SUMMARY
Previous studies examining the weather–bacillary dysentery association were of a large time scale (monthly or weekly) and examined the linear relationship without checking the linearity assumption. We examined this association in Beijing at a daily scale based on the exposure-response curves using generalized additive models. Our analyses suggested that there were thresholds for effects of temperature and relative humidity, with an approximately linear effect for temperature >12·5 °C [excess risk (ER) for 1 °C increase: 1·06%, 95% confidence interval (CI) 0·63–1·49 on lag day 3] and for relative humidity >40% (ER for 1% increase: 0·18%, 95% CI 0·12–0·24 at lag day 4); and there were linear effects of rainfall (ER for 1-mm increase: 0·22%, 95% CI 0·12–0·32), negative effects for wind speed (ER: −2·91%, 95% CI −4·28 to −1·52 at lag day 3) and sunshine duration (ER: −0·25% 95% CI −0·43 to −0·07 at lag day 4). This study suggests that there are thresholds for the effects of temperature and relative humidity on bacillary dysentery, and these findings should be considered in its prevention and control programmes.
Key words: Bacillary dysentery, meteorological factors, threshold, temperature
INTRODUCTION
Bacillary dysentery (BD) is a diarrhoeal disease caused by various species of Shigella bacteria, including S. dysenteriae, S. flexneri, S. boydii, and S. sonnei. Typical clinical characteristics of BD include bloody diarrhoea, fever, and stomach cramps with an incubation period of 1–2 days [1]. This infection can be transmitted via the faecal–oral route by contaminated water, food, or person-to-person contacts.
BD remains a major public health problem in both developed and developing countries [2, 3]. The incidence of this disease is relatively higher in developing countries than in developed countries, despite the fact that its incidence has decreased substantially during the past decades [4], it is a challenge to further reduce the incidence of BD in a changing environment [5]. According to the information from National Notifiable Diseases of China, there were about 269 299 BD cases reported in 2009, with an incidence rate of 20·28/100 000 [6]. It was the third leading notifiable disease in China, next only to tuberculosis and hepatitis B [6].
As a faecal–oral transmitted infectious disease, BD can be affected by changes in ambient environment [5]. The seasonality of BD incidence indicates that meteorological factors might play an important role in its epidemiology, which has gained increasing concerns in recent years, particularly in the context of global climate change [7, 8]. Ambient temperature may directly influence the replication and survival of the pathogens in the environment; rainfall, especially heavy rainfall events, may affect the frequency and level of contamination of drinking water. Weather variations may also affect population activities, including eating habits, which may indirectly influence the transmission of this infection [2].
A few studies have investigated the association between BD and meteorological factors, with inconsistent findings [2, 9]. For example, a time-series analysis in Jinan found that a 1 °C increase in monthly maximum temperature might relate to an ~11·40% [95% confidence interval (CI) 10·19–12·69] increase in BD [2]. In Shenyang, ambient temperature, precipitation and relative humidity were found to be positively associated with monthly incidence of BD [8]. Moreover, a large-scale study observed a strong association between extreme precipitation and water-borne infectious disease outbreaks with a 2-month lag in the United States [9]. These studies mainly used monthly data to investigate the association between temperature and BD transmission; however, more finer data, such as daily data, could make the estimation more accurate [10].
At the same time, most of the previous studies examined the linear association between weather variables and BD without checking the linear assumption [2, 8]. Although our recent study in Wuhan, and Zhou's study in Shanghai suggested that most of the meteorological factors had an approximate linear relationship with BD [11, 12], another study found that the thresholds for the effects of maximum and minimum temperatures were 17 °C and 8 °C in a northern Chinese city, but no thresholds were detected in a southern Chinese city [13].
Being the capital city of China, Beijing also had a relatively high prevalence of BD in recent years [14]; however, no study has been conducted to examine the effect of daily meteorological factors on the occurrence of BD in Beijing. This study quantified the association between daily meteorological factors and BD occurrence in Beijing; our analysis was at the daily level, and based on the exposure-response relationship.
MATERIALS AND METHODS
As the capital of China, Beijing is the political and financial centre of China with a total population of about 21·1 million over an area of 16 410 km2. This city has a continental monsoon climate, with cold and dry winters and hot summers.
