SUMMARY
Non-tuberculous mycobacteria (NTM) illness is an emerging life-threatening infection, and paediatric features have not been well studied. The objective of our study was to review the NTM isolates of hospitalized paediatric patients identified at our institution and to describe the characteristics of these cases. Our retrospective chart review from 2010 to 2013 identified 45 patients with 46 positive NTM cultures. Fifteen (33%) patients had received haematopoietic cell transplant, 13 (29%) had cystic fibrosis, and six (13%) were previously healthy. Twenty-seven (59%) NTM isolates were Mycobacterium chelonae/abscessus, 14 (30%) were M. avium intracellulare, and four (9%) were M. immunogenum. The majority (65%) of cases were community-acquired, and 20 (43%) patients were treated as infection. This case series identified a predominance of M. chelonae/abscessus, and includes a substantial number of haematopoietic cell transplant patients, which reflects the changing spectrum of NTM disease as molecular diagnostics improve and quaternary care facilities provide for a larger immunocompromised population.
Key words: Antibiotic resistance, cystic fibrosis, hospital-acquired (nosocomial) infections, immunocompromised patients, mycobacteria
INTRODUCTION
Non-tuberculous mycobacteria (NTM) have emerged as increasingly important pathogens in the last two decades [1, 2]. This increase has been most marked in patients with cystic fibrosis (CF), transplant recipients and other immunocompromised hosts. Unlike other environmental pathogens that are largely opportunistic in patients with malignancy, immunodeficiency, and transplant recipients, NTM can cause significant disease in otherwise healthy individuals. Moreover, children have specific clinical manifestations due to increased frequency of exposures, such as through pica ingestion, associated with their young age.
Changes in the microbiological quality of drinking water due to changes in chlorination methods have led to a documented increase in NTM in the water supply [3], and NTM have been increasingly recognized to cause disease. There have been few comprehensive reviews of NTM cases in hospitalized children to guide our understanding of the clinical and microbiological features of NTM in the paediatric population, particularly the immunocompromised population. The objective of our study was to determine the NTM isolates identified in hospitalized patients at our institution, to describe the characteristics and clinical outcomes of these cases and antimicrobial susceptibility patterns, with a focus on whether these were hospital- or community-acquired, colonization or infection.
METHODS
Clinical setting and study design
The University of Minnesota Masonic Children's Hospital is a 96-bed tertiary-care hospital that provides care in over 50 paediatric services, and has an active transplant service that performs over 50 haematopoietic cell transplants (HCTs) and 60 solid organ transplants a year.
This was a retrospective chart review from January 2010 to December 2013 of all paediatric patients admitted to the hospital who had a NTM-positive culture. All paediatric patients from 2 months up to 21 years of age were included. This study was approved by the Institutional Review Board of the University of Minnesota.
Case definition
A NTM-positive patient was defined as a patient with clinical samples from which NTM was recovered during the hospital stay. Community-acquired NTM was defined as isolation of this organism prior to, or within 72 h of, hospitalization. NTM isolates recovered after 72 h of hospitalization were considered hospital-acquired. If a patient had a NTM isolate recovered within 72 h of hospitalization, but had been recently discharged from a prolonged hospitalization and readmitted within 72 h, this was also considered a hospital-acquired infection. Colonization with NTM was defined as lack of evidence of clinical disease. Infection with NTM was defined as associated signs and symptoms of clinical disease that could not be attributed to another cause. NTM isolated from a sterile site was considered an infection. NTM isolated from a non-sterile site, such as sputum, was correlated with radiographic imaging for evidence of invasion, and with other clinical signs and symptoms, in order to be considered an infection. Whether the patient was treated with antibiotics directed against NTM was also considered in classifying the patient as being infected or colonized with NTM. If the investigators were unsure of the classification of the isolate as causing infection or colonization, it was classified as indeterminate. Medical records of all NTM-positive patients were reviewed for signs and symptoms of invasive disease and data extracted. All-cause inpatient mortality data were also extracted.
Microbiology
Patient samples were processed with sodium hydroxide containing N-acetylcysteine prior to inoculation into a mycobacterium growth indicator tube (Becton, Dickinson and Company, USA) and a 7H11 selective flask (Hardy Diagnostics, USA). For the next 6 weeks, samples were monitored hourly for growth at 37°C incubation. Positive isolates were confirmed by Kinyoun stain and these were subcultured onto Lowenstein–Jensen slant (Hardy Diagnostics), Columbia sheep blood agar plate (Hardy Diagnostics), and 7H9 broth. Selected isolates were identified by DNA probe (Gen-Probe, USA) or DNA sequencing. Susceptibility testing was performed using the broth microdilution method as recommended by the 2012 Clinical and Laboratory Standards Institute. Clarithromycin susceptibility testing was read for Mycobacterium fortuitum species at 3 days and 14 days, and for all other NTM species was read at 4 days and 14 days.
