SUMMARY
In Taiwan, avian influenza virus (AIV) subtypes H5N2, H6N1 and H7N3 have been identified in domestic poultry, and several strains of these subtypes have become endemic in poultry. To evaluate the potential of avian-to-human transmission due to occupational exposure, an exploratory analysis of AIV antibody status in poultry workers was conducted. We enrolled 670 poultry workers, including 335 live poultry vendors (LPVs), 335 poultry farmers (PFs), and 577 non-poultry workers (NPWs). Serum antibody titres against various subtypes of viruses were analysed and compared. The overall seropositivity rates in LPVs and PFs were 2·99% (10/335) and 1·79% (6/335), respectively, against H5N2; and 0·6% (2/335) and 1·19% (4/335), respectively, for H7N3 virus. Of NPWs, 0·35% (2/577) and 0·17% (1/577) were seropositive for H5N2 and H7N3, respectively. Geographical analysis revealed that poultry workers whose workplaces were near locations where H5N2 outbreaks in poultry have been reported face greater risks of being exposed to viruses that result in elevated H5N2 antibody titres. H6N1 antibodies were detected in only one PF, and no H7N9 antibodies were found in the study subjects. Subclinical infections caused by H5N2, H6N1 and H7N3 viruses were thus identified in poultry workers in Taiwan. Occupational exposure is associated with a high risk of AIV infection, and the seroprevalence of particular avian influenza strains in humans reflects the endemic strains in poultry in this region.
Key words: Avian influenza virus, poultry worker, seroprevalence
INTRODUCTION
Influenza A virus is a highly infectious respiratory pathogen that can infect both humans and animals; it poses a public health threat every year. This virus is a member of the family Orthomyxoviridae and is further classified into subtypes based on characteristics of two surface glycoproteins: haemagglutinin (HA) and neuraminidase (NA). Eighteen HA (H1–H18) and 11 NA (N1–N11) subtypes have been identified that circulate in wild birds and bats [1, 2]. Of these subtypes, only H1N1, H2N2 and H3N2 have been known to establish stable lineages in humans. These subtypes have caused sustained epidemics in human populations since 1918 [3]. In addition, the H5, H6, H7, H9 and H10 subtypes have caused infections in humans since 1959 [4–7]. Sporadic infections resulting from these subtypes have occurred mainly as a result of direct viral transmission from infected birds to humans through direct and indirect contacts [8, 9]. Human-to-human transmission of these influenza viruses of avian origin has rarely occurred.
Investigation of the relationship between poultry exposure and avian influenza infections in human populations is important for understanding possible transmission of the disease at the poultry–human interface. Previous epidemiological and virological reports have proposed that individuals with intense occupational exposure, especially poultry-farm and live-market workers, may be at an increased risk for avian influenza infection because environmental exposure may promote the transmission of avian influenza viruses (AIVs) [10–12]. To date, it remains unclear whether subclinical infections with regionally predominating AIVs have occurred in these high-risk populations through direct or indirect poultry contact. In Taiwan, several subtypes of AIVs, including H5N2, H6N1 and H7N3, have been identified in domestic poultry [13–15]. During the past decade, the H5N2 virus, which has low pathogenicity, has become the predominant infectious agent in chickens. Outbreaks caused by this virus were reported in 2003–2004 and 2008–2014 [16]. The highly pathogenic avian influenza (HPAI) A(H5N2) virus was first isolated in Taiwan in 2012; since then, this virus has caused subsequent outbreaks in several poultry farms [17]. Avian influenza A(H6N1) virus is frequently isolated from Taiwanese layers and broilers. It usually presents as a low pathogenic virus and continuously circulates as an endemic enzootic agent in animals. In 2013, this virus caused the first known human infection in Taiwan [6]. The low pathogenic H7N3 virus caused two outbreaks in domestic duck farms located in southern Taiwan in 2011 [15].
Few seroepidemiological studies of AIVs in high-risk populations in Taiwan have been performed. Recently, a study conducted by the Taiwan Centers for Disease Control (Taiwan CDC) reported that 1·4% of individuals in contact with H5N2-infected chickens were suspected to have been subclinically infected by the virus [18]. This finding emphasises that occupational exposure to infected poultry may pose a high risk of avian influenza infection in human populations. To better understand potential subclinical avian influenza infections in individuals who have frequent contact with poultry in Taiwan, we conducted an exploratory analysis in poultry workers for the presence of antibodies against H5N2, H6N1 and H7N3 viruses, all of which have caused infections in domestic poultry in Taiwan. Because four imported human cases of infection with the influenza A(H7N9) virus were confirmed in Taiwan between March 2013 and April 2014, this virus was also included in the study.