BD is a national legally notifiable infectious disease in China, all clinical and hospital doctors are required to report any BD case to local Centre for Disease Control and Prevention (CDC). This study included all BD cases that were diagnosed by authorized hospitals in Beijing and reported to Chinese Information System for Diseases Control and Prevention. Daily data on counts of BD cases covering the period 2007–2012 were obtained from the Chinese CDC. In addition to passive surveillance of infectious diseases, the local CDCs conduct active surveillance regularly to reduce the rate of misreporting and underreporting [15]. As BD is a very common disease in the study area, it is not difficult for the doctors to make a correct clinical diagnosis. Therefore, it is believed that the data quality is reliable [13].
Daily meteorological data for Beijing were extracted from China Meteorological Administration Climatic Dataset Centre. The meteorological variables included daily mean temperature (°C), relative humidity (%), rainfall (mm), wind speed (m/s) and duration of sunshine (h). Spearman's correlation coefficients were used to evaluate the interrelationships between the various weather factors.
We examined the short-term association between daily meteorological factors and BD occurrence using generalized additive models (GAM) with a quasi-Poisson link function to account for over-dispersion in daily BD count [16]. Consistent with previous time-series analyses, in all the models, we controlled for the day of the week (DOW) and public holidays using categorical indicator variables [17]. In addition, we used penalized smoothing splines to adjust for long-term trends and seasonal patterns in daily BD occurrence with degree of freedom (d.f.) selected a priori based on previous studies [11]. Specifically, we used 6 d.f. per year for time trend. For the smooth function of calendar time, 6 d.f. per year was chosen so that we filtered out the information at time scales of 2 months [18]. We set up a core model to remove the long-term trend, seasonality, and adjust for time-varying confounders as follows:
where E(Yt) is the expected daily disease count on day t, α is the model intercept, s() indicates a smoother based on penalized splines, DOW is an indicator for day of the week, PH presents a binary variable for public holidays, and β is the regression coefficient. We examined the residuals (the difference between fitted and observed values) of the core model to check whether there were discernible patterns and autocorrelation by means of residual plot and partial autocorrelation function (PACF) plot. The PACF of residuals of the core model was <0·1 for lags examined, indicating no serial autocorrelations in the residuals and sufficient confounder control [19, 20].
Univariate models were then fitted by adding each meteorological factor into the core model, and multivariate model was used to control the influence of other meteorological factors, meaning that for one specific meteorological variable, the other meteorological variables were treated as potential confounding factors; however, when there was high correlation (usually with correlation coefficient >0·8) between two variables, they were not included in the same model due to concerns regarding collinearity [21].
To examine the shape of the association between the logarithm of daily BD count and the weather variables, we first graphically examined exposure–response relationships derived using a smoothing function [22]. The initial analysis suggested that there was a threshold for some variables, e.g. for temperature, the exposure–response curve showed that there was no significant effect below a certain temperature and an approximately linear effect above that temperature. We used Akaike's Information Criterion (AIC) to determine that temperature threshold. Briefly, in the model multiple thresholds were tested based on the minimal AIC value of the model. For example, by visual inspection of the exposure–response curve, we could identify that the potential threshold might be within 10–13 °C, therefore we fitted two models with thresholds from 10 °C to 13 °C (in 0·1 °C increments) to identify the threshold with the minimum sum of the AIC of the two models.
Based on the threshold identified in the above procedure, we then estimated the association between various weather variables and BD for different lag structures, including current day (lag 0) up to 7 days before (lag 7), as this infection usually has a short incubation period [23]. We reported the result as excess risk (ER), defined as the percentage increase in daily BD for a 1-unit increase in each meteorological factor, with 95% CIs.
Because the risk estimates usually varied with the model specifications in time-series analyses [24, 25], we performed additional sensitivity analyses by changing the degrees of freedom (5, 7 and 8 d.f./year) for temporal adjustment. As another sensitivity analysis, we used a negative binomial link function to account for over-dispersion. We also included the logarithm of the annual population number in the model as the offset variable to check the effect on population change on the result estimation.
All statistical analyses were two-sided and values of P < 0·05 were considered statistically significant. The ‘mgcv’ package in R Software version 2.14·1 (R Development Core Team, Austria) was used to fit all models and estimate the exact standard errors of regression coefficients. The research protocol was reviewed and approved by the ethical committee of Guangdong Provincial CDC.
