SUMMARY
Limited information is available on the prevalence of hepatitis C virus (HCV) in the general population in China. A community-based epidemiological study was conducted in three counties in eastern China. A total of 149 175 individuals were investigated in 60 communities in three counties in Jiangsu province, eastern China, of whom 1175 subjects [0·79%, 95% confidence interval (CI) 0·74–0·83] were HCV antibody positive. The prevalence was low in children (0·09%, 95% CI 0·04–0·17), but increased progressively from adolescents (0·20%, 95% CI 0·15–0·28) to adults aged ⩾21 years (95% CI 0·15–1·64). Women had a higher prevalence of HCV infection than men in most age groups. In a multilevel regression analysis, age, sex, education, occupation, blood transfusion [odds ratio (OR) 2·91, 95% CI 1·09–5·37], invasive testing (OR 1·28, 95% CI 1·14–1·61), and dental therapy (OR 2·27, 95% CI 1·41–3·42) were associated with HCV infection. In conclusion, although the prevalence of HCV in this population was lower than reported from national levels, the total reservoir of infection is significant and warrants public health measures, such as health education to limit the magnitude of the problem.
Key words: Epidemiological study, general population, hepatitis C virus antibody, risk factor
INTRODUCTION
Hepatitis C virus (HCV) is a serious public health problem. The global prevalence of HCV infection is about 3%, representing 170 million people worldwide [1, 2]. Infection with HCV can cause slowly progressive liver damage that may lead to cirrhosis and/or liver cancer after many decades [3]. Effective therapy, with pegylated interferon and ribavirin, is available and can reduce mortality [4, 5]. China, still a developing country, has a high mortality rate due to hepatocellular carcinoma, and eastern China has a hepatocellular carcinoma mortality rate higher than that of China as a whole [3, 6]. The national prevalence rate in China for antibody HCV (anti-HCV) positivity is 2·20% [7].
HCV infection is mainly transmitted by percutaneous exposure to contaminated blood, perinatal exposure from a mother to her infant and unprotected sexual intercourse [2, 3]. The prevalence of HCV infection is higher in high-risk Chinese populations such as drug users (ranging from 18·30% to 66·97%), and patients undergoing regular dialysis (a pooled prevalence rate of 41·1%) [8–12]. Although a number of studies on high-risk populations are available, there is a lack of information on the prevalence of HCV infection in the general population. Meanwhile, few studies have reported on the relationship between socioeconomic status and HCV carriage status.
In this study, we planned a population-based serological study to determine the prevalence of anti-HCV in all age groups and to identify possible risk factors for acquiring the infections in eastern China. We hope the findings will eventually guide the development, adaptation, and evaluation of prevention strategies.
MATERIALS AND METHODS
Methods
This study was approved by the Institutional Review Board of Nanjing Medical University (Nanjing, China). Planning for this study began in January 2011 and data analysis was completed in December 2012. All field work was conducted from September 2011 to July 2012.
Participants
The target population was local residents in all age groups living in Jiangsu province, eastern China (Fig. 1). These subjects were selected as being representative of the population of eastern China; there was no statistical difference in demographics, the situation of the population or economic conditions between participants in this study and the whole of eastern China. All local residents with >6 months' residency at the time of the survey were selected. A list of residents was obtained from the residents' committee.
Fig. 1.
Map of Jiangsu, eastern China, showing the three counties (black areas) selected for sampling in the study.
Sampling method
The sample size was calculated as follows:
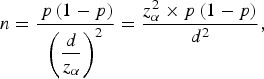 |
where p is the estimated prevalence rate, d represents power and was calculated by 0·1 × p and zα is the statistics of α. For this study, α was set at 0·05. Considering the variance of HCV prevalence in different age groups in the survey (0·4% for age <40 years and 1·2% for age ⩾40 years), the desired sample size was 132 534, and included 99 600 young people aged <40 years and 32 934 people aged ⩾40 years.
To represent the entire province's population, a multistage sampling method was used for this study. First, all the provinces were divided into three major regional groups (south, middle, north) where there was a large difference in earnings, education level, and awareness of healthcare and the healthcare system by the inhabitants. One county was randomly selected from each major regional group. As a result, Zhangjiagang, Danyang and Taixing were chosen to represent the south, middle and north sites, respectively (Fig. 1). Second, there were 887 communities in these three counties, and 20 communities were randomly chosen from each county. Finally, all local residents living in the 60 communities for >6 months at the time of the survey were the targeted subjects to be investigated by cluster sampling in this study.
