SUMMARY
The objective of this study was to identify the bioserotypes and virulence markers of Yersinia enterocolitica strains isolated from wild boars in Poland. Bacteriological examination of 302 rectal swabs from 151 wild boars resulted in the isolation of 40 Y. enterocolitica strains. The majority of the examined strains (n = 30), belonged to bioserotype 1A/NI. The presence of individual Y. enterocolitica strains belonging to bioserotypes 1B/NI (3), 1A/O:8 (2), 1A/O:27 (2), 2/NI (1), 2/O:9 (1) and 4/O:3 (1) was also demonstrated. Amplicons corresponding to ail and ystA genes were observed only in one Y. enterocolitica strain – bioserotype 4/O:3. The ail and ystB gene amplicons were noted in 11 Y. enterocolitica biotype 1A strains, although single amplicons of ystB gene were found in 28 of the tested samples. In four out of eight cases when two Y. enterocolitica strains were isolated from the same animal, the strains differed in biotype, serotype or virulence markers. The European population of wild boars continues to grow and spread to new areas, therefore, wild boars harbouring potentially pathogenic Y. enterocolitica 4/O:3 strains pose a challenge to public health.
Key words: Ail protein, bioserotype 4/O:3, enterotoxin Yst, hunted wild boars, Yersinia enterocolitica
INTRODUCTION
Yersiniosis is a zoonotic disease caused by Yersinia enterocolitica that occurs throughout the world. Y. enterocolitica has been divided into six biotypes based on its specific biochemical features: 1A, 1B, and 2–5. More than 70 serological groups of Y. enterocolitica have been identified based on chemical variations in the thermostable somatic O antigen. Most of the reported cases of yersiniosis in Poland are caused by bioserotypes 4/O:3, 2/O:9 and 1B/O:8. The presence of pYV (plasmid of Yersinia virulence), ail and ystA chromosomal genes has been noted in the above bioserotypes. The ail gene encodes the production of the Ail protein (attachment-invasion locus) responsible for adhesion to intestinal epithelial cells. By contrast, the ystA gene encodes the production of enterotoxin YstA, the main cause of diarrhoea during yersiniosis. Biotype 1A strains without pYV and major chromosomal virulence markers have been regarded as non-pathogenic [1, 2]. In recent years, however, a growing number of reports have revealed that Y. enterocolitica biotype 1A strains have been isolated in clinical cases of yersiniosis from patients with gastrointestinal complaints [3]. Although biotype 1A very rarely produces YstA enterotoxin, the ystB gene, which encodes the production of homologous and biologically active enterotoxin YstB, is found in more than 80% of cases. The above suggests that the potential pathogenicity of biotype 1A strains cannot be ruled out [4].
Numerous studies have demonstrated the presence of correlations between Y. enterocolitica strains isolated from clinically healthy pigs and from humans diagnosed with yersiniosis. The main source of human infection is raw or undercooked pork [2, 5, 6]. Outdoor pig farming is becoming increasingly popular, and wild boar populations have soared across Europe [7], which increases the risk of contact between wild boars and domestic pigs and contributes to the transmission of pathogenic Y. enterocolitica between animals. Until recently, there was a general paucity of information about wild boars as carriers of Y. enterocolitica. Wild boars are free-living animals that are closely related to swine, and they could constitute an important environmental reservoir of Y. enterocolitica. The growing popularity of foods that deliver health benefits, including game meat, increases the demand for the meat of wild animals. Evisceration of hunted animals without an appropriate hygiene protocol can expose hunters to Y. enterocolitica and lead to environmental contamination. Y. enterocolitica is able to survive and grow at low temperatures (e.g. in freezers where carcasses are stored), therefore, the consumption of contaminated meat poses a potential health risk for consumers [8].
The objective of this study was to identify the bioserotypes and virulence markers of Y. enterocolitica strains isolated from wild boars obtained during the 2012/2013 hunting season in Poland.