RESULTS
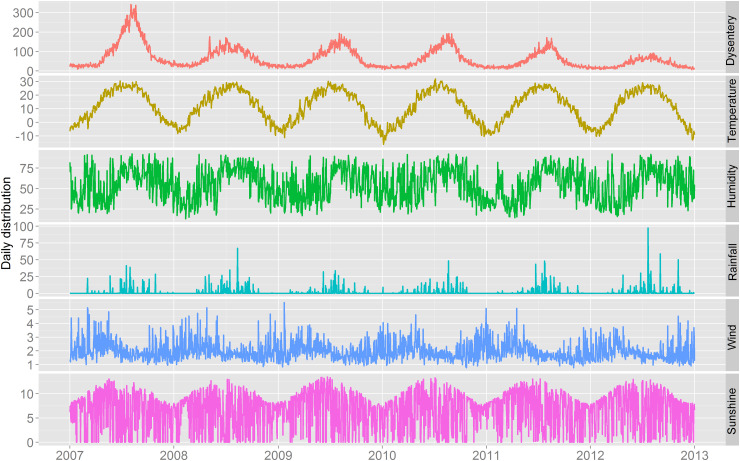
Between 1 January 2007 and 31 December 2012, a total of 142 065 BD cases were reported in Beijing. There were more male cases with a male:female sex ratio of 1·2:1 ( 77 661: 64 404). The descriptive statistics for weather factors and BD are given in Table 1. On average, there were 64·8 daily BD cases during the study period. The mean values of daily mean temperature, relative humidity, rainfall, wind speed and sunshine in Beijing were 11·8 °C, 55·2%, 1·6 mm, 2·0 m/s and 6·6 h, respectively. Figure 1 shows the time series of the daily BD count and weather variables in Beijing. There was obvious seasonal pattern in these factors. In particular, a summer epidemic peak was observed for the occurrence of BD, with a generally decreasing trend during the study period and the highest rate occurring in 2007.
Table 1.
Summary statistics of daily weather conditions and bacillary dysentery in Beijing, China, 2007–2012
| Variable | Minimum | Median | Mean | Maximum | s.d. |
|---|---|---|---|---|---|
| Daily cases | 6·0 | 45·0 | 64·8 | 342·0 | 54·4 |
| Temperature (°C) | −16·2 | 13·2 | 11·8 | 31·9 | 11·7 |
| Relative humidity (%) | 13·0 | 56·5 | 55·2 | 93·0 | 19·1 |
| Rainfall (mm) | 0·0 | 0·0 | 1·6 | 97·5 | 5·7 |
| Wind speed (m/s) | 0·8 | 1·8 | 2·0 | 5·5 | 0·7 |
| Sunshine (h) | 0·0 | 7·6 | 6·6 | 13·5 | 3·8 |
s.d., Standard deviation.
Fig. 1.
The time series of daily bacillary dysentery and meteorological factors in Beijing, 2007–2012. Temperature (°C), relative humidity (%), rainfall (mm), wind speed (m/s), and sunshine duration (h).
Table 2 depicts the correlations between daily BD and various meteorological factors in Beijing. All the factors were significantly correlated with each other with low to moderate correlation coefficients, e.g. between daily mean temperature and pressure (r = 0·41), between relative humidity and wind speed (r = −0·58), between relative humidity and duration of sunshine (r = −0·58), and between daily BD and temperature (r = 0·86). No high correlation coefficient was found in the meteorological factors, so all the variables were included in the same multivariate model.
Table 2.
Spearman's correlations between daily bacillary dysentery and weather variables in Beijing, 2007–2012
| Bacillary dysentery | Temperature | Humidity | Rainfall | Wind velocity | |
|---|---|---|---|---|---|
| Temperature | 0·86 | ||||
| Humidity | 0·46 | 0·41 | |||
| Rainfall | 0·29 | 0·29 | 0·52 | ||
| Wind velocity | −0·14 | −0·09 | −0·57 | −0·07 | |
| Sunshine | 0·04 | 0·12 | −0·58 | −0·39 | 0·33 |
P < 0·05 for all.
Supplementary Figure S1 shows the diagnostic graphs of the core model, which includ the plot of the residuals and the plot of the PACF. There were no discernible patterns and no autocorrelation in the residuals, showing that the core model was set up adequately to remove the potential confounding in the daily variations of the infection.