Investigation
In each community hospital of the 60 communities, there was a survey centre with a team of 20–25 trained staff comprising physicians, nurses, and community doctors to perform the investigation. Before the survey, community doctors issued a letter of physical examination notice to each household on the list of residents. The letter introduced the survey objective, examination items and matters requiring attention. Local residents willing to enrol in the study arrived at the survey centres in the community hospital at the appointed time. A standard questionnaire was used to obtain basic information including gender, birth date, education, occupation, marital status, smoking, drinking, history of hypertension and diabetes, and potential risk factors through a face-to-face interview with the study subject or parent (if the participant was aged <15 years). Definitions of education and occupation were developed according to the Chinese social classification criteria, and were only applicable to the population aged 15–59 years. The occupation of public service worker was defined as a person who works in a hotel, hospital, barbershop, transport centre, etc., and who has high frequency of contact with the public.
Serological testing
Blood samples collected from each study participant included 5 ml for the population aged >2 years and 2 ml for children aged ⩽2 years or less. Blood samples were collected without anticoagulant and were separated by centrifugation at room temperature. Serum samples were stored at −70 °C. These procedures were completed within 6 h of sample collection. All samples were collected and frozen according to standardized procedures and tested in a central laboratory. Anti-HCV antibodies were detected by enzyme-linked immunoassay with commercial kits (Beijing Wantai Biological Pharmacy Engineering Co. Ltd, China), according to the manufacturer's instructions. Each reaction plate included two negative controls, three positive controls, and one blank control. More than 10% of the samples for which anti-HCV antibody was positive were randomly selected for repeated assays, and the results were 100% concordant.
Statistical analysis
Differences in demographic characteristics between cases and non-cases were calculated using Student's t test or one-way analysis of variance (for continuous variables) and the χ2 test (for categorical variables). The associations of potential risk factors with HCV infection risk were estimated by computing the odds ratios (ORs) and their 95% confidence intervals (CIs) from both univariable and a multivariable multi-level logistic regression analyses with individuals, communities and counties as the different levels. All statistical analyses were performed with SAS v. 9·1·3 software (SAS Institute Inc., USA), and P < 0·05 in a two-sided test was considered statistically significant.
RESULTS
Basic characteristics
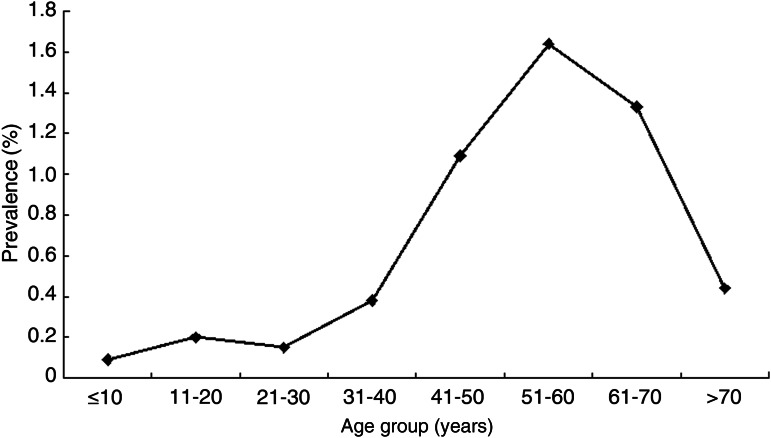
In this study, 149 175 participants was surveyed including 57 188 for Zhangjiagang, 48 422 for Danyang and 43 565 for Taixing. Significant differences were observed for age group in those three counties [mean 37·07 (s.d. = 20·56) years for Zhangjiagang, mean 44·52 (s.d. = 19·77) years for Danyang and mean 45·67 (s.d. = 18·10) years for Taixing; P < 0·001]. Distrubution of gender was not different in the three areas. Of the 149 175 subjects studied, 1175 were anti-HCV positive, while the overall prevalence of anti-HCV in this study population was 0·79% (95% CI 0·74–0·83): Zhangjiagang (0·13%, 95% CI 0·11–0·16), Danyang (0·32%, 95% CI 0·27–0·37) and Taixing (2·17%, 95% CI 2·04–2·32). There was a great variation of anti-HCV positivity in the different age groups (Table 1, Fig. 2). The prevalence was low in children (0·09%, 95% CI 0·04–0·17), but increased progressively from adolescents (0·20%, 95% CI 0·15–0·28) to adults aged ⩾21 years (0·15–1·64%). A gender difference was also observed for anti-HCV prevalence in participants. Women had a higher prevalence of HCV infection than men in most age groups; the prevalence of anti-HCV was 0·57 (95% CI 0·52–0·63) and 0·97 (95% CI 0·90–1·03) in males and females, respectively (Table 1).
Table 1.
