SUMMARY
Acute gastrointestinal illness (AGI) is an important public health priority worldwide. Few studies have captured the burden of AGI in developing countries, and even fewer have focused on Indigenous populations. This study aimed to estimate the incidence and determinants of AGI within a Batwa Pygmy Indigenous population in southwestern Uganda. A retrospective cross-sectional survey was conducted in January 2013 via a census of 10 Batwa communities (n = 583 participants). The AGI case definition included any self-reported symptoms of diarrhoea or vomiting in the past 2 weeks. The 14-day prevalence of AGI was 6·17% [95% confidence interval (CI) 4·2–8·1], corresponding to an annual incidence rate of 1·66 (95% CI 1·1–2·2) episodes of AGI per person-year. AGI prevalence was greatest in children aged <3 years (11·3%). A multivariable mixed-effects logistic regression model controlling for clustering at the community level indicated that exposure to goats [odds ratio (OR) 2·6, 95% CI 1·0–6·8], being a child aged <3 years (OR 4·8, 95% CI 1·2–18·9), and being a child, adolescent or senior Batwa in the higher median of wealth (OR 7·0, 95% CI 3·9–9·2) were significantly associated with having AGI. This research represents the first Indigenous community-census level study of AGI in Uganda, and highlights the substantial burden of AGI within this population.
Key words: Acute gastrointestinal illness, Batwa, burden of illness, diarrhoea, Indigenous health, risk factors, Uganda
INTRODUCTION
Acute gastrointestinal illness (AGI), transmitted by food, water, animals, or person-to-person contact, is a major health concern worldwide [1, 2]. AGI remains one of the top causes of morbidity and child mortality in developing nations, despite the widespread use of oral rehydration therapies and a greater comprehension of the pathogenesis of diarrhoea [1–3]. Furthermore, poor water supply, sanitation, and personal and domestic hygiene are among the greatest risk factors for death and disability adjusted life years (DALYs) in low-income countries [4]. Research on populations vulnerable to AGI has largely focused on children; studies estimate that 1731 billion cases of diarrhoea occur each year, of which half of the total attributed deaths (~700 000) were in sub-Saharan Africa [2, 3]. Yet AGI research is lacking for other vulnerable groups, such as Indigenous or minority populations. Despite an emergent scholarship of systematic and comparable population-based research on AGI in higher-income [5–18] and middle-income countries [19–21], there is limited literature on self-reported AGI for low-income countries [22], especially African countries. In high- and middle-income countries, population-based AGI research focuses primarily on under-reporting, estimating economic burden, and quantifying morbidity in a population [1, 10]. In lower-income regions and in vulnerable populations, where surveillance data are often poor or unavailable, even baseline data on population-based AGI prevalence are generally lacking.
Past and present social disadvantages experienced by some Indigenous populations can negatively impact overall health and can limit access to healthcare [23–25]. In sub-Saharan Africa, Indigenous populations experience some of the highest levels of infectious disease burden, yet negligible consideration is given to Indigenous inequalities in health [23, 26–28]. Standardized methodologies for estimating AGI burden have emerged in the scholarship, facilitating cross-country or regional comparisons [29], and recently, research from high-income regions indicated that Indigenous sub-populations may suffer from higher rates of AGI [30]. However, the use of these standardized methods for AGI burden estimates have not been extended to Indigenous populations in developing regions, and AGI research in Indigenous populations around the world is lacking. Herein, we seek to address this research gap by estimating the prevalence, incidence, and determinants of AGI within an Indigenous population in sub-Saharan Africa, i.e. the Batwa Pygmies of southwestern Uganda. Our research focuses on the Batwa of Kanungu District as a case study to contribute to the development of an evidence base to estimate the magnitude of AGI in highly vulnerable populations globally. To our knowledge, this research is the first published internationally comparable population-based estimate of self-reported AGI prevalence and determinants for Indigenous communities in Africa.
METHODS
Study population
The Batwa Pygmies of central sub-Saharan Africa are a highly marginalized population, who in the last century have been evicted from their ancestral forest homes and become landless [24, 27, 31]. The transition of the Batwa from historic hunter-gatherer forest livelihoods to settlement-based and agrarian livelihoods has been relatively unsuccessful, and the Batwa continue to experience substantially poorer health and socioeconomic status than neighbouring non-Indigenous populations in the region [23, 24, 26]. For instance, the average life expectancy at birth for the Batwa was 28 years compared to the Ugandan average of 53 years, and the Batwa under-five child mortality rate has been reported to be higher than the Ugandan average [26, 32, 33]. Furthermore, the Batwa in southwestern Uganda had higher prevalence of intestinal parasitic loads compared to their non-Indigenous neighbours [28, 34] and they reported diarrhoeal and vomiting diseases as a priority health concern within the population [24, 26].
