SUMMARY
Rapid and wide dispersal of passengers after flights makes investigation of flight-related outbreaks challenging. An outbreak of Salmonella Heidelberg was identified in a group of Irish travellers returning from Tanzania. Additional international cases sharing the same flight were identified. Our aim was to determine the source and potential vehicles of infection. Case-finding utilized information exchange using experts' communication networks and national surveillance systems. Demographic, clinical and food history information was collected. Twenty-five additional cases were identified from Ireland, The Netherlands, Norway, USA and Canada. We conducted a case-control study which indicated a significant association between illness and consumption of milk tart (OR 10·2) and an egg dish (OR 6) served on-board the flight. No food consumed before the flight was associated with illness. Cases from countries other than Ireland provided supplementary information that facilitated the identification of likely vehicles of infection. Timely, committed international collaboration is vital in such investigations.
Key words: Aircraft, food poisoning, in-flight catering, international travel, Salmonella
INTRODUCTION
Air travel for leisure and business purposes has markedly risen due to economic growth and increased globalization [1, 2]. Over one billion passengers travel by air annually all over the world. Although infrequently reported and difficult to assess accurately, there is a risk of disease transmission to passengers not only before and after the flight but also during [3–5].
In-flight catering is a highly specialized industry and a single large catering operation may produce tens of thousands of in-flight meals each day [6]. High-quality and safe in-flight catering relies on high standards of food preparation and storage; this applies at the airport kitchens (or at subcontractors' facilities), during transportation of food from the ground source to the aircraft and on-board the aircraft [7–10]. This is especially challenging in certain countries, where safeguarding temperature controls may be an issue [11].
The most commonly reported diseases associated with aircraft flights have been attributed to contaminated food [3, 12–15]. In-flight foodborne outbreaks, while uncommon, have been documented in the scientific literature. Salmonella is the most commonly reported foodborne pathogen transmitted on-board commercial flights [3, 16–18].
Salmonella enterica serotype Heidelberg (Salmonella Heidelberg) is an uncommon serovar in Europe. Data reported in The European Surveillance System (TESSy) shows that approximately 180 cases of S. Heidelberg are reported annually from European Union and European Economic Area (EEA)/European Free Trade Association (EFTA) countries. Of these, approximately one third were imported cases. From 2006 to 2010, 29% of imported cases originated in Thailand, 15% in Kenya and 15% in Tanzania [19].
Outbreaks of S. Heidelberg have been frequently reported. Investigations of these outbreaks have identified chicken, turkey, pork, eggs, milk and cheddar cheese as food vehicles associated with illness [20–25].
On 21 July 2011, the Health Protection Surveillance Centre (HPSC) in Ireland was notified of a cluster of salmonellosis cases in a group of travellers returning from Tanzania via a major European airport. We describe an outbreak of S. Heidelberg associated with a commercial flight and the international investigations carried out to determine the source and extent of the outbreak and to identify potential vehicles of infection.
METHODS
Outbreak identification
On 20 July 2011 a case of S. Heidelberg was notified to the Public Health Department in the Northwest (HSE NW) of Ireland. The index case had just returned from Tanzania as part of a group (32 Irish) that had climbed Mount Kilimanjaro (while staying in a hotel complex in Arusha, at the foot of Mount Kilimanjaro) and had flown back from Kilimanjaro, with a stopover in Dar-es-Salaam airport, to a major European airport on 6 July 2011. This case reported that a number of others on the return trip had also become ill with similar symptoms. On 21 July 2011, the HSE NW reported a cluster of six salmonellosis cases to the HPSC. The cluster occurred in the 18 Irish travellers (part of the group of 32) who had returned from their visit to Tanzania. A preliminary investigation carried out on this cohort suggested that either exposure on-board the return flight (from Tanzania to a major European airport) of 6 July 2011 (hereafter referred to as Flight X) or exposure in Tanzania prior to Flight X was responsible for the infection [26].
Case-finding
To raise awareness and to determine whether other countries had observed an increase in reported S. Heidelberg cases, an alert was issued on 22 July 2011 through the European Epidemic Intelligence Information System (EPIS) for the Food and Waterborne Diseases and Zoonoses (FWD) network. The Netherlands and Norway reported that each had identified, through their national surveillance systems, S. Heidelberg cases in groups of tourists who had returned from Tanzania. Following this, Ireland posted an alert through the European Commission Early Warning and Response System (EWRS) and shared pulsed-field gel electrophoresis (PFGE) profiles through EPIS FWD and with the USA and Canada through the Regional Office for Europe of the World Health Organization (WHO-EURO) to determine if related S. Heidelberg cases had been identified elsewhere. Subsequently, both the USA and Canada reported having S. Heidelberg cases with travel history to Tanzania on 6 July 2011 and a flight itinerary via the same European airport.