Age- and sex-specific prevalence of anti-HCV in eastern China
| Age group, years | Male | Female | Total |
|---|---|---|---|
| (no. positive/no. tested), % | (no. positive/no. tested), % | (no. positive/no. tested), % | |
| ⩽10 | 4/5 323 (0·08) | 5/4 749 (0. 11) | 9/1 0072 (0·09) |
| 11–20 | 26/1 1093 (0·23) | 15/9 044 (0·17) | 41/20 137 (0·20) |
| 21–30 | 8/5 628 (0·14) | 11/7 168 (0·15) | 19/12 796 (0·15) |
| 31–40 | 18/9 345 (0·19) | 67/13 270 (0·50) | 85/22 615 (0·38) |
| 41–50 | 68/11 508 (0·59) | 246/17 256 (1·43) | 314/28 764 (1·09) |
| 51–60 | 142/11 379 (1·24) | 274/13 980 (1·96) | 416/25 359 (1·64) |
| 61–70 | 96/8 319 (1·15) | 146/9 911 (1·47) | 242/18 230 (1·33) |
| >70 | 24/4 835 (0·50) | 25/6 367 (0·39) | 49/11 202 (0·44) |
| All ages | 386/67 430 (0·57) | 789/81 745 (0·97) | 1175/149 175 (0·79) |
Fig. 2.
Age-specific prevalence of anti-HCV in eastern China.
The prevalence of anti-HCV stratified by demographic and selected variables in this study population is described in Table 2. Significant differences were observed for age, sex, marital status, education, occupation, smoking and drinking status and body mass index (BMI) regarding the prevalence of anti-HCV. The prevalence of anti-HCV was higher in participants who were older, heavier, heavier smokers, drinkers or with a previous diagnosis of hypertension or diabetes (P< 0·05 for both comparisons). However, factors such as BMI, and previous diagnosis of hypertension or diabetes might not be truly related to HCV prevalence. Although the sample size of the study was large, any small difference could be detected significantly. HCV prevalence in subjects with a history of hospitalization, surgery, invasive testing, acupuncture, dental therapy and sharing a toothbrush was higher (Table 2).
Table 2.
Prevalence of anti-HCV stratified by demographic and selected variables in eastern China
| Variables | Anti-HCV negative | Anti-HCV positive | |
|---|---|---|---|
| (N = 148 000), n (%) | (N = 1175), n (%) | P value | |
| Age (years) | |||
| ⩽10 | 10 063 (99·91) | 9 (0·09) | <0·001 |
| 11–20 | 20 096 (99·80) | 41 (0·20) | |
| 21–30 | 12 777 (99·85) | 19 (0·15) | |
| 31–40 | 22 530 (99·62) | 85 (0·38) | |
| 41–50 | 28 450 (98·91) | 314 (1·09) | |
| 51–60 | 24 943 (98·36) | 416 (1·64) | |
| 61–70 | 17 988 (98·67) | 242 (1·33) | |
| >70 | 11 153 (99·56) | 49 (0·44) | |
| Sex | <0·001 | ||
| Male | 67 044 (99·43) | 386 (0·57) | |
| Female | 80 956 (99·03) | 789 (0·97) | |
| Marital status | <0·001 | ||
| Unmarried | 6 714 (99·75) | 17 (0·25) | |
| Married | 105 444 (99·00) | 1062 (1·00) | |
| Divorced | 6 228 (99·28) | 45 (0·72) | |
| Unknown | 442 | 2 | |
| Education | <0·001 | ||
| Primary school | 57 057 (98·90) | 637 (1·10) | |
| Middle school | 81 485 (99·38) | 508 (0·62) | |
| College school | 8704 (99·73) | 24 (0·27) | |
| Unknown | 754 | 6 | |
| Occupation | <0·001 | ||
| Agricultural worker | 39 912 (98·42) | 642 (1·58) | |
| Worker | 40 761 (99·28) | 294 (0·72) | |
| Student | 29 516 (99·84) | 47 (0·16) | |
| Civil servant | 5190 (99·77) | 12 (0·23) | |
| Shop assistant | 5274 (99·43) | 30 (0·57) | |
| Unemployed | 21 711 (99·48) | 114 (0·52) | |