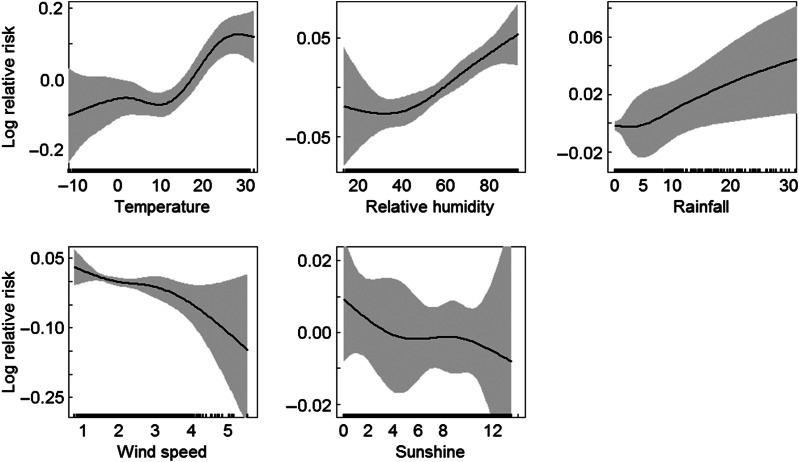
The exposure–response relationship for the meteorological variables with BD occurrence was illustrated in Figure 2. An almost linear relationship was observed for daily rainfall, wind speed and duration of sunshine; however, for temperature, it seemed that there was a threshold; our analysis suggested the best models with the threshold temperature at 12·5 °C, below which there was no significant effect, and above which there was a positive effect; similarly, for relative humidity, the threshold was found at about 40%, and there was an increasing trend of the occurrence of BD with higher relative humidity >40%. Therefore in subsequent analyses, we examined the linear effects of temperatures >12·5 °C and relative humidity >40%.
Fig. 2.
Smoothing plots of daily mean temperature, relative humidity, rainfall, wind speed and duration of sunshine against bacillary dysentery in Beijing. Confounding factors included time trend, day of week and public holidays.
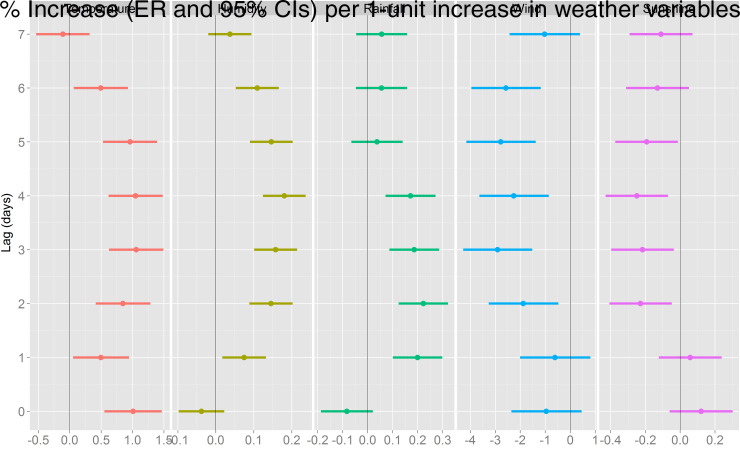
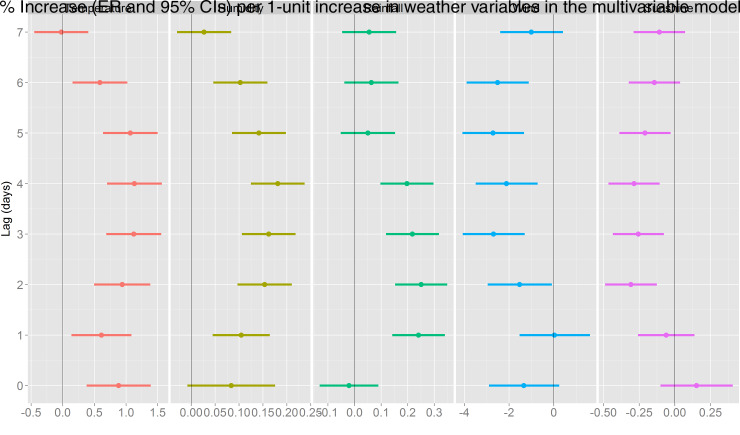
The association between various meteorological factors and BD in the univariate models is presented in Figure 3. A statistically significant positive effect was detected for temperature (at the current day and lag days 1–6), relative humidity (at lag days 1–6), and rainfall (at lag days 1–4), while negative effects were observed for wind speed (at lag days 2–6) and duration of sunshine (at lag days 2–5). The largest effect of temperature on BD was observed at lag day 3, the ER for a 1 °C increase in temperature >12·5 °C was 1·06% (95% CI 0·63–1·49); daily relative humidity had the largest effect at lag day 4 with an ER of 0·18% (95% CI 0·12–0·24) for a 1% increase in relative humidity >40%; and the largest effect of rainfall was at lag day 2, where a 1-mm increase was associated with an ER of 0·22% (95% CI 0·12–0·32) in BD; while the negative effects of wind speed and sunshine duration were found to be largest at lag day 3 (ER −2·91%, 95% CI −4·28 to −1·52) and lag day 4 (ER −0·25%, 95% CI −0·43 to −0·07), respectively. In the multivariate model including all the meteorological factors in the same model (as shown in Fig. 4), we that found the effect estimates for each meteorological factor remained similar.