METHODS
Study subjects
A total of 1247 subjects, including 670 poultry workers and 577 non-poultry workers (NPWs), were enrolled in the study. The poultry workers were further sub-classified into 335 live poultry vendors (LPVs) and 335 poultry farmers (PFs); the LPVs and PFs in this study were randomly selected from 1148 live poultry stalls and 11 296 poultry farms to be representative of the regional distribution of LPVs and PFs in 22 cities and counties in Taiwan. The 577 NPWs without a history of poultry vending or farming were selected as control subjects and were chosen to match the poultry workers by sex, age, and workplace for each farm or stall. Written informed consent was obtained from all subjects, and the study was reviewed and approved by the Institutional Review Board of the Taiwan CDC. During the study period from May 2012 to July 2012, participants were interviewed by staff members at Taiwan CDC and local health agencies. A written questionnaire was completed for each participant by one of these staff members to obtain the personal background information, previous poultry exposure histories, and influenza vaccination histories, among other information. In addition, a single whole blood specimen was collected from each subject for antibody measurements.
Viruses for antibody testing
Four AIVs, subtypes of H5N2, H6N1, H7N3 and H7N9, were used as the antigens for the haemagglutination inhibition (HI) test in this study. The A/Taiwan/2/2013(H6N1) and A/Taiwan/1/2013(H7N9) viruses were human strains isolated from clinical specimens of infected patients. The A/chicken/Taiwan/1209/2003(H5N2) and A/duck/Taiwan/A1741/2011(H7N3) viruses were provided by the Taiwan Animal Health Research Institute. All four viruses were propagated in the allantoic cavity of 9-day-old embryonated chicken eggs, according to standard procedures [19]. These viruses were selected for the following reasons. The A/chicken/Taiwan/1209/2003(H5N2) virus was the prototype and representative isolate of the H5N2 viruses circulating in Taiwanese chickens and was antigenically similar to the descendant chicken H5N2 viruses from 2003 to 2012 in Taiwan based on the results of HI tests conducted with ferret antisera (M. C. Cheng, unpublished data). Furthermore, phylogenetic analysis of A/chicken/Taiwan/1209/2003(H5N2) and other chicken H5N2 isolates in Taiwan has also indicated that these viruses grouped together forming two sub-clades [20]. The A/duck/Taiwan/A1741/2011(H7N3) virus was a representative isolate from the two low pathogenic outbreaks in domestic ducks in southern Taiwan. To determine the risks of human infection with the A/Taiwan/2/2013(H6N1)-like and A/Taiwan/1/2013(H7N9)-like viruses before these viruses were first identified, the two human isolates were used to test sera collected in 2012.
Serum specimen processing and HI assay
Whole blood samples were centrifuged at 1000 g for 10 min at 4 °C, and serum specimens were then collected and stored in aliquots at −20 °C. Before antibody measurements, serum specimens were incubated with receptor destroying enzyme (RDE, Denka Seiken, Japan) at a ratio of 1:3 at 37 °C overnight to remove non-specific HA and were then heat inactivated at 56 °C for 30 min. RDE-treated sera were further diluted with PBS to a final dilution of 1:10. The resulting sera were used in the HI assay at Taiwan CDC without prior adsorption with erythrocytes.
The HI assay was used to investigate the existence of specific antibodies against various AIVs in human sera and was performed as previously described [21]. Serial twofold dilutions of RDE-treated sera were prepared in 96-well V-bottom microtitre plates for the analysis of H5N2, H6N1 and H7N9 antibodies and in 96-well U-bottom plates for the analysis of H7N3 antibodies; 25 μl/well of the virus antigens (4 haemagglutination units) were added to their respective wells. After a 60-min incubation period at room temperature, 50 μl of 1% horse (for H5N2 subtype), 0·5% turkey (for H6N1 and H7N9) or 0·75% guinea pig (for H7N3) erythrocytes were added and mixed gently. The plates were incubated at room temperature for 60 min. HI titres were expressed as the reciprocal of the highest dilution of serum that inhibited virus-induced haemagglutination. Sera that tested negative at a dilution of 1:10 were indicated to have a titre of <10. Back titrations were also performed, and titres were only accepted when both replicates yielded matching results. When performing HI assays, human sera that had previously been shown to have elevated titres against H5N2 virus, mouse sera raised against H6N1 virus and ferret sera raised against H7N9 virus were used as positive controls to validate the test procedure. Pre-immune sera collected from naive mice were used as negative controls.
Statistical analysis
Questionnaire data were manually entered in duplicate, and data-entry problems, as well as inconsistencies, were verified. Pearson's χ2 test and Fisher's exact tests were used to compare categorical variables of demographic data. Logistic regression was used to calculate the odds ratio and P value. Statistical significance was considered when a P value of <0·05 was obtained. All tests were performed with SPSS v. 14 (SPSS Inc., USA) and were two-tailed. ArcGIS v. 10.0 software (ESRI, USA) was used to demonstrate the locations (districts/towns/villages) of poultry outbreaks and subjects with elevated antibody titres against AIVs.