Study design and sample
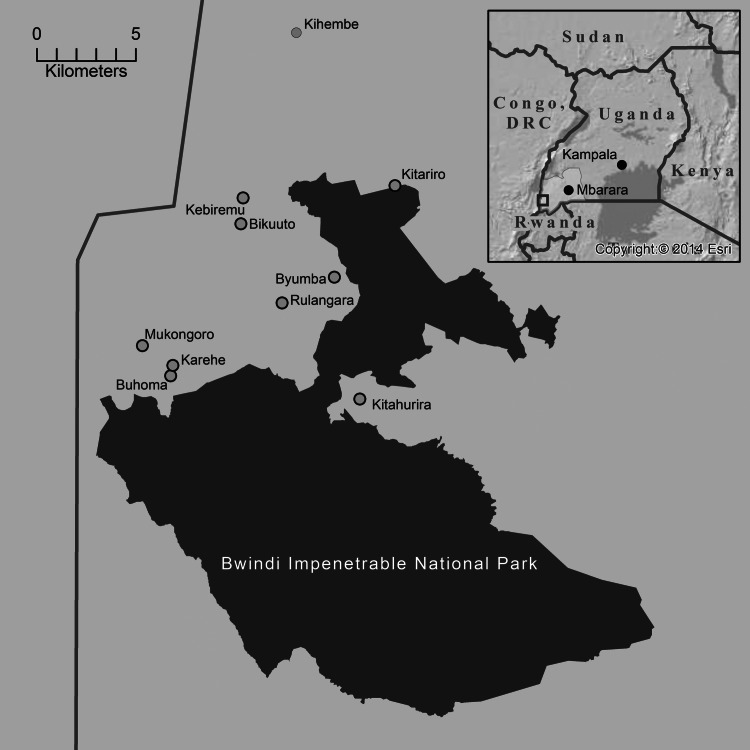
A retrospective cross-sectional, face-to-face survey was administered in 10 Batwa settlements in southwestern Uganda in January–February 2013. These 10 settlements represent the formal settlements of Batwa Pygmies in Kanungu District (1274 km2) (Fig. 1). Considering the small total population size (estimated at 750), a census was attempted of all Batwa present during the time of the survey and every individual in every household was invited to participate. The sampling frame for the study consisted of 589 people comprising all Batwa available in the settlements during the study period, of which six declined (response rate 99%). The number and composition of households in each community was verified by its respective village chairperson and household heads were asked to identify all of their co-habitants. Individuals not present in the communities at the survey time were either travelling outside the region or unavailable during the survey (e.g. in the fields, at the hospital, working elsewhere, visiting friends or relatives in the Democratic Republic of Congo). Late January in Uganda is classified as a dry to mild rainy season. Children and adolescents who attend boarding schools (primary or secondary) in neighbouring districts were on vacation and thus in their home villages during the survey.
Fig. 1.
Map of 10 Batwa communities in Kanungu District, Uganda and Bwindi Impenetrable Park.
Data collection
Respondents were interviewed in person in the predominant local language, Rukiga, by trained, local surveyors from Kanungu District. There were two questionnaires: (1) an individual-level health questionnaire for all participants of all ages (the AGI section can be viewed in the Supplementary material), and (2) a household questionnaire for the head of the household aged >18 years. The questionnaire design was based on previous international burden of AGI studies [20, 21] and adapted specifically for the cultural and geographical context of this study according to Berrang-Ford et al. [26]. Questionnaires were applied, revised, and validated with local research assistants, local partners, and the communities themselves during pilot testing in July and October 2012. The questionnaires captured data on: demographic and personal information, household information, exposure to animals, socioeconomic indicators, water sources, sanitation and hygiene, as well as occurrence of diarrhoea or vomiting in the previous 2 weeks, associated symptoms, and health-seeking behaviour. Parents or guardians answered questionnaires on behalf of children aged <12 years, while children aged 12–18 years answered questions with parental consent. Data were collected as hard copy and imported into a Microsoft Access database (Microsoft Corp., USA).
Case definition
The presence of any self-reported symptoms of vomiting or diarrhoea in the previous 14 days was used as a case definition for the outcome measure in this study. An alternative case definition, using stricter criteria proposed by Majowicz et al. [29], was used to facilitate international comparisons (⩾3 loose stools or any vomiting). A 2-week recall period was applied following pilot research and consultation with local partners regarding an appropriate, feasible, and reliable period for recall. Those with vomiting or diarrhoea were considered non-cases if participants reported the symptoms were due to any diagnosed chronic gastrointestinal illness, or diarrhoea caused by the use of medication, alcohol/drugs, or pregnancy. Since the duration of an episode of acute diarrhoea or vomiting can be short and the recall period was 2 weeks, an assumption was made that each case of acute diarrhoea contributed to only one new episode of acute diarrhoea in the preceding 2 weeks of the interview.
Statistical analysis
The 14-day prevalence of AGI was calculated as the number of identified cases fitting our case definition divided by the total number of respondents. An annual incidence rate was estimated to facilitate international comparability, as in previous AGI studies [20, 21]. Age groups (0–3, 4–12, 13–34, ⩾35) reflect the age distribution of the population – with a low average life expectancy – and natural breaks in the distribution of AGI cases by age. An asset-based index of wealth was calculated using principal component analysis; this method is recognized as a valid proxy for measuring wealth in health studies in rural Africa and elsewhere [35]. Data from participants responding ‘unsure’ or ‘refused to answer’ were excluded from the analysis of that question. We built a multivariable mixed logistic regression model with a random intercept to control for clustering at the community level. First, a series of univariable (i.e. one fixed effect) logistic regression models with a random intercept for community were conducted to explore potential unconditional associations between predictor variables and the outcome variable. Variables with P < 0·20 in univariable tests were considered for inclusion in the multivariable model. Potential collinearity between predictor variables was assessed using a Spearman's rank correlation coefficient, with a cut-off of 70%. Then, a manual backwards elimination process was used, and the best-fit model was determined by Bayesian Information Criterion (BIC) statistics of model comparison. We tested for potential confounding by removing and inserting plausible variables iteratively to assess for a change of ⩾25% in odds ratios and we tested for significant interactions between all final variables in the model. Finally, we graphically assessed the fit of the model by examining Pearson residuals, and assessing the assumptions of normality and homogeneity of variance for the best linear unbiased predictors (BLUPs). Data were analysed using Stata v. 11 (Stata Corp., USA).
Ethics
This study was approved by the McGill University Research Ethics Board and is consistent with the Canadian Tri-Council's Policies and requirements for the Ethical Conduct of Research Involving Human Subjects. Informed oral consent was obtained from all participants or the parent/guardian if the participant was a minor. This study is a part of the Indigenous Health Adaptation to Climate Change (IHACC) project, a larger international initiative with parallel field sites in the Canadian Arctic and the Peruvian Amazon (www.ihacc.ca).
