cellulitis cases, 76·3% bone infections, 29·8% joint infections).
Key words: Bone infections, epidemiology, joint infection, skin infections, streptococcal infections
INTRODUCTION
Group B streptococcus (GBS, Streptococcus agalactiae) emerged in the 1970s as the leading cause of neonatal sepsis and meningitis in the United States [1]. Although there has been a marked decrease in disease in infants, the incidence of GBS disease in adults has been rising for over a decade [2, 3]. Cases outside the perinatal risk period account for nearly 90% [4] of the national burden of disease, and patients in older age groups are significantly more likely to die from the infection than are infants [2, 5–8]. While clinical management guidelines have established antibiotic protocols for treatment [9, 10], there are currently no strategies in place to prevent GBS disease in adults.
Two studies suggest that invasive GBS is an increasing cause of infection in orthopaedic patients [11] and that the incidence of GBS-associated septic arthritis is rising [12]. Previously identified risk factors for invasive GBS disease include increasing age, black race, diabetes mellitus, and immune deficiency [7–8, 13]. Previous case series have identified diabetes mellitus, malignancy and chronic liver disease as risk factors for septic arthritis [14–15] and vertebral osteomyelitis caused by GBS [16]. To date, there have been no population-based studies that examine these risk factors specifically in patients with GBS infection of a bone or joint.
In this paper we explore the rate of GBS infections in adults in the Northern California counties of Alameda, Contra Costa and San Francisco. In addition, we examine the incidence of GBS soft tissue and bone infections and risk factors associated with these infections in the study population.
METHODS
Population-based surveillance
This is a retrospective study of data collected for active population-based surveillance for invasive GBS disease conducted from 1 January 1995 to 31 December 2012 as part of the Active Bacterial Core surveillance (ABCs) and the California Emerging Infections Program (CEIP), a collaboration between the Centers for Disease Control and Prevention (CDC), county health departments, the California Department of Public Health and the University of California, Berkeley School of Public Health. The ABCs methods have been described elsewhere [17]. The surveillance area included the Northern California counties of Alameda, Contra Costa and San Francisco. The total population under surveillance ranged from 2 965 009 in 1995 [representing 9·4% (2 965 009/31 493 525) of the California state population] to 3 460 180 in 2012 [representing 9·1% (3 460 180/38 041 430) of the California state population].
Demographic and clinical information was available only from 2006 to 2012 and was abstracted from medical records for cases occurring during these years. Outcome of illness was based on patient status at the time of hospital discharge; no information was collected on whether death was attributable to GBS infection and patients were not followed beyond the time of discharge. Information regarding clinical presentation, treatment and surgical intervention were not collected. Underlying conditions were similarly abstracted from medical records. Regular laboratory audits were conducted to identify cases missed by routine surveillance.
Definitions
Clinical syndromes were assigned based on physician diagnoses recorded in the patient's medical record. All cases which had GBS isolated from CSF were considered to be meningitis. Cases could be categorized into multiple syndromes, with the exception of bacteraemia without focus, which was defined as a positive blood culture result in the absence of another clinical syndrome. Disease was considered recurrent if a case patient had ⩾2 cultures positive for GBS that were performed ⩾30 days apart. Hospital-acquired disease was defined as isolation of GBS from a hospitalized patient ⩾3 days after hospital admission. Case patients were considered to be nursing home residents if they had a long-term care facility address at the time a specimen was obtained.
Race was classified in the following five categories in order to correspond with US Census categories: White, Black, American Indian or Alaskan Native, Asian or Native Hawaiian or Other Pacific Islander, or Unknown. Ethnicity was classified according to Hispanic or Latino origin as yes, no or unknown. Race and ethnicity were determined by medical record review and thus may represent self-identification or hospital staff identification.
Analytical methods
Incidence rate calculations used US Bureau of Census bridged-race intercensal population estimates as denominators for the years 1995–1999, 2001–2009, and 2011–2012 and census population estimates for the years 2000 and 2010. Incidence rates were based on Census-defined age groups. Trends over time were assessed using the χ2 test for linearity and the Cochran–Armitage test for trend. When data were not linear, the percentage change in incidence or proportion was reported. Percent change was calculated as [(later rate – baseline rate)/baseline rate] × 100. The case-fatality rate was calculated using the proportion of cases with known outcome as the denominator. Data were analysed using Stata version 12 (StataCorp., USA) and SAS version 9.3 [18].