RESULTS
Demographics
Detailed demographics of the 1247 study subjects are presented in Table 1. Of the poultry workers, 59% were male and more than 60% were aged ⩾50 years (mean age 52·3 years, range 17–83 years) in LPVs and 54·1 years (range 24–89 years) in PFs. Most of the subjects had worked in the poultry industry for more than 10 years (LPVs 86·6%, PFs 79·4%) and had close contact with poultry every day (LPVs 90·5%, PFs 94·0%). The majority of LPVs and PFs had not received H5N1 and/or seasonal influenza vaccines during the 2 years prior to the specimen collection date. For NPWs, the ages ranged from 21 to 88 years (mean age 53·2 years). Their education level was higher (P < 0·05) than that of poultry workers. More than 50% of the NPWs received seasonal influenza vaccines in 2010 and/or 2011, whereas the H5N1 vaccination coverage was still low. Overall, a higher proportion of PFs did not use personal protective equipment (PPE) compared to LPVs (LPVs: 2·1%, PFs: 12·5%; χ2 = 27·0, P < 0·0001). Of the PPE used, the most common were gloves, boots and masks.
Table 1.
Demographic characteristics of the 1247 study subjects
| Subjects | Live poultry vendors (N = 335) | Poultry farmers (N = 335) | Non-poultry workers (N = 577) | |||
|---|---|---|---|---|---|---|
| N | % | n | % | n | % | |
| Gender | ||||||
| Male | 171 | 51·04 | 226 | 67·46 | 339 | 58·75 |
| Female | 164 | 48·96 | 109 | 32·54 | 238 | 41·25 |
| Age, years | ||||||
| <20 | 1 | 0·30 | 0 | 0·00 | 0 | 0·00 |
| 20–29 | 7 | 2·09 | 7 | 2·09 | 12 | 2·08 |
| 30–39 | 39 | 11·64 | 43 | 12·84 | 60 | 10·40 |
| 40–49 | 82 | 24·48 | 68 | 20·30 | 138 | 23·92 |
| 50–59 | 112 | 33·43 | 99 | 29·55 | 191 | 33·10 |
| ⩾60 | 94 | 28·06 | 118 | 35·22 | 176 | 30·50 |
| Mean | 52·25 | − | 54·11 | − | 53·20 | − |
| Education level | ||||||
| Illiteracy | 24 | 7·16 | 35 | 10·45 | 13 | 2·25 |
| Elementary school | 100 | 29·85 | 99 | 29·55 | 65 | 11·27 |
| Junior high school | 82 | 24·48 | 66 | 19·70 | 49 | 8·49 |
| Senior high school | 112 | 33·43 | 100 | 29·85 | 168 | 29·12 |
| College | 17 | 5·07 | 35 | 10·45 | 282 | 48·87 |
| Years of working | ||||||
| <1 year | 5 | 1·49 | 11 | 3·28 | − | − |
| 1–5 years | 21 | 6·27 | 31 | 9·25 | − | − |
| 6–10 years | 19 | 5·67 | 27 | 8·06 | − | − |
| >10 years | 290 | 86·57 | 266 | 79·40 | − | − |
| Frequency of working | ||||||
| Seldom | 1 | 0·30 | 2 | 0·60 | − | − |
| Once per several month | 1 | 0·30 | 1 | 0·30 | − | − |
| Once per month | 0 | 0·00 | 4 | 1·19 | − | − |
| Once per week | 30 | 8·96 | 13 | 3·88 | − | − |
| Every day | 303 | 90·45 | 315 | 94·03 | − | − |
| Received H5N1 influenza vaccine | ||||||
| Never | 245 | 73·13 | 271 | 80·90 | 524 | 90·81 |
| 1 dose | 34 | 10·15 | 28 | 8·36 | 24 | 4·16 |
| 2 doses | 11 | 3·28 | 15 | 4·48 | 15 | 2·60 |
| >3 doses | 41 | 12·24 | 15 | 4·48 | 12 | 2·08 |
| Uncertain | 4 | 1·19 | 6 | 1·79 | 2 | 0·35 |
| Received seasonal influenza vaccine | ||||||
| 2011 | ||||||
| Yes | 64 | 19·10 | 107 | 31·94 | 291 | 50·43 |
| No | 270 | 80·60 | 225 | 67·16 | 285 | 49·39 |
| Uncertain | 1 | 0·30 | 3 | 0·90 | 1 | 0·17 |
| 2010 | ||||||
| Yes | 69 | 20·60 | 128 | 38·21 | 307 | 53·21 |
| No | 266 | 79·40 | 206 | 61·49 | 269 | 46·62 |