METHODS
Bacterial strains and culture conditions
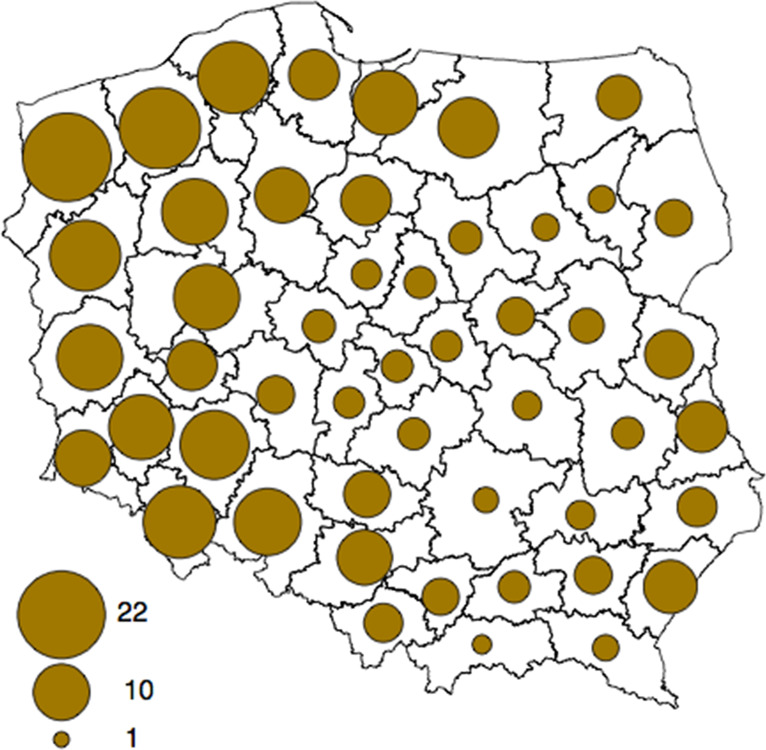
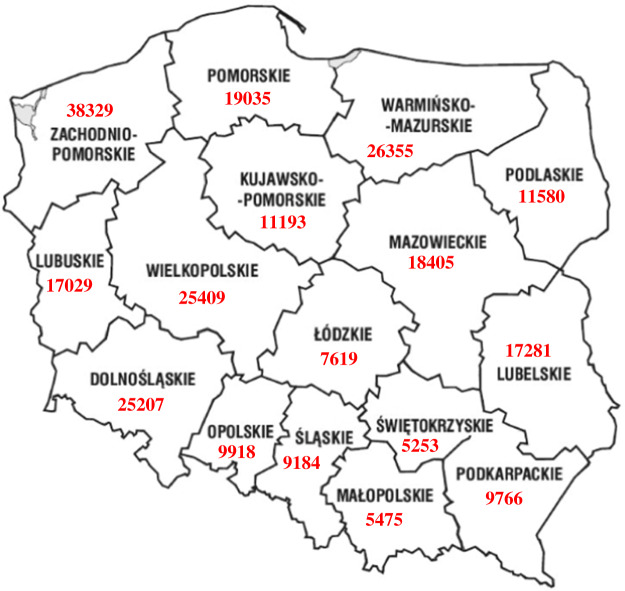
The materials for the study consisted of 302 rectal swabs obtained from 151 wild boars shot in the 2012/2013 hunting season in Poland (Fig. 1). Collection of the samples was not mandatory, it was based on voluntary cooperation with hunters. The samples were taken during both, individual and collective hunting, carried out under the natural shooting programme in Poland. The samples were taken from three different regions in Poland, where the wild boar population is large (Fig. 2). In northern Poland samples were taken from Warmińsko-Mazurskie voivodeship (province) (26255 head of wild boar) and Pomorskie voivodeship (19035 head of wild boar), in the central region of Poland from Łódzkie voivodeship (7619 head of wild boar) and in southern Poland from Lubelskie (17281 head of wild boar) and Podkarpackie (9766 head of wild boar) voivodeships. In central Poland, cooperation with hunters failed and we were only able to take samples from Łódzkie voivodeship. Two samples were taken from each animal, immediately after shooting and evisceration. One of the swabs was placed in a test tube containing 9 ml ITC (irgasan, ticarcillin and potassium chlorate medium) (warm culture, prepared according to PN-EN ISO 10273, incubated at 25 °C for 48 h). The other swab was placed simultaneously in a test tube containing 9 ml PSB (peptone, sorbitol and bile salts medium) (cold culture, prepared according to PN-EN ISO 10273, incubated at 4 °C for 3 weeks) to determine the ability of Y. enterocolitica to grow at low temperatures. Next, 0·5 ml of each culture was transferred to 4·5 ml of 0·5% KOH in 0·5% NaCl for 20 s, after which a loopful was streaked onto a CIN (cefsulodin, irgasan and novobiocin) plate and incubated at 30 °C for 48 h. Further biochemical identification of 1–5 typical colonies from each CIN plate was conducted according to the PN-EN ISO 10273 standard to select potentially pathogenic Y. enterocolitica strains.