Ethical standards
This project was reviewed and approved by the IRB committees of the California Health and Human Services Agency, Kaiser Permanente Northern California, the University of California, San Francisco, and the University of California, Berkeley.
The authors assert that all procedures contributing to this work comply with the ethical standards of the relevant national and institutional committees on human experimentation and with the Helsinki Declaration of 1975, as revised in 2008.
RESULTS
Trends over time
A total of 3917 cases of GBS disease were identified from 1995 to 2012. The median age for case-patients was 62 years (range 0–105 years); 897 (22·9%) were aged <40 years, 1395 (35·6%) of case-patients were aged 40–64 years, 865 (22·1%) were aged 65–79 years, and 760 (19·4%) were aged ⩾80 years. Of the cases, 2096 (53·0%) were male.
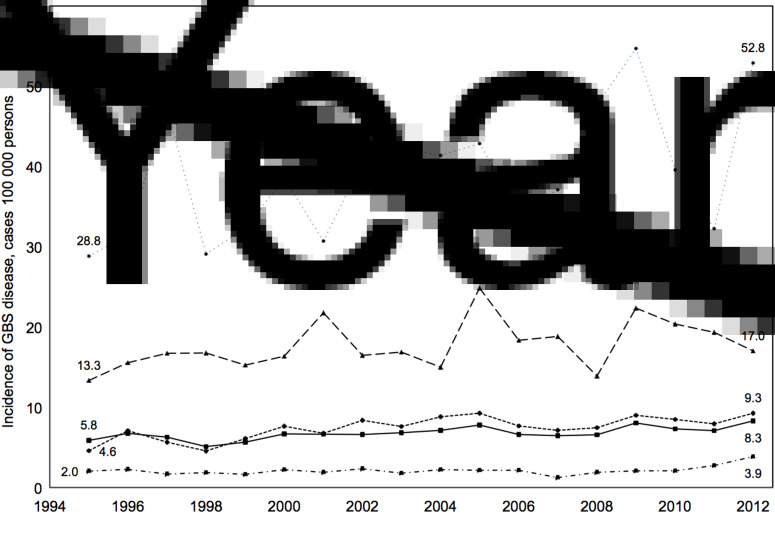
During the study period, the total incidence of GBS disease increased from 5·8 cases/100 000 persons in 1995 to 8·3 cases/100 000 persons in 2012 (P < 0·001) (Fig. 1). Between 1995 and 2012, the percent change in incidence of GBS disease in males was +50·4% and in females, +32·7%. Although overall incidence rates increased during the study period by 43%, the rate of change differed significantly between age groups. No change in incidence of GBS infection was observed in adults aged <40 years (P = 0·174), while incidence rates in age groups 40–64 (P = 0·002), 65–79 (P = 0·026), and ⩾80 (P = 0·030) years increased significantly during the study period (Fig. 1). There were no significant changes in case fatality over the study period (P = 0·154) with an average case-fatality rate of 9·5% and an incidence of 0·6 fatal cases/100 000 persons. In 2012, the case-fatality rate was 7·0% (20 deaths, 262 survivals, five unknown) with an incidence of 0·6 fatal cases/100 000 persons in the surveillance area.
Fig. 1.
Incidence of invasive group B streptococcus (GBS) disease/100 000 persons in surveillance area counties overall and by age group (1995–2012). ◾–––◾, Overall incidence; ◾· – ·◾, incidence in persons aged <40 years; • – – – – •, incidence in persons aged 40–64 years; ▴– –▴, incidence in persons aged 65–79 years; •·····•, incidence in persons aged ⩾80 years.
In addition to changes in age-specific incidence, the distribution of GBS-associated syndromes changed significantly over the study period. The incidence of meningitis in the population decreased from 0·3 to 0·1 cases/100 000 persons (P < 0·001). Of the 117 meningitis cases identified, 68 (58·1%) occurred in infants aged <7 days and 11 occurred in infants aged 7 days to 1 year. Of the remaining 38 cases of meningitis, the age distribution was as follows: 1–19 years age group (n = 1), 20–39 years (n = 6), 40–64 years (n = 21), 65–79 years (n = 8) and ⩾80 years (n = 2).