Case definition and epidemiological studies
The primary objective of our study was to determine where the exposure to infection occurred (prior to boarding or on-board Flight X) and the exposure vehicle. A descriptive epidemiological study was conducted using a sensitive (broad) case definition. We described cases by time, place and person.
The sensitive case definition used in the descriptive study defined a confirmed case as a person with laboratory-confirmed infection with S. Heidelberg and having diarrhoea during or after travel to Tanzania in July 2011. A probable case was a person with laboratory-confirmed infection with Salmonella spp. and having diarrhoea associated with travel to Tanzania in July 2011. A possible case was a person having diarrhoea associated with travel to Tanzania in July 2011 and an epidemiological link to a confirmed or probable case.
As further information became available from the results of the descriptive study, a specific (narrower) case definition was used for a subsequent analytical study.
A case-control study was performed involving 37 subjects (17 Irish, 12 Dutch, four Norwegian, three USA, one Canadian) identified as having been on-board the homeward Flight X. Of these 37, 22 were cases and 15 controls. Controls were identified as being travel companions of cases, and represented a convenience sample.
For the case-control study a confirmed case was defined as a person with laboratory-confirmed infection with S. Heidelberg demonstrating the XbaI JF6X01·0583 PFGE pattern and having diarrhoea with date of onset on or after 6 July 2011 and having been on-board Flight X. A probable case was a person with laboratory-confirmed infection with Salmonella spp., having diarrhoea with date of onset on or after 6 July 2011 and having been on-board Flight X. A possible case was a person with diarrhoea with date of onset on or after 6 July 2011, having been on-board Flight X and being a travel companion of a known case.
International coordination
In keeping with accepted practices in Europe where international outbreaks are investigated by member states and the responsibility of investigation rests with the national public health authority of the country that first identifies an outbreak, HPSC convened and chaired the International Outbreak Control Team (IOCT), while ECDC provided coordination and liaison, and facilitated international information exchange.
Data collection
A common questionnaire with images of the meals served on-board Flight X was developed to gather demographic, clinical and exposure information including travel and food history details. Food history was obtained for the 3 days prior to Flight X to explore potential previous exposures. In Ireland, the USA and Canada, questionnaires were administered by telephone; in The Netherlands and Norway these were completed electronically.
Questionnaires were given to 25 cases detected by national surveillance systems with travel history to Tanzania in July 2011 and, for the purpose of the analytical study, to 15 co-travellers identified as being part of the Irish and Dutch travel groups.
Microbiological investigation
All Salmonella isolates from the European cases were referred to the Irish National Salmonella, Shigella and Listeria Reference Laboratory (NSSLRL) for confirmation of serotype and molecular subtyping (PFGE by PulseNet protocol with XbaI). Canadian and USA isolates were typed at their National Reference Laboratories, respectively, using equivalent PulseNet protocols. PFGE profiles were subsequently compared with those from Ireland. Patterns with no discernible differences were considered indistinguishable.
Statistical analyses
We examined the association between exposure variables and illness using univariate and multivariate analyses. Exposures to food/drink associated with S. Heidelberg illness was explored and odd ratios (ORs), P values and 95% confidence intervals (CIs) were calculated. Age, sex and variables with a P value <0·2 in the univariate analysis were included in the multivariate model. SPSS package version 16.0 (SPSS Inc., USA) was used for the analyses.
RESULTS
Case-finding
In total 25 cases were identified by national surveillance systems; ten from the preliminary study conducted on the Irish cohort [26] and 15 additional cases from international case-finding. In total, 10 cases were from Ireland, five from The Netherlands, four from Norway, four from the USA and two from Canada.
Descriptive study
Twenty-five cases fitted the sensitive case definition, of whom 18 (72%) were confirmed, one (4%) was a probable and six (24%) were possible cases. The ages of the cases ranged from 15 to 76 years (median age 24 years). All 25 cases had travel history to Tanzania in June–July 2011, but itineraries differed between the travel groups. Most had travelled within Tanzania and climbed Mount Kilimanjaro but with different tour organizers and at different times (Table 1). In addition, places visited and hence places where food was consumed prior to the return flight differed considerably in the travel groups.
Table 1.