| Others | 4928 (99·44) | 28 (0·56) | |
| Unknown | 708 | 8 | |
| Smoking status | 0·005 | ||
| Never | 115 216 (99·22) | 901 (0·78) | |
| Current | 27 003 (99·17) | 227 (0·83) | |
| Past | 2699 (98·68) | 36 (1·32) | |
| Unknown | 3082 | 11 | |
| Drinking status | <0·001 | ||
| Never | 123 514 (99·28) | 897 (0·72) | |
| Frequently | 5902 (98·01) | 120 (1·99) | |
| Every day | 2124 (98·97) | 22 (1·03) | |
| Unknown | 16 460 | 136 | |
| Body mass index (kg/m2) | |||
| <18·5 | 18 541 (99·75) | 46 (0·25) | <0·001 |
| 18·5–24 | 70 942 (99·19) | 576 (0·81) | |
| 24–28 | 42 987 (98·99) | 439 (1·01) | |
| >28 | 15 530 (99·27) | 114 (0·73) | |
| P* for trend | <0·001 | ||
| History of hypertension | <0·001 | ||
| No | 127 805 (99·28) | 930 (0·72) | |
| Yes | 20 195 (98·80) | 245 (1·20) | |
| History of diabetes | 0·001 | ||
| No | 145 026 (99·22) | 1136 (0·78) | |
| Yes | 2974 (98·71) | 39 (1·29) | |
| Hospitalization | <0·001 | ||
| No | 101 478 (99·27) | 749 (0·73) | |
| Yes | 46 522 (99·09) | 426 (0·91) | |
| Surgery | <0·001 | ||
| No | 115 925 (99·28) | 844 (0·72) | |
| Yes | 32 075 (98·98) | 331 (1·02) | |
| Blood transfusion | <0·001 | ||
| No | 144 839 (99·25) | 1095 (0·75) | |
| Yes | 3161 (97·53) | 80 (2·47) | |
| Blood donation | 0·411 | ||
| No | 143 118 (99·21) | 1141 (0·79) | |
| Yes | 4882 (99·33) | 33 (0·67) | |
| Invasive testing | <0·001 | ||
| No | 135 049 (99·31) | 943 (0·69) | |
| Yes | 12 951 (98·24) | 232 (1·76) | |
| Haemodialysis | 0·404 | ||
| No | 147 926 (99·21) | 1173 (0·79) | |
| Yes | 174 (99·86) | 2 (1·14) | |
| Acupuncture | <0·001 | ||
| No | 137 970 (99·32) | 945 (0·68) | |
| Yes | 10 030 (97·76) | 230 (2·24) | |
| Dental therapy | <0·001 | ||
| No | 115 811 (99·35) | 757 (0·65) | |
| Yes | 32 189 (99·17) | 418 (1·28) | |
| Tattoos | 0·471 | ||
| No | 147 737 (99·21) | 1172 (0·79) | |
| Yes | 263 (98·87) | 3 (1·13) | |
| Piercing | 0·593 | ||
| No | 133 062 (99·22) | 1051 (0·78) | |
| Yes | 14 938 (99·18) | 124 (0·82) | |
| Sharing needles | 0·145 | ||
| No | 147 430 (99·21) | 1174 (0·79) | |
| Yes | 570 (99·82) | 1 (0·18) | |
| Sharing razors | 0·153 | ||
| No | 134 572 (99·20) | 1083 (0·80) | |
| Yes | 13 428 (99·32) | 92 (0·68) | |
| Sharing toothbrush | 0·011 | ||
| No | 147 477 (99·22) | 1165 (0·78) | |
| Yes | 523 (98·12) | 10 (1·88) |
P value of Cochran–Armitage's trend test.
Risk factors
The frequency of various risk factors associated with HCV infection and the calculated crude OR estimated by univariable analysis are shown in Table 3. Age, sex, marital status, education, occupation, smoking, drinking, BMI, history of hypertension and diabetes, hospitalization, surgery, blood transfusion, invasive test and dental therapy were associated with HCV infection. After adjusting for those variables, older age, female (OR 1·21, 95% CI 1·03–1·34), lower education, agricultural worker, blood transfusion (OR 2·91, 95% CI 1·09–5·37), invasive testing (OR 1·28, 95% CI 1·14–1·61), and dental therapy (OR 2·27, 95% CI 1·41–3·42) increased the risk of HCV infection (Table 4). All ORs were adjusted by the differences of the three regions.
Table 3.