Fig. 1.
Number of wild boars hunted in the hunting (forest) districts (units per 1000 ha of the total area). (Source: Polish Hunting Association.)
Fig. 2.
The structure of the wild boar population in Poland.
Serotype and biotype identification
The examined strains were serotyped by the slide agglutination test. Live bacterial cells from a 24-h blood agar culture (Graso Biotech, Poland) were used as the antigen, and the sera for the most common somatic antigens O:3, O:5, O:8, O:9 and O:27 were supplied by ITEST (Hradec Kralove, Czech Republic). The cells of the tested strains were suspended in a drop of 0·85% NaCl on a glass slide and mixed with a drop of serum using an inoculation loop. After shaking for 1 min, agglutination with one of the five sera used was regarded as a positive result. The strain was classified as non-identified (NI) in the absence of agglutination with any of the sera. The examined strains were biotyped based on pyrazinamidase and Tween esterase activity, esculin hydrolysis, indole production, and salicin, xylose and trehalose fermentation according to the PN-EN ISO 10273 standard.
DNA isolation
Genomic DNA isolation was performed with the Genomic Mini kit (A&A Biotechnology, Poland) according to the manufacturer's instructions and isolates were stored at -20 °C for further analyses.
Primers and triplex polymerase chain reaction (PCR) conditions
Triplex PCR included the amplification of three chromosomal genes: ail, ystA, and ystB. The sequences of the primers (synthesized in the DNA Sequencing Laboratory of the Biochemistry and Biophysics Institute of the Polish Academy of Sciences, Warsaw) and triplex PCR conditions have been described in a previous paper [9]. The only modification was starter connection temperature, which was set at 45 °C. Triplex PCR was performed using HotStarTaq Plus DNA polymerase (Qiagen GmbH, Germany) and the HotStarTaq Plus Master Mix kit (Qiagen). The 20-μl reaction mixture contained ~120 ng of isolated DNA (1–3 μl), 10 μl HotStarTaq Plus Master Mix 2x, 2 μl CoralLoad Concentrate 10x, and 0·1 μl of each primer (final concentration of 0·5 μm), and was supplemented with up to 20 μl RNase-free water. Three controls were applied in each reaction: two positive controls with DNA isolated from reference strains O:8 and O:5, and one negative control without DNA. After electrophoretic separation, the size of the products was evaluated by comparison with the standard mass of GeneRuler 100-bp Ladder Plus (Fermentas UAB, Lithuania). The following reaction products were searched: fragments of the ail gene (size 356 bp), the ystA gene (size 134 bp), and the ystB gene (size 180 bp). The specificity of some of the products was confirmed by purification with the CleanUp kit (A&A Biotechnology) and sequencing (Genomed, Poland).
RESULTS
Bacteriological examination of 302 rectal swabs from 151 wild boars resulted in the isolation of 40 Y. enterocolitica strains. Y. enterocolitica accounted for 13·2% of the analysed samples and was identified in 26·5% of the tested animals. The majority (80·0%) of the 32 strains originated from northern Poland, seven (17·5%) were sampled in the central part of the country and only one (2·5%) in southern Poland (Table 1). Twelve (30·0%) of the 40 identified strains originated from warm culture (ITC) and 28 (70·0%) from cold culture (PSB). Most (34, 85%) of the examined strains belonged to biotype 1A, only three (7·5%) were identified as biotype 1B, two (5·0%) as biotype 2 and one (2·5%) as biotype 4. The number of strain biotypes isolated from each type of culture is given in Table 2.