RESULTS
Magnitude
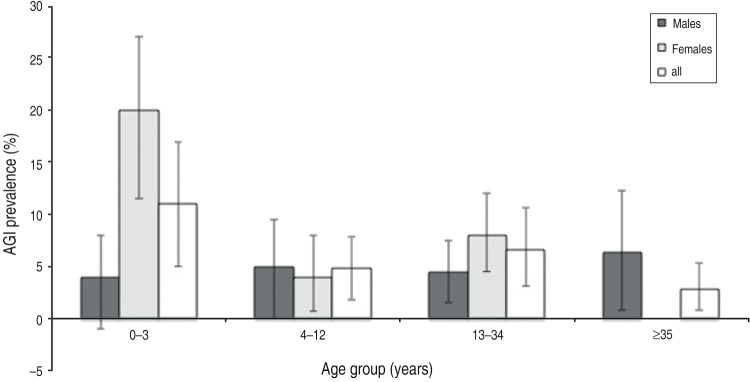
Individual-level surveys were obtained for 583 participants (99% response rate), and a total of 130 household-level surveys were linked to 553 participants (Table 1). Data are limited on the demographics of all Batwa in Kanungu. Based on available estimates, study participants reflect total Batwa population demographics, with study participants somewhat younger (mean age 20 years compared to 23 years in the total population) with more males (48·5% compared to 47% in the total population) [36]. Of the 583 individuals included in the study, 36 individuals reported having symptoms of vomiting or diarrhoea within the previous 14 days, corresponding to a prevalence of 6·17% [95% confidence interval (CI) 4·2–8·1], and an annual incidence rate of 1·66 (95% CI 1·1–2·2) episodes of AGI per person-year (52 new cases per month, 1·2 new cases per day). Using an alternative case definition [29], there were 34 cases of AGI in the previous 14 days, resulting in a prevalence of 5·83% (95% CI 4·0–8·5) and an annual incidence rate of 1·57 (95% CI 1·1–2·6) episodes of AGI per person-year. Children aged <3 years had a 14-day prevalence of 11·3% (95% CI 5·0–17·7) and the annual incidence rate was 3·13 (95% CI 1·3–5·1) cases per child per year (Fig. 2). Females had a 14-day prevalence of 7·3% (95% CI 4·3–10·2) and males, 4·9% (95% CI 2·4–7·5).
Table 1.
Results of the univariable (i.e. one fixed effect) logistic regression models, using a random intercept to control for community-level clustering for self-reported acute gastrointestinal illness in 10 Batwa settlements in southwestern Uganda, January 2013
| Characteristic | January 2013 surveyed participants (%) | No. of non-cases (%) | No. of cases (%) | OR* (95% CI) | P value* | Community- level clustering (P value)† |
|---|---|---|---|---|---|---|
| Social determinants | ||||||
| Sex | n = 582 | n = 546 | n = 36 | 0·04 | ||
| Male | 283 (49) | 269 (49) | 14 (39) | Ref. | Ref. | |
| Female | 299 (51) | 277 (51) | 22 (61) | 1·52 (0·74–3·02) | 0·30 | |
| Age group (years) | n = 581 | n = 545 | n = 36 | 0·04 | ||
| 0–3 | 97 (17) | 86 (16) | 11 (31) | 4·27 (2·14–15·99) | 0·03 | |
| 4–12 | 165 (28) | 157 (29) | 8 (22) | 1·70 (0·44–6·7) | 0·43 | |
| 13–34 | 211 (36) | 197 (36) | 14 (39) | 2·31 (0·65–8·43) | 0·21 | |
| ⩾35 | 107 (18) | 104 (19) | 3 (8) | Ref. | Ref. | |
| Highest education level in household‡ | n = 581 | n = 545 | n = 36 | 0·02 | ||
| No education | 192 (33) | 182 (33) | 10 (28) | Ref. | Ref. | |
| Primary education | 334 (58) | 312 (57) | 22 (61) | 1·20 (0·55–2·63) | 0·65 | |
| Beyond primary education | 55 (9) | 51 (10) | 4 (11) | 1·92 (0·54–6·86) | 0·31 | |
| Wealth (household assets) | n = 549 | n = 513 | n = 36 | 0·03 | ||
| Below median wealth | 274 (50) | 265 (52) | 9 (25) | Ref. | Ref. | |
| Above median wealth | 275 (50) | 248 (48) | 27 (75) | 3·12 (1·47–6·95) | <0·01 | |
| Environmental exposures | ||||||
| Use soap | n = 580 | n = 544 | n = 36 | 0·02 | ||
| No | 429 (74) | 398 (73) | 31 (84) | Ref. | Ref. | |
| Yes | 151 (26) | 146 (27) | 5 (14) | 0·44 (0·17–1·15) | 0·05 | |
| Own animals | n = 553 | n = 517 | n = 36 | 0·07 | ||
| No | 415 (75) | 394 (77) | 21 (58) | Ref. | Ref. | |
| Yes | 133 (25) | 118 (23) | 15 (42) | 2·38 (1·19–4·77) | 0·01 | |
| Exposure to goats | n = 553 | n = 517 | n = 36 | 0·07 | ||
| No | 493 (90) | 466 (90) | 27 (75) | Ref. | Ref. | |
| Yes | 58 (10) | 49 (10) | 9 (25) | 3·11 (1·3–6·91) | 0·02 | |
| Animals come inside the house | n = 553 | n = 517 | n = 36 | 0·06 | ||
| No | 478 (86) | 452 (88) | 26 (72) | Ref. | Ref. | |
| Yes | 73 (14) | 63 (12) | 10 (28) | 2·73 (1·25–5·91) | 0·01 | |
| Main water source | n = 539 | n = 505 | n = 34 | 0·13 | ||
| Standpipe/well | 319 (59) | 300 (59) | 19 (56) | Ref. | Ref. | |
| Surface or rain water | 220 (41) | 205 (41) | 15 (44) | 1·10 (0·53–2·28) | 0·78 | |
| Boil water | n = 551 | n = 515 | n = 36 | 0·05 | ||
| No | 347 (63) | 329 (64) | 18 (50) | Ref. | Ref. | |
| Yes | 204 (37) | 186 (36) | 18 (50) | 1·61 (0·83–3·40) | 0·15 | |
| Toilet facilities | n = 538 | n = 502 | n = 36 | 0·01 | ||
| Pit latrine covered | 395 (73) | 369 (74) | 26 (72) | Ref. | Ref. | |
| Pit latrine uncovered | 129 (24) | 120 (24) | 9 (25) | 1·10 (0·44–2·27) | 0·98 | |
| No facility | 14 (3) | 13 (2) | 1 (3) | 1·22 (1·14–10·3) | 0·85 | |
OR, Odds ratio; CI, confidence interval.