Univariable analysis of risk factors associated with anti-HCV positivity
| Variables | Unadjusted OR (95% CI) | P value |
|---|---|---|
| Age, years | ||
| ⩽10 | 1·00 | – |
| 11–20 | 1·38 (1·02–2·61) | 0·032 |
| 21–30 | 1·01 (0·62–2·81) | 0·615 |
| 31–40 | 3·23 (1·80–7·01) | <0·001 |
| 41–50 | 8·61 (3·09–18·59) | <0·001 |
| 51–60 | 11·85 (5·21–28·04) | <0·001 |
| 61–70 | 12·19 (6·90–21·13) | <0·001 |
| >70 | 5·21 (1·89–8·09) | <0·001 |
| Sex | ||
| Male | 1·00 | – |
| Female | 3·19 (1·79–7·10) | <0·001 |
| Marital status | ||
| Unmarried | 1·00 | – |
| Married | 4·78 (3·09–7·12) | <0·001 |
| Divorced | 1·65 (1·15–3·75) | <0·001 |
| Education | ||
| Primary school | 1·00 | – |
| Middle school | 0·81 (0·52–1·32) | 0·338 |
| College | 0·41 (0·19–0·65) | <0·001 |
| Occupation | ||
| Agricultural worker | 1·00 | – |
| Worker | 0·81 (0·59–1·37) | 0·418 |
| Student | 0·08 (0·03–0·19) | <0·001 |
| Civil servant | 0·56 (0·16–0·85) | <0·001 |
| Shop assistant | 0·51 (0·34–0·81) | <0·001 |
| Unemployed | 0·30 (0·19–0·59) | <0·001 |
| Others | 0·46 (0·27–0·73) | <0·001 |
| Smoking | ||
| Never | 1·00 | – |
| Current | 0·91 (0·78–1·53) | 0·469 |
| Past | 1·23 (1·05–2·23) | <0·001 |
| Drinking | ||
| Never | 1·00 | – |
| Frequently | 0·71 (0·48–1·04) | 0·078 |
| Every day | 1·31 (1·08–1·92) | <0·001 |
| Body mass index (kg/m2) | ||
| <18·5 | 1·00 | – |
| 18·5–24 | 1·57 (1·12–2·93) | <0·001 |
| 24–28 | 2·35 (1·89–3·77) | <0·001 |
| >28 | 3·09 (1·67–5·19) | <0·001 |
| History of hypertension | ||
| No | 1·00 | – |
| Yes | 1·93 (1·55–2·54) | <0·001 |
| History of diabetes | ||
| No | 1·00 | – |
| Yes | 1·37 (1·15–1·83) | <0·001 |
| Hospitalization | ||
| No | 1·00 | – |
| Yes | 1·39 (1·14–1·69) | 0·001 |
| Surgery | ||
| No | 1·00 | – |
| Yes | 1·48 (1·19–1·85) | <0·001 |
| Blood transfusion | ||
| No | 1·00 | – |
| Yes | 4·05 (2·92–5·63) | <0·001 |
| Blood donation | ||
| No | 1·00 | – |
| Yes | 1·72 (0·51–5·63) | 0·389 |
| Invasive testing | ||
| No | 1·00 | – |
| Yes | 3·13 (2·41–4·23) | <0·001 |
| Haemodialysis | ||
| No | 1·00 | – |
| Yes | 1·01 (0·81–1·27) | 0·753 |
| Acupuncture | ||
| No | 1·00 | – |
| Yes | 1·10 (0·74–1·75) | 0·681 |
| Dental therapy | ||
| No | 1·00 | – |
| Yes | 3·11 (1·76–4·46) | <0·001 |
| Tattoos | ||
| No | 1·00 | – |
| Yes | 1·96 (0·96–4·79) | 0·069 |
| Piercing | ||
| No | 1·00 | – |
| Yes | 0·62 (0·35–1·21) | 0·612 |
| Sharing needles | ||
| No | 1·00 | – |
| Yes | 1·03 (0·71–1·48) | 0·587 |
| Sharing razors | ||
| No | 1·00 | – |
| Yes | 0·67 (0·45–1·09) | 0·128 |
| Sharing tooth brush | ||
| No | 1·00 | – |
| Yes | 0·81 (0·75–1·12) | 0·431 |
OR, Odds ratio; CI, confidence interval.
Table 4.
Multivariable analysis of risk factors associated with anti-HCV positivity
| Variables | aOR (95% CI) | P value |
|---|---|---|
| Age, years | ||
| ⩽10 | 1·00 | – |
| 11–20 | 1·12 (0·78–1·87) | 0·413 |
| 21–30 | 0·93 (0·33–3·18) | 0·894 |
| 31–40 | 1·65 (0·61–7·92) | 0·276 |
| 41–50 | 2·16 (1·05–9·19) | <0·001 |
| 51–60 | 3·25 (1·67–12·14) | 0·003 |
| 61–70 | 1·44 (1·12–5·08) | <0·001 |
| >70 | 1·04 (0·41–9·13) | 0·615 |
| Sex | ||
| Male | 1·00 | – |
| Female | 1·21 (1·03–1·34) | <0·001 |
| Marital status | ||
| Unmarried | 1·00 | – |
| Married | 0·88 (0·48–1·62) | 0·818 |
| Divorced | 1·08 (0·45–1·91) | 0·436 |
| Education | ||
| Primary school | 1·00 | – |
| Middle school | 0·76 (0·51–1·80) | 0·716 |
| College | 0·61 (0·45–0·97) | 0·003 |
| Occupation | ||
| Agricultural worker | 1·00 | – |
| Worker | 1·05 (0·72–1·92) | 0·562 |
| Student | 0·31 (0·11–0·62) | <0·001 |
| Civil servant | 0·71 (0·24–1·02) | 0·074 |
| Shop assistant | 0·82 (0·63–1·02) | 0·062 |
| Unemployed | 0·52 (0·43–0·80) | <0·001 |
| Others | 0·68 (0·51–1·31) | 0·341 |
| Smoking | ||
| Never | 1·00 | – |
| Current | 1·31 (0·92–2·17) | 0·337 |
| Past | 1·17 (0·89–1·43) | 0·213 |
| Drinking | ||
| Never | 1·00 | – |
| Frequently | 0·98 (0·62–1·12) | 0·187 |
| Every day | 1·22 (0·69–1·24) | 0·538 |
| Body mass index (kg/m2) | ||
| <18·5 | 1·00 | – |
| 18·5–24 | 1·31 (0·84–2·01) | 0·298 |
| 24–28 | 1·04 (0·59–1·25) | 0·514 |
| >28 | 0·94 (0·51–1·21) | 0·623 |
| History of hypertension | ||
| No | 1·00 | – |
| Yes | 1·10 (0·91–1·29) | 0·081 |
| History of diabetes | ||
| No | 1·00 | – |
| Yes | 1·05 (0·73–1·42) | 0·482 |
| Hospitalization | ||
| No | 1·00 | – |