Number and percentages of confirmed and probable/possible cases by accommodation site in Tanzania and having been on-board the flight of 6 July 2011 (n = 25)
| On-board flight/accommodation site in Tanzania | Confirmed (N = 18) | Probable/possible (N = 7) | Total | (%) | ||
|---|---|---|---|---|---|---|
| n | (%) | n | (%) | |||
| Flight X | ||||||
| Yes | 17 | (94) | 7 | (100) | 24 | (96) |
| No | 1 | (6) | 0 | (0) | 1 | (4) |
| Lodge Hotel A (Arusha) | ||||||
| Yes | 7 | (39) | 3 | (43) | 10 | (40) |
| No | 11 | (61) | 4 | (57) | 15 | (60) |
| Lodge Hotel B (Arusha) | ||||||
| Yes | 2 | (11) | 3 | (43) | 5 | (20) |
| No | 16 | (89) | 4 | (57) | 20 | (80) |
| Hotel C | ||||||
| Yes | 3 | (17) | 1 | (14) | 4 | (16) |
| No | 15 | (83) | 6 | (86) | 21 | (84) |
| Others (hotels, private houses) | ||||||
| Yes | 6 | (33) | 0 | (0) | 6 | (24) |
| No | 12 | (67) | 7 | (100) | 19 | (76) |
All but one case departed from Tanzania on Flight X. This person flew on a different date (12 June 2011) through another country and used another airline carrier. Of the 24 who did take Flight X, 23 (96%) boarded at Kilimanjaro and case no. 24 joined Flight X on its final stopover in Dar-es-Salaam.
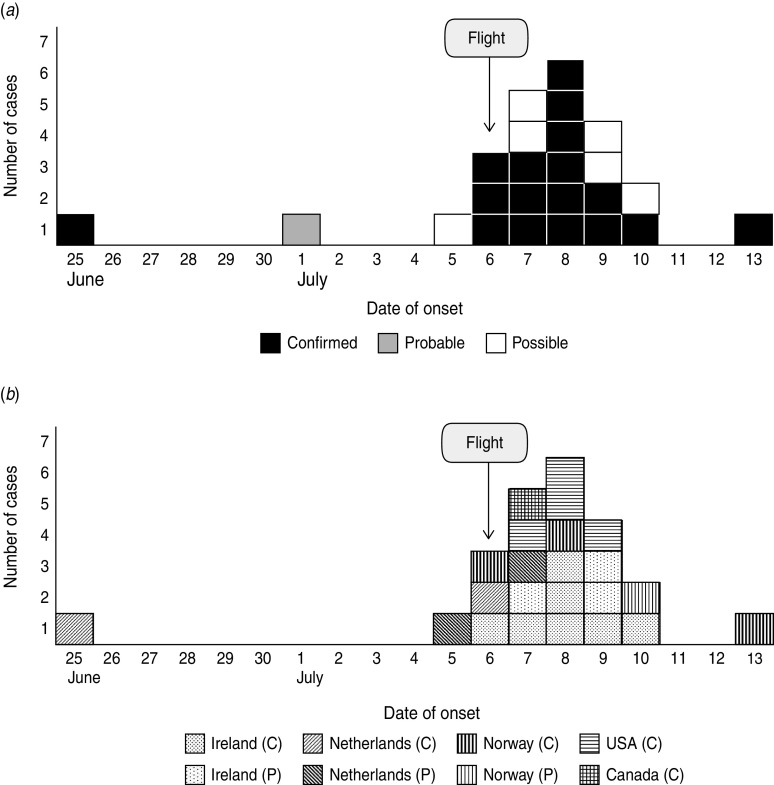
Illness onset dates ranged from 25 June to 13 July 2011 with a peak on 8 July. The confirmed cases that had onset of symptoms before boarding Flight X reported a range of mild symptoms on 25 June; however, one case developed fever and salmonellosis symptoms on 9 July (Fig. 1a). For the case that did not travel on Flight X, the date of onset was unclear. The epidemic curve for confirmed cases by country of destination is presented in Figure 1b.
Fig. 1.
(a) Cases of Salmonella Heidelberg with epidemiological link, by onset of symptoms and case definition, for travellers to Tanzania in June–July 2011 (n = 24). (b) Confirmed (C) and possible (P) cases of S. Heidelberg, by onset of symptoms and country of residence, for travellers to Tanzania in July 2011 (n = 23).
Although the exact time of onset of symptoms was not collected in the questionnaire, knowing the on-board meal serving time, we can infer that the incubation period ranged from about 6 to 108 hours (7 days).
Duration of symptoms ranged from 4 to 14 days (median 9 days). Main symptoms included: diarrhoea (91·3%), abdominal pain (78·3%), fever (69·6%), nausea (47·8%), vomiting (17·4%) and bloody diarrhoea (8·7%). Five cases were hospitalized (two Irish, two Norwegian, one USA). The length of stay in hospital ranged from 2 to 8 days (median 3 days). There were no deaths in the cases.