| Uncertain | 0 | 0·00 | 1 | 0·30 | 1 | 0·17 |
| Personal protective equipment use | ||||||
| None | 7 | 2·09 | 42 | 12·54 | − | − |
| Gloves | 251 | 74·93 | 170 | 50·75 | − | − |
| Mask | 111 | 33·13 | 200 | 59·70 | − | − |
| Hair cover | 16 | 4·78 | 44 | 13·13 | − | − |
| Goggle | 7 | 2·09 | 4 | 1·19 | − | − |
| Shoe cover | 33 | 9·85 | 38 | 11·34 | − | − |
| Boots | 301 | 89·85 | 249 | 74·33 | − | − |
| Water-resistant apron | 306 | 91·34 | 100 | 29·85 | − | − |
Seroprevalence of HI antibodies to various AIVs
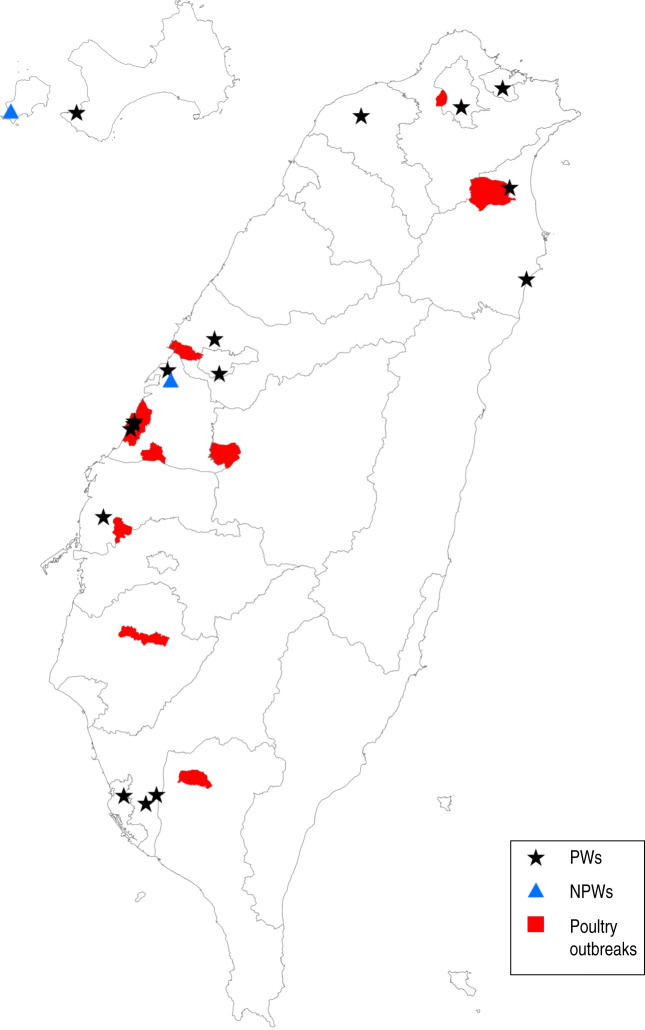
The distribution of HI titres against H5N2, H6N1, H7N3 and H7N9 viruses in all 1247 study subjects is shown in Table 2. Based on the results, poultry workers (LPVs or PFs) have antibody titres against the H5N2 virus (A/chicken/Taiwan/1209/2003) that are significantly higher than those of NPWs (P < 0·001). The overall seropositivity rates in LPVs, PFs and NPWs were 2·99% (10/335), 1·79% (6/335) and 0·35% (2/577), respectively, with a cut-off value of 1:80. Furthermore, geographical analysis revealed that poultry workers whose workplaces (districts/towns/villages) were near locations where H5N2 outbreaks in poultry were reported in 2012 had higher risks of virus exposure resulting in elevated H5N2 antibody titres (Fig. 1) [odds ratio (OR) 5·6, 95% confidence interval (CI) 1·5–20·8, P = 0·028]. Moreover, higher HI antibody titres to H5N2 virus were observed in LPVs (OR 8·85, 95% CI 1·1–67·5, P = 0·005) and PFs (OR 5·24, 95% CI 0·6–45·0, P = 0·043), than in NPWs. These results indicate that the persistently regional circulation of H5N2 viruses in poultry may potentially cause occupational exposure-related subclinical infections in humans. The vaccination histories of seasonal influenza vaccines in 2010 and 2011 in LPVs, PFs and NPWs who had H5N2 antibody titres ⩾1:40 were significantly different (χ2 = 20, P < 0·0001 for received 2010 seasonal influenza vaccine; χ2 = 21·4, P < 0·0001 for received 2011 seasonal influenza vaccine); no difference was observed for histories in the three groups with H5N2 antibody titres = 1:80 (χ2 = 0·4, P = 0·8 for received 2010 seasonal influenza vaccine; χ2 = 1·8, P = 0·4 for received 2011 seasonal influenza vaccine). For subtype H7, seropositive rates of antibody against H7N3 virus (A/duck/Taiwan/A1741/2011) in LPVs, PFs and NPWs were 0·6% (2/335), 1·19% (4/335) and 0·17% (1/577), respectively, with a cut-off value of 1:40. Higher rates of seropositivity were observed in LPVs and PFs compared to NPWs. However, the differences observed were not statistically significant (P = 0·14). None of the 1247 serum specimens were identified as being positive for antibodies against the H7N9 virus (A/Taiwan/1/2013) because they all had titres ⩽1:10. The seropositivity of H6N1 antibodies was also low in both poultry workers and NPWs. There was only one PF in southern Taiwan with an antibody titre of 1:40, while all the other subjects had titres ⩽1:10. Seasonal influenza vaccination histories of LPVs, PFs and NPWs were summarized based on the serological test results (Table 3). The serological test results, occupations, and vaccination histories of individuals with high HI titres against various AIVs are summarized in Table 4.