| Yes | 0·73 (0·51–1·12) | 0·632 |
| Surgery | ||
| No | 1·00 | – |
| Yes | 1·04 (0·61–1·31) | 0·564 |
| Blood transfusion | ||
| No | 1·00 | – |
| Yes | 2·91 (1·09–5·37) | <0·001 |
| Invasive testing | ||
| No | 1·00 | – |
| Yes | 1·28 (1·14–1·61) | <0·001 |
| Dental therapy | ||
| No | 1·00 | – |
| Yes | 2·27 (1·41–3·42) | <0·001 |
| Tattoos | ||
| No | 1·00 | – |
| Yes | 1·02 (0·95–3·82) | 0·105 |
aOR, Adjusted odds ratio; CI, confidence interval.
DISCUSSION
We report here a community-based epidemiologicak study of HCV infection in an eastern Chinese population with a large sample. This study differs from previous population-based epidemiological studies which mainly had smaller numbers of participants [13–15]. Previous studies on HCV prevalence were mostly derived from high-risk populations such as blood donors, men who have sex with men, and drug users in China and other countries [16–19]. However, owing to the low numbers of elderly and children investigated, these high-risk populations serve as a skewed general population [9]. Meanwhile, in addition to its large number of participants, the present study also used a standardized systematic sampling procedure. As shown in Figure 1, three counties were randomly chosen from three major regional groups as being representative of eastern China. Then 20 communities were randomly selected and each county investigated. In all, 163 462 individuals in 60 communities were identified, and 149 175 participated in the survey. This sampling procedure was aimed at eliminating bias from local social, economic or cultural factors that might be associated with the prevalence of HCV. However, there are clear issues with representativeness, as the sample is highly likely to underrepresent injecting drug users (IDUs). IDUs are difficult to survey and fail to appear in this survey, which inevitably leads to an underestimate of the true prevalence of the general population. However, this is a common problem in most population-based surveys [20, 21].
This community-based study in eastern China found an anti-HCV seroprevalence of 0·79% (95% CI 0·74–0·83), which was much lower than the reported average national prevalence (2·20%) [7]. Because local living conditions are high, well above the national average, the healthcare system is well-developed in this region. Moreover, as residents in this study are well-educated and have a good awareness of healthcare, they have a healthy lifestyle which helps minimize the risk of HCV infection.
Age-specific prevalence of HCV in our study was low in children (0·09%), but increased progressively from adolescents (0·20%) to adults aged ⩾21 years (0·15–1·64%). For subjects aged between 41 and 70 years, anti-HCV prevalence was much higher than for the younger groups. One of the most important reasons for this is that the elderly may have an increased risk of exposure to HCV infection such as multiple injections and a shortage of healthcare resources. In addition, with the improvement of healthcare, nosocomial infections through inadequately sterilized equipment, transplantation, and iatrogenic transmission by blood transfusion have reduced [22]. However, considering the many HCV-related hepatocellular carcinoma deaths in the >70 years age group, it is very reasonable that HCV prevalence was low in that group. The increasing anti-HCV seroprevalence with advanced age observed in this study was concordant with that found in previous study in China [22]. However, there is an interesting phenomenon in that the prevalence in those aged 21–30 years is less than in those aged 11–20 years. The reason may be that IDUs who are mainly in the 21–30 years age group were reluctant to participate in this survey, which may lead to an underestimation of the true prevalence in those aged 21–30 years. Another interesting phenomenon is that there are significantly fewer participants in the 21–30 years age group. There are two possible reasons why fewer young individuals participated in this study. First, is that people aged 21–30 years usually live or work in big cities such as Shanghai. Few of these individuals return to their home town. The second reason may be that this group has less interest in this type of survey than older age groups. Therefore, the 21–30 years age group is always likely to be underrepresented.