RESULTS
Patient characteristics
Between January 2010 and December 2013, a total of 45 patients had 46 positive NTM cultures. Patients had a median age of 9 years (range 8 months to 21 years), and 56% were male (Table 1). The duration from hospital admission to incident culture of NTM was a median of 2 days (range 0–91 days), and for patients with hospital-acquired infection it was a median of 27 days (range 17–90 days). Seventeen (38%) patients had chronic pulmonary conditions including 13 (29%) patients with CF, 15 (33%) patients had received HCT, 14 (31%) had some form of malignancy, three (7%) had chronic renal or pancreatic disease, one (2%) had an auto-islet pancreatic transplant, and one (2%) patient had a liver and kidney transplant. Six (13%) patients had no significant medical history.
Table 1.
Patient cases
| Patient no. | Age (years) | Sex | Diagnosis | Days post-BMT/other transplant | Mycobacterial species | Site | Community- or hospital-acquired | Infection, colonization, or uncertain | Treatment | Outcome |
|---|---|---|---|---|---|---|---|---|---|---|
| 1 | 10 months | F | History of pulmonary TB | n.a. | M. avium intracellulare | Gastric aspirate | CA | Colonization | None | Alive |
| 2 | 23 months | F | Parotid mass | n.a. | M. avium intracellulare | Parotid | CA | Infection | Removal of mass | Alive |
| 3 | 3 | F | Parotid swelling with lymph node dissection | n.a. | M. avium intracellulare | Submandibular node | CA | Infection | Removal of node | Alive |
| 4 | 3 | M | Parotid mass | n.a. | M. avium intracellulare | Parotid | CA | Infection | Removal of mass | Alive |
| 5 | 3 | F | Asthma | n.a. | M. avium intracellulare | Gastric fluid | CA | Colonization | None | Alive |
| 6 | 5 | M | ESRD, oto-palato-digital syndrome | n.a. | M. chelonae/ abscessus | Peritoneal fluid | CA | Infection | AZI, AMK, LIN | Alive |
| 7 | 6 | M | CF | n.a. | M. abscessus | Tracheal aspirate | CA | Colonization | None | Alive |
| 8 | 7 | F | Caroli disease | 20 | M. immunogenum | Pleural fluid, abdominal fluid | HA | Infection | LIN, PIP | Alive |
| 9* | 7 | M | ALD | 7 | M. immunogenum | Faeces | HA | Infection | CFZ, CTZ, LEV | Alive |
| 148 | M. chelonae | Blood | HA | Infection | TOB | Dead | ||||
| 10 | 8 | M | Anaplastic large B-cell lymphoma | 128 | M. chelonae | ET aspirate | CA | Colonization | None | Alive |
| 11 | 8 | F | Pericarditis | n.a. | M. arupense | Gastric aspirate | CA | Colonization | None | Alive |
| 12 | 8 | M | Metastatic glioblastoma multiforme | n.a. | M. fortuitum | Ear | CA | Infection | AMK, CLR | Alive |
| 13 | 9 | M | Hodgkin's lymphoma | 5 | M. immunogenum | Throat | HA | Colonization | None | Alive |
| 14 | 9 | M | T-cell ALL | 38 | M. chelonae | Sputum | HA | Colonization | None | Dead |
| 15 | 9 | M | Idiopathic pulmonary haemosiderosis | n.a. | M. chelonae | Sputum | CA | Colonization | None | Alive |
| 16 | 9 | M | CF | n.a. | M. avium intracellulare | Sputum | CA | Infection | CLR, MOX | Alive |
| 17 | 10 | M | ALD | 290 | M. chelonae | Sputum | HA | Colonization | None | Alive |
| 18 | 10 | F | Monosomy 7, myelodysplastic syndrome | 502 | M. chelonae | Sputum | CA | Infection | LEV | Dead |
| 19 | 11 | M | Pre B-cell ALL | 34 | M. immunogenum | Throat | HA | Colonization | None | Alive |
| 20 | 11 | F | ALL | 108 | M. chelonae | Sputum | HA | Infection | AZI, CTZ, MER | Dead |