Odds ratios and P values generated from univariable logistic models.
P values generated from a likelihood ratio test comparing the mixed logistic regression model (using a random intercept for community-level clustering) to logistic regression model.
Highest level of education of someone in the household aged >15 years.
Fig. 2.
Fourteen-day Prevalence of acute gastrointestinal illness (AGI) in age and gender groups, in the 2 weeks prior to interview in 10 Batwa settlements in southwestern Uganda, January 2013 (n = 583).
Description of disease
Of the 36 cases of AGI, about half had symptoms of vomiting (Table 2). The mean duration of vomiting episodes lasted for 2·79 days (95% CI 1·63–3·99) with an average of 2·80 maximum episodes in a 24-h period (95% CI 1·86–3·74). Over three-quarters of the cases had symptoms of diarrhoea, and the mean duration lasted 4·48 days (95% CI 3·37–5·59) with an average of 4·26 maximum loose stools in a 24-h period (95% CI 3·53–4·98). Of the 28 individuals who experienced diarrhoea, three (12%) had blood in their stool. The most commonly experienced secondary symptoms were stomach cramps, headache, and nausea. Half of the cases reported having to miss their daily activities as a result of their illness, and six cases reported that others had to take time off of their usual routines to provide care. Over two-thirds of cases reported seeking some form of healthcare, and the most common healthcare provider sought out was a physician or nurse (Table 3). The most recurrent reason for seeking healthcare was that symptoms lasted a long time, or that symptoms included diarrhoea. Thirty-one cases (86%) sought some form of treatment – both traditional and modern – with herbal remedies being the most common, followed by painkillers. For the cases that did not seek medical care, the dominant reasons were financial difficulty and distrust of doctors or nurses (Table 3).
Table 2.
Severity of primary symptoms, and associated secondary symptoms of acute gastrointestinal illness in 10 Batwa settlements in southwestern Uganda, January 2013
| Severity | No. of cases (%) | Average (95% CI) |
|---|---|---|
| Total cases (n = 36) | ||
| Experience any vomiting | 17 (49) | |
| Duration (days) of vomiting episodes | 2·79 (1·63–3·99) | |
| Maximum number of vomiting episodes in 24 h | 2·80 (1·86–3·74) | |
| Experience any diarrhoea | 28 (80) | |
| Duration (days) of diarrhoea | 4·48 (3·37–5·59) | |
| Maximum number of loose stools in 24 h | 4·26 (3·53–4·98) | |
| Had blood in stool | 3 (12) | |
| Associated symptoms (n = 36) | ||
| Nausea | 20 (56) | |
| Stomach cramps | 24 (67) | |
| Fever | 13 (36) | |
| Chills | 17 (47) | |
| Headache | 20 (56) | |
| Fatigue | 5 (14) | |
| Sore throat | 5 (14) | |
| Cough | 10 (28) | |
CI, Confidence interval.
Table 3.
Number and percent of cases (n = 36) that reported health-seeking behaviour, treatment sought, and reasons for no treatment of acute gastrointestinal illness in 10 Batwa settlements in southwestern Uganda, January 2013
| Variable | No. of cases (%) |
|---|---|
| Sought healthcare (n = 36) | |
| Yes | 25 (69) |
| No | 11 (31) |
| Healthcare provider visited (n = 25) | |
| Physician/nurse | 23 (92) |
| Herbalist | 2 (8) |
| Factors influencing decision to seek healthcare (n = 24) | |
| Symptoms lasted a long time | 14 (58) |
| Felt sick enough to go | 8 (33) |
| Had a fever | 5 (21) |
| Had vomiting | 8 (33) |
| Had diarrhoea | 10 (42) |
| Stools were bloody | 2 (8) |
| Wanted medication | 2 (8) |
| Type of medication to treat symptoms (n = 36) | |
| Herbal remedies from a garden | 17 (47) |
| Prescription medications | 7 (19) |
| Painkillers (e.g. acetaminophen, ibuprofen) | 14 (39) |
| Antidiarrhoeal | 7 (19) |
| Rehydration therapies | 1 (3) |
| Main reason medical care was not sought (n = 11) | |
| Financial difficulty | 6 (55) |
| Physician not available in the area | 2 (19) |
| Did not get around to it | 1 (9) |
| Transportation problems | 0 (0) |
| Dislike or distrust doctors or nurses | 3 (27) |
Multivariable model
The best-fit model retained respondent age, exposure to goats, and an interaction between wealth and adult ages (13–34 years) (Table 4). The odds of AGI for Batwa aged ⩽3 years were 4·8 times higher [odds ratio (OR) 4.83, 95% CI 1·24–18·95] than for Batwa aged ⩾35 years (P = 0·02). The only environmental predictor variable significantly associated with AGI was exposure to goats (P = 0·04), with the odds of AGI being 2·6 times greater in those exposed than those not exposed (OR 2·64, 95% CI 1·04–6·76). Interaction between a binary variable (middle-aged adults 13–34 years vs. all other age groups) and wealth indicated that there was a greater odds of AGI for non-middle-aged Batwa (children, adolescents or seniors, aged <13 or >34 years) and in a household with greater assets. Specifically, for children, adolescents or seniors (aged <13 or >34 years), those with greater than median wealth had significantly (P = 0·03) higher odds of AGI (OR 7·02, 95% CI 3·95–9·20) than those with lower than median wealth. In the wealthier Batwa, the odds of AGI was significantly (P = 0·03) higher for children, adolescents or seniors (aged <13 or >34 years) compared to middle-aged adults (13–34 years) (OR 3·12, 95% CI 1·16–6·85) and the odds of AGI was significantly (P = 0·02) higher for wealthier children, adolescents or seniors (<13 or >34 years) compared to non-wealthy middle-aged adults (13–34 years) (OR 4·00, 95% CI 1·26–7·43). Variables relating to soap and sanitation were significantly associated with cases of AGI in univariable tests only, and were not retained in the final multivariable model. None of the variables pertaining to water quality, water source or water treatments were significant in the univariable tests or multivariable model (Table 1). In the final multivariable model, we found no significant difference between models that did and did not control for community-level clustering (likelihood ratio test: P = 0·068, variance 0·26, median OR 1·62); however, we forced the random intercept into the model considering the structure of our data, and there was a minimal difference in fit of the model with and without the random intercept (based on BIC values). Post-estimation diagnostics indicated that the model was a good fit for the data.