Analytical study
Based on the descriptive study, it appeared that infection was most likely to be associated with Flight X. Twenty-four out of 25 cases fitted the specific case definition, 21 (88%) of whom had symptom onset dates consistent with exposure on-board Flight X. Of the three cases with earlier onset dates, one had a plausible later onset date of 9 July, bringing to 22 (92%) the number that could be explained by Flight X.
We undertook an analytical study to investigate possible exposures on-board the flight and prior to departure. We included the 22 cases that could be explained by Flight X and 15 controls (asymptomatic co-travellers) on-board Flight X were included in the case-control study (n = 37). Of these cases, 17 were confirmed, one probable, four possible and 15 were controls (the only controls identified were from Ireland and The Netherlands).
In univariate analysis, we calculated the strength of association between illness and exposure to food and drink items consumed at Kilimanjaro airport, brought on-board or served during the flight; these are shown in Table 2.
Table 2.
Univariate analysis of exposures to food/drink associated with Salmonella Heidelberg illness for confirmed and possible cases (crude odd ratios, confidence intervals and P values)
| Exposure | Cases (N = 22)* | Controls (N = 15)* | Crude OR | 95% CI | P | ||
|---|---|---|---|---|---|---|---|
| n | (%) | n | (%) | ||||
| First meal | 21 | (100) | 12 | (92·3) | ∞ | n.a. | 0·20 |
| Beef with rice | 10 | (55·6) | 4 | (28·6) | 3·13 | (0·71–13·81) | 0·27 |
| Chicken with rice | 10 | (55·6) | 7 | (50·0) | 1·25 | (0·31–5·07) | 0·76 |
| Dessert (milk tart) | 17 | (85·0) | 5 | (35·7) | 10·20 | (1·97–52·78) | <0·01 |
| Roll/bread | 17 | (85·0) | 12 | (80·0) | 1·42 | (0·24–8·26) | 0·70 |
| Butter | 15 | (71·4) | 8 | (53·3) | 2·10 | (0·55–8·76) | 0·27 |
| Cheese | 12 | (60·0) | 6 | (40·0) | 2·25 | (0·57–8·82) | 0·24 |
| Laughing cow | 12 | (57·1) | 5 | (35·7) | 2·40 | (0·60–9·67) | 0·21 |
| Crackers | 15 | (75·0) | 9 | (60·0) | 2·00 | (0·47–8·49) | 0·34 |
| Potato salad | 16 | (84·2) | 8 | (57·1) | 4·00 | (0·79–20·32) | 0·08 |
| Water from cooler | 10 | (66·7) | 5 | (38·5) | 3·20 | (0·68–15·07) | 0·14 |
| Breakfast | 20 | (90·9) | 10 | (76·9) | 3·00 | (0·43–20·95) | 0·25 |
| Musli | 13 | (65·0) | 8 | (53·3) | 1·63 | (0·41–6·39) | 0·49 |
| Fruit | 15 | (71·5) | 11 | (73·3) | 0·91 | (0·26–4·01) | 0·90 |
| Egg dish | 18 | (81·8) | 6 | (42·9) | 5·99 | (1·32–27·29) | <0·01 |
| Bread | 17 | (77·3) | 10 | (66·7) | 1·70 | (0·39–7·36) | 0·48 |
| Snacks on flight | 7 | (31·8) | 4 | (26·7) | 1·30 | (0·30–5·49) | 0·74 |
| Cashew nuts | 2 | (10·5) | 1 | (11·1) | 0·94 | (0·07–11·97) | 0·96 |
| Jellies | 4 | (22·2) | 2 | (25·0) | 0·86 | (0·12–6·01) | 0·88 |
| Eat at the airport | 3 | (13·6) | 2 | (13·3) | 1·60 | (0·14–7·02) | 0·98 |
| Packaged food | 2 | (66·7) | 1 | (33·3) | 1·17 | (0·09–15·32) | 0·91 |
OR, Odds ratio; CI, confidence interval; n.a., not applicable.
For several variables answers were not available for all participants (denominators in percentages may vary).
Consumption of food at Kilimanjaro airport prior to taking Flight X was explored. Of the 21 cases that boarded in Kilimanjaro airport (case no. 22 boarded in Dar-es-Salaam airport), 16 individuals did not consume any food at the airport while five did. Food items consumed included: pre-packaged cashew nuts and commercial jellied sweets.