| 21 | 12 | F | FTT, restrictive lung disease, fibronodular interstitial disease | n.a. | M. chelonae | Sputum | CA | Colonization | None | Alive |
| 22 | 12 | M | CF | n.a. | M. abscessus | BAL | CA | Infection | LEV, TOB | Alive |
| 23 | 13 | F | Asthma | n.a. | M. chelonae | Sputum | CA | Colonization | None | Alive |
| 24 | 13 | M | CF | n.a. | M. avium intracellulare | Bronchial | CA | Infection | AMK, AZI, MOX, RIF | Alive |
| 25 | 15 | M | AML | 11 | M. chelonae | Sputum | HA | Colonization | None | Dead |
| 26 | 15 | M | CF | n.a. | M. avium intracellulare | Sputum | CA | Colonization | None | Alive |
| 27 | 15 | F | CF | n.a. | M. avium intracellulare | Sputum | CA | Infection | LEV, TCV | Alive |
| 28 | 15 | F | CF | n.a. | M. avium intracellulare | Sputum | CA | Infection | LEV, PIP | Alive |
| 29 | 15 | M | Fanconi anaemia, myelodysplastic syndrome | 1642 | M. chelonae | Esophageal brush | HA | Infection | AZI, TOB | Alive |
| 30 | 15 | F | Osteoperosis | 22 | M. chelonae | Sputum | HA | Colonization | None | Dead |
| 31 | 15 | M | Chronic pancreatitis | n.a. | M. chelonae | Sputum | HA | Colonization | None | Alive |
| 32 | 15 | F | DiGeorge syndrome, Tetralogy of Fallot | n.a. | M. avium intracellulare | Gastric aspirate | HA | Colonization | None | Alive |
| 33 | 16 | F | Chronic pancreatitis | 2 | M. abscessus | Blood | CA | Colonization | None | Alive |
| 34 | 16 | F | CF | n.a. | M. abscessus | Sputum | CA | Colonization | None | Alive |
| 35 | 17 | F | Pneumonia | n.a. | M. chelonae | Sputum | CA | Infection | AZI, CTX | Alive |
| 36 | 17 | M | Pneumonia | n.a. | M. chelonae | Sputum | CA | Colonization | None | Alive |
| 37 | 17 | M | ALL | 90 | M. chelonae | Sputum | HA | Colonization | None | Dead |
| 38 | 17 | F | CF | n.a. | M. avium intracellulare | Sputum | CA | Colonization | None | Alive |
| 39 | 17 | F | Neuroblastoma | 15 | M. chelonae/ abscessus | Sputum | HA | Colonization | None | Dead |
| 40 | 19 | F | HLH | 182 | M. chelonae | BAL | HA | Infection | AZI, MER | Dead |
| 41 | 19 | M | CF | n.a. | M. chelonae/ abscessus | Bronchial lavage | CA | Colonization | None | Alive |
| 42 | 19 | M | CF | n.a. | M. abscessus | BAL | CA | Colonization | None | Alive |
| 43 | 20 | F | CF | n.a. | M. avium intracellulare | Sputum | CA | Colonization | None | Alive |
| 44 | 21 | M | CF | n.a. | M. avium intracellulare | Sputum | CA | Infection | LEV, MER, TOB | Alive |
| 45 | 21 | M | Hodgkin's lymphoma | 730 | M. chelonae | Sputum | CA | Infection | LIN, MER | Alive |
* Patient no. 9 had two non-tuberculous mycobacteria cultures positive in two separate hospitalizations.
ALD, Adrenoleukodystrophy; ALL, acute lymphoblastic leukaemia; AMK, amikacin; AML, acute myeloid leukaemia; AZI, azithromycin; BAL, bronchoalveolar lavage; BMT, bone marrow transplant; CA, community-acquired; CF, cystic fibrosis; CFZ, cefazolin; CLR, clarithromycin; CTX, ceftriaxone; CTZ, ceftazidime; DOX, doxycycline; ESRD, end-stage renal disease; ET, endotracheal; ETH, ethambutol; FTT, failure to thrive; HA, hospital-acquired, HLH, haemophagocytic lymphohistiocytosis; LEV, levofloxacin; LIN, linezolid; MER, meropenem; MOX, moxifloxacin; n.a., not applicable; PIP, piperacillin-tazobactam; RIF, rifampin; TCV, ticarcillin-clavulanate; TOB, tobramycin.