Women had a higher prevalence of HCV infection than men in this study, which differs from previous studies in Western countries but is in accord with previous surveys of six regions of China and Taiwan [15, 22]. One of the main reasons for this may be that IDUs are generally more likely to be male, but this group is difficult to survey and does not appear in this study, leading to an underestimation of the true prevalence of males. Second, unlike Western countries, the practice of commercial plasma donation led to a high prevalence of HCV infections in China in the 1980s [23]. During that period women in rural areas were the main donors, which may explain the prevalence of HCV in females being much higher than in males aged 41–70 in this survey. However, pursuant to the Law for Donating Blood being issued in 1998, blood transfusions and the use of serum-related products have become safer in recent years in China [24]. There was no such difference in the prevalence of HCV between males and females aged <40 years. In the >70 years age group, anti-HCV prevalence in females was lower, which may be because many HCV-related cirrhosis and hepatocellular carcinoma patients had died. Finally, obstetric measures were one important way of contracting HCV infection due to lack of healthcare in past decades [15].
In our study mixed-effects logistic regression revealed that blood transfusion, invasive testing and dental therapy increased the risk of HCV infection. A history of blood transfusions in particular was the greatest risk factor for HCV infection in this investigation. This may be associated with commercial plasma donation in rural areas of China in the 1980s [23]. For invasive testing and dental therapy, the main reason was that due to lack of systematic training programmes physicians were unable to understand HCV and sterilization procedures well enough in some underdeveloped villages. Moreover, in the past the general population had less education regarding healthcare and lacked knowledge regarding HCV transmission. Therefore, health education for the general population, especially the elderly, is very important, and may prove to be useful in preventive interventions in China [25].
A major limitation of this study is that lack of HCV RNA level information hinders the ability to identify individuals with persistent HCV infection and those requiring care and treatment, leading to a failure to control the spread of the virus. However, it is fairly well-established that about 25% of individuals spontaneously clear acute HCV infection, meaning that 75% of HCV infections persist [26]. According to the observed prevalence of antibodies in this study, an estimate of chronic prevalence is 0·59% (95% CI 0·55–0·63) [20]. As HCV genotypes were not tested in this study, it is difficult to explore the introduction, spread and evolution of HCV. Furthermore, we performed the simple sampling first, which may have caused bias in estimating the total prevalence of the whole province, i.e. the rate of 0·79% (95% CI 0·74–0·83) is probably narrow. Although the three counties are selected randomly, they represent three different regions which may have huge differences in living standards. Finally, the inclusion of so much risk information in the survey, may have induced a potential recall bias in this study.
Despite these limitations, this study is the first to investigate HCV infection with such a large general population utilizing a standardized systematic sampling method. Even with a lower prevalence of HCV infection, there may be millions of potential persons at risk of cirrhosis and hepatocellular carcinoma due to HCV. The epidemiological data in the present investigation may play an important role in focusing on the significance of public health in chronic HCV infection. As no vaccine exists currently for HCV, health education should be further strengthened to limit the spread of HCV infections.
ACKNOWLEDGEMENTS
We thank the students who have worked on the study, including Yingying Zhu, Qing Wang, Hui Zheng, Yuanyuan Zhang, Yin Xu, Wenzhe Ma. We also thank the staff from the Centers for Disease Control and Prevention of Jiangsu province for organization of the field investigation. This research would not have been possible without the consent and help of the participants.
This study was supported in part by National Natural Science Foundation of China (No. 81273146, No. 81001288 and No. 81473029), National S&T Major Project Foundation of China (No. 2011ZX10004-902 and No. 2012ZX10001-001), Priority Academic Program Development of Jiangsu Higher Education Institutions (PAPD), Jiangsu Province Health Development Project with Science and Education (No. ZX201109), National Science and Technology Support Program (No. 2011BAI09B02)), and Research and Innovation Project for College Graduates of Jiangsu Province of China (KYZZ_0265).
DECLARATION OF INTEREST
None.