The incidence of bacteremia showed no significant change during the study period (P = 0·9). On average, there were 74 cases/year and there were 83 cases in 2012, which corresponds to 2·3 cases/100 000 persons. The number of cellulitis cases increased from 21 to 65, which resulted in a change in incidence from 0·4 to 1·2 cases/100 000 persons (P = 0·002). Osteomyelitis increased from 0·2 to 0·9 cases/100 000 persons (P < 0·001) (seven cases in 1995, 32 in 2012); and septic arthritis increased from 0·2 to 0·4 cases/100 000 persons (P = 0·01) (five cases in 1995, 12 in 2012).
Given the observed increase of specific infection types, a focused analysis of GBS-associated cellulitis and infections of bone and joint was performed in adults aged ⩾40 years.
Cellulitis, bone and joint infections in adults aged ⩾40 years: trends over time
Of adults aged ⩾40 years, 1424 GBS cases met the study criteria for cellulitis or infection of a bone or joint from 1995 to 2012. The overall incidence of GBS increased from 100 to 231 cases or 8·5 to 14·2 cases/100 000 (P < 0·001) persons during the study period. The incidence of GBS cellulitis infection increased from 1·6 to 3·8 cases/100 000 (P < 0·001), the incidence of GBS bone infection increased from 0·7 to 2·4 cases/100 000 (P < 0·001), and the incidence of GBS joint infection remained approximately constant at an average rate of 1 case/100 000 (Table 1a, Fig. 2). The proportion of GBS cases attributable to infections of cellulitis, bone or joint infection increased from 26% (26/100 cases) to 43·3% (100/231 cases) during the study period.
Table 1a.
Cases of group B streptococcus cellulitis, bone infection and joint infection by sex, age group, and race (1995–2012)
| Population | Cellulitis cases | Bone infection cases | Joint infection cases | |||||
|---|---|---|---|---|---|---|---|---|
| Year | 1995 | 2012 | 1995 | 2012 | 1995 | 2012 | 1995 | 2012 |
| Total (age ⩾40 years) | 1 320 186 | 1 628 722 | 19 | 61 | 8 | 13 | 13 | 15 |
| Male (age ⩾40 years) | 618 790 | 783 785 | 11 | 35 | 5 | 8 | 8 | 8 |
| Female (age ⩾40 years) | 701 396 | 844 937 | 8 | 26 | 3 | 5 | 5 | 7 |
| 40–64 years | 826 556 | 1 186 403 | 6 | 29 | 2 | 6 | 6 | 8 |
| 65–79 years | 262 423 | 317 552 | 11 | 19 | 6 | 5 | 5 | 3 |
| ⩾80 years | 86 685 | 124 767 | 2 | 13 | 0 | 2 | 2 | 4 |
| Race (age ⩾40 years) | ||||||||
| Non-Hispanic white | 701 418 | 1 007 840 | 9 | 34 | 3 | 6 | 6 | 5 |
| Non-Hispanic black | 146 981 | 171 865 | 5 | 5 | 3 | 5 | 5 | 0 |
| Hispanic | 127 089 | 238 382 | 4 | 10 | 2 | 2 | 2 | 5 |
| Asian/Pacific Islander | 225 379 | 463 771 | 2 | 7 | 0 | 0 | 0 | 5 |
Fig. 2.
Incidence of invasive group B streptococcus (GBS) disease/100 000 persons in surveillance area counties by infection type (1995–2012). ▴ – – – – ▴, Cellulitis; ◾–––◾, bone infection; •·····•, joint infection.
The incidence of infection was significantly higher in men than women for all infection types and during all years of study. The largest increases in incidence infection for all three infection types were seen in persons aged ⩾80 years. Within this age group, the incidence of cellulitis increased from 2·3 to 10·4 cases/100 000, bone infection from 0 to 2·3 cases/100 000, and joint infection from 2·3 to 3·2 cases/100 000 persons. The incidence of cellulitis and bone infection increased in adults aged ⩾40 years; joint infection increased only in persons aged ⩾80 years and decreased in those aged 40–79 years (Table 1b).
Table 1b.