Mycobacterial species and clinical features
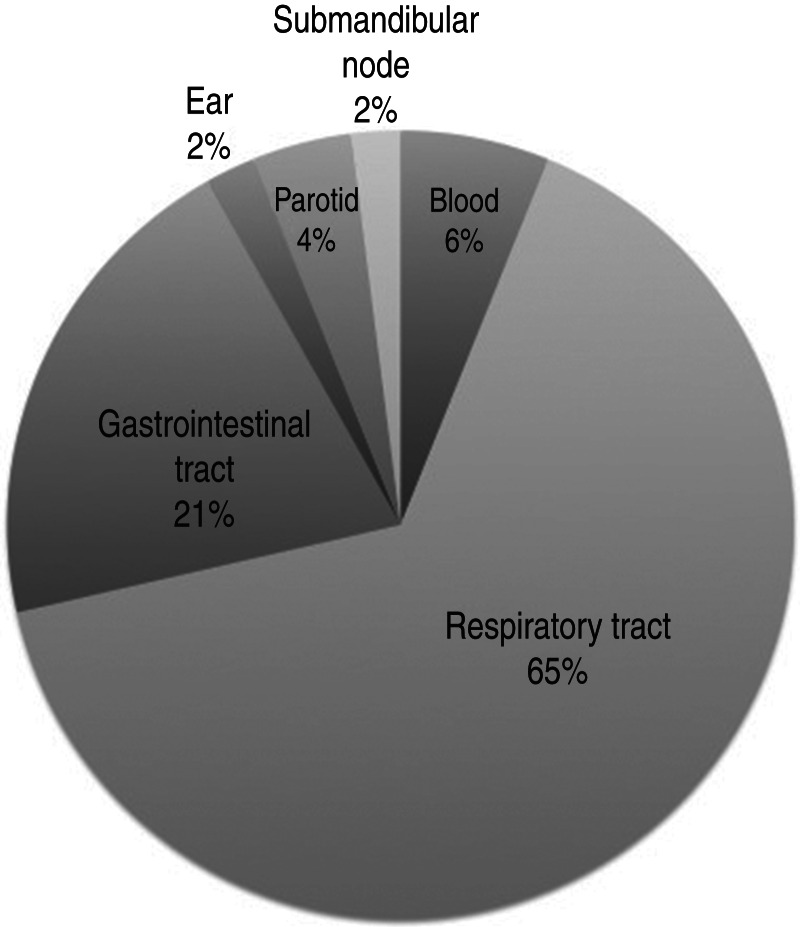
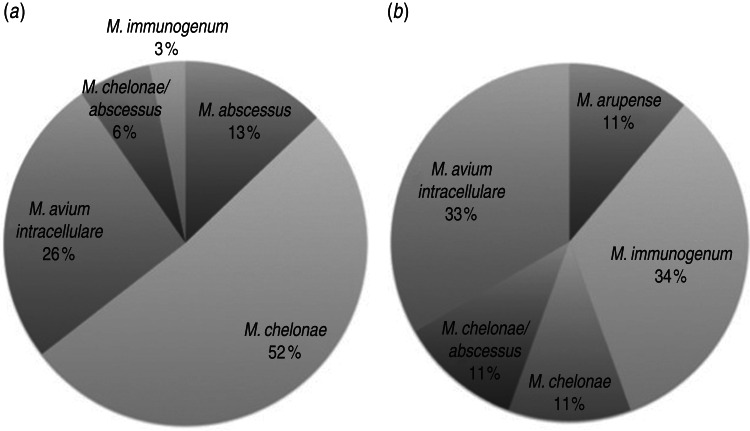
M. chelonae was isolated from 18/46 cases (39%), 14 (30%) cases were M. avium intracellulare, six (13%) cases were M. abscessus, and M. immunogenum was isolated in four (9%) cases. Three (7%) cases isolated M. chelonae/abscessus complex not further differentiated. One patient had both M. immunogenum and M. abscessus isolated on different occasions, and one had M. abscessus isolated on consecutive cultures. Culture sites were typically sputum or another respiratory tract sample (65%, Figs 1, 2a), but mycobacteria were also isolated from the gastrointestinal tract (21%, Fig. 2b), blood (6%), parotid (4%) and ear and submandibular lymph node (2% each).
Fig. 1.
Distribution of sites of non-tuberculous mycobacteria isolation (n = 46)
Fig. 2.
Non-tuberculous mycobacteria species isolated from (a) respiratory (n = 31) and (b) gastrointestinal tracts (n = 9). Not included are M. fortuitum from ear, M. avium-intracellulare from parotid gland and submandibular node, and M. abscessus and M. chelonae from blood.