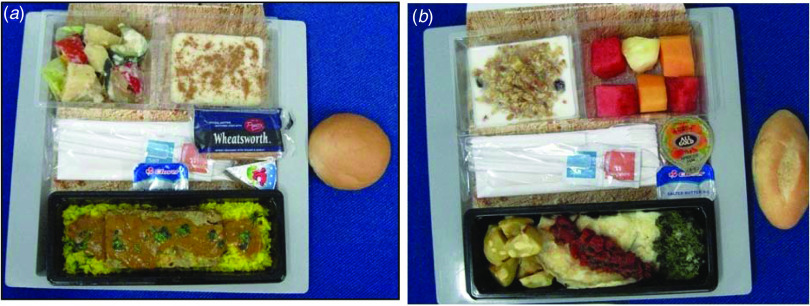
Two meals were served on Flight X; a supper with ten food items and a breakfast with four food items (Fig. 2). Exposure to milk tart served on-board Flight X as dessert during supper and an egg dish served later as part of the breakfast were significantly associated with illness (P < 0·01), (Table 2). The milk tart was more strongly associated with illness (OR 10·2, P < 0·01). No other food items served on-board or consumed at the airport, were significantly associated with illness.
Fig. 2.
[colour online]. (a) Evening meal: beef boboti or chicken pasta along with a salad, rolls, butter, cheese, and milk tart. (b) Breakfast: fruit salad, muesli and an egg dish (omelette) with crackers and bread roll.
When the same analysis was conducted with only confirmed cases (n = 32), consumption of the milk tart remained significantly associated with illness (OR 7·2, P = 0·02) while the egg dish became of equivocal significance (OR 4·3, P = 0·055).
A multivariate analysis was conducted using a logistic regression model. After adjusting for age, sex and other significant exposures, the milk tart (OR 12·1, P = 0·04) and the egg dish (OR 33·9, P = 0·02) remained significantly associated with illness; the egg dish being this time more strongly associated.
Laboratory investigation: molecular typing
PFGE profiling of S. Heidelberg isolates from all Irish, Dutch and Norwegian cases was performed by NSSLRL and all were indistinguishable. This PFGE pattern was previously identified in PulseNet USA and designated as ‘XbaI JF6X01·0583’. The four US cases and one of the Canadian cases were identified by USA CDC and Public Health Agency of Canada by searching in their own PFGE databases for the indistinguishable PFGE profile isolates from the European cases.
Actions taken
All unpackaged food served on the flight was locally produced in Tanzania by an in-flight catering services provider. This caterer was based in Dar-es-Salaam. The caterer was informed about the outbreak and the aircraft company requested time and temperature checks, microbiological analysis of base ingredients and final products from the implicated flight and from food available at the time of inspection. However, no food samples from Flight X were available for analysis.
As part of the internal in-flight food-hygiene quality-assurance programme, the carrier airline undertook the following actions: (a) records were requested from the local caterer in Dar-es-Salaam and these showed that the records for a range of processes encompassing food storage, temperature control, use-by date information, plating times and thermometer calibration were satisfactory; (b) laboratory testing of raw source materials and menu items was undertaken and results were all satisfactory and (c) suspected menu items as indicated by our analytical study were removed from subsequent menus.
DISCUSSION
Outbreaks resulting from exposures during air travel are particularly difficult to differentiate from illnesses attributable to pre-flight exposure [27]. In most instances the incubation period after an in-flight exposure exceeds the flight time, illness usually occurring after passengers have dispersed to different destinations. As a result, difficulties in recognition of outbreaks and identification of epidemiological links between cases arise, making investigation of such outbreaks especially challenging [3, 6, 11–14, 27].
In this paper, we describe an outbreak of S. Heidelberg infection affecting 24 persons of multiple nationalities; the primary objective of our investigation was to determine whether this outbreak was associated with some event on the ground in Tanzania or during Flight X.
The average incubation period for most Salmonella infections range from 6 to 72 h (generally 12–36 h) [28], and therefore, the distribution of the epidemic curve in the descriptive study suggests a common exposure around 5 and 6 July 2011. No other S. Heidelberg cases were reported by any countries from earlier or later flights in July 2011 and no signs of continuing exposure or transmission were reported suggesting a point-source outbreak.
The majority of cases can be explained by travelling on-board Flight X (22/25 cases), with no other common exposure identified. Both univariate and multivariate analysis identified a statistically significant association between the consumption of milk tart and/or the egg dish and illness; this held true for both confirmed and all cases. It was not possible to definitely determine if one or both items were responsible for the outbreak. If this had been the case, possible explanations could include: common ingredients, common production source, cross-contamination or residual confounders.
Four of the cases meeting the broad case definition (n = 25) could not be explained by Flight X; one person who flew back home through Zurich with another carrier, one possible and one probable case who were on-board the flight but had symptom onset before the flight. The fourth was the confirmed case who reported a range of mild symptoms on 25 June, but developed fever later, on 9 July, introducing the possibility that their initial symptoms were not in fact due to salmonellosis and that a super-infection on-board may have occurred; bringing to three the number that could not be explained by Flight X.