Table 2.
Distribution of antibody titres against various avian influenza viruses
| Virus antigen | Antibody titre* | No. of serum samples | ||
|---|---|---|---|---|
| Live poultry vendors (N = 335) n (%) | Poultry farmers (N = 335) n (%) | Non-poultry workers (N = 577) n (%) | ||
| H5N2 | <1:10 | 5 (1·49) | 10 (2·99) | 30 (5·20) |
| 1:10 | 23 (6·87) | 36 (10·75) | 100 (17·33) | |
| 1:20 | 170 (50·75) | 169 (50·45) | 296 (51·30) | |
| 1:40 | 127 (37·91) | 114 (34·03) | 149 (25·82) | |
| 1:80 | 10 (2·99) | 6 (1·79) | 2 (0·35) | |
| H7N3 | <1:10 | 307 (91·64) | 318 (94·93) | 551 (95·49) |
| 1:10 | 16 (4·78) | 9 (2·69) | 19 (3·29) | |
| 1:20 | 10 (2·99) | 4 (1·19) | 6 (1·04) | |
| 1:40 | 2 (0·60) | 4 (1·19) | 1 (0·17) | |
| H7N9 | <1:10 | 335 (100·00) | 335 (100·00) | 577 (100·00) |
| 1:10 | 0 | 0 | 0 | |
| H6N1 | <1:10 | 335 (100·00) | 334 (99·70) | 577 (100·00) |
| 1:10 | 0 | 0 | 0 | |
| 1:20 | 0 | 0 | 0 | |
| 1:40 | 0 | 1 (0·30) | 0 | |
The cut-off antibody titre (bold font) of seropositivity was 1:80 for H5N2 and 1:40 for H7N3, H7N9 and H6N1 viruses.
Fig. 1.
Locations of workplaces of poultry workers (PWs) with elevated H5N2 antibody titres and poultry farms where H5N2 outbreaks were reported in 2012. Workplaces (districts/towns/villages) of PWs and non-poultry workers (NPWs) with antibody titres against H5N2 virus ⩾1:80 are indicated by black stars and blue triangles, respectively. Locations of H5N2 outbreaks in poultry that occurred in 2012 in Taiwan are indicated in red.
Table 3.
Seasonal influenza vaccination histories of live poultry vendors, poultry farmers and non-poultry workers based on the serological test results
| Virus antigen | Antibody titre* | Seasonal influenza vaccination history in past 2 years | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Live poultry vendors (N = 335) | Poultry farmers (N = 335) | Non-poultry workers (N = 577) | ||||||||
| 2010 | 2011 | None | 2010 | 2011 | None | 2010 | 2011 | None | ||
| H5N2 | <1:10 | 1 | 1 | 4 | 4 | 4 | 6 | 21 | 20 | 8 |
| 1:10 | 6 | 5 | 16 | 15 | 12 | 20 | 61 | 60 | 32 | |
| 1:20 | 40 | 36 | 124 | 72 | 62 | 93 | 152 | 145 | 125 | |
| 1:40 | 20 | 20 | 102 | 33 | 27 | 76 | 73 | 66 | 70 | |
| 1:80 | 2 | 2 | 8 | 4 | 2 | 2 | 0 | 0 | 2 | |
| H7N3 | <1:10 | 64 | 59 | 231 | 123 | 104 | 185 | 293 | 280 | 225 |
| 1:10 | 2 | 2 | 14 | 4 | 2 | 5 | 9 | 6 | 10 | |
| 1:20 | 2 | 2 | 8 | 0 | 0 | 4 | 5 | 5 | 1 | |
| 1:40 | 1 | 1 | 1 | 1 | 1 | 3 | 0 | 0 | 1 | |
| H7N9 | <1:10 | 69 | 64 | 254 | 128 | 107 | 197 | 307 | 291 | 237 |
| 1:10 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | |
| H6N1 | <1:10 | 69 | 64 | 254 | 128 | 107 | 196 | 307 | 291 | 237 |
| 1:10 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | |
| 1:20 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | |
| 1:40 | 0 | 0 | 0 | 0 | 0 | 1 | 0 | 0 | 0 | |
The cut-off antibody titre (bold font) of seropositivity was 1:80 for H5N2 and 1:40 for H7N3, H7N9 and H6N1 viruses.
Table 4.