Table 1.
Yersinia enterocolitica strains isolated from various sampling sites
| Region/forest district | No. of wild boars | No. of samples | No. of isolated Y. enterocolitica strains | No. of wild boars | No. of samples | No. of isolated Y. enterocolitica strains | ||
|---|---|---|---|---|---|---|---|---|
| No. | (%) | No. | (%) | |||||
| Northern Poland | ||||||||
| Górowo Iławeckie | 4 | 8 | 0 | (0·0) | 99 | 198 | 32 | (16·1) |
| Miłomłyn | 19 | 38 | 4 | (10·5) | ||||
| Mrągowo | 26 | 52 | 3 | (5·8) | ||||
| Olsztyn | 4 | 8 | 2 | (25·0) | ||||
| Trzebielino | 46 | 92 | 23 | (25·0) | ||||
| Central Poland | ||||||||
| Spała | 25 | 50 | 7 | (14·0) | 25 | 50 | 7 | (14·0) |
| Southern Poland | ||||||||
| Rudnik | 8 | 16 | 0 | (0·0) | 27 | 54 | 1 | (1·8) |
| Tomaszów | 19 | 38 | 1 | (2·6) | ||||
Table 2.
Biotyping results for Yersinia enterocolitica strains isolated from wild boars
| No. of strains | Biotype | Total | |||
|---|---|---|---|---|---|
| 1A | 1B | 2 | 4 | ||
| Total | 34 (85·0%) | 3 (7·5%) | 2 (5·0%) | 1 (2·5%) | 40 (100·0%) |
| Warm culture | 9 (75·0%) | 1 (8·3%) | 2 (16·7%) | 0 (0·0%) | 12 (100·0%) |
| Cold culture | 25 (89·3%) | 2 (7·1%) | 0 (0·0%) | 1 (3·6%) | 28 (100·0%) |
Agglutination with diagnostic serum for somatic antigen O:3 was observed only in the Y. enterocolitica biotype 4 strain (2·5%). A similar, single positive result was reported in a biotype 2 strain agglutinated with O:9 serum. Reactions with diagnostic serum for somatic antigen O:8 were observed in two (5·0%) Y. enterocolitica biotype 1A strains, and a positive result of two Y. enterocolitica biotype 1A strains for O:27 serum were also noted. Agglutination with diagnostic serum for somatic antigen O:5 was not observed, the remaining 85·0% of the examined Y. enterocolitica strains were regarded as NI due to the absence of agglutination with any of the analysed sera.
The presence of genes directly linked with the pathogenicity of Y. enterocolitica was evaluated by triplex PCR with primers for ail, ystA, and ystB gene fragments. Amplicons corresponding to ail and ystA genes were observed only in Y. enterocolitica biotype 4, serotype O:3 strain (2·5%). Amplicons corresponding to ail and ystB genes were noted in 11 (27·5%) Y. enterocolitica biotype 1A strains, including one (2·5%) serotype O:27 strain and 10 (25·0%) NI strains. The products whose size corresponded only to the ystB gene were found in 28 (70·0%) of the tested samples, including 20 bioserotype 1A/NI strains; two bioserotype 1A/O:8 strains; one bioserotype 1A/O:27 strain; three bioserotype 1B/NI strains; one bioserotype 2/NI strain and one bioserotype 2/O:9 strain. The results of triplex PCR, including the number, biotype and serotype characteristics of the isolated Y. enterocolitica strains are presented in Table 3.
Table 3.