McMullen et al. [11] described 43 outbreaks of food poisoning associated with aviation travel for the period 1947–2007. Representing 39·5% of all reported outbreaks, Salmonella was the most commonly identified foodborne pathogen transmitted on-board commercial flights, with 15 documented outbreaks (some involving numerous flights), resulting in almost 4000 passengers becoming ill and seven deaths [3, 11–18]. Different serotypes of S. enterica have been reported; however, this is the first time that S. Heidelberg has been implicated in an in-flight food-poisoning outbreak. Of the 43 travel-related food-poisoning outbreaks reported, the most frequently implicated foodstuffs were dessert items (crème anglaise, custard, chocolate éclairs, etc.) (19%), cooked meat (16%) and seafood (14%) [11, 18].
Both milk and eggs have previously been reported as vehicles of transmission in S. Heidelberg outbreaks [22–25, 29]. Moreover, in the case of the egg dish on Flight X, this would be consistent with the anecdotal observations offered by some passengers during interview that the egg dish tasted ‘funny’.
Both of these food items implicated in this outbreak were supplied by a local company in Tanzania; S. Heidelberg is known to be a common serovar in East Africa [20]. Data from previous studies have suggested that contamination with Salmonella was more likely when food was sourced from Africa and Asia [16–18, 28, 30], which may be attributable to high local ambient temperature, suboptimal refrigeration facilities and long-haul flights [11, 16–18].
Moreover, had there been a sizable point source in Tanzania, we might have expected to see significant numbers of S. Heidelberg cases associated with other flights from Tanzania to other European and North American locations, but despite investigations, we could find no evidence of this.
The possibility of exposure on the ground in Tanzania was explored. Although most of the cases climbed Mount Kilimanjaro and stayed in Arusha before the flight, they stayed in different lodges and hotels, making Arusha an unlikely explanation of the source of the infection. Exposures at Kilimanjaro airport were also considered but no meals or unpackaged foods were consumed here. Branded packaged jellies and cashew nuts were bought and consumed at the airport but neither appeared to be significantly associated with illness. Furthermore, one confirmed case boarded Flight X later on in Dar-es-Salaam never having been to Kilimanjaro airport, making the possibility of a common exposure at Kilimanjaro airport less likely.
The possibility of an infectious person spreading the illness to others while on the plane was also considered. However, person-to-person spread of Salmonella in such a setting was not considered to be likely, as it would have required the plane to be heavily contaminated with either human faecal matter or contaminated food to permit the exposure of so many passengers. Transmission following contact with human faecal matter of such an extent to explain 24 cases of human illness would only be likely in the aircraft toilet following heavy soiling and there was no evidence of such soiling on board the aircraft.
Although results of microbiological testing of food samples from later flights taken at the catering company in Tanzania were satisfactory, McMullan et al. [11] noted that samples taken from the catering units at the end of production rather than on-board the aircraft may give an indication of the microbiological quality of the foods at the end of production but not at the time of service. Therefore, conditions, length of time and temperature of storage prior to delivery and service on-board the aircraft may not give a true picture of conditions pertaining during the flight [11, 16–18, 30].
This study emphasized the importance of seeking and identifying international cases when investigating outbreaks where a common exposure is not easy to ascertain. In the Irish study, which included only Irish cases, it was not possible to definitively identify the location of the outbreak as Flight X [26]. However, by including international cases in the investigation, we had a stronger evidence base implicating Flight X. Moreover, with the additional evidence provided by these international cases, we were then able to identify two particular food items that were the likely source of infection. This would not have been possible using only the information obtained from the Irish passengers. However, our study also highlights the difficulties in finding and tracing such cases, these is mainly due to the rapid and wide dispersal of passengers following a flight and the fact that passengers who develop symptoms of food-poisoning may readily attribute these to an alternative exposure on the ground rather than on-board the flight, a consequence of which might be that the attending physician may not suspect an outbreak and thus may not report it as such, making case-finding and traceback more difficult [11]. This highlights the benefits of real-time international collaboration and the utility of experts' communication networks.
Given the low pathogenicity of S. Heidelberg and the low severity of its illness, it was decided early on in the investigation that contact tracing using the passenger manifest would not be of added value and that the resources necessary to do so would outweigh the benefits of implementing it. However, the extent of this outbreak is likely to have been greater than identified, because not all affected persons may have been identified by a physician, or have submitted stools for culture [27, 31]. Case-finding was conducted primarily by means of passive surveillance with a small amount of active case-finding in travel companions of the Irish, Dutch and Norwegian cases, thus potentially weakening our analysis and limiting precision of the estimates in the analytical study. In total, there were 258 passengers on-board Flight X. The proportion of passengers included in the study is 14% (37/258). However, this cannot be considered to be a participation rate.