Serological test results, occupation and vaccination histories of individuals with high haemagglutination inhibition titres against various avian influenza viruses
| Subject no. | Tested antigens | Vaccination in past 2 years | Occupation | ||||
|---|---|---|---|---|---|---|---|
| H5N2 | H7N3 | H7N9 | H6N1 | H5N1 | Seasonal flu | ||
| 1 | 80 | <10 | <10 | <10 | − | + | Live poultry vendor |
| 2 | 80 | <10 | <10 | <10 | − | − | Poultry farmer |
| 3 | 80 | <10 | <10 | <10 | − | − | Live poultry vendor |
| 4 | 80 | <10 | <10 | <10 | − | − | Poultry farmer |
| 5 | 80 | <10 | <10 | <10 | − | − | Live poultry vendor |
| 6 | 80 | 20 | <10 | <10 | − | − | Live poultry vendor |
| 7 | 80 | <10 | <10 | <10 | − | − | Poultry farmer |
| 8 | 80 | <10 | <10 | <10 | − | − | Poultry farmer |
| 9 | 80 | <10 | <10 | <10 | − | + | Poultry farmer |
| 10 | 80 | <10 | <10 | <10 | + | − | Live poultry vendor |
| 11 | 80 | <10 | <10 | <10 | − | + | Poultry farmer |
| 12 | 80 | <10 | <10 | <10 | − | − | Live poultry vendor |
| 13 | 80 | <10 | <10 | <10 | − | − | Live poultry vendor |
| 14 | 80 | <10 | <10 | <10 | − | + | Live poultry vendor |
| 15 | 80 | <10 | <10 | <10 | − | − | Live poultry vendor |
| 16 | 80 | <10 | <10 | <10 | + | − | Live poultry vendor |
| 17 | 80 | <10 | <10 | <10 | − | − | Non-poultry worker |
| 18 | 80 | <10 | <10 | <10 | − | − | Non-poultry worker |
| 19 | 40 | 40 | <10 | <10 | − | − | Live poultry vendor |
| 20 | 40 | 40 | <10 | <10 | − | − | Poultry farmer |
| 21 | 40 | 40 | <10 | <10 | − | + | Poultry farmer |
| 22 | 40 | 40 | <10 | <10 | − | − | Poultry farmer |
| 23 | 20 | 40 | <10 | <10 | − | + | Live poultry vendor |
| 24 | 40 | 40 | <10 | <10 | − | − | Poultry farmer |
| 25 | 40 | 40 | <10 | <10 | − | − | Poultry farmer |
| 26 | 20 | <10 | <10 | 40 | − | − | Poultry farmer |
DISCUSSION
This study provides evidence that possible subclinical avian influenza infections may have occurred in poultry workers (LPVs or PFs) and that these poultry workers have a higher risk of acquiring infections compared to the general public. The study also demonstrates that infected poultry are the principal source of human exposures to AIVs, as evidenced by the elevated HI antibody titres in poultry workers. The endemicity of various subtypes of viruses in poultry in particular countries/regions may contribute to their ability to infect local residents. Close contact, such as consuming uncooked and infected poultry products, or handling or caring for infected avian species, is considered to be a source for avian influenza infection [9]. It has been reported that 10% of poultry workers were seropositive for H5N1 viruses, and 3·1% of government workers who were involved in the culling of infected poultry also tested positive during the outbreak in Hong Kong [10]. In the 2003 poultry outbreaks that occurred in The Netherlands, 49% of poultry cullers had serological evidence of H7N7 infection [21]. In the USA, 0·8% and 0·3% of agricultural workers experienced a ⩾fourfold rise in antibodies against avian H5N2 and H9N2 viruses, respectively [22]. Another seroprevalence study conducted in veterinarians exposed to birds demonstrated significantly elevated antibody titres against the H5N2, H6N2, and H7N2 AIVs compared to healthy subjects [23]. In Japan, 5% (13/257) of poultry workers living in Ibaraki, where the H5N2 virus was isolated from chickens, had a ⩾fourfold increase in neutralizing antibodies against avian H5N2 viruses [24]. These data consistently show that occupational exposure to infected poultry may serve as a potential transmission route of avian influenza.
In Taiwan, both avian influenza H5N2 and H6N1 viruses have been co-circulating persistently in poultry and have developed into unique and local lineages [14, 20]. However, based on data from the surveillance of AIVs in Taiwan since 1998, only low pathogenic avian influenza (LPAI) H7N3 virus was detected from the two low pathogenic outbreaks in domestic ducks in southern Taiwan [15]. The novel H7N9-like viruses, which have been identified in China since 2013, have not been detected in poultry in Taiwan. The results of the present study suggest that the H5N2 virus is an important zoonotic agent at the chicken–human interface in Taiwan. However, the lower seropositivity observed in LPVs and PFs against H7N3 virus, compared to that of the H5N2 virus, may be related to the endemic nature of H5N2 compared with the limited detection of H7N3 in Taiwanese domestic ducks in 2011. No seroreactivity for antibodies specific to the novel H7N9 virus currently circulating in China was detected in the subjects, which is consistent with the observation that no H7N9 virus has been reported to date in Taiwanese poultry. We were surprised to find that only one subject (LPV) showed seropositivity to the H6N1 virus in the study, as the H6N1 virus is frequently isolated in Taiwanese chickens and has formed a unique lineage [14]. A previous study showed that only two (18·1%, 2/11) volunteers were experimentally infected even when a high infective dose of duck-derived H6N1 virus was used, and none of the volunteers had a detectable antibody response [25]. Moreover, the first human H6N1 virus-infected case had low HI titres (1:80) in convalescent serum [6]. These observations may indicate that the H6N1 virus exhibits poor immunogenicity in human populations, which may explain the low seroprevalence of H6N1 antibodies detected in the present study. However, the responses can be variable as data from another study revealed that HA antibodies were detected in subjects tested against a turkey-origin H6 antigen [26]. Cross-reactive heterosubtypic antibodies elicited from heterologous influenza viruses, such as receiving H5N1 and seasonal influenza vaccines, may confound the interpretation of seropositivity [27, 28]. To evaluate this potential influence, previous influenza vaccination histories, including those of both H5N1 and seasonal influenza vaccines, of all the subjects were reviewed, and a correlation between vaccine administration and serum antibody titres against H5N2 and H7N3 viruses was analysed. No statistical antibody titre differences were observed between vaccinated and non-vaccinated subjects. However, 25·82% of the NPWs had HI titres of 1:40 against H5N2 virus. As these subjects reported no exposure history to poultry in their daily lives, the detected antibody titres were suggested as basal-level titres that may be related to their previous exposure to human seasonal influenza viruses.