Fig. 3.
Excessive risk (ER) with 95% confidence intervals (CIs) in bacillary dysentery in Beijing per 1-unit increase in daily meteorological factors for the current day (lag 0) to 7 days before the current day (lag 7), results obtained from univariate regression models.
Fig. 4.
Excessive risk (ER) with 95% confidence intervals (CIs) in bacillary dysentery in Beijing per 1-unit increase in daily meteorological factors for the current day (lag 0) to 7 days before the current day (lag 7), results obtained from multivariate regression models including all the meteorological variables.
In the sensitivity analyses, we changed the d.f. (5, 7, 8) for calendar time in order to control for seasonality and long-term trend, which produced similar results. When we controlled for the logarithm of the population number as the offset variable in the model, the result remained similar (Supplementary Fig. S2). The sensitivity analysis using a negative binomial link function to account for over-dispersion also yielded consistent result (Supplementary Fig. S3).
DISCUSSION
BD and other diarrhoeal diseases are still important public health concerns in developing countries largely due to unsafe water supply, inadequate sanitation, poor hygiene, and particularly global climate change [26]. Our study examined the association between the occurrence of BD and meteorological factors at a daily time scale in Beijing. We observed positive effects of temperature and relative humidity with thresholds of 12·5 °C and 40%, respectively, and a positive effect of rainfall and negative effects of wind speed and duration of sunshine without threshold in Beijing.
Global climate change has already caused and will continue to pose great challenges to public health, particularly communicable diseases [27]. The effects of meteorological factors on transmission of enteric infection have attracted less attention compared to vector-borne diseases, e.g. malaria, dengue fever, and haemorrhagic fever with renal syndrome [28–30]. Our study showed that weather variables are also important risk factors of BD, the identification of thresholds suggests that there could be more cases expected in the near future if the a temperature continues to rise, which has significant implications for public health practitioners and policy makers [23].
A few studies have reported that temperature was one key environmental factor in the transmission of BD. Another study [2] has suggested that there is a threshold in the association between temperature and BD, above which, the relationship was linear, e.g. a study found that the thresholds for the effects of maximum and minimum temperatures were 17 °C and 8 °C in Jinan, a northern Chinese city, but no thresholds were detected in Shenzhen, a southern Chinese city [13]; while one study from Denmark found the threshold of average temperature at 8 °C [31]. Our study suggests that the threshold for daily mean temperature in Beijing is at 12·5 °C, which is in accordance with that in Jinan. The magnitude of the effect of temperature of the present study is generally in agreement with previous reports. In China, for instance, a study in Wuhan also reported a positive association between daily temperature and BD, the excess risk for 1 °C increase in daily temperature increase was 0·94% with a lag of 2 days [11]; however, studies at the monthly time scale showed a larger effect estimate, e.g. a 1 °C rise of temperature corresponded to an increase of 3·60% in the monthly number of BD cases in Guangzhou [32], and increases of 12% in Jinan and 16% in Shenzhen [13]. In Peru, each 1 °C increase in temperature was found to be associated with an 8% increase in the risk of severe child diarrhoea [33]. The underlying mechanism might be that higher temperature may increase the reproduction of the bacteria along the food chain and water supply, promote the growth of the bacteria, and lengthen the survival of the bacteria in the environment and contaminated food, and thus increase human exposure to the pathogens [33]. It is also possible that high temperature may be associated with the behavioural pattern of the population, such as increased demand for water and less conscientious health-related activities, which could facilitate the transmission of this disease [34]. In the context of global climate change, it has been projected that the temperature will continue to rise in the future [35], more specific interventions should be considered at this stage to adapt and mitigate the possible impacts on the transmission of BD.