Incidence rates (per 100 000 persons) of group B streptococcus cellulitis, bone infection and joint infection by sex, age group, and race (1995–2012)
| Cellulitis | Bone infection | Joint infection | |||||||
|---|---|---|---|---|---|---|---|---|---|
| Year | 1995 | 2012 | P | 1995 | 2012 | P | 1995 | 2012 | P |
| Total (age ⩾40 years) | 1·6 | 3·8 | <0·01 | 0·7 | 2·4 | <0·01 | 1·1 | 0·9 | 0·8 |
| Male (age ⩾40 years) | 1·8 | 4·5 | 0·01 | 0·8 | 3·7 | <0·01 | 1·3 | 1·0 | 0·6 |
| Female (age ⩾40 years) | 1·1 | 3·1 | 0·01 | 0·4 | 0·9 | 0·2 | 0·7 | 0·8 | 0·8 |
| 40–64 years | 0·7 | 2·4 | <0·01 | 0·2 | 2·0 | <0·01 | 0·7 | 0·7 | 0·8 |
| 65–79 years | 4·2 | 6·0 | 0·3 | 2·3 | 1·9 | 0·7 | 1·9 | 0·9 | 0·3 |
| ⩾80 years | 2·3 | 10·4 | 0·02 | 0·0 | 5·6 | 0·04 | 2·3 | 3·2 | 0·7 |
| Race (age ⩾40 years) | |||||||||
| Non-Hispanic white | 1·3 | 3·4 | <0·01 | 0·4 | 1·6 | 0·02 | 0·9 | 0·5 | 0·4 |
| Non-Hispanic black | 3·4 | 2·9 | 0·8 | 2·0 | 3·5 | 0·4 | 3·4 | 0·0 | <0·01 |
| Hispanic | 3·1 | 4·2 | 0·6 | 1·6 | 2·5 | 0·5 | 1·6 | 2·1 | 0·7 |
| Asian/Pacific Islander | 0·9 | 1·5 | 0·5 | 0·0 | 1·1 | 0·1 | 0·0 | 1·1 | 0·1 |
The highest incidence rates for all infection types were observed in non-Hispanic blacks and Hispanics. Cellulitis increased in all ethnic and racial groups between 1995 and 2012, while bone infection increased only in non-Hispanic whites and non-Hispanic blacks, and remained constant in Hispanics. The rate of joint infection decreased in non-Hispanic whites and non-Hispanic blacks, but increased in Hispanics and Asians/Pacific Islanders.
Cellulitis, bone and joint infections: descriptive epidemiology, 2006–2012
From 2006 to 2012, 334 cases of cellulitis and 330 cases of invasive GBS infections of bone or joint were identified. Of these, 208 cases were infections of a bone and 128 cases were infections of a joint; six cases had infections of both bone and joint.
Of the 165 cases with a clinical diagnosis of osteomyelitis, 99 (60%) had GBS isolated from bone, 60 (36%) from blood, and six from a joint (4%). Of the 86 cases with a clinical diagnosis of septic arthritis, 63 (73%) had GBS isolated from a joint, 21 (24%) from blood, and two (2%) from bone.
Most case-patients (Table 2). were hospitalized (95·2% cellulitis cases, 85·8% bone infections, 83·5% joint infections) and the median length of hospital stay was 8 days. Of cases between 2011 and 2012, 8·6% were admitted to the intensive care unit (ICU); data regarding ICU admission were not collected prior to 2011. In cases of bone infection, 20·0% met criteria for hospital onset infection as opposed to 9·1% of joint infections and 3·3% of cellulitis cases. Rates of recurrent infection were similar between infection types (7·8% of cellulitis cases, 5·3% of bone infections, 4·1% of joint infections).
Table 2.