HI, neutralization (NT) and the later modified microneutralization (MN) methods are considered to be the most current and commonly used serological assays for antigenic characterization. Due to labour intensity and complex technical requirements, the HI assay has become the most widely used surrogate to screen human sera for antibodies against influenza viruses. Antibody titres obtained from the HI method have been demonstrated to correlate well with those detected by MN in detecting antibodies against human and AIVs [26, 29–32]. Different red blood cells (RBCs) have their own preferences to agglutinate with specific influenza viruses [33]. Turkey, guinea pig and human RBCs were recommended for use in HI tests to detect human antibodies against human influenza viruses. However, for antibodies against avian subtype H5 influenza viruses, several studies have reported that the sensitivity of the HI assay was elevated when horse RBCs were used compared to those of guinea pigs, turkeys, humans, or chickens [26, 34]. Therefore, we used horse RBCs in our HI assay to increase the sensitivity of detection of antibodies against the H5N2 virus, which is also reported to have high agreement and reproducibility for the detection of H5 antibodies [26].
This study has some limitations when interpreting the serological test results from the field studies. First, there was no available previous history of influenza-like illness in the subjects. Hence, the association of seropositivity with clinical symptoms, as well as the severity of clinical illness caused by specific avian influenza infections, remains unknown. Second, influenza vaccination histories of the study subjects were obtained through questionnaires and, thus, may not be accurate. Third, because there were no reliable or referenced cut-off values of seropositivity for different subtypes in previous studies, cut-off values of seropositivity were set at an HI titre of 40 for H6N1, H7N3 and H7N9 subtypes and at 1:80 for H5N2. In conclusion, this study indicates that poultry workers have a higher risk of exposure to AIVs during occupational activities and consistently supports the results reported previously by other studies [10, 21–24]. Therefore, active surveillance for the early detection and intervention of viral infections in live poultry should be conducted continuously. These screenings can improve the control of measures to prevent AIV-induced human illnesses. For poultry workers, especially LPVs, the use of appropriate PPE during their occupational activities is also suggested to mitigate the risk of exposure to AIVs.
ACKNOWLEDGEMENTS
The authors are grateful to the medical officers and colleagues in the Regional Centers of Taiwan CDC who participated in the sample collection and onsite investigation.
This study was financially supported by the Department of Health, Taiwan (DOH101-DC-2013).
DECLARATION OF INTEREST
None.
REFERENCES
- 1.Fouchier RA, et al. Characterization of a novel influenza A virus hemagglutinin subtype (H16) obtained from black-headed gulls. Journal of Virology 2005; 79: 2814–2822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Tong S, et al. New World bats harbor diverse influenza A viruses. PLoS Pathogens 2013; 9: e1003657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Cox NJ, Subbarao K. Global epidemiology of influenza: past and present. Annual Review of Medicine 2000; 51: 407–421. [DOI] [PubMed] [Google Scholar]
- 4.Nicholson KG, Wood JM, Zambon M. Influenza. Lancet 2003; 362: 1733–1745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Claas EC, et al. Human influenza A H5N1 virus related to a highly pathogenic avian influenza virus. Lancet 1998; 351: 472–477. [DOI] [PubMed] [Google Scholar]
- 6.Wei SH, et al. Human infection with avian influenza A H6N1 virus: an epidemiological analysis. Lancet Respiratory Medicine 2013; 1: 771–778. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Chen H, et al. Clinical and epidemiological characteristics of a fatal case of avian influenza A H10N8 virus infection: a descriptive study. Lancet 2014; 383: 714–721. [DOI] [PubMed] [Google Scholar]
- 8.Tweed SA, et al. Human illness from avian influenza H7N3, British Columbia. Emerging Infectious Diseases 2004; 10: 2196–2199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Van Kerkhove MD, et al. Highly pathogenic avian influenza (H5N1): pathways of exposure at the animal-human interface, a systematic review. PLoS ONE 2011; 6: e14582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Bridges CB, et al. Risk of influenza A (H5N1) infection among poultry workers, Hong Kong, 1997–1998. Journal of Infectious Diseases 2002; 185: 1005–1010. [DOI] [PubMed] [Google Scholar]
- 11.Koopmans M, et al. Transmission of H7N7 avian influenza A virus to human beings during a large outbreak in commercial poultry farms in the Netherlands. Lancet 2004; 363: 587–593. [DOI] [PubMed] [Google Scholar]
- 12.Kayali G, et al. Evidence of previous avian influenza infection among US turkey workers. Zoonoses and Public Health 2010; 57: 265–272. [DOI] [PubMed] [Google Scholar]
- 13.Cheng MC, et al. Isolation and characterization of potentially pathogenic H5N2 influenza virus from a chicken in Taiwan in 2008. Avian Diseases 2010; 54: 885–893. [DOI] [PubMed] [Google Scholar]
- 14.Lee MS, et al. Genetic and pathogenic characterization of H6N1 avian influenza viruses isolated in Taiwan between 1972 and 2005. Avian Diseases 2006; 50: 561–571. [DOI] [PubMed] [Google Scholar]
- 15.OIE. Low pathogenic avian influenza (poultry), Chinese Taipei (Follow-up Report 1: 22 April 2011) (http://www.oie.int/wahis_2/temp/reports/en_fup_0000010506_20110422_133554.pdf). Accessed 9 December 2014.