Inconsistent findings have been reported in previous studies about the association between relative humidity, rainfall and BD. This study found that relative humidity and rainfall is positively associated with risk of BD in Beijing. Consistent with our study, one study in northeast China observed a positive effect of relative humidity and rainfall on BD [8], a study in Fiji also found a positive association between diarrhoea and rainfall [36], similar findings have also been reported in Taiwan [37], United States [38], and Bangladesh [39]. On the other hand, one study found that shortage of rainfall in the dry season increased the prevalence of diarrhoeal diseases across Sub-Saharan Africa [40], and a study in Wuhan found a negative effect of relative humidity and rainfall on BD [11], while studies in two southern and northern Chinese cities did not detect a significant effect of relative humidity and rainfall [2, 13]. This discrepancy might be due to the difference in socioeconomic status, such as water and sanitation infrastructure in different regions, as well as human hygiene behaviours and population characteristics.
Wind speed may also affect the reproduction and survival of pathogens in the environment and the contamination of drinking water, and thus affect the transmission of BD [31]. In this study, we found a negative association between wind speed and BD in Beijing, contrary to some previous studies, e.g. studies from two Chinese cities, Changsha and Wuhan, did not find significant association between wind speed and BD [5, 11].
Longer duration of sunshine was found to correspond with lower risk of BD in this study; the underlying mechanism might be that more sunshine could inhibit the growth and survival of the pathogens in the environment. However, one study from Denmark reported an opposite result, suggesting that sunshine was associated with high risk of BD [31]. More studies are warranted to further investigate the association between sunshine and this infection.
Many environmental factors could affect the transmission of Shigella and the dynamics of BD, including people's dietary pattern, hygiene behaviours, susceptibility to different pathogen strains, and sensitivity to the available drugs as well as local weather conditions [8]. The results of this study could help to better understand the seasonality of this disease. During the high epidemic months (June–September), daily mean temperature, relative humidity and rainfall were 23·8 °C, 70·9%, 3·7 mm, respectively, which were statistically higher than that in other months (7·7 °C, 49·9% and 0·8 mm), while the wind speed and duration of sunshine were statistically lower in summer months (1·6 m/s, 6·2 h) than other months (2·1 m/s and 6·7 h). These meteorological factors might have played an important role in the seasonality of BD.
Understanding the lag pattern of the effects of meteorological factors is essential for policy makers and community leaders to develop response plans and prevention measures. Lagged effects of meteorological factors on enteric infectious diseases have been reported to range from a few days to weeks [2, 36]. This study found a relatively acute effect within a few days, which was more biologically plausible due to the short incubation period of this disease [1]. The discrepancy with previous studies, particularly the magnitude of the effect of temperature, might be due to different observation scales [2, 13, 36]. Two factors should be considered to choose the time scale in time-series analysis [10]. The most important factor is the biological pathway of the association between weather factors and the disease of interest. When there is an acute effect of the weather factors on the disease occurrence, such as within a few days, use of a daily scale should be considered; otherwise, weekly or monthly data should be used. The second factor is the number of cases of a disease at a time-specific unit, for instance, when there are too many days without disease occurrence, it will be better to perform the analysis based on weekly or monthly observations, or use other appropriate statistical methods. For the study of BD, we suggest using daily scale data in future endeavours to examine the effect of weather factors on its transmission.
Our study had two major strengths. First, this study investigated the impacts of meteorological factors on BD at a daily scale in Beijing, this daily-level analysis provided more accurate estimate of the association between various meteorological factors and BD. Second, our study used a novel method to detect the threshold of the effect of meteorological factors on BD, and indicated that there were thresholds of the effects of temperature and relative humidity on BD, which had not been done in previous studies.