Characteristics of patients with group B streptococcus infection associated with cellulitis, bone infection and joint infection, in those aged ⩾40 years, 2006–2012
| Cellulitis | Bone infection | Joint infection | |
|---|---|---|---|
| (N = 334) | (N = 190) | (N = 121) | |
| n (%) | n (%) | n (%) | |
| Male sex | 187 (56·0) | 143 (75·3) | 79 (65·3) |
| Race | |||
| White | 204 (61·1) | 75 (39·5) | 68 (56·2) |
| Black | 38 (11·4) | 45 (23·7) | 14 (11·6) |
| Hispanic | 33 (9·9) | 35 (18·4) | 17 (14·0) |
| Asian/Pacific Islander | 28 (8·4) | 14 (7·4) | 11 (9·1) |
| American Indian | 4 (1·2) | 2 (1·1) | 0 (0) |
| Unknown | 27 (8·1) | 19 (10·0) | 21 (17·1) |
| Death | 8 (2·4) | 2 (1·1) | 4 (3·3) |
| Hospitalization | 318 (95·2) | 163 (85·8) | 101 (83·5) |
| ICU admission* | 27 (8·1) | 9 (4·7) | 8 (6·6) |
| Hospital onset | 11 (3·3) | 38 (20·0) | 11 (9·1) |
| Recurrent infection | 26 (7·8) | 10 (5·3) | 5 (4·1) |
| Underlying condition | |||
| Diabetes mellitus | 171 (51·2) | 145 (76·3) | 36 (29·8) |
| Atherosclerotic vascular disease | 61 (18·3) | 26 (13·7) | 16 (13·2) |
| Cerebrovascular accident | 21 (6·3) | 12 (6·3) | 7 (5·8) |
| Cancer | 62 (18·6) | 10 (5·3) | 17 (14·0) |
| Obesity | 112 (33·5) | 28 (14·7) | 24 (19·8) |
| Renal disease/dialysis | 34 (10·2) | 30 (15·8) | 6 (5·0) |
| Immunosuppressive therapy | 28 (8·4) | 4 (2·1) | 8 (6·6) |
| Current smoker | 38 (11·4) | 30 (15·8) | 16 (13·2) |
| Alcohol use | 20 (6·0) | 18 (9·5) | 5 (4·1) |
| Intravenous drug use | 10 (3·0) | 8 (4·2) | 5 (4·1) |
* Data only collected from years 2011, 2012.
Of the cases, 64·7% of patients had ⩾1 underlying condition (Table 2). Diabetes was the most common especially in cases of bone infection (51·2% cellulitis cases, 76·3% bone infections, 29·8% joint infections), followed by obesity (33·5% cellulitis cases, 14·7% bone infections, 19·8% joint infections), smoking (11·4% cellulitis, 15·8% bone infections, 13·2% joint infections), atherosclerotic cardiovascular disease (18·3% cellulitis, 13·7% bone infections, 13·2% joint infections), renal disease (10·2% cellulitis, 15·8% bone infections, 5·0% joint infections) and cancer (18·6% cellulitis, 5·3% bone infections, 14·0% joint infections). Other common underlying conditions are given in Table 2.
DISCUSSION
The overall incidence of invasive GBS disease increased by 43% (from 5·8 to 8·3 cases/100 000 persons) in our surveillance area between 1995 and 2012. This increase was observed only in persons aged ⩾40 years. The incidence did not change significantly for persons aged 7 days to 39 years and declined for newborns aged <7 days. The decrease in GBS disease in newborns is probably due to improved implementation of the neonatal GBS disease prevention programme, promulgated in 1996 [19].
The increased incidence of invasive GBS disease in adults aged ⩾40 years reflects an increase over previously reported rates in non-pregnant adults [1, 20]. The highest incidence rates were seen in non-Hispanic blacks. Although the average incidence in black persons was nearly three times the rate in non-blacks, the difference in incidence rates between blacks and non-blacks remained constant (data not shown), which is consistent with previously observed racial disparities reported in other surveillance areas of the United States [8, 20]. It is unclear why this disparity has persisted and remained stable over time. Race may reflect differences in income, access to care, and prevalence of underlying conditions.
GBS is a growing cause of cellulitis and invasive bone infections in adults aged ⩾40 years. Although previous studies have noted an increase in GBS infections of bone, this is the first longitudinal study to examine trends of cellulitis, bone and joint infections and describe patients' characteristics. In addition to the significant increase in surveillance area incidence, the percentage of all GBS cases classified as cellulitis, bone or joint infections increased from 25·9% (45/174 total cases) to 43·3% (124/287 total cases) in adults aged ⩾40 years during the study period. Patients with any of these three infection types had a median hospitalization of 8 days and more than 80% were hospitalized. These figures indicate a growing burden of disease and suggest that the cost of caring for these patients may be substantial.