Thirty (65%) cases were community-acquired. The most commonly isolated NTM in community-acquired cases were M. avium intracellulare (43%), followed by M. chelonae (27%) and M. abscessus (17%). M. chelonae was the predominant isolate in hospital-acquired cases (59%), followed by M. immunogenum (24%) and M. avium intracellulare (12%). Of the 26 cases of colonization, M. chelonae was most commonly identified (42%), then M. avium intracellulare (23%) and M. abscessus (15%). Of the 20 cases of infection, the predominant NTM were split evenly between M. avium intracellulare and M. chelonae (40% each), followed by M. immunogenum (10%).
Of the 13 CF patients with NTM-positive isolates, eight (62%) were M. avium intracellulare, four (30%) were M. abscessus, and one (8%) was M. chelonae/abscessus complex. About half (46%) of these cases were determined to be infection, of which 83% were M. avium intracellulare followed by one (17%) case of M. abscessus. Other pulmonary conditions included asthma (4%), restrictive lung disease and idiopathic pulmonary haemosiderosis (2% each), and M. chelonae was isolated in 75% of these cases from the respiratory tract.
The 15 HCT patients had 16 positive cultures for rapidly growing NTM species, including 12 (75%) cases of M. chelonae, three (19%) cases of M. immunogenum, and one (6%) M. chelonae/abscessus complex. One patient had M. immunogenum and M. chelonae isolated in separate hospitalizations. These patients had received HCT a median of 38 days prior to NTM isolation, with a range of 5–1642 days. For the two solid organ transplant recipients with positive NTM cultures, the median duration from transplant to NTM isolation was 9 days (range 2–20 days). The patient with NTM isolated at 2 days was classified as community-acquired colonization (patient no. 33, Table 1). The majority of positive cultures for HCT patients were detected in the sputum or another respiratory tract sample (69%), although throat or oesophagus (19%), blood and faeces (6% each) were also sites. Thirteen (81%) cases in HCT patients were hospital-acquired, resulting in four cases of infection. Three (19%) cases were community-acquired, resulting in two cases of infection. After a thorough outbreak investigation, the hospital-acquired cases were attributed to elevated levels of NTM species in the hospital drinking water and ice machines [4].
Of the 31 patients with NTM isolated from respiratory sites, nine were symptomatic with respiratory symptoms and of these four had fever. One patient with M. immunogenum isolated from both pleural and abdominal fluids also had Enterococcus isolated from the same sites, but her fevers improved once the enterococcal infection was treated (patient no. 8, Table 1). However, as NTM was isolated from several sterile sites, and as she was treated for her NTM isolate, the case was determined to be an infection. One patient had M. chelonae and M. mucogenicum isolated from peritoneal dialysis fluid at another hospital prior to transfer to our institution (patient no. 6, Table 1).
Nine patients died (20% all-cause mortality), although NTM isolation was determined to be an infection in less than half of these patients. M. chelonae was the predominant isolate (89%) followed by M. chelonae/abscessus (11%). All except one case (89%) was classified as hospital-acquired.
Microbiological features
Antimicrobial susceptibility testing was done on up to 41 isolates (89%, Table 2). All NTM isolates had susceptibility testing to clarithromycin, linezolid and moxifloxacin, and results were 95%, 37% and 7% susceptibility, respectively. Other antimicrobials were tested in a limited range of isolates, such as tobramycin which had 88% susceptibility to 19 isolates. Notably, imipenem had 0% susceptibility against all isolates tested, although 75% of tested isolates were intermediately susceptible (data not shown). Similarly, cefoxitin had 0% susceptibility although 67% of tested isolates were intermediately susceptible. Susceptibility varied based on the mycobacterial species, with 59% of M. chelonae isolates susceptible to linezolid compared to 0% for M. immunogenum; and 100% of M. immunogenum isolates susceptible to amikacin compared to 12% for M. chelonae.
Table 2.