Meanwhile, a few limitations should be acknowledged when interpreting findings from this study. Our study was ecological in study design, which did not allow us to explore individual-based associations and limited our capacity of causal inference [25]. Our analysis was preliminary and exploratory, we could not exclude the possibility of a spurious finding or unmeasured confounding factors that might be associated with both weather variables and BD occurrence. Furthermore, this analysis was based on the cases diagnosed based on clinical symptoms, lack of laboratory-confirmed information, particularly the disease caused by each species, might be cause for concern. However, since it is a common disease in the study population, it is not difficult for local doctors to make a correct diagnosis, so this concern should not have distorted the results to a great extent.
In conclusion, our study suggests that there is a threshold in the association between temperature, relative humidity and BD, above which, there are positive effects of these two factors on BD; rainfall is also positively associated with BD, while wind speed and duration of sunshine are negatively associated with this disease in Beijing. Future BD prevention and control strategies should consider these meteorological factors.
ACKNOWLEDGEMENTS
This work was supported in part by the National Sci-tec Key Project of China (2013ZX10004218) and the ‘863’ Project of China (2014AA021404). The sponsors had no role in the study design, data collection, analysis and interpretation of data, the writing of the manuscript, or in the decision to submit the manuscript for publication. We thank the anonymous reviewers for their valuable suggestions and comments.
DECLARATION OF INTEREST
None.
Supplementary material
For supplementary material accompanying this paper visit https://doi.org/10.1017/S0950268815001156.
click here to view supplementary material
REFERENCES
- 1.Niyogi SK. Shigellosis. Journal of Microbiology, 2005; 43: 133. [PubMed] [Google Scholar]
- 2.Zhang Y, Peng B. Hiller JE. Weather and the transmission of bacillary dysentery in Jinan, northern China: a time-series analysis. Public Health Reports 2008; 123: 61–66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Walker CLF, et al. Estimating diarrhea mortality among young children in low and middle income countries. PLoS ONE 2012; 7: e29151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Wang X, et al. Trend and disease burden of bacillary dysentery in China (1991–2000). Bulletin of the World Health Organization 2006; 84: 561–568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Gao L, et al. Meteorological variables and bacillary dysentery cases in Changsha City, China. American Journal of Tropical Medicine and Hygiene 2014; 90: 697–704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Sui J, et al. Surveillance of bacillary dysentery in China, 2009. Disease Surveillance 2010; 25: 947–950. [Google Scholar]
- 7.Guan P, et al. Bacillary dysentery and meteorological factors in northeastern china: a historical review based on classification and regression trees. Japanese Journal of Infectious Diseases 2008; 61: 356–360. [PubMed] [Google Scholar]
- 8.Huang D, et al. Investigating the effects of climate variations on bacillary dysentery incidence in northeast China using ridge regression and hierarchical cluster analysis. BMC Infectious Diseases 2008; 8: 130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Curriero FC, et al. The association between extreme precipitation and waterborne disease outbreaks in the United States, 1948–1994. American Journal of Public Health 2001; 91: 1194–1199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Wu H, et al. The effect of meteorological factors on adolescent hand, foot, and mouth disease and associated effect modifiers. Global Health Action 2014; 7: 24664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Li Z, et al. Identifying high-risk areas of bacillary dysentery and associated meteorological factors in Wuhan, China. Scientific Reports 2013; 3: 3239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Zhou X, et al. High temperature as a risk factor for infectious diarrhea in Shanghai, China. Journal of Epidemiology 2013. 23: 418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Zhang Y, et al. Climate variations and bacillary dysentery in northern and southern cities of China. Journal of Infection 2007; 55: 194–200. [DOI] [PubMed] [Google Scholar]
- 14.Xu Z, et al. Spatiotemporal pattern of bacillary dysentery in china from 1990 to 2009: what is the driver behind? PloS ONE 2014; 9: e104329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Wang L, et al. Emergence and control of infectious diseases in China. Lancet 2008; 372: 1598–1605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Dominici F, et al. On the use of generalized additive models in time-series studies of air pollution and health. American Journal of Epidemiology 2002; 156: 193–203. [DOI] [PubMed] [Google Scholar]