Authors of other studies evaluating trends in invasive GBS disease have speculated that the increasing burden of chronic underlying conditions, especially diabetes and obesity, may be responsible for the rising incidence of GBS disease [8, 11, 21]. Due in part to their chronic immunocompromised state [22], diabetics are known to be at increased risk for skin and soft tissue infections. In 2011, the CDC reported an estimated prevalence of diagnosed diabetes of 7·7%, 8·3% and 6·8% in Alameda, Contra Costa and San Francisco counties, respectively [23]. The percentage of patients with GBS infection of a bone and a diagnosis of diabetes was 76·3%. In patients with cellulitis or infection of a joint, the percentages diagnosed with diabetes were 51·2% and 29·8%, respectively (Table 2). These values are significantly higher than the estimated prevalence in the community and suggest that diabetes is a risk factor for invasive GBS disease in general and especially for infections of bone. Moreover, the prevalence of diabetes in California is high in older age groups, which may explain the age distribution of cases observed in this study [23]. Other causes of immunosuppression may also play a role: a case-control study identified neutropenia and recent glucocorticoid use as risk factors for GBS infection in adults [2].
The reasons for the increased incidence in bone and joint infection found in this study are not known but may be related to an increase in the prevalence of diabetes, as well as a rise in elective arthroplasty in elderly persons and those with significant underlying conditions [24, 25]. However, additional data are needed to evaluate this relationship. Another factor contributing to the increased incidence of skin infections may be the rise in obesity prevalence. Obesity is responsible for multiple changes in skin barrier function, including impaired microcirculation and poor wound healing, and may also be a risk factor for GBS infection [26].
There are several limitations to this study. It was based on longitudinal data, collected prospectively and analysed retrospectively and as such, there was no control group and it was not possible to evaluate risk factors directly. The variables assessed were based on pre-determined surveillance-driven methods and data were not collected for many factors that may be predictive of bone and joint infection (such as a recent history of trauma or arthroplasty). Similarly, a lack of data made it impossible to evaluate the role of socioeconomic factors. Denominator data for adults with specific underlying conditions in our population were not available. The lack of isolate collection for GBS in this surveillance area did not allow examination of specific bacterial strains or evaluation of patterns of antimicrobial resistance. Data on treatment were also unavailable.
This study may help describe risk factors for GBS infections of soft tissue and bone in adult patients. Additional studies are needed to distinguish independent risk factors, characterize the molecular epidemiology of adult GBS infections, and identify possible prevention strategies. Multi-serotype conjugate GBS vaccines for the prevention of perinatal GBS infection are currently in development [27]. If successful, use of such a vaccine could potentially be expanded to at-risk non-pregnant adults.
ACKNOWLEDGEMENTS
We thank the local public health and infection control practitioners and laboratorians throughout Alameda, Contra Costa and San Francisco counties for their participation in the surveillance programme. We also thank Mirasol Apostol, Pam Daily, Joelle Nadle, and Gretchen Rothrock of the California Emerging Infections Program (CEIP) for their careful review of this manuscript and CEIP surveillance staff for their assistance with data collection.
This work was supported by CDC Cooperative Agreement no. 5U50CK000201–02.
DECLARATION OF INTEREST
None.
REFERENCES
- 1.Schuchat A. Epidemiology of group B streptococcal disease in the United States: shifting paradigms. Clinical Microbiology Reviews 1998; 11: 497–513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Sunkara B, et al. Group B Streptococcus infections in non-pregnant adults: the role of immunosuppression. International Journal of Infectious Diseases 2012; 16: e182–186. [DOI] [PubMed] [Google Scholar]
- 3.Farley MM. Group B streptococcal disease in nonpregnant adults. Clinical Infectious Diseases 2001; 33: 556–561. [DOI] [PubMed] [Google Scholar]
- 4.Centers for Disease Control and Prevention. Emerging Infections Program Active Bacterial Core surveillance (ABCs) Report: Group B streptococcus. http://www.cdc.gov/abcs/reports-findings/survreports/gbs11.html. Accessed 3 June 2013.