Table 4.
Final multivariable mixed-effects logistic regression model using a random intercept to control for community level clustering for risk factors of acute gastrointestinal illness in 10 Batwa settlements in southwestern Uganda, January 2013
| Predictor variable | OR | 95% CI | P value |
|---|---|---|---|
| Exposure to goats | 2·64 | 1·04–6·76 | 0·04 |
| Age group (yr) | |||
| 0–3 | 4·83 | 1·24–18·95 | 0·02 |
| 4–12 | 1·72 | 0·43–6·97 | 0·44 |
| 13–34* | – | – | – |
| ⩾35 | Ref. | Ref. | Ref. |
| Wealth (households assets) × middle age (13–34 yr) | |||
| Interaction term | |||
| No wealth + child, adolescent or senior (<13 or >34 yr) | Ref. | Ref. | Ref. |
| Wealth + child, adolescent or senior (<13 or >34 yr) | 7·02 | 3·95–9·20 | 0·03 |
| Interaction term | |||
| No wealth + middle aged (13–34 yr) | Ref. | Ref. | Ref. |
| Wealth + child, adolescent or senior (<13 or >34 yr) | 4·00 | 1·26–7·43 | 0·02 |
| Interaction term | |||
| Wealth + middle aged (13–34 yr) | Ref. | Ref. | Ref. |
| Wealth + child, adolescent or senior (<13 or >34 yr) | 3·12 | 1·16–6·85 | 0·03 |
| Clustering at the community level for the model† | 0·068 | ||
| Area level variance (s.e.) | 0·26 (0·3) | 0·03–2·18 | |
| Median OR‡ | 1·62 | ||
| Interclass correlation coefficient | 0·08 | ||
OR, Odds ratio; CI, confidence interval.
The middle-aged adult group (13–34 years) cannot be interpreted on its own, as it significantly interacts with the wealth variable.
Likelihood ratio test comparing the mixed logistic regression model (using a random intercept for community-level clustering) to logistic regression model.
DISCUSSION
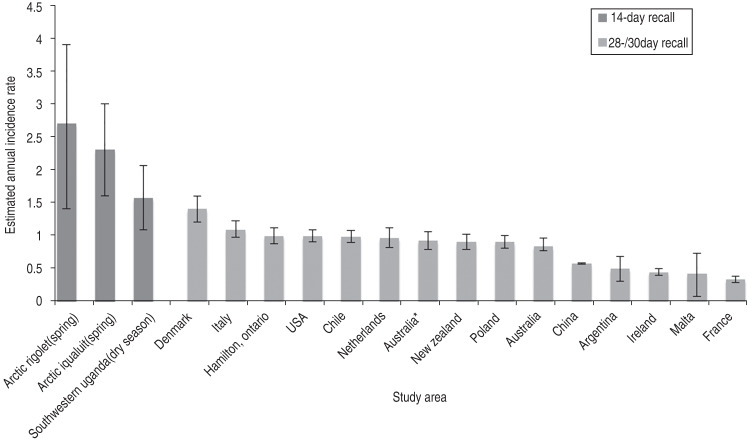
In the Batwa, the estimated incidence of AGI (1·66) was lower than results from parallel AGI research in Indigenous Inuit in the Canadian Arctic (2·9–3·8) using the same methodology, similar recall period, and case definition [30]. This finding might be explained by surveying during a potentially lower-risk season (dry season) in Uganda, which could also result in under-estimates of annual incidence. The estimated incidence of AGI from the dry season is, however, still higher than comparable international estimates. Batwa AGI estimated incidence using an internationally comparable case definition [29] was higher than incidences reported in studies using the same case definition but using a 28- or 30-day recall period in Denmark [12], Italy [11], Ontario [16], United States [10], Chile [20], The Netherlands [8], Australia [10, 13], New Zealand [15], Poland [6], China [14], Argentina [21], Ireland [10], Malta [17], and France [7] (Fig. 3). These higher Batwa AGI rates were expected given the conditions of high poverty among the Batwa and the already high rates of mortality and morbidity due to diarrhoeal diseases in central sub-Saharan Africa [37]. Some AGI studies have observed that longer recall periods can result in underreporting and missed cases [20, 38]. Notably, we used a 14-day recall period for Batwa participants in an attempt to reduce underreporting due to recall bias.