REFERENCES
- 1.Lavanchy D. Chronic viral hepatitis as a public health issue in the world. Best Practice & Research Clinical Gastroenterology 2008; 22: 991–1008. [DOI] [PubMed] [Google Scholar]
- 2.Shepard CW, et al. Global epidemiology of hepatitis C virus infection. Lancet Infectious Diseases 2005; 5: 558–567. [DOI] [PubMed] [Google Scholar]
- 3.El-Serag HB. Epidemiology of viral hepatitis and hepatocellular carcinoma. Gastroenterology 2012; 142: 1264–1273.e1261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Muir AJ, et al. Phase 1b study of pegylated interferon lambda 1 with or without ribavirin in patients with chronic genotype 1 hepatitis C virus infection. Hepatology 2010; 52: 822–832. [DOI] [PubMed] [Google Scholar]
- 5.Asselah T, et al. Hepatitis C: viral and host factors associated with non-response to pegylated interferon plus ribavirin. Liver International 2010; 30: 1259–1269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bruix J, Sherman M. Management of hepatocellular carcinoma: an update. Hepatology 2011; 53: 1020–1022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Lavanchy D. Evolving epidemiology of hepatitis C virus. Clinical Microbiology and Infection 2011; 17: 107–115. [DOI] [PubMed] [Google Scholar]
- 8.Zhou YH, et al. Comparison of HIV-, HBV-, HCV- and co-infection prevalence between Chinese and Burmese intravenous drug users of the China-Myanmar border region. PLoS ONE 2011; 6: e16349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Nelson PK, et al. Global epidemiology of hepatitis B and hepatitis C in people who inject drugs: results of systematic reviews. Lancet 2011; 378: 571–583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Su Y, et al. Prevalence and risk factors of hepatitis C and B virus infections in hemodialysis patients and their spouses: A multicenter study in Beijing, China. Journal of Medical Virology 2013; 85: 425–432. [DOI] [PubMed] [Google Scholar]
- 11.Ruan Y, et al. Evaluation of harm reduction programs on seroincidence of HIV, Hepatitis B and C, and syphilis among intravenous drug users in southwest China. Sexually Transmitted Diseases 2013; 40: 323–328 [DOI] [PubMed] [Google Scholar]
- 12.Patel PR, et al. Epidemiology, surveillance, and prevention of Hepatitis C virus infections in hemodialysis patients. American Journal of Kidney Diseases 2010; 56: 371–378. [DOI] [PubMed] [Google Scholar]
- 13.Charlebois A, et al. Factors associated with HCV antiviral treatment uptake among participants of a community-based HCV programme for marginalized patients. Journal of Viral Hepatitis 2012; 19: 836–842. [DOI] [PubMed] [Google Scholar]
- 14.Cozzolongo R, et al. Epidemiology of HCV infection in the general population: a survey in a southern Italian town. American Journal of Gastroenterology 2009; 104: 2740–2746. [DOI] [PubMed] [Google Scholar]
- 15.Lee M-H, et al. Community and personal risk factors for hepatitis C virus infection: a survey of 23 820 residents in Taiwan in 1991–2. Gut 2011; 60: 688–694. [DOI] [PubMed] [Google Scholar]
- 16.Ng M-H, et al. High prevalence but low awareness of hepatitis C virus infection among heroin users who received methadone maintenance therapy in Taiwan. Addictive Behaviors 2013; 38: 2089–2093. [DOI] [PubMed] [Google Scholar]
- 17.van der Helm J, et al. Effect of HCV infection on cause-specific mortality after HIV seroconversion, before and after 1997. Gastroenterology 2013; 144: 751–760.e752. [DOI] [PubMed] [Google Scholar]
- 18.Bao YP, Liu ZM. Systematic review of HIV and HCV infection among drug users in China. International Journal of STD & AIDS 2009; 20: 399–405. [DOI] [PubMed] [Google Scholar]
- 19.Dai S, et al. Seroprevalence of HIV, syphilis, and hepatitis C virus in the general population of the Liangshan Prefecture, Sichuan Province, China. Journal of Medical Virology 2012; 84: 1–5. [DOI] [PubMed] [Google Scholar]
- 20.De Angelis D, et al. An evidence synthesis approach to estimating Hepatitis C prevalence in England and Wales. Statistical Methods in Medical Research 2009; 18: 361–379. [DOI] [PubMed] [Google Scholar]
- 21.Hickman M, et al. Injecting drug use in Brighton, Liverpool, and London: best estimates of prevalence and coverage of public health indicators. Journal of Epidemiology and Community Health 2004; 58: 766–771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Lu J, et al. General epidemiological parameters of viral hepatitis A, B, C, and E in six regions of China: a cross-sectional study in 2007. PLoS ONE 2009. 4: e8467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Rao HY, et al. Outcome of hepatitis C virus infection in Chinese paid plasma donors: a 12–19-year cohort study. Journal of Gastroenterology and Hepatology 2012; 27: 526–532. [DOI] [PubMed] [Google Scholar]
- 24.Lu F, Zhuang H. Prevention of hepatitis B in China: achievements and challenges. Chinese Medical Journal (English) 2009; 122: 2925–2927. [PubMed] [Google Scholar]
- 25.Mitchell AE, et al. Institute of medicine recommendations for the prevention and control of hepatitis B and C. Hepatology 2010; 51: 729–733. [DOI] [PubMed] [Google Scholar]
- 26.Micallef JM, Kaldor JM, Dore GJ. Spontaneous viral clearance following acute hepatitis C infection: a systematic review of longitudinal studies. Journal of viral hepatitis 2006; 13: 34–41. [DOI] [PubMed] [Google Scholar]