- 5.Muñoz P, et al. Group B streptococcus bacteremia in nonpregnant adults. Archives of Internal Medicine 1997; 157: 213–216. [DOI] [PubMed] [Google Scholar]
- 6.Farley MM, et al. A population-based assessment of invasive disease due to group B Streptococcus in nonpregnant adults. New England Journal of Medicine 1993; 328: 1807–1811. [DOI] [PubMed] [Google Scholar]
- 7.Schwartz B, et al. Invasive group B streptococcal disease in adults: a population-based study in metropolitan Atlanta. Journal of the American Medical Association 1991; 266: 1112–1114. [PubMed] [Google Scholar]
- 8.Skoff TH et al. Increasing burden of invasive group B streptococcal disease in nonpregnant adults, 1990–2007. Clinical Infectious Diseases 2009; 49: 85–92. [DOI] [PubMed] [Google Scholar]
- 9.Stevens DL, et al. Infectious Diseases Society of America. Practice guidelines for the diagnosis and management of skin and soft-tissue infections. Clinical Infectious Diseases 2005; 41: 1373–1406. [DOI] [PubMed] [Google Scholar]
- 10.Osmon DR, et al. Infectious Diseases Society of America. Executive summary: diagnosis and management of prosthetic joint infection: clinical practice guidelines by the Infectious Diseases Society of America. Clinical Infectious Diseases 2013; 56: 1–10. [DOI] [PubMed] [Google Scholar]
- 11.Jenkins PJ, et al. J. Invasive group B streptococcal disease in an orthopaedic unit. Journal of Hospital Infection 2010; 76: 231–233. [DOI] [PubMed] [Google Scholar]
- 12.Binard A, et al. Group B streptococcal arthritis. Joint, Bone and Spine 2006; 73: 465–468. [DOI] [PubMed] [Google Scholar]
- 13.Phares CR, et al. Active Bacterial Core surveillance/Emerging Infections Program Network. Epidemiology of invasive group B streptococcal disease in the United States, 1999–2005. Journal of the American Medical Association 2008; 299: 2056–2065.18460666 [Google Scholar]
- 14.Nolla JM, et al. Group B streptococcus (Streptococcus agalactiae) pyogenic arthritis in nonpregnant adults. Medicine (Baltimore), 2003; 82: 119–28. [DOI] [PubMed] [Google Scholar]
- 15.Dubost JJ, et al. Streptococcal septic arthritis in adults. A study of 55 cases with a literature review. Joint Bone and Spine 2004; 71: 303–311. [DOI] [PubMed] [Google Scholar]
- 16.Solís-Garcia del Pozo J, Martinez-Alfaro E, Abad L, Solera J. Vertebral osteomyelitis caused by Streptococcus agalactiae. Journal of Infection 2000; 41: 84–90. [DOI] [PubMed] [Google Scholar]
- 17.Schuchat A, et al. Active Bacterial Core surveillance of the Emerging Infections Program Network. Emerging Infectious Diseases, 2001; 7: 92–99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.SAS Institute Inc. 2011. SAS® 9.3 System Options: Reference, 2nd edn. Cary, NC: SAS Institute Inc. (http://support.sas.com/software/93/index.html). Accessed January 2015.
- 19.Schrag S, et al. Prevention of perinatal group B streptococcal disease. Morbidity and Mortality Weekly Report 2002; 51: 1–22. [PubMed] [Google Scholar]
- 20.Sendi P, Johansson L, Norrby-Teglund A. Invasive group B Streptococcal disease in non-pregnant adults: a review with emphasis on skin and soft-tissue infections. Infection 2008; 36:100–111. [DOI] [PubMed] [Google Scholar]
- 21.Jackson LA, et al. Risk factors for group B streptococcal disease in adults. Annals of Internal Medicine 1995; 123: 415–420. [DOI] [PubMed] [Google Scholar]
- 22.Crook M. Type 2 diabetes mellitus: a disease of the innate immune system? An update. Diabetes Medicine 2004; 21: 203–207. [DOI] [PubMed] [Google Scholar]
- 23.Centers for Disease Control and Prevention. Diabetes Interactive Atlas. County-level data 2011: estimates of diabetes and its risk factors. (http://www.cdc.gov/diabetes/atlas/countydata/atlas.html). Accessed November 2013.
- 24.Cram P, et al. Total knee arthroplasty volume, utilization, and outcomes among Medicare beneficiaries, 1991–2010. Journal of the American Medical Association 2012; 308:1227–1236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Wolf BR, et al. Adverse outcomes in hip arthroplasty: long-term trends. Journal of Bone and Joint Surgery 2012; 94: e103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Yosipovitch G, DeVore A, Dawn A. Obesity and the skin: skin physiology and skin manifestations of obesity. Journal of the American Academy of Dermatology 2007; 56: 901–916. [DOI] [PubMed] [Google Scholar]
- 27.Health PT, Feldman RG. Vaccination against group B streptococcus. Expert Reviews of Vaccines 2005; 4:207–218. [DOI] [PubMed] [Google Scholar]