Species and antimicrobial susceptibility profile of mycobacterial isolates
| Antimicrobial | Susceptible mycobacterial isolates (%) | ||||||
|---|---|---|---|---|---|---|---|
| Overall susceptibility (%) | M. abscessus (n = 5) | M. arupense (n = 1) | M. avium intracellulare (n = 12) | M. chelonae (n = 17) | M. chelonae/ abscessus (n = 2) | M. immunogenum (n = 4) | |
| Amikacin | 27·6 | 20 | 100 | n.d. | 11·8 | 50 | 100 |
| Cefoxitin | 0 | 0 | n.d. | n.d. | n.d. | n.d. | 0 |
| Ciprofloxacin | 6·9 | 0 | 100 | n.d. | 0 | 0 | 25 |
| Clarithromycin | 95·1 | 80 | 100 | 100 | 100 | 50 | 100 |
| Ethambutol | 100 | n.d. | 100 | n.d. | n.d. | n.d. | n.d. |
| Imipenem | 0 | 0 | n.d. | n.d. | 0 | 0 | 0 |
| Linezolid | 36·6 | 20 | 100 | 16·7 | 58·8 | 50 | 0 |
| Moxifloxacin | 7·3 | 0 | 100 | 16·7 | 0 | 0 | 0 |
| Rifampin | 100 | n.d. | 100 | n.d. | n.d. | n.d. | n.d. |
| Rifabutin | 100 | n.d. | 100 | n.d. | n.d. | n.d. | n.d. |
| Tobramycin | 88·2 | n.d. | n.d. | n.d. | 88·2 | 50 | n.d. |
| Trimethroprim-sulfamethoxazole | 3·4 | 0 | 100 | n.d. | 0 | 0 | 0 |
n.d., Not done or not interpretable; non-susceptible: doxycycline, minocycline.
DISCUSSION
NTM have long been recognized as environmental bacteria which are found in soil and water and can be human pathogens or colonize humans without causing disease. Organisms can be found in water production and distribution systems [5], and some NTM such as M. xenopi and M. simiae are associated almost exclusively with municipal water sources rather than other environmental sources [2]. Human disease is suspected to be acquired from environmental exposures, and human-to-human transmission of NTM has rarely been reported [6, 7]. NTM can result in asymptomatic colonization and symptomatic infection, and can be community-acquired, hospital-acquired or post-surgical [8–11]. In recent years NTM have emerged as an important cause of opportunistic infections in immunocompromised hosts, including solid organ and HCT recipients and patients with immunodeficiency [4, 12, 13]. Our institution experienced outbreaks of mycobacterial infections in HCT patients that were traced to the hospital water supply [4, 14]. We surmised that our first outbreak was related to a temporary drop in chloramine levels in the city water supply [14]; the second outbreak was believed to be related to plumbing changes in the new building and the ice/drinking water machines [4].
There has been increasing interest in NTM disease based on major recent trends associating NTM infection with AIDS, and the increasing frequency of pulmonary NTM disease in non-AIDS populations [2, 15]. In addition, the number of identified NTM species has more than doubled in the last two decades, with over 160 species documented [16]. Improved microbiological techniques for isolating NTM from clinical specimens, as well as advanced molecular techniques such as 16S rRNA sequencing, have superseded traditional biochemical and phenotypic methods and contributed to the identification of new species. The use of more discriminatory molecular techniques led to the recognition of M. triplex, M. lentiflavum, M. celatum and M. conspicuum, among others, as species separate from M. avium complex [17], as well as the revelation that M. abscessus complex consists of three genomospecies equivalent to M. abscessus subsp. abscessus, M. abscessus subsp. bolletii, and M. abscessus subsp. massiliense [18].
That NTM can colonize the respiratory tract without causing disease can partly be attributed to the ability of mycobacteria to form biofilm [19, 20]. This is similar to the biofilm-forming ability of M. tuberculosis in pulmonary infection [21], which explains why NTM can be difficult to eradicate in patients once infection has been established [2]. In our review, 13 patients with CF had NTM isolated, all were community-acquired and just under half of these cases were determined to be infection. As NTM isolated from sputum in CF patients generally reflect chronic colonization [22], this rate of infection was surprisingly high based on the current literature, although this may be biased by our focus on hospitalized patients and hence those more likely to have significant disease.
Antimicrobial therapy for NTM infections can be challenging as NTM are relatively resistant to many of the first- and second-line drugs used to treat tuberculosis, and duration of treatment can be up to 12 months or more [23]. A macrolide-based multidrug regimen has been part of NTM therapy since the 1990s [24], and macrolide resistance has been associated with worse pulmonary status as well as fatal pulmonary infections in CF patients [25]. In vitro susceptibility testing has not been a reliable predictor of clinical response to therapy for various NTM species, unlike for M. tuberculosis. While NTM such as M. kansasii, M. marinum and M. fortuitum are susceptible to multiple antimicrobials based on in vitro testing, M. avium complex has limited in vitro susceptibility with clinical response correlating with macrolides only, compared with M. abscessus and M. simiae which have limited in vitro susceptibility and poor correlation with clinical response [2]. NTM exhibit a diverse antimicrobial susceptibility spectrum, as evidenced in the susceptibility profile of our isolates (Table 2), which constitutes part of the challenges in management.