Fig. 3.
Graphical representation of 14-day and 28-/30-day recall period estimated annual incidence rates of acute gastrointestinal illness (AGI) per person per year in the Batwa and by comparable retrospective international population-based AGI studies using an international case definition [27] of ⩾3 diarrhoea episodes and/or any vomiting [5–7, 9–16, 18, 19, 28].
The severity of AGI was greater in the Batwa in terms of the duration of illness, with a mean duration of diarrhoea (4·5 days) and vomiting (2·8 days), compared to the Indigenous Inuit in the Canadian North [30], Cuba [22], China [14], Canadian communities [9, 18], Chile [20], Argentina [21], New Zealand [15], and the United States [5]. The mean maximum number of vomiting and diarrhoea episodes for Batwa within a 24-h period are within range of studies performed in Cuba [22] but greater than reported in the Inuit in the Canadian North [30] and in China [14]. Generally, this greater magnitude in AGI severity in the Batwa could be due to differential exposures to pathogens more or less abundant in different geographical or local environments. While the annual incidence reported in the Indigenous Inuit in the Canadian North was greater than that reported in the Batwa, the Batwa reported a greater severity of AGI symptoms, implying that the consequence of AGI in this population is more serious. Greater debilitating effects of AGI in the Batwa are probable, based on the greater morbidity and mortality of diarrhoea in sub-Saharan Africa compared to higher-income countries [37].
A high proportion of cases reported seeking some form of healthcare; however, sample sizes were low and precluded inferential analysis. The Batwa had a greater proportion of cases seeking care from a physician or nurse compared to cases in the Canadian North [30], Poland [6], Argentina [21], New Zealand [15], Denmark [12], France [7], Italy [11] and China [14]. This can be explained by the recent efforts of international and local organizations, seeking to improve Batwa access to medical care through subsidized healthcare and outreach programmes [39]. The Batwa more often utilized traditional forms of treatment such as herbal remedies than taking prescription or modern medicines. Past research has documented the prevalent use of herbal diarrhoeal remedies by Indigenous populations in Africa [40]; however, this form of treatment has received negligible attention in the comparable AGI literature [5–17, 20, 21, 30]. Thus, the Batwa maintain traditional Indigenous practices to address AGI episodes in conjunction with utilizing contemporary forms of clinical consultation, with implications for the development of health interventions and strategies.
The gender and age distribution of AGI in the Batwa had the same pattern observed in other studies [7, 9, 10, 12, 14, 15, 17], with a high prevalence in females and in children. Our results are in contrast to studies that observed a higher incidence in young males [21, 22], and it is possible that there are cultural differences with respect to gender-related exposures such as food preparation [16–18], water collection [41], and animal rearing, that could put female Batwa at higher risk. This study found a significant association of higher AGI incidence in children aged ⩽3 years (3·13 cases per person-year) which is on a par with the estimated incidence of childhood diarrhoea in Africa (3·3 cases per child per year), confirming studies that indicate AGI is a substantial threat to children globally, and particularly in low-income regions [2]. Children may have a greater vulnerability to AGI due to lower levels of immunological protection when exposed to enteric pathogens [10, 12, 14] and the behaviour of young children may also increase their exposure via person-person contact, environmental transmission, decreased hygiene, and increased hand-to-mouth contact [3, 14].
Of the potential environmental risk factors, exposure to goats significantly increased the odds of AGI (OR 2·64). Similar results of exposure to animals as a risk factor for AGI have been found in studies in the Inuit in the Canadian North [30], Canada [18], Chile [20], and Argentina [21]. AGI symptoms common to humans and animals living in close proximity were noted by previous studies in rural western Uganda [42]. Goats are known to sometimes carry multiple pathogens, including Escherichia coli, Cryptosporidium, and rotavirus [42], and human contact with animal faeces is often associated with gastrointestinal illness [42]. Direct transmission of pathogens from animals to humans (or vice versa) can occur through petting, touching or feeding [42]. Pathogens can also survive for long periods of time in the environment and transmission can occur from a contaminated environment long after animals have been removed [43]. Some of the Batwa own or raise animals, and goats are found in or near the communities for both economic and consumption purposes. We did not, however, test animals or the environment for AGI-related pathogens; this warrants further research.
Wealth in the model, derived from household asset scores, significantly interacted with a middle-aged adult category (13–34 years), changing the directionality of odds for Batwa in this age group (13–34 years) or outside of it (<13 or >34 years). Wealth seemed to affect the odds of AGI differently for middle-aged adults (13–34 years) compared to non-middle-aged adults (children, adolescents or seniors, <34 or >34 years), putting children, adolescents or seniors at greater risk of illness. Children, adolescent or seniors (<13 or >34 years) in the higher half of the wealth classification had an increased odds of AGI compared to children, adolescents or seniors in the lower half of the wealth classification. The association between increased wealth and increased odds of AGI was also recorded in studies in Australia [13] and the United States [5] that adopted the hypothesis that higher socioeconomic status could be related to high-risk behaviour regarding health; however, the opposite relationship was found in a study of children aged <5 years in Uganda [44]. There was low socioeconomic variation in households within the communities in this study and income was an invalid measure of wealth given the predominance of subsistence livelihoods. As such, we used household assets as a proxy for other related socioeconomic risk factors. It is plausible that households with greater assets are more likely to be exposed to animals, making wealth a proxy for increased exposure to potential pathogens. Moreover, larger households with potentially more income earners could also generate more asset-based wealth for the household, but at the same time increase over-crowding. Studies have confirmed the important role that over-crowding and increased human-to-human contact can have in increasing the odds of AGI [6, 14, 45].