Triplex PCR results, including the number, biotype and serotype characteristics of the isolated Yersinia enterocolitica strains
| Bioserotype | Virulence markers | No. of strains | ||
|---|---|---|---|---|
| ail | ystA | ystB | ||
| 1A/NI | + | 20 | ||
| + | + | 10 | ||
| 1A/O:8 | + | 2 | ||
| 1A/O:27 | + | 1 | ||
| + | + | 1 | ||
| 1B/NI | + | 3 | ||
| 2/NI | + | 1 | ||
| 2/O:9 | + | 1 | ||
| 4/O:3 | + | + | 1 | |
NI, Non-identified.
In four (50·0%) out of eight cases when two Y. enterocolitica strains were isolated from the same animal, the strains differed in bioserotype (2/O:9 and 1A/NI; 1A/O:27 and 1A/NI; 1B/NI and 1A/NI), and in both bioserotype and virulence markers (2/NI ystB and 1A/NI ail, ystB). The above results could suggest that the gastrointestinal tract was colonized by more than one type of Y. enterocolitica strain.
DISCUSSION
The growing significance of Y. enterocolitica as an aetiological factor in human diarrhoea has prompted research into the pathogen's prevalence in various species of wild animals [9–12]. Currently, more than 70 serotypes and six biotypes of Y. enterocolitica have been identified. Bacteriological analyses, in particular cold culture, involve a lengthy waiting period, but provides highly valuable information and should not be omitted. In this study, 12 Y. enterocolitica strains were isolated from warm culture, and 28 strains from cold culture. The results of other studies also suggest that both types of culture should be examined, in particular cold culture, which is most specific for Y. enterocolitica [13–15].
The bioserotype concept has been introduced to describe the correlations between biotype and serotype. In this study, the predominant bioserotype was 1A/NI. The serological variations in biotype 1A have been researched extensively [1, 2, 16, 17]. The presence of single Y. enterocolitica strains belonging to bioserotypes 1A/O:8, 1A/O:27, 1B/NI, 2/NI, 2/O:9 and 4/O:3 was also demonstrated. The prevalence of Y. enterocolitica in wild boars has rarely been studied in Poland, Europe and other regions of the world. One of the first studies investigating the presence of Yersinia spp. in wild boars in Poland was performed by Koronkiewicz et al. [8] who identified one out of 45 strains isolated from wild boar faeces based on biochemical markers for Yersinia spp. Serological and molecular identification was not performed, and the strain could not be verified as Y. enterocolitica. The analysis of swabs from wild boar carcasses produced negative results [8]. In northeastern Germany, Al Dahouk et al. [18] identified antibodies against Yop proteins in Yersinia spp. pathogenic strains in 478 (62·6%) out of 763 examined serum samples. By contrast, in the work of Koppel et al. [7], who evaluated the prevalence of infectious diseases in wild boars in Switzerland, the results of bacteriological tests for Y. enterocolitica were negative. Y. enterocolitica strains were not biotyped or serotyped in the above studies. In Bulgaria, Nikolova et al. [19] isolated 46 Yersinia spp. strains from different organs of 37 animals. Fifteen of the isolated strains were identified as serotype O:3, two (5·4%) as serotype O:5, two (5·4%) as serotype O:8 and two (5·4%) as serotype O:9. The cited study did not involve a molecular analysis, but virulence-associated properties were detected in 21 Y. enterocolitica strains in calcium dependence and autoagglutination assays and in a pyrazinamidase activity test.