- 17.Lin HL, et al. Short term effects of El Nino-Southern Oscillation on hand, foot, and mouth disease in Shenzhen, China. PLoS ONE 2013; 8: e65585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Curriero FC, et al. Temperature and mortality in 11 cities of the eastern United States. American Journal of Epidemiology 2002; 155: 80–87. [DOI] [PubMed] [Google Scholar]
- 19.Wong CM, et al. Public health and air pollution in Asia (PAPA): a multicity study of short-term effects of air pollution on mortality. Environmental Health Perspectives 2008; 116: 1195–1202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Qiu H, et al. Greater temperature variation within a day associated with increased emergency hospital admissions for asthma. Science of the Total Environment 2015; 505: 508–513. [DOI] [PubMed] [Google Scholar]
- 21.Belsley DA, Kuh E. Welsch RE. Regression Diagnostics: Identifying Influential Data and Sources of Collinearity, vol. 571. Wiley-Interscience, 2005. [Google Scholar]
- 22.Kan H, et al. Differentiating the effects of fine and coarse particles on daily mortality in Shanghai, China. Environment International 2007; 33: 376–384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ma W, et al. Applied mixed generalized additive model to assess the effect of temperature on the incidence of bacillary dysentery and its forecast. PLoS ONE 2013; 8: e62122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Gasparrini A. Armstrong B. Time series analysis on the health effects of temperature: Advancements and limitations. Environmental Research 2010; 110: 633–638. [DOI] [PubMed] [Google Scholar]
- 25.Peng RD, Dominici F. Louis TA. Model choice in time series studies of air pollution and mortality. Journal of the Royal Statistical Society: Series A 2006; 169: 179–203. [Google Scholar]
- 26.Alexander KA, et al. Climate change is likely to worsen the public health threat of diarrheal disease in Botswana. International Journal of Environmental Research and Public Health 2013; 10: 1202–1230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Shuman EK. Global climate change and infectious diseases. New England Journal of Medicine 2010; 362: 1061–1063. [DOI] [PubMed] [Google Scholar]
- 28.Lin H, et al. Time series analysis of Japanese encephalitis and weather in Linyi City, China. International Journal of Public Health 2012; 57: 289–296. [DOI] [PubMed] [Google Scholar]
- 29.Lin HL, et al. Spatial and temporal distribution of falciparum malaria in China. Malaria Journal 2009; 8: 130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Lu L, et al. Time series analysis of dengue fever and weather in Guangzhou, China. BMC Public Health 2009; 9: 395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Patrick ME, et al. Effects of climate on incidence of Campylobacter spp. in humans and prevalence in broiler flocks in Denmark. Applied and Environmental Microbiology 2004; 70: 7474–7480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Li T, Yang Z. Wang M. Temperature and atmospheric pressure may be considered as predictors for the occurrence of bacillary dysentery in Guangzhou, Southern China. Revista da Sociedade Brasileira de Medicina Tropical 2014; 47: 382–384. [DOI] [PubMed] [Google Scholar]
- 33.Checkley W, et al. Effects of EI Niño and ambient temperature on hospital admissions for diarrhoeal diseases in Peruvian children. Lancet 2000; 355: 442–450. [DOI] [PubMed] [Google Scholar]
- 34.Kovats R, et al. The effect of temperature on food poisoning: a time-series analysis of salmonellosis in ten European countries. Epidemiology and Infection 2004; 132: 443–453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Lean JL. Rind DH. How will Earth's surface temperature change in future decades? Geophysical Research Letters 2009; 36: L15708. [Google Scholar]
- 36.Singh RB, et al. The influence of climate variation and change on diarrheal disease in the Pacific Islands. Environmental Health Perspectives 2001; 109: 155–159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Chou WC, et al. Modeling the impact of climate variability on diarrhea-associated diseases in Taiwan (1996–2007). Science of The Total Environment 2010; 409: 43–51. [DOI] [PubMed] [Google Scholar]
- 38.Auld H, MacIver D. Klaassen J. Heavy rainfall and waterborne disease outbreaks: the Walkerton example. Journal of Toxicology and Environmental Health Part A 2004; 67: 1879–1887. [DOI] [PubMed] [Google Scholar]
- 39.Hashizume M, et al. Association between climate variability and hospital visits for non-cholera diarrhoea in Bangladesh: effects and vulnerable groups. International Journal of Epidemiology 2007; 36: 1030–1037. [DOI] [PubMed] [Google Scholar]
- 40.Bandyopadhyay S, Kanji S. Wang L. The impact of rainfall and temperature variation on diarrheal prevalence in Sub-Saharan Africa. Applied Geography 2012; 33: 63–72. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
For supplementary material accompanying this paper visit https://doi.org/10.1017/S0950268815001156.
click here to view supplementary material