- 16.OIE. Exceptional epidemiological events (http://www.oie.int/wahis_2/public/wahid.php/Countryinformation/Countryreports). Accessed 9 December 2014.
- 17.OIE. Highly pathogenic avian influenza, Chinese Taipei (Follow-up Report 2: 19 March 2012) (http://www.oie.int/wahis_2/temp/reports/en_fup_0000011766_20120319_182044.pdf). Accessed 9 Dec 2014.
- 18.Wu HS, et al. Influenza A(H5N2) virus antibodies in humans after contact with infected poultry, Taiwan, 2012. Emerging Infectious Diseases 2014; 20: 857–860. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.WHO. Manual for the laboratory diagnosis and virological surveillance of influenza (http://whqlibdoc.who.int/publications/2011/9789241548090_eng.pdf). Accessed 24 April 2014.
- 20.Lee CC, et al. Emergence and evolution of avian H5N2 influenza viruses in chickens in Taiwan. Journal of Virology 2014; 88: 5677–5686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Meijer A, et al. Measurement of antibodies to avian influenza virus A(H7N7) in humans by hemagglutination inhibition test. Journal of Virological Methods 2006; 132: 113–120. [DOI] [PubMed] [Google Scholar]
- 22.Gray GC, et al. Evidence for avian influenza A infections among Iowa's agricultural workers. Influenza and Other Respiratory Viruses 2008; 2: 61–69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Myers KP, et al. Infection due to 3 avian influenza subtypes in United States veterinarians. Clinical Infectious Diseases 2007; 45: 4–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Ogata T, et al. Human H5N2 avian influenza infection in Japan and the factors associated with high H5N2-neutralizing antibody titer. Journal of Epidemiology 2008; 18: 160–166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Beare AS, Webster RG. Replication of avian influenza viruses in humans. Archives of Virology 1991; 119: 37–42. [DOI] [PubMed] [Google Scholar]
- 26.Kayali G, et al. Testing human sera for antibodies against avian influenza viruses: horse RBC hemagglutination inhibition vs. microneutralization assays. Journal of Clinical Virology 2008; 43: 73–78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Corti D, et al. Heterosubtypic neutralizing antibodies are produced by individuals immunized with a seasonal influenza vaccine. Journal of Clinical Investigation 2010; 120: 1663–1673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Li GM, et al. Pandemic H1N1 influenza vaccine induces a recall response in humans that favors broadly cross-reactive memory B cells. Proceedings of the National Academy of Sciences USA 2012; 109: 9047–9052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Qi W, et al. Antibodies against H10N8 avian influenza virus among animal workers in Guangdong Province before November 30, 2013, when the first human H10N8 case was recognized. BMC Medicine 2014; 12: 205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Dong L, et al. A combination of serological assays to detect human antibodies to the avian influenza A H7N9 virus. PLoS ONE 2014; 9: e95612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Puzelli S, et al. Serological analysis of serum samples from humans exposed to avian H7 influenza viruses in Italy between 1999 and 2003. Journal of Infectious Diseases 2005; 192: 1318–1322. [DOI] [PubMed] [Google Scholar]
- 32.Di Trani L, et al. Serosurvey against H5 and H7 avian influenza viruses in Italian poultry workers. Avian Diseases 2012; 56: 1068–1071. [DOI] [PubMed] [Google Scholar]
- 33.Ito T, et al. Receptor specificity of influenza A viruses correlates with the agglutination of erythrocytes from different animal species. Virology 1997; 227: 493–499. [DOI] [PubMed] [Google Scholar]
- 34.Stephenson I, et al. Detection of anti-H5 responses in human sera by HI using horse erythrocytes following MF59-adjuvanted influenza A/Duck/Singapore/97 vaccine. Virus Research 2004; 103: 91–95. [DOI] [PubMed] [Google Scholar]