Fast and reliable molecular methods are required to overcome the problems associated with the identification of Y. enterocolitica strains. In a previous study [10], the authors performed one of the first molecular analyses of Y. enterocolitica strains isolated from wild boars, and the results were used in extended research. The above study [10] analysed the occurrence of genes directly linked to the pathogenicity of Y. enterocolitica strains isolated from wild boars hunted in the 2007/2008 hunting season in northeastern Poland. Palatine tonsils from 46 wild boars of various ages were subjected to bacteriological tests. The DNA of two Y. enterocolitica strains with bioserotypes 1A/O:5 and 1A/NI was isolated, and ail, ystA and ystB genes were targeted by triplex PCR. Both strains (4·34%) possessed the ystB gene fragment, whereas ail and ystA genes were not detected in any of the tested samples. This study, extended to other Polish regions, showed that the prevalence of Y. enterocolitica in wild boars in northern and central Poland was higher than Y. enterocolitica prevalence in wild boars in the southern region of Poland (Supplementary Fig. S1). However, more comprehensive studies are necessary to confirm this observation. Sannö et al. [20] relied on a combination of cultivation and PCR analyses to isolate 18 Y. enterocolitica strains from 88 examined wild boars, which implies that 20% of individuals tested positive for the pathogen. Pathogenic Y. enterocolitica strains were also isolated by Fredriksson-Ahomaa et al. [21] from 9% of wild boars and were molecularly examined. Bioserotypes 2/O:5,27, 2/O:9 and 4/O:3 were identified in Y. enterocolitica strains isolated from three, four and five animals, respectively. The prevalence of anti-Yersinia spp. antibodies was higher than in the study by Al Dahouk et al. [18], and was determined at 65·0%. ail-positive Y. enterocolitica strains were isolated from the tonsils of 14 (9%) animals and were identified as belonging to bioserotypes 2/O:5,27, 2/O:9 and 4/O:3. Wacheck et al. [22] identified ail gene fragments in Y. enterocolitica strains isolated from 35% of the examined wild boar tonsils by real-time PCR. On the other hand, Y. enterocolitica was detected in only 5% of the examined faecal samples, and serotypes O:5,27, O:9, and O:3 were identified in three (21·0%), four (29·0%), and five (36·0%) strains, respectively. According to above studies, wild boars appear to be an important reservoir of Y. enterocolitica.
Our most important finding was the identification of the first pathogenic Y. enterocolitica 4/O:3 strain in a wild boar in Poland. Its pathogenicity was confirmed by triplex PCR that detected ail and ystA gene fragments based on product size. According to the European Food Safety Authority (EFSA), bioserotype 4/O:3 is widely distributed, and it is responsible for most cases of human yersiniosis in Europe. This bioserotype had been previously detected in wild boars [5, 6], but the ystA gene was not identified. The pathogenic, ail- and ystA-positive Y. enterocolitica O:3 strain isolated from a hunted wild alpine ibex in 2012 was identified in only one study [11]. In a study of wild boars and domestic pigs conducted by Fredriksson-Ahomaa et al. [6], five (36·0%) out of 14 ail-positive Y. enterocolitica strains isolated from wild boars belonged to bioserotype 4/O:3. In wild boars, bioserotypes 2/O:9 and 2/O:5·27 were identified in four (29·0%) and three (21·0%) strains, respectively. Two ail-positive Y. enterocolitica strains isolated from wild boars were not biotyped or serotyped. All Y. enterocolitica strains belonging to bioserotypes that are pathogenic for humans carried yst (without diversification into types A, B or C) and hreP genes associated with virulence. The above genes were not found in two ail-positive Y. enterocolitica strains that could not be biotyped or serotyped. This could indicate lower pathogenicity of the ail gene, and it could suggest that the presence of the ail gene alone is not a sufficient virulence marker for identifying human pathogenic strains. The above observations are consistent with the results of the present study where ail gene fragments were detected in 12 out of 40 Y. enterocolitica strains. However, fragments of the ail gene as well as the ystA gene, which is highly associated with Y. enterocolitica pathogenicity, were identified in the analysed strains in only one case. The remaining ail gene fragments were detected together with ystB gene fragments in 10 strains with the 1A/NI bioserotype and in one strain with the 1A/O:27 bioserotype. Half of all examined Y. enterocolitica strains belonging to the 1A/NI bioserotype had the ail gene fragment. In the work of Sihvonen et al. [23], the partial ail gene nucleotide sequences of two Y. enterocolitica biotype 1A strains were identical and similar in 99·7% (one difference per 394 bp) to the ail gene sequence of Y. enterocolitica biotype 1B serotype O:8 strain 8081 (GenBank accession no. M29945). The alignment of the complete ail coding DNA sequences (CDS), made by Kraushaar et al. [24], revealed that the ail sequence of the Y. enterocolitica biotype 1A strain examined by these authors is slightly different to the ail CDS of the pathogenic strains. Although the isolated Y. enterocolitica biotype 1A strains rarely harboured the ail gene and were free of most classical virulence genes, their pathogenic potential cannot be ruled out [24].