The high number of controls exposed to the incriminated food items could be explained by universal exposure due to the fact that almost all passengers consumed all food items served on-board Flight X. No dose–response effect could be explored because information required to perform this analysis was not included in the questionnaire. Other studies have shown that during in-flight outbreaks the exposures of cases and controls may be so similar that food-specific ORs may not implicate any particular food item [18].
Because we provided a complete menu for Flight X including photographs of the two main meals, it is unlikely that recall bias would have a large effect, although this remains a possibility as some of the passengers were interviewed up to 3 weeks later.
Although it is the standard policy of this particular carrier to request all their caterers to regularly submit samples of base ingredients and final products for microbiological analysis, these samples are taken on a random basis and no food samples were available from base ingredients and final products used on-board Flight X. Therefore, the only evidence for the association between the foodstuffs with illness was epidemiological.
Finally, this outbreak demonstrated the benefits of identifying international cases and the value of international collaboration and coordination in doing so. Our study highlights the value of rapid, committed, real-time international collaboration and the usefulness of dedicated communication networks of experts such as EPIS, EWRS, and the PulseNet database in such investigations, facilitating the sharing of information and the effective identification of potential outbreak-related cases.
Recommendations
This investigation emphasizes the importance of international collaboration in outbreaks involving holiday destinations, particularly those where pathogens unusual in Europe are common. Collaboration between national public health agencies and laboratories is critical for addressing disease threats effectively. The use of European and international information systems and networks such as EPIS and EWRS can facilitate the sharing of common questionnaires along with the sharing of the results of PFGE and other definitive molecular profiling, making the investigation more efficient and effective. It is essential that such collaboration is maintained and reinforced.
Clinicians who treat patients with gastroenteritis and a history of recent travel should consider including airline meals as possible sources of infection. Travel history and potential in-flight exposures should be routinely gathered and communicated in a timely manner to local public health departments, as this can be extremely useful in identifying outbreaks and determining imported cases, particularly those strains that are uncommon locally.
The availability of the passenger manifest can be extremely valuable for investigating in-flight disease outbreaks where more pathogenic agents might be involved. Active case-finding for all passengers will increase the likelihood of confirming associations between exposure to food and illness, allowing for the identification of potential vehicles of infection and the prevention of future in-flight outbreaks. The ability to easily access such manifests should be explored in the future.
ACKNOWLEDGMENTS
We thank the following for their essential contribution to the investigation of this outbreak: all cases and passengers who answered the questionnaire and provided information. Liz Comerford, Fiona Kenny and staff from HSE West (Northwest). Niall DeLappe and Jean O'Connor from NSSLRL for performing the PFGE on the European strains. Berend van Welzen, Centre for Infectious Disease Control, National Institute for Public Health and the Environment, The Netherlands. Sietze Felix, Schipphol Medical Center, The Netherlands. Jukka Pukkila, Alert and Response of WHO European Region and contact point of WHO AFRO. Pasha Marcynuk from the Centre for Food-borne, Environmental and Zoonotic Infectious Diseases, Public Health Agency of Canada, Alberta Health Services Environmental Public Health and the Government of Alberta's Ministry of Health, Alberta, Canada; Fraser Health Authority, British Columbia, Canada.
DECLARATION OF INTEREST
None.
REFERENCES
- 1.Jeoum NK, Byung ML. Risk factors, health risks, and risk management for aircraft personnel and frequent flyers. Journal of Toxicology and Environmental Health, Part B 2007; 10: 223–234. [DOI] [PubMed] [Google Scholar]
- 2.Royal L, McCoubrey I. International spread of disease by air travel. American Family Physician 1989; 40: 129–136. [PubMed] [Google Scholar]
- 3.Mangili A, Gendreau MA. Transmission of infectious diseases during commercial air travel. Lancet 2005; 365: 989–996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.DeHart RL. Health issues of air travel. Annual Review of Public Health 2003; 24: 133–151. [DOI] [PubMed] [Google Scholar]