Our study was limited in that we did not examine a history of macrolide use in CF patients, nor did we look at allergic bronchopulmonary aspergillosis or steroid use, variables that have been found to increase NTM rates [26]. Our laboratory did not further distinguish M. avium intracellulare into M. avium and M. intracellulare, although there is evidence that these species have differing clinical features and in vitro susceptibility profiles [27]. In addition, our determination of colonization vs. infection was influenced by whether the patient received antimicrobial therapy directed against the NTM and whether the consulting infectious disease physicians determined the patient to be infected or colonized by NTM. Our high infection rate may reflect the inclusion of hospitalized patients who were symptomatic, as well as the number of immunocompromised patients whom physicians decided to treat conservatively with antimicrobials. Inclusion of outpatients may provide a more accurate picture of NTM pathogens within the community. Our goal, however, was to characterize NTM infections in hospitalized paediatric patients, and while other countries have reported on trends in NTM infection in children [28, 29], this is one of the larger case series at a single institution with a sizable immunocompromised patient population.
In conclusion, features such as the ability of mycobacteria to form biofilm and the challenge of effective antimicrobial therapy are contributing factors to the emergence of NTM as a pathogen. In addition, new diagnostic techniques and the evolution of quaternary care providing for a larger immunocompromised populations have broadened the diversity of mycobacterial species being identified in children. Our report provides a deeper understanding of NTM features and characteristics in an inpatient, predominantly immunocompromised, population, and patients by and large do well as reflected in our 80% survival rate. The predominance of M. chelonae and M. abscessus in our study reflects changing environmental exposures with public health implications. US regulatory changes to lower disinfectant byproducts in drinking water has impacted chlorination methods and the subsequent microbiological quality of municipal water sources, and led to increasing dominance and distribution of mycobacteria in water distribution systems [3]. These changes suggest that mycobacteria levels will continue be detectable in the environment, and that NTM in children will continue to be a concern.
ACKNOWLEDGEMENTS
We thank the Infectious Diseases Diagnostic Laboratory at University of Minnesota Medical Center, Fairview, for their assistance with processing isolates.
Research reported in this publication was supported by the National Center for Advancing Translational Sciences of the National Institutes of Health Award Number UL1TR000114.
DECLARATION OF INTEREST
None.
REFERENCES
- 1.Bar-On O, et al. Increasing nontuberculous mycobacteria infection in cystic fibrosis. Journal of Cystic Fibrosis 2015; 14: 53–62. [DOI] [PubMed] [Google Scholar]
- 2.Griffith DE, et al. An official ATS/IDSA statement: diagnosis, treatment, and prevention of nontuberculous mycobacterial diseases. American Journal of Respiratory and Critical Care Medicine 2007; 175: 367–416. [DOI] [PubMed] [Google Scholar]
- 3.Pryor M, et al. Investigation of opportunistic pathogens in municipal drinking water under different supply and treatment regimes. Water Science and Technology 2004; 50: 83–90. [PubMed] [Google Scholar]
- 4.Iroh Tam PY, et al. Rapidly growing mycobacteria among pediatric hematopoietic cell transplant patients traced to the hospital water supply. Pediatric Infectious Disease Journal 2014; 33: 1043–1046. [DOI] [PubMed] [Google Scholar]
- 5.Thomson R, et al. Mycobacterium abscessus isolated from municipal water – a potential source of human infection. BMC Infectious Diseases 2013; 13: 241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Aitken ML, et al. Respiratory outbreak of Mycobacterium abscessus subspecies massiliense in a lung transplant and cystic fibrosis center. American Journal of Respiratory and Critical Care Medicine 2012; 185: 231–232. [DOI] [PubMed] [Google Scholar]
- 7.Ricketts WM, O'Shaughnessy TC, van Ingen J. Human-to-human transmission of Mycobacterium kansasii or victims of a shared source? European Respiratory Journal 2014; 44: 1085–1087. [DOI] [PubMed] [Google Scholar]
- 8.Anon. Tattoo-associated nontuberculous mycobacterial skin infections – multiple states, 2011–2012. Morbidity and Mortality Weekly Report 2012; 61: 653–656. [PubMed] [Google Scholar]