An interesting observation was made in four (50·0%) out of eight cases when two Y. enterocolitica strains isolated from the same animal differed in biotype, serotype and virulence markers. Similar results were reported by Nikolova et al. [19] who isolated Y. enterocolitica strains from different organs of the same animal. The above observation could indicate that the gastrointestinal tract was colonized by more than one type of Y. enterocolitica strain. Our results also suggest that wild boars are a reservoir and a potential vector of Y. enterocolitica infection in humans.
Numerous studies have demonstrated that wild boars may constitute a reservoir of the bacteria and a potential source of Y. enterocolitica infection in humans. The risk of infection is particularly high in people who come into direct contact with wild boars or their meat, including hunters, veterinary practitioners and consumers. For this reason, yersiniosis is a zoonotic disease with significant implications for public health. The correlations between Y. enterocolitica strains isolated from wild boars and the prevalence of yersiniosis in humans have not been fully explained and require more extensive research. The presence of Y. enterocolitica 4/O:3 strains in wild boars, confirmed molecularly in this study, suggests that the analysed animal species is a carrier responsible for shedding pathogenic Y. enterocolitica into the environment. It also indicates that similarly to pigs, wild boars are an important reservoir of enteropathogenic Y. enterocolitica and a source of infection for humans by contaminated meat.
ACKNOWLEDGEMENTS
This study was supported by the National Centre for Research and Development (NCBiR, grant no. NR12-0126-10).
DECLARATION OF INTEREST
None.
Supplementary material
For supplementary material accompanying this paper visit https://doi.org/10.1017/S0950268814003872.
click here to view supplementary material
REFERENCES
- 1.Tennant SM, Grant TH, Robins-Browne RM. Pathogenicity of Yersinia enterocolitica biotype 1A. FEMS Microbiological Letters 2003; 38: 127–137. [DOI] [PubMed] [Google Scholar]
- 2.Singh I, Virdi JS. Production of Yersinia stable toxin (YST) and distribution of yst genes in biotype 1A strains of Yersinia enterocolitica. Journal of Medical Microbiology 2004; 53: 1065–1068. [DOI] [PubMed] [Google Scholar]
- 3.Batzilla J, Heesemann J, Rakin A. The pathogenic potential of Yersinia enterocolitica 1A. International Journal of Medical Microbiology 2011; 301: 556–61. [DOI] [PubMed] [Google Scholar]
- 4.Grant T, Bennett-Wood V, Robins-Browne RM. Identification of virulence-associated characteristics in clinical isolates of Yersinia enterocolitica lacking classical virulence markers. Infection and Immunity 1998; 66: 1113–1120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Fredriksson-Ahomaa M, Stolle A, Stephan R. Prevalence of pathogenic Yersinia enterocolitica in pigs slaughtered at a Swiss abattoir. International Journal of Food Microbiology 2007; 119: 207–212. [DOI] [PubMed] [Google Scholar]
- 6.Fredriksson-Ahomaa M, et al. Different enteropathogenic Yersinia strains found in wild boars and domestic pigs. Foodborne Pathogens and Disease 2011; 8: 733–737. [DOI] [PubMed] [Google Scholar]
- 7.Koppel C, et al. Serosurveillance for selected infectious disease agents in wild boars (Sus scrofa) and outdoor pigs in Switzerland. European Journal of Wildlife Research 2007; 53: 212–220. [Google Scholar]
- 8.Koronkiewicz A, et al. Game animals as carriers of enteric pathogens. Folia Universitatis Agriculturae Stetinensis Scientia Alimentaria 2004; 238: 79–84. [Google Scholar]