The absence of longitudinal and seasonal data, which stems from the cross-sectional design of the survey, is a limitation in this study. Our survey captures cases in the dry season, making the estimated annual incidence rate more conservative than would be expected in the wet season and may underestimate the actual annual incidence of AGI in this population. A low number of observations for health-seeking behaviour estimates hinder the comparability across the literature, and subsequent studies on the burden of disease and access to healthcare systems should be conducted to understand these trends more fully. Another limitation, similar to other studies examining self-reported illness, is that self-reported surveys, by their nature, may result in over- or under-estimates of disease due to potential reporting biases.
In conclusion, this study provides estimates on the magnitude and determinants of AGI within an Indigenous Batwa population in southwestern Uganda. The results highlight the high prevalence of AGI in this population relative to other comparable international studies. Of the potential environmental, socio-demographic, or economic factors that can increase the odds of AGI, we found that being aged <3 years, exposure to goats, and being a child, adolescent or senior with greater wealth were significantly associated with increased odds of AGI. Knowledge of these risk factors is important for resource allocation and public health interventions in these communities. The results of this study support the need for an increase in systematic population or community-based AGI studies to further investigate the differential burden of illness in vulnerable or Indigenous populations.
ACKNOWLEDGEMENTS
We thank the 10 Batwa communities in Kanungu District: Buhoma, Byumba, Bikuto, Karehe, Kitahuria, Kihembe, Kebiremu, Mukongoro, Rulangala, and Kitariro, and all the Batwa community members for engagement with the project. Thanks to all the surveyors from Kanungu District, and other members of the IHACC Team (Mr Jamen Kasumba, Mr Martin Kigozi, Ms. Christine Nantongo and Ms. Fortunate Twebaze, Mr Emmanuel Eloku from the Kanungu District Administration, Mr Hubert Nkabura from the Bwindi Community Hospital, and Mr Sabastian Twesigomwe from the Batwa Development Programme). Thanks to Dr Blanaid Donnelly, for assistance in interpreting the zoonosis portion of this research. Thanks to Kate Thomas for advice and expertise on AGI research. This research is part of an international project entitled the ‘Indigenous Health Adaptation to Climate Change’ (IHACC) project (www.ihacc.ca), with parallel field study sites in the Canadian Arctic and Peru. Funding was provided by CIHR/NSERC/SSHRC and IDRC Tri-Council Initiative on Adaptation to Climate Change, Indigenous Health Adaptation to Climate Change (IHACC), IDRC File nos. 106372-003, 004, 005; CIHR Open Operating Grant, Adaptation to the health effects of climate change among Indigenous peoples in the global south (IP-ADAPT), Application no. 298312.
APPENDIX
Indigenous Health Adaptation to Climate Change Research Team: Lea Berrang-Ford, Cesar Carcamo, Victoria Edge, James Ford, Sherilee Harper, Alejandro Llanos, Shuaib Lwasa, Didacus Namanya.
DECLARATION OF INTEREST
None.
Supplementary material
For supplementary material accompanying this paper visit https://doi.org/10.1017/S0950268814003124.
click here to view supplementary material
REFERENCES
- 1.Cheng AC, et al. Infectious diarrhea in developed and developing countries. Journal of Clinical Gastroenterology 2005; 39: 757–773. [DOI] [PubMed] [Google Scholar]
- 2.Walker CLF, et al. Global burden of childhood diarrhoea and pneumonia. Lancet 2013; 381: 1405–1416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Thapar N, Sanderson IR. Diarrhoea in children: an interface between developing and developed countries. Lancet 2004; 36: 641–653. [DOI] [PubMed] [Google Scholar]
- 4.WHO. Global Health Risks: Mortality and Burden of Disease Attributable to Selected Major Risks. Geneva: World Health Organization, 2009. [Google Scholar]
- 5.Jones TF, et al. A population-based estimate of the substantial burden of diarrhoeal disease in the United States; FoodNet, 1996–2003. Epidemiology and Infection 2007; 135: 293–301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Baumann-Popczyk A, et al. Incidence of self-reported acute gastrointestinal infections in the community in Poland: a population-based study. Epidemiology and Infection 2012; 140: 1173–1184. [DOI] [PubMed] [Google Scholar]
- 7.Van Cauteren D, et al. Burden of acute gastroenteritis and healthcare-seeking behaviour in France: a population-based study. Epidemiology and Infection 2012; 140: 697–705. [DOI] [PubMed] [Google Scholar]
- 8.Doorduyn Y, Van Pelt W, Havelaar AH. The burden of infectious intestinal disease (IID) in the community: a survey of self-reported IID in The Netherlands. Epidemiology and Infection 2012; 140: 1185–1192. [DOI] [PubMed] [Google Scholar]
- 9.Thomas MK, et al. Population distribution and burden of acute gastrointestinal illness in British Columbia, Canada. BMC Public Health 2006; 6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Scallan E, et al. Prevalence of diarrhoea in the community in Australia, Canada, Ireland, and the United States. International Journal of Epidemiology 2005; 34: 454–460. [DOI] [PubMed] [Google Scholar]
- 11.Scavia G, et al. The burden of self-reported acute gastrointestinal illness in Italy: a retrospective survey, 2008–2009. Epidemiology and Infection 2012; 140: 1193–1206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Müller L, Korsgaard H, Ethelberg S. Burden of acute gastrointestinal illness in Denmark 2009: a population-based telephone survey. Epidemiology and Infection 2012; 140: 290–298. [DOI] [PubMed] [Google Scholar]
- 13.Hall G V, et al. Frequency of infectious gastrointestinal illness in Australia, 2002: regional, seasonal and demographic variation. Epidemiology and Infection 2006; 134: 111–118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Chen Y, et al. Burden of self-reported acute gastrointestinal illness in China: a population-based survey. BMC Public Health 2013; 13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Adlam SB, et al. Acute gastrointestinal illness in New Zealand: a community study. Epidemiology and Infection 2011; 139: 302–308. [DOI] [PubMed] [Google Scholar]
- 16.Sargeant JM, Majowicz SE, Snelgrove J. The burden of acute gastrointestinal illness in Ontario, Canada, 2005–2006. Epidemiology and Infection 2008; 136: 451–460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Gauci C, et al. The magnitude and distribution of infectious intestinal disease in Malta: a population-based study. Epidemiology and Infection 2007; 135: 1282–1289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Majowicz SE, et al. Magnitude and distribution of acute, self-reported gastrointestinal illness in a Canadian community. Epidemiology and Infection 2004; 132: 607–617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Gurpreet K, et al. Incidence and determinants of acute diarrhoea in Malaysia: a population-based study. Journal of Health, Population, and Nutrition 2011; 29: 103–112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Thomas MK, et al. Burden of acute gastrointestinal illness in the Metropolitan region, Chile, 2008. Epidemiology and Infection 2011; 139: 560–571. [DOI] [PubMed] [Google Scholar]
- 21.Thomas MK, et al. Burden of acute gastrointestinal illness in Gálvez, Argentina, 2007. Journal of Health, Population, and Nutrition 2010; 28: 149–158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Prieto AP, et al. Burden of self-reported acute gastrointestinal illness in Cuba. Journal of Health, Population, and Nutrition 2009; 27: 345–357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ohenjo N, et al. Indigenous Health 3: health of Indigenous people in Africa. Lancet 2006; 367: 1937–1946. [DOI] [PubMed] [Google Scholar]
- 24.Jackson D. The health situation of women and children in central African Pygmy peoples. Forest Peoples Programme, 2006.