- 9.Duarte RS, et al. Epidemic of postsurgical infections caused by Mycobacterium massiliense. Journal of Clinical Microbiology 2009; 47: 2149–2155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Galil K, et al. Abscesses due to Mycobacterium abscessus linked to injection of unapproved alternative medication. Emerging Infectious Diseases 1999; 5: 681–687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Leao SC, et al. Epidemic of surgical-site infections by a single clone of rapidly growing mycobacteria in Brazil. Future Microbiology 2010; 5: 971–980. [DOI] [PubMed] [Google Scholar]
- 12.Haverkamp MH, van de Vosse E, van Dissel JT. Nontuberculous mycobacterial infections in children with inborn errors of the immune system. Journal of Infection 2014; 68 (Suppl. 1): S134–150. [DOI] [PubMed] [Google Scholar]
- 13.Longworth SA, et al. Risk factors for nontuberculous mycobacterial infections in solid organ transplant recipients: a case-control study. Transplant Infectious Disease 2014; 16: 76–83. [DOI] [PubMed] [Google Scholar]
- 14.Kline S, et al. An outbreak of bacteremias associated with Mycobacterium mucogenicum in a hospital water supply. Infection Control and Hospital Epidemiology 2004; 25: 1042–1049. [DOI] [PubMed] [Google Scholar]
- 15.Horsburgh CR Jr., Selik RM. The epidemiology of disseminated nontuberculous mycobacterial infection in the acquired immunodeficiency syndrome (AIDS). American Review of Respiratory Disease 1989; 139: 4–7. [DOI] [PubMed] [Google Scholar]
- 16.Hatzenbuehler LA, Starke JR. Common presentations of nontuberculous mycobacterial infections. Pediatric Infectious Disease Journal 2014; 33: 89–91. [DOI] [PubMed] [Google Scholar]
- 17.Uchiya K, et al. Comparative genome analysis of Mycobacterium avium revealed genetic diversity in strains that cause pulmonary and disseminated disease. PLoS ONE 2013; 8: e71831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Sassi M, Drancourt M. Genome analysis reveals three genomospecies in Mycobacterium abscessus. BMC Genomics 2014; 15: 359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.El Helou G, et al. Rapidly growing mycobacterial bloodstream infections. Lancet Infectious Diseases 2013; 13: 166–174. [DOI] [PubMed] [Google Scholar]
- 20.Wallace RJ Jr., et al. Absence of Mycobacterium intracellulare and presence of Mycobacterium chimaera in household water and biofilm samples of patients in the United States with Mycobacterium avium complex respiratory disease. Journal of Clinical Microbiology 2013; 51: 1747–1752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kulka K, Hatfull G, Ojha AK. Growth of Mycobacterium tuberculosis biofilms. Journal of Visualized Experiments 2012; 60: e3820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Leung JM, Olivier KN. Nontuberculous mycobacteria: the changing epidemiology and treatment challenges in cystic fibrosis. Current Opinion in Pulmonary Medicine 2013; 19: 662–669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Johnson MM, Odell JA. Nontuberculous mycobacterial pulmonary infections. Journal of Thoracic Disease 2014; 6: 210–220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Griffith DE. Therapy of nontuberculous mycobacterial disease. Current Opinion in Infectious Diseases 2007; 20: 198–203. [DOI] [PubMed] [Google Scholar]
- 25.Sanguinetti M, et al. Fatal pulmonary infection due to multidrug-resistant Mycobacterium abscessus in a patient with cystic fibrosis. Journal of Clinical Microbiology 2001; 39: 816–819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Mussaffi H, et al. Nontuberculous mycobacteria in cystic fibrosis associated with allergic bronchopulmonary aspergillosis and steroid therapy. European Respiratory Journal 2005; 25: 324–328. [DOI] [PubMed] [Google Scholar]
- 27.Renvoise A, et al. Significant difference in drug susceptibility distribution between Mycobacterium avium and Mycobacterium intracellulare. Journal of Clinical Microbiology 2014; 52: 4439–4440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Haverkamp MH, et al. Nontuberculous mycobacterial infection in children: a 2-year prospective surveillance study in the Netherlands. Clinical Infectious Diseases 2004; 39: 450–456. [DOI] [PubMed] [Google Scholar]
- 29.Pham-Huy A, et al. Current trends in nontuberculous mycobacteria infections in Canadian children: A pediatric investigators collaborative network on infections in Canada (PICNIC) study. Paediatrics & Child Health 2010; 15: 276–282. [DOI] [PMC free article] [PubMed] [Google Scholar]