- 5.Webster CH. Airline operating realities and the global spread of infectious diseases. Asia Pacific Journal of Public Health 2010; 22 (3 Suppl.): 137S–143S. [DOI] [PubMed] [Google Scholar]
- 6.Sockett P, Ries A, Wieneke AA. Food poisoning associated with in-flight meals. Communicable Disease Report Review 1993; 3: R103–104. [PubMed] [Google Scholar]
- 7.Dal Fabro G. Hygiene in airline catering [in Italian]. Minerva Medica 1977; 68: 4121–4124. [PubMed] [Google Scholar]
- 8.Castellani P, Frugoni G. Hygiene in airline catering [in Italian]. Minerva Medica 1983; 74; 1925–1932. [PubMed] [Google Scholar]
- 9.Beers KN, Mohler SR. Food poisoning as an in-flight safety hazard. Aviation Space and Environmental Medicine 1985; 56: 594–597. [PubMed] [Google Scholar]
- 10.Burslem CD, Kelly MJ, Preston FS. Food poisoning – a major threat to airline operations. Journal of the Society of Occupational Medicine 1990; 40: 97–100 [DOI] [PubMed] [Google Scholar]
- 11.McMullan R, et al. Food-poisoning and commercial air travel. Travel Medicine and Infectious Disease 2007; 5: 276–286. [DOI] [PubMed] [Google Scholar]
- 12.Eberhart-Phillips E et al. An outbreak of cholera from food served on an international aircraft. Epidemiology and Infection 1996; 116: 9–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Eisenberg MS, et al. Staphylococcal food poisoning aboard a commercial aircraft. Lancet 1975; 2: 595–599. [DOI] [PubMed] [Google Scholar]
- 14.Holmes JD, Simmons GC. Gastrointestinal illness associated with a long-haul flight. Epidemiology and Infection 2009; 137: 441–447. [DOI] [PubMed] [Google Scholar]
- 15.Sutton RG. An outbreak of cholera in Australia due to food served in flight on an international aircraft. Journal of Hygiene (London) 1974; 72: 441–451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hatakka M. Salmonella outbreak among railway and airline passengers. Acta Veterinaria Scandinavica 1992; 33: 253–260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hatakka M, Asplund K. The occurrence of Salmonella in airline meals. Acta Veterinaria Scandinavica 1993; 34: 391–396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Tauxe RV, et al. Salmonellosis outbreak on transatlantic flights; foodborne illness on aircraft: 1947–1984. American journal of Epidemiology 1987; 125: 150–157. [DOI] [PubMed] [Google Scholar]
- 19.The European Surveillance System. TESSy data. ECDC (personal communication)
- 20.Layton M, et al. A mixed foodborne outbreak with Salmonella Heidelberg and Campylobacter jejuni in a nursing home. Infection Control and Hospital Epidemiology 1997; 18: 115–121. [DOI] [PubMed] [Google Scholar]
- 21.O'Mahony M, et al. An outbreak of Salmonella heidelberg infection associated with a long incubation period. Journal of Public Health Medicine 1990; 12: 19–21. [DOI] [PubMed] [Google Scholar]
- 22.Centers for Disease Control and Prevention. Salmonella Heidelberg outbreak at a convention – New Mexico. Morbidity and Mortality Weekly Report 1986; 35: 91. [PubMed] [Google Scholar]
- 23.Schonei J, et al. Growth and penetration of Salmonella Enteritidis, Salmonella Heidelberg, and Salmonella Typhimurium in eggs. International Journal of Food Microbiology 1995; 24: 385–396. [DOI] [PubMed] [Google Scholar]
- 24.Thomas W, et al. Egg consumption is the principal risk factor for sporadic Salmonella serotype Heidelberg infections: a case-control study in FoodNet sites. Clinical Infectious Diseases 2004: 38 (Suppl. 3); 238–243. [DOI] [PubMed] [Google Scholar]
- 25.Knox WA, et al. A milk-borne outbreak of food poisoning due to Salmonella Heidelberg. Journal of Hygiene (London) 1963; 61: 175–185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Ryan A, Cullen L. Final report: Outbreak of Salmonella Heidelberg associated with an Irish group expedition to climb Mount Kilimanjaro in July 2011. Health Service Executive North West, Ireland, 2011.
- 27.Gaynor K, et al. International foodborne outbreak of Shigella sonnei infection in airline passengers. Epidemiology and Infection 2009; 137: 335–341. [DOI] [PubMed] [Google Scholar]
- 28.Hennessy TW, et al. Egg consumption is the principal risk factor for sporadic Salmonella serotype Heidelberg infections: a case-control study in FoodNet sites. Clinical Infectious Diseases 2004; 38 (Suppl. 3): S237–243. [DOI] [PubMed] [Google Scholar]
- 29.Miller SI, Pegues DA. Salmonella species, including Salmonella typhi. In: Mandell GL, Bennett JE, Dolin R, eds. Mandell, Douglas, and Bennett's Principles and Practice of Infectious Diseases, 5th edn. Philadelphia: Churchill Livingstone, 2000, pp. 2345–2363. [Google Scholar]
- 30.Hatakka M. Hygienic quality of foods served on aircraft. Thesis of Faculty of Veterinary Medicine, University of Helsinki, 2000. [Google Scholar]
- 31.Hedberg CW, et al. An international foodborne outbreak of shigellosis associated with a commercial airline. Journal of the American Medical Association 1992; 268: 3208–3212. [PubMed] [Google Scholar]