- 25.Gracey M, King M. Indigenous health part 1: determinants and disease patterns. Lancet 2009; 374: 65–75. [DOI] [PubMed] [Google Scholar]
- 26.Berrang-Ford L, et al. Vulnerability of indigenous health to climate change: a case study of Uganda's Batwa Pygmies. Social Science and Medicine 2012; 75: 1067–1077. [DOI] [PubMed] [Google Scholar]
- 27.Baker W. Uganda: The Marginalization of Minorities. Minority Rights Group, 2001.
- 28.Jackson D. Twa women, Twa rights in the Great Lakes Region of Africa. Minority Rights Group, 2003.
- 29.Majowicz SE, et al. A common, symptom-based case definition for gastroenteritis. Epidemiology and Infection 2008; 13: 886–894. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Harper S. Gastrointestinal illness in Canada’ s North: implications of climate change on current and future Inuit health (dissertation). Guelph, Ontario, Canada: University of Guelph, 2013, 275 pp. [Google Scholar]
- 31.Lewis J. The Batwa pygmies of the Great Lakes region. Minority Rights Group, 2000.
- 32.Kabananukye K, Wily L. Report on a study of the Abayanda pygmies of South Western Uganda for the Mgahinga and Bwindi Impenetrable Forest Conservation Trust, 1996.
- 33.Uganda Bureau of Statistics. Ugandan Demographic and Health Survey 2006. Kampala, 2007.
- 34.Cheverud J, Cavalli-Sforza L. Cultural transmission among Aka pygmies. American Anthropologist 1986; 88. [Google Scholar]
- 35.Balen J, et al. Comparison of two approaches for measuring household wealth via an asset-based index in rural and peri-urban settings of Hunan province, China. Emerging Themes in Epidemiology 2010; 7: 7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Bwindi Community Hospital (BCH). In-patient database. 30 August 2014.
- 37.WHO. The Global Burden of Disease: 2004 Update. Geneva: World Health Organisation, 2008. [Google Scholar]
- 38.Cantwell LB, et al. The effect of different recall periods on estimates of acute gastroenteritis in the United States, FoodNet Population Survey 2006–2007. Foodborne Pathogens and Disease 2010; 7: 1225–1228. [DOI] [PubMed] [Google Scholar]
- 39.The Kellermann Foundation. Annual report 2012 (http://www.kellermannfoundation.org/annual_report.pdf). 2012, pp. 1–4.
- 40.Bisi-Johnson MA, et al. A survey of indigenous herbal diarrhoeal remedies of O.R. Tambo district, Eastern Cape Province, South Africa. African Journal of Biotechnology 2010; 9: 1245–54. [Google Scholar]
- 41.UNICEF. Gender and water, sanitation and hygiene (WASH) (http://www.unicef.org/esaro/7310_Gender_and_WASH.html). Accessed 7 June 2014.
- 42.Rwego IB, et al. High rates of Escherichia coli transmission between livestock and humans in rural Uganda. Journal of Clinical Microbiology 2008; 46: 3187–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Rwego IB, et al. Gastrointestinal bacterial transmission among humans, mountain gorillas, and livestock in Bwindi Impenetrable National Park, Uganda. Conservation Biology 2008; 22: 1600–1607. [DOI] [PubMed] [Google Scholar]
- 44.Bbaale E. Determinants of diarrhoea and acute respiratory infection among under-fives in Uganda. Australasian Medical Journal 2011; 4: 400–409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Wikswo M, Hall A. Outbreaks of acute gastroenteritis transmitted by person-to-person contact – United States, 2009–2010. Morbidity and Mortality Weekly Reports. Surveillance Summaries 2012; 6: 1–12. [PubMed] [Google Scholar]
- 46.Merlo J, et al. A brief concept tutorial of multilevel analysis in social epidemiology: using measures of clustering in multilevel logistic regression to investigate contextual phenomena. Journal of Epidemiology and Community Health 2006; 60: 290–297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Lebel A, et al. Geographic variability in the association between socioeconomic status and BMI in the USA and Canada. PLoS ONE 2014; 9. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
For supplementary material accompanying this paper visit https://doi.org/10.1017/S0950268814003124.
click here to view supplementary material