- 9.Bancerz-Kisiel A, et al. Bioserotypes and virulence markers of Yersinia enterocolitica strains isolated from mallards (Anas platyrhynchos) and pheasants (Phasianus colchicus). Journal of Food Protection 2012; 75: 2219–222. [DOI] [PubMed] [Google Scholar]
- 10.Bancerz-Kisiel A, et al. Application of multiplex PCR for the evaluation of the occurrence of ail, ystA and ystB genes in Yersinia enterocolitica strains isolated from wild boars (Sus scrofa). Bulletin of the Veterinary Institute in Pulawy 2009; 53: 351–355. [Google Scholar]
- 11.Joutsen S, et al. Pathogenic Yersinia enterocolitica O:3 isolated from a hunted wild alpine ibex. Epidemiology and Infection 2012; 141: 612–617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Bancerz-Kisiel A, et al. Bioserotypes and virulence markers of Y. enterocolitica strains isolated from roe deer (Capreolus capreolus) and red deer (Cervus elaphus). Polish Journal of Veterinary Sciences 2014; 17: 315–319. [DOI] [PubMed] [Google Scholar]
- 13.Kot B, Trafny EA, Jakubczak A. Application of multiplex PCR for monitoring colonization of pig tonsils by Yersinia enterocolitica, including biotype 1A, and Yersinia pseudotuberculosis. Journal of Food Protection 2007; 70: 1110–1115. [DOI] [PubMed] [Google Scholar]
- 14.Sihvonen LM, et al. Yersinia enterocolitica and Y. enterocolitica-like species in clinical stool specimens of humans: identification and prevalence of bio/serotypes in Finland. European Journal of Clinical Microbiology & Infectious Diseases 2009; 28: 757–765. [DOI] [PubMed] [Google Scholar]
- 15.Laukkanen R, et al. Evaluation of isolation methods for pathogenic Yersinia enterocolitica from pig intestinal content. Journal of Applied Microbiology 2010; 108: 956–964. [DOI] [PubMed] [Google Scholar]
- 16.Singh I, Virdi JS. Interaction of Yersinia enterocolitica biotype 1A strains of diverse origin with cultured cells in vitro. Japanese Journal of Infectious Diseases 2005; 58: 31–33. [PubMed] [Google Scholar]
- 17.Tennant SM, et al. Homologues of insecticidal toxin complex genes in Yersinia enterocolitica biotype 1A and their contribution to virulence. Infection and Immunity 2005; 73: 6860–6867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Al Dahouk S, et al. Seroprevalence of brucellosis, tularemia and yersiniosis in wild boar (Sus scrofa) from north-eastern Germany. Journal of Veterinary Medicine B, 2005; 52: 444–455. [DOI] [PubMed] [Google Scholar]
- 19.Nikolova S, et al. Isolation of pathogenic Yersiniae from wild animals in Bulgaria. Journal of Veterinary Medicine B 2001; 48: 203–209. [DOI] [PubMed] [Google Scholar]
- 20.Sannö A, et al. Presence of Salmonella spp., Yersinia enterocolitica, Yersinia pseudotuberculosis and Escherichia coli O157:H7 in wild boars. Epidemiology and Infection 2014; 6: 1–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Fredriksson-Ahomaa M, et al. Prevalence of pathogenic Yersinia enterocolitica and Yersinia pseudotuberculosis in wild boars in Switzerland. International Journal of Food Microbiology 2009; 135: 199–202. [DOI] [PubMed] [Google Scholar]
- 22.Wacheck S, et al. Wild boars as an important reservoir for foodborne pathogens. Foodborne Pathogens and Diseases 2010; 3: 307–312. [DOI] [PubMed] [Google Scholar]
- 23.Sihvonen LM, et al. The ail gene is present in some Yersinia enterocolitica biotype 1A strains. Foodborne Pathogens and Diseases 2011; 8: 455–457. [DOI] [PubMed] [Google Scholar]
- 24.Kraushaar B, et al. Characterization of a Yersinia enterocolitica biotype 1A strain harbouring an ail gene. Journal of Applied Microbiology 2011; 111: 997–1005. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
For supplementary material accompanying this paper visit https://doi.org/10.1017/S0950268814003872.
click here to view supplementary material